Abstract
α-Scorpion toxins constitute a family of peptide modulators that induce a prolongation of the action potential of excitable cells by inhibiting voltage-gated sodium channel inactivation. Although they all adopt a conserved structural scaffold, the potency and phylogentic preference of these toxins largely vary, which render them an intriguing model for studying evolutionary diversification among family members. Here, we report molecular characterization of a new multigene family of α-toxins comprising 13 members (named MeuNaTxα-1 to MeuNaTxα-13) from the scorpion Mesobuthus eupeus. Of them, five native toxins (MeuNaTxα-1 to -5) were purified to homogeneity from the venom and the solution structure of MeuNaTxα-5 was solved by nuclear magnetic resonance. A systematic functional evaluation of MeuNaTxα-1, -2, -4, and -5 was conducted by two-electrode voltage-clamp recordings on seven cloned mammalian voltage-gated sodium channels (Nav1.2 to Nav1.8) and the insect counterpart DmNav1 expressed in Xenopus oocytes. Results show that all these four peptides slow inactivation of DmNav1 and are inactive on Nav1.8 at micromolar concentrations. However, they exhibit differential specificity for the other six channel isoforms (Nav1.2 to Nav1.7), in which MeuNaTxα-4 shows no activity on these isoforms and thus represents the first Mesobuthus-derived insect-selective α-toxin identified so far with a half maximal effective concentration of 130 ± 2 nm on DmNav1 and a half maximal lethal dose of about 200 pmol g−1 on the insect Musca domestica; MeuNaTxα-2 only affects Nav1.4; MeuNaTxα-1 and MeuNaTxα-5 have a wider range of channel spectrum, the former active on Nav1.2, Nav1.3, Nav1.6, and Nav1.7, whereas the latter acting on Nav1.3–Nav1.7. Remarkably, MeuNaTxα-4 and MeuNaTxα-5 are two nearly identical peptides differing by only one point mutation at site 50 (A50V) but exhibit rather different channel subtype selectivity, highlighting a switch role of this site in altering the target specificity. By the maximum likelihood models of codon substitution, we detected nine positively selected sites (PSSs) that could be involved in functional diversification of Mesobuthus α-toxins. The PSSs include site 50 and other seven sites located in functional surfaces of α-toxins. This work represents the first thorough investigation of evolutionary diversification of α-toxins derived from a specific scorpion lineage from the perspectives of sequence, structure, function, and evolution.
Venomous animals, such as sea anemones, scorpions, spiders, cone snails, jellyfish, and snakes, represent a distinct group of organisms that evolve toxins to help them capture prey and defend themselves from predators (1). These animals occupy diverse ecological niches and have different evolutionary histories and food sources, but they convergently select voltage-gated sodium channels (VGSCs)1 as molecular targets for their toxins that can strongly modify VGSCs' functions to alter electrical excitability of an animal's neuromuscular system and thus cause rapid immobilization of both prey and competitors (2, 3). VGSCs play vital roles in the normal functioning of excitable tissues (neurons, muscles, and heart). These large, complex membrane proteins are composed of one principal α-subunit of 220–260 kDa associated with one or more auxiliary β-subunits of ∼33–36 kDa in some tissues of mammals and the tipE subunit in insects (4–6). The α-subunit of VGSCs includes four homologous domains (DI-DIV) connected by intracellular and extracellular loops, each domain containing six helical transmembrane segments (S1–S6), and a hairpin-like P loop between S5 and S6 that forms the ion selectivity filter. Thus far, at least seven distinct receptor sites have been identified on the α-subunits for various animal-derived toxins that either affects ion permeation or voltage-dependent gating properties (7), of which receptor sites 3, 4, and 6 are targeted by several types of water-soluble polypeptide toxins from scorpions, spiders, sea anemone, and cone snails, whereas site 1 is shared by the peptide toxin μ-conotoxin from cone snails and tetrodotoxin (TTX), a heterocyclic guanidine alkaloid harbored naturally by bacteria and animals of phylogenetically diverse origins (e.g. fish, octopus, and newt) (8, 9).
It is known that Drosophila has a single VGSC α-subunit (DmNav1) encoded by para whereas mammals have at least nine α-subunit isoforms (Nav1.1–Nav1.9) that constitute a single gene family of proteins with more than 70% amino acid sequence identity in their transmembrane segments (4–6). Members in the VGSC family have different tissue distributions and developmental expression patterns (10). For example, Nav1.2 and Nav1.3 are mainly expressed in central nervous system whereas Nav1.7, Nav1.8, and Nav1.9 are specific to peripheral nervous system. Nav1.1 and Nav1.6 expression is common to central nervous system and peripheral nervous system. Nav1.4 is expressed in skeletal muscles whereas Nav1.5 is the main isoform in cardiac muscle. Based on sensitivity to TTX, these channels are distinguished into TTX-sensitive (TTX-S) and TTX-resistant (TTX-R) subtypes (11). The former includes Nav1.1, Nav1.2, Nav1.3, Nav1.4, Nav1.6, and Nav1.7 and the latter consists of Nav1.5, Nav1.8, and Nav1.9. The recognition of these channel subtypes and elucidation of their structure, pharmacological function, and biological role is technically difficult because it requires highly selective molecular probes.
Scorpion toxins specific for VGSCs (NaScTxs) are essential components identified with their functions associated to venom toxicity (12, 13). These molecules comprise a family of evolutionarily conserved gating modulators that are composed of 61–76 residues and are cross-linked by four disulfide bridges (14). NaScTxs belong to the superfamily of cysteine-stabilized α-helical and β-sheet (CSαβ) motif-containing proteins (15) and most of them adopt the same secondary structure topology (βαββ). NaScTxs have been divided into two principle pharmacological types based on their electrophysiological effects and binding characteristics (16, 17). α-Toxins that were exclusively found in Old World scorpions slow the VGSC inactivation to induce prolongation of action potentials by binding to the channel receptor site 3, a key binding regions shared by sea-anemone toxins and some spider toxins. It has been proposed that such binding inhibits the outward movement of the S4 segment of domain IV and thereby prevents conformational changes required for fast inactivation (14). β-Toxins that are mainly derived from New World scorpions enhance the activation of VGSCs by binding to receptor site 4 to shift the voltage dependence of activation to more negative membrane potentials. Despite similar functional properties, α-toxins show remarkable differences in preference for insect and mammalian VGSCs and accordingly they can be further divided into three subtypes: (1) Classical α-toxins that are highly selective on mammals and nontoxic to insects (e.g. AaHII and Lqh2); (2) Insect α-toxins that are weakly toxic on mammals but especially toxic for insects (e.g. LqhαIT and Lqq3); and (3) α-Like toxins that act on both mammalian and insect VGSCs (e.g. BmKM1 and Lqh3) (14, 18). Similarly, β-toxins also include several different pharmacological subtypes with various affinities toward insect or mammalian VGSCs (19), in which depressant and excitatory β-toxins primarily bind to insect sodium channels. The observed phylogenetic preference provides rationality for studying structure-function relationship of NaScTxs and for designing highly selective insecticidal peptides for pest control (20).
Here, we report a systematic investigation of a new multigene family of α-toxins (named MeuNaTxαs) in Mesobuthus eupeus, a sibling species of the Chinese scorpion Mesobuthus martensii (21), through molecular cloning, biochemical purification, nuclear magnetic resonance analysis, functional characterization, and evolutionary study. We found that despite high sequence identity and overall structural similarity, each MeuNaTxα member has different selectivity on various VGSCs, which could be a consequence of positive selection for adaptation of channel alterations during an evolutionary arms race of Mesobuthus and its prey/competitor.
MATERIALS AND METHODS
Isolation of cDNA and Genomic Clones
Total RNA and genomic DNA of M. eupeus were isolated from the venom gland and legs, respectively, according to previously described methods (22). All the primers used here were synthesized by SBS Genetech (Beijing, China) (supplemental Table S1, provided as Electronic Supplementary Material). For 3′ rapid amplification of cDNA ends (3′ RACE) of scorpion α-toxins, total RNA was reverse-transcribed into the first-strand cDNAs using RT-PreMix Kit (SBS Genetech) and a universal oligo(dT)-containing adaptor primer (dT3AP). For 5′ RACE, the first-strand cDNAs were tailed by terminal transferase and dCTP (Takara, Dalian, China). PCR amplification of cDNAs or genomic DNAs was carried out by TaKaRa LA Taq (Takara), a DNA polymerase with 3′→5′ exonuclease proofreading activity, under standard PCR conditions. Primer pairs are provided in supplemental Table S2 and their locations indicated in Fig. 1A. All PCR products were ligated into pGM-T and resultant recombinant plasmids were transformed into Escherichia coli DH5α.
Fig. 1.
The multigene family of α-toxins in M. eupeus. A, Strategy for isolating cDNAs of a complete α-toxin multigene family in M. eupeus by combination of different primers (represented by arrows) whose sequence information and pairs for PCR amplification are provided in supplemental Table S1 and S2. Different regions of the tailed first-strand cDNA encoding an α-toxin are shown in color. UTR, untranslational region. SP, signal peptide. MP, mature peptide; B, Multiple sequence alignment of α-toxins. Cysteines are shadowed in yellow and residues conserved across the alignment in color (Basic: blue; hydrophobic: green; polar: cyan). Extra C-terminal amino acids are underlined once and amidated residues are boldfaced. Secondary structure elements (arrow: β-strand; cylinder: α-helix) and four disulfide bridges (indicated by lines) extracted from the NMR structure of MeuNaTxα-5 are shown at the bottom of the alignment. PSSs predicted from the codon-substitution model are indicated by red arrows. N-turn and four loops (J, M, B, and F) are shown at the top of the alignment. g, m, and p represent genomic, mRNA and protein, respectively. * indicates MeuNaTxα, where native peptides were isolated from the venom.
Recombinant clones were sequenced through the chain termination method using T7 primer. Nucleotide sequences for genes reported here have been deposited in the GenBank database (http://www.ncbi.nlm.nih.gov/) under accession numbers provided in supplemental Table S3.
Biochemical Purification
Purification approaches used have been described previously (23). Briefly, M. eupeus crude venom collected by an electrical stimulation method was suspended in 0.1% trifluoroacetic acid (TFA, v/v) and directly subjected to reversed-phase high-performance liquid chromatography (RP-HPLC) isolation. All well-defined peaks were collected separately and rerun on the same column for further purification. The purity and molecular mass of MeuNaTxαs were determined by MALDI-TOF mass spectra on a Kratos PC Axima CFR plus (Shimadzu Co. LTD, Kyoto). The automated Edman degradation by ABI Procise 492cLC protein sequencer (Shanghai GeneCore BioTechnologies Co., Ltd., Shanghai, China) were used to determine amino-terminal sequences of MeuNaTxα-1, -2, -4 and -5 (Table I).
Table I. Molecular and functional properties of Mesobuthus eupeus α-toxins. —*, C-terminal amidation; TR, retention time; †, determined by MALDI-TOF; ¶, determined by Edman degradation; pI, theoretical isoelectric point; —, not determined.
| TR (min) | Theoretical MW (Da) | Experimental MW (Da)† | N-terminal sequence¶ | pI | |
|---|---|---|---|---|---|
| MeuNaTxα-1* | 38.2 | 7177.12 | 7178.27 | VRDGY | 8.14 |
| MeuNaTxα-2* | 42.2 | 7233.19 | 7233.68 | ARDAYIANDR | 8.19 |
| MeuNaTxα-3 | 43.5 | 8298.31 | 8299.90 | — | 6.27 |
| MeuNaTxα-4 | 44.0 | 7199.27 | 7199.55 | ARDAYI | 8.19 |
| MeuNaTxα-5/5' | 44.4 | 7171.22 | 7170.47 | ARDAYIAKPHNCVYECFDAF | 8.19 |
| MeuNaTxα-6 | 43.5 | 7113.18 | — | — | 8.48 |
| MeuNaTxα-7* | — | 7149.07 | — | — | 8.16 |
| MeuNaTxα-8 | — | 7099.15 | — | — | 8.48 |
| MeuNaTxα-9* | — | 7276.22 | — | — | 8.19 |
| MeuNaTxα-10 | — | 7169.25 | — | — | 8.19 |
| MeuNaTxα-11 | — | 7156.25 | — | — | 8.19 |
| MeuNaTxα-12 | — | 7050.00 | — | — | 8.20 |
| MeuNaTxα-13 | — | 7287.25 | — | — | 6.05 |
NMR Data Acquisition and Analysis
MeuNaTxα-5 was dissolved at a concentration of 1 mm in 50 mm KH2PO4, pH 4.0, 10% (v/v) D2O. The sample was then freeze-dried and dissolved in 100% D2O for measurements of amide protons exchange rate. NMR data were acquired at 298 K employing a Varian Inova spectrometer operating at 600 MHz proton frequency. The TOCSY spectra were recorded with mixing times of 80 and 120 ms, and the NOESY spectra were recorded with mixing times of 100, 150, 200, and 250 ms. All two-dimensional-NMR spectra were collected as 4096 × 512 data point matrices using 64–128 scans. NMR data were processed using vnmr or NMRPipe and a shifted sine window function and zero filling were applied prior to Fourier transformation. Data analysis was performed with Sparky 3 (http://www.cgl.ucsf.edu/home/sparky/).
Structure Calculation
Seven hundred and ten distance and 29 dihedral angle constraints were mainly derived from cross-peaks in NOESY (mix = 200 ms). 3JHN-Ha and 3JHa-Hb coupling constants obtained from DQF-COSY spectra were converted into dihedral angle constraints (24). Structures were generated using XPLOR-NIH (2.19 version), employing simulated annealing algorithms. Twenty-six hydrogen bond constraints were introduced based on the unexchanged backbone amide protons identified in DQF-COSY acquired in D2O and the assigned inerratic NOE pattern belonging to the α-helix and β-sheet conformations. A total of 100 structures were calculated, and a final ensemble of 20 structures was selected without NOE violation > 0.3 Å, on the basis of the lowest XPLOR energies, to represent the final solution structure. Structure statistics for the 25 structures with the lowest energy were summarized in Table II. Structure evaluation was performed with the program PROCHECK-NMR. Three-dimensional conformations were inspected in MOLMOL. The chemical shifts and coordinates of MeuNaTxα-5 are deposited in the Biological Magnetic Resonce Bank (BMRB entry, 17988) and the Protein Data Bank (PDB entry, 2LKB), respectively.
Table II. NMR structural statistics for MeuNaTxα-5 calculation. r.m.s.d., root-mean-square deviation.
| NMR distance and dihedral constraints | |
|---|---|
| Intra-residue | 216 |
| Sequential (|i-j| = 1) | 255 |
| Medium range (1 < |i-j| ≤5) | 122 |
| Long range (|i-j| > 5) | 117 |
| Hydrogen bonds | 26 |
| Total NOE | 710 |
| Dihedral angels | 29 |
| r.m.s.d. versus the mean structure (Å) | |
| All backbone atoms | 1.50 ± 0.20 Å |
| All heavy atoms | 2.11 ± 0.20 Å |
| Backbone atoms (2nd structural region) | 0.52 ± 0.15 Å |
| Heavy atoms (2nd structural region) | 0.92 ± 0.20 Å |
| Structural statistics | |
| r.m.s.d. versus the mean structure (Å) | |
| NOE distances (Å) | 0.034 ± 0.044 |
| Dihedral angles (deg.) | 0.49 ± 0.079 |
| r.m.s.d. from idealized geometry | |
| Bonds (Å ) | 0.0016 ± 0.00011 |
| Angles (deg.) | 0.30 ± 0.014 |
| Impropers (deg.) | 0.26 ± 0.013 |
| Structure Analysis | |
| % of residues in most favored regions | 77.2 |
| % of residues in additionally allowed regions | 16.9 |
| % of residues in generously allowed regions | 5.4 |
| % of residues in disallowed regions | 0.6 |
Expression in Xenopus Oocytes
For the expression in Xenopus oocytes (25), the rNav1.2/pLCT1, rNav1.3/pLCT2, rNav1.4/pUI-2, mNav1.6/pLCT1, and hNav1.8/pBSTA vectors were linearized with NotI. The rNav1.7/pBSTA, and hβ1/pGEM-HE vectors were linearized with SacII and NheI, respectively. These vectors were transcribed with the T7 mMESSAGE-mMACHINE transcription kit (Ambion, Austin, TX). The hNav1.5/pSP64T and rβ1/pSP64T constructs were linearized with XbaI and EcoRI, respectively, and transcribed with the SP6 mMESSAGE-mMACHINE transcription kit (Ambion) (supplemental Table S4). The harvesting of stage V-VI oocytes from anesthetized female Xenopus laevis frog was as described previously. Oocytes were injected with 50 nl of cRNA at a concentration of 1 ng/nl using a micro-injector (Drummond Scientific, Broomall, PA). The oocytes were incubated in a solution containing (in mm): NaCl, 96; KCl, 2; CaCl2, 1.8; MgCl2, 2 and HEPES, 5 (pH 7.4), supplemented with 50 mg/l gentamycin sulfate and 180 mg/l theophylline.
Electrophysiological Recordings
Two-electrode voltage-clamp recordings were performed at room temperature (18–22 °C) using a Geneclamp 500 amplifier (Molecular Devices, Sunnyvale, CA) controlled by a pClamp data acquisition system (Axon Instruments, Union City, CA). Whole cell currents from oocytes were recorded 1–4 days after injection. Bath solution composition was (in mm): NaCl, 96; KCl, 2; CaCl2, 1.8; MgCl2, 2; and HEPES, 5 (pH 7.4). Voltage and current electrodes were filled with 3 m KCl. Resistances of both electrodes were kept at 0.8–1.8 MΩ. The elicited currents were filtered at 1 kHz and sampled at 20 kHz using a four-pole low-pass Bessel filter. Leak subtraction was performed using a –P/4 protocol. In order to avoid overestimation of a potential toxin-induced shift in the current-voltage relationships of inadequate voltage control when measuring large sodium currents in oocytes, only data obtained from cells exhibiting currents with peak amplitude below 2 μA were considered for analysis.
For the electrophysiological analysis, a number of protocols were applied from a holding potential of –90 mV with a start-to-start interval of 0.2 Hz. Sodium current traces were evoked by 100 ms depolariarions to Vmax (the voltage corresponding to maximal sodium current in control conditions). The current-voltage relationships were determined by 50-ms step depolarization between –90 and 70 mV, using 5-mV increments. The sodium conductance (gNa) was calculated from the currents according to Ohm's law: gNa = INa/(V-Vrev), where INa represents the Na+ current peak amplitude at a given test potential V, and Vrev is the reversal potential. The values of gNa were plotted as a function of voltage and fitted using the Boltzmann equation: gNa/gmax = [1 + (exp(Vg-V)/k)]-1, where gmax represents maximal gNa, Vg is the voltage corresponding to half-maximal conductance and k is the slope factor. Toxin-induced effects on the steady-state inactivation were investigated with a standard two-step protocol. In this protocol, 100-ms conditioning 5-mV step prepulses ranging from –90 to 70 mV were followed by a 50-ms test pulse to –30 or –10 mV. Data were normalized to the maximal Na+ current amplitude, plotted against prepulse potential and fitted using the Boltzmann equation: INa/Imax = [(1-C)/(1 + exp((V-Vh)/k))] + C, where Imax is the maximal INa, Vh is the voltage corresponding to half-maximal inactivation, V is the test voltage, k is the slope factor, and C is a constant representing a noninactivating persistent fraction (close to zero in control). The recovery from inactivation was assayed with a double-pulse protocol, where a 100-ms conditioning pulse to –30 or –10 mV was followed by a 50-ms test pulse to the same voltage. Both pulses were interspersed by a repolarization to –90 mV for a gradually increasing time interval (1–40 ms). The INa obtained in the test pulse was normalized to the INa obtained in the conditioning pulse, plotted against the corresponding time interval and fitted with the following exponential equation: f(t) = Ae−t/τ + C, where t represents the time, A is the amplitude of the current, τ is the time constant for the fast inactivation, and C is a constant representing a noninactivating persistent fraction (close to zero in control).
To assess the concentration dependence of the MeuNaTxαs-induced effects, dose-response curves were constructed. To quantify the toxin induced slowing down of inactivation, the relative I30ms/Ipeak values were plotted against the applied concentrations. The data were fitted with the Hill equation: y = 100/[1 + (EC50/[toxin])h], where y is the amplitude of the toxin-induced effect, EC50 is the toxin concentration at half maximal efficacy, [toxin] is the toxin concentration and h is the Hill coefficient. The time constants (τ) of the VGSC fast inactivation were measured directly from the decay phase of the recorded Na+ current using a single exponential fit. Comparison of two sample means was made using a paired Student's t test (p < 0.05). All data are presented as means ± S.E. (S.E.) of at least five independent experiments (n ≥ 5). All data was analyzed by pClamp Clampfit 10.0 (Molecular Devices) and Origin 7.5 software (Originlab, Northampton, MA).
Insect Toxicity Assays
Insect toxicity assays were performed according to the method of Maggio and King (26). Briefly, MeuNaTxα-4 with twofold dilution by insect saline was injected into house flies (Musca domestica TJS, deltamethrin-sensitive strain; and M. domestica BJD, deltamethrin-resistant strain) (27) for toxicity assays. Each fly (about 10 mg) was injected with 1 μl of toxin at a dose of 0.14, 0.28, 0.56, 1.12, 2.25, 4.5, 9, or 18 pmol and the flies injected with 1 μl of insect saline were used as control. Ten flies were used for each toxin dose. Experiments were performed in duplicate. Half maximal lethal dose (LD50) values at 24 h postinjection were calculated from the dose-response data by OriginPro 7.5.
Maximum Likelihood Analysis
The α-toxins used here include 34 sequences: 13 M. eupeus sequences reported here and 21 M. martensii sequences retrieved from the GenBank database (http://www.ncbi.nlm.nih.gov/). Nucleotide sequences of these toxins were aligned according to their amino acid alignment, where sites containing gaps were excluded in this analysis. Aligned nucleotide sequences of Mesobuthus α-toxins for analysis of positive selection are provided in the supplementary Appendix. Ratios of nonsynonymous to synonymous rates of substitution (ω = dN/dS) between pairs of genes were calculated by the method of Nei and Gojobori 1986 (28). A neighbor-joining tree constructed by MEGA 3.1 (http://www.megasoftware.net) was used for further statistical analysis. The CODEML program of the PAML software package (http://abacus.gene.ucl.ac.uk/software/paml.html) (29) was used to estimate ω values, in which four models were used to make two likelihood ratio tests (LRTs) by M1a/M2a and M7/M8: M1a (nearly neutral model) constraints a proportion p0 of conserved sites with 0<ω0 < 1, whereas a proportion p1 = 1 – p0 of neutral sites with ω1 = 1; M2a (positive selection model) adds an extra class of sites with the proportion p2 = 1 – p0 – p1 and with ω2 estimated from the data. M7 (β distribution model) does not allow for positively selected sites (PSSs) and M8 (β and ω model) adds an extra class of sites to M7, allowing for ω>1, which means the presence of PSSs. Upon detection of the positively selected signals, the calculation of posterior probabilities was completed using the empirical Bayes method.
Homology Modeling of Structures of DmNav1
For modeling the structure of DmNav1, sequences of segments S1-S6 of this channel domains I-IV (residues Asn140–Ala439 for DI; Phe816-Asn1042 for DII; Tyr1302-Gly1557 for DIII; Arg1607-Gln1871 for DIV) were aligned using ClustalX software with the paddle-chimaera channel, a chimeric voltage-gated potassium channel, in which the voltage-sensor paddle has been transferred from Kv2.1 to Kv1.2 (30). All the alignments were then manually adjusted using secondary structure element information predicted from SOMPA (http://www.expasy.org) and the paddle-chimaera channel voltage sensor structure. To obtain a model of each domain, aligned sequences (for details, see supplemental Fig. S1) were input into Homer, a fully automated protein structure homology-modeling server (http://protein.cribi.unipd.it/homer/). The DmNav1 structure comprising S1-S6 of all the four domains were assembled in a clockwise arrangement manner according to the architecture of the paddle-chimaera channel (pdb entry 2R9R).
RESULTS
Isolation of a Multigene Family of α-Toxins in M. eupeus
To isolate a multigene family of α-toxins in M. eupeus, we designed multiple forward and reverse primers based on known sequence information from different regions of α-toxins for 3′ and 5′ RACE (Fig. 1A) and genomic amplification, from which a total of 13 different toxin genes were cloned and their sequences were shown in Fig. 1B. We name these toxins MeuNaTxα-1 to MeuNaTxα-13, all of which contain 8 conserved cysteines that form 4 disulfide bridges. In addition to the cysteines, there are 13 other residues (Arg2, Ile6, Gly30, Ala31, Ser33, Gly34, Gly43, Asn44, Leu51, Pro52, Val55, Pro56, and Ile57, all sites of toxins described in this study numbered according to MeuNaTxα-1) which are exactly conserved across the alignment of the multigene family. Most residues identified here are likely associated with structural stabilization of α-toxins. For example, Arg2 and Val55 have been found to form hydrogen bonds between their side-chains; Ile6, Leu51, Pro52, and Val55 constitute part of the hydrophobic core of α-toxins; Ala31 acts as a key residue that is involved in the tight turn connecting the α-helix to the β–sheet; Gly34, a highly conserved residue in scorpion toxins and insect defensins, has an important role in minimizing the steric hindrance involved in the CSαβ motif (31). Mutations at sites Arg2, Ile6, Gly34, Leu51, and Pro52 of BmKM1 have been found to markedly affect the structural stability of this toxin (32).
Despite structural residue conservation, the carboxyl termini of MeuNaTxαs exhibit extensive sequence diversity, in which four peptides (MeuNaTxα-1, -2, -7, and -9) may be amidated because of the presence of Gly-Arg or Gly in the C terminus of their precursors, as previously observed in other animal toxins, e.g. the scorpion sodium channel toxin TsV (33) and bee venom peptides apamin and melittin (34, 35). For most other MeuNaTxαs, the C-terminal 1–2 arginine residues will be removed. In addition, MeuNaTxα-3 contains an unusual C-terminal sequence His-Leu-Gln, as compared with other α-toxins. Based on the size of the J-loop preceding the conserved α-helix, MeuNaTxαs can be divided into three distinct groups: (1) MeuNaTxα-1, -2, -7, -9 and -12, which all have a five-residue J-loop, as oberved in most known α-toxins; (2) MeuNaTxα-4, -5, -6, -8, -10, -11, and -13, which contains an insertion of two hydrophobic residues (Ala-Phe) in the J-loop relative to the first group; (3) MeuNaTxα-3 whose J-loop extends a hydrophilic eight-residue segment (19Glu-Tyr-Thr-Tyr-Ile-Gly-Thr-Ser26). Given the J-loop carries crucial functional residues in several α-toxins (e.g. LqhαIT and Lqh2) (36, 37), it is assumed that these changes in size could be associated with the divergence of MeuNaTxαs, if any, by introducing a novel chemical environment (hydrophilic) into this binding loop of α-toxins, as previously observed in convergent evolution of novel function in serine proteases of shrew and lizard venom (38).
We also determined the genomic organization of three MeuNaTxαs, i.e. MeuNaTxα-1, MeuNaTxα-2, and MeuNaTxα-5′, which all share a conserved exon-intron structure, as observed previously for some known α-toxins, where an intron disrupts the coding-region of the signal peptide end (39). Despite differing in size, the sequences around 5′ and 3′ splice signals of these toxins are highly similar (Fig. 2A and 2B).
Fig. 2.
Exon-intron structure of M. eupeus α-toxins. A, Genomic organization conservation among MeuNaTxα-1, MeuNaTxα-2 and MeuNaTxα-5′. Signal peptide sequences are italicized and residues split by a phase I intron are boxed. Identical amino acids and nucleotides are shadowed in gray. Extra residues are underlined once; B, Conservation of 5′- and 3′-splicing sites of introns.
Phylogenetic Analysis of Mesobuthus NaScTxs
To elucidate the evolutionary relationship of Mesobuthus NaScTxs, we reconstructed a phylogeny of the toxins from their nucleotide sequences using the Neighbor-Joining algorithm with the Maximum Composite Likelihood model and 1000 bootstrap replicates (Fig. 3). From the topology of the phylogeny, we can recognize five well-defined clades (I to V), all with over 90% bootstrap support except for clade I. Of them, I and V are two large clades containing multiple toxins from both M. eupeus and M. martensii, supporting their orthologous relationship, whereas II, III, IV consist of less sequences. Clade I includes most of M. eupeus toxins and several toxins from M. martensii, in which BmKM1, an α-like toxin with activity on both insect and mammalian VGSCs, can be considered as the prototype of this clade (32). Members in clade III have been found to evolve distinct functional feature that exhibits nitrergic actions (40). Clade 4 only includes two M. eupeus toxins (MeuNaTxα-2 and -9) with the closest distance to Clade 5 that contains BmKαIV, a functional hybrid of α-toxins (41).
Fig. 3.
Phylogenetic tree of Mesobuthus α-toxin genes. The tree is a bootstrap consensus tree based upon 1000 replicates of the Neighbor-Joining (NJ) algorithm with the Maximum Composite Likelihood model of DNA substitutions. The scale bar shows total nucleotide divergence. > 50% bootstrap values are shown at nodes. Branches derived from M. eupeus are in red. Similar tree topology was obtained with the Minimum Evolution (ME) or Unweighted Pair Group Method Algorithm (UPGMA) (data not shown).
Purification and Characterization of Four MeuNaTxαs
To structurally and functionally characterize M. eupeus α-NaScTxs, we isolated five native peptides from M. eupeus venom by RP-HPLC, which respectively correspond to MeuNaTxα-1 to MeuNaTxα-5 predicted from their gene sequences (Fig. 1). MeuNaTxα-1 to MeuNaTxα-5 were eluted as single peaks at retention time (TR) of 38.2, 42.2, 43.5, 44.0, and 44.4 min, respectively (Fig. 4). Their purities and molecular masses were confirmed by MALDI-TOF (Table I; supplemental Fig. S2). Experimental masses for these peptides are 7178.27, 7233.68, 8299.90, 7199.55, and 7170.47 Da, respectively, perfectly matching the theoretical masses calculated from their primary sequences (7177.12, 7233.19, 8298.31, 7199.27, and 7171.22 Da, respectively). Amino-terminal sequences of MeuNaTxα-1, -2, -4, and -5 were determined by Edman degradation, which are completely identical to those deduced from their gene sequences (Table I; Fig. 1).
Fig. 4.
Purification of scorpion α-toxins from the M. eupeus crude venom by RP-HPLC. Fractions corresponding to MeuNaTxα-1 - MeuNaTxα-5 and those to three known peptides (MeuTXKα1, MeuTx3B and BeKm-1) (23) are indicated by arrows. The Agilent semi-prep Zorbax 300SB-C18 (9.2 × 250 mm, 5 μm) was equilibrated with 0.1% TFA in water (v/v) and peptide components were eluted from the column with a linear gradient from 0 to 60% acetonitrile in 0.1% TFA in water (v/v) within 60 min with a flow rate of 1 ml/min. The UV absorbance trace was followed at 225 nm.
NMR Structure of MeuNaTxα-5
The solution structure of MeuNaTxα-5 was solved by proton NMR spectroscopy at pH 4.0. Twenty models superposed with all backbone atoms (N, Cα, and C) have a root-mean-square deviation (r.m.s.d.) of 1.50 ± 0.20 Å to the mean structure, and this value is 2.11 ± 0.20 Å when all heavy atoms are superimposed (Table II). The corresponding values for secondary structural region are 0.52 ± 0.15 Å and 0.92 ± 0.20 Å, indicating that these regions are more stable than the loops. Among the 20 converged structures (Fig. 5A), 77.2% of the residues fall within the most favored regions of the Ramachandran plot, 16.9% within additional allowed regions, and 5.4% within generously allowed regions, and only 0.6% within disallowed regions. Not surprisingly, the overall fold of MeuNaTxα-5 is similar to other scorpion α-toxins, which is composed of an α-helix (Tyr23 to Thr29) and three anti-parallel β-strands (β1: Ala1 to Asp3; β2: Ser35 to Ile40; β3: Asn46 to Leu53), and is stabilized by four disulfide bridges (Cys12–Cys65, Cys16–Cys38, Cys24-Cys48 and Cys28–Cys50) (Fig. 5B).
Fig. 5.
Solution structure of MeuNaTxα-5. A, Stereo view of the 20 NMR models, superimposed for a minimum r.m.s.d. to the (C, Cα, N) atoms from residues Ala1 to His66; B, Ribbon representation of the toxin. Disulfide bridges (SS1-SS4) are shown in a blue stick model. Secondary structure regions are assigned by STRIDE (http://webclu.bio.wzw.tum.de/stride/). Beginning and ending amino acids of each secondary structure element (α-helix in red and β-strand in cyan) are labeled according to the peptide sequence.
Channel Subtype Selectivity of MeuNaTxαs
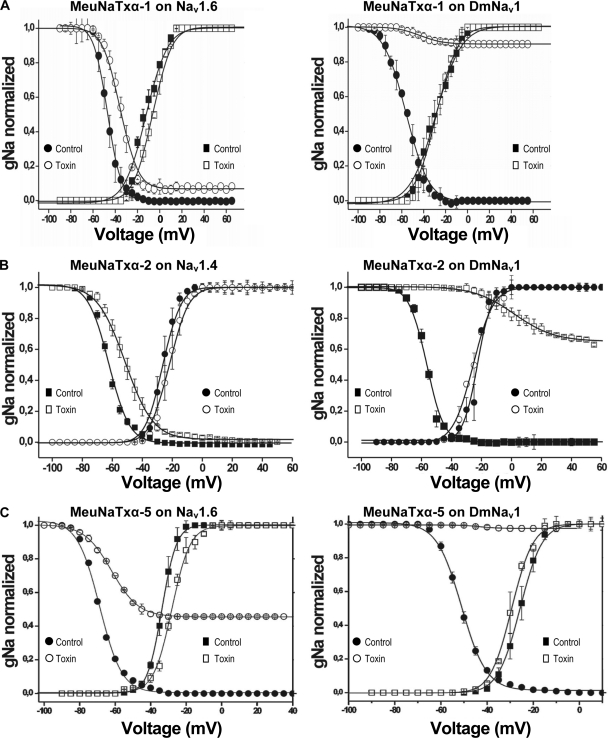
The eight cloned VGSCs (Nav1.2 - Nav1.8 from mammals and DmNav1 from Drosophila) expressed in Xenopus laevis oocytes were used for evaluation of pharmacological functions of four MeuNaTxαs (-1, -2, -4, and -5) by using the two-electrode voltage clamp technique. MeuNaTxα-3 is not functionally evaluated because of its low abundance in the venom. As shown in Fig. 6, DmNav1 is the most sensitive channel of MeuNaTxαs since its inactivation process is completely impaired by all the four peptides at 1–2 μm concentration. At these two concentrations, all the peptides did not affect Nav1.8 but differentially affected other six mammalian VGSC isoforms (Fig. 6). MeuNaTxαs induced slowing of inactivation of VGSCs in a concentration-dependent manner (Fig. 7). The 50% effective concentration (EC50) values of these toxins on sensitive channels were determined with a Boltzmann sigmoidal fit of the obtained data. For DmNav1, the EC50 values are 1.17 ± 0.15 μm for MeuNaTxα-1; 0.22 ± 0.13 μm for MeuNaTxα-2; 0.13 ± 0.02 μm for MeuNaTxα-4; 0.28 ± 0.04 μm for MeuNaTxα-5 (Table III). MeuNaTxα-1 and -5 obviously lack selectivity because they display some affinity for multiple mammalian VGSC isoforms. MeuNaTxα-1 impaired the inactivation process of Nav1.2, 1.3, 1.6, 1.7 whereas MeuNaTxα-5 affected Nav1.3, 1.4, 1.5, 1.6, 1.7. The EC50 values of MeuNaTxα-1 for Nav1.6 are 3.10 ± 1.14 μm and MeuNaTxα-5 for Nav1.6 is 0.79 ± 0.06 μm. Among seven mammalian VGSCs, Nav1.4 is the only one isoform affected by MeuNaTxα-2 with an EC50 of 2.23 ± 0.45 μm. MeuNaTxα-4 is the only one insect-specific toxin characterized in the Mesobuthus lineage. Its insect toxicity was verified by in vivo assays on Musca domestica that showed a half lethal dose (LD50) values of 204.18 ± 23.73 pmol g−1 on M. domestica TJS (deltamethrin-sensitive strain) and 194.57 ± 12.56 pmol g−1 on M. domestica BJD (deltamethrin-resistant strain, kdr and super-kdr). Although kdr (Leu1014Phe) and super-kdr (M918T) mutations in the BJD Na+ channel may be a cause of resistance to deltamethrin (27), these two changes did not influence the binding of MeuNaTxα-4 to the BJD Na+ channel, indicating the receptor difference between scorpion α-toxins and the pesticide deltamethrin (site 3 versus site 7). Overall, MeuNaTxα-1,-2 and -5 can be considered as α-like toxins but with a preference for the insect VGSC.
Fig. 6.
Differential effects of MeuNaTxα-1, -2, -4, and -5 on Nav isoforms expressed in X. leavis oocytes. Representative whole cell Na+ current traces of oocytes expressing cloned Nav isoforms (Nav1.2–Nav1.8, and DmNav1) are shown. The dotted line indicates the zero-current level. The * indicates the steady-state current peak amplitude in the presence of 1–2 μm toxin. CNS: central nervous system; PNS: peripheral nervous system.
Fig. 7.
Dose-response curves of M. eupeus α-toxins. The curves were obtained by plotting the relative I30 ms/Ipeak values of the channels in function of the toxin concentrations. All the curves were fitted with the Hill equation. A, MeuNaTxα-1 on DmNav1 and Nav1.6; B, MeuNaTxα-2 on DmNav1 and Nav1.4; C, MeuNaTxα-4 on DmNav1; D, MeuNaTxα-5 on DmNav1 and Nav1.6.
Table III. Effects of Mesobuthus eupeus toxins on mammalian Nav1.2-Nav1.8 and Drosophila sodium channels. N.A. means no activity at 1 μm for MeuNaTxα-1 - MeuNaTxα-3 and 2 μm for MeuNaTxα-4. “Weak” indicates far smaller than 50% steady-state current peak amplitude at 1 μm for MeuNaTxα-1 - MeuNaTxα-3, and 2 μm for MeuNaTxα-4 and MeuNaTxα-5. The 50% effective concentration (EC50, μm) values indicating high affinity are boldfaced.
| Nav1.2 | Nav1.3 | Nav1.4 | Nav1.5 | Nav1.6 | Nav1.7 | Nav1.8 | DmNav1 | |
|---|---|---|---|---|---|---|---|---|
| MeuNaTxα-1 | weak | weak | N.A. | N.A. | 3.10 ± 1.14 | weak | N.A. | 1.17 ± 0.15 |
| MeuNaTxα-2 | N.A. | N.A. | 2.23 ± 0.45 | N.A. | N.A. | N.A. | N.A. | 0.22 ± 0.13 |
| MeuNaTxα-4 | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | 0.13 ± 0.02 |
| MeuNaTxα-5 | N.A. | weak | weak | weak | 0.79 ± 0.06 | weak | N.A. | 0.28 ± 0.04 |
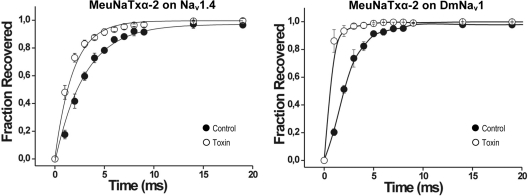
Effects of MeuNaTxαs on the voltage dependence of activation and steady-state inactivation of sensitive VGSCs were also investigated (Fig. 8). The results show that 1 μm toxin (MeuNaTxα-1 on Nav1.6 and DmNav1; MeuNaTxα-2 on Nav1.4 and DmNav1; and MeuNaTxα-5 on Nav1.6 and DmNav1) do not significantly (p < 0.05) shift the midpoint of activation at the channels, but the same concentration of toxin induced a significant (p < 0.05) depolarizing shift in the V1/2 of inactivation of the channels. In addition, we examined the recovery from fast inactivation in the absence or presence of MeuNaTxα-2 (as an example) and found that 1 μm accelerates the recovery from fast inactivation in Nav1.4 and DmNav1 (Fig. 9).
Fig. 8.
Effects of M. eupeus α-toxins on the voltage dependence of activation and steady-state inactivation curves of Nav channels. The steady-state currents of the channels in control and after the addition of 1 μm toxin are shown. A, MeuNaTxα-1: Nav1.6 (left) and DmNav1 (right); B, MeuNaTxα-2: Nav1.4 (left) and DmNav1 (right); C, MeuNaTxα-5: Nav1.6 (left) and DmNav1 (right).
Fig. 9.
Effects of MeuNaTxα-2 on the recovery from fast inactivation of Nav channels. Nav1.4 (left) and DmNav1 (right) in control and in the presence of 1 μm peptide are shown.
Positive Selection in Mesobuthus α-Toxins
To study whether positive selection has driven adaptive substitutions of α-toxins in the Mesobutus lineage, we first analyze all currently available toxin sequences from two sibling species (M. eupeus and M. martensii) (Fig. 3). Nucleotide sequence alignment was first used for pairwise analysis to calculate ω values between two toxins. Overall, the majority of the ω values observed are > 0.5 and even some > 1 (Fig. 10), which are significantly larger than those calculated from genes under neutral evolution (about 0.2) (42). Our observation thus indicates that Mesobuthus α-scorpion toxins evolve in an accelerated manner. To pinpoint which sites suffering positive selection and to test whether the functionally important substitutions identified by site-directed mutagenesis reside at these sites, we employed maximum likelihood models of codon substitution where five models (M0, M1a, M2a, M7, and M8) were implemented to construct two likelihood ratio tests (M1a/M2a and M7/M8). The M0 assumes that all sites have a ω ratio of 0.906, however, it fits the data worse than any other model. Two selection models (M2a and M8) do detect the presence of a substantial proportion (0.2–0.3) of PSSs in Mesobuthus α-scorpion toxins with a similar ω (2.6–2.9) (Table IV). The LRT statistics (2Δl) are 34.1 for M1a/M2a and 39.5 for M7/M8, which both are much greater than the χ2 distribution critical values (p < 0.001), indicating the existence of positive selection in Mesobuthus α-toxins. Furthermore, M2a and M8 both convergently detected nine identical PSSs (8, 9, 15, 18, 20, 38, 39, 41, and 50) (Fig. 1; Table IV), of which sites 8 and 9 are located on the amino-terminal five-residue-turn; sites 15 and 18 preceding the α-helix; site 20 on the α-helix; sites 38 and 39 on the end of the second β-strand; site 41 on the β-turn linking the second and third β-strands; site 50 on the end of the last β-strand. When locating these PSS residues on the three-dimensional (3D) structure, we found that most of them spatially cluster on one face of the toxin molecule despite these sites are not contiguous in the primary sequence (Fig. 11). The only exception is site 50 that appears in the opposite of the surface. Functional significance of these PSSs will be discussed below.
Fig. 10.
Pairwise ω estimation of Mesobuthus α-toxins. The ω values, estimated by the method of Nei and Gojobori (28) implemented in CODEML (29), are shadowed in different colors: ω<0.5 (gray); 0.5≤ω≤1 (yellow); 1 < ω < 10 (red); ∞ (blue).
Table IV. Maximum likelihood estimates of parameters and sites inferred to be under positive selection for α-toxins in the Mesobuthus lineage. S represents tree length; p is the number of parameters in the ω distribution; l is the log likelihood; κ is transition/transversion rate ratio. Twice the log likelihood difference (2Δl) between null models (M1a and M7) and their alternative models (M2a and M8): M1a/M2a = 34.114 (χ2 significant value: p < 0.001); M7/M8 = 39.502 (p < 0.001). Positively selected sites identified by the Bayes Empirical Bayes (BEB) and Naive Empirical Bayes (NEB) methods under M2a and M8 with P (posterior probabilities) ≥0.99 are indicated by ** and ≥0.95 by *. Residues are numbered according to MeuNaTxα-1. Two ω values in models M2a and M8 as indicators of positive selection are boldfaced.
| Model | S | p | l | κ | Estimates of parameters | Positively selected sites |
|---|---|---|---|---|---|---|
| M0 (one-ratio) | 5.71 | 1 | −1812.815 | 1.357 | ω = 0.906 | None |
| M1a (neutral) | 6.28 | 2 | −1733.642 | 1.228 | p0 = 0.490 (p1 = 0.510) | Not allowed |
| ω0 = 0.076 (ω1 = 1) | ||||||
| M2a (selection) | 6.70 | 4 | −1716.585 | 1.420 | p0 = 0.458 | 8D, 9D**, 15F**,18R**, 20A* |
| p1 = 0.358 (p2 = 0.184) | 38W**, 39A**, 41Q, 50K | |||||
| ω0 = 0.094 (ω1 = 1) | ||||||
| ω2 = 2.940 | ||||||
| M7 (beta) | 6.42 | 2 | −1738.610 | 1.185 | p = 0.223, q = 0.202 | Not allowed |
| M8 (beta&ω) | 6.55 | 4 | −1718.859 | 1.440 | p0 = 0.696 (p1 = 0.304) | 8D**, 9D**, 15F**,18R**, 20A** |
| p = 0.364, q = 0.606 | 38W**, 39A**, 41Q*, 50K* | |||||
| ωs = 2.645 |
Fig. 11.
Structural and functional features of PSSs in Mesobuthus α-toxins. A, Mapping of the PSSs on the crystal structure of BmK M1 (pdb entry 1SN1), where PSSs are in shown in red. Different amino acid types in PSSs are highlighted in color: blue, positively charged; red, negatively charged; cyan, polar; green, hydrophobic; B, Comparison of functional sites (FSs) of four well-identified α-toxins and PSSs estimated from different sequence sources [2004 (52); 2010 (47)]. βT: β-turn; NT: N-terminal five-residue turn; “o” represents positions of FSs or PSSs. PSSs commonly predicted in three different studies are shadowed in yellow and PSSs reported here associated with functions are shown in red. *residue numberings in Lqh3 are 39, 45, 59, 64, and 66, respectively. Identical FSs or PSSs are boxed.
DISCUSSION
MeuNaTxαs Are New VGSC Ligands
So far, about sixty α-toxins have been isolated or cloned from scorpions (13), however, little information is available with regard to their selectivity toward specific VGSCs due to the lack of a complete subtype targets for testing. BmKM1 and OD1 are two examples tested for multiple channel isoforms, the former active on Nav1.2, 1.3, 1.5, 1.6, and DmNav1 (32, 43), and the latter affecting Nav1.3, 1.5, 1.7, and DmNav1 (44, 45). These two toxins were found to bind to the channels with differential affinities. To the best of our knowledge, the work presented here represents the first report that systemically evaluated the effects of four paralogous α-toxins on eight VGSCs, including seven mammalian isoforms and the Drosophila counterpart DmNav1. The differential selectivity of these toxins on different channel subtypes renders them useful for identification of the channels at various tissues and act as molecular probes for studying structure-function relationships of site 3 of the sensitive VGSCs. In this regard, MeuNaTxα-2 and MeuNaTxα-4 are of particular interest due to their high selectivity. MeuNaTxα-2 specifically inhibits the inactivation process of Nav1.4 that is the only one VGSC subtype expressed in skeletal muscles, whereas MeuNaTxα-4 shows a prospect in developing a new pest control agent because of its selective on DmNav1 and its killing activity on insects. Among all scorpion α-toxins previously identified, LqhαIT from Leiurus quinquestriatus is considered as the best candidate for developing an agent for pest control because of its strong insecticidal activity (46). However, this has been challenged by its absence of selectivity on mammalian VGSCs. Unlike LqhαIT that only shows about 80-fold selectivity for DmNav1 over rNav1.4 (47), MeuNaTxα-4 shows more than 15385-fold selectivity for DmNav1 over seven mammalian VGSCs (Nav1.2-Nav1.8).
Possible Roles of Positive Selection Sites in Functional Diversification of Mesobuthus α-Toxins
Identifying individual codons of a gene family that undergoes advantageous substitutions is evidence for adaptive evolution which frequently occurs in proteins implicated in evolutionary arms race (48). Some such examples include the major histocompatibility complex (MHC) (49), the retroviral inhibitor TRIM5α in primates (50), neurotoxins from cone snails and scorpions (51, 52), sperm lysin (53), and testes-specific α4 proteasome subunit in Drosophila (54). As an important toxic component, scorpion α-toxins constitute the target of positive selection for adapting to evolutionary alteration of VGSCs in the course of scorpion and its prey interaction. Thus this class of toxins has become a focus of research in evolutionary biology. Thanks to extensive site-directed mutagenesis of four α-toxins (BmKM1, Lqh3, LqhαIT, and Lqh2) from different functional subgroups (32, 36, 37, 55–57), we can now draw structural or functional significance of these PSSs. As shown in Fig. 11, despite overall there are two clear functional domains comprising NC-domain and Core-domain in LqhαIT and BmKM1, they both only share eight identical functional sites (2, 8, 10, 38, 41, 57, 58, and 59). Moreover, the functionally important five-residue turn region in LqhαIT and BmKM1 are excluded in the functional surfaces of Lqh2 and Lqh3. These observations suggest that sites composed of functional surfaces may differ among toxins, consistent with the proposal that all α-toxins bind to a partially overlapping region within site 3 of VGSCs by a similar but nonidentical functional surface located on their exteriors.
In our early work (52), we analyzed α-toxins from four different scorpion species (M. martensii, Androctonus australis, L. quinquestriatus, and Buthus occitanus) by using the maximum likelihood models of codon substitution that detected seven PSSs (10, 17, 18, 37, 39, 41, and 56), in which 10, 17, and 18 were found in the functional regions of α-toxins (36). Subsequently, Weinberger et al. used the same method to analyze sequences from more scorpion species and identified four PSSs (10, 18, 39, and 41) identical to those mentioned above and confirmed functional roles of 39 and 41 by mutagenesis experiments (47). Based on a complete data from two Mesobuthus species, we identified a total of nine PSSs (8, 9, 15, 18, 20, 38, 39, 41, and 50) by the same method, which include three functional sites (18, 39, and 41) common to those predicted previously (47, 52) (Fig. 11B). Of nine PSSs derived from the Mesobuthus lineage seven were found in the functional surface of toxins. It is striking that all these sites are located on three structurally variable and functionally important regions (36, 47, 58), in which two (residues 8–12 and 37–43) are associated with phylogenetic preference of α-toxins, and one (residues 18–21) are implicated in the receptor binding. Our analysis identified six new PSSs, in which five (8, 9, 15, 38, and 50) fall into the functional region. The first finding that sites 8 and 9 undergoing positive selection is of particular interest because in LqhαIT, these two sites were found to be directly involved in binding to the loop connecting S3-S4 of DIV (abbreviated as LDIV:S3-S4) (59) whereas in BmKM1, the residue couplet (8, 9) has been proposed to act as a molecular switch where the substitution at site 8 with specific residue can redirect the α-like characteristics of BmKM1 to either insect or much higher specificity to mammalian VGSCs, whereas P9N becomes highly specific for mammals (60); Site 15 is a key determinant whose mutations profoundly affect affinities of Lqh2 and Lqh3 on mammalian rather than insect VGSCs (37, 57); Site 38 was also identified in the interface of LqhαIT and LDIV:S3–34 of DmNav1 (59) and its functional importance has been revealed in all the four toxins (32, 36, 37, 55–57) (Fig. 11). Although functional importance of site 50 has not been experimentally evaluated so far, it is highlighted by two natural mutants (MeuNaTxα-4 and MeuNaTxα-5) where the mutation V50A results in activity on multiple mammalian VGSC subtypes. This suggests a switch role of this site in discriminating between mammalian and insect VGSCs. These observations support the importance of these new PSSs in the divergence of Mesobuthus toxins and thus these amino acid sites constitute good starting points in evolution-guided design of novel VGSC-targeted molecules with improved potency and phylogenetic selectivity.
Molecular Basis for Channel Subtype Selectivity of MeuNaTxαs
It is shown that scorpion α-toxins exhibit phylogenetic preference and differential affinity toward different VGSCs. Such functional divergence is associated with mutations in bioactive surfaces on the toxins and with variations in site 3 of VGSCs (18). Extensive studies suggest that three extracellular loops comprise site 3. These loops include LDIV:S3-S4, LDI:S5-S6, and LDIV:S5-S6. To observe the locations of these three loops on the channel, we reconstructed a model structure of DmNav1, in which LDI:S5-S6 was found to be located between LDIV:S5-S6 and LDIV:S3-S4, making these three loops form a contiguous region (Fig. 12A and 12B) for toxin binding. Among these three loops, LDIV:S3-S4 is considered as the most important region because some mutations (e.g. Glu1613, Lys1617, and Pro1622) in it can cause significant effects on binding of scorpion α-toxin (LqTx) or sea anemone (ATX II) on rNav1.2. Moreover, recent NMR analysis identified an interface between LqhαIT and LDIV:S3-S4 of DmNav1, in which a functional surface of the toxin comprising Lys8, Asn9, Cys12, Val13, Arg18, and Trp38 is involved (59). Of these functional residues, three (8, 9, and 18) have been identified here as PSSs, suggesting that this loop constitutes a critical driving force for the adaptive evolution of Mesobuthus α-scorpion toxins. To elucidate which residues of the loop of various VGSCs are possibly implicated in interacting with MeuNaTxαs, we compared the amino acid sequences of LDIV:S3-S4 of all VGSCs used in this study and their pharmacological sensitivity pattern to these toxins (Fig. 12C). We found that an acidic residue at position 1616 (Glu1616, all positions of VGSCs described here numbered according to rNav1.2) is shared by five sensitive VGSCs (rNav1.2, rNav1.3, mNav1.6, rNav1.7, and DmNav1) to MeuNaTxα-1. In the resistant channels, this position is occupied by Gln1616 in rNav1.4 and hNav1.5 or Lys1616 in rNav1.8. DmNav1 is the only target of MeuNaTxα-4 and possesses four specific residues at positions Leu1607, Leu1625, Val1628, and Val1630, which all differ from those in the resistant channels. For MeuNaTxα-2, the only mammalian VGSC target is rNav1.4 that contains a specific residue at position Ala1610. For MeuNaTxα-5, no sensitive channel-specific residues were recognized in LDIV:S3-S4, and in this region rNav1.2 and rNav1.3 have completely identical sequences, but their sensitivity on this toxin is different, suggesting that additional loops such as LDI:S5-S6; LDIV:S5-S6 are possibly involved (6). The resistance of hNav1.8 on all four MeuNaTxαs may be associated with its unique amino acid sequences, such as a two-amino acid (Ser-Leu) insertion; mutations at positions 1607–1608 (Ala-Ser in hNav1.8 but Leu/Val-Gly in other subtypes and DmNav1), 1611 (Phe in hNav1.8 but Leu in other subtypes and DmNav1), 1613 (Ala in hNav1.8 but Asp or Glu in other subtypes and DmNav1), 1617–1618 (Gln-Ser in hNav1.8 but Lys-Tyr in other subtypes and DmNav1). At these positions, several have been identified as key determinants for toxin binding. For example, the mutation from a Lys to a Thr at position 1617 (Lys1617Thr) was found to result in decreased affinity of the toxin on Nav1.7 and Lys1617Ser in the scorpion VGSC is considered as a key mutation for the scorpion's adaptive insensitivity (61). In addition, Glu/Asp1613 has been verified to be the most determinant for toxin binding possibly through electrostatic interaction with a positively charged residue in the toxin (6, 7). In the sensitive VGSCs of MeuNaTxα-1, Glu1616 could also participate in such electrostatic interaction because of its adjacent position to Glu/Asp1613. Further elucidation and comparison of bioactive surfaces of these four distinct α-toxins could facilitate identification of key determinants that control specific binding of the toxins to phylogenetically distinct VGSCs. This will enable design of selective agents for pest control and disease treatment by regulating excitability of neurons, muscles, and heart (62).
Fig. 12.
Molecular basis for channel subtype selectivity of MeuNaTxαs. A, The model structure of DI-DIV domains of DmNav1. All the four domains, shown in different colors (DI, green; DII, purple; DIII, blue; DIV, red), were build by Homer, a comparative modeling server (http://protein.cribi.unipd.it/homer/) and the atomic structure of a mammalian chimeric VGPC (pdb entry 2R9R) was used as template. Domain assembly was performed by Swiss PDB Viewer (http://spdbv.vital-it.ch/); B, Structural representation of the DmNav1 site 3 comprising three loops connecting S3 and S4 in domain IV (LDIV:S3-S4) shown as molecular surface, and S5-S6 in DI and DIV (LDI:S5-S6, LDIV:S5-S6); C, Toxin sensitivity of different VGSCs associated with sequences in LDIV:S3-S4. α: MeuNaTxα. Identical amino acids to DmNav1 are shadowed in yellow. The phylogeny was reconstructed based on the sequence of site 3 listed here. Bootstrap values are shown at nodes. +, activity; -, no activity.
Acknowledgments
We thank A. L. Goldin (University of California, Irvine, USA) for rNav1.2, rNav1.3, mNav1.6; G. Mandel (Stony Brook University, Stony Brook, NY) for rNav1.4; R.G. Kallen (Univ. of Pennsylvania, Philadelphia, USA) for hNav1.5. A. George, Jr. (Vanderbilt University, Nashville, TN) for hNav1.5; P. Dietrich (Roche Applied Science) for rNav1.7 and hNav1.8; L. Isom (University of Michigan, Ann Arbor); S. H. Heinemann (Friedrich-Schiller-Universität, Jena, Germany) for rβ1; S. C. Cannon (University of Texas, Dallas) for hβ1; and M.S. Williamson (IACR-Rothamsted, Harpenden, UK) for DmNav1 and tipE. We also thank X. Qiu (Institute of Zoology, CAS, Beijing, China) for providing Musca domestica TJS and Musca domestica BJD for quantitative evaluation of MeuNaTxα-4 insect toxicity.
Footnotes
* This work was supported by grants from The National Natural Science Foundation of China (30730015 and 30921006) to S.Z.; The National Basic Research Program of China (2010CB945300) to S.Z.; Bilateral Cooperation for the 16th Session of the Sino-Belgian S&T Mixed Commission to S.Z.; and G.0330.06 and G.0257.08 (F.W.O.-Vlaanderen), OT-05-64 (K.U.Leuven), P6/31 (Interuniversity attraction Poles Program- Belgian State- Belgian Science Policy) and BIL 07/10 (China) to J.T.
 This article contains supplemental Figs. S1 and S2 and Tables S1 to S4 and Appendix.
This article contains supplemental Figs. S1 and S2 and Tables S1 to S4 and Appendix.
The authors have declared no conflicts of interest.
Authors' contributions to this work: S.Z. designed the research; B.G. and S.Z. performed molecular cloning, peptide isolation and characterization, and insect toxicity assays; S.P. and J.T. performed electrophysiological experiments; X.L. and C.C. determined the NMR structure of MeuNaTxα-5; S.Z. performed evolutionary analysis and comparative modeling; S.Z., C.C. and J.T. wrote the paper.
1 The abbreviations used are:
- VGSC
- voltage-gated sodium channel
- 3′-UTR
- 3′-untranslational region
- CSαβ
- cysteine-stabilized α-helical and β-sheet
- MALDI-TOF MS
- matrix-assisted laser desorption ionization time-of-flight mass spectrometry
- MeuNaTxα
- Mesobuthus eupeus α-scorpion toxin
- PSS
- positively selected site
- RACE
- rapid amplification of cDNA end
- RP-HPLC
- reversed-phase high-performance liquid chromatography
- TTX
- tetrodotoxin.
REFERENCES
- 1. Rochat H., Martin-Eauclaire M. F. (2000) Animal Toxins: Facts and Protocols. Birkhauser Verlag, Basel, Boston, Berlin [Google Scholar]
- 2. Billen B., Bosmans F., Tytgat J. (2008) Animal peptides targeting voltage-activated sodium channels. Curr. Pharm. Design. 14, 2492–2502 [DOI] [PubMed] [Google Scholar]
- 3. Lewis R. J., Garcia M. L. (2003) Therapeutic potential of venom peptides. Nat. Rev. Drug Discov. 2, 790–802 [DOI] [PubMed] [Google Scholar]
- 4. Catterall W. A. (1995) Structure and function of voltage-gated ion channels. Annu. Rev. Biochem. 64, 493–531 [DOI] [PubMed] [Google Scholar]
- 5. Catterall W. A. (2000) From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron 26, 13–25 [DOI] [PubMed] [Google Scholar]
- 6. Catterall W. A., Cestèle S., Yarov-Yarovoy V., Yu F. H., Konoki K., Scheuer T. (2007) Voltage-gated ion channels and gating modifier toxins. Toxicon 49, 124–141 [DOI] [PubMed] [Google Scholar]
- 7. Cestèle S., Catterall W. A. (2000) Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie 82, 883–892 [DOI] [PubMed] [Google Scholar]
- 8. Geffeney S., Brodie E. D., Jr., Ruben P. C., Brodie E. D., 3rd (2002) Mechanisms of adaptation in a predator-prey arms race: TTX-resistant sodium channels. Science 297, 1336–1339 [DOI] [PubMed] [Google Scholar]
- 9. Geffeney S. L., Fujimoto E., Brodie E. D., 3rd, Brodie E. D., Jr, Ruben P. C. (2005) Evolutionary diversification of TTX-resistant sodium channels in a predator-prey interaction. Nature 434, 759–763 [DOI] [PubMed] [Google Scholar]
- 10. Dib-Hajj S. D., Black J. A., Waxman S. G. (2009) Voltage-gated sodium channels: therapeutic targets for pain. Pain Med. 10, 1260–1269 [DOI] [PubMed] [Google Scholar]
- 11. Tate S., Benn S., Hick C., Trezise D., John V., Mannion R. J., Costigan M., Plumpton C., Grose D., Gladwell Z., Kendall G., Dale K., Bountra C., Woolf C. J. (1998) Two sodium channels contribute to the TTX-R sodium current in primary sensory neurons. Nat. Neurosci. 1, 653–655 [DOI] [PubMed] [Google Scholar]
- 12. Possani L. D., Becerril B., Delepierre M., Tytgat J. (1999) Scorpion toxins specific for Na+-channels. Eur. J. Biochem. 264, 287–300 [DOI] [PubMed] [Google Scholar]
- 13. Rodriguez de la Vega R. C., Possani L. D. (2005) Overview of scorpion toxins specific for Na+ channels and related peptides: biodiversity, structure-function relationships and evolution. Toxicon 46, 831–844 [DOI] [PubMed] [Google Scholar]
- 14. Bosmans F., Tytgat J. (2007) Voltage-gated sodium channel modulation by scorpion alpha-toxins. Toxicon 49, 142–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhu S., Gao B., Tytgat J. (2005) Phylogenetic distribution, functional epitopes and evolution of the CSαβ superfamily. Cell. Mol. Life Sci. 62, 2257–2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jover E., Couraud F., Rochat H. (1980) Two types of scorpion neurotoxins characterized by their binding to two separate receptor sites on rat brain synaptosomes. Biochem. Biophys. Res. Comm. 95, 1607–1614 [DOI] [PubMed] [Google Scholar]
- 17. Wheeler K. P., Watt D. D., Lazdunski M. (1983) Classification of Na channel receptors specific for various scorpion toxins. Pflügers Archiv. 397:164–165 [DOI] [PubMed] [Google Scholar]
- 18. Gordon D., Karbat I., Ilan N., Cohen L., Kahn R., Gilles N., Dong K., Stühmer W., Tytgat J., Gurevitz M. (2007) The differential preference of scorpion α-toxins for insect or mammalian sodium channels: Implications for improved insect control. Toxicon. 49, 452–472 [DOI] [PubMed] [Google Scholar]
- 19. Schiavon E., Sacco T., Cassulini R. R., Gurrola G., Tempia F., Possani L. D., Wanke E. (2006) Resurgent current and voltage sensor trapping enhanced activation by a beta-scorpion toxin solely in Nav1.6 channel. Significance in mice Purkinje neurons. J. Biol. Chem. 281, 20326–20337 [DOI] [PubMed] [Google Scholar]
- 20. Gurevitz M., Karbat I., Cohen L., Ilan N., Kahn R., Turkov M., Stankiewicz M., Stühmer W., Dong K., Gordon D. (2007) The insecticidal potential of scorpion β-toxins. Toxicon 49, 473–489 [DOI] [PubMed] [Google Scholar]
- 21. Goudet C., Huys I., Clynen E., Schoofs L., Wang D. C., Waelkens E., Tytgat J. (2001) Electrophysiological characterization of BmK M1, an alpha-like toxin from Buthus martensi Karsch venom. FEBS Lett. 495, 61–65 [DOI] [PubMed] [Google Scholar]
- 22. Zhu S., Gao B. (2006) Molecular characterization of a possible progenitor sodium channel toxin from the Old World scorpion Mesobuthus martensii. FEBS Lett. 580, 5979–5987 [DOI] [PubMed] [Google Scholar]
- 23. Gao B., Peigneur S., Dalziel J., Tytgat J., Zhu S. (2011) Molecular divergence of two orthologous scorpion toxins affecting potassium channels. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 159, 313–321 [DOI] [PubMed] [Google Scholar]
- 24. Wuthrich K. (1986) NMR of proteins and nucleic acids, John Wiley & Sons [Google Scholar]
- 25. Liman E. R., Tytgat J., Hess P. (1992) Subunit stochiometry of a mammalian K+ channel determined by construction of multimeric cDNAs Neuron 9:861–871 [DOI] [PubMed] [Google Scholar]
- 26. Maggio F., King G. F. (2002) Scanning mutagenesis of a Janus-faced atracotoxin reveals a bipartite surface patch that is essential for neurotoxic function. J. Biol. Chem. 277, 22806–22813 [DOI] [PubMed] [Google Scholar]
- 27. Qiu X., Li M., Luo H., Fu T. (2007) Molecular analysis of resistance in a deltamethrin-resistant strain of Musca domestica from China. Pest. Biochem. Physiol. 89, 146–150 [Google Scholar]
- 28. Nei M., Gojobori T. (1986) Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol. Biol. Evol. 3, 418–426 [DOI] [PubMed] [Google Scholar]
- 29. Yang Z. (2007) PAML 4: Phylogenetic Analysis by Maximum Likelihood. Mol. Biol. Evol. 24, 1586–1591 [DOI] [PubMed] [Google Scholar]
- 30. Long S. B., Tao X., Campbell E. B., MacKinnon R. (2007) Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature 450, 376–382 [DOI] [PubMed] [Google Scholar]
- 31. Bontems F., Roumestand C., Gilquin B., Menez A., Toma F. (1991) Refined structure of charybdotoxin: common motifs in scorpion toxins and insect defensins. Science 254, 1521–1523 [DOI] [PubMed] [Google Scholar]
- 32. Liu L. H., Bosmans F., Maertens C., Zhu R. H., Wang D. C., Tytgat J. (2005) Molecular basis of the mammalian potency of the scorpion alpha-like toxin, BmK M1. FASEB J. 19, 594–596 [DOI] [PubMed] [Google Scholar]
- 33. Martin-Eauclaire M. F., Céard B., Ribeiro A. M., Diniz C. R., Rochat H., Bougis P. E. (1994) Biochemical, pharmacological and genomic characterisation of Ts IV, an α-toxin from the venom of the South American scorpion Tityus serrulatus. FEBS Lett. 342, 181–184 [DOI] [PubMed] [Google Scholar]
- 34. Gmachl M., Kreil G. (1995) The precursors of the bee venom constituents apamin and MCD peptide are encoded by two genes in tandem which share the same 3′-exon. J. Biol. Chem. 270, 12704–12708 [DOI] [PubMed] [Google Scholar]
- 35. Kreil G. (1973) Biosynthesis of melittin, a toxic peptide from bee venom. Amino-acid sequence of the precursor. Eur. J. Biochem. 33, 558–566 [DOI] [PubMed] [Google Scholar]
- 36. Karbat I., Frolow F., Froy O., Gilles N., Cohen L., Turkov M., Gordon D., Gurevitz M. (2004) Molecular basis of the high insecticidal potency of scorpion α-toxins. J. Biol. Chem. 279, 31679–31686 [DOI] [PubMed] [Google Scholar]
- 37. Karbat I., Kahn R., Cohen L., Ilan N., Gilles N., Corzo G., Froy O., Gur M., Albrecht G., Heinemann S. H., Gordon D., Gurevitz M. (2007) The unique pharmacology of the scorpion α-like toxin Lqh3 is associated with its flexible C-tail. FEBS J. 274, 1918–1931 [DOI] [PubMed] [Google Scholar]
- 38. Aminetzach Y. T., Srouji J. R., Kong C. Y., Hoekstra H. E. (2009) Convergent evolution of novel protein function in shrew and lizard venom. Curr. Biol. 19, 1925–1931 [DOI] [PubMed] [Google Scholar]
- 39. Delabre M. L., Pasero P., Marilley M., Bougis P. E. (1995) Promoter structure and intron-exon organization of a scorpion alpha-toxin gene. Biochemistry 34, 6729–6736 [DOI] [PubMed] [Google Scholar]
- 40. Gong J., Kini R. M., Gwee M. C., Gopalakrishnakone P., Chung M. C. (1997) Makatoxin I, a novel toxin isolated from the venom of the scorpion Buthus martensi Karsch, exhibits nitrergic actions. J. Biol. Chem. 272, 8320–8324 [DOI] [PubMed] [Google Scholar]
- 41. Chai Z. F., Zhu M. M., Bai Z. T., Liu T., Tan M., Pang X. Y., Ji Y. H. (2006) Chinese-scorpion (Buthus martensi Karsch) toxin BmKαIV, a novel modulator of sodium channels: from genomic organization to functional analysis. Biochem. J. 399, 445–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ohno M., Ménez R., Ogawa T., Danse J. M., Shimohigashi Y., Fromen C., Ducancel F., Zinn-Justin S., Le Du M. H., Boulain J. C., Tamiya T., Ménez A. (1998) Molecular evolution of snake toxins: is the functional diversity of snake toxins associated with a mechanism of accelerated evolution? Progr. Nucleic Acid Res. Mol. Biol. 59, 307–364 [DOI] [PubMed] [Google Scholar]
- 43. He H., Liu Z., Dong B., Zhou J., Zhu H., Ji Y. (2010) Molecular determination of selectivity of the site 3 modulator (BmK I) to sodium channels in the CNS: a clue to the importance of Nav1.6 in BmK I-induced neuronal hyperexcitability. Biochem. J. 431, 289–298 [DOI] [PubMed] [Google Scholar]
- 44. Jalali A., Bosmans F., Amininasab M., Clynen E., Cuypers E., Zaremirakabadi A., Sarbolouki M. N., Schoofs L., Vatanpour H., Tytgat J. (2005) OD1, the first toxin isolated from the venom of the scorpion Odonthobuthus doriae active on voltage-gated Na+ channels. FEBS Lett. 579, 4181–4186 [DOI] [PubMed] [Google Scholar]
- 45. Maertens C., Cuypers E., Amininasab M., Jalali A., Vatanpour H., Tytgat J. (2006) Potent modulation of the voltage-gated sodium channel Nav1.7 by OD1, a toxin from the scorpion Odonthobuthus doriae. Mol. Pharmacol. 70, 405–414 [DOI] [PubMed] [Google Scholar]
- 46. Zilberberg N., Gordon D., Pelhate M., Adams M. E., Norris T. M., Zlotkin E., Gurevitz M. (1996) Functional expression and genetic alteration of an α-scorpion neurotoxin. Biochemistry 35, 10215–10222 [DOI] [PubMed] [Google Scholar]
- 47. Weinberger H., Moran Y., Gordon D., Turkov M., Kahn R., Gurevitz M. (2010) Positions under positive selection–key for selectivity and potency of scorpion α-toxins. Mol. Biol. Evol. 27, 1025–1034 [DOI] [PubMed] [Google Scholar]
- 48. Yang Z. (2006) Computational Molecular Evolution. Oxford University Press, Oxford, England [Google Scholar]
- 49. Swanson W. J., Yang Z., Wolfner M. F., Aquadro C. F. (2001) Positive Darwinian selection in the evolution of mammalian female reproductive proteins. Proc. Natl. Acad. Sci. U.S.A. 98, 2509–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sawyer S. L., Wu L. I., Emerman M., Malik H. S. (2005) Positive selection of primate TRIM5alpha identifies a critical species-specific retroviral restriction domain. Proc. Natl. Acad. Sci. U.S.A. 102, 2832–2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Duda T. F., Jr., Palumbi S. R. (1999) Molecular genetics of ecological diversification: duplication and rapid evolution of toxin genes of the venomous gastropod Conus. Proc. Natl. Acad. Sci. U.S.A. 96, 6820–6823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhu S., Bosmans F., Tytgat J. (2004) Adaptive evolution of scorpion sodium channel toxins. J. Mol. Evol. 58, 145–153 [DOI] [PubMed] [Google Scholar]
- 53. Clark N. L., Findlay G. D., Yi X., MacCoss M. J., Swanson W. J. (2007) Duplication and selection on abalone sperm lysin in an allopatric population. Mol. Biol. Evol. 24, 2081–2090 [DOI] [PubMed] [Google Scholar]
- 54. Torgerson D. G., Singh R. S. (2004) Rapid evolution through gene duplication and subfunctionalization of the testes-specific α4 proteasome subunits in Drosophila. Genetics 168, 1421–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sun Y. M., Bosmans F., Zhu R. H., Goudet C., Xiong Y. M., Tytgat J., Wang D. C. (2003) Importance of the conserved aromatic residues in the scorpion α-like toxin BmK M1. J. Biol. Chem. 278, 24125–24131 [DOI] [PubMed] [Google Scholar]
- 56. Wang C. G., Gilles N., Hamon A., Le Gall F., Stankiewicz M., Pelhate M., Xiong Y. M., Wang D. C., Chi C. W. (2003) Exploration of the functional site of a scorpion α-like toxin by site-directed mutagenesis. Biochemistry 42, 4699–4708 [DOI] [PubMed] [Google Scholar]
- 57. Kahn R., Karbat I., Ilan N., Cohen L., Sokolov S., Catterall W. A., Gordon D., Gurevitz M. (2009) Molecular requirements for recognition of brain voltage-gated sodium channels by scorpion α-toxins. J. Biol. Chem. 284, 20684–20691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Krimm I., Gilles N., Sautière P., Stankiewicz M., Pelhate M., Gordon D., Lancelin J. M. (1999) NMR structures and activity of a novel α-like toxin from the scorpion Leiurus quinquestriatus hebraeus. J. Mol. Biol. 285, 1749–1763 [DOI] [PubMed] [Google Scholar]
- 59. Schnur E., Turkov M., Kahn R., Gordon D., Gurevitz M., Anglister J. (2008) NMR analysis of interaction of LqhαIT scorpion toxin with a peptide corresponding to the D4/S3-S4 loop of insect para voltage-gated sodium channel. Biochemistry 47, 911–921 [DOI] [PubMed] [Google Scholar]
- 60. Ye X., Bosmans F., Li C., Zhang Y., Wang D. C., Tytgat J. (2005) Structural basis for the voltage-gated Na+ channel selectivity of the scorpion α-like toxin BmKM1. J. Mol. Biol. 353, 788–803 [DOI] [PubMed] [Google Scholar]
- 61. Zuo X. P., He H. Q., He M., Liu Z. R., Xu Q., Ye J. G., Ji Y. H. (2006) Comparative pharmacology and cloning of two novel arachnid sodium channels: Exploring the adaptive insensitivity of scorpion to its toxins. FEBS Lett. 580, 4508–4514 [DOI] [PubMed] [Google Scholar]
- 62. Wood J. N., Boorman J. (2005) Voltage-gated sodium channel blockers; target validation and therapeutic potential. Curr. Top. Med. Chem. 5, 529–537 [DOI] [PubMed] [Google Scholar]