Abstract
The identification of oncogenic alterations in subsets of non-small cell lung cancers (NSCLC) is transforming clinical care. Genomic rearrangements in the anaplastic lymphoma kinase (ALK) are detected in 3 to 7% of NSCLC patients. The ALK tyrosine kinase inhibitor crizotinib has demonstrated clinical efficacy in ALK rearranged NSCLC patients and was recently approved by the FDA. Crizotinib is currently under additional phase III clinical development as both initial and second line therapy for advanced ALK rearranged NSCLC. However new challenges in the diagnosis and treatment of this subset of NSCLC have emerged. These include the most effective means of diagnosing ALK rearranged NSCLC and the emergence of acquired drug resistance to crizotinib. In this review we will review current treatment and diagnosis as well as the present knowledge on acquired resistance mechanisms to crizotinib and discuss the strategies presently underway to clinically overcome acquired drug resistance.
Keywords: Non-small cell lung carcinoma, translocation, kinase inhibitor, drug resistance, mutation
Background
The anaplastic lymphoma kinase (ALK) is a receptor tyrosine kinase initially identified as a result of a translocation in anaplastic large cell lymphoma (ALCL) in 1998 (1). ALK plays a role during development and is not expressed in most, if any, adult tissues. In 2007, a fusion of ALK with echinoderm microtubule-associated protein-like 4 (EML4) was first identified in a non-small cell lung cancer (NSCLC) patient (2). This occurs due to a chromosomal inversion on chromosome 2p resulting in the formation of the EML4-ALK fusion oncogene (2). The chromosomal inversion does not always occur in the exact same location and multiple EML4-ALK variants have been identified (3). All involve the intracellular tyrosine kinase domain of ALK beginning at the portion encoded by exon 20. EML4, however, is variably truncated and gives rise to various variants of EML4-ALK (3). The most common variants were E13;A20 (the nomenclature refers to the exons in EML4 (E) that are fused to ALK (A)) and E6a/b;A20, which are also referred to as variants 1 and 3a/b, respectively. In addition to at least 9 variants have been reported to date, rare non-EML4 fusions partners have also been described (herein collectively referred to as ALK rearranged NSCLC) (3).
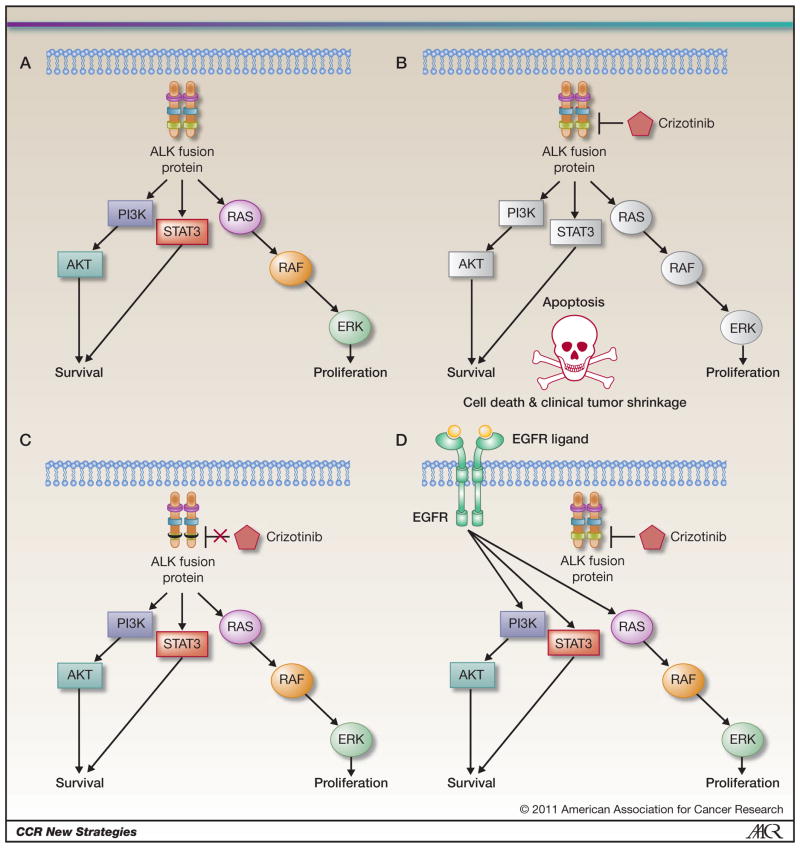
The EML4-ALK fusion protein is oncogenic both in vitro and in vivo (2, 4, 5). EML4-ALK fusions result in protein dimerization and constitutive activation of ALK kinase activity and of critical downstream signaling proteins involved in cell survival and proliferation (Fig 1A) (2, 6). Inhibition of ALK kinase activity, using small molecule ALK kinase inhibitors, leads to apoptosis in EML4-ALK NSCLC cell lines and in tumor shrinkage in murine models of EML4-ALK NSCLC (4, 5, 7).
Figure 1. ALK signaling in drug sensitive and resistant ALK rearranged cancers.
A. Oncogenic ALK activates downstream signaling pathways including the PI3K/Akt, STAT3 and RAS/RAF/ERK signaling pathways. B. Crizotinib inhibits ALK kinase activity leading to inhibition of downstream signaling pathways and resulting in apoptosis. C. ALK secondary mutations (depicted as a black box) prevent crizotinib from inhibiting ALK kinase activity D. EGFR signaling can independently activate downstream signaling pathways even in the presence of ALK inhibition by crizotinib.
Lung cancers harboring ALK rearrangements represent a unique subpopulation of lung cancer patients. The frequency of ALK rearrangements ranges from 3% to 7% in unselected NSCLC patients (3, 7, 8). Similar to epidermal growth factor receptor (EGFR) mutations, the frequency of this genetic alteration is higher in NSCLC patients with adenocarcinomas, and in those that are never or light (defined as ≤ 10 pack years) cigarette smokers. ALK rearrangements tend also to be mutually exclusive with EGFR and KRAS mutations and tend to have a lower frequency of p53 mutations (7, 8). Most tumors with ALK rearrangements are positive for thyroid transcription factor -1 (TTF-1). A variety of histologic features of ALK rearranged NSCLC have been reported including acinar, papillary, cribriform, mucin production (intra- and extra cytoplasmic), and signet-ring patterns (9).
Current Diagnosis and Treatment Strategies for ALK rearranged NSCLC
Clinical Diagnosis of ALK rearranged NSCLC
Detection of the ALK rearrangements can be challenging and several methods are currently being evaluated including fluorescence in situ hybridization (FISH), reverse transcription-polymerase chain reaction (RT-PCR) and immunohistochemistry (IHC). Each technique is associated with specific strengths and weaknesses for the screening of samples for ALK rearrangements.
Given the recent FDA approval of critozinib the evaluation for an ALK rearrangement should take place early on the in the treatment course. Routine molecular diagnostics need to include evaluation for both epidermal growth factor receptor (EGFR) mutations and for ALK rearrangements as the therapies are different for these two genetically defined subsets of lung cancer.
FISH
Current clinical trials of the ALK kinase inhibitor crizotinib use FISH as the diagnostic test for detecting an ALK rearrangement. This test is also recently FDA approved. In this assay, the 5′ and 3′ ends of the ALK gene are differently labeled with red and green fluorescent probes. Under normal circumstances, the two probes are close together. However, in the presence of an ALK rearrangement they are further apart (hence the term “break-apart” probe) red and green signals (8). Using a cut-off of >15% of cells and examining four or more fields (approximately 60 cells), high sensitivity and specificity have been reported (10–12). This assay detects ALK rearrangements regardless of the ALK fusion partner or the specific EML4-ALK variant. However, as EML4-ALK results from a chromosomal inversion on chromosome 2p and because the two genes are normally only about 12 Mb apart, the split signal can sometimes be very subtle and challenging to detect. In addition, as the assay requires specialized technical resources and expertise, it is not available in all pathology laboratories. Furthermore, FISH is a relatively low throughput assay and costly which is not ideal for detecting a relatively infrequent subset of NSCLC patients. Recent studies have compared ALK chromogenic in situ hybridization (CISH) to FISH and have demonstrated > 95% agreement between the two methods (13). CISH has potential advantages over FISH as it can be performed without the need for a fluorescence microscope and the signals are stable over long periods of time.
RT-PCR
As an alternative method of screening and confirming an ALK rearrangement, RT-PCR can also be used and offers some unique advantages. In the absence of contamination, this assay is very specific and the resulting PCR product can be sequenced to help define the specific type of translocation present (14). It is the only technique capable of definitively defining both the ALK fusion partner and the precise fusion variant. This technique can also be applied to samples with limited tissue, for example sputum specimens or material obtained by bronchoscopic biopsies (15). While RT-PCR is potentially the most sensitive assay, it requires adequate and sufficient quality RNA, which can be difficult to obtain from routine clinical samples such as formalin fixed paraffin embedded (FFPE) tumor specimens. In addition, given the large number of potential EML4-ALK variants, and non-EML4 ALK fusions, several PCR primer sets are necessary to cover all of the potential ALK rearrangements.
IHC
Immunohistochemistry is a diagnostic tool available in every clinical pathology laboratory. In addition, IHC is cost effective, can be incorporated into the existing sample flow for pathologists and an ideal tool to screen for a subpopulation of patients such as those harboring ALK rearrangements. Furthermore, as ALK is not normally expressed in the lung or in adults, any degree of ALK expression may be a sign of aberrant ALK expression due to an ALK rearrangement. The initial attempts to use ALK antibodies, used in to diagnose ALCL, in NSCLC were disappointing as in lung cancer ALK expression is much lower than in ALCL harboring an ALK translocation (9, 16). However, since the initial efforts, the detection of ALK by IHC has improved due to incorporation of techniques to enhance the IHC signal and to the development of more sensitive ALK antibodies (9, 16). Methods to amplify the IHC signaling using existing antibodies include the intercalated antibody-enhanced polymer (iAEP) approach (used with the ALK antibody 5A4) and tyramide amplification (using the ALK antibody ALK1) (9, 16). For example, the tyramide amplification method improves the ability to detect ALK by IHC from 40% to 80% of specimens harboring ALK rearrangements defined by FISH (9). Both techniques have been used successfully as a screening method in surgically resected specimens, as well as in smaller samples obtained by transbronchial needle biopsies (17). The mouse monoclonal antibody (D5F3) for ALK appears to offer sufficient sensitivity (100%) and specificity (99%), as compared with ALK FISH, and maybe able to be developed into a clinical diagnostic tool (11). However this is not yet commercially available thus limiting the ability to use it as a screening test. It is anticipated that the optimization of both IHC methods and identification of novel ALK antibodies will continue to evolve. IHC holds the greatest hope for a widely available, cost effective and rapid screening tool for the identification of patients with ALK rearranged NSCLC.
Treatment of ALK rearranged NSCLC
Since the ALK tyrosine kinase activity is necessary for its transforming activity and oncogenicity, its inhibition by a kinase inhibitor represents a potential therapeutic approach for ALK rearranged NSCLC (Fig 1B). Several ALK inhibitors have been identified and are being evaluated in pre-clinical models both in vitro and in vivo as potential clinical therapies (4, 18–20). In such models, ALK inhibitors lead to apoptosis in vitro and to tumor shrinkage in vivo thus demonstrating the phenomenon of “oncogene addiction” (4, 5, 18, 21) (Fig 1B). This is further highlighted by the dramatic clinical studies to date. Crizotinib (PF-02341066), an oral kinase inhibitor initially designed as a MET inhibitor, is a clinically effective ALK inhibitor in NSCLC patients harboring ALK rearrangements (10). In the phase I clinical trial, the response rate to crizotinib was 57% in the 82 treated patients (10). The study also highlighted the screening necessary to identify the patients. Over 1500 patients needed to be screened to identify the 82 patients with ALK rearrangements (10). As our targeted therapies are increasingly focusing on subsets of cancer patients the screening programs necessary to identify such patients also need to evolve. The ALK example highlights the value of this approach both for drug development and as means of identifying patients that will derive the greatest benefit from crizotinib treatment. Based on the dramatic clinical activity crizotinib has recently been approved by the FDA for ALK rearranged NSCLC. The clinical development has occurred over a remarkably short period of time, from the initial identification of the EML4-ALK translocation as oncogene in 2007, to validation as a clinical target in NSCLC in 2010 and to FDA approval in 2011 (2, 10).
Two randomized phase III clinical trials are currently underway to compare the use of crizotinib to standard of care (systemic chemotherapy) in patients with advanced ALK rearranged NSCLC. These include a phase III registration trial testing crizotinib versus second-line therapy (Pemetrexed or Docetaxel) as in ALK rearranged advanced NSCLC (NCT00932893). Another phase III clinical trial is testing crizotinib versus first line therapy (pemetrexed/cisplatin or pemetrexed/carboplatin) in treatment naïve ALK rearranged patients with advanced NSCLC (PROFILE 1014, NCT01154140). Based on the recent FDA approval of crizotinib, the National Comprehensive Cancer Network (NCCN) guidelines already recommend crizotinib as first line systemic therapy to patients with ALK rearranged NSCLC(22).
On the Horizon
Acquired Resistance to Crizotinib
ALK tyrosine kinase inhibitors are emerging as effective clinical therapies for ALK translocated cancers. However, as has been observed with other targeted therapies, including EGFR kinase inhibitors, their efficacy will ultimately be limited by the development of acquired drug resistance (23). The mechanistic understanding of drug resistance may help in the development effective subsequent clinical treatments and/or rational combination therapeutic strategies (Figure 2). How to best treat patients that develop acquired resistance to crizotinib has not yet been defined. However, several recent studies have begun to identify and study crizotinib resistance mechanisms. These include secondary mutations in the target of the kinase itself which abrogate the inhibitory activity of the drug and activation of alternative signaling pathways that bypass the continued requirement for inhibition of the original target (Fig 1C and D) (19, 24–26). The fraction of crizotinib resistance that is mediated by a secondary mutation as compared to activation by an alternative signaling pathway is currently not known.
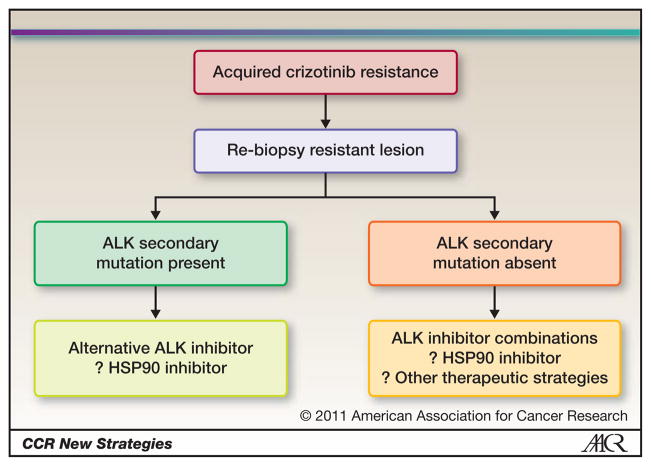
Figure 2. Potential therapeutic strategies for critozinib resistant ALK rearranged NSCLC.
NSCLC patients that develop acquired resistance can be divided into two major categories based on the presence or absence of an ALK secondary mutation detected in the resistant tumor specimen. For patients with secondary ALK mutations, a different ALK inhibitor, capable of overcoming the resistance mutation, may be an effective treatment strategy. However, if acquired resistance to crizotinib is not mediated by a secondary mutation, combination therapy strategies or HSP90 inhibitors may represent alternative therapeutic approaches.
Secondary mutations in kinases are a common mechanism of acquired drug resistance to kinase inhibitors (27). To date four acquired drug resistance mutations, all identified from crizotinib treated NSCLC or inflammatory myofibroblastic tumor (IMT) patients, have been reported (Fig 1C) (24–26). These mutations either involve the “gatekeeper” residue (L1196M) or residues away from crizotinib binding (L1152R, C1156Y and F1174L) (24–26). In vitro, cells engineered to express these secondary mutations were resistant to crizotinib. It is not clear how these secondary mutations actually lead to crizotinib resistance. Some possibilities include steric hindrance (for the L1196M mutation), promotion of a conformational change disfavoring critozinib binding and by increasing the affinity for ATP (24). Structural and biochemical studies of each of these mutations will be necessary to further understand how they lead to crizotinib resistance. In addition such studies may provide insight into the potential efficacy of next generation ALK kinase inhibitors.
Data is also emerging on mechanisms of crizotinib resistance that result from activation of an alternative signaling pathway. A recent study reported that activation of the EGFR signaling pathway can bypass the continued requirement for inhibition of ALK contributes to ALK inhibitor resistance (26). In some of these models, EGFR is activated by a ligand mediated process (Fig 1D). Concurrent inhibition of both EGFR and ALK is therapeutically effective in such resistant models (26). Additional studies are needed to evaluate changes in EGFR signaling from crizotinib treated tumor specimens and also to determine whether activation of other receptor tyrosine kinases can also contribute to crizotinib resistance.
Understanding the specific mechanism(s) of drug resistance is critical in selecting and evaluating subsequent therapeutic approaches. It is imperative that any new therapeutic strategy for patients that have developed acquired resistance to crizotinib incorporate tumor biopsies as part of the early clinical trials. This will be the only way to understand the potential benefits and limitations of a new therapeutic approach.
Treatment of ALK rearranged tumors with HSP90 Inhibitors
A second class of agents that has demonstrated clinical efficacy in ALK rearranged NSCLC patients is heat shock protein 90 (HSP90) inhibitors. EML4-ALK associates in complex with multiple cellular chaperones including HSP90 (5). Inhibitors of HSP90 disrupt this complex, lead to degradation of EML4-ALK and to tumor regression in xenograft and genetically engineered models of EML4-ALK NSCLC (5). In addition, cell lines bearing the crizotinib resistance mutations (L1196M and F1174L) remain equally sensitive to HSP90 inhibitors compared to ones without the secondary mutations (19, 25).
Clinical studies of two HSP90 inhibitors, retaspimycin (IPI-504) and ganetespib (STA-9090) have also demonstrated efficacy in ALK NSCLC patients (28, 29). Neither study was specifically designed to evaluate ALK rearranged patients and included NSCLC patients with other genotypes (such as EGFR and KRAS mutant). However, a substantial proportion of the ALK rearranged patients had partial responses which was not observed in patients with other genotypes (28, 29). Why EML4-ALK is a particularly good HSP90 client protein clinically remains to be determined. Additional clinical trials are underway to further evaluate HSP90 inhibitors in ALK rearranged NSCLC. Of note, the studies to date have predominately treated crizotinib naïve patients and whether HSP90 inhibitors will also be clinically effective in patients that have developed acquired resistance to crtizotinib is not presently known.
Next generation ALK kinase inhibitors and novel therapeutic combinations
Several new ALK kinase inhibitors have been developed and are currently entering early clinical development (Table 1). Some of these agents, including CH5424802, AP26113 and X-396, have been tested, and have been demonstrated pre-clinical efficacy, in models bearing crizotinib resistance mutations (18–20). Many of these new ALK inhibitors are also more potent inhibitors of ALK than crizotinib which was originally identified as a MET inhibitor (18, 20). Whether this increase in potency will translate into an increase in clinical efficacy is can only be determined from future clinical trials. Two drug development strategies are likely going to be employed. These include treating patients that have developed acquired resistance to crizotinib and patients that are crizotinib naïve. The former approach assumes that clinical acquired resistance to crizotinib will be mediated by an ALK dependent process (e.g. secondary mutation). However, the studies so far from actual patients that have developed acquired crizotinib resistance, to define the fraction of crizotinib resistance that is mediated by secondary mutations, are limited.
Table 1.
ALK tyrosine kinase inhibitors in development
| Drug | Company | Clinical stage | Notes |
|---|---|---|---|
| Crizotinib | Pfizer | Phase III and II for NSCLC | |
| CEP-37440 | Cephalon | Preclinical | |
| AP-26113 | Ariad Pharmaceuticals | Preclinical; IND expected 2011 | Effective against L1196M, S1206R & G1269S mutations |
| NMS-E628 | Nerviano Medical | Preclinical | |
| X-276/396 | Xcovery | Preclinical | Effective against L1196M ALK secondary mutations |
| TAE684 | Novartis | Not a clinical candidate | Effective against L1196M, F1174L ALK secondary mutations |
| CH5424802 | Chugai Pharmaceuticals | Preclinical; Phase I trial ongoing in Japan | Effective against L1196M, C1156Y & F1174L secondary mutations |
| LDK378 | Novartis | Phase I trial ongoing | |
| ASP3026 | Astellas | Phase I trial ongoing |
Combination treatment strategies may also be effective against crizotinib resistance. At the present time, there is an ongoing phase I clinical trial of crizotinib and the irreversible EGFR/HER2 inhibitor PF299804 (NCT01121575). This study was originally designed to evaluate the therapeutic benefit of inhibiting MET (crizotinib is a potent MET inhibitor) and EGFR T790M in erlotinib resistant EGFR mutant NSCLC patients (30). However, given recent pre-clinical studies, which suggest that the combination of crizotinib and PF299804 may represent a rational therapeutic strategy for a subset of patients that develop crizotinib resistance, this clinical trial may offer a rationale treatment strategy.
Acknowledgments
This study was supported by NIH R01CA136851 (P.A.J.), by the NCI Lung SPORE P50CA090578 (P.A.J.) and the Cammarata Family Foundation Research Fund (P.A.J.).
References
- 1.Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263:1281–4. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 2.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–67. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 3.Sasaki T, Rodig SJ, Chirieac LR, Janne PA. The biology and treatment of EML4-ALK non-small cell lung cancer. Eur J Cancer. 2010;46:1773–80. doi: 10.1016/j.ejca.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soda M, Takada S, Takeuchi K, Choi YL, Enomoto M, Ueno T, et al. A mouse model for EML4-ALK-positive lung cancer. Proc Natl Acad Sci U S A. 2008;105:19893–7. doi: 10.1073/pnas.0805381105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Z, Sasaki T, Tan X, Carretero J, Shimamura T, Li D, et al. Inhibition of ALK, PI3K/MEK, and HSP90 in murine lung adenocarcinoma induced by EML4-ALK fusion oncogene. Cancer Res. 2010;70:9827–36. doi: 10.1158/0008-5472.CAN-10-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takezawa K, Okamoto I, Nishio K, Janne PA, Nakagawa K. Role of ERK-BIM and STAT3-survivin signaling pathways in ALK inhibitor-induced apoptosis in EML4-ALK-positive lung cancer. Clin Cancer Res. 2011;17:2140–8. doi: 10.1158/1078-0432.CCR-10-2798. [DOI] [PubMed] [Google Scholar]
- 7.Koivunen JP, Mermel C, Zejnullahu K, Murphy C, Lifshits E, Holmes AJ, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14:4275–83. doi: 10.1158/1078-0432.CCR-08-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, Heist RS, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27:4247–53. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodig SJ, Mino-Kenudson M, Dacic S, Yeap BY, Shaw A, Barletta JA, et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res. 2009;15:5216–23. doi: 10.1158/1078-0432.CCR-09-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mino-Kenudson M, Chirieac LR, Law K, Hornick JL, Lindeman N, Mark EJ, et al. A novel, highly sensitive antibody allows for the routine detection of ALK-rearranged lung adenocarcinomas by standard immunohistochemistry. Clin Cancer Res. 2010;16:1561–71. doi: 10.1158/1078-0432.CCR-09-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camidge DR, Kono SA, Flacco A, Tan AC, Doebele RC, Zhou Q, et al. Optimizing the detection of lung cancer patients harboring anaplastic lymphoma kinase (ALK) gene rearrangements potentially suitable for ALK inhibitor treatment. Clin Cancer Res. 2010;16:5581–90. doi: 10.1158/1078-0432.CCR-10-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim H, Yoo SB, Choe JY, Paik JH, Xu X, Nitta H, et al. Detection of ALK Gene Rearrangement in Non-small Cell Lung Cancer: A Comparison of Fluorescence In Situ Hybridization and Chromogenic In Situ Hybridization with Correlation of ALK Protein Expression. J Thorac Oncol. 2011 doi: 10.1097/JTO.0b013e31821cfc73. [DOI] [PubMed] [Google Scholar]
- 14.Takeuchi K, Choi YL, Soda M, Inamura K, Togashi Y, Hatano S, et al. Multiplex reverse transcription-PCR screening for EML4-ALK fusion transcripts. Clin Cancer Res. 2008;14:6618–24. doi: 10.1158/1078-0432.CCR-08-1018. [DOI] [PubMed] [Google Scholar]
- 15.Nakajima T, Kimura H, Takeuchi K, Soda M, Mano H, Yasufuku K, et al. Treatment of lung cancer with an ALK inhibitor after EML4-ALK fusion gene detection using endobronchial ultrasound-guided transbronchial needle aspiration. J Thorac Oncol. 2010;5:2041–3. doi: 10.1097/JTO.0b013e3181f77b58. [DOI] [PubMed] [Google Scholar]
- 16.Takeuchi K, Choi YL, Togashi Y, Soda M, Hatano S, Inamura K, et al. KIF5B-ALK, a novel fusion oncokinase identified by an immunohistochemistry-based diagnostic system for ALK-positive lung cancer. Clin Cancer Res. 2009;15:3143–9. doi: 10.1158/1078-0432.CCR-08-3248. [DOI] [PubMed] [Google Scholar]
- 17.Sakairi Y, Nakajima T, Yasufuku K, Ikebe D, Kageyama H, Soda M, et al. EML4-ALK fusion gene assessment using metastatic lymph node samples obtained by endobronchial ultrasound-guided transbronchial needle aspiration. Clin Cancer Res. 2010;16:4938–45. doi: 10.1158/1078-0432.CCR-10-0099. [DOI] [PubMed] [Google Scholar]
- 18.Sakamoto H, Tsukaguchi T, Hiroshima S, Kodama T, Kobayashi T, Fukami TA, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell. 2011;19:679–90. doi: 10.1016/j.ccr.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Katayama R, Khan TM, Benes C, Lifshits E, Ebi H, Rivera VM, et al. Therapeutic strategies to overcome crizotinib resistance in non-small cell lung cancers harboring the fusion oncogene EML4-ALK. Proc Natl Acad Sci U S A. 2011;108:7535–40. doi: 10.1073/pnas.1019559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lovly CM, Heuckmann JM, de Stanchina E, Chen H, Thomas RK, Liang C, et al. Insights into ALK-Driven Cancers Revealed through Development of Novel ALK Tyrosine Kinase Inhibitors. Cancer Res. 2011;71:4920–31. doi: 10.1158/0008-5472.CAN-10-3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinstein IB. Cancer. Addiction to oncogenes--the Achilles heal of cancer. Science. 2002;297:63–4. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- 22.www.nccn.org
- 23.Engelman JA, Janne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2008;14:2895–9. doi: 10.1158/1078-0432.CCR-07-2248. [DOI] [PubMed] [Google Scholar]
- 24.Choi YL, Soda M, Yamashita Y, Ueno T, Takashima J, Nakajima T, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med. 2010;363:1734–9. doi: 10.1056/NEJMoa1007478. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki T, Okuda K, Zheng W, Butrynski J, Capelletti M, Wang L, et al. The neuroblastoma-associated F1174L ALK mutation causes resistance to an ALK kinase inhibitor in ALK-translocated cancers. Cancer Res. 2010;70:10038–43. doi: 10.1158/0008-5472.CAN-10-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasaki T, Koivunen J, Ogino A, Yanagita M, Nikiforow S, Zheng W, et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-11-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janne PA, Gray N, Settleman J. Factors underlying sensitivity of cancers to small-molecule kinase inhibitors. Nat Rev Drug Discov. 2009;8:709–23. doi: 10.1038/nrd2871. [DOI] [PubMed] [Google Scholar]
- 28.Sequist LV, Gettinger S, Senzer NN, Martins RG, Janne PA, Lilenbaum R, et al. Activity of IPI-504, a novel heat-shock protein 90 inhibitor, in patients with molecularly defined non-small-cell lung cancer. J Clin Oncol. 2010;28:4953–60. doi: 10.1200/JCO.2010.30.8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong K, Koczywas M, Goldman JW, Paschold EH, Horn L, Lufkin JM, et al. An open-label phase II study of the Hsp90 inhibitor ganetespib (STA-9090) as monotherapy in patients with advanced non-small cell lung cancer (NSCLC) Journal of Clinical Oncology. 2011;29 abstr 7500. [Google Scholar]
- 30.Turke AB, Zejnullahu K, Wu YL, Song Y, Dias-Santagata D, Lifshits E, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17:77–88. doi: 10.1016/j.ccr.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]