Abstract
Metal ions are required by all organisms in order to execute an array of essential molecular functions. They play a critical role in many catalytic mechanisms and structural properties. Proper homeostasis of ions is critical; levels that are aberrantly low or high are deleterious to cellular physiology. To maintain stable intracellular pools, metal ion-sensing regulatory (metalloregulatory) proteins couple metal ion concentration fluctuations with expression of genes encoding for cation transport or sequestration. However, these transcriptional-based regulatory strategies are not the only mechanisms by which organisms coordinate metal ions with gene expression. Intriguingly, a few classes of signal-responsive RNA elements have also been discovered to function as metalloregulatory agents. This suggests that RNA-based regulatory strategies can be precisely tuned to intracellular metal ion pools, functionally akin to metalloregulatory proteins. In addition to these metal-sensing regulatory RNAs, there is a yet broader role for metal ions in directly assisting the structural integrity of other signal-responsive regulatory RNA elements. In this chapter, we discuss how the intimate physicochemical relationship between metal ions and nucleic acids is important for the structure and function of metal ion- and metabolite-sensing regulatory RNAs.
1. INTRODUCTION
Metal ions serve many important roles in biology. They are required as enzymatic cofactors and help stabilize protein structures and macromolecular interactions. In particular, metal ions exhibit a crucial influence on the structure and function of cellular nucleic acids [1-3]. Indeed, a metal ion ‘atmosphere’ surrounds nucleic acids, mostly through weak nonspecific interactions with the polymer backbone. Such charge-screening ions function to neutralize the polyanionic backbone, thereby reducing the electrostatic penalty incurred when oligonucleotide strands are brought in close proximity. As a result, nucleic acid secondary structure features are highly dependent on the presence of metal ions. Mg2+ and K+ are the metal ions with the highest intracellular abundance; correspondingly, they are the predominant nonspecific ions closely associated with nucleic acids. In addition to these nonspecific interactions, individual metal ions also associate specifically to nucleic acids, sometimes with profound structural consequences accompanying these interactions. Unlike DNA, which exists almost exclusively in B-form double helices, biological RNAs often adopt complex three-dimensional folds. One outcome of these higher-order structural features is the dramatic variation in the local surface electrostatic potential of the folded molecule. This can result in formation of highly electronegative pockets, which can act as metal binding sites. Depending on the features of these individual sites, they may localize metal ions nonspecifically but with moderate affinity, or, alternatively, exhibit high selectivity toward a cognate metal ion. For the former, electrostatic interactions alone may govern metal binding. In other instances, metal ions can be bound via hydrogen bonding interactions of RNA functional groups with metal-associated waters. For a subset of RNA-associated metal ions, RNA groups chelate directly to the metal ion. These inner-sphere coordination interactions result in a high degree of metal ion selectivity. A variety of RNA functional groups have been found to bind metal ions via these inner-sphere coordinations. The primary atoms that mediate these interactions are nonbridging phosphate oxygens, the 2' hydroxyl, and a subset of nucleobase nitrogens and oxygens [3].
These different types of metal ion-binding sites appear to be generally important for structured RNAs. For example, an analysis of metal ions associated with the large ribosomal subunit revealed the presence of 116 and 88 Mg2+ and monovalent ions, respectively [4]. The majority of these metals were found to include at least one inner-sphere coordination to an RNA functional group. In many such instances, these metal ion-RNA interactions appear to specifically position the backbone and nucleoside atoms in order to facilitate noncanonical interactions or long-range tertiary contacts. These different types of metal ion sites are likely to be generally important for other structured RNAs, in addition to the ribosome. Indeed, three-dimensional structural analyses of an increasingly diverse array of noncoding RNA elements have confirmed this.
One of the fastest growing categories of noncoding RNAs is that of bacterial riboswitches. Riboswitches are cis-acting regulatory RNAs, mostly residing within the untranslated regions (UTRs) of mRNAs, which bind specific cellular metabolites and, in response, control gene expression [5-6]. Over 20 riboswitch classes have been identified, which respond to a wide variety of metabolite ligands, including enzyme cofactors, nucleobases, nucleosides, amino acids a second messenger, and an amino sugar [6-7]. Riboswitches, which have been subjected to extensive structural analyses in recent years, are comprised largely of two portions, a metabolite-sensing domain (aptamer) and downstream regulatory sequences that couple genetic control to the aptamer domain. The riboswitch aptamer domains associate with their target ligands both with high affinity and high selectivity. This is accomplished using an assortment of structural features that together create the riboswitch metabolite-binding pockets. As we discuss in this chapter, metal ions play an indispensable role in promoting aptamer tertiary structures. Moreover, in many instances, RNA-bound metals directly assist formation of the ligand-binding pockets. Study of these structures has expanded the functional repertoire of RNA-associated metal ions and has begun to provide new insights into the mechanisms that guide RNA folding pathways.
Metal ions are required for many cellular functions, in addition to their roles in stabilizing nucleic acid structures, and their absence is deleterious to the cell. However, inappropriately high intracellular concentrations of metal ions are also associated with cellular toxicity [8]. Therefore, proper homeostasis mechanisms are paramount to cell survival, a task that can be daunting given the considerable fluctuations of extracellular metal ion concentrations to which cells are exposed. The ability to accrue or export metals is largely accomplished through membrane-bound transporters [9-10]. Some of these transporter complexes regulate metal ion import through metal-responsive gating mechanisms involving cytoplasmic protein domains. However, as an additional layer of genetic control, microorganisms rely on many individual regulatory factors that control the acquisition, storage, and detoxification of metal ions [11]. These factors are mostly comprised of metal ion sensor proteins that regulate gene expression (metalloregulatory proteins) in response to metal ion fluctuations. The selectivity of these proteins for their target metal ions is achieved largely through recognition of the coordination chemistry of the metal-ion [12]. Binding of the appropriate metal ion to the metalloregulatory protein triggers changes in protein structure, which in turn alter the ability of the protein to activate or inhibit gene expression. Many examples of such metalloregulatory proteins have been discovered and characterized [9], mostly for control of transition metal ions. Less is known regarding regulation of other metals, such as magnesium [13].
In bacteria, metalloregulatory proteins are also often coupled to posttranscriptional regulatory circuits. For example, upon conditions of excessive iron the E. coli protein Fur (ferric uptake regulator) inhibits expression of iron uptake genes and inhibits the synthesis of certain small regulatory RNAs [14]. The decreased abundance of the latter then promotes de-repression of target mRNAs. However, for some organisms, control of metal ion homeostasis might not require metalloregulatory proteins at all. Instead, in these organisms RNA-based mechanisms may directly sense metal ion concentrations in order to regulate gene expression. As we discuss in this chapter, two categories of riboswitch-like RNA elements have been discovered that regulate magnesium ion transport genes for a variety of bacteria [15-16]. What is unique about these particular riboswitch RNA classes is the fact that they do not require a metabolite ligand for genetic control. Instead, metal ion-induced structural changes are directly coupled to control of downstream gene expression. In general, the discovery and characterization of these RNAs argues that bacteria are likely to have evolved both protein- and RNA-based mechanisms for metal ion homeostasis. In this chapter, we discuss both the roles of metal ions in metabolite-sensing riboswitches and the mechanisms of metal ion-responsive regulation by magnesium-sensing riboswitches.
2. METAL IONS THAT ASSIST RECOGNITION OF RIBOSWITCH LIGANDS
Some of the most compelling evidence for the direct participation of metal ions in the recognition of their small molecule ligands by riboswitches comes from X-ray crystallography. In evaluating the significance of these findings, it is necessary to appreciate their experimental basis. X-ray crystallography reveals the average location and degree of order of the electrons of the molecules that comprise the crystal (the electron density map). The crystallographer interprets features of the electron density map to correspond to, for instance, RNA, small molecule ligands, ordered water molecules, or metal ions. Interpretation is aided by several factors, but is more reliable when high-resolution data (better than ~1.8 Å) are available. First, the chemical composition of the crystal will limit the possibilities. Second, the electron density will be proportional to the number of electrons. Thus, a Mn2+ ion will be more electron dense than a water molecule. However, the degree of order (B-factor and occupancy) of the ion or molecule producing the electron density has to be taken into account. Third, if the resolution is sufficient to resolve all the ligands of an ion, its coordination geometry (bond lengths and angles) may be diagnostic. Fourth, the anomalous scattering properties (the wavelength-dependent absorption of X-rays) of different ions can be diagnostic. Thus, at typical X-ray wavelengths, Ca2+ and K+ may have anomalous signal, but not Mg2+ or Na+. Fifth, substitution experiments may be informative. For example, Mn2+ may be able to replace Mg2+ in crystals grown in the presence of the former ion. As with all experiments of this nature, however, the ability of Mn2+ to bind to a site in the RNA does not prove it to be a Mg2+ binding site, because the two ions have different chemical properties.
2.1 Participation of magnesium ion in binding pockets of phosphate-containing ligands
Crystal structures indicate that several riboswitches recognize small molecules containing a phosphate moiety through Mg2+ ions. In addition to helping neutralize the negative charge of the ligand to facilitate its binding to the anionic riboswitch, the specific chemical properties of the Mg2+ ion may play a role in small molecule recognition. At the time of writing, RNAs that appear to use this molecular recognition strategy are the thiamine-pyrophosphate (TPP) binding thi-box riboswitch, the glmS riboswitch-ribozyme, the flavin mononucleotide (FMN) specific or RFN riboswitch, and the cyclic diguanylate (c-di-GMP) riboswitch.
The 2.05 Å resolution structure of the E. coli thiM riboswitch aptamer domain cocrystallized with TPP in the presence of Mg2+ revealed that this RNA employs two partially hydrated Mg2+ ions to mediate its interactions with the pyrophosphate moiety of its ligand, which is bound in an extended conformation (Figure 1A). The RNA makes only two direct contacts to non-bridging oxygens of the pyrophosphate; all other RNA-pyrophosphate contacts are mediated by the bound Mg2+ ions or their waters of hydration [17]. Cocrystallization of the riboswitch in the presence of Mn2+, Ca2+, or Ba2+ revealed that these ions can all take the place of the two Mg2+ ions that coordinate the pyrophosphate of TPP. The identity of the ions was established by their anomalous diffraction signal [18]. These experiments indicate that the TPP riboswitch structure adapts to accommodate the different ionic radii of TPP-chelating ions, ranging from 0.81 Å (hexacoordinate Mn2+) to 1.49 Å (hexacoordinate Ba2+). Even more dramatic evidence of plasticity emerged from the crystal structure of the riboswitch bound to thiamine-monophosphate (TMP). The single phosphate of this ligand was observed to coordinate to two partially hydrated Mg2+ ions. The two metal ions allow the riboswitch to recognize the phosphate and pyrophosphate of TMP and TPP, respectively, using essentially equivalent interactions [18]. Nonetheless, this accommodation results in considerably weaker binding of TMP (Kd ~260 nM) than TPP (Kd ~ 8.4 nM, both measured at 10 mM Mg2+ concentration) [19]. The structure of the TPP riboswitch is conserved between bacteria and eukarya, and a 2.25 Å resolution structure confirmed that the eukaryotic riboswitch binds TPP in an identical manner, employing two Mg2+ ions to coordinate and recognize the pyrophosphate [20].
Figure 1.
Mg2+ ions mediate recognition of phosphate-containing riboswitch ligands. A. TPP is bound to its cognate riboswitch chelated to two Mg2+ ions 1. B. The phosphate group of FMN binds to single Mg2+ ion in the binding site of the cognate riboswitch 12.
In contrast to the plastic TPP riboswitch, crystallographic studies of the glmS riboswitch-ribozyme in multiple functional states revealed a rigid, pre-organized RNA [21]. This RNA from Gram-positive bacteria controls gene expression by binding to glucosamine-6-phosphate (GlcN6P). Binding of GlcN6P to the RNA uncovers a latent self-cleavage activity of the riboswitch [22]. The riboswitch is part of the 5'-UTR of the mRNA encoding the enzyme GlcN6P synthase, and self cleavage of the riboswitch ultimately results in destruction of the mRNA, thereby completing a negative feedback regulatory loop [23]. GlcN6P binds to the ribozyme in an open, solvent-accessible pocket with its amine group in contact with the scissile phosphate [21]. Biochemical and crystallographic experiments indicate that the amine group, in concert with the G40 nucleobase of the RNA catalyzes the cleavage reaction [21,24-25]. The phosphate group in GlcN6P appears to serve mainly to increase the affinity of the activator to the riboswitch. The phosphate hydrogen bonds to the G1 nucleobase of the riboswitch, and also coordinates fully hydrated Mg2+ ions that in turn hydrogen bond to the RNA. Comparison of the GlcN6P complex structures of glmS riboswitches from B. anthracis [26] and T. tengcongensis [27] at 2.5 Å and 1.7 Å resolution, respectively, indicates that the direct interactions of the activator with the RNA are highly conserved. In contrast, the position and number of the Mg2+ ions near the phosphate to GlcN6P varies from structure to structure. This, and the fact that all the Mg2+ interactions with phosphate of GlcN6P on one hand and the riboswitch on the other are mediated by waters (i.e. are outer-sphere) suggests that, in this case, the metal ions function in Coulombic stabilization, but not directly in molecular recognition.
The 2.95 Å resolution structure of a FMN riboswitch bound to its cognate ligand revealed a complex RNA fold with internal pseudo-2-fold symmetry. The cofactor is bound asymmetrically, and its phosphate group is coordinated by a Mg2+ ion that makes direct (i.e. inner-sphere) coordination with a non-bridging phosphate oxygen and the N7 nitrogen of a guanine base (Figure 1B). Due to the low resolution, the other ligands of the Mg2+ ion, presumed to be water, were not built into the crystallographic model [28]. The phosphate of FMN plays an important role in recognition by the riboswitch. Solution binding studies indicate that riboflavin, the unphosphorylated form of FMN, binds to the RNA ~1000 times more weakly in the presence of physiological monovalent and divalent ion concentrations (100 mM K+ and 2 mM Mg2+). A crystal structure of riboflavin bound to the riboswitch shows absence of a Mg2+ near the location occupied by the ion in the cognate complex [28]. Crystallization experiments show that Ba2+, Ca2+, or Mn2+ can replace the Mg2+ ion that binds to the phosphate of FMN. However, the monovalent ion Cs+ (binding of which can be monitored by its anomalous scattering, even at modest resolution) does not support high-affinity binding in solution and does not occupy the position of the Mg2+ ion that coordinates the phosphate of the ligand [28]. These experiments argue for the importance of the divalent cation that coordinates the phosphate group of FMN in recognition, and reveal a degree of flexibility of the binding pocket reminiscent of that of the TPP riboswitch. Solution and crystallization experiments show that the FMN riboswitch can even employ cobalt (III) hexammine instead of Mg2+ to bind FMN (the respective Kds differ by less than 2-fold, in the presence of 100 mM K+) [28]. Cobalt (III) hexammine is isosteric with hexahydrated Mg2+, but the amine ligands are very tightly coordinated to Co3+, and therefore the complex ion is unable to make inner-sphere coordinations [29]. The ability of the riboswitch to recognize FMN employing cobalt (III) hexammine indicates that the inner-sphere coordinations seen with Mg2+ (Figure 1C) are not essential for recognition. However, there are steric limits to the flexibility of the binding site: iridium (III) hexammine, which is bulkier than cobalt (III) hexammine, does not support binding and does not bind to the riboswitch-FMN complex near the phosphate of the ligand [28]. In addition, it is important to note that cobalt (III) hexammine is not present in biological systems, so that the riboswitch would not be expected to have evolved selectivity against it.
The c-di-GMP riboswitch is the first known gene-regulatory RNA to bind to a second messenger [30]. Solution studies show that the riboswitch has a strong requirement for Mg2+ to adopt the ligand-bound conformation in the presence of saturating c-di-GMP [31]. At present, two cocrystal structures have been reported, at 3.2 and 2.7 Å resolution. Both show an RNA assembled around a three-helix junction, with the symmetric second messenger bound asymmetrically at the junction. The two guanine bases of c-di-GMP are recognized by the riboswitch through canonical and non-canonical base pairing and stacking interactions. The electron density maps of the lower resolution structure were deemed too ambiguous to indicate the presence or position of metal ions bound to the ligand [31]. The authors of the 2.7 Å resolution structure located an iridium (III) hexammine ion coordinating to one of the phosphates of c-di-GMP, and electron density suggestive of a hydrated Mg2+ ion near the other phosphate [32]. Definitive identification of Mg2+ ion binding sites in this complex awaits higher resolution structures.
2.2 Other metal ions that participate in ligand recognition and selectivity
Crystallographic studies have implicated metal ions in ligand recognition by riboswitches that bind specifically to small molecules that lack a phosphate group. These are the riboswitches responsive to lysine and preQ1. Although it falls beyond the subject matter of this review, an RNA evolved in vitro to bind to tetracycline that has been shown to function as an artificial riboswitch in vivo has also been found to depend critically on binding of Mg2+ to a non-phosphate moiety of its ligand for recognition [33].
The lysine riboswitch has one of the most complex riboswitch structures determined to date, and consists of a three-helix bundle and a two-helix bundle radiating from a five-way junction [34]. Solution studies reveal that the overall structure of the riboswitch is insensitive to the presence of metal ions [34-36]. In contrast, binding of lysine is strongly dependent on the presence of K+ ions. The affinity of the RNA for lysine is decreased by 50 – 100-fold if Na+ or Mg2+ are present instead [34]. The 1.9 Å resolution structure of the riboswitch reveals that lysine is bound in an extended conformation, with its carboxylate coordinated to a K+ ion (Figure 2A) [34]. The identity of the ion was confirmed by its anomalous signal. In addition, it could be replaced very efficiently by Tl+, which is a well-documented mimic of K+ [37]. Mn2+ does not support lysine binding, and this ion was not observed to chelate the carboxylate of bound lysine in crystals. A structure of the lysine-free riboswitch was also determined, and the binding site was found to be devoid both of the lysine and of the K+ ion. In aggregate, the foregoing experiments provide strong evidence that this riboswitch recognizes its cognate ligand as a K+ ion chelate.
Figure 2.
Cation-mediated recognition of riboswitch ligands without phosphate groups. A. A K+ ion coordinates lysine when the amino acid is bound by its cognate riboswitch 18. B. The characteristic exocyclic aminomethyl group of preQ1 engages in hydrogen bonding with a water of hydration of a structural Ca2+ ion 26.
Several tRNAs of bacteria and eukarya have an essential hypermodified nucleotide called Quoeuosine (Q) at the wobble position of the anticodon. The precursor tRNAs are transcribed with G at this position. A specific transglycosylase replaces this guanine with a modified purine base called preQ1. After attachment to tRNA, preQ1 is further elaborated enzymatically to result in Q. Consistent with its being the last small molecule intermediate in the synthesis of Q, preQ1 was found to regulate the expression of enzymes involved in synthesis and transport of Q in bacteria through at least two different classes of riboswitches [38-39]. The ligand binding domain of the class I preQ1 riboswitch is the most compact one known in nature (~ 34 nt). Solution and crystallographic studies revealed it to fold into a canonical pseudoknot [40], with the ligand bound at the interface of two helices [41-43]. The preQ1 riboswitch discriminates strongly against guanine, from which preQ1 differs primarily in having an exocyclic aminomethyl group pendant from position 7. The 2.2 Å resolution cocrystal structure of a class I preQ1 riboswitch suggests that this selectivity depends on a bound metal ion. The structure shows that the G-like portion of the ligand is recognized by Watson-Crick and Hoogsteen base pairing. These interactions would not discriminate against G. The discrimination arises from three hydrogen bonds made by the aminomethyl group that is characteristic of preQ1, which bonds to the carbonyl group of a G of the RNA, to a non-bridging phosphate oxygen, and to a water of hydration of a tightly-bound Ca2+ ion (Figure 2B). This cation appears to be an intrinsic structural component of the RNA, as it is likely to be required to stabilize the pseudoknot fold of the riboswitch [42]. The cation selectivity of the preQ1 riboswitch remains to be analyzed experimentally.
3. METAL IONS AND RIBOSWITCH FOLDING
Like all structured RNAs, the three-dimensional structures of riboswitches undergo stabilization in the presence of cations. To a first approximation, metal ions can be thought to function in two modes [44]. First, fully solvated metal ions accumulate near the RNA and thereby neutralize its charge (counterion condensation). These ions remain diffuse, thereby not paying the full entropic cost of specific binding, and they remain hydrated, so thereby not paying the enthalpic cost of desolvation. This type of counterion is not readily apparent in high-resolution structures. Second, some ions may be specifically chelated by RNA. These are the types of cations discussed above in section 2. These bound cations will have lost most of their translational degrees of freedom, and may be partially or fully desolvated. In the study of global folding of RNAs in general, and riboswitches in particular, the former, diffuse class of counterions is more important (see chapter by Tan and Chen in this volume), although, as indicated above, ligand recognition by many riboswitches relies on specific chelation of counterions.
3.1 Experimental considerations
A variety of methods have been employed to study the role of metal ions in RNA folding (see chapters by Sigel and by Johnson-Buck et al. in this volume). These can be classified into methods that detect some property that is a surrogate for folding, and those that directly study folding (that is, the change in size and shape of RNA as it adopts its native conformation). In the former category are, for instance, spectroscopic methods and enzymatic and chemical probing methods. In the latter category are, primarily, hydrodynamic and solution scattering techniques. The application of analytical ultracentrifugation to the study of riboswitch folding is discussed in more detail in section 4. The following emphasizes the results of studies employing small-angle X-ray scattering (SAXS).
An important consideration regarding folding studies of riboswitches is the nature of the RNA construct employed. Riboswitches are not free-standing RNA molecules. Rather, they are functional units embedded within a mRNA. For many riboswitches, it is possible to define a minimal RNA sequence that is capable of specific, high-affinity binding to its cognate ligand. This sequence segment also commonly corresponds to the region of RNA with the highest phylogenetic conservation. This operationally defined segment is the aptamer domain. In order to function in regulating gene expression, this domain must interface with the transcription, translation, or post-transcriptional RNA processing machinery. This often employs downstream sequences that collectively are called the expression platform [45]. Thus, study of the full functional riboswitch requires analysis of ligand- and ion-dependent folding of both moieties. However, both because of experimental expediency and because it is the segment with highest sequence conservation, the majority of riboswitch folding studies to date have studied the aptamer domains in isolation.
3.2 Monovalent ions and riboswitch folding
In vivo, K+ is the most abundant counterion for nucleic acids. In E. coli, its intracellular activity ranges from ~100 mM to ~1 M, depending on the osmolarity of the growth medium [46]. However, there are few studies that examine the role of K+ in riboswitch folding. Often, K+ is present as part of the “native” buffer condition to which divalent cations and ligand molecules are added to examine their effects on folding, and therefore the role of K+ is not explicitly addressed. Another reason for this is that, for a given concentration, monovalent ions are less effective in promoting RNA folding than polyvalent ions. This phenomenon can be understood in terms of the entropy loss incurred by the cation in associating, loosely or tightly, with the nucleic acid. The loss of translational entropy is the same for a monovalent or a polyvalent ion. However, whereas the monovalent ion can only neutralize the charge of one phosphate, the polyvalent ion can neutralize several phosphates [44]. Thus, Mg2+ promotes RNA folding better than K+, and the trivalent cobalt (III) hexammine is even more stabilizing than Mg2+. Monovalent ions at very high (molar) concentration may allow RNAs to fold and even be catalytically active, see e.g. ref. [47], but the physiological significance of such conditions is open to debate.
A recent study examined the relationship between the size of the monovalent cation and its effect on folding of RNAs by through thermal denaturation. Lambert et al. found that for most RNAs examined, including the purine riboswitch aptamer domain, the smaller Li+ and Na+ ions are more stabilizing than the larger Cs+ ion by as much as 3 kcal/mol [48]. This effect may be explained by the increased ability of smaller cations to approach the RNA surface, especially in the case of intricately folded RNA molecules, such as riboswitches. However, the authors also noted a case in which an RNA structural domain has evolved specifically to coordinate a K+ ion (analogous to the case of the lysine riboswitch discussed above). This RNA had a preference of the same order for Na+ or K+ over smaller or larger ions. Thus, RNAs have the capacity to evolve to be selective for a particular monovalent cation, and riboswitches, being intracellular molecules, presumably have some selectivity for K+.
3.3 Magnesium ion and compaction of metabolite-binding riboswitches
Compaction of several complex RNA molecules induced by addition of Mg2+ has been observed employing SAXS, a technique that is sensitive to aggregation state, size and shape of macromolecules free in solution. For instance, studies of the Group I intron in which folding was initiated by addition of Mg2+ and folding followed by time-resolved SAXS indicated that the ribozyme compacts at least 20-fold faster than folding to the native state (revealed by the onset of catalytic activity), indicating that a rapid collapse is followed by local structural rearrangements [49]. Other SAXS studies of this and other RNAs suggest that the most compact state of an RNA is its most stable conformation [49-52]. Because of these precedents, it was expected that riboswitches would be partially stabilized in the presence of physiologic Mg2+ concentrations, and that ligand binding would then lead to maximum stability (and maximum compaction, as detected by SAXS, for instance).
SAXS experiments on a variety of riboswitch aptamer domains reveals that this expectation was unfounded (Table 1). Some riboswitches behave in the expected manner. Thus, the radius of gyration (Rg) of the glycine riboswitch decreases from 55 Å in monovalent ions alone to 45 Å in the presence of Mg2+. Addition of its cognate ligand further compacts the riboswitch to an Rg of 39 Å [53]. The c-di-GMP and TPP riboswitches exhibit similar behavior [31,36]. In contrast the lysine and SAM-I riboswitches compact upon addition of Mg2+, but do not compact further when ligand is added [36]. This suggests that the overall fold of the RNA is insensitive to ligand binding, and only local rearrangements take place upon specific complex formation [34-35]. Even more divergent from expectation is the behavior of the FMN riboswitch. At the level of global conformation analyzed by SAXS, this RNA is insensitive to the presence of Mg2+ or ligand. Thus, the overall fold of the RNA is established in monovalent ions alone, and structural accommodation upon ligand binding must be limited to the binding pocket [36]. Enzymatic probing data support this interpretation [28]. The data summarized in Table 1 indicate that the effect of Mg2+ on the global folding of riboswitches (strictly, of their aptamer domains) is highly idiosyncratic, as is the effect of their respective cognate ligands. This could indicate that the degree of stabilization of an aptamer domain that results from ligand binding has been fine-tuned to correspond to the regulatory needs of specific genomic loci. Alternatively, the effect of Mg2+ and ligand binding on the rates of folding of riboswitch aptamer domains, rather than their stability, may be biologically critical.
Table 1.
Cation and ligand induced compaction of selected riboswitches
| Rg (Å) | |||
|---|---|---|---|
| Riboswitch(*) | K+/Na+ and no/low Mg2+(*) | K+/Na+ and Mg2+(*) | K+, Mg2+ and ligand(*) |
| c-di-GMP15 | 31.6 ± 1 | 28.5 ± 1 | 23.9 ± 1 |
| FMN20 | 29.4 ± 1 | 29.1 ± 1 | 28.6 ± 1 |
| glycine37 | 55 ± 2 | 45 ± 1 | 39 ±1 |
| lysine20 | 46.8 ± 1 | 39.1 ± 1 | 39.2 ± 1 |
| SAM-I20 | 29.9 ± 1 | 27.5 ± 1 | 26.4 ± 1 |
| TPP20 | 27.5 ± 1 | 24.4 ± 1 | 22.1 ± 1 |
See references for experimental conditions.
4. MAGNESIUM-SENSING RIBOSWITCHES: Salmonella mgtA
4.1 The bacterial metallome
The cellular metallome is defined as the composite of the distribution and equilibrium concentrations of metals within cells [54]. Many of these metals are associated with proteins. Indeed, it has been estimated that approximately one third of intracellular proteins contain a metal cofactor [55]. Most often, these protein-bound metals are transition metals, such as Mn, Cu, Fe, Zn, and Mo, although many cellular proteins associate instead with Mg2+. For example, Fe2+ is frequently associated with proteins in the electron transfer chain, bound via iron-sulfur clusters or heme cofactors, while Zn2+ associates with DNA and RNA polymerase enzymes, zinc finger proteins and ribosomes. The other transition metals associate with myriad abundant, but specific, classes of proteins. For example, cobalt is present in adenosylcobalimin-containing proteins and manganese associates with superoxide dismutase enzymes.
Metals in the highest intracellular abundance are K+, Mg2+, Ca2+, and Na+. With the exception of sporulating bacteria, Ca2+ is generally maintained at submicromolar levels [54]. Mg2+ is present at higher levels (10-20 mM free and ~100 mM total) and is required by DNA and RNA polymerases, as well as many ATPases and kinases. K+ is also retained at high levels, at a total concentration of approximately 100-200 mM, and is important for osmotic balance and pH homeostasis [56-57]. Trace metal ions, which consist mostly of the transition metals plus Zn2+, are maintained at much lower concentrations. The fact that the cytoplasm is a reducing environment alters both the oxidation state of some metals and their intracellular availability. The predominant alkali (K+, Na+) and alkaline (Mg2+, Ca2+) earth metals frequently exist in the free state as they do not bind strongly to ligands. In contrast, the trace metals largely do not occur as free ions inside cells [12,54]. Instead, they mostly remain associated with proteins and certain metabolites. In general, the affinities between proteins and metals correlate with a universal order of preference. For divalents, this order is referred to as the Irving-Williams series (Mg2+ and Ca2+ < Mn2+ < Fe2+ < Co2+ < Ni2+ < Cu2+ > Zn2+) [58]. Assuming equal accessibility for all of these metals, this order would predict that all proteins would simply bind Cu2+, if given an equal choice. However, that is obviously not the case, and there is an abundance of evidence that collectively argue against equal access to intracellular metals. Therefore, proteins and metabolites that bind metals must employ specialized strategies to exclude metals at the top of the Irving-Williams series. In part, this is accomplished through specific compartmentalization of metals, for example, through sequestration of Cu2+ [59]. By corollary, proper localization of proteins can influence which metals can be selected and retained during protein folding. In general, the total numbers of intracellular metals are restricted such that binding of metals to proteins is dictated by their relative affinities to the target proteins [60]. The reduced levels of metals in the cytoplasm are achieved in part by an array of import and export transport proteins, and through sequestration by metallochaperone proteins [9,59]. The quantitative analysis of these respective intracellular metal concentrations, i.e., metallomics, is a rapidly burgeoning research discipline [61]. Some of these emerging data suggest that the metallome is likely to be similar in composition and in overall constancy between microorganisms [61-62]. This fact implies that bacteria are likely to contain elaborate regulatory mechanisms to maintain metal homeostasis. Indeed, many examples of metal-responsive transcription factors have been discovered for control of metal homeostasis [12,59].
4.2 Control of magnesium homeostasis and bacterial pathogenesis by the mgtA RNA
The control of intracellular metal ion pools and other adaptive responses to extracellular metal fluctuations may be particularly important for bacterial pathogens. The human host presents, at minimum, a two-fold challenge to pathogenic bacteria: (1) metal ions are efficiently retained by host cells, such that invading bacteria must use aggressive techniques for metal acquisition, and (2) bacteria oftentimes encounter strongly oxidative and nitrosative stress conditions within host cells, requiring elaborate adaptive techniques for intracellular survival [9,63]. These responses are best characterized in regards to iron acquisition by pathogenic bacteria, which must rely on multiple iron scavenging mechanisms in an attempt to recruit the essential metal, including secretion of iron-gathering proteins and metabolites [64]. Genes encoding for import of other metals, including Cu+, Mn2+, and Zn2+, have also been demonstrated to be required for virulence of certain pathogens [12]. Mg2+ too, can be a critical component in the battle between bacterial pathogens and host cells. The importance of metals, including Mg2+, during pathogenesis has been particularly well studied for Salmonella enterica.
Salmonella enterica are Gram-negative, facultative anaerobes, which reside in part within the intestinal microbiome, and are a frequent cause of food-born gastroenteritis. Salmonella is an intracellular pathogen, and enters both phagocytic and non-phagocytic cells through pathways that require type III secretion systems or through standard phagocytosis [65]. Once internalized, Salmonella modify the location and environment of the phagosome to maximize their survival and replication. The modified phagosome, called the Salmonella-containing vacuole (SCV), is the product of many bacterial virulence factors, which find passage into the host cell through multiple type I and type III secretion systems [66]. Salmonella prevents the fusion of the bacteria-containing vacuole with the lysosome, and ultimately reconfigures the SCV into a modified, microtubule-associated vesicular system called Salmonella-induced filaments [67].
In response to bacterial invasion, host cells employ multiple strategies to combat Salmonella. This includes utilization of an NADPH oxidase (NOX2) enzyme within the phagosome for production of reactive oxygen species, which is in turn neutralized by a Salmonella-produced superoxide dismutase enzyme [68]. In addition to this attempt at creating a “toxic environment” within phagosomes, host cells also try to subject the bacteria to nutrient poor conditions. Such nutrient deprivation strategies are likely to also include access to metals. For example, macrophages produce metal transporters such as Nramp1 and transferrin and, as a consequence, severely reduce the general availability of iron. To counteract the depletion of this and other essential metals, Salmonella produces a combination of iron-sequestering siderophores and proteins that transport Mn2+, K+, Zn2+, and Mg2+, which are collectively necessary for optimal virulence [66].
The importance of Mg2+ concentrations during Salmonella pathogenesis has been particularly well studied, and is influenced in part by a two component regulatory system, PhoP-PhoQ [69]. PhoQ is the cognate sensor kinase for the DNA-binding transcription factor, PhoP. This basic regulatory system is widespread in enteric bacteria, and can affect expression of many operons [70]. Several signals have been reported to activate the system, including low Mg2+, low pH, and antimicrobial peptides [71-73]. Activation of PhoP results in expression of many genes, including those encoding for Mg2+ transport, resistance to cationic antimicrobial peptides, modification of the cellular envelope, and other factors that are important for virulence [74-75]. In fact, PhoP regulates ~3% of total genes in Salmonella enterica serovar Typhimurium [76]. PhoQ is an integral membrane protein, which projects a sensor domain into the periplasmic space. An acidic patch on the outside face of PhoQ is close to the membrane and has been proposed to mediate the response to metal ions and antimicrobial peptides [77-78]. The interaction of divalent ions with the negatively charged patch has been postulated to alter positioning of the sensor domain relative to the membrane; these conformational changes are believed to control the transmembrane signaling pathway. In Salmonella enterica, low Mg2+ and low pH activate different sets of genes, in accordance with adaptation to the respective signals [79]. Low-Mg2+-activated PhoP induces expression of many genes including but not limited to hilA (transcription factor for invasion genes), pmrAB (Fe3+-responsive regulatory system), prgHIJK (type III secretion system components), mgtA (magnesium transport), mgtB (magnesium transport), and mgtC (intramacrophage survival) [74].
Although it is a debated subject [80-81], the magnesium level within the phagosome is generally believed to be very low. This is in good agreement with the upregulation of multiple Mg2+ transporters upon activation of PhoP, which is required for phagosomal survival. It has been proposed that Salmonella ascertains its subcellular location by measuring the surrounding Mg2+ via the PhoP-PhoQ system. Low Mg2+ would indicate the intracellular environment within the phagosome; high Mg2+ would indicate the extracellular environment. Further demonstrating the importance of Mg2+ homeostasis in this microorganism, it was recently discovered that expression of the mgtA transcript is controlled by a second Mg2+-responsive pathway, in addition to control of transcription initiation by PhoP [82]. Identification of a second Mg2+ regulatory mechanism agreed with earlier data showing that mgtA expression was still modulated in response to Mg2+ in the context of a constitutively active PhoP mutant; also, the promoter region for mgtA was previously found to engender Mg2+-responsive control over a reporter gene even in the absence of the PhoP-PhoQ system [83-84]. Upon further inspection, the second regulatory mechanism was discovered to occur post-transcription initiation, and to involve a regulatory RNA element. Specifically, the mgtA transcript was found to house a long 5′ leader region that regulates expression of the downstream gene [82]. Northern blotting of mgtA transcripts revealed that abundance of the leader region decreased as extracellular Mg2+ was raised above 0.1 mM, most likely as a reflection of PhoP control over initiation. However, abundance of the downstream mgtA coding region decreased at lower Mg2+. When the PhoP-responsive promoter was replaced with a heterologous promoter, high Mg2+ still triggered a decrease in abundance for the coding sequence, but not for the leader region [82].
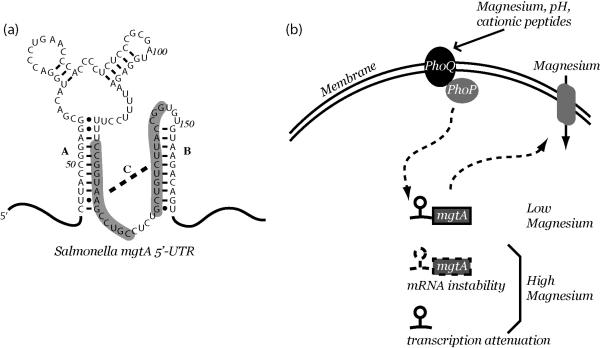
Many different types of cis-acting regulatory RNAs have been identified previously, which respond to a diverse range of signals, including temperature, proteins, soluble RNAs, and metabolites [85]. However, a regulatory RNA element that was explicitly evolved for the purposes of sensing metal ions had not yet been observed. Examination of the mgtA leader region by a secondary structure prediction program, Mfold, revealed several potential stem-loop structures, which could be involved in genetic control (Figure 3). Importantly, a similar combination of stem-loop structures and interspersed primary sequence elements could be identified upstream of mgtA in other enterics. Deletion or mutagenesis of these structural elements resulted in mgtA expression that was either constitutively high or constitutively low, depending on which elements were removed. In aggregate, the mutagenesis data suggested that a single stem-loop (“C”) was formed during conditions of low Mg2+, conditions that promote downstream expression. In contrast, a mutually exclusive arrangement of two stem-loops (“A”, “B”) is favored in high Mg2+, conditions that disallow downstream expression (Figure 3). Limited biochemical support for the A, B, and C helices was obtained from interpretation of partial RNase digestion patterns, using RNase T1 for cleavages of unpaired guanosines. These data were interpreted as suggesting that association of Mg2+ with the mgtA leader RNA in vitro directly promoted the A + B conformation.
Figure 3.
Genetic regulation by the mgtA riboswitch. A. Secondary structure of the Salmonella enterica mgtA riboswitch. B. Under low Mg2+ conditions the PhoP protein activates transcription of the mgtA transcript. Under high Mg2+ conditions, a riboswitch located within the 5’ leader region activates destabilization of the mgtA transcript and reduces the efficiency of transcription elongation of the mgtA gene.
4.3 Mechanistic models for regulation by the mgtA RNA
While it is clear from published data that the mgtA riboswitch controls gene expression in a Mg2+-responsive manner, the molecular mechanism through which this is accomplished is unresolved. The riboswitch has been proposed to exert regulatory control over transcription elongation, in a Mg2+-dependent manner [82]. This model is based on in vitro transcription reactions using a DNA template encoding the mgtA leader region. In these reactions, a truncated transcription intermediate was observed, which appeared to be preferentially synthesized in response to increased Mg2+. However, the exact site of transcription attenuation has not yet been conclusively resolved. Also, the components typically involved in promoting transcription termination (Rho-dependent termination sites and intrinsic terminator hairpins) appear to be absent from the mgtA RNA sequence. Therefore, it is still unclear exactly how the mgtA riboswitch affects transcription elongation activity in vitro. More recently, the mgtA riboswitch has been implicated in controlling stability of the overall mgtA transcript [86]. Specifically, high Mg2+ was shown to stimulate degradation of the mgtA transcript in vivo through a mechanism that was dependent on both the mgtA riboswitch and the global endoribonuclease, RNase E. The structural relationship between the riboswitch and RNase E has not yet been determined. Therefore, it is currently unclear whether the mgtA riboswitch controls transcript abundance by regulating transcription elongation, mRNA stability, or both. Nor has the molecular basis of Mg2+ recognition been resolved for the riboswitch. It is currently unclear which portions of the mgtA leader RNA might comprise a Mg2+ aptamer domain, if indeed a discrete aptamer domain exists within the RNA element.
Interestingly, recent data provide some clues as to why Salmonella may have evolved two overlapping Mg2+-based regulatory mechanisms. In short, these individual regulatory mechanisms may respond to different ranges of intracellular metals. For example, PhoP-mediated control of mgtA transcription initiation occurs over a broad range of extracellular Mg2+, whereas the mgtA riboswitch upregulates mgtA abundance only when Mg2+ is added at <50 μM [87]. Also, recent data suggest that the mgtA RNA element may not even be the only magnesium riboswitch in Salmonella. A long 5′ leader region has also been identified upstream of mgtC, and was postulated to incorporate another Mg2+-sensing regulatory RNA [16]. MgtC is a protein of unknown function that is somehow involved in adaptation to low-Mg2+ environments, and has been particularly implicated in the survival of Salmonella inside human cells [80]. Similar to the mgtA riboswitch, the mechanistic basis for Mg2+-sensing by the mgtC leader region remains to be discovered. Together, the emerging data on regulation of mgtA and mgtC argue that Mg2+-responsive regulatory mechanisms are essential for survival of Salmonella in macrophages, and are likely to occur in other bacteria for control of Mg2+ homeostasis. Given that little is known regarding the mechanisms, or even the overall need, for control of Mg2+ homeostasis in all three domains of life [13], elucidation of the molecular mechanisms underlying post-transcriptional control of Salmonella Mg2+ homeostasis remains an important goal.
5. MAGNESIUM-SENSING RIBOSWITCHES: THE M-BOX RNA
5.1 Identification and distribution of the M-box RNA
Many different classes of metabolite-sensing riboswitches have been discovered, mostly as an outcome of bioinformatics-based searches for conserved bacterial RNA motifs. Individual riboswitch classes have been discovered that sense nucleobase-containing metabolites, enzyme cofactors, amino acids, a signaling metabolite (cyclic di-guanosine monophosphate), and an amino sugar (glucosamine-6-phosphate) [7]. In all instances, a portion of these regulatory RNAs comprises a discrete aptamer domain, responsible for high affinity association with the target metabolite. Typically, the aptamer domains are followed by sequence and structural elements that coordinate the aptamer with downstream genetic control. The majority of bacterial riboswitches regulate gene expression by either controlling access to a downstream translation initiation site or through formation of a transcription termination site (transcription attenuation). Also, most riboswitches are used for feedback inhibition of downstream genes, although some are used for other regulatory purposes, such as substrate-mediated activation of catabolism genes [7].
In addition to the known classes of riboswitches, there is a rapidly increasingly catalog of ‘orphan’ riboswitches [7,88-93]. These are evolutionarily conserved, structured RNA elements that resemble riboswitches, but whose metabolite ligands remain unidentified. Thus far, the most successful approach in identifying orphan riboswitch ligands is to examine whether their downstream genes exhibit functional trends consistent with certain signaling cues. For example, TPP-sensing riboswitches are most often utilized for genetic regulation of TPP synthesis and transport; therefore, it is reasonable to assume that a new regulatory RNA upstream of these gene categories is likely to be a TPP riboswitch. Unfortunately, some orphan riboswitches co-occur with complex combinations of gene categories, such that the prediction of metabolite ligand candidates is difficult [94]. For orphan riboswitch classes that are positioned solely upstream of unknown, uncharacterized genes, it is impossible to predict their metabolite ligands. A 2004 search for riboswitches resulted in discovery of several new putative riboswitch classes [88]. Most of the metabolite ligands for these riboswitch classes have been subsequently identified; however, several still remain to be discovered. Interestingly, at least three of the putative riboswitch classes from this study appeared to regulate metal transport genes, suggesting a relationship between metals and their regulatory mechanisms. One of these riboswitches, later renamed the M-box, was found to function as a metal-responsive genetic element [15].
At the time of the preparation of this article, approximately 600 individual M-box sequences from 355 bacterial species had been listed on Rfam, a curated databse of evolutionarily conserved RNA families [92]. Comparative sequence alignments revealed that the M-box RNA is approximately ~160 nucleotides in length. The molecule is characterized by extensive primary sequence conservation and includes five conserved helical portions. It also includes several common secondary structural motifs, including the UAA/GAN motif, and a GNRA tetraloop, which are likely to be essential for stabilization of long-range interactions. The majority of the M-box examples that have been identified are located upstream of putative Mg2+ transport genes.
Mg2+ is readily available on Earth, located in mineral deposits, as a salt or a free cation in aqueous solutions. For example, in oceanic environments Mg2+ is present at an average concentration of 55 mM [95]. It is also the most abundant divalent cation in living organisms, present at approximately 10 – 20 mM for the free metals. Due to the abundance of the metal within cells, it was previously presumed that specific regulatory mechanisms would not be required for maintaining intracellular Mg2+ concentrations, as they are for trace metals. Recent evidence suggests that this assumption is incorrect; indeed, control of Mg2+ homeostasis has now been clearly demonstrated to be essential for growth and bacterial virulence [13]. For example, structural characterization of Mg2+ transport proteins has revealed that bacteria employ gating mechanisms to modulate Mg2+ transport activity. Furthermore, the discoveries of the PhoP-PhoQ regulatory pathway and the mgtA and M-box riboswitches have demonstrated that organisms also utilize dedicated genetic regulatory mechanisms for control of Mg2+ pools.
There are three classes of Mg2+ transport genes, which are present in all three domains of life [13,96]. The first to be discovered was CorA, an ion channel widely represented in bacterial genomes. It is not subjected to genetic regulatory control in Salmonella, leading to the hypothesis that it is usually constitutively expressed in bacteria as a ‘housekeeping’ Mg2+ transporter. MgtE is a Mg2+ channel protein that is widespread in bacteria, and has also been clearly identified in eukaryotes (SLC41A1-3). Both MgtE and CorA contain cytoplasmic domains that regulate gating of the ion-conducting pore. However, the two transporters may function at different ion concentrations. CorA transports Mg2+, Co2+, and Ni2+ with apparent affinities of 15 μM, 20-40 μM, and 200-400 μM, respectively, and does not transport Mn2+, Ca2+, Zn2+, or Fe2+. MgtE imports Mg2+ and Co2+ with an apparent Km of 300-700 μM for Mg2+, but not Ni2+, which acts as a potent inhibitor of Mg2+ transportation. The third class of dedicated Mg2+ transporters, called MgtA/B, is a subset of P-type ATPase transporters. In contrast to other P-type ATPase transporters, MgtA/B mediate influx of Mg2+ along its electrochemical gradient, rather than against it. Also, they exhibit an apparent affinity of 10 μM for import of the ion, similar in principle to CorA. Interestingly, all three classes of transporters are subjected to control by M-box riboswitches [97]. However, of the M-box RNAs that have been identified, most appear to be present upstream of homologues of genes for MgtE and MgtA/B [97]. There are only a few examples where the M-box is arranged upstream of CorA homologues. The distribution of these gene categories varies extensively between bacterial species. So, too, does the distribution of M-box RNAs. Most bacteria contain multiple homologues of the different Mg2+ transport classes, but only a subset of these genes appear to be subjected to genetic control by an M-box riboswitch. For example, Listeria monocytogenes contains three homologues of corA and a single copy of mgtA; only the mgtA gene is regulated by an M-box riboswitch. In contrast, Bacillus halodurans contains four mgtE homologues, each under control of a separate M-box RNA, along with an mgtA that lacks the riboswitch. For some microorganisms, the M-box RNA appears to regulate genes other than Mg2+ transporters. For example, in rare instances, the M-box riboswitch is predicted to regulate expression of genes encoding for MgtC, NRAMP family transporters, an unknown transcription factor, uncharacterized ABC type transport genes, cell surface PPE/PE family proteins (in Mycobacterium species) and a few unknown, uncharacterized genes [97].
To test whether a regulatory connection is shared between Mg2+ pools and expression of M-box-associated genes, a representative B. subtilis M-box riboswitch was transcriptionally fused to a lacZ reporter gene. Cells expressing this riboswitch-reporter fusion were cultured in media with varying levels of Mg2+. Expression of the lacZ reporter was increased upon depletion of Mg2+, but not other divalents, suggesting that the riboswitch indeed specifically responds to Mg2+ fluctuations in vivo.
5.2 A mechanistic model for metal-responsive regulation by the M-box RNA
Demonstration of Mg2+-responsive regulation by the M-box RNA in vivo suggested that one of two possibilities was likely to occur: (1) the M-box aptamer domain constitutes a direct sensor for Mg2+ ions, functionally similar to metabolite-sensing aptamers, or (2) the M-box aptamer domain senses a metabolite whose levels fluctuate as a function of intracellular Mg2+ pools. All available data suggest the former, that the M-box RNA directly senses intracellular Mg2+, without aid of accessory factors or indirect signaling pathways. This is accomplished using a mechanistic strategy that closely mirrors that of metabolite-sensing riboswitches, and that exploits the propensity of metals to encourage compacted RNA conformations [15,36,98].
To ascertain whether the conserved portion of M-box riboswitches functions as a distinct Mg2+ sensory domain, conserved residues were individually altered by site-directed mutagenesis and downstream expression of a lacZ reporter was assayed at varying Mg2+ concentrations. These mutations disrupted the in vivo Mg2+ regulatory response, as did disruption of a downstream putative intrinsic terminator site, suggesting that, indeed, the putative aptamer domain coupled a a Mg2+-responsive signal with control of transcription attenuation. Indeed, most M-box riboswitches contain putative intrinsic terminator sites downstream of the aptamer domain, suggesting that control of transcription attenuation is the primary mode of genetic regulation for this riboswitch class. To rule out a role for protein accessory factors in the regulatory mechanism, transcription reactions of the M-box riboswitch were assessed in vitro, using only purified components (RNA polymerase, DNA templates, Mg2+, and nucleotides). Mg2+-responsive control of transcription attenuation could be recapitulated in these reactions, demonstrating that Mg2+ alone constitutes the signal that is perceived by the M-box aptamer domain. Structural probing of the M-box aptamer domain revealed a concerted, metal-induced conformational change, that occurred with a half-maximal concentration of ~0.5 mM Mg2+ [15]. Importantly, cobalt (III) hexamine was unable to elicit this conformational change, suggesting that compaction was dependent on partially dehydrated divalent ions. Furthermore, molar quantities of monovalent ions were unable to trigger the metal-bound conformation alone; divalent ions were required even in the presence of 2.1 M monovalents. A significant decrease in hydrodynamic radius accompanied the metal-induced conformational change, as evidenced by an increase in sedimentation velocity. Indeed, a ‘compact’ conformation could be physically separated from the population of low-Mg2+, ‘extended’ conformation(s) by chromatography or nondenaturing polyacrylamide gel electrophoresis.
The 2.6 Å structure of the Mg2+-bound M-box was solved by X-ray crystallography, using crystals grown in the presence of 10 mM Mg2+ [15]. This revealed a compact structure, mostly comprising of three closely packed, nearly parallel helices. Inter-helical packing interactions are stabilized by long-range hydrogen bonding interactions between nucleosides, including multiple A-minor motifs. A total of six well-ordered Mg2+ were observed in the structural model, all of which involved at least one inner-sphere interaction. Indeed, one of the metals included a total of four inner-sphere contacts to RNA oxygen groups. For each of these metals, the Mg2+ ions appeared to assist positioning of neighboring residues in order to promote long-range interactions. Almost all of the RNA-metal contacts in the structural model occurred with nonbridging phosphate oxygens. To functionally probe the importance of these interactions, nonbridging phosphate oxygens were randomly replaced with phosphorothioate groups, by standard nucleotide analog interference methods. The individual phosphorothioate substitutions that were found to restrict the RNA to the extended conformation agreed well with the sites of inner-sphere interactions that were proposed by the structural model. Therefore, the compact, metal-bound conformation is dependent upon association of a network of Mg2+ ions within specific electronegative pockets. Biochemical studies show this transition occurs over a very narrow range of metal concentrations. The regulatory link between metal-mediated compaction and genetic control is similar in concept to metabolite-sensing riboswitches. In the compact conformation, nucleotides at the 5' and 3' termini of the aptamer pair together to form the P1 helix. Specifically, tertiary contacts and base-stacking interactions that further stabilize the P1 helix are formed in the compact conformation. In contrast, the right half of the P1 helix interacts with downstream sequences to form a mutually exclusive helix in low Mg2+. This ‘antiterminator’ competes with formation of the intrinsic terminator, resulting in control of attenuation. In total, these data demonstrate how an RNA element can function as a metalloregulatory agent, to couple metal concentrations to control of gene expression. Together, the discovery of both the M-box and Salmonella mgtA riboswitches suggest that there are likely to be additional mechanisms utilized for control of Mg homeostasis that still await discovery.
6. ARE THERE ADDITIONAL CLASSES OF METAL-SENSING RIBOSWITCHES?
Orphan riboswitches are structured RNA elements encoded in bacterial genomes that share basic features with known riboswitches, but whose metabolite ligands remain unidentified [7,88-93]. Individual orphan riboswitch classes are usually upstream of a common collection of genes, although a few classes are located upstream of genes of diverse functions. Additionally, orphan riboswitches exhibit secondary structure and primary sequence conservation patterns resembling established riboswitches. They are also generally co-transcribed with obvious sequence and structural features that are predicted to regulate translation initiation or transcription termination, again, akin to known riboswitches. Indeed, many of the current, established riboswitches were initially described as orphan riboswitches. A 2004 search for riboswitches resulted in discovery of several new putative riboswitch classes [88]. Metabolite ligands for at least two of these classes, which appear to regulate expression of metal transport genes, have not yet been identified. For example, the so-called ‘yybP’ orphan most often regulates expression of a wide variety of metal transport genes, including Mg2+ transporters, an assortment of metal efflux genes, and toxic anion resistance genes. The so-called ‘ydaO’ orphan riboswitch most often regulates K+ transport genes, uncharacterized transport genes, and cell wall hydrolases. It is not yet clear from existing data whether these orphan riboswitches respond to metal ion-based signals, or to metabolites that fluctuate in concert with certain metal ions. Also, it is possible that a subset of M-box RNAs could function differently from the B. subtilis variant that has been examined. A small subset of M-box RNAs has been predicted to regulate NRAMP genes, which typically transport Fe3+ or Mn2+ [97]. From this observation, one can speculate that certain M-box RNAs may respond specifically to Mn2+, or, rather that certain microorganisms have evolved a regulatory interrelationship between Mg2+ and Mn2+ homeostasis.
Figure 4.
Genetic regulation by the M-box riboswitch. A. Classes of genes that are regulated y the M-box riboswitch. B. Shown in this figure is a schematic depiction of genetic regulation by the M-box riboswitch. Under conditions of high Mg2+, the P1 helix at the base of the aptamer domain is stabilized, which allows for formation of a downstream termination site. Under conditions of low Mg2+, the right half of the P1 helix associates with downstream sequences to prevent terminator formation, and thereby allow for transcription elongation to continue synthesis of downstream genes. C. Secondary structure of the B. subtilis mgtE M-box aptamer. Symbols denote locations of metal ions that form interactions with the RNA, as observed within the crystallographically determined structural model (D). Circled residues denote sites of phosphorothioate interferences, which prevent the compacted metal-bound conformation. E. One of the six RNA-bound Mg2+ ions appears to be particular important for the compacted RNA conformation, as evidenced by strong phosphorothioate interference and four inner-sphere metal coordinations.
ACKNOWLEDGEMENTS
The authors thank past and current members of the their laboratories for their many contributions. Riboswitch research in the authors’ laboratories has been funded by the Bill and Melinda Gates Foundation, the Damon Runyon Cancer Research Foundation, the Howard Hughes Medical Institute (HHMI), the National Institutes of Health (GM63576 and RR15943), and the W.M. Keck Foundation (A.R.F) and ... (W.C.W.) A.R.F. is an Investigator of the HHMI.
References
- 1.Draper DE, Grilley D, Soto AM. Ions and RNA folding. Annu Rev Biophys Biomol Struct. 2005;34:221–243. doi: 10.1146/annurev.biophys.34.040204.144511. [DOI] [PubMed] [Google Scholar]
- 2.Misra VK, Shiman R, Draper DE. A thermodynamic framework for the magnesium-dependent folding of RNA. Biopolymers. 2003;69:118–36. doi: 10.1002/bip.10353. [DOI] [PubMed] [Google Scholar]
- 3.Sigel RK, Pyle AM. Alternative roles for metal ions in enzyme catalysis and the implications for ribozyme chemistry. Chem Rev. 2007;107:97–113. doi: 10.1021/cr0502605. [DOI] [PubMed] [Google Scholar]
- 4.Klein DJ, Moore PB, Steitz TA. The contribution of metal ions to the structural stability of the large ribosomal subunit. RNA. 2004;10:1366–79. doi: 10.1261/rna.7390804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winkler WC, Breaker RR. Regulation of bacterial gene expression by riboswitches. Annu Rev Microbiol. 2005;59:487–517. doi: 10.1146/annurev.micro.59.030804.121336. [DOI] [PubMed] [Google Scholar]
- 6.Edwards TE, Klein DJ, Ferré-D'Amaré AR. Riboswitches: small-molecule recognition by gene regulatory RNAs. Curr Opin Struct Biol. 2007;17:273–279. doi: 10.1016/j.sbi.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Dambach MD, Winkler WC. Expanding roles for metabolite-sensing regulatory RNAs. Curr Opin Microbiol. 2009;12:161–169. doi: 10.1016/j.mib.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finney LA, O'Halloran TV. Transition metal speciation in the cell: insights from the chemistry of metal ion receptors. Science. 2003;300:931–936. doi: 10.1126/science.1085049. [DOI] [PubMed] [Google Scholar]
- 9.Ma Z, Jacobsen FE, Giedroc DP. Coordination chemistry of bacterial metal transport and sensing. Chem Rev. 2009;109:4644–4681. doi: 10.1021/cr900077w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maguire ME. Magnesium transporters: properties, regulation and structure. Front Biosci. 2006;11:3149–63. doi: 10.2741/2039. [DOI] [PubMed] [Google Scholar]
- 11.O'Halloran TV. Transition metals in control of gene expression. Science. 1993;261:715–25. doi: 10.1126/science.8342038. [DOI] [PubMed] [Google Scholar]
- 12.Giedroc DP, Arunkumar AI. Metal sensor proteins: nature's metalloregulated allosteric switches. Dalton Trans. 2007;29:3107–20. doi: 10.1039/b706769k. [DOI] [PubMed] [Google Scholar]
- 13.Moomaw AS, Maguire ME. The unique nature of Mg2+ channels. Physiology. 2008;23:275–285. doi: 10.1152/physiol.00019.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massé E, Salvail H, Desnoyers G, Arguin M. Small RNAs controlling iron metabolism. Curr Opin Microbiol. 2007;10:140–145. doi: 10.1016/j.mib.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Dann CE, Wakeman CA, Sieling CL, Baker SC, Irnov I, Winkler WC. Structure and mechanism of a metal-sensing regulatory RNA. Cell. 2007;130:878–892. doi: 10.1016/j.cell.2007.06.051. [DOI] [PubMed] [Google Scholar]
- 16.Groisman EA, Cromie MJ, Shi Y, Latifi T. A magnesium-responding RNA that controls the expression of a magnesium transporter. Cold Spring Harb Symp Quant Biol. 2006;71:251–8. doi: 10.1101/sqb.2006.71.005. [DOI] [PubMed] [Google Scholar]
- 17.Serganov A, Polonskaia A, Phan AT, Breaker RR, Patel DJ. Structural basis for gene regulation by a thiamine pyrophosphate-sensing riboswitch. Nature. 2006;441:1167–1171. doi: 10.1038/nature04740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards TE, Ferré-D'Amaré AR. Crystal structures of the thi-box riboswitch bound to thiamine pyrophosphate analogs reveal adaptive RNA-small molecule recognition. Structure. 2006;14:1459–1468. doi: 10.1016/j.str.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Kulshina N, Edwards TE, Ferré-D'Amaré AR. Thermodynamic analysis of ligand binding and ligand binding-induced tertiary structure formation by the thiamine pyrophosphate riboswitch. RNA. 2010;16:186–196. doi: 10.1261/rna.1847310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thore S, Frick C, Ban N. Structural basis of thiamine pyrophosphate analogues binding to the eukaryotic riboswitch. J Am Chem Soc. 2008;130:8116–8117. doi: 10.1021/ja801708e. [DOI] [PubMed] [Google Scholar]
- 21.Klein DJ, Ferré-D'Amaré AR. Structural basis of glmS ribozyme activation by glucosamine-6-phosphate. Science. 2006;313:1752–1756. doi: 10.1126/science.1129666. [DOI] [PubMed] [Google Scholar]
- 22.Winkler WC, Nahvi A, Roth A, Collins JA, Breaker RR. Control of gene expression by a natural metabolite-responsive ribozyme. Nature. 2004;428:281–286. doi: 10.1038/nature02362. [DOI] [PubMed] [Google Scholar]
- 23.Collins JA, Irnov I, Baker S, Winkler WC. Mechanism of mRNA destabilization by the glmS ribozyme. Genes Dev. 2007;21:3356–3368. doi: 10.1101/gad.1605307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarthy TJ, et al. Ligand requirements for glmS ribozyme self-cleavage. Chem. Biol. 2005;12:1221–1226. doi: 10.1016/j.chembiol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Klein DJ, Been MD, Ferré-D'Amaré AR. Essential role of an active-site guanine in glmS ribozyme catalysis. J. Am. Chem. Soc. 2007;129:14858–14859. doi: 10.1021/ja0768441. [DOI] [PubMed] [Google Scholar]
- 26.Cochrane JC, Lipchock SV, Strobel SA. Structural investigation of the GlmS ribozyme bound to its catalytic cofactor. Chem. Biol. 2007;14:97–105. doi: 10.1016/j.chembiol.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein DJ, Wilkinson SR, Been MD, Ferré-D'Amaré AR. Requirement of helix P2.2 and nucleotide G1 for positioning of the cleavage site and cofactor of the glmS ribozyme. J Mol Biol. 2007;373:178–189. doi: 10.1016/j.jmb.2007.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serganov A, Huang L, Patel D. Coenzyme recognition and gene regulation by a flavin mononucleotide riboswitch. Nature. 2009;457:233–237. doi: 10.1038/nature07642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cowan JA. Metallobiochemistry of RNA. Co(NH3)6 3+ as a probe for Mg2+ (aq) binding sites. J. Inorg. Biochem. 1993;49:171–175. doi: 10.1016/0162-0134(93)80002-q. [DOI] [PubMed] [Google Scholar]
- 30.Sudarsan, et al. Riboswitches in eubacteria sense the second messenger cyclic di-GMP. Science. 2008;321:411–413. doi: 10.1126/science.1159519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kulshina N, Baird NJ, Ferré-D'amaré AR. Recognition of the bacterial second messenger cyclic diguanylate by its cognate riboswitch. Nat Struct Mol Biol. 2009;16:1212–1217. doi: 10.1038/nsmb.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith KD, et al. Structural basis of ligand binding by a c-di-GMP riboswitch. Nat Struct Mol Biol. 2009;16:1218–1223. doi: 10.1038/nsmb.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao H, Edwards TE, Ferré-D'Amaré AR. Structural basis for specific, high-affinity tetracycline binding by an in vitro evolved aptamer and artificial riboswitch. Chem Biol. 2008;15:1125–1137. doi: 10.1016/j.chembiol.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serganov A, Huang L, Patel D. Structural insights into amino acid binding and gene control by a lysine riboswitch. Nature. 2008;6 doi: 10.1038/nature07326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garst AD, Héroux A, Rambo RP, Batey RT. Crystal structure of the lysine riboswitch regulatory mRNA element. J Biol Chem. 2008;283:22347–22351. doi: 10.1074/jbc.C800120200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baird NJ, Ferré-D'Amaré AR. Idiosyncratically tuned switching behavior of riboswitch aptamer domains revealed by comparative small-angle X-ray scattering analysis. RNA. 2010;16:598–609. doi: 10.1261/rna.1852310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basu S, et al. A specific monovalent metal ion integral to the A-A platform of the RNA tetraloop receptor. Nature Struct. Biol. 1998;5:986–992. doi: 10.1038/2960. [DOI] [PubMed] [Google Scholar]
- 38.Roth, et al. A riboswitch selective for the queuosine precursor preQ1 contains an unusually small aptamer domain. Nat Struct Mol Biol. 2007;14:308–317. doi: 10.1038/nsmb1224. [DOI] [PubMed] [Google Scholar]
- 39.Meyer MM, Roth A, Chervin SM, Garcia GA, Breaker RR. Confirmation of a second natural preQ1 aptamer class in Streptococcaceae bacteria. RNA. 2008;14:685–695. doi: 10.1261/rna.937308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rieder U, Lang K, Kreutz C, Polacek N, Micura R. Evidence for pseudoknot formation of class I preQ1 riboswitch aptamers. ChemBioChem. 2009;10:1141–1144. doi: 10.1002/cbic.200900155. [DOI] [PubMed] [Google Scholar]
- 41.Kang M, Peterson R, Feigon J. Structural insights into riboswitch control of the biosynthesis of queuosine, a modified nucleotide found in the anticodon of tRNA. Mol Cell. 2009;33:784–790. doi: 10.1016/j.molcel.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Klein D, Edwards T, Ferré-D'Amaré A. Cocrystal structure of a class I preQ1 riboswitch reveals a pseudoknot recognizing an essential hypermodified nucleobase. Nat Struct Mol Biol. 2009;16:343–344. doi: 10.1038/nsmb.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spitale R, Torelli A, Krucinska J, Bandarian V, Wedekind J. The structural basis for recognition of the PreQ0 metabolite by an unusually small riboswitch aptamer domain. J Biol Chem. 2009;284:11012–11016. doi: 10.1074/jbc.C900024200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Draper DE. A guide to ions and RNA structure. RNA. 2004;10:335–343. doi: 10.1261/rna.5205404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winkler W, Nahvi A, Breaker RR. Thiamine derivatives bind messenger RNAs directly to regulate bacterial gene expression. Nature. 2002;419:952–956. doi: 10.1038/nature01145. [DOI] [PubMed] [Google Scholar]
- 46.Cayley S, Lewis BA, Guttman HJ, Record MT. Characterization of the cytoplasm of Escherichia coli K-12 as a function of external osmolarity. Implications for protein-DNA interactions in vivo. J Mol Biol. 1991;222:281–300. doi: 10.1016/0022-2836(91)90212-o. [DOI] [PubMed] [Google Scholar]
- 47.Murray JB, Seyhan AA, Walter NG, Burke JM, Scott WG. The hammerhead, hairpin and VS ribozymes are catalytically proficient in monovalent cations alone. Chem. Biol. 1998;5:587–595. doi: 10.1016/s1074-5521(98)90116-8. [DOI] [PubMed] [Google Scholar]
- 48.Lambert D, Leipply D, Shiman R, Draper DE. The Influence of Monovalent Cation Size on the Stability of RNA Tertiary Structures. J Mol Biol. 2009;390:791–804. doi: 10.1016/j.jmb.2009.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Russell R, Millett IS, Doniach S, Herschlag D. Small angle X-ray scattering reveals a compact intermediate in RNA folding. Nat Struct Biol. 2000;7:367–370. doi: 10.1038/75132. doi:10.1038/75132. [DOI] [PubMed] [Google Scholar]
- 50.Fang X, et al. Mg2+-dependent compaction and folding of yeast tRNAPhe and the catalytic domain of the B. subtilis RNase P RNA determined by small-angle X-ray scattering. Biochemistry. 2000;39:11107–11113. doi: 10.1021/bi000724n. [DOI] [PubMed] [Google Scholar]
- 51.Baird N, Westhof E, Qin H, Pan T, Sosnick T. Structure of a folding intermediate reveals the interplay between core and peripheral elements in RNA Folding. J Molec Biol. 2005;352:712–722. doi: 10.1016/j.jmb.2005.07.010. doi:10.1016/j.jmb.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 52.Chauhan S, et al. RNA tertiary interactions mediate native collapse of a bacterial group I ribozyme. J Mol Biol. 2005;353:1199–1209. doi: 10.1016/j.jmb.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 53.Lipfert J, et al. Structural transitions and thermodynamics of a glycine-dependent riboswitch from Vibrio cholerae. J Mol Biol. 2007;365:1393–1406. doi: 10.1016/j.jmb.2006.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams RJP, Frausto da Silva JJR. The distribution of elements in cells. Coord Chem Rev. 2000;200-202:247–348. [Google Scholar]
- 55.Tainer JA, Roberts VA, Getzoff ED. Curr Opin Biotechnol. 1991;2:582. doi: 10.1016/0958-1669(91)90084-i. [DOI] [PubMed] [Google Scholar]
- 56.Booth IR. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985;49:359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wood JM. Osmosensing by bacteria: signals and membrane-based sensors. Microbiol Mol Biol Rev. 1999;63:230–262. doi: 10.1128/mmbr.63.1.230-262.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Irving H, Williams RJP. Order of stability of metal complexes. Nature. 1948;162:746–747. [Google Scholar]
- 59.Waldron KJ, Rutherford JC, Ford D, Robinson NJ. Nature. 2009;460:823–830. doi: 10.1038/nature08300. [DOI] [PubMed] [Google Scholar]
- 60.Robinson NJ. A more discerning zinc exporter. Nature Chem Biol. 2007;3:692–693. doi: 10.1038/nchembio1107-692. [DOI] [PubMed] [Google Scholar]
- 61.Mounicou S, Szpunar J, Lobinski R. Metallomics: the concept and methodology. Chem Soc Rev. 2009;38:1119–38. doi: 10.1039/b713633c. [DOI] [PubMed] [Google Scholar]
- 62.Barton LL, Goulhen F, Bruschi M, Woodards NA, Plunkett RM, Rietmeijer FJ. The bacterial metallome: composition and stability with specific reference to the anaerobic bacterium Desulfovibrio desulfuricans. Biometals. 2007;20:291–302. doi: 10.1007/s10534-006-9059-2. [DOI] [PubMed] [Google Scholar]
- 63.Forbes JR, Gros P. Divalent-metal transport by NRAMP proteins at the interface of host-pathogen interactions. Trends Microbiol. 2001;9:397–403. doi: 10.1016/s0966-842x(01)02098-4. [DOI] [PubMed] [Google Scholar]
- 64.Wandersman C, Delepelaire P. Bacterial iron sources: from siderophores to hemophores. Annu Rev Microbiol. 2004;58:611–647. doi: 10.1146/annurev.micro.58.030603.123811. [DOI] [PubMed] [Google Scholar]
- 65.Salcedo SP, Noursadeghi M, Cohen J, Holden DW. Intracellular replication of Salmonella typhimurium strains in specific subsets of splenic macrophages in vivo. Cell Microbiol. 2001;3:587–597. doi: 10.1046/j.1462-5822.2001.00137.x. [DOI] [PubMed] [Google Scholar]
- 66.Ibarra JA, Steele-Mortimer O. Salmonella – the ultimate insider. Salmonella virulence factors that modulate intracellular survival. Cell Microbiol. 2009;11:1579–1586. doi: 10.1111/j.1462-5822.2009.01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brumell JH, Grinstein S. Salmonella redirects phagosomal maturation. Curr Opin Microbiol. 2004;7:78–84. doi: 10.1016/j.mib.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 68.Krishnakumar R, Kim B, Mollo EA, Imlay JA, Slauch JM. Structural properties of periplasmic SodCI that correlate with virulence in Salmonella enterica serovar Typhimurium. J Bacteriol. 2007;189:4343–4352. doi: 10.1128/JB.00010-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Groisman EA. The pleiotropic two-component regulatory system PhoP-PhoQ. J Bacteriol. 2001;183:1835–1842. doi: 10.1128/JB.183.6.1835-1842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perez JC, Shin D, Zwir I, Latifi T, Hadley TJ, Groisman EA. Evolution of a bacterial regulon controlling virulence and Mg2+ homeostasis. PLoS Genetics. 2009;5:e1000428. doi: 10.1371/journal.pgen.1000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garcia Vescovi E, Soncini FC, Groisman EA. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell. 1996;84:165–174. doi: 10.1016/s0092-8674(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 72.Prost LR, Daley ME, Le Sage V, Bader MW, Le Moual H, Klevit RE, Miller SI. Activation of the bacterial sensor kinase PhoQ by acidic pH. Mol Cell. 26:165–174. doi: 10.1016/j.molcel.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 73.Bader M,W, Sanowar S, Daley ME, Schneider AR, Cho U, Xu W, Klevit RE, Le Moual H, Miller SI. Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell. 2005;122:461–472. doi: 10.1016/j.cell.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 74.Kato A, Groisman EA. The PhoQ/PhoP regulatory network of Salmonella enterica. Adv Exp Med Biol. 2008;631:7–21. doi: 10.1007/978-0-387-78885-2_2. [DOI] [PubMed] [Google Scholar]
- 75.Prost LR, Sanowar S, Miller SI. Salmonella sensing of anti-microbial mechanisms to promote survival within macrophages. Immunol Rev. 2007;219:55–65. doi: 10.1111/j.1600-065X.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- 76.Zwir I, Shin D, Kato A, Nishino K, Latifi T, Solomon F, Hare JM, Huang H, Groisman EA. Dissecting the PhoP regulatory network of Escherichia coli and Salmonella enterica. Proc Natl Acad Sci. 2005;102:2862–2867. doi: 10.1073/pnas.0408238102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cho US, Bader MW, Amaya MF, Daley ME, Klevit RE, Miller SI, Xu W. Metal bridges between the PhoQ sensor domain and the membrane regulate transmembrane signaling. J Mol Biol. 2006;356:1193–1206. doi: 10.1016/j.jmb.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 78.Cheung J, Bingman CA, Reyngold M, Hendrickson WA, Waldburger CD. Crystal structure of a functional dimmer of the PhoQ sensor domain. J Biol Chem. 2008;283:13762–13770. doi: 10.1074/jbc.M710592200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Choi E, Groisman EA, Shin D. Activated by different signals, the PhoP/PhoQ two-component system differentially regulates metal uptake. J Bacteriol. 2009;191:7174–7181. doi: 10.1128/JB.00958-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alix E, Blanc-Potard A. MgtC: a key player in intramacrophage survival. Trends Microbiol. 2007;15:252–256. doi: 10.1016/j.tim.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 81.Groisman EA, Mouslim C. Sensing by bacterial regulatory systems in host and non-host environments. Nat Rev Microbiol. 2006;4:705–709. doi: 10.1038/nrmicro1478. [DOI] [PubMed] [Google Scholar]
- 82.Cromie MJ, Shi Y, Latifi T, Groisman EA. An RNA sensor for intracellular Mg2+. Cell. 2006;125:71–84. doi: 10.1016/j.cell.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 83.Chamnongpol S, Groisman EA. Acetyl-phosphate-dependent activation of a mutant PhoP response regulator that functions independently of its cognate sensor kinase. J Mol Biol. 2000;300:291–305. doi: 10.1006/jmbi.2000.3848. [DOI] [PubMed] [Google Scholar]
- 84.Tao T, Grulich PF, Kucharski LM, Smith RL, Maguire ME. Magnesium transport in Salmonella typhimurium: biphasic magnesium and time dependence of the transcription of the mgtA and mgtCB loci. Microbiol. 1998;144:655–664. doi: 10.1099/00221287-144-3-655. [DOI] [PubMed] [Google Scholar]
- 85.Irnov, Kertsburg A, Winkler WC. Genetic control by cis-acting regulatory RNAs in Bacillus subtilis: general principles and prospects for discovery. Cold Spring Harb Symp Quant Biol. 2006;71:239–249. doi: 10.1101/sqb.2006.71.021. [DOI] [PubMed] [Google Scholar]
- 86.Spinelli SV, Pontel LB, Garcia Vescovi E, Soncini FC. FEMS Microbiol Lett. 2008;280c:226–234. doi: 10.1111/j.1574-6968.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- 87.Cromie MJ, Groisman EA. Promoter and riboswitch control of the Mg2+ transporter MgtA from Salmonella enterica. J Bacteriol. 2010;192:604–607. doi: 10.1128/JB.01239-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barrick JE, Corbino KA, Winkler WC, Nahvi A, Mandal M, Collins J, et al. New RNA motifs suggest an expanded scope for riboswitches in bacterial genetic control. Proc Natl Acad Sci USA. 2004;101:6421–6426. doi: 10.1073/pnas.0308014101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Corbino KA, Barrick JE, Lim J, Welz R, Tucker BJ, Puskarz I, et al. Evidence for a second class of S-adenosylmethionine riboswitches and other regulatory RNA motifs in alpha-proteobacteria. Genome Biol. 2005;6:R70. doi: 10.1186/gb-2005-6-8-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weinberg Z, Barrick JE, Yao Z, Roth A, Kim JN, Gore J, et al. Identification of 22 candidate structured RNAs in bacteria using the CMfinding comparative genomics pipeline. Nucleic Acids Res. 2007;35:4809–4819. doi: 10.1093/nar/gkm487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weinberg Z, Wang JX, Bogue J, Yang J, Corbino K, Moy RH, Breaker RR. Comparative genomics reveals 104 candidate structured RNAs from bacteria, archaea, and their metagenomes. Genome Biol. 2010;11:R31. doi: 10.1186/gb-2010-11-3-r31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gardner PP, Daub J, Tate JG, Nawrocki EP, Kolbe DL, Lindgreen S, Wilkinson AC, Finn RD, Griffiths-Jones S, Eddy SR, Bateman A. Rfam: updates to the RNA families database. Nucleic Acids Res. 2009;37:D136–40. doi: 10.1093/nar/gkn766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu X, Ji Y, Stormo GD. Discovering cis-regulatory RNAs in Shewanella genomes by Support Vector Machines. PLoS Comput Biol. 2009;5:e1000338. doi: 10.1371/journal.pcbi.1000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Block KF, Hammond MC, Breaker RR. Evidence for widespread gene control function by the ydaO riboswitch candidate. J Bacteriol Epub ahead of print. 2010 doi: 10.1128/JB.00450-10. Doi:10.1128/JB.00450-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maguire ME, Cowan JA. Magnesium chemistry and biochemistry. BioMetals. 2002;15:203–210. doi: 10.1023/a:1016058229972. [DOI] [PubMed] [Google Scholar]
- 96.Kehres DG, Maguire ME. Structure, properties and regulation of magnesium transport proteins. BioMetals. 2002;15:261–270. doi: 10.1023/a:1016078832697. [DOI] [PubMed] [Google Scholar]
- 97.Ramesh A, Winkler WC. Magnesium-sensing riboswitches in bacteria. RNA Biol. 2010;7:77–83. doi: 10.4161/rna.7.1.10490. [DOI] [PubMed] [Google Scholar]
- 98.Wakeman CA, Ramesh A, Winkler C. J Mol Biol. 2009;392:723–735. doi: 10.1016/j.jmb.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]