Abstract
Purpose
After neoadjuvant chemoradiation (CXRT) for esophageal cancer, surgery has traditionally been recommended to be performed within 8 weeks. However, surgery is often delayed for various reasons. Data from other cancers suggests that delaying surgery may increase the pathologic complete response rate. However, there are theoretical concerns that waiting longer after radiation may lead to a more difficult operation and more complications. The optimal timing of esophagectomy after CXRT is unknown.
Methods
From a prospective database, we analyzed 266 patients with resected esophageal cancer who were treated with neoadjuvant CXRT from 2002–2008. Salvage resections were excluded from this analysis. We compared patients who had surgery within 8 weeks of CXRT and those who had surgery after 8 weeks. We used multivariable analysis to determine whether increased interval between chemoradiation and surgery was independently associated with perioperative complication, pathologic response, or overall survival.
Results
150 patients were resected within 8 weeks and 116 were resected greater than 8 weeks after completing CXRT. Mean length of operation, intraoperative blood loss, anastomotic leak rate, and perioperative complication rate were similar for the two groups. Pathologic complete response rate and overall survival were also similar for the two groups (p=NS). In multivariable analysis, timing of surgery was not an independent predictor of perioperative complication, pathologic complete response, or overall survival.
Conclusion
The timing of esophagectomy after neoadjuvant CXRT is not associated with perioperative complication, pathologic response, or overall survival. It may be reasonable to delay esophagectomy beyond 8 weeks for patients who have not yet recovered from chemoradiation.
Keywords: Adjuvant/neoadjuvant therapy, Esophageal Cancer, Radiation Therapy, Esophageal Surgery
INTRODUCTION
Successful treatment of locally advanced esophageal cancer remains challenging. Randomized trials have shown that the addition of neoadjuvant chemoradiation (CXRT) leads to a survival benefit compared to surgery alone for the treatment of locally advanced esophageal cancer.[1–3] Most of the clinical trials evaluating neoadjuvant therapy have stipulated that surgery be performed within a defined time-frame after completion of therapy. Traditionally, this has been within 3–8 weeks after completing radiation therapy.[2–7] An extrapolation from this tradition is that surgery is recommended within a pre-defined duration whether patients are on or off clinical protocol. However, for a variety of reasons, many patients do not undergo surgery within that time frame. Some patients have suffered adverse events from medical therapy or simply have not adequately recovered from their neoadjuvant therapy. Other patients have their surgery delayed for personal or logistic reasons.
Radiation-induced tumor necrosis may increase over time and studies of neoadjuvant radiotherapy for rectal cancer suggest that a longer interval between radiation and surgery may actually result in improved pathologic complete response and decreased postoperative morbidity.[8,9] On the other hand, there are theoretical concerns that waiting longer could make the dissection more difficult due to increased radiation fibrosis.[10] There is also a possibility that delaying surgery could allow for tumor regrowth, increasing the risk of recurrence. The optimal timing of surgery after CXRT for esophageal cancer is unknown. We thus decided to analyze patients who underwent esophagectomy after neoadjuvant CXRT at our institution to explore what effect the timing of surgery had on perioperative complications, pathologic response, and long term outcomes.
PATIENTS AND METHODS
We retrospectively analyzed a prospectively maintained database of patients with esophageal cancer who were treated with neoadjuvant CXRT followed by surgery at our institution from 2002–2008. This study was approved by our Institutional Review Board (IRB number). Individual consent was waived due to the retrospective nature of the study. Patients with either squamous cell or adenocarcinoma histology were included. We excluded all emergency cases, redo esophagectomies, patients with distant metastatic disease, and patients with recent metachronous or synchronous primary cancers. Salvage cases were also excluded. Cases were designated as salvage if they underwent planned definitive chemoradiation. Those patients who received neoadjuvant chemoradiation, had a complete clinical response, and chose to undergo surveillance rather than surgery were also considered salvage if they went on to have surgery after evidence of recurrence.
Patients were divided into two groups: those that had surgery within 8 weeks of completing CXRT and those that had surgery greater than 8 weeks after completing CXRT. We compared the rates of major perioperative complications, pathologic response, and overall survival for the two groups. Perioperative death was defined as death occurring during initial hospitalization regardless of length or within 30 days after esophagectomy. We also examined the estimated blood loss, transfusion requirement, and length of operation as surrogate markers for the difficulty of the operation. We then performed a multivariable analysis to determine which factors were independently associated with perioperative complication, pathologic response, and overall survival. Those variables with a significance of <0.25 on univariable analysis were included in the regression. For the multivariable analysis, timing of surgery was examined both as a continuous variable and a dichotomous variable (patients with surgery within 8 weeks of completing CXRT and patients with surgery > 8 weeks after CXRT).
Overall, 100% of patients had endoscopic ultrasound and 100% had PET. Staging was according to the 6th edition AJCC guidelines. Patients who were stage IVa were included in the study. Platinum based chemotherapy was given to 69% of patients. 83% of patients received 45 Gy or a higher dose of radiation.
After completing chemoradiotherapy, all patients were restaged with PET/CT or CT and repeat endoscopy was also performed. Patients were then reassessed by the surgeon.
Surgical technique was determined by tumor location and surgeon preference.
All resected specimens underwent routine histopathology. If no tumor cells were identified in the specimen, including lymph nodes, patients were classified as having a pathologic complete response (pCR). All other patients were classified according to the AJCC guidelines. Patients were followed periodically for at least 5 years or until death. Median potential follow-up was 55 months (14–99 months).
SUBGROUP ANALYSIS
We found that there were more patients with squamous cell histology in the longer interval group. In order to eliminate this potential selection bias, we performed a subgroup analysis using only patients with adenocarcinoma histology.
STATISTICAL ANALYSIS
Survival probability analyses were performed using the Kaplan-Meier method and were calculated from the date of diagnosis to the date of death or most recent follow-up. Statistical significance was assessed by the log-rank test or Mann-Whitney test. Univariate analyses were performed by chi-square analysis. Factors independently associated with perioperative complication and long-term survival were determined by logistic regression or Cox regression analysis. Two-tailed probability values (P values) of .05 or less were considered significant. Data were analyzed by the statistical software SPSS (Chicago, IL).
RESULTS
From 2002–2008, 570 esophagectomies were performed for either squamous cell or adenocarcinoma at our institution. 383 patients received neoadjuvant chemoradiation. 266 patients were identified who met all inclusion criteria. 150 (57%) had surgery within 8 weeks and 116 (43%) had urgery greater than 8 weeks after completing chemoradiotherapy (Figure 1). Among patients in the short interval group, the median interval between completion of radiotherapy and surgery was 46 days (21–56 days). The median interval in the delayed group was 87 days (57–322 days), p < .001.
Figure 1.

Distribution of timing of surgery after CXRT.
The majority of patients (76% in the short interval group and 65% in the delayed group) underwent an Ivor Lewis approach with a separate laparotomy and thoracotomy with intrathoracic anastomosis as shown in Table 1. The remaining patients were split relatively evenly among three-field (9%), transhiatal (10%), and minimally invasive (11%) approaches. 4 patients in each group (3% of total) underwent primary jejunal interposition and were classified as three-field.
Table 1.
Baseline characteristics of patients with ≤ 8 week interval between radiation and surgery and patients with > 8 week interval between radiation and surgery.
| Variable | Interval ≤ 8 weeks N=150 |
Interval > 8 weeks N=116 |
P value |
|---|---|---|---|
| Mean Age | 57 | 60 | 0.03 |
| Male | 135 (90%) | 97 (84%) | 0.14 |
| Caucasian | 132 (88%) | 103 (89%) | 0.85 |
| BMI ≥ 25 | 112 (75%) | 62 (53%) | 0.001 |
| BMI 18.5 – 24.9 | |||
| BMI < 18.5 | |||
| Adenocarcinoma | 145 (97%) | 101 (87%) | 0.003 |
| Lower Third/GE Junction | 145 (97%) | 109 (94%) | 0.38 |
| Zubrod performance status ≥ 2 | 85 (57%) | 77 (66%) | 0.13 |
| Zubrod 1 | |||
| Zubrod 0 | |||
| Coronary Artery Disease | 11 (7%) | 20 (17%) | 0.02 |
| COPD | 6 (4%) | 3 (3%) | 0.74 |
| Weight loss within 3 months | 72 (48%) | 61 (53%) | 0.54 |
| Diabetes | 21 (14%) | 14 (12%) | 0.72 |
| Radiation dose > 45 Gy | 127 (85%) | 92 (82%) | 0.62 |
| Type of Operation | |||
| Ivor Lewis | 114 (76%) | 76 (66%) | 0.28 |
| Transhiatal | 13 (9%) | 14 (12%) | |
| Three-Field | 10 (7%) | 9 (8%) | |
| Minimally Invasive | 13 (9%) | 17 (15%) | |
| Tumor grade | 0.49 | ||
| Well and moderately differentiated | 45 (34%) | 40 (39%) | |
| Poor and undifferentiated | 87 (66%) | 62 (61%) | |
| Clinical Stage | 0.34 | ||
| II | 58 (39%) | 40 (35%) | |
| III | 88 (59%) | 69 (60%) | |
| IVa | 4 (3%) | 7 (6%) |
There was no significant difference between the groups for: alcohol use, smoking history, ASA class, type of chemotherapy received, pulmonary function test (p>.05).
Patients in the delayed group had a higher mean age (60 vs. 57 years), were more likely to have squamous cell histology (13% vs. 3%), and were less likely to be overweight (53% vs. 74%) as shown in Table 1. They also had a higher incidence of coronary artery disease (17% vs 7%, p = .02). Otherwise, there were no statistically significant differences in the baseline characteristics of the two groups of patients.
The operative outcomes for the two groups were similar in terms of average length of operation, the number of lymph nodes removed during the operation, and the mean estimated blood loss, although patients in the delayed group did have a higher rate of intraoperative transfusion (22% vs 13%, p = .05) as shown in Table 2. There were no statistically significant differences in postoperative mortality, pulmonary complication, anastomotic leak, or median length of stay. The overall complication rate was 39%. The anastomotic leak rate was 11% in the short interval group and 16% in the delayed group (p = 0.28). In univariable analysis, increased time to surgery, histology, ASA classification, and recent weight loss were associated with perioperative complication. In multivariable analysis, however, only recent weight loss was found to be associated with complication (p = 0.02) as shown in Table 3.
Table 2.
Postoperative outcomes of patients with ≤ 8 week interval between radiation and surgery and patients with > 8 week interval between radiation and surgery.
| Outcome | Interval ≤ 8 weeks N=150 |
Interval > 8 weeks N=116 |
P value |
|---|---|---|---|
| Operation Characteristics | |||
| Intraoperative Transfusion | 19 (13%) | 26 (22%) | 0.05 |
| Mean Estimated Blood Loss (mL) | 502 ± 53 | 478 ± 29 | 0.44 |
| Mean Surgery Time (minutes) | 390 ± 8 | 398 ± 10 | 0.37 |
| Mean Number of Lymph Nodes Removed | 21 ± 0.8 | 20 ± 0.9 | 0.21 |
| Pathologic Stage | 0.79 | ||
| Path Complete Response | 32 (21%) | 26 (22%) | |
| Stage I | 19 (13%) | 10 (9%) | |
| Stage II | 65 (43%) | 51 (44%) | |
| Stage III | 33 (22%) | 27 (23%) | |
| Stage IV | 1 (1%) | 2 (2%) | |
| Postoperative Complications | |||
| Mortality | 3 (2%) | 4 (3%) | 0.70 |
| Pulmonary Complication | 47 (31%) | 41 (35%) | 0.51 |
| Anastomotic Leak | 17 (11%) | 19 (16%) | 0.28 |
| Median Length of Stay (days) | 11 | 11 | 0.41 |
| Median Survival (months) | 53 [30–77] | 39 [28–50] | 0.23 |
Mean values expressed with ± SEM. Median survival expressed with 95% confidence interval.
Table 3.
Multivariable analysis of independent predictors of: complication, pathologic complete response, and overall survival.
| Endpoint | Independent Predictor | OR/HR | 95% CI | p value |
|---|---|---|---|---|
| Complication | Weight loss | 1.84 | 1.10–3.06 | .02 |
| Path CR | Female Gender | 2.51 | 1.09–5.82 | .03 |
| BMI≥25 | 2.69 | 1.24–5.84 | .01 | |
| Squamous Cell Histology | 4.87 | 1.65–14.34 | .004 | |
| Overall Survival | Age | 1.026 | 1.008–1.045 | .005 |
| Number of Lymph Nodes Positive | 1.115 | 1.049–1.185 | < .001 | |
| Pathologic Stage IV | 5.105 | 1.504–17.32 | .009 |
Path CR = Pathologic complete response. OR = odds ratio, used for complication and Path CR endpoints. HR = hazard ratio, used for overall survival endpoint. CI = confidence interval.
The shorter interval group and the delayed group had similar rates of complete pathologic response (21% vs. 22%) with a similar distribution of pathologic stage (Table 2). The multivariable analysis also failed to demonstrate an association between timing of surgery and pathologic response (Table 3).
Overall survival was similar for the two groups (p = 0.23) as shown in Figure 2. Median survival for the short interval group was 53 months (95% CI 30–77 months) and 39 months for the delayed group (95% CI 28–50 months). Likewise, there was no significant difference in disease free survival for the two groups as shown in Figure 3. On multivariable analysis, age, pathologic stage, and number of positive lymph nodes were significantly associated with survival (p < .05) as shown in Table 3.
Figure 2.
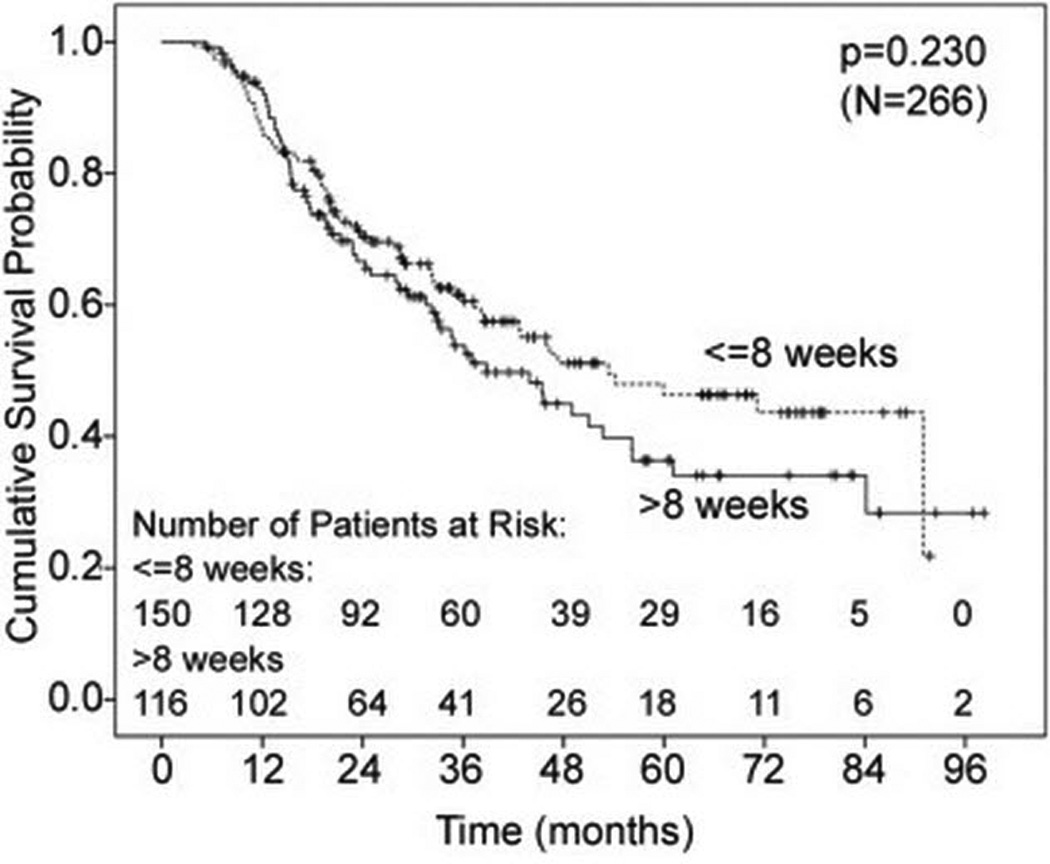
Kaplan-Meier curve for overall survival. p = 0.23.
Figure 3.

Kaplan-Meier curve for disease free survival. p=0.17.
In the subgroup analysis of patients with only adenocarcinoma histology, those patients who had surgery ≤ 8 weeks after CXRT and those who had surgery > 8 weeks after CXRT had similar rates of anastomotic leak (11% vs. 15%, p = 0.44), perioperative complication (35% vs. 41%, p = 0.42), and pathologic complete response (20% vs. 19%, p = 0.76). Median survival was greater for those in the short interval group (53 vs. 36 months), but this difference was not statistically significant (p = 0.21).
In the other subgroup analysis, patients who had surgery ≤ 8 weeks after CXRT were compared to the 41 patients who had surgery > 12 weeks after CXRT. The patients in the two groups had similar rates of perioperative complication (36% vs. 42%, p = 0.59) and pathologic complete response (21% vs. 24%, p = 0.55). The anastomotic leak rate was 11% in the short interval group compared to 17% in the > 12 week group, but this difference was not statistically significant (p = 0.42). The median survival was 53 months in the short interval group compared to 46 months in the > 12 week group, but this also was not statistically significant (p = 0.33).
COMMENT
In this study, a longer interval between neoadjuvant CXRT and surgery was not associated with a difference in postoperative morbidity, pathologic response, or overall survival. By the criteria we measured (length of operation, estimated blood loss, and number of lymph nodes retrieved), a longer interval did not appear to affect the conduct of the operation. The longer interval group did have a higher incidence of intraoperative transfusion, however, this may only reflect the older age or increased incidence of coronary artery disease in this group of patients.
Studies on neoadjuvant chemoradiation for rectal cancer have shown conflicting data about the impact of the interval between chemoradiation and surgery. Whereas some studies have shown no differences in postoperative complications, others have shown increased rates of anastomotic leak with shorter intervals.[8,9,11–16] We did not find a statistically significant difference in anastomotic leak rates between patients with longer and shorter intervals. The overall leak rate of 13% is comparable to other studies of esophagectomy after neoadjuvant chemoradiation.[2,7] The complication rates and postoperative mortality rates were similar for the two groups. The median length of stay (a marker for serious complication) also was similar for the two groups.
Some data from rectal cancer has also shown an increased rate of pCR with a longer interval between chemoradiation and surgery. In contrast, we did not find a difference in the rate of pCR. The overall pCR rate of 21% is comparable with other studies of neoadjuvant chemoradiation and surgery.[17] We did not find that the timing of surgery was associated with a difference in long term survival or disease free survival. The median survival of 46 months compares favorably with other studies of neoadjuvant chemoradiation for esophageal cancer.[17]
There was a significantly higher percentage of patients with squamous cell carcinoma in the delayed group, but eliminating the bias of histology in the subgroup analysis did not reveal any significant differences in outcome.
In summary, our results do not support a longer interval after neoadjuvant chemoradiation for esophageal cancer in order to increase pathologic response. On the other hand, we also did not show any increase in operative morbidity for patients with longer intervals, thus it may be reasonable to delay surgery for patients who have not yet recovered from chemoradiation. These results should be interpreted with caution because we only included patients who underwent resection. This represents only a selected group of patients who received neoadjuvant therapy. We did not include patients who ultimately did not go on to surgery because of metastatic disease, poor performance status, or patient choice. It is certainly possible that some patients with lower performance status were found to have had a complete clinical response on restaging studies and chose not to undergo surgery. We also excluded salvage cases. Data from our experience suggests that salvage operations have a higher morbidity.[18] Including salvage cases would also obviously decrease the rate of pCR in the delayed group.
Another possible weakness in the study is the inherent bias in the selection of patients with delayed surgery. We did not include data on adverse events from CXRT, therefore, a delay in surgery could represent a surrogate endpoint. In addition, small differences in survival would be difficult to detect with this relatively small sample size.
In conclusion, we recommend that surgery be undertaken at the earliest opportunity after adequate recovery from neoadjuvant therapy. But if it is necessary, our data suggests that an additional delay of surgery beyond 8 weeks is not associated with significantly increased perioperative morbidity or mortality. Furthermore, although our data suggests that there is not an increase in pCR with a longer interval to surgery, validation of these findings through a prospective trial may be warranted.
Footnotes
Meeting Presentation: STS Annual Meeting, San Diego, CA. Feb. 1, 2011. Oral slide presentation.
References
- 1.Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol. 2007;8(3):226–234. doi: 10.1016/S1470-2045(07)70039-6. [DOI] [PubMed] [Google Scholar]
- 2.Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26(7):1086–1092. doi: 10.1200/JCO.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335(7):462–467. doi: 10.1056/NEJM199608153350702. [DOI] [PubMed] [Google Scholar]
- 4.Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med. 1997;337(3):161–167. doi: 10.1056/NEJM199707173370304. [DOI] [PubMed] [Google Scholar]
- 5.Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6(9):659–668. doi: 10.1016/S1470-2045(05)70288-6. [DOI] [PubMed] [Google Scholar]
- 6.Nygaard K, Hagen S, Hansen HS, et al. Pre-operative radiotherapy prolongs survival in operable esophageal carcinoma: a randomized, multicenter study of pre-operative radiotherapy and chemotherapy. The second Scandinavian trial in esophageal cancer. World J Surg. 1992;16(6):1104–1109. doi: 10.1007/BF02067069. discussion 1110. [DOI] [PubMed] [Google Scholar]
- 7.Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19(2):305–313. doi: 10.1200/JCO.2001.19.2.305. [DOI] [PubMed] [Google Scholar]
- 8.Francois Y, Nemoz CJ, Baulieux J, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. 1999;17(8):2396. doi: 10.1200/JCO.1999.17.8.2396. [DOI] [PubMed] [Google Scholar]
- 9.Tulchinsky H, Shmueli E, Figer A, et al. An interval >7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Ann Surg Oncol. 2008;15(10):2661–2667. doi: 10.1245/s10434-008-9892-3. [DOI] [PubMed] [Google Scholar]
- 10.Delanian S, Lefaix JL. Current management for late normal tissue injury: radiation-induced fibrosis and necrosis. Semin Radiat Oncol. 2007;17(2):99–107. doi: 10.1016/j.semradonc.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Dolinsky CM, Mahmoud NN, Mick R, et al. Effect of time interval between surgery and preoperative chemoradiotherapy with 5-fluorouracil or 5-fluorouracil and oxaliplatin on outcomes in rectal cancer. J Surg Oncol. 2007;96(3):207–212. doi: 10.1002/jso.20815. [DOI] [PubMed] [Google Scholar]
- 12.Kerr SF, Norton S, Glynne-Jones R. Delaying surgery after neoadjuvant chemoradiotherapy for rectal cancer may reduce postoperative morbidity without compromising prognosis. Br J Surg. 2008;95(12):1534–1540. doi: 10.1002/bjs.6377. [DOI] [PubMed] [Google Scholar]
- 13.Lim SB, Choi HS, Jeong SY, et al. Optimal surgery time after preoperative chemoradiotherapy for locally advanced rectal cancers. Ann Surg. 2008;248(2):243–251. doi: 10.1097/SLA.0b013e31817fc2a0. [DOI] [PubMed] [Google Scholar]
- 14.Moore HG, Gittleman AE, Minsky BD, et al. Rate of pathologic complete response with increased interval between preoperative combined modality therapy and rectal cancer resection. Dis Colon Rectum. 2004;47(3):279–286. doi: 10.1007/s10350-003-0062-1. [DOI] [PubMed] [Google Scholar]
- 15.Pahlman L. Optimal timing of surgery after preoperative chemoradiotherapy for rectal cancer. Nat Clin Pract Oncol. 2009;6(3):128–129. doi: 10.1038/ncponc1325. [DOI] [PubMed] [Google Scholar]
- 16.Tran CL, Udani S, Holt A, et al. Evaluation of safety of increased time interval between chemoradiation and resection for rectal cancer. Am J Surg. 2006;192(6):873–877. doi: 10.1016/j.amjsurg.2006.08.061. [DOI] [PubMed] [Google Scholar]
- 17.Courrech Staal EF, Aleman BM, Boot H, et al. Systematic review of the benefits and risks of neoadjuvant chemoradiation for oesophageal cancer. Br J Surg. 97(10):1482–1496. doi: 10.1002/bjs.7175. [DOI] [PubMed] [Google Scholar]
- 18.Swisher SG, Wynn P, Putnam JB, et al. Salvage esophagectomy for recurrent tumors after definitive chemotherapy and radiotherapy. J Thorac Cardiovasc Surg. 2002;123(1):175–183. doi: 10.1067/mtc.2002.119070. [DOI] [PubMed] [Google Scholar]


