Abstract
Background:
The World Trade Center (WTC) collapse on September 11, 2001, produced airflow obstruction in a majority of firefighters receiving subspecialty pulmonary evaluation (SPE) within 6.5 years post-September 11, 2001.
Methods:
In a cohort of 801 never smokers with normal pre-September 11, 2001, FEV1, we correlated inflammatory biomarkers and CBC counts at monitoring entry within 6 months of September 11, 2001, with a median FEV1 at SPE (34 months; interquartile range, 25-57). Cases of airflow obstruction had FEV1 less than the lower limit of normal (LLN) (100 of 801; 70 of 100 had serum), whereas control subjects had FEV1 greater than or equal to LLN (153 of 801; 124 of 153 had serum).
Results:
From monitoring entry to SPE years later, FEV1 declined 12% in cases and increased 3% in control subjects. Case subjects had elevated serum macrophage derived chemokine (MDC), granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor, and interferon inducible protein-10 levels. Elevated GM-CSF and MDC increased the risk for subsequent FEV1 less than LLN by 2.5-fold (95% CI, 1.2-5.3) and 3.0-fold (95% CI, 1.4-6.1) in a logistic model adjusted for exposure, BMI, age on September 11, 2001, and polymorphonuclear neutrophils. The model had sensitivity of 38% (95% CI, 27-51) and specificity of 88% (95% CI, 80-93).
Conclusions:
Inflammatory biomarkers can be risk factors for airflow obstruction following dust and smoke exposure. Elevated serum GM-CSF and MDC levels soon after WTC exposure were associated with increased risk of airflow obstruction in subsequent years. Biomarkers of inflammation may help identify pathways producing obstruction after irritant exposure.
On September 11, 2001, the World Trade Center (WTC) in New York City collapsed after a terrorist attack, producing a dust cloud with a particulate matter (PM) concentration of 100,000 μg/m3 that overwhelmed lung-protective mechanisms.1 Even 1 month after the event, concentration of PM up to 2.5 μm in size was 196 μg/m3, three times the National Ambient Air Quality Standards 24-h standard.2 WTC dust was caustic. Fires burned for months after September 11, 2001, exposing rescue and recovery workers to products of combustion. New or increased self-reported respiratory symptoms occurred in large percentages of Fire Department City of New York (FDNY) rescue workers, other WTC workers, and exposed residents.3‐10 FDNY rescue workers have suffered from decline in FEV1, dyspnea, cough, sinusitis, and acid reflux.3,7,11‐13 Longitudinal evaluation of lung function showed firefighters who had never smoked lost, on average, 439 mL of FEV1 in the first year post-September 11, 2001, followed by a mean annualized reduction in FEV1 of 26 mL/y in the 6.5 years following September 11, 2001.3,14 A majority of the 1,720 symptomatic FDNY rescue workers who presented for subspecialty pulmonary evaluation (SPE) had airflow obstruction as their manifestation of lung disease.15 There was a significant interindividual variation in lung function, with some patients recovering and others suffering accelerated decline.
Airflow obstruction from PM-induced inflammation and smoke is poorly understood.16 PM concentration in ambient air remained high in the months after September 11, 2001. Studies related to high-ambient PM exposure show that there is a significant decrease in FEV1 after 5 to 7 days of sustained ambient PM exposure.17,18 Upper respiratory symptoms increased by 3% per 10 μg/m3 of PM exposure.17,19 Although the intensity of exposure produced by the WTC collapse was far greater than that produced by most other ambient sources, the effects of WTC dust on lung function appear to be similar to those produced by PM from other sources. A large proportion of exposed firefighters had cough and dyspnea with subsequent development of airflow obstruction and reactive airway dysfunction syndrome.7
FDNY rescue workers form a well-characterized cohort that has been a powerful resource for documenting the impact of WTC exposure on the lung. Active workers (92%) had serial pulmonary function testing, mostly from before their WTC exposure. A well-organized medical monitoring and treatment program helped to minimize longitudinal dropout. Serum samples were drawn on a majority of the cohort from September 2001 to February 2002, at initial development of airflow obstruction.
Using a nested case-control study design, we identified cytokines and chemokines in the serum of FDNY rescue workers obtained within 6 months of September 11, 2001. We hypothesized that individuals highly susceptible to airflow obstruction, induced by environmental irritants, would express different levels of proinflammatory cytokines than similarly exposed individuals with an average susceptibility to irritants. Differentially expressed cytokines might serve as biomarkers of vulnerability to accelerated decline of lung function produced by an irritant exposure and direct future research into development of airflow obstruction.
Materials and Methods
Study Design and Participants
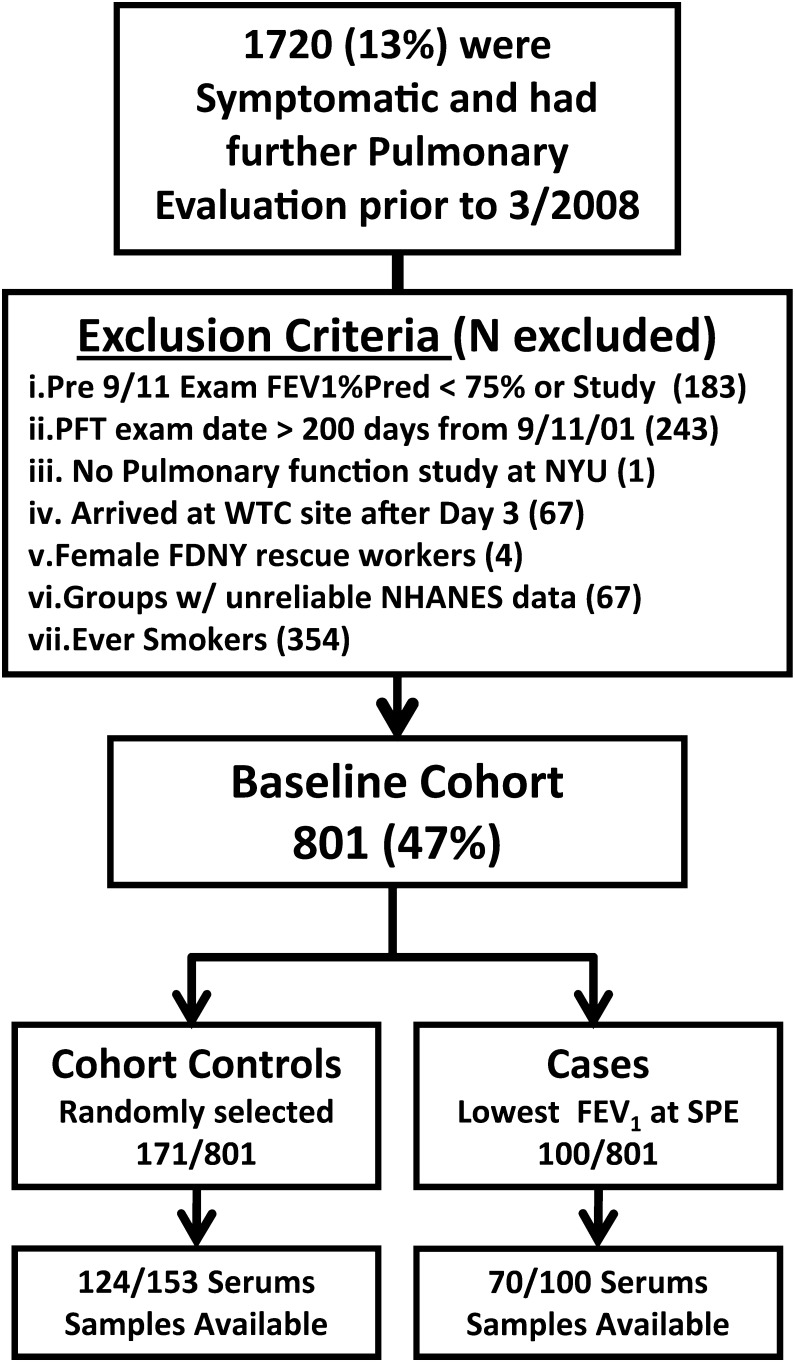
The study cohort was derived from subjects (N = 1,720) entering SPE between October 1, 2001, and March 10, 2008,15 if they met the following criteria: never smokers (consistently reported not smoking on all health screens); male; had reliable National Health and Nutrition Examination Survey (NHANES) normative data for predicted FEV1; had post-September 11, 2001, FDNY-Bureau of Health Services pulmonary function test (PFTs) within 200 days of September 11, 2001; and had pre-September 11, 2001, FEV1 > 75% predicted (801 of 1,720; 47%). A control cohort was derived from the study cohort by random sampling of the 801 after stratification based on BMI and FEV1 at monitoring entry (N = 171 of 801). The 75th percentile of study cohort biomarker expression was defined in this random cohort (138 of 171 with serum available). Cases of airflow obstruction were identified as the 100 (70 of whom had serum available) who had the lowest FEV1 at the time of SPE. These individuals also had an FEV1 below the lower limit of normal (LLN) as calculated by NHANES III. For this case-control study, the control subjects are all individuals in the random sample control cohort who did not meet criteria to be an airflow obstruction case (N = 153 of 171, 124 of 153 of whom had serum). Subjects signed informed institutional review board-approved consent at the time of enrollment allowing analysis of their information and samples for research (Montefiore Medical Center #07-09-320 and New York University #11-00439).
Demographics
Age, sex, race/ethnicity, and years of service at FDNY were obtained from the FDNY-WTC-monitoring database. BMIs were calculated from height and weight measured at the time of medical monitoring entry (MME) and SPE. Degree of exposure was self-reported at the first FDNY-WTC monitoring and was categorized using the FDNY-WTC Exposure Intensity Index (Arrival Time): (1) present on the morning of September 11, 2001; (2) arrived after noon on September 11, 2001; (3) arrived on September 12, 2001.15 Those arriving after day 3 were excluded from analysis as a result of their low numbers and significant demographic differences as previously described.15
Serum Sampling and FEV1 Measurement
Blood drawn at the first post-September 11, 2001, FDNY-WTC monitoring examination was allowed to stand for 1 h at room temperature before being centrifuged at 1,800 × g for 10 min. Serum was stored at −80°C (Bio-Reference Laboratories, Inc). Serum was thawed once at 4°C and assayed using proinflammatory panel (39-Plex) according to manufacturer’s instructions (Millipore) and analyzed on a Luminex 200IS (Luminex Corporation). Data were analyzed with MasterPlex QT software (version 1.2; MiraiBio, Inc). The dynamic range of the assay was 3.2 to 10,000 pg/mL. Each batch of samples processed contained control subjects and cases in an approximate 12:7 ratio. Subjects underwent spirometry at the first post-September 11, 2001, FDNY-WTC monitoring examination and at SPE as previously described.15,20,21
Statistical Analysis
Database management and statistics were performed using SPSS 18 (IBM), S+ (TIBCO Spotfire), STATA (StataCorp LP), and SAS (SAS Institute). Normally distributed data were expressed as means and SD. Data not distributed normally and multianalyte comparisons were evaluated using a Mann-Whitney U test. Analytes that were significantly different between cases and control subjects were used to construct logistic regression models with case status as the outcome variable. The models were adjusted for potential confounders of arrival time, BMI, polymorphonuclear neutrophil (PMN) concentration, and age. Significance was assessed by P < .05.
Results
Participants
This nested case-control study was drawn from a population of 801 never smokers with normal pre-September 11, 2001, PFTs. Derivation of baseline cohort, cases, and control subjects from the cohort that received SPE is described in Figure 1. Control subjects had similar FEV1 at MME when compared with the larger never smoker baseline cohort (n = 801) and to the original SPE cohort (n = 1,720). Airflow obstruction case and control subjects had similar WTC exposure; time from September 11, 2001, to monitoring entry; time from September 11, 2001, to SPE; BMI at monitoring entry; years of service; age at September 11, 2001; and adequate representation of the highest exposure group. Serum drawn at the first monitoring examination was available for 124 of 153 (81%) of the control subjects and 70 of 100 (70%) of airflow obstruction case subjects. The demographics of the total groups were similar to those of control subjects and case subjects with serum available.
Figure 1.
Study design. FDNY = Fire Department City of New York; NHANES = National Health and Nutrition Examination Survey; NYU = New York University; PFT = pulmonary function test; Pred = predicted; SPE = subspecialty pulmonary evaluation; WTC = World Trade Center.
Control subjects had higher FEV1 % predicted than case subjects at pre-September 11, 2001 (104% vs 88%), monitoring entry (93% vs 78%), and subsequent pulmonary evaluation entry (96% vs 72%, P < .001 for all time points) (Table 1). Lung function in control subjects increased from the monitoring examination to pulmonary evaluation entry (93%-96%), whereas the FEV1 of case subjects continued to decline between the two PFTs (78% to 72%; P < .001 for the difference in FEV1 observed between control subjects and case subjects). To confirm that the median FEV1 in control and case subjects represented individual improvement in control subjects and deterioration in case subjects, we used patients as their own control subjects. The mean ratio of FEV1 (pulmonary evaluation/monitoring examination) was 1.03 in control subjects and 0.87 in cases (P < .0001, by Student t test). Case subjects had slightly higher BMI than control subjects at pulmonary evaluation (30 vs 29, P < .02).
Table 1.
—Demographics and FEV1 of SPE Cohorts
| Control Subcohort |
Cases |
|||||
| Characteristics | Total | Baseline Never Smokers | All | Serum Availablea | All | Serum Availablea |
| No. | 1,720 | 801 | 153 | 124 | 100 | 70 |
| WTC arrival time | ||||||
| Morning of September 11, 2001 | 404 (24) | 197 (25) | 32 (21) | 21 (17) | 30 (30) | 18 (26) |
| Afternoon of September 11, 2001 | 1,022 (59) | 498 (62) | 105 (68) | 89 (72) | 49 (49) | 37 (53) |
| Anytime on September 12, 2001 | 294 (17) | 106 (13) | 16 (11) | 14 (11) | 21 (21) | 15 (21) |
| September 11, 2001 to evaluation, mo | ||||||
| MME | 3.0 (2-4) | 2.7 (2-3.8) | 2.7 (2-4) | 2.6 (2-3) | 2.7 (2-4) | 2.7 (2-4) |
| SPE | 33.6 (23-56) | 33.8 (25-57) | 35.5 (25-56) | 35.5 (26-56) | 32.1 (20-54) | 32.6 (21-53) |
| BMI, kg/m2 | ||||||
| MME | 28.1 (26-31) | 28.0 (26-30) | 27.9 (26-30) | 28.0 (26-31) | 29.0 (27-31) | 29.0 (27-31) |
| SPE | 29.0 (27-32) | 28.9 (27-31) | 29.0 (27-31) | 29.0 (27-31) | 30.0 (28-34) | 29.6 (27-34) |
| Years of service at September 11, 2001 | 19.2 (12-24) | 18.3 (11-23) | 16.5 (11-23) | 16.5 (11-22) | 18.7 (14-22) | 18.8 (14-22) |
| Age at September 11, 2001 | 42.0 (37-46) | 40.0 (36-45) | 42 (37-46) | 45.0 (40-49) | 40.0 (36-46) | 41.2 (36-46) |
| FEV1 | ||||||
| Pre September 11, 2001 | ||||||
| % | 99.0 (89-108) | 100.0 (91-109) | 104 (92-113) | 103.5 (92-113) | 88.0 (82-97) | 87.5 (81-96) |
| L | 4.2 (3.7-4.7) | 4.3 (3.8-4.8) | 4.3 (3.9-4.9) | 4.3 (4-5) | 3.8 (3.5-4.3) | 3.8 (3.5-4.2) |
| MME | ||||||
| % | 89.0 (80-99) | 90.0 (82-100) | 93 (84-101) | 92.5 (84-99) | 79 (72-88) | 77.5 (71-89) |
| L | 3.7 (3.3-4.2) | 3.84 (3.4-4.3) | 3.8 (3.4-4.4) | 3.8 (3-4) | 3.4 (3.1-3.7) | 3.4 (3.1-3.7) |
| SPE | ||||||
| % | 91.0 (81-100) | 94 (84-102) | 96 (87-105) | 96.1 (88-104) | 73 (66-75) | 72.3 (66-75) |
| L | 3.8 (3.3-4.2) | 3.9 (3.5-4.3) | 4.0 (3.6-4.3) | 3.9 (4-4) | 3.0 (2.7-3.2) | 3.0 (2.7-3.2) |
Data are presented as No. (%) or median (IQR). IQR = interquartile range; MME = medical monitoring entry; SPE = subspecialty pulmonary examination; WTC = World Trade Center.
Available serum biomarkers.
Biomarkers of Airflow Obstruction
Serum levels of 39 cytokines/chemokines were measured using a multiplexed assay. Cases had significant elevations by Mann-Whitney U test compared with control subjects of (median concentration in pg/mL) granulocyte-macrophage colony-stimulating factor (GM-CSF) (32 vs 27, P = .02), granulocyte colony-stimulating factor (G-CSF) (27 vs 23, P = .03), macrophage-derived chemokine (MDC) (1,701 vs 1,403, P = .002), and interferon-inducible protein-10 (IP-10) (283 vs 227, P = .01). Control subjects had significant elevations compared with cases of IL-6 (5.6 vs < 3.2, P = .047) and IL-15 (4.4 vs < 3.2, P = .03) (Table 2). Because WBCs are routinely available inflammatory biomarkers, we assessed if control subjects and case subjects had different leukocyte concentrations. Surprisingly, there was a trend toward increased leukocyte concentration (6.6 ± 1.8 cells/103 μL vs 6.2 ± 1.5 cells/103 μL, P = .17) in control subjects and a significant increase in the concentration of PMN (3.9 ± 1.4 cells/103 μL vs 3.4 ± 1.1 cells/103 μL, P = .02), so PMN concentration could confound the analysis. There was also no significant difference in serum chemistries and liver function between these two groups (data not shown).
Table 2.
—Biomarkers of Inflammation (No. = 39)
| Biomarker | Control Subjects (n = 124) | Case Subjects (n = 70) | P Value |
| G-CSF | 22.89 (16-38) | 26.87 (20-38) | .03a |
| GM-CSF | 26.52 (19-44) | 32.39 (23-72) | .02a |
| IL-6 | 5.59 ( < 3.2-19) | < 3.2 ( < 3.2-10) | .03a |
| IL-15 | 4.41 ( < 3.2-8) | < 3.2 ( < 3.2-6) | .05a |
| MDC | 1,403.50 (1,069-1,756) | 1,701.36 (1,290-2,012) | .002a |
| IP-10 | 227.37 (193-301) | 282.67 (206-373) | .01a |
| EGF | 65 (38-151) | 82 (29-140) | .80 |
| Eotaxin | 129 (95-171) | 132 (97-200) | .37 |
| FGF-2 | 101 (61-129) | 83 (56-117) | .09 |
| Flt-3 ligand | 8 ( < 3.2-33) | 6 ( < 3.2-29) | .54 |
| Fractalkine | 67 (37-164) | 66 (25-156) | .28 |
| GRO | 669 (486-908) | 712 (529-1095) | .43 |
| IFN-α2 | < 3.2 ( < 3.2-17) | < 3.2 ( < 3.2-29) | .66 |
| IFN-γ | 9 (5-22) | 7 (5-17) | .25 |
| IL-10 | 6 ( < 3.2-17) | 5 ( < 3.2-14) | .42 |
| IL-12 (p40) | 20 (6-50) | 18 ( < 3.2-42) | .48 |
| IL-12 (p70) | 7 ( < 3.2-10) | 7 (4-12) | .19 |
| IL-1α | 5 ( < 3.2-29) | 4 ( < 3.2-27) | .41 |
| IL-1β | < 3.2 ( < 3.2-8) | < 3.2 ( < 3.2-5) | .26 |
| IL-1ra | 9 ( < 3.2-32) | 10 ( < 3.2-25) | .82 |
| IL-2 | < 3.2 ( < 3.2- < 3.2) | < 3.2 ( < 3.2- < 3.2) | .16 |
| IL-3 | < 3.2 ( < 3.2- < 3.2) | < 3.2 ( < 3.2- < 3.2) | .08 |
| IL-4 | < 3.2 ( < 3.2-8) | 3.2 ( < 3.2-6) | .80 |
| IL-5 | < 3.2 ( < 3.2- < 3.2) | < 3.2 ( < 3.2-3.4) | .23 |
| IL-7 | 8 ( < 3.2-20) | 9 ( < 3.2-20) | .97 |
| IL-8 | 16 (10-29) | 14 (12-28) | .86 |
| IL-9 | < 3.2 ( < 3.2-4) | < 3.2 ( < 3.2-4) | .59 |
| IL-13 | 4 ( < 3.2-14) | < 3.2 ( < 3.2-15) | .09 |
| IL-17 | 3 ( < 3.2-11) | < 3.2 ( < 3.2-8) | .07 |
| MCP-1 | 537 (387-642) | 531 (425-732) | .38 |
| MCP-3 | 8 (6-14) | 7 (6-12) | .56 |
| MIP-1α | 23 (14-34) | 24 (12-32) | .88 |
| MIP-1β | 56 (39-81) | 49 (31-63) | .05 |
| sCD40L | 8,003 (4,558- > 10,000) | 9,711 (5,224- > 10,000) | .26 |
| sIL-2Rα | 3.2 ( < 3.2-17) | 3.2 ( < 3.2-18) | .53 |
| TGF-α | 8 ( < 3.2-16) | 7 ( < 3.2-14) | .23 |
| TNF-α | 6 (5-9) | 7 (6-9) | .10 |
| TNF-β | < 3.2 ( < 3.2-6) | < 3.2 ( < 3.2-4) | .17 |
| VEGF | 146 (85-226) | 147 (87-271) | .47 |
Data are presented as median (IQR) in pg/mL. Values < 3.2 indicate levels below the limit of detection. EGF = epidermal growth factor; FGF = fibroblast growth factor; G-CSF = granulocyte colony-stimulating factor; GM-CSF = granulocyte-macrophage colony-stimulating factor; GRO = growth regulated oncogene; IFN = interferon; IP-10 = interferon inducible protein-10; MCP = monocyte chemotactic protein; MDC = macrophage-derived chemokine; MIP = macrophage inflammatory protein; TGF = transforming growth factor; TNF = tumor necrosis factor; VEGF = vascular endothelial growth factor. See Table 1 legend for expansion of other abbreviation.
Significant P < .05 by Mann-Whitney U test.
All airflow obstruction case subjects had FEV1 less than LLN, whereas all control subjects had FEV1 greater than or equal to LLN. We used logistic regression to further assess if inflammatory biomarkers significantly different between case and control subjects increased the risk of abnormal FEV1 in subsequent years. We used the upper quartile of cytokine/chemokine expression as the standard cutpoint. The upper quartiles were 53.2 pg/mL for GM-CSF, 1,819 pg/mL for MDC, 306 pg/mL for IP-10, 38.7 pg/mL for G-CSF, 17.8 pg/mL for IL-6, and 8.7 pg/mL for IL-15. In a single analyte logistic regression model, only GM-CSF and MDC had a significant OR after adjusting for BMI, PMN, age, and arrival time (Table 3). GM-CSF and MDC increased the risk of developing abnormal FEV1 by 2.5-fold and 2.95-fold, respectively. These associations did not decline in a model that included both GM-CSF and MDC. The final model was robust when assessed with a likelihood ratio of 210.0 and Hosmer-Lemeshow goodness-of-fit test, χ2 value of 7.59 and P = .47. We assessed the impact of multiple comparisons of the six analytes tested in logistic models. The false discovery rate was 0.012 for MDC and 0.04 for GM-CSF in the final model. The interaction terms between GM-CSF and MDC were not significant (data not shown). The logistic regression model with MDC, GM-CSF, BMI, age, PMN, and WTC arrival time had a sensitivity of 38% (95% CI, 27-51) and a specificity of 88% (95% CI, 80-93).
Table 3.
—Logistic Regression Models
Discussion
This study was designed to identify risk factors of susceptibility to WTC-related airflow obstruction early in the disease process. We measured biomarkers in blood from the first post-September 11, 2001, monitoring evaluation done within 6 months of exposure and used subsequent lung function to define disease status. Case subjects had FEV1 less than LLN at pulmonary evaluation within 6.5 years of September 11, 2001, whereas control subjects had FEV1 greater than or equal to LLN. From the first post-September 11, 2001 monitoring evaluation to pulmonary evaluation, case subjects’ FEV1 continued to decline, whereas control subjects’ lung function remained stable. Elevation in GM-CSF and MDC increased the risk of subsequent airflow obstruction by 250% and 295%, respectively. Elevated GM-CSF and MDC within 6 months of September 11, 2001, predicted airflow obstruction over the next 6.5 years with a specificity of 88% and sensitivity of 38%. The relatively low sensitivity of the model suggests the presence of independent pathways to airflow obstruction not identified in this analysis. These results were not due to weight as measured by BMI, WTC exposure intensity, age, or PMN concentration, because the logistic model adjusted for these confounders. This investigation is a step toward defining inflammatory biomarkers of increased risk for airflow obstruction with the goal of identifying subgroups in future disasters that may benefit from intensive monitoring and treatment.
Elevations of the two inflammatory biomarkers could be due to different exposures or different sensitivities to exposure between case subjects and control subjects. The proportion of cases with high exposure is slightly larger, but there is adequate representation of high exposure in case subjects and control subjects (26% for case subjects vs 17% for control subjects) (Table 1). The logistic regression model also adjusted for exposure intensity and was found to be nonsignificant. Thus, the more likely explanation for the large differences in these biomarkers may be differential inflammatory response to irritant exposure. Importantly, cases had lower pre-September 11, 2001, FEV1 % predicted than control subjects (88% vs 104%), suggesting that cases may have manifested their increased susceptibility to airflow obstruction before WTC exposure. There was no pre-September 11, 2001, serum available, so it is not possible to investigate if altered pre-exposure biomarker levels accounted for the differences in pre-exposure FEV1.
The 1,720 individuals who received SPE within 6.5 years post-September 11, 2001, formed an extensively evaluated cohort. We excluded patients with a history of smoking or abnormal lung function before September 11, 2001. The resulting study cohort of 801 patients had similar average lung function and WTC exposure as the parent pulmonary evaluation and monitoring cohorts. Two FDNY pulmonologists provided assessment and treatment of those with low FEV1 at any monitoring examination as well as for patients with normal lung function who were presented for evaluation of respiratory symptoms. As a result, the pulmonary evaluation cohort has considerable variation in severity of airflow obstruction as measured by FEV1. Case subjects had accelerated decline in FEV1, suggesting that the inflammatory process initiated by WTC exposure impacted lung function over the subsequent years.
Roles for GM-CSF and MDC in airway injury are biologically plausible. Human bronchial epithelial cells produce GM-CSF in response to PM up to 2.5 μm in size, and MDC (CCL22) is elevated in BAL of patients with asthma.22‐24 Cigarette smoke-induced lung inflammation elevates MDC.25 The association between case status and elevated levels of both GM-CSF and MDC was analyzed in a logistic model. The interaction between the two analytes was statistically nonsignificant, suggesting that they are independent risk factors for accelerated decline in FEV1. Therefore, inflammation leading to obstructive lung disease may be mediated by GM-CSF and MDC.
This study has several limitations. It uses a single cohort of WTC-exposed FDNY rescue workers. Serum collected soon after intense exposure to WTC from resident or other worker cohorts is unavailable. Therefore, replicating the biomarker findings in other cohorts is not feasible. Some analytes are increased in control subjects and may be protective factors, but because a majority of the analyte levels were below the limits of detection, measurement with higher sensitivity assays are needed to investigate this possibility. We have recently identified moderate reductions of α1-antitrypsin as another risk factor for accelerated decline of lung function after WTC exposure.26 The sensitivity of the model containing GM-CSF and MDC is low, so biomarkers defining other pathways to disease are likely undiscovered. Other pathways to disease need to be defined for serum biomarkers to be clinically useful in directing therapy designed to prevent airflow obstruction after irritant exposure. Finally, the 39 analytes tested are not biomarkers of exposure, since all individuals with serum stored during the monitoring had WTC exposure.
The results of this study emphasize the importance of rapidly mobilizing resources to conduct a medical monitoring and serum banking after a disaster exposes large populations to smoke, dust, and chemicals. Elevations of serum MDC and GM-CSF in individuals with irritant-induced airflow obstruction identify immune pathways that warrant further investigation as mediators of susceptibility to PM-induced lung injury. This insight into protein expression may guide future genetic polymorphism studies in MDC, GM-CSF, or other regulatory elements. Candidate susceptibility polymorphisms from this cohort could then be assayed in other WTC cohorts to assess their generalizability as risk factors for airflow obstruction following irritant exposure.
Acknowledgments
Author contributions: Drs Nolan and Weiden had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Nolan: contributed to study design; data collection and analysis; manuscript writing and preparation; and reviewing, editing, and approving the manuscript.
Dr Naveed: contributed to data collection and analysis; and reviewing, editing, and approving the manuscript.
Ms Comfort: contributed to data collection and reviewing, editing, and approving the manuscript.
Dr Ferrier: contributed to data collection and reviewing, editing, and approving the manuscript.
Dr Hall: contributed to study design, data analysis, and reviewing, editing, and approving the manuscript.
Ms Kwon: contributed to data collection and analysis and reviewing, editing, and approving the manuscript.
Ms Kasturiarachchi: contributed to data collection and reviewing, editing, and approving the manuscript.
Dr Cohen: contributed to study design, data analysis, and reviewing, editing, and approving the manuscript.
Ms Zeig-Owens: contributed to data collection and analysis and reviewing, editing, and approving the manuscript.
Ms Glaser: contributed to data collection and analysis and reviewing, editing, and approving the manuscript.
Dr Webber: contributed to data collection and analysis and reviewing, editing, and approving the manuscript.
Dr Aldrich: contributed to data collection and reviewing, editing, and approving the manuscript.
Dr Rom: contributed to manuscript writing and preparation; and reviewing, editing, and approving the manuscript.
Dr Kelly: contributed to participant recruitment and reviewing, editing, and approving the manuscript.
Dr Prezant: contributed to participant recruitment; manuscript writing and preparation; and reviewing, editing, and approving the manuscript.
Dr Weiden: contributed to study design; participant recruitment; data analysis; manuscript writing and preparation; and reviewing, editing, and approving the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Abbreviations
- FDNY
Fire Department City of New York
- G-CSF
granulocyte colony-stimulating factor
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- IP-10
interferon inducible protein-10
- LLN
lower limit of normal
- MDC
macrophage-derived chemokine
- MME
medical monitoring entry
- NHANES
National Health and Nutrition Examination Survey
- PFT
pulmonary function test
- PM
particulate matter
- PMN
polymorphonuclear neutrophil
- SPE
subspecialty pulmonary evaluation
- WTC
World Trade Center
Footnotes
For editorial comment see page 278
Funding/Support: This work was supported by the National Institutes of Health [Grants K23HL084191 (Dr Nolan), K24A1080298 (Dr Weiden), UL1 RR029893, T32-ES007267 (Drs Naveed and Ferrier), U01CA008617, RO1HL090316 (Dr Rom), and 1UL1RR029893 (New York University, Clinical and Translational Science Institute)] and the National Institute for Occupational Safety and Health [Grants U10-OH008243, U10-OH008242 (Dr Prezant)].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Lioy PJ, Georgopoulos P. The anatomy of the exposures that occurred around the World Trade Center site: 9/11 and beyond Ann N Y Acad Sci. 2006;107654–79. [DOI] [PubMed] [Google Scholar]
- 2.Rom WN, Reibman J, Rogers L, et al. Emerging exposures and respiratory health: World Trade Center dust Proc Am Thorac Soc. 2010;72142–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prezant DJ, Weiden M, Banauch GI, et al. Cough and bronchial responsiveness in firefighters at the World Trade Center site N Engl J Med. 2002;34711806–815. [DOI] [PubMed] [Google Scholar]
- 4.Banauch GI, Dhala A, Alleyne D, et al. Bronchial hyperreactivity and other inhalation lung injuries in rescue/recovery workers after the World Trade Center collapse Crit Care Med. 2005;33(supp 1):S102–S106. [DOI] [PubMed] [Google Scholar]
- 5.Banauch GI, Hall C, Weiden M, et al. Pulmonary function after exposure to the World Trade Center collapse in the New York City Fire Department Am J Respir Crit Care Med. 2006;1743312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldman DM, Baron SL, Bernard BP, et al. Symptoms, respirator use, and pulmonary function changes among New York City firefighters responding to the World Trade Center disaster Chest. 2004;12541256–1264. [DOI] [PubMed] [Google Scholar]
- 7.Banauch GI, Alleyne D, Sanchez R, et al. Persistent hyperreactivity and reactive airway dysfunction in firefighters at the World Trade Center Am J Respir Crit Care Med. 2003;168154–62. [DOI] [PubMed] [Google Scholar]
- 8.Reibman J, Lin S, Hwang SA, et al. The World Trade Center residents’ respiratory health study: new-onset respiratory symptoms and pulmonary function Environ Health Perspect. 2005;1134406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.From the Centers for Disease Control and Prevention Self-reported increase in asthma severity after the September 11 attacks on the World Trade Center–Manhattan, New York, 2001 JAMA. 2002;288121466–1467. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Self-reported increase in asthma severity after the September 11 attacks on the World Trade Center—Manhattan, New York, 2001 MMWR Morb Mortal Wkly Rep. 2002;5135781–784. [PubMed] [Google Scholar]
- 11.Kelly KJ, Connelly E, Reinhold GA, Byrne M, Prezant DJ. Assessment of health effects in New York City firefighters after exposure to polychlorinated biphenyls (PCBs) and polychlorinated dibenzofurans (PCDFs): the Staten Island Transformer Fire Health Surveillance Project Arch Environ Health. 2002;574282–293. [DOI] [PubMed] [Google Scholar]
- 12.Prezant DJ, Levin S, Kelly KJ, Aldrich TK. Upper and lower respiratory diseases after occupational and environmental disasters Mt Sinai J Med. 2008;75289–100. [DOI] [PubMed] [Google Scholar]
- 13.Webber MP, Gustave J, Lee R, et al. Trends in respiratory symptoms of firefighters exposed to the World Trade Center disaster: 2001-2005 Environ Health Perspect. 2009;1176975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aldrich TK, Gustave J, Hall CB, et al. Lung function in rescue workers at the World Trade Center after 7 years N Engl J Med. 2010;362141263–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiden MD, Ferrier N, Nolan A, et al. Obstructive airways disease with air trapping among firefighters exposed to World Trade Center dust Chest. 2010;1373566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnes PJ. Chronic obstructive pulmonary disease: effects beyond the lungs PLoS Med. 2010;73e1000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoek G, Brunekreef B. Acute effects of a winter air pollution episode on pulmonary function and respiratory symptoms of children Arch Environ Health. 1993;485328–335. [DOI] [PubMed] [Google Scholar]
- 18.Brunekreef B, Kinney PL, Ware JH, et al. Sensitive subgroups and normal variation in pulmonary function response to air pollution episodes Environ Health Perspect. 1991;90189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostro BD, Lipsett MJ, Mann JK, Krupnick A, Harrington W. Air pollution and respiratory morbidity among adults in southern California Am J Epidemiol. 1993;1377691–700. [DOI] [PubMed] [Google Scholar]
- 20.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes Eur Respir J. 2005;263511–522. [DOI] [PubMed] [Google Scholar]
- 21.Herbert R, Moline J, Skloot G, et al. The World Trade Center disaster and the health of workers: five-year assessment of a unique medical screening program Environ Health Perspect. 2006;114121853–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu L, Jarjour NN, Busse WW, Kelly EA. Enhanced generation of helper T type 1 and 2 chemokines in allergen-induced asthma Am J Respir Crit Care Med. 2004;169101118–1124. [DOI] [PubMed] [Google Scholar]
- 23.Reibman J, Hsu Y, Chen LC, et al. Size fractions of ambient particulate matter induce granulocyte macrophage colony-stimulating factor in human bronchial epithelial cells by mitogen-activated protein kinase pathways Am J Respir Cell Mol Biol. 2002;274455–462. [DOI] [PubMed] [Google Scholar]
- 24.Hartl D, Griese M, Nicolai T, et al. Pulmonary chemokines and their receptors differentiate children with asthma and chronic cough J Allergy Clin Immunol. 2005;1154728–736. [DOI] [PubMed] [Google Scholar]
- 25.Ritter M, Göggel R, Chaudhary N, et al. Elevated expression of TARC (CCL17) and MDC (CCL22) in models of cigarette smoke-induced pulmonary inflammation Biochem Biophys Res Commun. 2005;3341254–262. [DOI] [PubMed] [Google Scholar]
- 26.Banauch GI, Brantly M, Izbicki G, et al. Accelerated spirometric decline in New York City firefighters with α1-antitrypsin deficiency Chest. 2010;13851116–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]