Abstract
Idiotype (Id) protein in combination with GM-CSF has been used as vaccines for immunotherapy of patients with myeloma and B-cell tumors and the results have been disappointing. To search for better immune adjuvants to improve the efficacy of Id-based immunotherapy in myeloma, we evaluated and compared the efficacy of vaccination of Id protein in combination with CpG or IFN-α, or GM-CSF as a control, in the 5TGM1 myeloma mouse model. Our results showed that Id vaccine combined with CpG or IFN-α, but not GM-CSF, not only efficiently protected mice from developing myeloma but also eradicated established myeloma. The therapeutic responses were associated with an induction of strong humoral immune responses including anti-Id antibodies, and cellular immune responses including Id- and myeloma-specific CD8+ cytotoxic T lymphocytes (CTLs), CD4+ type-1 T-helper (Th1) cells and memory T cells in mice receiving Id vaccine combined with CpG or IFN-α. Furthermore, Id vaccine combined with CpG or IFN-α induced Id- and tumor-specific memory immune responses that protected surviving mice from tumor rechallenge. Thus, our study clearly shows that CpG or IFN-α are better immune adjuvants than GM-CSF. This information will be important for improving the strategies of Id-based immunotherapy for patients with myeloma and other B-cell tumors.
Keywords: Multiple myeloma, Idiotype, Adjuvants, Vaccination, Immunotherapy
Introduction
Multiple myeloma (MM) is a B-cell malignancy, characterized by an accumulation of malignant plasma cells within the bone marrow. Myeloma cells secrete, in most cases, a monoclonal immunoglobulin (m-protein or idiotype protein; Id) and induce skeletal destruction and hypercalcemia. Despite the progress in therapy of the disease, MM still remains an incurable malignancy in most of the patients [1, 2]. Therefore, there is a great need for new treatments to stabilize or eradicate minimal residual tumors achieved after high-dose chemotherapy supported by autologous stem-cell transplantation [3].
Idiotype protein is a tumor-specific antigen because of the unique antigenic structure in its variable regions. Id-based immunotherapy has been explored in patients with MM and other B-cell tumors for inducing or enhancing Id-specific immune responses that could control the tumor cells [4, 5]. Recent studies have shown that although Id proteins were efficient in protecting mice from subsequent tumor challenge, the antigen by itself is not enough to promote the differentiation of naïve T cells into effector cells [6]. Tumor-associated antigens, which are poorly immunogenic, elicit anergic or regulatory T cells, when administered alone without appropriate immunostimulatory molecules. Thus, immunological adjuvant is one of the important strategies to enhance the desired immune responses to weak antigens. It will be particularly important for therapeutic vaccines targeting tumor antigens, where adjuvants are necessary to overcome various tolerance mechanisms, elicit activation of antigen presenting cells (APCs) in the presence of tumor antigen, and facilitate induction of cytotoxic T lymphocytes (CTLs) that can directly lyse tumor cells [7]. Thus far, many investigators have examined vaccination with Id protein in combination with immunological adjuvants, such as granulocyte-monocyte colony-stimulating factor (GM-CSF), interleukin (IL)-12 and aluminum, for the treatment of patients with MM as well as other B-cell malignancies [8–10], and results have been rather disappointing. Therefore, there is a great need to search for more potent immunological adjuvants to efficiently enhance myeloma-specific immune responses after vaccination.
In this study, we explored and compared the effects of Id protein-based immunotherapy in combination with various adjuvants, such as GM-CSF, interferon (IFN)-α, and synthetic oligodeoxnucleotides containing unmethylated CG dinucleotides (CpG), to enhance the immunogenicity of Id protein in the 5TGM1 myeloma mouse model originally derived from 5T33 myeloma cells [11, 12]. Such a study is important for selection and use of more potent adjuvants for future clinical immunotherapy studies in patients.
Materials and methods
Mice and cell lines
The C57BL/KaLwRij mice were purchased from Harlan CPB (Zeist, The Netherland) and maintained in an American Association of Laboratory Animal Care-accredited animal facility. This study was approved by the Institutional Animal Care and Use Committee of The University of Texas, MD Anderson Cancer Center. All mice were 6–8 weeks old at the beginning of each experiment. The 5TGM1 murine myeloma cell line was cultured in Iscove’s modified dulbecco’s media (IMDM; Invitrogen, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Thermo Scientific, Rockford, IL), 100 U/mL penicillin–streptomycin, and 2 mM l-glutamine (both from Invitrogen). The B16 melanoma cell line, originated from C57BL/6 mice, was purchased from American Type Culture Collection (ATCC; Rockville, MD) and cultured in IMDM.
Preparation of idiotype protein
The 5TGM1 myeloma cells were cultured in Hybridoma-serum-free medium (Invitrogen) and mouse IgG2b Id protein, secreted by the 5TGM1 myeloma cells, was purified from cell culture supernatant using Hi-Trap Protein A affinity chromatography (GE Healthcare, Piscataway, NJ) as described previously [13]. Id protein and keyhole limpet hemocyanin (KLH; EMD Biosciences, La Jolla, CA) conjugate was made using 0.1% of glutaraldehyde (Sigma, St. Louis, MO) to enhance the immunogenicity of the Id protein as described previously [14].
Vaccination of mice
Each experiment included four groups of mice and was repeated two times. In prophylaxis studies, mice were subcutaneously vaccinated by a weekly injection of 100 μg of Id-KLH protein vaccine for a total of three injections. Following each vaccination, GM-CSF (200 ng/day per mouse; R&D Systems, Minneapolis, MN) or IFN-α (5,000 U/day per mouse; PBL InterferonSource, Piscataway, NJ) was injected subcutaneously adjacent to the vaccination sites for three consecutive days. CpG-ODN-1826 (CpG; TCCATGACGTTCCTGACGTT; InvivoGen, San Diego, CA) was injected at dose of 50 μg/mouse mixed with Id-KLH protein vaccine. Control mice received injections of phosphate-buffered saline (PBS) mixed with Id-KLH protein vaccine. One week after the final vaccination, mice were challenged intravenously with 1 × 106 5TGM1 myeloma cells and tumor burden was monitored by measuring serum IgG2b Id protein. In therapeutic studies, mice were injected intravenously with 1 × 106 5TGM1 myeloma cells. Ten days later when myeloma growth is established, mice were treated with 100 μg of Id-KLH protein vaccine followed by injected with either GM-CSF, IFN-α, or CpG as described previously.
Detection of IgG2b Id protein and anti-Id or anti-KLH antibodies
Enzyme-linked immunoabsorbent assay (ELISA) was used to measure titers of anti-Id and anti-KLH antibodies, as described previously [14]. When detecting anti-Id antibodies, horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Southern Biotech, Birmingham, AL) was pre-absorbed against Id protein to reduce unspecific binding. The same assay was also used to measure the serum IgG2b Id protein for the monitoring of tumor burden as described previously [6].
Immunophenotyping
Fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, or allophycocyanin (APC)-conjugated monoclonal antibodies (mAbs) against CD4, CD8, and CD44 (all from eBioscience, San Diego, CA) were added to splenocytes, incubated for 30 min at 4°C, washed twice, and analyzed using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA).
Detection of cytokine production
Intracellular staining of IFN-γ was performed using a Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer’s instruction. Mice were subcutaneously vaccinated once every week for a total of three times with Id-KLH protein in combination with either GM-CSF, IFN-α or CpG, and PBS was injected as control. One week later, spleens were harvested from the mice and mashed through a cell strainer (BD Biosciences) with a plunger of syringe to generate single splenocytes. The splenocytes were cultured with irrelevant mouse IgG2b, Id protein or irradiated (80 Gy) 5TGM1 myeloma cells for 24 h, and GolgiPlug (BD Biosciences) was added for the final 6 h before staining to inhibit cytokine secretion. T cells were stained with FITC-conjugated anti-CD8 or CD4 mAbs, followed by fixation and permeabilization, stained with APC-conjugated anti-IFN-γ mAbs (eBiosciences), and after washing, analyzed using a FACSCalibur flow cytometer.
In some experiments, ELISA was used to measure secreted cytokine of IFN-γ from T cells. Splenocytes were restimulated for 5 days with irradiated 5TGM1 myeloma cells. Supernatants were collected and the amounts of cytokines were quantified using commercially available ELISA kits (eBioscience) according to the manufacturer’s instruction. All assays were performed in duplicate.
Antigen-specific T cell proliferation assay
Splenocytes were pre-labeled with 5 μM of 5(6)-carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen) for 10 min at 37°C. After washing, labeled cells were seeded and restimulated with irrelevant mouse IgG2b, Id protein or irradiated 5TGM1 myeloma cells for 5 days. After that, the cells were incubated with PE-conjugated anti-CD8 or CD4 mAbs for 30 min, washed, and flow cytometric analysis was used to detect dilution of CFSE.
Cytotoxicity assay
The standard 51Cr-release assay was performed to measure the cytotoxicity of the T cells against 5TGM1 myeloma cells [15]. As no myeloma cell lines from C57BL mice are available, B16 melanoma cells were used as control target cells. Target cells were labeled with 50 μCi of 51Cr-sodium chromate (PerkinElmer, Waltham, MA) for 1 h and incubated with various numbers of T cells in 96-well U-bottom tissue culture plates in RPMI-1640 complete medium. After 4 h, 50% of the supernatants were collected, and radioactivity was measured by a gamma-counter. All assays were performed in triplicate. Results are shown as mean percentage 51Cr-release calculated as follows:
 |
In some experiments, dendritic cells (DCs) were generated from murine bone marrow stem cells as described previously [16]. At day 8, immature DCs were pulsed with Id protein at a concentration of 50 μg/mL, followed by addition of tumor necrosis factor-α (TNF-α; 10 ng/mL) and IL-1β (10 ng/mL; both from R&D Systems) for 48 h to induce DC maturation. Mature DCs were collected and used as target cells. Unpulsed DCs were used as control target cells.
Statistical analysis
Student’s t test was used to compare various experimental groups. P < 0.05 was considered statistically significant. Survival was evaluated from the day of tumor injection until death, and Kaplan–Meier test used to compare mouse survival between the groups. All data are shown as mean and SD.
Results
Idiotype protein vaccine in combination with CpG or IFN-α but not GM-CSF protects mice from developing myeloma
In the prophylactic study, as shown in Fig. 1a, mice were subcutaneously vaccinated by a weekly injection of 100 μg Id-KLH protein in combination with either GM-CSF, IFN-α, or CpG for a total of three injections. Control mice were received injection of PBS. One week after the final vaccination, 1 × 106 5TGM1 myeloma cells were challenged intravenously, and tumor burden was monitored by measuring circulating IgG2b Id protein. Mice were humanely sacrificed when moribund or hind-leg paralysis developed. As shown in Fig. 1b, Id-KLH protein vaccines in combination with the adjuvants had some protective effects; one out of five mice receiving GM-CSF (P < 0.05, compared with mice receiving PBS), three out of five mice receiving IFN-α (P < 0.01), and all mice receiving CpG (P < 0.01) displayed no increase in serum IgG2b Id protein and showed no sign of myeloma. In contrast, all mice receiving injections of PBS developed myeloma. The same results were obtained with repeated experiment with 5 mice per group. Mouse survival data summarizing all 10 mice per group are shown in Fig. 1c. All mice receiving PBS died within 60 days after tumor injection, whereas 20 and 60% of mice receiving GM-CSF and IFN-α, respectively, survived without detectable tumors. Moreover, all mice receiving CpG survived without tumor burden. The Kaplan–Meier test showed that mice receiving CpG had better survival than those treated with GM-CSF (P < 0.01), while the difference between mice receiving GM-CSF and IFN-α was no statistically significant. These results show that Id protein vaccine in combination with CpG, and probably with IFN-α, provides better protection than that with GM-CSF in mice against developing myeloma.
Fig. 1.
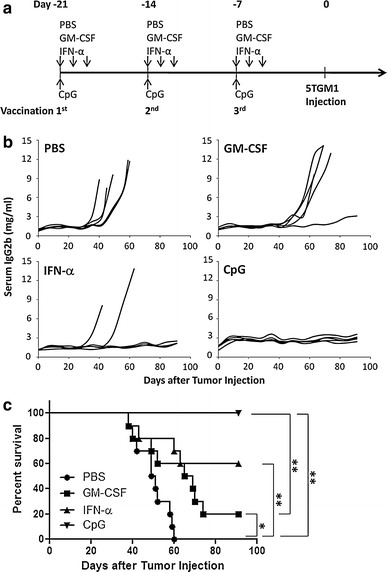
In vivo protective effect of Id protein vaccine in combination with different adjuvants. a Schedule of mouse vaccination for protective effect. C57BL/KaLwRij mice were subcutaneously vaccinated by a weekly injection of Id-KLH protein vaccine together with either GM-CSF, IFN-α or CpG for a total of three injections. PBS served as control. One week after the third vaccination, all mice were challenged intravenously with 1 × 106 5TGM1 myeloma cells. Serum samples were collected weekly to monitor the tumor burden. b Tumor burden measured as levels of serum IgG2b Id protein in mice (5 per group) receiving Id-KLH protein vaccine in combination with PBS, GM-CSF, IFN-α or CpG. Representative results from one of two experiments performed are shown. c Survival data of mice (10 per group, summarized from two experiments) receiving Id-KLH protein vaccine in combination with PBS, GM-CSF, IFN-α or CpG. *P < 0.05; **P < 0.01
Idiotype protein vaccine in combination with CpG or IFN-α, but not GM-CSF, was therapeutic against established myeloma
To examine and compare the efficacy of the Id protein vaccine in combination with the adjuvants in treating established myeloma, as shown in Fig. 2a, mice were first challenged with 5TGM1 myeloma cells intravenously. Ten days later, vaccinations were given to tumor-bearing mice once every week for a total of three times. As shown in Fig. 2b, myeloma-bearing mice receiving injections of PBS or GM-CSF all died of myeloma with large tumor burdens, whereas one out of five mice receiving IFN-α (P < 0.01, compared with mice receiving PBS) and four out of five mice receiving CpG (P < 0.01, compared with mice receiving PBS) displayed no increase in serum IgG2b Id protein and showed no sign of myeloma. The same results were obtained with repeated experiment with 5 mice per group. Based on the survival curve (Fig. 2c) from all 10 mice per each group, mice receiving PBS and GM-CSF all died within 53 and 73 days, respectively, after tumor injection, while 20 and 80% of mice receiving IFN-α and CpG, respectively, survived without detectable tumors. Mice receiving CpG (P < 0.01) or IFN-α (P < 0.01) had better survival than mice receiving GM-CSF, and mice receiving CpG (P < 0.05) had better survival than mice receiving IFN-α. These results demonstrate that Id protein vaccine in combination with either CpG or IFN-α efficiently retarded tumor growth and induced tumor regression.
Fig. 2.
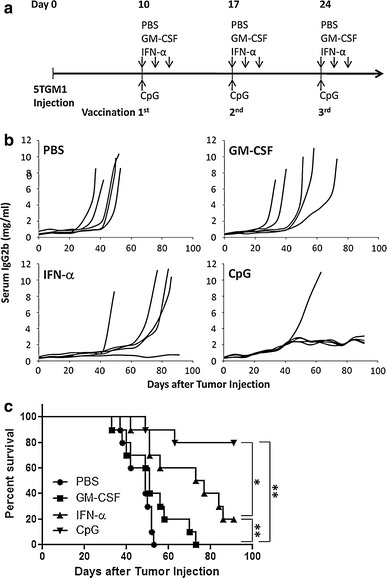
In vivo therapeutic effect of Id protein vaccine in myeloma-bearing mice. a Schedule of mouse vaccination for therapeutic effect. C57BL/KaLwRij mice were challenged with 1 × 106 5TGM1 myeloma cells, and 10 days after tumor injection, the vaccinations with Id-KLH protein vaccine in combination with GM-CSF, IFN-α or CpG were given to the mice once every week for a total of three times. PBS served as control. Serum samples were collected weekly to monitor the tumor burden. b Tumor burden measured as levels of serum IgG2b Id protein in mice (5 per group) receiving Id-KLH protein vaccine in combination with PBS, GM-CSF, IFN-α or CpG. Representative results from one of two experiments performed are shown. c Survival curve of mice (10 per group, summarized from two experiments) receiving Id-KLH protein vaccine in combination with PBS, GM-CSF, IFN-α or CpG. *P < 0.05; **P < 0.01
Idiotype protein vaccine in combination with CpG or IFN-α was potent at inducing Id- and KLH-specific antibody responses in vivo
To elucidate the immunological mechanisms underlying these phenomena, we first examined vaccine-induced humoral immune responses. Serum samples were collected from mice after the third vaccination, and titers of anti-Id and anti-KLH antibodies were measured by ELISA. Id-KLH protein vaccine in combination with CpG, IFN-α and GM-CSF induced significantly higher titers of anti-Id (Fig. 3a) and anti-KLH (Fig. 3b) antibodies than PBS controls in treated tumor-free mice (P < 0.01, compared with mice receiving PBS). Mice receiving CpG had significantly higher titers of anti-Id antibodies than those receiving GM-CSF (P < 0.01) or IFN-α (P < 0.05), while the difference between mice receiving GM-CSF and IFN-α was no statistically significant. In tumor-bearing mice (Fig. 3c), Id protein vaccine in combination with the adjuvants also induced significantly higher titers of anti-Id antibodies than PBS control (P < 0.01), and mice receiving CpG had significantly higher titers of anti-Id antibodies than those receiving either GM-CSF (P < 0.01) or IFN-α (P < 0.01). However, compared with tumor-free mice, the titers of anti-Id antibodies in tumor-bearing mice were generally lower, while the titers of anti-KLH antibodies (Fig. 3d) were comparable to those found in tumor-free mice. These results suggest a role for the humoral immune responses in protecting mice from developing myeloma induced by the Id protein vaccine in combination with the adjuvants. Moreover, the lower titers of anti-Id antibodies in tumor-bearing than tumor-free mice may be the result of binding and neutralizing of the antibodies by the large amounts of circulating IgG2b Id protein, rather than the inability of the mice to mount humoral immune responses against the antigens.
Fig. 3.

Vaccination-induced humoral immune responses. Titers of serum anti-Id (a) and anti-KLH (b) antibodies in tumor-free mice; and titers of anti-Id (c) and anti-KLH (d) antibodies in tumor-bearing mice after three subcutaneous injections of Id-KLH protein vaccine in combination with PBS, GM-CSF, IFN-α or CpG. Shown are titers of the antibodies in mice (5 per group) 1 week after the third vaccination, and the bars represent the mean titers of the antibodies in each group. Representative results from one of two independent experiments are shown. **P < 0.01, compared with PBS control
Idiotype protein vaccine in combination with CpG or IFN-α induced strong IFN-γ T-cell immune responses against myeloma cells
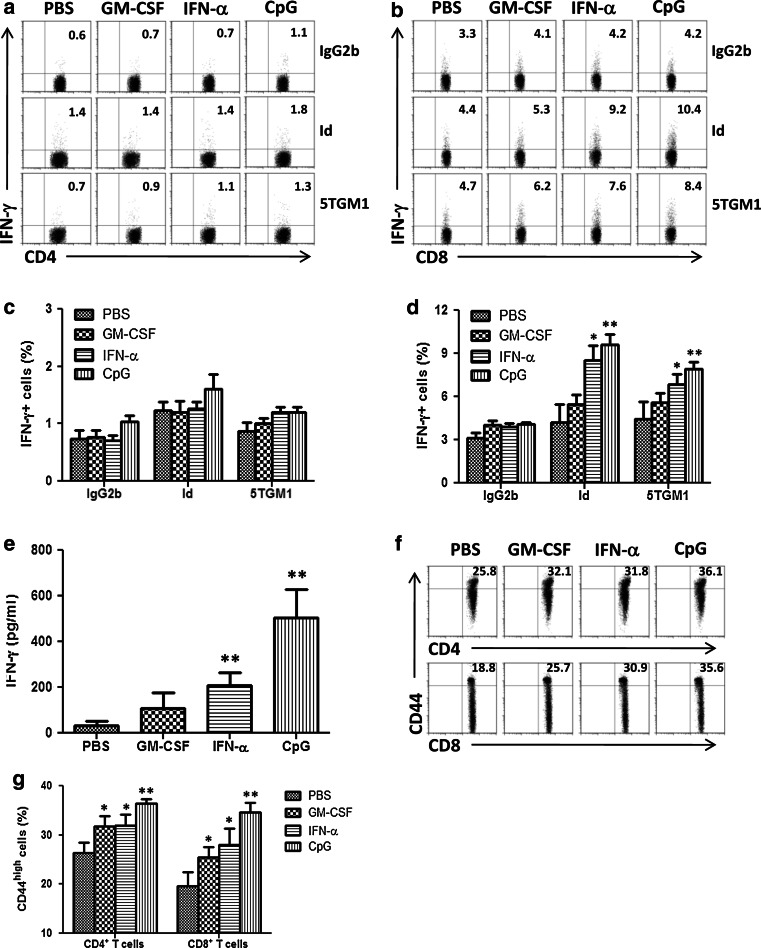
To examine the vaccine-induced cellular immune responses, we analyzed the type of T-cell responses induced by the vaccine in tumor-free mice after vaccination with Id-KLH protein in combination with the adjuvants. Splenocytes were collected and restimulated in vitro with Id protein, irradiated 5TGM1 myeloma cells or irrelevant mouse IgG2b, as control, for 24 h. As shown in Fig. 4b, intracellular cytokine staining showed that Id-KLH protein vaccine in combination with either IFN-α (P < 0.05, compared with PBS control) or CpG (P < 0.01; compared with PBS control), but not GM-CSF, induced increased percentages of IFN-γ-expressing CD8+ T cells in response to Id protein or 5TGM1 myeloma cells, whereas IFN-γ-expressing CD4+ T cells slightly increased in response to Id protein or 5TGM1 myeloma cells (Fig. 4a). In contrast, IFN-γ-expressing CD4+ or CD8+ T cells did not increase in response to irrelevant mouse IgG2b. In addition, the numbers of Id-stimulated, IFN-γ-secreting CD8+ T cells in mice receiving IFN-α (P < 0.05) or CpG (P < 0.01) were significantly higher than mice treated with GM-CSF. The percentages of IFN-γ-expressing CD4+ (Fig. 4c) and CD8+ T cells (Fig. 4d) were summarized from three independent experiments. Similar results were obtained with ELISA assay measuring the secretion of IFN-γ of splenocytes in response to 5TGM1 myeloma cells from the 4 different groups of mice (Fig. 4e). Flow cytometric analysis showed that both CD4+ and CD8+ (CD44high) memory T cells were increased in mice receiving Id-KLH protein vaccine in combination with either GM-CSF, IFN-α, or CpG (Fig. 4f). Moreover, CpG induced significantly higher percentages of CD44high memory T cells than GM-CSF or IFN-α (P < 0.05). Quantitative expression of these molecules was summarized from three independent experiments in Fig. 4g. These findings demonstrate that Id protein vaccine in combination with either IFN-α or CpG not only induced predominantly tumor-specific IFN-γ CD8+ T-cell responses but also generated CD4+ or CD8+ memory T cells.
Fig. 4.
Vaccination-induced cellular immune responses. Flow cytometry analyses showing the expression of IFN-γ in gated CD4+ (a) or CD8+ (b) T cells in response to irrelevant mouse IgG2b (IgG2b), Id protein (Id) or irradiated 5TGM1 myeloma cells (5TGM1). Values in each dot plot represent the percentages of CD4+ or CD8+ T cells expressing IFN-γ. Summarized data for percentages of IFN-γ-expressing CD4+ (c) or CD8+ (d) T cells from three different experiments are shown. In these studies, tumor-free mice (3 per group) were vaccinated with three subcutaneous injections of Id-KLH protein vaccine in combination with GM-CSF, IFN-α or CpG. PBS served as control. One week later, splenocytes were isolated, pooled, and restimulated with irrelevant mouse IgG2b, Id protein or irradiated 5TGM1 myeloma cells for 24 h. e Amount of secreted IFN-γ by tumor-specific T cells in splenocytes of mice that were restimulated with irradiated 5TGM1 myeloma cells for 5 days. Cytokine in cell culture media was quantified by ELISA. f Flow cytometry analyses showing the expression of CD4+ or CD8+ T cell surface marker and CD44 memory T cell marker. Values in each dot plot represent the percentages of CD44high T cells on gated CD4+ or CD8+ T cells. g Summarized data for percentages of CD44high T cells on gated CD4+ or CD8+ from three different experiments are shown. Representative results from one of three independent experiments are shown. The error bars represent SD of three independent experiments. *P < 0.05;**P < 0.01, compared with PBS control
To examine the functional properties of T cells in mice receiving Id protein vaccine in combination with the adjuvants, we examined T-cell proliferative responses induced in response to Id protein or irradiated 5TGM1 myeloma cells by using CFSE dilution assay. Splenocytes from mice receiving Id-KLH protein vaccine in combination with adjuvants were collected, labeled with 5 μM CFSE, and restimulated in vitro with Id protein, irradiated 5TGM1 myeloma cells, or irrelevant mouse IgG2b as control, for 5 days. An Id-specific CD4+ (Fig. 5a) or CD8+ T-cell proliferative response (Fig. 5b) was induced in mice receiving Id-KLH protein vaccine in combination with either IFN-α (P < 0.01), CpG (P < 0.01), or GM-CSF (P < 0.05), as compared with PBS controls. Moreover, the T-cell responses in mice receiving IFN-α or CpG were significantly stronger than that induced by GM-CSF (P < 0.05). Similar results were also obtained in T cells in response to 5TGM1 myeloma cells. In contrast, CD4+ or CD8+ T-cell proliferative response was not induced in response to irrelevant mouse IgG2b. Cultures of CD4+ or CD8+ T cells from mice receiving Id-KLH protein vaccine in combination with either IFN-α or CpG resulted in high percentages of proliferating T cells cultured with irradiated 5TGM1 myeloma cells. The percentages of proliferating CD4+ (Fig. 5c) and CD8+ T cells (Fig. 5d) were summarized from three independent experiments. These results confirm that Id- and tumor-specific CD4+ or CD8+ T cells were generated in mice receiving Id protein vaccine in combination with adjuvants, especially IFN-α or CpG.
Fig. 5.
Proliferative and cytotoxic activity of T cells in mice receiving Id protein vaccine. CFSE-dilution assay showing the percentages of proliferating CD4+ (a) and CD8+ (b) T cells in splenocytes of mice after restimulation in vitro with irrelevant mouse IgG2b (IgG2b), Id protein (Id) or irradiated 5TGM1 myeloma cells (5TGM1) for 5 days. Values in each histogram represent the percentages of dividing CD4+ or CD8+ T cells. Summarized data for percentages of proliferating CD4+ (c) or CD8+ (d) T cells from three different experiments are shown. Also shown is cytotoxicity activity against 5TGM1 myeloma cells (e), DCs pulsed with Id protein (f), B16 melanoma cells (g), or unpulsed DCs (h) of splenocytes of mice after restimulation with irradiated 5TGM1 myeloma cells for 7 days. In these studies, tumor-free mice (3 per group) were vaccinated with three subcutaneous injections of Id-KLH protein vaccine in combination with PBS, GM-CSF, IFN-α or CpG. Splenocytes were isolated, pooled, pre-labeled with 5 μM CFSE, restimulated with irrelevant mouse IgG2b, Id protein or irradiated 5TGM1 cells for 5 days, and measured for CSFE dilution, or splenocytes were isolated, pooled, and restimulated with irradiated 5TGM1 myeloma cells for 7 days, and the percentage of cytotoxicity was measured by 51Cr-release assay. Representative results from one of three experiments are shown. The error bars represent SD of three independent experiments. *P < 0.05; **P < 0.01, compared with PBS control
To evaluate the induction of tumor-specific CTL responses in these vaccinated mice, the standard 51Cr-release assay was used. Splenocytes from mice receiving Id-KLH protein vaccine in combination with adjuvants were restimulated with irradiated 5TGM1 myeloma cells for 7 days and subjected to analysis. T cells from mice receiving Id-KLH protein vaccine in combination with IFN-α (P < 0.01), CpG (P < 0.01), or GM-CSF (P < 0.05) efficiently and specifically lysed 5TGM1 myeloma cells (Fig. 5e) and DCs pulsed with Id protein (Fig. 5f) as compared with PBS control, although the T cells in mice receiving IFN-α or CpG displayed stronger cytotoxic activity compared with that from mice receiving GM-CSF (P < 0.05). No killing was observed against B16 melanoma cells (Fig. 5g) and unpulsed DCs (Fig. 5h). These results indicate that the Id protein vaccine in combination with either IFN-α or CpG was able to induce therapeutic myeloma-specific CTL responses in vivo.
Idiotype protein vaccine in combination with IFN-α or CpG protected surviving mice from tumor rechallenge
To examine whether the vaccine and adjuvant promotes an induction of tumor-specific immunity, mice that survived without tumor burden in the prophylactic and therapeutic experiments were rechallenged with the 5TGM1 myeloma cells 4 months after the first tumor inoculation. As controls, naïve mice were also injected with the tumor cells. As shown in Fig. 6a, all naïve mice developed myeloma, whereas all four mice receiving vaccine and IFN-α (P < 0.01, compared with naïve mice) and six out of eight mice receiving vaccine and CpG (P < 0.01, compared with naïve mice) displayed no increase in serum IgG2b Id protein and showed no sign of myeloma growth. Mouse survival data are shown in Fig. 6b; all naïve mice died within 50 days from tumor injection, whereas 100 and 70% of mice receiving IFN-α and CpG, respectively, survived without detectable tumor burden after tumor rechallenge. These results demonstrate that Id protein vaccine in combination with IFN-α or CpG induced myeloma-specific memory immune responses, which efficiently protected mice from tumor rechallenge.
Fig. 6.

Id protein vaccine in combination with CpG or IFN-α protects surviving mice against tumor rechallenge. Tumor burden measured as levels of serum IgG2b Id protein (a) and survival curve (b) of naïve mice (n = 5) or surviving mice from prior prophylactic and therapeutic studies of Id-KLH protein vaccine in combination with IFN-α (n = 4) or CpG (n = 8) after tumor rechallenge. In the studies, surviving mice were rechallenged 4 months later with intravenous injection of 1 × 106 5TGM1 myeloma cells and followed for tumor burden and survival. Naïve mice (n = 5) served as the control and were injected with the same number of 5TGM1 cells. **P < 0.01
Discussion
Idiotype protein, secreted by myeloma cells, has been the main target for immunotherapy in B-cell malignancies including MM, as it is the best-defined tumor-specific antigen. Unfortunately, the Id protein by itself is too weak to promote myeloma-specific immune responses in vivo. Id protein vaccines in combination with local injections of immunomodulatory molecules, especially GM-CSF, have been used as vaccines for active immunotherapy against MM and other B-cell malignancies. Although GM-CSF has previously been shown to be able to induce potent immunological and clinical responses in both preclinical and clinical studies [9, 14, 17–19], it is not enough to maintain the tumor-specific responses with large amounts of tumor burden. Thus, there is a great need for new and more potent adjuvants to enhance the immunogenicity of Id protein vaccines.
In this study we have chosen and used IFN-α and CpG as adjuvants to promote, accelerate, and prolong tumor-specific immune responses in combination with Id protein vaccine. IFN-α, produced primarily by plasmacytoid dendritic cells (pDCs), is a powerful polyclonal B-cell activator that induces a strong primary humoral immune responses and stimulate DC maturation by increasing the expression of costimulatory molecules including CD40, CD80, and CD86 and MHC antigens [20, 21]. Moreover, IFN-α not only acts directly on memory T cells to enhance their survival and indirectly through stimulation of IL-15 production by APCs that contributes to the survival of memory T cells, but also plays a role indirectly on proliferation and survival of CD4+ or CD8+ T cells through enhancing antigen presentation, costimulation and cross-priming [22–25]. Therefore, IFN-α has been used as a single agent as well as the immunological adjuvant in combination with vaccines for immunotherapy of cancers, such as leukemia, B-cell lymphoma, and melanoma [26–29]. Furthermore, IFN-α has been used in the maintenance treatment of MM [30, 31]. Thus, there is a good rationale for using IFN-α as an immunoadjuvant for Id-based vaccine in MM.
Synthetic oligodeoxynucleotides expressing CpG motifs, similar to those found in bacterial DNA, stimulate the immune responses. CpG can act as vaccine adjuvants by improving APC function, inducing cytokines/chemokines in microenvironment supportive of antigen-specific immunity and promoting the induction of antigen-specific adoptive immune responses [32, 33]. Indeed, activation of B cells and pDCs by CpG initiates an immunostimulatory cascade that culminates in the maturation, differentiation, and proliferation of natural killer (NK) cells, T cells, and monocytes/macrophages [34–36]. Therefore, CpG also has been used as an immunological adjuvant for promoting the immunogenicity of co-administered antigens in cancer immunotherapy [37–40].
In this study, we evaluated the capacity of Id protein vaccine in combination with either IFN-α or CpG to prevent and treat myeloma in the 5TGM1 myeloma murine model, which manifests similarly to human myeloma, including monoclonal gammopathy, marrow replacement, osteolytic bone lesions, and hypercalcemia [41, 42]. Our results clearly showed that Id-KLH protein vaccine in combination with either IFN-α or CpG, but not GM-CSF, efficiently protected mice against developing myeloma and protected surviving mice from tumor rechallenge, and retarded tumor growth and induced tumor regression against established tumor. In prophylactic and therapeutic experiments of Id vaccination, 100 and 80%, respectively, of mice receiving CpG as adjuvant survived of myeloma, 60 and 20%, respectively, of mice receiving IFN-α survived of myeloma, while only 20 and 0%, respectively, of mice receiving GM-CSF survived of myeloma without increase tumor burden. These results clearly showed that the adjuvants, especially CpG, are much better than GM-CSF for Id-based vaccines in myeloma. Our mechanistic studies showed that CpG induced a significantly stronger anti-myeloma immunity than IFN-α and GM-CSF. NK cells, T cells, and monocyte/marcrophage, which are stimulated by CpG, may secrete numerous cytokines and chemokines, such as IL-1, IL-6, IL-18, and TNF-α, that creates a pro-inflammatory microenvironment favoring the induction of tumor-specific Th1 and CTL responses [43, 44]. Therefore, it is possible that these various cytokines, chemokines, and immune cell subsets resulted in a synergistic anti-myeloma response.
We also demonstrated that Id protein vaccine in combination with the adjuvants induced significantly high titers of Id- and KLH-specific antibodies than vaccine alone, and CpG was more potent at inducing specific antibody responses in vivo than other adjuvants. We noticed that the titers of anti-Id antibodies were lower in tumor-bearing mice as compared with vaccinated tumor-free mice. This phenomenon can be explained by the presence of large amount of circulating Id proteins in tumor-bearing mice that likely neutralize the antibodies. Therefore, the role of anti-Id antibodies in controlling myeloma growth in vivo is unclear. Nevertheless, the fact that we can still detect free anti-Id antibodies in tumor-bearing mice suggest that these Id-specific antibodies may play a role in mediating the killing of myeloma cells in vivo directly or indirectly with the help of killer cells and/or complements in this murine model. This notion is supported by our previous finding that anti-Id antibodies can bind with the myeloma cells [6]. Moreover, we showed that the efficacy of Id protein vaccine in combination with either CpG or IFN-α to eradicate established myeloma was associated with an induction and expansion of potent tumor-specific CD8+ T-cell responses, increasing capacity of tumor-specific CD8+ CTLs to lyse myeloma cells, and generation of CD4+ and CD8+ memory T cells.
In conclusion, our study shows that Id protein vaccine in combination with CpG or IFN-α efficiently protected mice and eradicated tumor in the myeloma murine model. The therapeutic immunity against myeloma induced by the vaccines was associated with an induction of humoral immune response such as Id-specific antibodies, and cellular immune responses including myeloma-specific CTLs and Th1 cells. In addition, the vaccines induced specific immune memory responses that were able to protect surviving mice from tumor rechallenge. Thus, this study clearly shows that newer adjuvants such as CpG or IFN-α are much better than GM-CSF for Id-based immunotherapy in MM and this information is important for improving the efficacy of Id-based immunotherapy for patients with MM and possibly other B-cell tumors.
Acknowledgments
This study was supported by grants from the University of Texas M.D. Anderson Cancer Center, National Cancer Institute grants R01 s (CA96569, CA103978, CA138402, and CA138398) and P50 CA142509, the Leukemia and Lymphoma Society Translational Research Grants, Multiple Myeloma Research Foundation, and Commonwealth Foundation for Cancer Research.
Conflict of interest
The authors declare that they have no conflict of interest.
Abbreviations
- GM-CSF
Granulocyte-monocyte colony-stimulating factor
- IFN-α
Interferon-α
- CpG
Synthetic oligodeoxnucleotides containing unmethylated CG dinucleotides
References
- 1.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111(6):2962–2972. doi: 10.1182/blood-2007-10-078022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yi Q. Novel immunotherapies. Cancer J. 2009;15(6):502–510. doi: 10.1097/PPO.0b013e3181c51f0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yi Q. Immunotherapy in multiple myeloma: current strategies and future prospects. Expert Rev Vaccines. 2003;2(3):391–398. doi: 10.1586/14760584.2.3.391. [DOI] [PubMed] [Google Scholar]
- 5.Houot R, Levy R. Vaccines for lymphomas: idiotype vaccines and beyond. Blood Rev. 2009;23(3):137–142. doi: 10.1016/j.blre.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Wang S, Hong S, Wezeman M, Qian J, Yang J, Yi Q. Dendritic cell vaccine but not idiotype-KLH protein vaccine primes therapeutic tumor-specific immunity against multiple myeloma. Front Biosci. 2007;12:3566–3575. doi: 10.2741/2335. [DOI] [PubMed] [Google Scholar]
- 7.Dubensky TW, Jr, Reed SG. Adjuvants for cancer vaccines. Semin Immunol. 2010;22(3):155–161. doi: 10.1016/j.smim.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Bergenbrant S, Yi Q, Osterborg A, Bjorkholm M, Osby E, Mellstedt H, Lefvert AK, Holm G. Modulation of anti-idiotypic immune response by immunization with the autologous M-component protein in multiple myeloma patients. Br J Haematol. 1996;92(4):840–846. doi: 10.1046/j.1365-2141.1996.419959.x. [DOI] [PubMed] [Google Scholar]
- 9.Osterborg A, Yi Q, Henriksson L, Fagerberg J, Bergenbrant S, Jeddi-Tehrani M, Ruden U, Lefvert AK, Holm G, Mellstedt H. Idiotype immunization combined with granulocyte-macrophage colony-stimulating factor in myeloma patients induced type I, major histocompatibility complex-restricted, CD8- and CD4-specific T-cell responses. Blood. 1998;91(7):2459–2466. [PubMed] [Google Scholar]
- 10.Hansson L, Abdalla AO, Moshfegh A, Choudhury A, Rabbani H, Nilsson B, Osterborg A, Mellstedt H. Long-term idiotype vaccination combined with interleukin-12 (IL-12), or IL-12 and granulocyte macrophage colony-stimulating factor, in early-stage multiple myeloma patients. Clin Cancer Res. 2007;13(5):1503–1510. doi: 10.1158/1078-0432.CCR-06-1603. [DOI] [PubMed] [Google Scholar]
- 11.Garrett IR, Dallas S, Radl J, Mundy GR. A murine model of human myeloma bone disease. Bone. 1997;20(6):515–520. doi: 10.1016/S8756-3282(97)00056-2. [DOI] [PubMed] [Google Scholar]
- 12.Mundy G. Preclinical models of bone metastases. Semin Oncol. 2001;28(11):2–8. doi: 10.1016/S0093-7754(01)90225-8. [DOI] [PubMed] [Google Scholar]
- 13.Liso A, Stockerl-Goldstein KE, Auffermann-Gretzinger S, Benike CJ, Reichardt V, van Beckhoven A, Rajapaksa R, Engleman EG, Blume KG, Levy R. Idiotype vaccination using dendritic cells after autologous peripheral blood progenitor cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2000;6(6):621–627. doi: 10.1016/S1083-8791(00)70027-9. [DOI] [PubMed] [Google Scholar]
- 14.Kwak LW, Young HA, Pennington RW, Weeks SD. Vaccination with syngeneic, lymphoma-derived immunoglobulin idiotype combined with granulocyte/macrophage colony-stimulating factor primes mice for a protective T-cell response. Proc Natl Acad Sci USA. 1996;93(20):10972–10977. doi: 10.1073/pnas.93.20.10972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen YJ, Min R, Tricot G, Barlogie B, Yi Q. Tumor lysate-specific cytotoxic T lymphocytes in multiple myeloma: promising effector cells for immunotherapy. Blood. 2002;99(9):3280–3285. doi: 10.1182/blood.V99.9.3280. [DOI] [PubMed] [Google Scholar]
- 16.Hong S, Qian J, Yang J, Li H, Kwak LW, Yi Q. Roles of idiotype-specific t cells in myeloma cell growth and survival: Th1 and CTL cells are tumoricidal while Th2 cells promote tumor growth. Cancer Res. 2008;68(20):8456–8464. doi: 10.1158/0008-5472.CAN-08-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massaia M, Borrione P, Battaglio S, Mariani S, Beggiato E, Napoli P, Voena C, Bianchi A, Coscia M, Besostri B, Peola S, Stiefel T, Even J, Novero D, Boccadoro M, Pileri A. Idiotype vaccination in human myeloma: generation of tumor-specific immune responses after high-dose chemotherapy. Blood. 1999;94(2):673–683. [PubMed] [Google Scholar]
- 18.Bendandi M, Gocke CD, Kobrin CB, Benko FA, Sternas LA, Pennington R, Watson TM, Reynolds CW, Gause BL, Duffey PL, Jaffe ES, Creekmore SP, Longo DL, Kwak LW. Complete molecular remissions induced by patient-specific vaccination plus granulocyte-monocyte colony-stimulating factor against lymphoma. Nat Med. 1999;5(10):1171–1177. doi: 10.1038/13928. [DOI] [PubMed] [Google Scholar]
- 19.Neelapu SS, Kwak LW, Kobrin CB, Reynolds CW, Janik JE, Dunleavy K, White T, Harvey L, Pennington R, Stetler-Stevenson M, Jaffe ES, Steinberg SM, Gress R, Hakim F, Wilson WH. Vaccine-induced tumor-specific immunity despite severe B-cell depletion in mantle cell lymphoma. Nat Med. 2005;11(9):986–991. doi: 10.1038/nm1290. [DOI] [PubMed] [Google Scholar]
- 20.Le Bon A, Schiavoni G, D’Agostino G, Gresser I, Belardelli F, Tough DF. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14(4):461–470. doi: 10.1016/S1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 21.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 22.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189(3):521–530. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8 + T cells in vivo by IL-15. Immunity. 1998;8(5):591–599. doi: 10.1016/S1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 24.Cho HJ, Hayashi T, Datta SK, Takabayashi K, Van Uden JH, Horner A, Corr M, Raz E. IFN-alpha beta promote priming of antigen-specific CD8+ and CD4+ T lymphocytes by immunostimulatory DNA-based vaccines. J Immunol. 2002;168(10):4907–4913. doi: 10.4049/jimmunol.168.10.4907. [DOI] [PubMed] [Google Scholar]
- 25.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202(5):637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sikora AG, Jaffarzad N, Hailemichael Y, Gelbard A, Stonier SW, Schluns KS, Frasca L, Lou Y, Liu C, Andersson HA, Hwu P, Overwijk WW. IFN-alpha enhances peptide vaccine-induced CD8+ T cell numbers, effector function, and antitumor activity. J Immunol. 2009;182(12):7398–7407. doi: 10.4049/jimmunol.0802982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Pucchio T, Pilla L, Capone I, Ferrantini M, Montefiore E, Urbani F, Patuzzo R, Pennacchioli E, Santinami M, Cova A, Sovena G, Arienti F, Lombardo C, Lombardi A, Caporaso P, D’Atri S, Marchetti P, Bonmassar E, Parmiani G, Belardelli F, Rivoltini L. Immunization of stage IV melanoma patients with Melan-A/MART-1 and gp100 peptides plus IFN-alpha results in the activation of specific CD8(+) T cells and monocyte/dendritic cell precursors. Cancer Res. 2006;66(9):4943–4951. doi: 10.1158/0008-5472.CAN-05-3396. [DOI] [PubMed] [Google Scholar]
- 28.Gutterman JU. Cytokine therapeutics: lessons from interferon alpha. Proc Natl Acad Sci USA. 1994;91(4):1198–1205. doi: 10.1073/pnas.91.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smalley RV, Andersen JW, Hawkins MJ, Bhide V, O’Connell MJ, Oken MM, Borden EC. Interferon alfa combined with cytotoxic chemotherapy for patients with non-Hodgkin’s lymphoma. N Engl J Med. 1992;327(19):1336–1341. doi: 10.1056/NEJM199211053271902. [DOI] [PubMed] [Google Scholar]
- 30.Schaar CG, Kluin-Nelemans HC, Te Marvelde C, le Cessie S, Breed WP, Fibbe WE, van Deijk WA, Fickers MM, Roozendaal KJ, Wijermans PW. Interferon-alpha as maintenance therapy in patients with multiple myeloma. Ann Oncol. 2005;16(4):634–639. doi: 10.1093/annonc/mdi125. [DOI] [PubMed] [Google Scholar]
- 31.Blade J, San Miguel JF, Escudero ML, Fontanillas M, Besalduch J, Gardella S, Arias J, Garcia-Conde J, Carnero M, Marti JM, Rozman C, Estape J, Montserrat E. Maintenance treatment with interferon alpha-2b in multiple myeloma: a prospective randomized study from PETHEMA (Program for the study and treatment of hematological malignancies, Spanish Society of Hematology) Leukemia. 1998;12(7):1144–1148. doi: 10.1038/sj.leu.2401039. [DOI] [PubMed] [Google Scholar]
- 32.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374(6522):546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 33.Shirota H, Sano K, Hirasawa N, Terui T, Ohuchi K, Hattori T, Shirato K, Tamura G. Novel roles of CpG oligodeoxynucleotides as a leader for the sampling and presentation of CpG-tagged antigen by dendritic cells. J Immunol. 2001;167(1):66–74. doi: 10.4049/jimmunol.167.1.66. [DOI] [PubMed] [Google Scholar]
- 34.Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci USA. 1996;93(7):2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun S, Zhang X, Tough DF, Sprent J. Type I interferon-mediated stimulation of T cells by CpG DNA. J Exp Med. 1998;188(12):2335–2342. doi: 10.1084/jem.188.12.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stacey KJ, Sweet MJ, Hume DA. Macrophages ingest and are activated by bacterial DNA. J Immunol. 1996;157(5):2116–2122. [PubMed] [Google Scholar]
- 37.Weiner GJ, Liu HM, Wooldridge JE, Dahle CE, Krieg AM. Immunostimulatory oligodeoxynucleotides containing the CpG motif are effective as immune adjuvants in tumor antigen immunization. Proc Natl Acad Sci USA. 1997;94(20):10833–10837. doi: 10.1073/pnas.94.20.10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gendron KB, Rodriguez A, Sewell DA. Vaccination with human papillomavirus type 16 E7 peptide with CpG oligonucleotides for prevention of tumor growth in mice. Arch Otolaryngol Head Neck Surg. 2006;132(3):327–332. doi: 10.1001/archotol.132.3.327. [DOI] [PubMed] [Google Scholar]
- 39.Mukherjee P, Pathangey LB, Bradley JB, Tinder TL, Basu GD, Akporiaye ET, Gendler SJ. MUC1-specific immune therapy generates a strong anti-tumor response in a MUC1-tolerant colon cancer model. Vaccine. 2007;25(9):1607–1618. doi: 10.1016/j.vaccine.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim SK, Ragupathi G, Cappello S, Kagan E, Livingston PO. Effect of immunological adjuvant combinations on the antibody and T-cell response to vaccination with MUC1-KLH and GD3-KLH conjugates. Vaccine. 2000;19(4–5):530–537. doi: 10.1016/S0264-410X(00)00195-X. [DOI] [PubMed] [Google Scholar]
- 41.Radl J, De Glopper ED, Schuit HR, Zurcher C. Idiopathic paraproteinemia. II. Transplantation of the paraprotein-producing clone from old to young C57BL/KaLwRij mice. J Immunol. 1979;122(2):609–613. [PubMed] [Google Scholar]
- 42.Asosingh K, Radl J, Van Riet I, Van Camp B, Vanderkerken K. The 5TMM series: a useful in vivo mouse model of human multiple myeloma. Hematol J. 2000;1(5):351–356. doi: 10.1038/sj.thj.6200052. [DOI] [PubMed] [Google Scholar]
- 43.Ballas ZK, Rasmussen WL, Krieg AM. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J Immunol. 1996;157(5):1840–1845. [PubMed] [Google Scholar]
- 44.Halpern MD, Kurlander RJ, Pisetsky DS. Bacterial DNA induces murine interferon-gamma production by stimulation of interleukin-12 and tumor necrosis factor-alpha. Cell Immunol. 1996;167(1):72–78. doi: 10.1006/cimm.1996.0009. [DOI] [PubMed] [Google Scholar]