This review summarizes the results of percutaneous radiofrequency ablation and cryoablation in the treatment of renal neoplasms.
Abstract
Percutaneous ablation in the kidney is now performed as a standard therapeutic nephron-sparing option in patients who are poor candidates for resection. Its increasing use has been largely prompted by the rising incidental detection of renal cell carcinomas with cross-sectional imaging and the need to preserve renal function in patients with comorbid conditions, multiple renal cell carcinomas, and/or heritable renal cancer syndromes. Clinical studies to date indicate that radiofrequency ablation and cryoablation are effective therapies with acceptable short- to intermediate-term outcomes and with a low risk in the appropriate setting, with attention to pre-, peri-, and postprocedural detail. The results following percutaneous radiofrequency ablation and cryoablation in the treatment of renal cell carcinoma are reviewed in this article, including those of several larger scale studies of ablation of T1a tumors. Clinical and technical considerations unique to ablation in the kidney are presented, and potential complications are discussed.
© RSNA, 2011
Introduction
More than 60 000 new cases of renal cell carcinoma (RCC) have been predicted to occur in 2011 in the United States, with 13 120 deaths from the disease estimated to occur over the same time period (1,2). With the increasing use of cross-sectional imaging, the incidental detection of RCC has become more common (3,4). More than half of new cases are detected incidentally, and overall detection incidence has more than doubled during the past 50 years (3–7). Preservation of renal function is paramount in patients with RCC with comorbid conditions, multiple RCCs, and/or heritable renal cancer syndromes (4). This need has driven the development of minimally invasive therapeutic alternatives to conventional open nephrectomy (4). The incidental detection of RCC has furthered interest in minimally invasive ablative techniques, since many incidental tumors are smaller, of lower grade, and are associated with longer disease-free survival than those identified in symptomatic patients (8). Some have argued that small incidental renal masses in the elderly should undergo active imaging surveillance rather than immediate treatment due to the slow growth rate in general and the low metastatic potential for tumors smaller than 4 cm, making it unlikely that they will influence the health of many elderly patients (9). Nevertheless, there remains widespread interest in minimally invasive ablative therapies. This is due, in part, to the anxiety generated by using serial imaging without treatment, as well as the need to ultimately manage many of these small tumors once they grow. The choice of radiofrequency (RF) ablation or cryoablation is often driven by practice patterns. Care must be taken when interpreting the literature or attempting to compare results from RF ablation and cryoablation, due to patient selection bias, local practice issues, and nonuniform techniques or differences in follow-up.
In this review, we summarize the results of percutaneous RF ablation and cryoablation in the treatment of renal neoplasms. Data concerning the effectiveness of percutaneous microwave ablation and high-intensity focused ultrasound may also be of interest to the reader, although these data are preliminary at present (10–14). Since RF ablation was first proposed in the 1990s as a new treatment for RCC, several investigators have reported the short-term and midterm effectiveness of both RF ablation and cryoablation of small renal masses (8,15) (Figs 1,2). A select number of groups have also since reported the results for larger cohorts of more than 100 tumors treated with either RF ablation or cryoablation, and there are emerging series of patients in whom 5-year follow-up data are available and are presented (8). Clinical and technical considerations unique to ablation in the kidney are also presented.
Figure 1a:
Early treatment of RCC with percutaneous RF ablation. (a) Axial contrast-enhanced CT scan of left kidney shows 2-cm RCC arising from posterior midportion of the left kidney. (b) Axial contrast-enhanced CT scan 5 years after technically successful percutaneous RF ablation shows no evidence for enhancement in the ablation zone. Local control was sustained on follow-up.
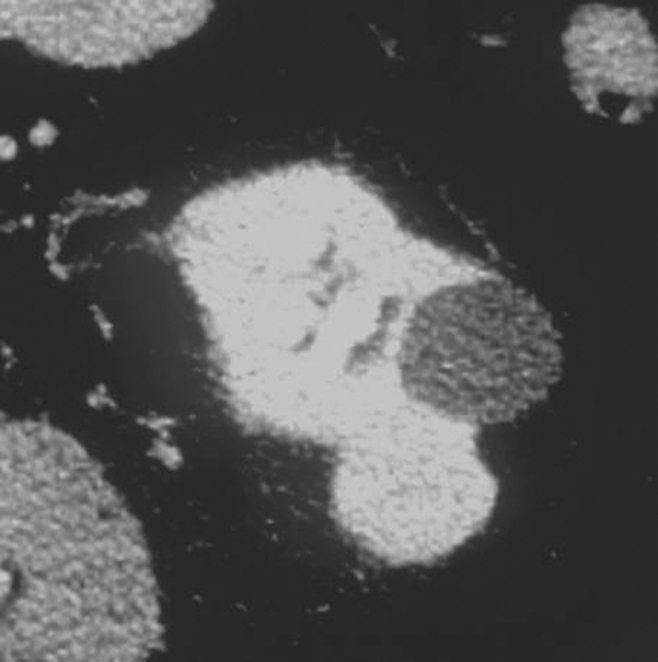
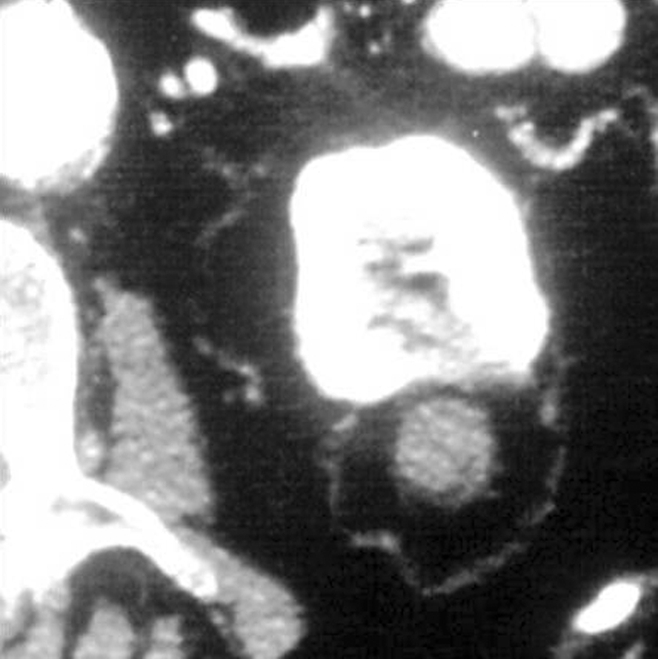
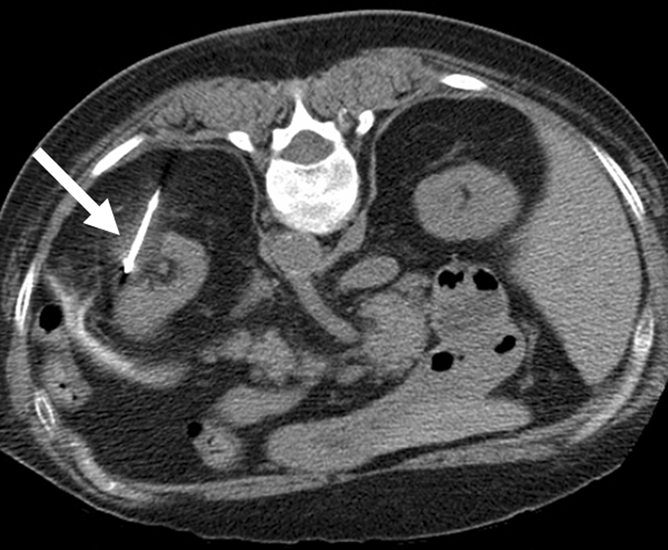
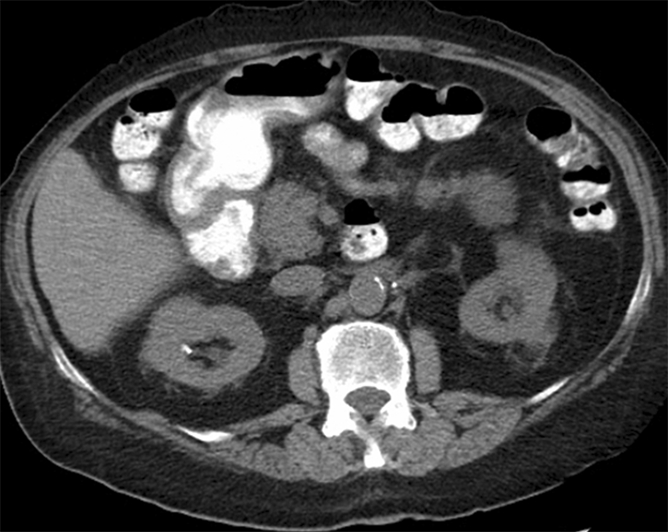
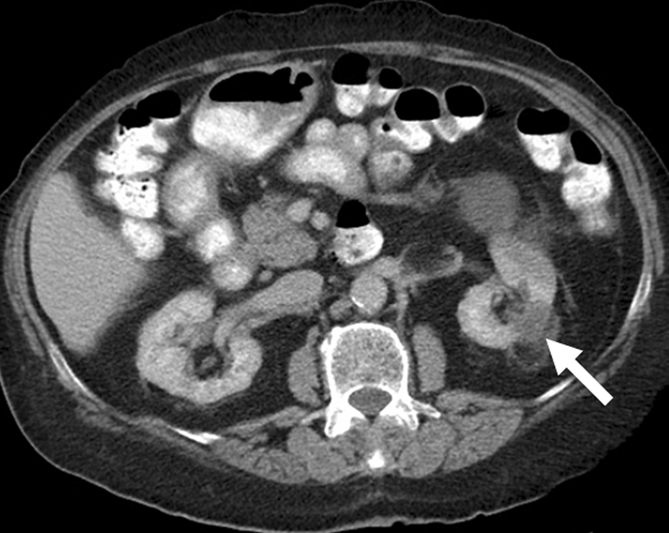
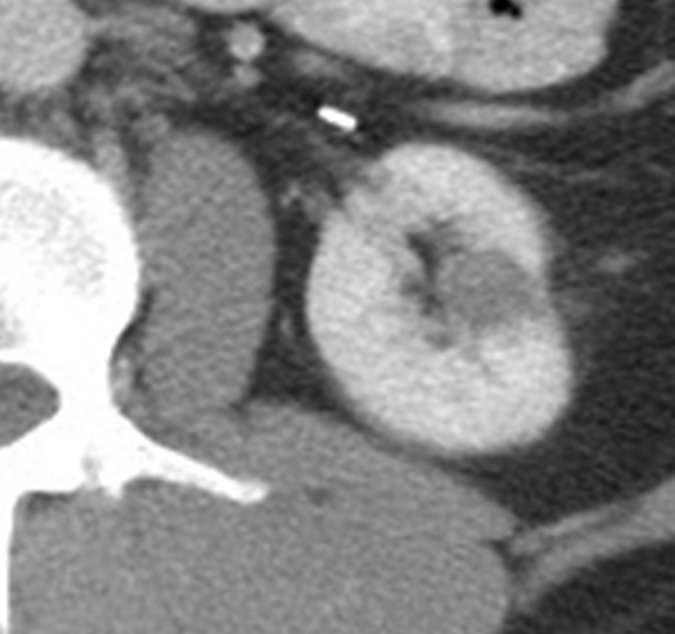
Figure 2a:
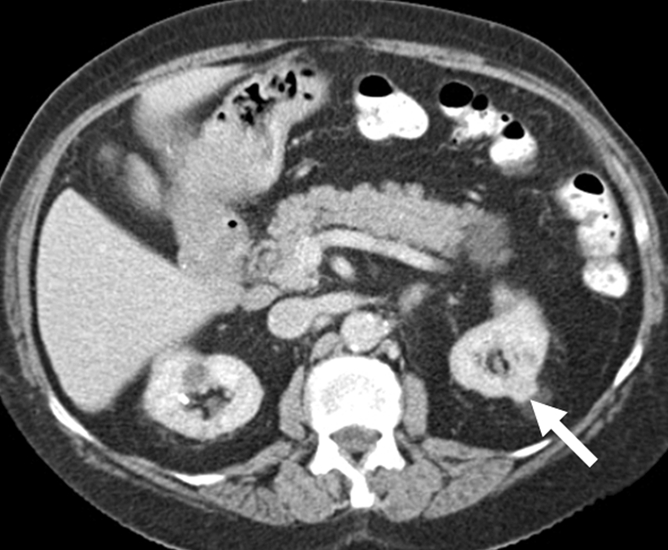
Cryoablation of RCC. (a) Axial contrast-enhanced CT scan in 78-year-old woman shows 1.4-cm enhancing left RCC (arrow). (b) Intraprocedural nonenhanced CT scan obtained in prone position at time of cryoablation shows cryoprobe within left renal mass (arrow). (c) Nonenhanced and (d) contrast-enhanced CT scans 3 months after ablation show nonenhancing ablation zone (arrow).
Figure 1b:
Early treatment of RCC with percutaneous RF ablation. (a) Axial contrast-enhanced CT scan of left kidney shows 2-cm RCC arising from posterior midportion of the left kidney. (b) Axial contrast-enhanced CT scan 5 years after technically successful percutaneous RF ablation shows no evidence for enhancement in the ablation zone. Local control was sustained on follow-up.
Figure 2b:
Cryoablation of RCC. (a) Axial contrast-enhanced CT scan in 78-year-old woman shows 1.4-cm enhancing left RCC (arrow). (b) Intraprocedural nonenhanced CT scan obtained in prone position at time of cryoablation shows cryoprobe within left renal mass (arrow). (c) Nonenhanced and (d) contrast-enhanced CT scans 3 months after ablation show nonenhancing ablation zone (arrow).
Figure 2c:
Cryoablation of RCC. (a) Axial contrast-enhanced CT scan in 78-year-old woman shows 1.4-cm enhancing left RCC (arrow). (b) Intraprocedural nonenhanced CT scan obtained in prone position at time of cryoablation shows cryoprobe within left renal mass (arrow). (c) Nonenhanced and (d) contrast-enhanced CT scans 3 months after ablation show nonenhancing ablation zone (arrow).
Figure 2d:
Cryoablation of RCC. (a) Axial contrast-enhanced CT scan in 78-year-old woman shows 1.4-cm enhancing left RCC (arrow). (b) Intraprocedural nonenhanced CT scan obtained in prone position at time of cryoablation shows cryoprobe within left renal mass (arrow). (c) Nonenhanced and (d) contrast-enhanced CT scans 3 months after ablation show nonenhancing ablation zone (arrow).
Technical Considerations
As noted by Zagoria (16), formal indications regarding the use of percutaneous ablation in the treatment of renal malignancy have yet to be validated with prospective scientific studies. Clinical observational studies performed to date, however, indicate that both RF ablation and cryoablation in the kidney are effective and have a low risk of substantial complications (4,8,16–20). Details concerning the basic science mechanisms and technical details essential to successful percutaneous RF ablation and cryoablation will be covered in a separate review in this series. A brief summary of the basic principles governing RF ablation and cryoablation is provided in this review.
Thermal RF ablation is achieved by converting electrical current in the RF range into heat (21). RF ablation is accomplished by creation of a closed-loop circuit, with a generator, grounding pads, a patient, and an electrode placed in series (22). Application of current results in marked agitation of the ions present in the tissues that immediately surround the electrode, with resultant frictional heat and thermal damage to the surrounding tissues. The extent of thermal damage is dependent on the tissue temperature generated and the duration of heating (22). For adequate destruction of tumor tissue, the entire volume of an index tumor must be treated with temperatures that are above the threshold for cell death, typically 50°–60°C (22).
Cryoablation has been used in the treatment of skin, breast, liver, brain, and bone tumors during the past 4 decades (23–30). During cryoablation, the freezing and thawing process destroys cell membranes and organelles due to the mechanical stresses associated with phase change and ice formation (24). As noted by Weber and Lee (31), cryoablation freeze and thaw cycling causes intracellular ice crystal formation, microvascular and cell membrane injury, and hypotonic cell disruption. Experimental data have shown that temperatures of −40° to −50°C are required to induce complete cell death (32,33).
Both RF ablation and cryoablation may be used with either a percutaneous or laparoscopic approach. At present most cryoablations are performed by using a laparoscopic approach, with RF ablation being more commonly administered percutaneously (34). With the more recent introduction of thin probes used with argon systems, minimally invasive percutaneous cryoablation techniques are being utilized (34,35). In general, anteriorly located tumors may not be amenable to a percutaneous approach given their proximity to the bowel, although, in clinical practice, this is rarely an absolute contraindication (36).
A recent study (34) has compared laparoscopic and percutaneous approaches for cryoablation. Finley et al (36) compared results of percutaneous cryoablation in 18 patients (19 masses) with those of laparoscopic cryosurgery in 19 patients (24 masses). The mean tumor size was 2.9 cm (range, 1.1–5.4 cm). The procedure time, narcotic requirement, and length of hospital stay were significantly greater with the laparoscopic approach. Bandi et al (37) described results of laparoscopic cryoablation in 58 patients (68 masses), percutaneous cryoablation in 20 patients (20 masses), and percutaneous RF ablation in 15 patients (15 masses). The mean patient age was 66 years (range, 24–86 years), with no significant difference in the mean age, body mass index, and median American Society of Anesthesiologists scores between the groups. The mean diameter of the treated mass was slightly larger in the laparoscopic ablation group (2.6 cm), compared with masses in the percutaneous cryoablation (2.2 cm) and percutaneous RF ablation (2.2 cm) groups. The mean diameter in the percutaneous cryoablation and RF ablation groups was the same (2.2 cm). Compared with laparoscopic cryoablation, percutaneous cryoablation was associated with use of fewer probes per lesion, shorter mean anesthesia time, shorter mean hospital stay, earlier return to activity, and shorter time to complete recovery. Compared with laparoscopic cryoablation, percutaneous RF ablation was associated with shorter mean anesthesia time and earlier returns to physical activity and work. No difference was noted in the median opioid analgesic requirement or in patient satisfaction measured on a 0–5 scale between the various groups. Hinshaw et al (38) described outcomes in 30 percutaneous renal cryoablations (mean tumor size, 2.1 cm) in 30 patients and 60 laparoscopic renal cryoablations (mean tumor size, 2.5 cm) in 46 patients. Both approaches in the percutaneous and laparoscopic groups demonstrated comparable success rates, with no significant difference in rates of residual disease. One renal mass treated with laparoscopic cryoablation had a local recurrence, but none of the masses treated with percutaneous cryoablation had a recurrence. The disease-specific survival was 100% in both groups, with no significant difference in the mean follow-up time or major complication rate.
For the treatment of solid renal masses, percutaneous cryoablation was associated with 40% lower hospital charges (mean, $14 175 vs $23 618) and a shorter hospital stay than laparoscopic cryoablation (38). Badwan et al (39) have also described a cost comparison of laparoscopic versus percutaneous cryoablation, demonstrating that the percutaneous approach (median, $6861) was more cost-effective than laparoscopic cryoablation (median, $29 617). Similar findings concerning the effectiveness and complication rates of ablation of RCC have been found with meta-analysis. Hui et al (40) conducted a meta-analysis of 46 series from the English-language renal ablation literature (28 percutaneous, 18 surgical) published between January 1996 through August 2006. Meta-analysis was performed on primary effectiveness, secondary effectiveness, and major complication rates. Primary effectiveness rate in the percutaneous group (87%) was significantly lower than that in the surgical group (94%), although the secondary effectiveness rate in the percutaneous treatment group was not significantly different from that in the surgical treatment group (92% vs 95%). The major complication rate in the percutaneous treatment group (3%) was significantly lower than that in the surgical treatment group (7%), leading the authors to conclude that a percutaneous approach was safer than an open or laparoscopic approach and as effective, although more than one procedure might be needed to treat the tumor completely.
Anatomic Considerations and Related Adjunctive Techniques
Several anatomic and physiologic considerations unique to the kidney must be kept in mind for effective and safe percutaneous ablation. As advocated by Ogan et al (41), it is recommended that the tines of the ablative probe be deployed to ablate 0.5–1.0 cm beyond the deepest margin of the tumor to achieve effective ablation and avoid the presence of viable tumor cells at the periphery of treated tumors. The anatomic location of the kidney also warrants close inspection for proximity between the renal tumor and nearby vulnerable structures such as the ureters, genitofemoral or ilioinguinal nerves, the psoas major muscle, small and large bowel, and the adrenal gland.
Physiologic Considerations
The unique physiology of the kidney also has important implications in ablation planning. The kidney is a highly perfused organ, with approximately four times the perfusion of the liver (42,43). This difference in perfusion significantly alters the results of the bioheat equation, which estimates the amount of energy that can be deposited in tissue, by increasing the energy lost (42,43). Convection of heat due to overall perfusion and to perfusion by large central vessels can also contribute to a heat-sink effect that may result in under-treatment of renal tumors (43). This phenomenon is particularly likely when ablation algorithms are used for renal ablation that were developed specifically for liver or other less perfused tissue (43). It may be advisable to consider repeated ablation cycles in highly perfused RCCs that show delayed impedance rises or lower temperatures during or immediately after RF ablation.
Tumor Characteristics
Tumor size and location have implications for the likelihood of success. Gervais et al (4), in a multivariate analysis of 100 percutaneous RCC RF ablations, found that tumor size and location were independent predictors of the results of RF ablation. Small size (≤3 cm) and noncentral location were individual predictors of complete necrosis after a single ablation session. Forty-four percent (16 of 36) of tumors between 3 and 5 cm required more than one ablation session to achieve complete necrosis in this series. Noncentral location was a significant predictor of complete necrosis after any number of sessions, which is attributed to the insulating effect of the perirenal fat and the heat-sink effect from larger central blood vessels (4,44,45). Because over-treating the central kidney may risk collecting system or vascular complication, the lower success rate achieved with central tumors may reflect less aggressive ablation in that location. Similar findings concerning the influence of index tumor size and location on ablation success have been observed in a larger scale study using cryoablation. In a retrospective review of cryoablation in 115 renal tumors, Atwell et al (19) achieved technical success, defined as lack of tumor enhancement on postablation images, in 112 of 115 (97%) treated tumors. Two of the three technical failures occurred in tumors measuring 4.0 and 4.3 cm in diameter. All three technical failures occurred in central tumors, likely secondary to technical challenges associated with the proximity of these tumors to major vessels and the collecting system.
Patient Selection and Treatment
All patients who are candidates for percutaneous or laparoscopic renal ablation are ideally evaluated by an interdisciplinary team consisting of interventional radiologists and urologists. In selected cases, evaluation by an oncologist may be beneficial. Patients should discuss with members of this team the appropriateness, risks, benefits, and alternatives to percutaneous ablation for their individual case. Patients who are believed to be appropriate candidates for percutaneous ablation should undergo preprocedural imaging, including multiphase contrast material–enhanced computed tomography (CT) or magnetic resonance (MR) imaging with gadolinium for tumor staging. To achieve curative results, patients should have no tumor spread beyond Gerota fascia, into the renal vein, or to lymph nodes (stage I or II in the Robson classification system or stage T1–T3a in the TNM classification system) (16,46). Extension of the tumor into veins, adjacent organs, or lymph nodes or distant metastases may preclude curative intent. In the setting of advanced disease, alternate reasons to pursue ablation may include tumor debulking or palliative intent to control pain and hematuria (47). At a minimum, laboratory values obtained for all patients should include a blood count, prothrombin time, partial thromboplastin time, and creatinine level. Patients should discontinue the use of aspirin, other antiplatelet agents, and anticoagulants far enough in advance of the procedure for coagulation parameters and function to return to normal (16). Candidates for percutaneous renal ablation should have a histopathologic diagnosis of RCC, a heritable renal cancer syndrome, and/or imaging findings characteristic of the same. The need for a preablation biopsy should be considered; details regarding the role of preablation biopsy are discussed in further detail below.
In most instances, RF ablation or cryoablation are considered suitable in patients with a renal tumor compatible with RCC on the basis of cross-sectional imaging and those who have marginal renal function, comorbidities that make them high-risk surgical candidates, multiple renal tumors, or a high risk of developing additional RCCs (eg, patients with von Hippel–Lindau disease or familial RCC) (16). Patients at significant risk for impaired renal function, such as those with RCC and a solitary functioning kidney, prior partial or total nephrectomy, or tumors within a renal transplant may also be ideal candidates for percutaneous ablation (19). Percutaneous ablation has also served a palliative role in preserving renal function in patients with metastatic RCC, in those with transfusion-dependent, tumor-related hematuria, and in those who voluntarily prefer ablation despite surgery being a treatment option (19,48). Absolute contraindications to percutaneous renal ablation include uncorrected coagulopathy and acute illness, such as sepsis (16). Candidates unwilling or unable to comply with follow-up requirements are also not ideal ablation candidates. Serious comorbidities (such as congestive heart failure) should not be considered an absolute contraindication to ablation, and procedural risk should be evaluated on an individual basis (16).
There is no consensus about the effectiveness of prophylactic antibiotics for patients undergoing RF ablation in the kidney. In the absence of definitive scientific evidence, some practitioners continue to empirically use prophylaxis, and others use them only in selected cases (49–51). At one of our institutions, all patients undergoing percutaneous renal RF ablation receive a single dose of intravenous levofloxacin prior to the procedure. At the other institution, no antibiotics are routinely given. Instances in which patients are at an increased risk of infection warrant careful evaluation; these cases may necessitate prolonged or extended antibiotic coverage. These cases include patients with a history of ileal conduit and ureteral stent placement, each of which increases the likelihood of bacterial colonization with the upper urinary tract. Additional conditions that may warrant consideration of extended antibiotic prophylaxis include diabetes mellitus and chronic immunosuppression (52).
Imaging Guidance
The majority of renal ablations are guided by using ultrasonography (US), CT, or MR imaging (53,54). To our knowledge, no published trials exist evaluating the utility of US versus CT or MR imaging for imaging guidance and treatment monitoring during RF ablation or cryoablation; therefore, the selection of any single modality as the preferred modality remains controversial and at the discretion of the interventional radiologist. There are advantages and disadvantages in the use of each of these modalities for renal RF ablation and cryoablation.
The advantages of using US for monitoring during renal ablation are its real-time capability and its lack of ionizing radiation. As noted by Maybody and Solomon (54), however, imaging with US is highly operator-dependent and may be compromised in certain settings, such as in patients with a large body habitus, the presence of abundant bowel gas, and when the tumor is near a focal loop of bowel that needs to be avoided. Use of US is also associated with limitations regarding reliable intraprocedural monitoring, because hyperechoic bubbles produced by vaporization during the procedure substantially disturb image quality (53). CT is associated with fewer changes in the imaging pattern of ablated renal masses, although intratumoral hyperdensity and gas generated during ablation may limit visualization (53). Residual tumor tissue can be detected at CT with the administration of contrast material because viable tissue will exhibit signal enhancement, whereas coagulation necrosis does not (53,55). As noted by Boss et al (53), MR imaging is well suited for imaging guidance during RF ablation due to its intrinsically high soft-tissue contrast, the capability of true multiplanar imaging, and the possibility of applicator targeting with MR fluoroscopic sequences. Rapid T1-weighted gradient-echo sequences and T2-weighted steady-state free precession sequences allow for rapid imaging of applicator placement (53). MR imaging is the only modality providing near real-time monitoring of the ablation procedure. Discrete postablation changes are visible on T2-weighted MR images. Complete tumor ablation is achieved in areas where signal loss on T2-weighted images replaces the isointense or hyperintense signal of the index tumor (53). In the acute phase after ablation, the hypointense area of complete necrosis is typically surrounded by a small hyperintense rim, which corresponds to the zone of inflammation and hemorrhage (53). Residual tumor tissue can be detected due to its persisting isointense or hyperintense signal (53). Repeated assessment of ablation outcome without repeated injection of contrast material offers an advantage of MR guidance over other imaging modalities (53).
Percutaneous image-guided cryoablation can be performed with US, CT, or MR guidance. The ice ball generated during cryoablation consists of multiple isotherms (54). The central layer is the coldest isotherm. Freezing has a more consistent cytotoxic effect when cell temperatures are lowered to –20°C or less, with the outer layer of the ice ball (0°C isotherm) not necessarily cytotoxic (54). None of the three imaging monitoring modalities available for guidance are capable of differentiating isotherms from each other. Instead, with current imaging, the ice appears as a homogeneous structure. To achieve the best result, special attention should be paid to ensure that the desired –20°C isotherm covers the entire tumor and the expected rim of the surrounding parenchyma (54). The ice ball must extend at least 3 mm beyond the tumor margin to achieve a tissue temperature of −20°C at the margin, and the goal should be an ice ball margin of at least 5 mm to avoid residual or untreated tumor (56). US guidance and monitoring during cryoablation may be limited due to the generation of acoustic shadowing on the far side of the ice ball that prevents complete visualization (54). CT is less operator-dependent and its wide field of view enables visualization of critical organs and structures that need to be avoided during cryoablation (54). MR imaging is the least commonly used imaging modality for percutaneous cryoablation because of the need for special arrangements to accommodate cryoablation and for MR-compatible equipment (54). Image-guided percutaneous cryoablation can be performed by using a dedicated interventional magnet or a conventional magnet with a larger gantry, with the ice ball visualized as a zone of decreased signal intensity on T1-weighted images (54,56).
Role of Preablation Biopsy
Percutaneous biopsy of renal masses, particularly those less than 3 cm, has been described as having a limited role, given difficulties with respect to index tumor targeting and sampling error, which may confound equivocal or negative results (57). Some investigators suggest that biopsy does not significantly alter diagnosis or treatment in the majority of cases, given the likelihood of diagnosis on the basis of noninvasive imaging and the lack of need for adjuvant therapy for early-stage (stage I) RCC (47). With the increased use of percutaneous ablative techniques to treat renal cancer, the role of confirmatory biopsy is being revisited, as ablation lacks the opportunity for histopathologic analysis afforded with surgical resection (57). Several published trials of percutaneous tumor ablation of RCC have included patients who did not undergo a percutaneous biopsy, but who were treated with a presumptive diagnosis of renal cancer on the basis of imaging features (11–14,17,18). If benign index tumors were included in these data, including angiomyolipomas or oncocytomas, which may mimic solid RCCs, the treatment effectiveness may have been over-estimated and unnecessary follow-up may have been prescribed (57,58). Tuncali et al (57) performed percutaneous biopsy in 27 patients referred for MR-guided cryotherapy because of suspected RCC. Masses ranged in size from 1.0 to 4.6 cm (mean, 2.2 cm); CT, MR imaging, and percutaneous biopsy findings were tabulated and compared with surgical and imaging follow-up findings. A substantial proportion of patients (37%) had a benign renal mass, including three angiomyolipomas, which showed no evidence of fat at CT or MR imaging. The masses in the remaining 17 patients were ablated, with the investigators advocating pretreatment biopsy in all patients in whom imaging cannot characterize an index tumor as benign. Physicians performing RCC ablation should give very serious consideration to performing biopsy before RF ablation, perhaps with the exception of hereditary RCC. Renal biopsy is increasingly being performed immediately prior to ablation, with results available after completion of the ablation, given patients’ preference for a definitive diagnosis, the fact that ablation does not yield a resection specimen, and to inform follow-up imaging planning (16).
Results
Percutaneous renal ablative techniques have the potential for parenchymal preservation with less morbidity compared with open surgical or even laparoscopic tumor resection (59). Although long-term oncologic data are currently not available, current 5-year follow-up data suggest that renal RF ablation and cryoablation are effective treatments with acceptable short- to intermediate-term effectiveness and a low risk of complications (59). A summary of the results of a larger series of renal tumors (>30 tumors) treated with percutaneous RF ablation and cryoblation has been provided in Tables 1 and 2, respectively.
Table 1.
Results of Larger Series of Renal Tumors Treated with Percutaneous RF Ablation

Numbers are the range; numbers in parentheses are the mean.
Defined as complete necrosis at imaging after a single ablation session.
Five elderly patients were not re-treated on the basis of clinical status; 12 of 14 remaining tumors were successfully re-treated.
One large (>8 cm) RCC was excluded from analysis due to absence of baseline enhancement.
Table 2.
Results of Larger Series of Renal Tumors Treated with Percutaneous Cryoablation
Numbers are the range; numbers in parentheses are the mean.
Defined as complete necrosis at imaging after a single ablation session.
In the report by Gervais et al (4) of RF ablation of 100 tumors in 85 patients, all 52 tumors less than 3 cm in size and all 68 exophytic tumors underwent complete necrosis, defined by tumor nonenhancement. This result was sustained over a mean follow-up period of 2.3 years (range, 3.5 months to 6 years). Up to 50% of large tumors (>3 cm) required more than one ablation session to achieve complete necrosis; however, only one case of local tumor progression was detectable 14 months after RF ablation in a patient whose viable tumor was not detectable immediately after RF ablation. This local tumor progression was successfully treated with RF ablation, with complete necrosis sustained up to 1.5 years. Eleven complications were encountered after 126 ablation sessions, of which two were major complications of hemorrhage and requred transfusion. Noncentral location was also found to be a predictor of complete necrosis after any number of ablation sessions, with small size showing a trend toward prediction of treatment success that did not quite reach statistical significance.
Breen et al (60) used RF ablation in the treatment of 105 renal tumors; 83 tumors were completely treated in a single session (79%). Twelve of the remaining tumors were successfully re-treated, with a clinical decision made not to retreat five patients, most of whom were elderly, with small residual foci of viable tumor at contrast-enhanced CT. Comparable to the 90% tumor necrosis rate in the Gervais et al study, Breen et al achieved technical success in 90.5% of patients, which was sustained over a mean follow-up of 16.7 months (range, 1–76 months). Five complications were sustained after 120 ablation sessions. Multivariate analysis revealed that tumor size was the only predictor of procedure outcome, with tumors less than 3 cm being associated with complete treatment in a single ablation session (60).
Zagoria et al (18) have reported similar results in their experience with RF ablation of 125 RCCs in 104 patients. Of 125 treated tumors, 116 (93%) were completely ablated (109 in a single ablation session, seven after a second ablation session), with a mean follow-up of 13.8 months. All 95 RCCs less than 3.7 cm were completely ablated. Smaller tumors (<3.7 cm) were significantly associated with complete tumor eradication (P < .001). Twenty-one of 30 larger (>3.7 cm) tumors were completely ablated after a repeat ablation session, with nine showing evidence of residual viable tumor on follow-up scans. Eight complications were encountered, none of which resulted in long-term morbidity.
Atwell et al (19) have described comparable technical feasibility, safety, and outcome for cryoablation of renal tumors. In their retrospective review of 115 renal tumors in 110 patients treated with percutaneous cryoablation, technical success was achieved in 112 (97%) of the treated tumors, with three residual tumors seen at 3-month follow-up imaging. The mean tumor size was 3.3 cm (range, 1.5–7.3 cm), with 29 treated tumors measuring 4.0 cm or larger. No local progression was observed in 80 tumors followed up 3 months or longer (mean, 13.3 months). Seven major complications were associated with 113 cryoablation procedures.
The aforementioned results have included small number of patients with hereditary forms of renal cancer. Of interest, similar success rates have been observed in a larger series of patients with hereditary renal cancer syndromes. Hwang et al (43) have described results of the largest series of patients treated with RF ablation for renal tumors in the setting of hereditary renal cancer and have reported intermediate follow-up data for 17 patients with a total of 24 hereditary-based renal tumors and a minimum of 1-year follow-up. All but two patients in this series had von Hippel–Lindau disease; one each had Birt-Hogg-Dube syndrome and hereditary RCC. Treatment eligibility criteria included an average tumor diameter of less than 3.0 cm, tumor growth during the 1st year, and solid appearance with contrast enhancement (change > 20 HU) at CT. A percutaneous approach was considered unsuitable if kidney tumors were contiguous to the bowel, ureter, or large vessels, and these tumors were approached laparoscopically. A total of 15 renal tumors were ablated laparoscopically, 13 of which were in direct contact with the bowel and two of which were abutting the ureter. Postoperative follow-up consisted of CT without and with intravenous contrast material and renal function assessment at regular intervals. At the minimum 1-year follow-up, 23 of 24 ablated tumors lacked contrast material uptake at CT, meeting radiographic criteria for successful RF ablation treatment. One centrally located tumor adjacent to the renal hilum exhibited enhancement (change >10 HU) and met radiographic criteria for local tumor progression.
In a recent meta-analysis, therapeutic approaches to renal cancer, including percutaneous RF ablation and cryoablation, were reviewed and compared. Kunkle et al (64) reviewed treatment modalities for small (<4 cm) renal masses, including partial nephrectomy, cryoablation, RF ablation, and observation. A total of 99 prospective and retrospective single and multi-institutional studies representing 6471 lesions were analyzed. Significant differences in mean patient age, tumor size, and follow-up duration were detected among treatment modalities and studies. Of note, in the study, local recurrence for cryoablation and RF ablation was defined as radiographic or pathologic evidence of residual disease following initial treatment, regardless of time to recurrence. For lesions treated with partial nephrectomy, local recurrence was considered to have occurred if there was radiographic or pathologic evidence of tumor in the ipsilateral kidney adjacent to the site of previous resection. Compared with nephron-sparing surgery, significantly increased local progression rates were calculated for cryoablation and RF ablation, although no significant differences were detected in the incidence of metastatic progression, regardless of whether the lesions were excised, ablated, or observed. Significant major limitations to the study included a lack of standardized inclusion and treatment criteria and resultant differences in lesion selection, institutional biases for a particular therapeutic approach, the type of anesthesia (general vs conscious sedation), and follow-up criteria (65). Furthermore, as noted by Raman and Cadeddu (65), a metachronous tumor recurrence following partial nephrectomy is a distinct entity from radiographic evidence of incomplete primary thermal ablation. Defining these two distinct phenomena as indicative of local tumor recurrence has the potential to inflate the recurrence rates for ablation (65). As suggested by Raman and Cadeddu¸ one of the advantages of image-guided percutaneous RF ablation is ease of repeatability, allowing for caution by the treating physician to avoid over-aggressive therapy that might otherwise potentially contribute to complications.
A separate meta-analysis of existing published literature on cryoablation and RF ablation was reported by Kunkle and Uzzo (66), reflecting a subset of studies from a prior publication comparing surgery, thermal ablation, and observation for small renal masses. Forty-seven studies representing 1375 renal lesions treated with cryoablation or RF ablation were analyzed. Cryoablation was largely performed laparoscopically (65%), whereas 94% of lesions treated with RF ablation were approached percutaneously. Of note, local recurrence for cryoablation and RF ablation was defined as for the prior meta-analysis (64), with radiographic or pathologic evidence of residual disease following initial treatment regardless of time to recurrence. Repeat ablation was performed more often after RF ablation (8.5% vs 1.3%), with rates of local tumor progression being significantly higher for RF ablation (12.9% vs 5.2%) than for cryoablation, with the authors concluding that cryoablation results in fewer re-treatments and improved local tumor control (66). As noted by Caddedu and Raman (67), limitations of the meta-analysis by Kunkle and Uzzo include the authors’ definition of local tumor recurrence, since an incomplete primary thermal ablation is not typically considered clinically synonymous with a local tumor recurrence. Furthermore, the outcomes observed between cryoablation and RF ablation do not allow distinction between the effictiveness of the ablation technology itself and the technique used (67). Differences in the technical approach are notable in this meta-analysis, since the majority of cryoablation cases evaluated were performed laparoscopically or with open procedures, while 94% of RF ablation procedures were performed percutaneously, often as an outpatient procedure (67). As noted by Caddedu and Raman (67), physicians may be more judicious with needle placement and ablation zone dimension with a percutaneous ablation knowing that repeat ablation for residual tumor may be readily performed, is not a sign of local tumor progression or recurrence, and remains less morbid than either laparoscopic or open surgery. Conversely, a direct-vision open or laparoscopic approach for cryotherapy or even RF ablation is not as easily repeatable, which may mandate a more aggressive strategy (67). In addition, because RF ablation has been utilized clinically over a longer period of time than was cryoablation, it is possible that populations undergoing cryoablation have benefited from the techniques and experience reported previously in the RF ablation literature.
Although promising intermediate-term results and meta-analysis data are available for both renal RF ablation and cryoablation, it should be emphasized that few direct comparisons between the treatment effectiveness of RF ablation and cryoablation have been reported to date. The validity of comparative results between multiple single-center reports with small numbers, limited experience, and diverse patient populations remains questionable. Practical clinical practice guidelines exist that summarize existing literature concerning treatment modalities for stage 1a tumors (68). However, these do not obviate randomized controlled clinical trials that enable rigorous comparative analysis of the ablation technologies.
Effect of Percutaneous Ablation on Renal Function and the Solitary Kidney
Percutaneous ablation is increasingly used in patients with reduced renal function because it avoids the potential for renal ischemia that may be sustained during surgery (69). Literature regarding the effect of percutaneous ablation on renal function also suggests that percutaneous ablation can be performed successfully with minimal negative impact on renal function, even in patients with a solitary kidney (69). Johnson et al (70) evaluated the impact of renal tissue ablation on the function of the remaining kidney parenchyma in 25 patients with 26 tumors, ranging in size from 0.8 to 4 cm, who were treated and followed for a minimum of 6 months. Blood pressure measurements and serum creatinine levels were obtained at preoperative and postoperative office visits. Values of preoperative and postoperative systolic blood pressure, diastolic pressure, serum creatinine level, and calculated serum creatinine clearance for each patient before and after treatment were compared. No patient experienced new-onset hypertension or worsening of existing hypertension, and no changes in mean serum level and estimated creatinine clearance were observed. These results are similar to those published in studies of cryoablation in the kidney, which have shown no significant negative effects on renal function or blood pressure following therapy. Carvalhal et al (71) evaluated the effect of cryoablation in 22 patients followed up a minimum of 6 months and a mean of 20.6 months. No significant differences were found between preoperative and postoperative serum creatinine levels, systolic and diastolic blood pressure values, or in the estimated creatinine clearance. The dose required and the number of antihypertensive medications did not change during the follow-up period for any patient. In three patients with a solitary kidney, blood pressure and serum values also remained unchanged. Hegarty et al (72) presented results of 164 cryoablations and 82 RF ablations performed at their institution and compared them retrospectively, including their impact on renal function. Comparison of cryotherapy versus RF ablation revealed that the mean tumor size was similar (2.56 vs 2.51 cm) and complication rates were minimal in both groups. Renal function remained essentially unchanged at 5-year follow-up in the cryoablation group and at 2-year follow-up in the RF ablation group. Specifically, no significant difference was seen between preoperative and postoperative serum creatinine levels, which included those obtained immediately after ablation; 1, 3, and 6 months after ablation; and annually thereafter.
Patients with a solitary kidney may sustain the greatest benefit from minimally invasive tumor ablation compared with partial nephrectomy given their reduced renal reserve (69). Jacobsohn et al (69) evaluated clinical outcomes in 16 patients with a solitary kidney and solid renal mass treated with RF ablation. In these patients, the median preoperative creatinine level and creatinine clearance were 1.4 mg/dL and 76.7 mL/min, respectively. The median percentage of change in creatinine clearance was 13.3% within 1 week of ablation and 9.1% at a mean follow-up of 15.3 months. Major complications occurred in four patients, including three cases of clot obstruction and associated azotemia treated with ureteral stent placement and one case of perinephric hemorrhage treated with blood transfusion. All kidneys functioned postoperatively, with all but one patient maintaining renal function as of the last follow-up visit.
Hereditary Renal Neoplasms
Patients with hereditary or familial neoplasms require special consideration. The procedural goals may need to be modified, with a smaller treatment margin achieved (eg, several millimeters instead of ≥10 mm), to remain nephron sparing, given the propensity for metachronous tumors in these patients. Prior ablation or surgery is common in this patient population and creates adhesions, which may raise the risks for subsequent intervention (73). Consultation with a multidisciplinary team will ensure that all possible options have been fully considered (open, laparoscopic, or percutaneous and RF ablation vs cryoablation or partial nephrectomy). The decision of when to intervene may be especially difficult or controversial in these patients, in whom metastases are rare for tumors less than 3 cm (74). However, it may not be advisable to always wait for this 3-cm threshold for intervention. Some index tumors may be best approached when much smaller, especially if their location forecasts a more difficult ablation in the future. There is also minimal impact on the glomerular filtration rate as measured by using 24-hour urine creatinine levels before and after ablation in patients with hereditary renal cancers (75).
Percutaneous Ablation of the Cystic Renal Neoplasm
There is relatively little information in the published literature concerning the effectiveness of RF ablation or cryoablation on cystic RCC. Ablation of the cystic renal neoplasm is of practical importance, since cystic RCC may be found in sporadic cases, as well as in patients with heritable RCC syndromes, where an evolution from mainly cystic components to solid tumor may be observed over time. Park et al (76) performed RF ablation in nine patients with 14 Bosniak category III (n = 5) or IV (n = 9) cystic renal tumors that had a mean size of 2.5 cm ± 0.6 (standard deviation). Of the 14 index tumors treated, 13 required one session and one required two sessions for complete ablation, with no evidence of local tumor progression after a follow-up period of 8 months (range, 1–19 months). The ablation time ranged from 1 to 12 minutes per tumor (mean, 6 minutes). These preliminary findings suggest that percutaneous ablation may be successfully performed in the treatment of cystic renal neoplasms, with effectiveness demonstrated over a short-term follow-up period. These data also suggest that ablation of these index tumors may be achieved with a shorter duration of applied energy than that used for solid renal tumors of the same size. In our experience, this may not be universally true, particularly for larger cystic index tumors (≥3 cm), which may be difficult to heat.
Side Effects and Complications
A relatively common anticipated side effect following percutaneous RF ablation of abdominal tumors is known as postablation syndrome, which includes low-grade fever (37.5°–38.5°C), delayed pain, nausea, vomiting, malaise, and myalgias (77). In a retrospective review by Carrafiello et al (77), postablation syndrome was observed in 32% of 53 patients who had undergone percutaneous ablation of an abdominal tumor. These symptoms are notable for being self-limited, responding well to acetaminophen or nonsteroidal anti-inflammatory agents, and otherwise requiring no procedural intervention (78). The symptoms reported with cryoablation are similar, with pain and fever being among the most common (79). Additional anticipated side effect specific to renal ablation includes mild hematuria, which is also self-limited and requires no transfusion. These signs and symptoms should be distinguished from complications, which are uncommon and have been reported to occur in approximately 1% of patients (16,80). In a multi-institutional review of 180 percutaneous renal RF ablation and cryoablation procedures, the most common complication of ablation was pain or paresthesias at the percutaneous probe insertion site (50).
Hematuria occurs infrequently and is typically self-limited, resolving within 24 hours of therapy (81). The likelihood of hematuria following percutaneous ablation is increased with ablation of centrally located tumors. Rarely, bladder outlet obstruction may result from clot formation; this is typically treated with bladder irrigation and serial hematocrits (81). Perinephric hematomas may also occur during ablation, with Rhim et al (81) noting a prevalence of post–RF ablation hematoma of less than 5%. Hematomas of 1 cm or smaller are common and typically of no clinical consequence, and even larger perinephric hematomas are, for the most part, self-limited (Fig 3). Renal biopsy performed prior to ablation may induce bleeding, which can be treated with subsequent RF ablation (81). Cauterization during electrode withdrawal can also limit back bleeding, as well as tumor seeding (81). Of note, cryoablation does not cauterize or coagulate vessels with the ablation, in contrast to other heat-based ablation modalities such as RF ablation (54). Therefore, hemorrhage at the ablation zone immediately after probe removal is a risk of cryoablation that requires alternate management. Hemorrhage risk as a result of cryoablation can be avoided in most cases by strictly correcting patients’ coagulation profiles before the procedure and avoiding removal of probes at the end of the procedure in the presence of any resistance (54). Injection of gel foam or thrombin down the cryoprobe tract to the site of bleeding can also promote hemostasis (54,82).
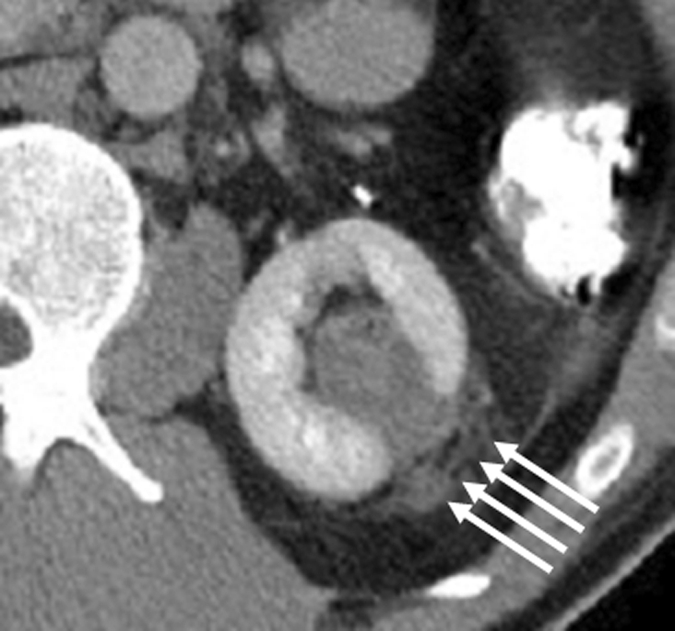
Figure 3a:
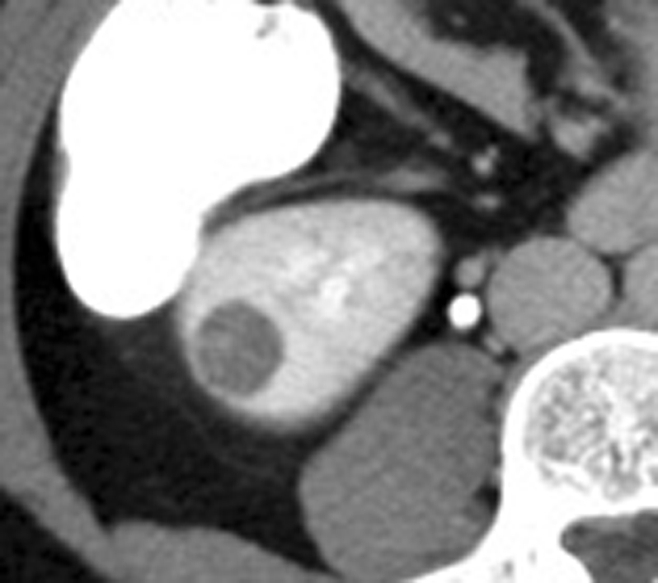
Incidental perinephric bleeding during percutaneous renal RF ablation. (a) Axial contrast-enhanced CT scan shows 1.5-cm RCC in lower pole of right kidney. (b) Intraprocedural axial contrast-enhanced CT scan shows electrode within mass in lower pole of right kidney, with adjacent small (<2 cm) perinephric hemorrhage (arrow). This finding is frequently seen and typically is self-limited; it resolved at follow-up imaging.
Figure 3b:
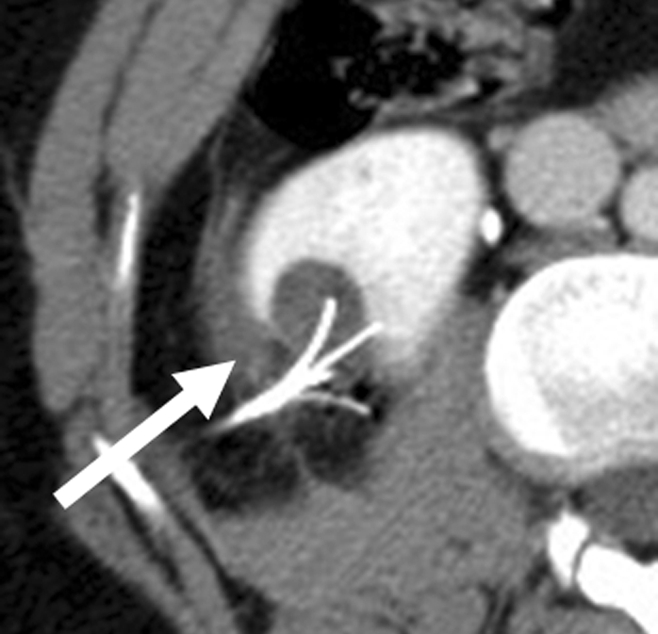
Incidental perinephric bleeding during percutaneous renal RF ablation. (a) Axial contrast-enhanced CT scan shows 1.5-cm RCC in lower pole of right kidney. (b) Intraprocedural axial contrast-enhanced CT scan shows electrode within mass in lower pole of right kidney, with adjacent small (<2 cm) perinephric hemorrhage (arrow). This finding is frequently seen and typically is self-limited; it resolved at follow-up imaging.
Additional risks of percutaneous renal ablation include the risk of thermal injury to the adjacent structures, including the ureters, the genitofemoral nerve, the psoas major muscle, the small and large bowel, and the adrenal gland. In general, these risks should be mitigated by careful choice of probes, careful probe placement, and configuration. Straight RF electrodes may be favored over multiple array probes for greater ease and accuracy of placement, which may be especially true if real-time monitoring of array deployment is not available (83). During cryoablation, careful monitoring of the ice ball generated is advisable to ensure that it is not in contact with vulnerable regional anatomy.
Inadvertent injury of the proximal ureter during percutaneous renal ablation has been associated with urinoma, ureteral obstruction, and chronic stricture (17). To avoid these potential complications, some authors advocate the use of intraprocedural retrograde pyeloperfusion. Cantwell et al (84) have reported about early experience with the use of cooled 5% dextrose in water (D5W) instilled by means of retrograde pyeloperfusion during RF ablation of RCCs located within 1.5 cm of the ureter. All 19 ablations for which cooled D5W pyeloperfusion was used were technically successful; no patient developed ureteral stricture or hydronephrosis during a mean follow-up of 14 months. In three patients, residual tumor was seen at the first follow-up imaging study, and all three underwent complete ablation after a second RF ablation session.
Genitofemoral nerve injury is also a recognized risk of renal ablation. Thermal injury to the nerve, which originates from the upper part of the lumbar plexus and descends laterally along the psoas major muscle, has resulted in chronic pain and diminished sensitivity in the ipsilateral groin (85). Injury to the psoas muscle itself can result in isolated impairment of hip flexion (86).
Bowel perforation secondary to renal ablation is especially serious. Colonic perforation and colonephric fistula have also been reported following percutaneous renal ablation (28–30). As noted by Rhim et al, at least 5 mm of mesenteric fat should be noted between the target tumor and the adjacent bowel when using single-tine RF electrodes, to adequately insulate the bowel from injury (81). Strategic patient positioning and hydrodissection are additional adjunctive techniques that should be used to protect the bowel, as needed. Although small-bowel injury has yet to be reported in humans following renal ablation, duodenal thermal injury has been reported in a porcine model for renal RF ablation (56,87,88). Anteriorly located tumors and left renal tumors may be in particularly close relationship to the small bowel.
Physical separation of the target tumor from vulnerable regional anatomy may mitigate the risk of complications. This may be accomplished by strategic patient positioning, such as the decubitus position, which is more likely to separate the kidney from the bowel, as shown in the study of Gervais et al with ablation of 100 tumors (17). Safe ablation was achieved among 21 tumors within 1 cm of the bowel with cautious electrode and patient positioning and without bowel displacement techniques, although bowel displacement is helpful in cases where careful positioning alone cannot achieve adequate separation between the tumor and the vulnerable anatomy.
Hydrodissection may be performed to prevent complications by injecting 300–500 mL of 5% dextrose before and during ablation to maintain space between the tumor and the vulnerable anatomy (Fig 4a) (89). Hydrodissection is the most commonly used technique for bowel displacement, although other successful techniques used during renal ablation include percutaneous interpostioning of a balloon or injection of carbon dioxide between the tumor and the vulnerable regional anatomy (Fig 4b) (89). Hydrodissection may separate index tumors abutting or in close proximity to the psoas muscle and prevent muscular or nerve damage (86). However, since the tumor-to-bowel distance may decrease during ablation as water dissects through the tissues, repeated or continuous instillations may be warranted in long procedures (17). The use of ionic solutions, such as saline, should be avoided for hydrodissection during RF ablation, given their propensity to conduct rather than insulate current (90). Ionic solutions such as saline would be safe for hydrodissection prior to cryoablation, however.
Figure 4a:
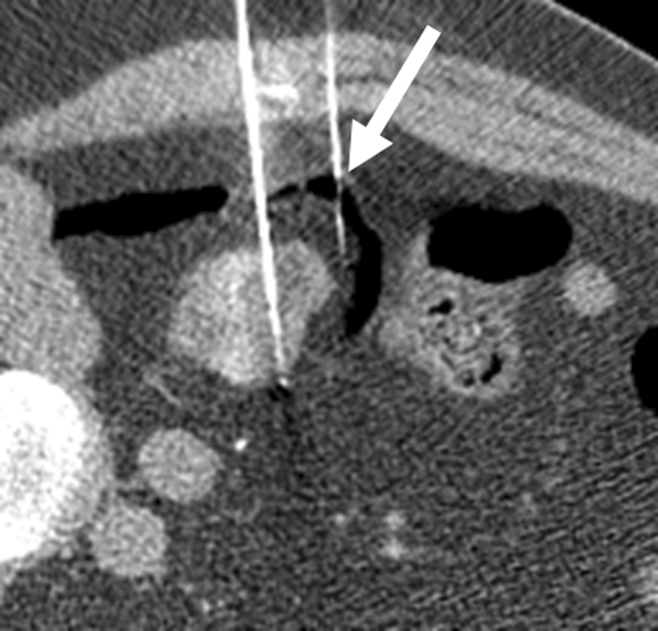
Protective maneuvers used during percutaneous ablation of RCC. (a) Intraprocedural axial contrast-enhanced CT scan of 1.5-cm RCC in lower pole of right kidney, with instillation of CO2 (arrow) to displace renal mass from adjacent right colon. (b) Intraprocedural axial nonenhanced CT scan of 3.5-cm left RCC, with right lateral decubitus positioning and use of hydrodissection (arrow) to separate kidney from right lateral chest wall and intercostal nerve.
Figure 4b:
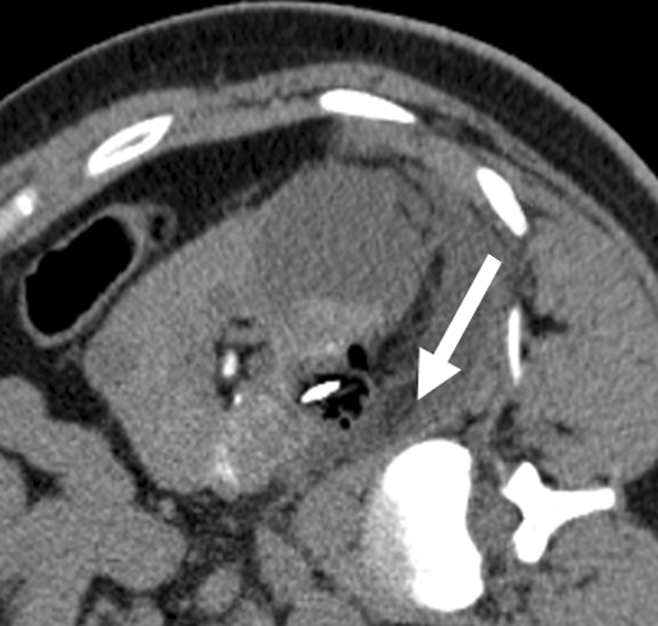
Protective maneuvers used during percutaneous ablation of RCC. (a) Intraprocedural axial contrast-enhanced CT scan of 1.5-cm RCC in lower pole of right kidney, with instillation of CO2 (arrow) to displace renal mass from adjacent right colon. (b) Intraprocedural axial nonenhanced CT scan of 3.5-cm left RCC, with right lateral decubitus positioning and use of hydrodissection (arrow) to separate kidney from right lateral chest wall and intercostal nerve.
If hydrodissection is performed prior to RF ablation or cryoablation, an awareness of the theoretical complication of hyponatremia syndrome is important (91). Hyponatremia syndrome is thought to be dilutional and secondary to absorption of electrolyte-free irrigation fluids, causing acute hypervolemia and hyponatremia (91). Clinical consequences range from an isolated laboratory abnormality to cardiac arrhythmias, seizures, coma, and death. As noted by Lee et al (92), hyponatremia syndrome has not been reported in the setting of ablative procedures using hydrodissection. However, use of large volumes of fluid (≥1 L) for hydrodissection during renal ablation may present a theoretical risk for hyponatremia analogous to that encountered during irrigation in endoscopic interventions. Serial evaluation of serum sodium during the periprocedural period may therefore be warranted (92).
Hypertensive crisis due to ipsilateral adrenal injury during renal ablation should also be considered when evaluating patients for ablation. Although this has not been reported in the setting of renal ablation, it has been reported to occur during ablation of posterior right lobe hepatic tumors (93). It is possible that treatment of exophytic upper pole renal tumors may place patients at similar risk for adrenal injury. Hydrodissection may be used to separate and insulate the adrenal gland from the upper pole of the kidney. Careful intraprocedural anesthesia monitoring and administration of α– and β–adrenergic inhibitors may also be warranted. A laparoscopic or open approach should be considered for any patient for whom the aforementioned maneuvers are believed to be unlikely to adequately separate the tumor from vulnerable regional anatomy (81).
Tract seeding is a rare complication of percutaneous renal biopsy and ablation. Tumor seeding of the applicator track after percutaneous renal tumor ablation has been reported in only one case, to our knowledge (50). However, tumor seeding is an important complication that must be screened for at follow-up imaging. It is important to be aware that benign inflammatory tissue may mimic applicator track seeding after percutaneous renal RF ablation or cryoablation (Fig 5). Lokken et al (94) reported a 1.9% incidence of rim-enhancing or ill-defined nodules along the electrode track, suggesting tumor seeding, after percutaneous renal tumor ablation procedures. This prompted biopsy after 1.3% of their procedures, which revealed acute or chronic inflammatory changes in all cases and no evidence for tumor (94).
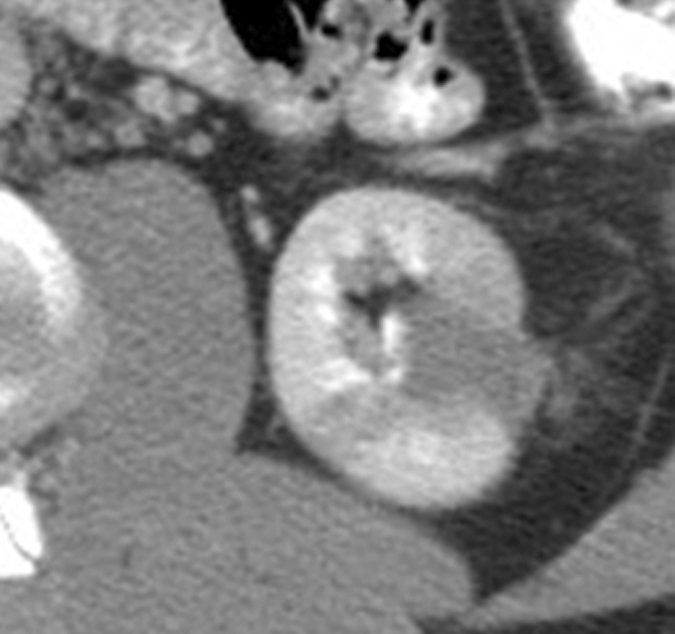
Figure 5a:
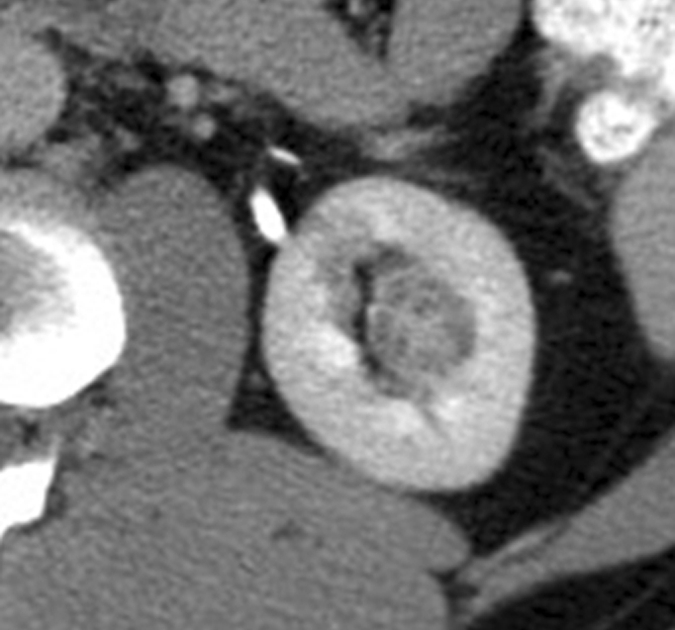
Expected evolution of imaging findings prior to and following percutaneous RF ablation. (a) Axial contrast-enhanced CT scan shows 1.5-cm enhancing RCC abutting left renal collecting system. (b) Axial intraprocedural nonenhanced CTscan shows electrode (arrow) within the tumor. (c) Axial contrast-enhanced CT scan 2 months after ablation shows trace left perinephric fat stranding and nonenhancement in the ablation zone. Nodularity within left perinephric fat (arrows), when observed along the electrode track, may mimic tumor seeding. (d) Axial contrast-enhanced CT scan 6 months after percutaneous RF ablation shows decreased size of ablation zone and decreased left perinephric fat stranding. Axial contrast-enhanced CT scans (e) 12 and (f) 18 months after ablation show continued decrease in size of ablation zone and continued decrease in left perinephric fat stranding and nodularity.
Figure 5b:
Expected evolution of imaging findings prior to and following percutaneous RF ablation. (a) Axial contrast-enhanced CT scan shows 1.5-cm enhancing RCC abutting left renal collecting system. (b) Axial intraprocedural nonenhanced CTscan shows electrode (arrow) within the tumor. (c) Axial contrast-enhanced CT scan 2 months after ablation shows trace left perinephric fat stranding and nonenhancement in the ablation zone. Nodularity within left perinephric fat (arrows), when observed along the electrode track, may mimic tumor seeding. (d) Axial contrast-enhanced CT scan 6 months after percutaneous RF ablation shows decreased size of ablation zone and decreased left perinephric fat stranding. Axial contrast-enhanced CT scans (e) 12 and (f) 18 months after ablation show continued decrease in size of ablation zone and continued decrease in left perinephric fat stranding and nodularity.
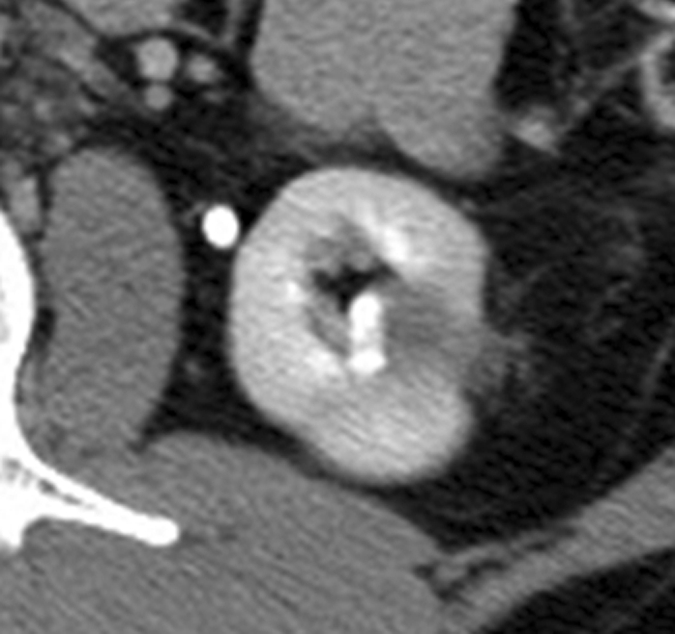
Figure 5c:
Expected evolution of imaging findings prior to and following percutaneous RF ablation. (a) Axial contrast-enhanced CT scan shows 1.5-cm enhancing RCC abutting left renal collecting system. (b) Axial intraprocedural nonenhanced CTscan shows electrode (arrow) within the tumor. (c) Axial contrast-enhanced CT scan 2 months after ablation shows trace left perinephric fat stranding and nonenhancement in the ablation zone. Nodularity within left perinephric fat (arrows), when observed along the electrode track, may mimic tumor seeding. (d) Axial contrast-enhanced CT scan 6 months after percutaneous RF ablation shows decreased size of ablation zone and decreased left perinephric fat stranding. Axial contrast-enhanced CT scans (e) 12 and (f) 18 months after ablation show continued decrease in size of ablation zone and continued decrease in left perinephric fat stranding and nodularity.
Figure 5d:
Expected evolution of imaging findings prior to and following percutaneous RF ablation. (a) Axial contrast-enhanced CT scan shows 1.5-cm enhancing RCC abutting left renal collecting system. (b) Axial intraprocedural nonenhanced CTscan shows electrode (arrow) within the tumor. (c) Axial contrast-enhanced CT scan 2 months after ablation shows trace left perinephric fat stranding and nonenhancement in the ablation zone. Nodularity within left perinephric fat (arrows), when observed along the electrode track, may mimic tumor seeding. (d) Axial contrast-enhanced CT scan 6 months after percutaneous RF ablation shows decreased size of ablation zone and decreased left perinephric fat stranding. Axial contrast-enhanced CT scans (e) 12 and (f) 18 months after ablation show continued decrease in size of ablation zone and continued decrease in left perinephric fat stranding and nodularity.
Figure 5e:
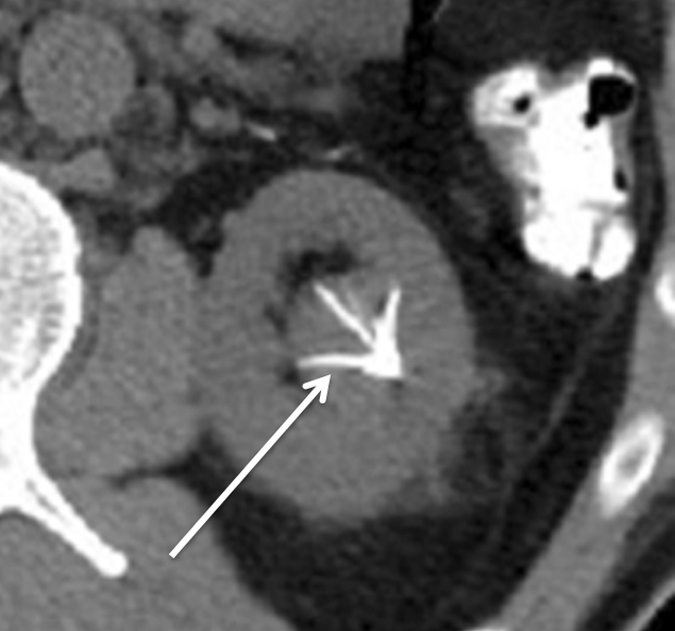
Expected evolution of imaging findings prior to and following percutaneous RF ablation. (a) Axial contrast-enhanced CT scan shows 1.5-cm enhancing RCC abutting left renal collecting system. (b) Axial intraprocedural nonenhanced CTscan shows electrode (arrow) within the tumor. (c) Axial contrast-enhanced CT scan 2 months after ablation shows trace left perinephric fat stranding and nonenhancement in the ablation zone. Nodularity within left perinephric fat (arrows), when observed along the electrode track, may mimic tumor seeding. (d) Axial contrast-enhanced CT scan 6 months after percutaneous RF ablation shows decreased size of ablation zone and decreased left perinephric fat stranding. Axial contrast-enhanced CT scans (e) 12 and (f) 18 months after ablation show continued decrease in size of ablation zone and continued decrease in left perinephric fat stranding and nodularity.
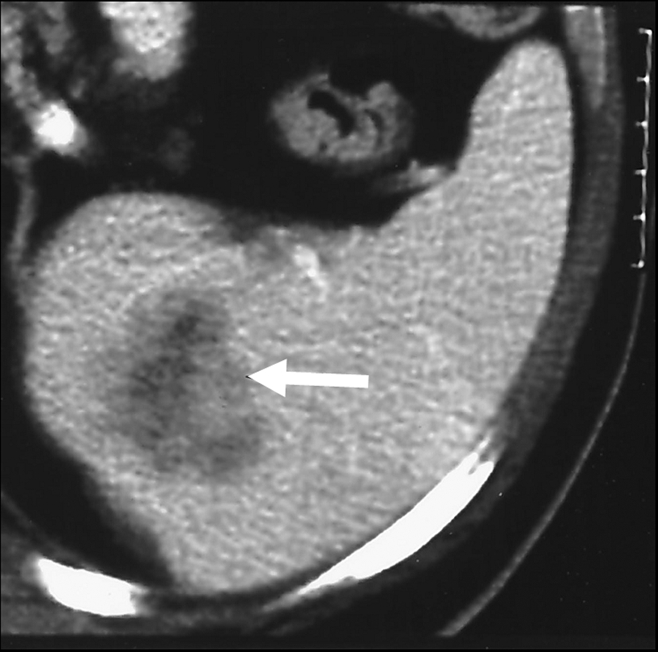
Figure 5f:
Expected evolution of imaging findings prior to and following percutaneous RF ablation. (a) Axial contrast-enhanced CT scan shows 1.5-cm enhancing RCC abutting left renal collecting system. (b) Axial intraprocedural nonenhanced CTscan shows electrode (arrow) within the tumor. (c) Axial contrast-enhanced CT scan 2 months after ablation shows trace left perinephric fat stranding and nonenhancement in the ablation zone. Nodularity within left perinephric fat (arrows), when observed along the electrode track, may mimic tumor seeding. (d) Axial contrast-enhanced CT scan 6 months after percutaneous RF ablation shows decreased size of ablation zone and decreased left perinephric fat stranding. Axial contrast-enhanced CT scans (e) 12 and (f) 18 months after ablation show continued decrease in size of ablation zone and continued decrease in left perinephric fat stranding and nodularity.
Imaging Follow-up
Since renal tumors undergoing percutaneous ablation remain in situ following treatment, follow-up imaging surveillance is mandatory to screen for residual or recurrent malignancy. Controversies exist concerning how often and for how long follow-up imaging should be performed. There are no prospective clinical studies to validate a particular imaging schedule. However, most investigators are in agreement about the importance of long-term postablation cross-sectional imaging follow-up as a crucial element of overall treatment success. Follow-up imaging provides a baseline for future imaging, helps document technical success and procedural complications, and depicts local tumor progression. Many radiologists recommend obtaining unenhanced and contrast-enhanced CT or MR images 1–3 months after ablation (16). Some investigators also advocate the utility of immediate (within 48 hours) postablation imaging (19). In their review of 100 renal RF ablations, Gervais et al (17) performed CT or MR imaging with and without contrast material at 1, 3, and 6 months after RF ablation. Subsequent imaging follow-up depended on the clinical condition of the patient and comorbid conditions, but was generally at 6- to 12-month intervals, with a mean follow-up duration of 2.3 years (range, 3.5–6 years). Similar intervals are described in Atwell et al (19) review of 115 renal cryoablations. They performed immediate postablation imaging within 24 hours of cryoablation using unenhanced and enhanced CT or contrast-enhanced MR imaging in patients with reduced renal function or history of allergy to iodinated contrast material. Additional follow-up imaging with contrast-enhanced CT or MR imaging was performed at 3, 6, and 12 months following ablation, and yearly thereafter. Breen et al (60), in their review of 105 renal RF ablations, describe imaging follow-up with contrast-enhanced CT within 10 days after ablation and at 6-month intervals thereafter.
Renal tumors treated with RF ablation or cryoablation appear as low-attenuation regions at CT and, relative to renal parenchyma, and are generally hypointense on T2-weighted MR images and iso- to hyperintense on T1-weighted images (95). Ablated tumors often have internal areas of increased attenuation and increased signal intensity on CT and MR images, respectively, corresponding to proteinaceous debris and/or products of hemorrhage (16). This is most notable within the first 6 months following ablation. Use of intravenous contrast material is essential for the evaluation of possible residual or recurrent tumors (95). At follow-up CT or MR imaging, successfully treated renal tumors appear as focal masses that demonstrate no evidence of enhancement and that frequently decrease in size over time, whereas residual or recurrent tumors can be detected as abnormal foci of contrast enhancement (95). Areas of contrast enhancement at initial follow-up (≥10 HU or ≥15% with CT and MR imaging, respectively) usually indicate residual viable RCC and primary treatment failure (8,16). The pattern of residual or recurrent tumors often manifests as a focus of nodular or crescentic enhancement on postablation contrast-enhanced CT scans and MR images (8,16). Of note, attenuation of 10–20 HU can sometimes be seen following successful ablation and, in cases of cryoablation, a thin peripheral rim of enhancement often persists for several months following therapy (96). As noted by Ganguli et al (97), a decrease in tumor size may be observed immediately after treatment, thus findings at follow-up imaging studies should ideally be compared with a baseline tumor size measured as soon as possible after ablation. Over time, decrease in perinephric stranding and nodularity is also expected (Fig 5). There should be no enhancement of nonviable tumor after intravenous administration of contrast material (16). Enhancement or enlargement at subsequent imaging after initial negative imaging findings is considered indicative of local tumor progression, and patients should be counseled about additional treatment, including repeat ablation, partial nephrectomy, radical nephrectomy, and observation, as appropriate (8).
Surveillance Biopsy
An ongoing challenge to the success of ablative therapies is the possibility of tumor cell viability after ablation (98). As noted by Rendon et al (88), the therapeutic effectiveness of ablative techniques depends on the equivalence between percutaneous tumor ablation and surgical excision by means of partial or radical nephrectomy. This has led to support the addition of a postablation “surveillance biopsy” to confirm treatment success (88,99).
The data regarding cell viability following RF ablation and cryoablation have been mixed in terms of the effectiveness of percutaneous RF ablation to achieve complete necrosis. Park et al (76) performed histologic sampling with fine-needle aspiration in 37 patients 6 months following percutaneous RF ablation and compared these findings with those of core biopsy performed after laparoscopic cryoablation in 97 patients. The specimens were assessed with hematoxylin-eosin staining. At 6 months, success in the RF ablation cohort, defined by absence of malignant or atypical cells, decreased to 64.8% (24 cases), while cryoablation success remained high at 93.8% (91 cases). The authors concluded that post–RF ablation follow-up biopsy depicts substantial incidence of residual RCC, including in patients who lack imaging evidence of treatment failure. Limitations to this study include the underlying histologic definition of success, since atypical cells, including inflammatory cells, may be seen in post–RF ablation specimens without clinical or radiographic evidence of incomplete local therapy. Current data furthermore suggest that neither hematoxylin-eosin nor nicotinamide adenine dinucleotide staining may be a reliable means of assessing tissue viability immediately after a thermal insult, not even within the first several months of treatment (4,5,12). An additional confounding factor involves the differing methods of sampling from the two cohorts in the study and the potential for sampling error, since findings from fine-needle aspiration were evaluated for the RF ablation cohort, while core biopsy specimens were evaluated for the cryoablation cohort (76).
Interestingly, Raman et al (98) have reported different results for patients with no radiographic evidence of active disease who underwent biopsy more than 1 year following percutaneous RF ablation. Pre- and postablation biopsies were performed in 20 patients who underwent percutaneous renal tumor RF ablation, with preablation biopsies helping diagnose 17 of 20 tumors as RCCs and the remaining three tumors as oncocytomas. Postablation biopsies were performed to evaluate cell viability in the ablation zone. All patients had no clinical evidence of residual disease, defined by absent index tumor growth and contrast enhancement 1 year or more after ablation. Histologic comparison of pre- and postablation specimens was performed by using hematoxylin-eosin staining. At least four 18-gauge core biopsy specimens were obtained from each ablated index tumor. Histopathologic examination of these tumors confirmed absence of viable tumor in all specimens, with post–RF ablation histopathologic changes ranging from extensive coagulative necrosis with preserved tumor cytoskeletal architecture to total replacement by acellular necrotic debris with or without dystrophic calcifications and inflamed hyalinized fibroconnective tissue.
The means by which to assess viability versus necrosis and the appropriate time point at which to perform surveillance biopsy are ongoing controversies, for which further study is needed. At the present time, many investigators continue to rely solely on cross-sectional imaging features, including presence of enhancement or postablation index tumor growth as markers for residual or recurrent tumor warranting additional treatment.
Other Applications
Percutaneous ablation has been used for other applications to treat RCC. Both Rhim et al (81) and Wood et al (100) have described use of RF ablation for the treatment of refractory, transfusion-dependent hematuria secondary to large RCCs that had failed to resolve with standard interventions, including renal artery embolization. Percutaneous RF ablation and cryoablation have also been used in the treatment of recurrent and metastatic RCC, including the treatment of renal, adrenal, musculoskeletal, and hepatic metastases (101). Current case series in the literature describe effective local tumor control and/or palliation of symptoms; the experience with percutaneous ablation for the treatment of metastatic RCC, while preliminary, remains promising (Fig 6) (4).
Figure 6a:
Use of percutaneous RF ablation to treat metastatic RCC. (a) Axial contrast-enhanced CT scan shows heterogeneous enhancing 3.50-cm intrasplenic mass (arrow). Biopsy confirmed metastatic RCC. (b) Axial contrast-enhanced CT scan 6 months after percutaneous RF ablation shows decreased nonenhancing ablation zone (arrow) and no evidence of residual tumor. (Reprinted, with permission, from reference 102.)
In summary, extensive clinical experience supports the role of percutaneous RF ablation and cryoablation in the treatment of early-stage RCC in patients who are nonsurgical candidates or unwilling to undergo surgery. Both RF ablation and cryoablation are well-tolerated procedures that have demonstrated acceptable short- to intermediate-term outcomes and a low rate of complications. With appropriate periprocedural management, patients at risk for renal compromise may undergo this procedure effectively with preservation of their renal function. RF ablation has demonstrated a role in the treatment of refractory hematuria, and both RF ablation and cryoablation have been used effectively to treat recurrent and metastatic disease. Ongoing areas of clinical investigation include evaluation of the long-term effectiveness of percutaneous ablation for early-stage RCC and follow-up data on the effectiveness of percutaneous ablation for treatment of metastatic disease. Additional management controversies for further study include the appropriate frequency and duration of follow-up imaging in these patients and the best means by which to assess tumor viability after ablation.
Figure 6b:
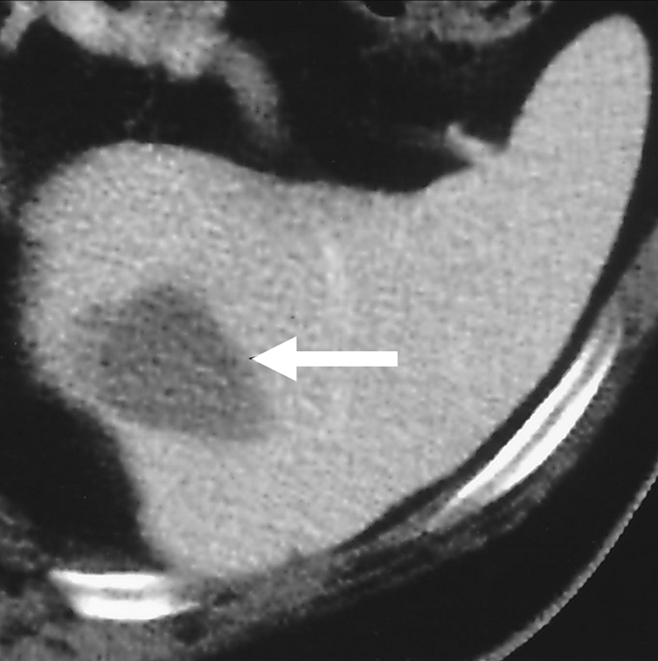
Use of percutaneous RF ablation to treat metastatic RCC. (a) Axial contrast-enhanced CT scan shows heterogeneous enhancing 3.50-cm intrasplenic mass (arrow). Biopsy confirmed metastatic RCC. (b) Axial contrast-enhanced CT scan 6 months after percutaneous RF ablation shows decreased nonenhancing ablation zone (arrow) and no evidence of residual tumor. (Reprinted, with permission, from reference 102.)
Received July 16, 2009; revision requested September 18; revision received February 10, 2010; accepted March 5; final version accepted April 15.
Funding: A.M.V. and B.J.W. are employees of National Institutes of Health.
D.A.G. has received a research grant from Covidien.
Abbreviations:
- RCC
- renal cell carcinoma
- RF
- radiofrequency
References
- 1.Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med 2005;353(23):2477–2490 [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute Kidney cancer home page. http://www.cancer.gov/cancertopics/types/kidney. Accessed September 4, 2011
- 3.Pantuck AJ, Zisman A, Belldegrun AS. The changing natural history of renal cell carcinoma. J Urol 2001;166(5):1611–1623 [PubMed] [Google Scholar]
- 4.Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Radiofrequency ablation of renal cell carcinoma. I. Indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. AJR Am J Roentgenol 2005;185(1):64–71 [DOI] [PubMed] [Google Scholar]
- 5.Zagoria RJ, Hawkins AD, Clark PE, et al. Percutaneous CT-guided radiofrequency ablation of renal neoplasms: factors influencing success. AJR Am J Roentgenol 2004;183(1):201–207 [DOI] [PubMed] [Google Scholar]
- 6.Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology 1998;51(2):203–205 [DOI] [PubMed] [Google Scholar]
- 7.Chow WH, Devesa SS, Warren JL, Fraumeni JF., Jr Rising incidence of renal cell cancer in the United States. JAMA 1999;281(17):1628–1631 [DOI] [PubMed] [Google Scholar]
- 8.Levinson AW, Su L-M, Agarwal D, et al. Long-term oncological and overall outcomes of percutaneous radio frequency ablation in high risk surgical patients with a solitary small renal mass. J Urol 2008;180(2):499–504; discussion 504 [DOI] [PubMed] [Google Scholar]
- 9.Mattar K, Jewett MA. Watchful waiting for small renal masses. Curr Urol Rep 2008;9(1):22–25 [DOI] [PubMed] [Google Scholar]
- 10.Carrafiello G, Mangini M, Fontana F, et al. Single-antenna microwave ablation under contrast-enhanced ultrasound guidance for treatment of small renal cell carcinoma: preliminary experience. Cardiovasc Intervent Radiol 2010;33(2):367–374 [DOI] [PubMed] [Google Scholar]
- 11.Liang P, Wang Y, Zhang D, Yu X, Gao Y, Ni X. Ultrasound guided percutaneous microwave ablation for small renal cancer: initial experience. J Urol 2008;180(3):844–848; discussion 848 [DOI] [PubMed] [Google Scholar]
- 12.Terai A, Ito N, Yoshimura K, et al. Laparoscopic partial nephrectomy using microwave tissue coagulator for small renal tumors: usefulness and complications. Eur Urol 2004;45(6):744–748 [DOI] [PubMed] [Google Scholar]
- 13.Klatte T, Marberger M. High-intensity focused ultrasound for the treatment of renal masses: current status and future potential. Curr Opin Urol 2009;19(2):188–191 [DOI] [PubMed] [Google Scholar]
- 14.Marberger M. Ablation of renal tumours with extracorporeal high-intensity focused ultrasound. BJU Int 2007;99(5 Pt B):1273–1276 [DOI] [PubMed] [Google Scholar]
- 15.Zlotta AR, Wildschutz T, Raviv G, et al. Radiofrequency interstitial tumor ablation (RITA) is a possible new modality for treatment of renal cancer: ex vivo and in vivo experience. J Endourol 1997;11(4):251–258 [DOI] [PubMed] [Google Scholar]
- 16.Zagoria RJ. Imaging-guided radiofrequency ablation of renal masses. RadioGraphics 2004;24(Suppl 1):S59–S71 [DOI] [PubMed] [Google Scholar]
- 17.Gervais DA, Arellano RS, McGovern FJ, McDougal WS, Mueller PR. Radiofrequency ablation of renal cell carcinoma. II. Lessons learned with ablation of 100 tumors. AJR Am J Roentgenol 2005;185(1):72–80 [DOI] [PubMed] [Google Scholar]
- 18.Zagoria RJ, Traver MA, Werle DM, Perini M, Hayasaka S, Clark PE. Oncologic efficacy of CT-guided percutaneous radiofrequency ablation of renal cell carcinomas. AJR Am J Roentgenol 2007;189(2):429–436 [DOI] [PubMed] [Google Scholar]
- 19.Atwell TD, Farrell MA, Leibovich BC, et al. Percutaneous renal cryoablation: experience treating 115 tumors. J Urol 2008;179(6):2136–2140; discussion 2140–2141 [DOI] [PubMed] [Google Scholar]
- 20.McDougal WS, Gervais DA, McGovern FJ, Mueller PR. Long-term followup of patients with renal cell carcinoma treated with radio frequency ablation with curative intent. J Urol 2005;174(1):61–63 [DOI] [PubMed] [Google Scholar]
- 21.Parikh AA, Curley SA, Fornage BD, Ellis LM. Radiofrequency ablation of hepatic metastases. Semin Oncol 2002;29(2):168–182 [DOI] [PubMed] [Google Scholar]
- 22.Rhim H, Goldberg SN, Dodd GD, 3rd, et al. , Essential techniques for successful radio-frequency thermal ablation of malignant hepatic tumors. RadioGraphics 2001;21(Spec No):S17–S35; discussion S36–S39 [DOI] [PubMed] [Google Scholar]
- 23.Staren ED, Sabel MS, Gianakakis LM, et al. Cryosurgery of breast cancer. Arch Surg 1997;132(1):28–33; discussion 34 [DOI] [PubMed] [Google Scholar]
- 24.Goel RK, Kaouk JH. Probe ablative treatment for small renal masses: cryoablation vs radio frequency ablation. Curr Opin Urol 2008;18(5):467–473 [DOI] [PubMed] [Google Scholar]
- 25.Korpan NN. Hepatic cryosurgery for liver metastases: long-term follow-up. Ann Surg 1997;225(2):193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faraci RP, Bagley DH, Marrone JA, Beazley RM. The effect of curative cryosurgery on the tumor-specific immune response of C57 mice. Cryobiology 1975;12(2):175–179 [DOI] [PubMed] [Google Scholar]
- 27.Lee FT, Jr, Mahvi DM, Chosy SG, et al. Hepatic cryosurgery with intraoperative US guidance. Radiology 1997;202(3):624–632 [DOI] [PubMed] [Google Scholar]
- 28.Schreuder HW, van Egmond J, van Beem HB, Veth RP. Monitoring during cryosurgery of bone tumors. J Surg Oncol 1997;65(1):40–45 [DOI] [PubMed] [Google Scholar]
- 29.Crisp WE, Asadourian L, Romberger W. Application of cryosurgery to gynecologic malignancy. Obstet Gynecol 1967;30(5):668–673 [PubMed] [Google Scholar]
- 30.Rubinsky B, Gilbert JC, Onik GM, Roos MS, Wong ST, Brennan KM. Monitoring cryosurgery in the brain and in the prostate with proton NMR. Cryobiology 1993;30(2):191–199 [DOI] [PubMed] [Google Scholar]
- 31.Weber SM, Lee FT., Jr Expanded treatment of hepatic tumors with radiofrequency ablation and cryoablation. Oncology (Williston Park) 2005;19(11 Suppl 4):27–32 [PubMed] [Google Scholar]
- 32.Hoffmann NE, Bischof JC. The cryobiology of cryosurgical injury. Urology 2002;60(2 Suppl 1):40–49 [DOI] [PubMed] [Google Scholar]
- 33.Johnson DB, Nakada SY. Cryosurgery and needle ablation of renal lesions. J Endourol 2001;15(4):361–368; discussion 375–376 [DOI] [PubMed] [Google Scholar]
- 34.Kutikov A, Kunkle DA, Uzzo RG. Focal therapy for kidney cancer: a systematic review. Curr Opin Urol 2009;19(2):148–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tatli S, Acar M, Tuncali K, Morrison PR, Silverman S. Percutaneous cryoablation techniques and clinical applications. Diagn Interv Radiol 2010;16(1):90–95 [DOI] [PubMed] [Google Scholar]
- 36.Finley DS, Beck S, Box G, et al. Percutaneous and laparoscopic cryoablation of small renal masses. J Urol 2008;180(2):492–498; discussion 498 [DOI] [PubMed] [Google Scholar]
- 37.Bandi G, Hedican SP, Nakada SY. Current practice patterns in the use of ablation technology for the management of small renal masses at academic centers in the United States. Urology 2008;71(1):113–117 [DOI] [PubMed] [Google Scholar]
- 38.Hinshaw JL, Shadid AM, Nakada SY, Hedican SP, Winter TC, 3rd, Lee FT., Jr Comparison of percutaneous and laparoscopic cryoablation for the treatment of solid renal masses. AJR Am J Roentgenol 2008;191(4):1159–1168 [DOI] [PubMed] [Google Scholar]
- 39.Badwan K, Maxwell K, Venkatesh R, et al. Comparison of laparoscopic and percutaneous cryoablation of renal tumors: a cost analysis. J Endourol 2008;22(6):1275–1277 [DOI] [PubMed] [Google Scholar]
- 40.Hui GC, Tuncali K, Tatli S, Morrison PR, Silverman SG. Comparison of percutaneous and surgical approaches to renal tumor ablation: metaanalysis of effectiveness and complication rates. J Vasc Interv Radiol 2008;19(9):1311–1320 [DOI] [PubMed] [Google Scholar]
- 41.Ogan K, Jacomides L, Dolmatch BL, et al. Percutaneous radiofrequency ablation of renal tumors: technique, limitations, and morbidity. Urology 2002;60(6):954–958 [DOI] [PubMed] [Google Scholar]
- 42.Duck FA. Appendix A: tissue perfusion rates. In: Physical properties of tissue. London, England: Academic Press, 1990; 319–329 [Google Scholar]
- 43.Hwang JJ, Walther MM, Pautler SE, et al. Radio frequency ablation of small renal tumors: intermediate results. J Urol 2004;171(5):1814–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology 1999;210(3):655–661 [DOI] [PubMed] [Google Scholar]
- 45.Goldberg SN, Hahn PF, Tanabe KK, et al. Percutaneous radiofrequency tissue ablation: does perfusion-mediated tissue cooling limit coagulation necrosis? J Vasc Interv Radiol 1998;9(1 Pt 1):101–111 [DOI] [PubMed] [Google Scholar]
- 46.Zagoria RJ, Bechtold RE. The role of imaging in staging renal adenocarcinoma. Semin Ultrasound CT MR 1997;18(2):91–99 [DOI] [PubMed] [Google Scholar]
- 47.Zagoria RJ. Imaging of small renal masses: a medical success story. AJR Am J Roentgenol 2000;175(4):945–955 [DOI] [PubMed] [Google Scholar]
- 48.Neeman Z, Sarin S, Coleman J, Fojo T, Wood BJ. Radiofrequency ablation for tumor-related massive hematuria. J Vasc Interv Radiol 2005;16(3):417–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryan JM, Ryan BM, Smith TP. Antibiotic prophylaxis in interventional radiology. J Vasc Interv Radiol 2004;15(6):547–556 [DOI] [PubMed] [Google Scholar]
- 50.Mayo-Smith WW, Dupuy DE, Parikh PM, Pezzullo JA, Cronan JJ. Imaging-guided percutaneous radiofrequency ablation of solid renal masses: techniques and outcomes of 38 treatment sessions in 32 consecutive patients. AJR Am J Roentgenol 2003;180(6):1503–1508 [DOI] [PubMed] [Google Scholar]
- 51.Shawker TH, Kluge RM, Ayella RJ. Bacteremia associated with angiography. JAMA 1974;229(8):1090–1092 [PubMed] [Google Scholar]
- 52.Dupuy DE, Goldberg SN. Image-guided radiofrequency tumor ablation. II. Challenges and opportunities. J Vasc Interv Radiol 2001;12(10):1135–1148 [DOI] [PubMed] [Google Scholar]
- 53.Boss A, Clasen S, Kuczyk M, Schick F, Pereira PL. Image-guided radiofrequency ablation of renal cell carcinoma. Eur Radiol 2007;17(3):725–733 [DOI] [PubMed] [Google Scholar]
- 54.Maybody M, Solomon SB. Image-guided percutaneous cryoablation of renal tumors. Tech Vasc Interv Radiol 2007;10(2):140–148 [DOI] [PubMed] [Google Scholar]
- 55.Crowley JD, Shelton J, Iverson AJ, Burton MP, Dalrymple NC, Bishoff JT. Laparoscopic and computed tomography-guided percutaneous radiofrequency ablation of renal tissue: acute and chronic effects in an animal model. Urology 2001;57(5):976–980 [DOI] [PubMed] [Google Scholar]
- 56.Campbell SC, Krishnamurthi V, Chow G, Hale J, Myles J, Novick AC. Renal cryosurgery: experimental evaluation of treatment parameters. Urology 1998;52(1):29–33 [DOI] [PubMed] [Google Scholar]
- 57.Tuncali K, vanSonnenberg E, Shankar S, Mortele KJ, Cibas ES, Silverman SG. Evaluation of patients referred for percutaneous ablation of renal tumors: importance of a preprocedural diagnosis. AJR Am J Roentgenol 2004;183(3):575–582 [DOI] [PubMed] [Google Scholar]
- 58.Schmidbauer J, Remzi M, Memarsadeghi M, et al. Diagnostic accuracy of computed tomography-guided percutaneous biopsy of renal masses. Eur Urol 2008;53(5):1003–1011 [DOI] [PubMed] [Google Scholar]
- 59.Dominguez-Escrig JL, Sahadevan K, Johnson P. Cryoablation for small renal masses. Adv Urol 2008:479495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Breen DJ, Rutherford EE, Stedman B, et al. Management of renal tumors by image-guided radiofrequency ablation: experience in 105 tumors. Cardiovasc Intervent Radiol 2007;30(5):936–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farrell MA, Charboneau WJ, DiMarco DS, et al. Imaging-guided radiofrequency ablation of solid renal tumors. AJR Am J Roentgenol 2003;180(6):1509–1513 [DOI] [PubMed] [Google Scholar]
- 62.Su LM, Jarrett TW, Chan DY, Kavoussi LR, Solomon SB. Percutaneous computed tomography-guided radiofrequency ablation of renal masses in high surgical risk patients: preliminary results. Urology 2003;61(4 Suppl 1):26–33 [DOI] [PubMed] [Google Scholar]
- 63.Georgiades CS, Hong K, Bizzell C, Geschwind JF, Rodriguez R. Safety and efficacy of CT-guided percutaneous cryoablation for renal cell carcinoma. J Vasc Interv Radiol 2008;19(9):1302–1310 [DOI] [PubMed] [Google Scholar]
- 64.Kunkle DA, Egleston BL, Uzzo RG. Excise, ablate or observe: the small renal mass dilemma—a meta-analysis and review. J Urol 2008;179(4):1227–1234 [DOI] [PubMed] [Google Scholar]
- 65.Raman JD, Cadeddu JA. Re: Excise, ablate or observe: the small renal mass dilemma—a meta-analysis and review D. A. Kunkle, B. L. Egleston and R. G. Uzzo J Urol 2008; 179: 1227-1234. J Urol 2008;180(4):1567–1568 [DOI] [PubMed] [Google Scholar]
- 66.Kunkle DA, Uzzo RG. Cryoablation or radiofrequency ablation of the small renal mass: a meta-analysis. Cancer 2008;113(10):2671–2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cadeddu JA, Raman JD. Renal tumor ablation is a function of patient selection and technique—not the ablation technology. Cancer 2008;113(10):2623–2626 [DOI] [PubMed] [Google Scholar]
- 68.Novick AC, Campbell SC, Belldegrun AS, et al. Guideline for management of the clinical stage 1 renal mass. American Urological Association. http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines/main-reports/renalmass09.pdf. Published 2009. Accessed September 4, 2011 [DOI] [PubMed]
- 69.Jacobsohn KM, Ahrar K, Wood CG, Matin SF. Is radiofrequency ablation safe for solitary kidneys? Urology 2007;69(5):819–823; discussion 823 [DOI] [PubMed] [Google Scholar]
- 70.Johnson DB, Taylor GD, Lotan Y, Sagalowsky AI, Koenemann KS, Cadeddu JA. The effects of radio frequency ablation on renal function and blood pressure. J Urol 2003;170(6 Pt 1):2234–2236 [DOI] [PubMed] [Google Scholar]
- 71.Carvalhal EF, Gill IS, Meraney AM, Desai MM, Schweizer DK, Sung GT. Laparoscopic renal cryoablation: impact on renal function and blood pressure. Urology 2001;58(3):357–361 [DOI] [PubMed] [Google Scholar]
- 72.Hegarty NJ, Gill IS, Desai MM, Remer EM, O’Malley CM, Kaouk JH. Probe-ablative nephron-sparing surgery: cryoablation versus radiofrequency ablation. Urology 2006;68(1 Suppl):7–13 [DOI] [PubMed] [Google Scholar]
- 73.Kowalczyk KJ, Hooper HB, Linehan WM, Pinto PA, Wood BJ, Bratslavsky G. Partial nephrectomy after previous radio frequency ablation: the National Cancer Institute experience. J Urol 2009;182(5):2158–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Herring JC, Enquist EG, Chernoff A, Linehan WM, Choyke PL, Walther MM. Parenchymal sparing surgery in patients with hereditary renal cell carcinoma: 10-year experience. J Urol 2001;165(3):777–781 [PubMed] [Google Scholar]
- 75.Wood B, Locklin J, Neeman Z, et al. Radiofrequency ablation of small < 4 cm hereditary or familial kidney tumors: long term results > 5 years. Presented at the 31st Annual Scientific Meeting of the Society of Interventional Radiology, Toronto, Ontario, Canada, April 1, 2006 [Google Scholar]
- 76.Park BK, Kim CK, Lee HM. Image-guided radiofrequency ablation of Bosniak category III or IV cystic renal tumors: initial clinical experience. Eur Radiol 2008;18(7):1519–1525 [DOI] [PubMed] [Google Scholar]
- 77.Carrafiello G, Laganà D, Ianniello A, et al. Post-radiofrequency ablation syndrome after percutaneous radiofrequency of abdominal tumours: one centre experience and review of published works. Australas Radiol 2007;51(6):550–554 [DOI] [PubMed] [Google Scholar]
- 78.Gervais DA, Arellano RS, Mueller PR. Percutaneous ablation of kidney tumors in nonsurgical candidates. Oncology (Williston Park) 2005;19(11 Suppl 4):6–11 [PubMed] [Google Scholar]
- 79.Pearson AS, Izzo F, Fleming RY, et al. Intraoperative radiofrequency ablation or cryoablation for hepatic malignancies. Am J Surg 1999;178(6):592–599 [DOI] [PubMed] [Google Scholar]
- 80.Johnson DB, Solomon SB, Su LM, et al. Defining the complications of cryoablation and radio frequency ablation of small renal tumors: a multi-institutional review. J Urol 2004;172(3):874–877 [DOI] [PubMed] [Google Scholar]
- 81.Rhim H, Dodd GD, 3rd, Chintapalli KN, et al. Radiofrequency thermal ablation of abdominal tumors: lessons learned from complications. RadioGraphics 2004;24(1):41–52 [DOI] [PubMed] [Google Scholar]
- 82.Permpongkosol S, Nicol TL, Bagga HS, Kohanim S, Kavoussi L, Solomon SB. Prophylactic gelatin sponge tract injection to prevent bleeding after percutaneous renal cryoablation in a swine model. J Vasc Interv Radiol 2006;17(9):1505–1509 [DOI] [PubMed] [Google Scholar]
- 83.Chopra S, Dodd GD, 3rd, Chanin MP, Chintapalli KN. Radiofrequency ablation of hepatic tumors adjacent to the gallbladder: feasibility and safety. AJR Am J Roentgenol 2003;180(3):697–701 [DOI] [PubMed] [Google Scholar]
- 84.Cantwell CP, Wah TM, Gervais DA, et al. Protecting the ureter during radiofrequency ablation of renal cell cancer: a pilot study of retrograde pyeloperfusion with cooled dextrose 5% in water. J Vasc Interv Radiol 2008;19(7):1034–1040 [DOI] [PubMed] [Google Scholar]
- 85.Boss A, Clasen S, Kuczyk M, et al. Thermal damage of the genitofemoral nerve due to radiofrequency ablation of renal cell carcinoma: a potentially avoidable complication. AJR Am J Roentgenol 2005;185(6):1627–1631 [DOI] [PubMed] [Google Scholar]
- 86.Lee SJ, Choyke LT, Locklin JK, Wood BJ. Use of hydrodissection to prevent nerve and muscular damage during radiofrequency ablation of kidney tumors. J Vasc Interv Radiol 2006;17(12):1967–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weizer AZ, Raj GV, O’Connell M, Robertson CN, Nelson RC, Polascik TJ. Complications after percutaneous radiofrequency ablation of renal tumors. Urology 2005;66(6):1176–1180 [DOI] [PubMed] [Google Scholar]
- 88.Rendon RA, Gertner MR, Sherar MD, et al. Development of a radiofrequency based thermal therapy technique in an in vivo porcine model for the treatment of small renal masses. J Urol 2001;166(1):292–298 [PubMed] [Google Scholar]
- 89.Yamakado K, Nakatsuka A, Akeboshi M, Takeda K. Percutaneous radiofrequency ablation of liver neoplasms adjacent to the gastrointestinal tract after balloon catheter interposition. J Vasc Interv Radiol 2003;14(9 Pt 1):1183–1186 [DOI] [PubMed] [Google Scholar]
- 90.Tateishi R, Shiina S, Teratani T, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma: an analysis of 1000 cases. Cancer 2005;103(6):1201–1209 [DOI] [PubMed] [Google Scholar]
- 91.Hahn RG. Fluid absorption in endoscopic surgery. Br J Anaesth 2006;96(1):8–20 [DOI] [PubMed] [Google Scholar]
- 92.Lee SJ, Locklin JK, Wood BJ. Sodium alterations after irrigation fluid for adjacent organ protection in radiofrequency ablation. J Vasc Interv Radiol 2006;17(8):1366–1367 [DOI] [PubMed] [Google Scholar]
- 93.Onik GM, Onik C, Medary I, et al. Life-threatening hypertensive crises in two patients undergoing hepatic radiofrequency ablation. AJR Am J Roentgenol 2003;181(2):495–497 [DOI] [PubMed] [Google Scholar]
- 94.Lokken RP, Gervais DA, Arellano RS, et al. Inflammatory nodules mimic applicator track seeding after percutaneous ablation of renal tumors. AJR Am J Roentgenol 2007;189(4):845–848 [DOI] [PubMed] [Google Scholar]
- 95.Kawamoto S, Permpongkosol S, Bluemke DA, Fishman EK, Solomon SB. Sequential changes after radiofrequency ablation and cryoablation of renal neoplasms: role of CT and MR imaging. RadioGraphics 2007;27(2):343–355 [DOI] [PubMed] [Google Scholar]
- 96.Wile GE, Leyendecker JR, Krehbiel KA, Dyer RB, Zagoria RJ. CT and MR imaging after imaging-guided thermal ablation of renal neoplasms. RadioGraphics 2007;27(2):325–339; discussion 339–340 [DOI] [PubMed] [Google Scholar]
- 97.Ganguli S, Brennan DD, Faintuch S, Rayan ME, Goldberg SN. Immediate renal tumor involution after radiofrequency thermal ablation. J Vasc Interv Radiol 2008;19(3):412–418 [DOI] [PubMed] [Google Scholar]
- 98.Raman JD, Stern JM, Zeltser I, Kabbani W, Cadeddu JA. Absence of viable renal carcinoma in biopsies performed more than 1 year following radio frequency ablation confirms reliability of axial imaging. J Urol 2008;179(6):2142–2145 [DOI] [PubMed] [Google Scholar]
- 99.Weight CJ, Kaouk JH, Hegarty NJ, et al. Correlation of radiographic imaging and histopathology following cryoablation and radio frequency ablation for renal tumors. J Urol 2008;179(4):1277–1281; discussion 1281–1283 [DOI] [PubMed] [Google Scholar]
- 100.Wood BJ, Grippo J, Pavlovich CP. Percutaneous radio frequency ablation for hematuria. J Urol 2001;166(6):2303–2304 [PMC free article] [PubMed] [Google Scholar]
- 101.Rohde D, Albers C, Mahnken A, Tacke J. Regional thermoablation of local or metastatic renal cell carcinoma. Oncol Rep 2003;10(3):753–757 [PubMed] [Google Scholar]
- 102.Wood BJ, Bates S. Radiofrequency thermal ablation of a splenic metastasis. J Vasc Interv Radiol 2001;12(2):261–263 [DOI] [PMC free article] [PubMed] [Google Scholar]


