Abstract
Cyclic nucleotide phosphodiesterases (PDEs) that specifically inactivate the intracellular messengers cAMP and cGMP in a compartmentalized manner represent an important enzyme class constituted by 11 gene-related families of isozymes (PDE1 to PDE11). Downstream receptors, PDEs play a major role in controlling the signalosome at various levels of phosphorylations and protein/protein interactions. Due to the multiplicity of isozymes, their various intracellular regulations and their different cellular and subcellular distributions, PDEs represent interesting targets in intracellular pathways. Therefore, the investigation of PDE isozyme alterations related to various pathologies and the design of specific PDE inhibitors might lead to the development of new specific therapeutic strategies in numerous pathologies.
This manuscript (i) overviews the different PDEs including their endogenous regulations and their specific inhibitors; (ii) analyses the intracellular implications of PDEs in regulating signalling cascades in pathogenesis, exemplified by two diseases affecting cell cycle and proliferation; and (iii) discusses perspectives for future therapeutic developments.
Keywords: cAMP, cGMP, cyclic nucleotide phosphodiesterases, regulation, expression, distribution, PDE inhibitors, pathologies, therapeutic strategies, signalosome
Cyclic nucleotide phosphodiesterases (PDEs)
Cyclic nucleotide phosphodiesterases play a critical role in intracellular signalling by selectively hydrolysing the second messengers cAMP and cGMP that control cAMP- and cGMP-regulated proteins and transcription factors. In the 1970s, ‘the cyclic nucleotide system’ concept was developed as providing a powerful tool to enhance the opportunity for drug discovery (Amer, 1977). This system encompassed adenylyl and guanylyl cyclases, PDEs and cAMP-dependent and cGMP-dependent kinases. Very early, the cAMP- and cGMP-PDEs have been considered as targets for drug development (Pang, 1988). At that time, only three types of PDE (PDEI, PDEII and PDEIII) were described in cardiovascular tissues on the basis of their chromatographic elution order (Lugnier et al., 1986; Weishaar et al., 1987; Pang, 1988). Nowadays, advances in PDE methodology and technology (see book: Lugnier, 2005) have favoured the development of knowledge concerning PDE families (Beavo and Brunton, 2002; and see book: Beavo et al., 2006). Presently, PDEs represent an important enzyme class constituted by 11 gene-related families of isozymes (PDE1 to PDE11), giving rise to a multiplicity of isozymes. Each PDE family encompasses one to four distinct genes, giving rise to more than 20 genes in mammals that encode more than 50 different PDE proteins that are all probably expressed in mammalian cells (Lugnier, 2006; Conti and Beavo, 2007; Francis et al., 2011). PDE isozymes generally exist as dimers. Their monomeric structure has common features with three distinct domains. The N-terminal regulatory domain characterizes each family and their variants. The catalytic domain is quite conserved, sharing 25% to 52% homology with other mammalian PDE catalytic domains, and contains a Zn2+ binding site. The C-terminal domain can be prenylated (Anant et al., 1992) or phosphorylated by MAPK (Baillie et al., 2000). New mechanisms for cAMP action have been highlighted with the findings of GAF domains (cGMP-activated PDE, adenylyl cyclase- and Fh1A-binding domain) (Heikaus et al., 2009; Schultz, 2009), exchange protein activated by cAMP (EPAC), cAMP-regulated guanine nucleotide exchange factors (cAMP-GEFS), and cyclic nucleotide gated channels (CNGC; Cukkemane et al., 2011). This has resulted in expanding and diversifying cyclic-nucleotide-related pathways (for review, Borland et al., 2009; Gloerich and Bos, 2010).
In many pathologies including inflammation, cardiovascular diseases, neurodegenerescence and cancer, alterations of intracellular signalling related to PDE isozyme deregulation might contribute in explaining the difficulties observed in the prevention and treatment of these pathologies. The multiplicity of biochemical and structural properties of PDEs, their specific subcellular compartments, their transcriptional and posttranscriptional regulation make possible to envision to target only the altered PDE isozymes, thus avoiding and/or decreasing the adverse effects induced by non-specific treatments.
Below are described the 11 PDE families, their contribution in physiological functions and the therapeutical use of some specific PDE inhibitors (Table 1).
Table 1.
Cyclic nucleotide phosphodiesterase isozyme families: properties, tissue distribution and reference inhibitors
| Family | Specificities | Km (µM) cAMP/cGMP | Tissue distribution | Inhibitors |
|---|---|---|---|---|
| PDE1 (3) | Ca2+/calmodulin-stimulated | 0.3–124/0.6–6 | Heart, brain, lung, smooth muscle | Nimodipine, IC86340, IC224, IC295, dioclein |
| PDE2 (1) | cGMP-stimulated | 15/15 | Adrenal gland, heart, lung, liver, platelets, endothelial cells | EHNA, BAY-60–7750, PDP, IC933, oxindole, ND7001 |
| PDE3 (2) | cAMP-selective, cGMP-inhibited | 0.2/0.1 | Heart, smooth muscle, lung, liver, platelets, adipocytes, immunocytes | Cilostamide, milrinone, siguazodan, cilostazol |
| PDE4 (4) | cAMP-specific, cGMP-insensitive | 2/>300 | Brain, Sertolli cells, kidney, liver, heart, smooth muscle, lung, endothelial cells, immunocytes | Rolipram, roflumilast, cilomast, NCS 613 |
| PDE5 (1) | cGMP-specific | 150/1 | Lung, platelets, smooth muscle, heart, endothelial cells, brain, | Zaprinast, DMPPO, sildenafil tadalafil, vardenafil |
| PDE6 (3) | cGMP-specific, transducin activated | 2000/60 | Photoreceptors, pineal gland, lung | Zaprinast, DMPPO, sildenafil, vardenafil |
| PDE7 (2) | cAMP-specific, high-affinity rolipram-insensitive | 0.2/>1000 | Skeletal muscle, heart, kidney, brain, pancreas, T lymphocytes | BRL 50481, IC242, ASB16165 |
| PDE8 (2) | cAMP-selective, IBMX insensitive rolipram-insensitive | 0.06/NA | Testes, eye, liver, skeletal muscle, heart, kidney, ovary, brain, T lymphocytes, thyroid | PF-04957325 |
| PDE9 (1) | cGMP-specific, IBMX insensitive | NA/0.07–0.17 | Kidney, liver, lung, brain | BAY-73–6691, PF-04447943 |
| PDE10 (1) | cGMP-sensitive, cAMP-selective | 0.02–1/13 | Testes, brain, thyroid | Papaverine, TP-10, MP-10 |
| PDE 11 (1) | cGMP-sensitive, dual specificity | 0.5–2/0.3–1 | Skeletal muscle, prostate, pituitary gland, liver, heart | None selective |
(N) = gene numbers. Adapted from Bender and Beavo (2006), Francis et al. (2009), Lugnier (2006). NA, non applicable.
PDE1
Previously named calmodulin-stimulated PDE (CaM-PDE), PDE1 is the unique PDE family that is Ca2+-dependently regulated via calmodulin (CaM, a 16 kDa Ca2+-binding protein) complexed with four Ca2+ (for review, Stoclet et al., 1988; Kakkar et al., 1999; Bender, 2006). Thus, this family represents an interesting regulatory link between cyclic nucleotides and intracellular Ca2+, as shown in olfactory mucosa in which Ca2+ stimulation of PDE1 might be necessary for termination of olfactory signals (Borisy et al., 1992). The PDE1 family is encoded by three genes: PDE1A (mapped on human chromosome 2q32), PDE1B (human chromosome location, hcl: 12q13) and PDE1C (hcl: 7p14.3). They have alternative promoters and give rise to a multitude of proteins by alternative splicing. More than 10 human isoforms are identified (Bolger, 2006). Their molecular weights vary from 58 to 86 kDa per monomer. The N-terminal regulatory domain that contains two Ca2+/CaM binding domains and two phosphorylation sites differentiate their corresponding proteins and modulate their biochemical functions. PDE1A and PDE1B preferentially hydrolyse cGMP, whereas PDE1C hydrolyses cAMP and cGMP with similar Km values (see Table 1). The association of Ca2+/CaM complex changes PDE1 isozyme conformation, increasing their Vmax for cyclic nucleotide hydrolysis without modifying their Km. The sensitivity of PDE1 to Ca2+ activation, expressed in EC50, varies from 0.27 µM for PDE1A1 to 3.01 µM for PDE1C1 (Yan et al., 1996). Phosphorylation of PDE1A1 (59 kDa) and PDE1A2 (61 kDa) by PKA and phosphorylation of PDE1B1 by CaM kinase II decrease their calmodulin and Ca2+ sensitivities, resulting in a decrease of PDE1 activity. Interestingly, PDE1A2 isozyme is sensitive to endogenous proteolysis by calpain that activates PDE1. This could be an alternative mechanism for the permanent activation of PDE1A2 (for review, see Sharma et al., 2006). PDE1 is mainly present in brain, cardiac tissues and smooth muscles. Its presence in isolated cardiomyocytes was recently reported (Keravis et al., 2007).
Initially, PDE1 activity has been described as cytosolic. It now appears that PDE1A is not restricted to the cytosol but is also present in the nucleus where it contributes to the regulation of transcription factors (Nagel et al., 2006). This opens a new field of research in transcriptional regulation. For example, we recently showed that PDE1A controls the expression of the epigenetic integrator UHRF1 (Abusnina et al., 2011). Changes in PDE1 location associated with cell differentiation might contribute to compartmental signalling (Nagel et al., 2006).
Until now, very few inhibitors of PDE1 were available for evaluating the contribution of PDE1 in tissue and cell function. Vinpocetine (Ahn et al., 1989) and 8-methoxymethyl-IBMX (Ahn et al., 1997) are common PDE1 inhibitors. However, these compounds are not specific for PDE1; they also inhibit PDE5 at lower concentrations and vinpocetine inhibits PDE7B with an IC50 of 59 µM (Sasaki et al., 2004). Furthermore, vinpocetine directly inhibits BKCa channels (Wu et al., 2001), and recently vinpocetine was shown to inhibit NF-κB-dependent inflammation via an IKK-dependent but PDE-independent mechanism (Jeon et al., 2010). Previously, we showed that nimodipine, a dihydropyridine Ca2+ antagonist, selectively inhibits PDE1 in basal- and calmodulin-activated states (Lugnier et al., 1984), indicating that this compound is a helpful tool to assess the contribution of PDE1 in tissue or cell homogenates. Indeed, the use of nimodipine in cardiac tissue showed that PDE1 is up-regulated in early cardiac hypertrophy induced by angiotensin II (Mokni et al., 2010). Additionally, this compound, despite its Ca2+ antagonist effect, has been used to assess the participation of PDE1 in relaxation of noradrenaline-contracted smooth muscle (Noguera et al., 2001). Use of two potent and specific PDE1 inhibitors, namely IC86340 (IC50 from 0.06 to 0.44 µM depending on PDE1 variants; Nagel et al., 2006) and IC295 (Vandeput et al., 2007) synthesized by ICOS Corporation clearly established the contribution of PDE1 in cardiovascular function. Notably, PDE1 inhibition by IC86340 or down-regulation of PDE1A using siRNA allowed demonstrating that PDE1A may regulate specific cGMP pools with elevations of [Ca2+]i during hypertrophic stimulation (Miller et al., 2009). Unfortunately, these compounds are no longer available to the scientific community. We recently showed that dioclein selectively inhibits PDE1 (IC50 of 0.62 ± 0.14 µM for basal PDE1 and of 0.55 ± 0.07 µM for activated PDE1) and induces PKG-dependent vasodilatation (Gonçalves et al., 2009), indicating that this compound is helpful for studying basal- and stimulated-PDE1 contributions in cellular signalling.
PDE1 was reported to offer a new target for therapeutic intervention in pulmonary hypertension (PHT). Indeed, a strong up-regulation of PDE1C and PDE1A in pulmonary arterial smooth muscle cells (PASMCs) was noted, respectively, in idiopathic pulmonary arterial hypertension (IPAH) lungs and in lungs from animal models of PHT, and inhibition of PDE1 was reversing structural lung vascular remodelling and right heart hypertrophy in two animal models (Schermuly et al., 2007). The expression of both PDE1A and PDE1C was enhanced in PASMCs from IPAH and secondary pulmonary hypertension (SPH) patients compared with control PASMCs (Murray et al., 2007). PDE1C has been reported to be expressed in proliferating human SMCs but to be absent in quiescent human aorta and to be expressed in vivo in human foetal aorta containing proliferating SMCs, making PDE1C a marker of human SMC proliferation ex vivo and in vivo (Rybalkin et al., 2002). Furthermore, inhibition of PDE1C in SMCs isolated from normal aorta or from lesions of atherosclerosis was resulting in suppression of SMC proliferation, making PDE1C a good target to inhibit proliferating SMCs in lesions of atherosclerosis or restenosis (Rybalkin et al., 2002).
PDE2
PDE2 hydrolyses both cAMP and cGMP. It is encoded by a single gene PDE2A (hcl: 11q 13.4) and expressed as PDE2A1, PDE2A2 and PDE2A3. These isozymes differ by their N-terminal residues allowing different subcellular locations: PDE2A1 is cytosolic, whereas PDE2A2 and PDE2A3 are membrane-associated. We showed that PDE2 is associated with the nuclear envelope (Lugnier et al., 1999) and to purified Golgi endosomal fraction, and its phosphorylation by PKC increases its activity (Geoffroy et al., 1999). The N-terminal domain of the monomer has two cGMP-binding domains, GAF-A and GAF-B. The GAF-A domain mediates PDE2 dimerization (Martinez et al., 2002). Recently, crystal structure studies extended this function to GAF-A and GAF-B domains (Heikaus et al., 2009).
The binding of cGMP (1–5 µM) to GAF-B domain allosterically and positively stimulates up to a 30-fold increase in cAMP hydrolysis, inducing Michaelian behaviour with Km values from 10–30 µM (for review, Martinez, 2006). For this reason, PDE2 was first named cGMP-stimulated PDE (cGS-PDE). This specific regulation gives a major feedback role for PDE2 by restoring the basal cyclic nucleotide level in response to elevated cGMP (Bender and Beavo, 2006), notably following NO, ANP or BNP production. PDE2 is thus able to induce a crosstalk between cAMP and cGMP pathways (Lugnier, 2006).
When activated by cGMP, PDE2 is selectively inhibited by EHNA in comparison with other PDEs (Duncan et al., 1982; Podzuweit et al., 1995) with an IC50 value of 2 µM for activated PDE2 and 38 µM for basal PDE2 (Keravis et al., 2006). However, EHNA is also a potent adenosine deaminase inhibitor (Ki= 10 nM; Schaeffer and Schwender, 1974). Bay 60–7550 (Boess et al., 2004), IC933 (Snyder et al., 2005), PDP (Seybold et al., 2005) and oxindole (Chambers et al., 2006) are new potent and specific PDE2 inhibitors; however, only Bay 60–7550 is available. Interestingly, it was reported in a cell-based assay that EHNA, Bay 60–7550 and PDP increase cGMP levels only when combined with ANP, indicating that these inhibitors work only on cGMP-stimulated PDE2 (Wunder et al., 2009). Recently, our group designed and patented ND7001 as a PDE2 inhibitor that inhibits with the same potency basal and cGMP-stimulated PDE2 (Bourguignon et al., 2004). This compound antagonizes the anxiogenic effects of restraint stress on behaviour in mouse, similarly to Bay 60–7550, and in primary cultures of rat cerebral cortical neurons, ND7001 increases cGMP levels similarly to Bay 60–7550 (Masood et al., 2009). These data confirm that ND7001 is efficacious both in vivo and in vitro. Interestingly, in a mouse model of acute lung injury, LPS by intratracheal (i.t.) administration increases lung PDE2A mRNA and protein expression. PDE2A knockdown with an adenovirus administrated i.t. 3 days before LPS decreases PDE2A protein expression and attenuates alveolar inflammation and protein leak (Rentsendorj et al., 2011).
For complementary information, an extensive and up-to-date review on PDE2A has recently appeared online (Gesellchen and Zaccolo, 2011).
PDE3
PDE3 hydrolyses both cAMP and cGMP. The Vmax for cAMP hydrolysis is 10-fold higher than for cGMP hydrolysis and the substrate affinity for cGMP being higher than for cAMP, cGMP behaves as a competitive inhibitor for cAMP hydrolysis and therefore participates in cAMP/cGMP crosstalk (in platelets: Maurice and Haslam, 1990; in vascular smooth muscle: Komas et al., 1991; Eckly and Lugnier, 1994). Accordingly, PDE3 was ‘cGMP-inhibited PDE’ (cGI-PDE; Beavo, 1995). PDE3 is encoded by two genes: PDE3A (hcl: 12p12) and PDE3B (hcl: 11p15.1). Three variants are expressed for PDE3A (Wechsler et al., 2002): PDE3A1 (136 kDa), PDE3A2 (118 kDa) and PDE3A3 (94 kDa), whereas only a single PDE3B1 variant has been identified (Bolger, 2006) even if PDE3B of various sizes have been reported. These variants possess N-terminal hydrophobic membrane association regions (NHRs) and a 44-amino acid insert in the catalytic domain. A PKA phosphorylation site is present on PDE3A1 and PDE3A2, whereas an Akt/protein kinase B (PKB) phosphorylation site is only present on PDE3A1. PDE3A3 lacks PKA and PKB phosphorylation sites (Wechsler et al., 2002). PKA phosphorylation activates PDE3A, inducing a negative-feedback regulation in cAMP signalling. PKA phosphorylation of human PDE3B promotes 14-3-3-protein binding and inhibits phosphatase-catalysed inactivation (Palmer et al., 2007). PKB-dependent phosphorylation activates PDE3A and PDE3B. PKC phosphorylates and activates PDE3A (Pozuelo Rubio et al., 2005). PDE3A1 contains two membrane-associated domains. PDE3A2 contains only one membrane-associated domain, whereas PDE3A3 lacks membrane-associated domains. PDE3 is associated with cardiac sarcoplasmic reticulum (Lugnier et al., 1993), cardiac nuclear envelope near nucleopore complexes (Lugnier et al., 1999) as well as with liver Golgi endosomal fraction (Geoffroy et al., 2001). In primary adipocytes, PDE3B is associated with caveolae (Nilsson et al., 2006). PDE3B is present in hepatocyte caveolae and smooth endoplasmic reticulum (Berger et al., 2009) and adipocyte caveolae, endoplasmic reticulum and Golgi (Ahmad et al., 2009).
PDE3A is mainly present in heart, platelet, vascular smooth muscle and oocytes, whereas PDE3B is mainly associated with adipocytes, hepatocytes and spermatocytes. Reviews have appeared on the implications of PDE3 isoforms in heart failure and hypertension (Movsesian and Smith, 2006) and the role of PDE3B in energy homeostasis (Degerman and Manganiello, 2006). In adipocytes, insulin-mediated phosphorylation/activation of membrane-associated PDE3B leads to a reduction in PKA activity and inhibition of lipolysis. Inhibition of adipocyte PDE3B blocks the antilipolytic action of insulin and reduces insulin-stimulated lipogenesis and glucose uptake (Zmuda-Trzebiatowska et al., 2006).
PDE3B has the ability to act as an important scaffold protein with PI3K. A PDE3B-based signalling complex integrates EPAC1 and PI3K signals in human arterial endothelial cells, and this signalosome may be of importance in wound repair and angiogenesis (Wilson et al., 2011). Also, a recent study (Perino et al., 2011) shows that p110γ-anchored PKA activates PDE3B thus enhancing cAMP degradation and phosphorylating p110γ to inhibit PIP3 production, and this provides a local feedback control of PIP3 and cAMP signalling events. The authors show that pharmacological inhibition of p110γ normalizes β-adrenergic receptor density and improves contractility in failing hearts.
The first potent and selective inhibitor for PDE3 was cilostamide, shown as a platelet anti-aggregant (Hidaka et al., 1979). PDE3 was considered as a cardiotonic target. Therefore, medicinal chemistry has been focusing on PDE3 inhibitors, giving rise to amrinone, milrinone and enoximone. Milrinone was the first PDE3 inhibitor given in heart failure. However, due to some mortality in chronic treatment related to tachyarrhythmia and tachycardia, its prescription in humans is now only for a short period. In these conditions, milrinone does not induce exacerbation of myocardial injury in patients with severe heart failure and low cardiac output but instead is associated with a reduced inflammatory and apoptotic signalling (Lanfear et al., 2009). Nevertheless, continuous milrinone therapy use is shown to be safe as bridge to transplant in patients with short waiting time (<100 days) (Assad-Kottner et al., 2008). Recently, the PDE3 inhibitor cilostazol was marketed as Pletal® for treatment of intermittent claudication, the most common symptom of peripheral artery disease in which the narrowing and hardening of the arteries that supply legs leads to decreased blood flow. When a patient with peripheral artery disease exercises, the insufficient blood flow can produce muscle ischemia, and walking becomes problematic.
Pharmacological therapy for intermittent claudication in patients with peripheral artery disease is limited. Along with pentoxyfilline, cilostazol is the only medication approved by the Food and Drug Administration (FDA) for intermittent claudication. Cilostazol inhibits platelet aggregation and is a direct arterial vasodilator. Its main effects are dilation of the arteries supplying blood to the legs and decreasing platelet coagulation. Interestingly, cilostazol might increase O2 delivery by prediabetes erythrocytes whose PDE3B is up-regulated by insulin (Hanson et al., 2010). Long-term safety of cilostazol has been questioned because other PDE3 inhibitors, notably milrinone, have been previously associated with excess mortality in patients with heart failure (Movsesian and Kukreja, 2011). However, a recent study has shown that cilostazol demonstrated no increased risk of all-cause mortality (Pande et al., 2010).
PDE4
The PDE4 family that selectively hydrolyses cAMP is the most extended family, encoded by four genes, namely PDE4A (hcl: 19p13.2), PDE4B (hcl: 1p31), PDE4C (hcl: 19p13.1) and PDE4D (hcl: 5p12) that have different promoters and give rise to a multitude of proteins by alternative splicing. More than 25 human isoforms (from 50 to 125 kDa) are identified (Bolger, 2006). These proteins contain a unique amino acid signature region called upstream conserved regions 1 and 2 (UCR1 and UCR2; Bolger et al., 1993). Long PDE4 isozymes exhibit both UCR1 and UCR2, short PDE4 isozymes lack UCR1 and super-short isozymes contain only half of UCR2. UCR1 contains a PKA phosphorylation site that upon phosphorylation attenuates the ability of UCR1 to interact with UCR2 and thereby activates PDE4 activity (Beard et al., 2000). The carboxyl-terminal end of the catalytic region contains an ERK phosphorylation site; its phosphorylation induces the activation of PDE4 short forms and the inhibition of PDE4 long forms (Baillie et al., 2000). PDE4, like other PDEs, may exist as a dimer. Since the UCR modules mediate dimerization, only long-form PDE4 splice variants containing UCR1 and UCR2 are dimerized (Richter and Conti, 2004). PDE4A1 is a super-short isoform that possesses a specific N-terminal region entirely associated with membranes (Scotland and Houslay, 1995) and formed from two helices separated by a mobile hinge. The helix-2 contains a TAPAS-1 microdomain that allows rapid Ca2+-triggered membrane association with phosphatidic acid (Baillie et al., 2002). The helix-1 is important for intracellular targeting of PDE4A1 in living cells, facilitating membrane association, targeting to the trans-Golgi stack and conferring Ca2+-stimulated intracellular redistribution in a manner that is dependent on the phospholipase-D-mediated generation of phosphatidic acid (Huston et al., 2006). PDE4 variants are specifically localized by A-kinase anchoring protein (AKAPs; Skroblin et al., 2010; Welch et al., 2010) in subcellular compartments nearby PKA, which regulates functional responses (Dodge et al., 2001; for review see Houslay, 2010). We have shown that PDE4 is associated with cardiac sarcoplasmic reticulum (Lugnier et al., 1993), and that PDE4B and PDE4D are associated with the nuclear envelope (Lugnier et al., 1999). There is a predominant co-localisation of PDE4D3 with centrosome-associated AKAP450 (McCahill et al., 2005). Furthermore, PDE4s interact directly with many other intracellular proteins, thereby defining the PDE4 interactome (for a detailed recent review, see Houslay, 2010). PDE4D tethering EPAC1 regulates both the activity and subcellular localization of EPAC1 and controls cAMP-mediated vascular permeability (Rampersad et al., 2010). Furthermore, a relationship between PDE4A and PDE4B expression and the regulation of [Ca2+]i was shown in human endothelial cells (Campos-Toimil et al., 2008). PDE4s are present in brain, smooth muscle, heart, kidney, endothelial cells and immunocytes.
Rolipram, an antidepressant compound (Schwabe et al., 1976), specifically binds with high affinity to membrane brain fractions (Schneider et al., 1986). Rolipram was shown to be a potent inhibitor of cAMP hydrolysis in brain homogenates. Rolipram and Ro 20-1724 are highly selective for PDE4 (Lugnier et al., 1983, 1986; Komas et al., 1989). Accordingly, PDE4 became an archetype for the synthesis of new potent and selective PDE4 inhibitors (Marivet et al., 1989). PDE4 subtypes are expressed in a number of cell types that are considered suitable drug targets for the treatment of respiratory diseases such as asthma and COPD (for review, see: Spina, 2008). Suppression of human inflammatory cell function by subtype-selective PDE4 inhibitors correlates with inhibition of PDE4A and PDE4B (Manning et al., 1999). Interestingly, PDE4A4 is significantly upregulated in lung macrophages from smokers with COPD when compared with control smokers (Barber et al., 2004). Pharmaceutical companies synthesized many PDE4 inhibitors as anti-inflammatory agents for asthma and COPD, since they decreased production of TNF-α and cytokines (for review, Houslay et al., 2005; Boswell-Smith et al., 2006; Lugnier, 2006; Pagès et al., 2009). The PDE4 inhibitor NCS 613, a potent adenine analogue synthesized by our group, inhibits TNFα production in human lymphocytes; interestingly, this compound does not stimulate gastric acid secretion in rats (Boichot et al., 2000). Today, roflumilast is the first PDE4 inhibitor on the market (as Daxas®) for COPD treatment (Hatzelmann et al., 2010). Recently, a very potent and subtype-specific PDE4D inhibitor was reported (Aspiotis et al., 2010) and PDE4D allosteric modulators for enhancing cognition with improved safety have been designed (Burgin et al., 2010).
PDE5
PDE5 specifically hydrolyses cGMP and is encoded by one gene PDE5A (hcl: 4q27) with three variants being expressed: PDE5A1 (≍100 kDa), PDE5A2 (≍95 kDa) and PDE5A3 (≍95 kDa). Their N-terminal domain contains GAF-A and GAF-B domains. The GAF-A domain is responsible for allosteric binding of cGMP that promotes phosphorylation of PDE5 (Turko et al., 1998). Such phosphorylation activates catalytic activity and it increases cGMP-binding affinity and converts PDE5 into a stably activated form. The GAF-B domain modulates cGMP binding by GAF-A, thereby contributing to PDE5 dimerization (Francis et al., 2006). Despite a similar overall folding of the two GAF domains, only one (GAF-A) binds cGMP. A recent study suggests a complicated and cooperative process of cGMP signalling, which is composed of conformation changes in the GAF-A pocket upon cGMP binding, relay of the cGMP signal from GAF-A to GAF-B, and involvement of N-terminal segment 101–127 in the cGMP signalling (Wang et al., 2010). The first three-dimensional structure resolved by electron microscopic analysis of a native PDE revealed a dimeric arrangement for PDE5 having GAF-A, GAF-B and catalytic domains (Kameni-Tcheudji et al., 2001). PDE5 is cytosolic in vascular smooth muscle cells (Lugnier et al., 1986) and is mainly present in lung, smooth muscles, platelets and corpus cavernosum. Initially, no detectable levels of PDE5 in cardiac ventricles were reported (Komas et al., 1989; Wallis et al., 1999). PDE5 is associated with Z-bands in cardiac ventricle tissues (Takimoto et al., 2005) and with membrane fractions in isolated cardiomyocytes (Keravis et al., 2007). PDE5 is localized in endothelial caveolae, where it modulates NOS3 activity (Gebska et al., 2011).
Zaprinast (M&B 22948) was the first selective PDE5 inhibitor described (Lugnier et al., 1986), and it was used to investigate various functional implications of PDE5. Sildenafil was the first potent and selective PDE5 inhibitor marketed (Viagra™, Pfizer Inc.) for male erectile dysfunction (Francis et al., 2008). Thereafter, compounds more potent were developed. Tadalafil (Cialis™, Lilly-ICOS) was synthetized with greater selectivity for PDE5 versus PDE1-4 than sildenafil and with the highest long-lasting effect (>7h) (Daugan et al., 2003). Vardenafil (Levitra ™, Bayer-GSK) was synthetized with the lowest IC50. Both sildenafil and vardenafil are also potent PDE6 inhibitor and induce visual perturbation (Cote, 2006). Furthermore, tadalafil although inhibiting PDE11 might not induce cross-reaction in patients (Weeks et al., 2005). Pulmonary hypertension (Wilkens et al., 2001; Ghofrani et al., 2002; Michelakis et al., 2002; for review, Croom and Curran, 2008), high altitude mountain sickness (Bates et al., 2007), memory dysfunctions (Puzzo et al., 2008) and cardiovascular diseases (Kass et al., 2007; for reviews, Kumar et al., 2009 and Tsai and Kass, 2009) are new therapeutic fields for PDE5 inhibitors. Recently, it has been reported that, human heart PDE5 being present at much lower levels than those seen in animal models, the applicability of PDE5 inhibitors in treating heart failure could be questioned (Movsesian and Kukreja, 2011). However, PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure (Guazzi et al., 2011).
Moreover, PDE5 plays a role in cancer. In melanoma cells, oncogenic BRAF induces invasion through down-regulation of PDE5A (Arozarena et al., 2011; Houslay, 2011). Nevertheless, PDE5 inhibition is responsible for the breast tumour cell growth inhibitory activity and apoptosis inducing activity of sulindac sulphite and may contribute to the chemopreventive properties of sulindac (Tinsley et al., 2009).
PDE6
In contrast to other PDE families, the PDE6 family is restricted to retinal rod and cone cells (Miki et al., 1975; Ridge et al., 2003) and to pineal gland (Morin et al., 2001). PDE6 family, that hydrolyses cGMP, is a key component in the visual transduction cascade and is known as the photoreceptor phosphodiesterase (Cote, 2006). PDE6 is composed of two large catalytic subunits (α and β in rod, 100 and 98 kDa, respectively, and two α′ in cones, 99 kDa) associated with two copies of small inhibitory subunits (γ in rods, 9.5 kDa and γ′ in cones, 8.9 kDa; Cote, 2006). The catalytic subunits are encoded by PDE6A for rod α subunit, PDE6B for rod β subunit and PDE6C for cone α′ subunit. The inhibitory subunits are encoded by PDE6G (hcl: 17q25) and PDE6H (hcl: 12p13), for γ and γ′ respectively. The PDE6D (hcl: 2q35-q36) gene that encodes a δ subunit (17 kDa) co-purified with PDE6 was recently excluded from PDE6 family and revised as a prenyl binding protein (PrBP/δ). Contrary to PDE6, PrBP/δ is present in numerous tissues and may interact with other proteins (Cote, 2006). The structure for the membrane-bound rod PDE6 holoenzyme is αβγ2, whereas it is α′2γ2 for cone PDE6 (Artemyev et al., 1996). Each catalytic subunit contains the cGMP-binding GAF-A and GAF-B domains. The GAF-A domain may allow dimerization and binding of the inhibitory subunit γ′. The binding of cGMP is required for high affinity binding of γ subunit. PDE6 activation, mediated by phototransduction, decreases cGMP levels, and it induces the closure of cyclic nucleotide-gated channels (CNGC), thereby converting a light signal into an electrical signal (Wensel, 2008). For now, there is no specific PDE6 inhibitor. However, some PDE5 inhibitors also inhibit PDE6 (for review, Francis et al., 2009).
Subtypes of PDE6 have been found outside the eye, especially PDEγ in human embryonic kidney 293 cells (Wan et al., 2001) and in mouse lung (Tate et al., 2002), which seems to have a critical role in regulating p42/p44 MAPK signalling. PDEγ is also in pulmonary vessels from rats as well as in cultured human pulmonary SMCs, both maintained under chronic hypoxic conditions (Murray et al., 2003). Recently, PDE6D and PDE6G/H subtypes have been shown to be present in alveolar epithelial cells and to be altered in lung fibrosis (Nikolova et al., 2010). These studies are encouraging to investigate whether some PDE6 subtypes could be therapeutic targets in pulmonary hypertension.
PDE7
The PDE7 family specifically hydrolyses cAMP and is insensitive to rolipram, the specific inhibitor of the PDE4 family, but is sensitive to isobutyl-methylxanthine (IBMX). There is no known regulatory domain on the N-terminal region. This family includes two genes PDE7A (hcl: 8q13) and PDE7B (hcl: 6q23-q24), with alternative splicing for PDE7A (Michaeli, 2006) giving rise to PDE7A1 (57 kDa), PDE7A2 (50 kDa) and PDE7A3 (50 kDa). PDE7A1, expressed in lymphocytes and proinflammatory cells, is a bifunctional protein. In addition to its cAMP hydrolytic activity, its N-terminal domain, by interacting directly with the catalytic subunit of PKA, inhibits kinase activity with high affinity. PDE7A2, present in skeletal and cardiac muscles, has a hydrophobic N-terminus. PDE7A3 lacks a part of the catalytic domain structure and retains the capacity of PDE7A1 to interact and inhibit the catalytic subunit of PKA. Although no endogenous PDE7B proteins have been yet detected, four alternative splice PDE7B transcripts have been identified: PDE7B1, PDE7B2, PDE7B3 and an additional variant found in GenBank (Michaeli, 2006). Two putative phosphorylation sites for PKA would be on PDE7B1 and PDE7B3. PDE7B1 is the only variant to be activated by D1 agonist through the cAMP/PKA/CREB pathway in striatal neuron, arguing for a role for PDE7B1 in memory function and promoting PDE7B1 as a possible target for Parkinson and Huntington diseases (Sasaki et al., 2004). There are few PDE7 inhibitors. IC242 was the first reported selective PDE7A inhibitor with an IC50 value of 0.37 µM (Lee et al., 2002). BRL 50481was discovered as a PDE7 inhibitor (Ki= 180 nM) and has an acceptable in vitro selectivity (Smith et al., 2004). The thiadiazoles is a structural class of potent and selective PDE7 inhibitors, synthetized by Pfizer and acting in the nanomolar range (Vergne et al., 2004). Lastly, ASB16165, a novel inhibitor for PDE7A (IC50= 15 nM) was reported to suppress IL-12-induced IFN-γ production by mouse activated T lymphocytes (Kadoshima-Yamaoka et al., 2009).
PDE7 family is a pharmacological target in chronic lymphocytic leukaemia (CLL). Indeed, PDE7A is present in human splenic B lymphocytes from either healthy donors or B-lineage malignancy CLL patients as well as in WSU-CLL, a CLL-derived cell line (Lee et al., 2002). Amongst the different cAMP-PDE isozymes present in WSU-CLL, only PDE7A levels increase after a treatment with methylxanthines; theophylline, IBMX and IC242 inhibit PDE7A activity with respective IC50 values of 343.5, 8.6 and 0.84 µM. These data might explain the observation that theophylline, a non-specific methylxanthine PDE inhibitor, induces apoptosis in CLL B-lymphocytes in vitro (Mentz et al., 1995). Another study has shown that PDE7B of peripheral blood mononuclear cells (PBMC) was increased at both mRNA (23-fold increase) and protein (10-fold increase) levels in patients with CLL compared with healthy donors and that the PDE7 inhibitors BRL-50481 and IR-202 selectively increased apoptosis in CLL cells compared with normal PBMC or B cells (Zhang et al., 2008).
PDE8
The PDE8 family specifically hydrolyses cAMP with the highest affinity amongst all PDEs and is insensitive to rolipram, IBMX and cGMP (Vasta, 2006). It is encoded by PDE8A (hcl: 15q25.3) and PDE8B (hcl: 5q13.3). PDE8A mRNA has the highest expression in testis, followed be eye, liver, skeletal muscle, heart, kidney ovary and brain, in decreasing order. The primary structure of PDE8 includes N-terminal REC and PAS domains whose regulatory functions for PDE8 are unknown. Three putative PKA and PKG phosphorylation sites are located between the PAS domain and the catalytic domain. One to five splice variants of PDE8 were cloned from testis and T cells. PDE8A1, the longest (93 kDa) and most frequently expressed variant, contains REC and PAS domains. PDE8A2 lacks the PAS domain, whereas PDE8A3 and the truncated PDE8A4 and PDE8A5 lack both REC and PAS domains (Wang et al., 2001). Five variants of PDE8B have been identified (Gamanuma et al., 2003). PDE8B1 and PDE8B4 contain both REC and PAS domains, whereas PDE8B2 and PDE8B3 have a deletion in PAS domain. RT-PCR studies showed that PDE8B1 is mainly present in thyroid gland, PDE8B1 and PDE8B3 are equally expressed in placenta and PDE8B3 is the most abundant form in brain (Hayashi et al., 2002). Interestingly, PDE8 regulates excitation–contraction coupling in ventricular myocytes, revealing its participation in cardiac function (Patrucco et al., 2010). PF-04957325, the recently produced PDE8 inhibitor (Vang et al., 2010) will be helpful for studying the role of PDE8 in cardiovascular pathologies.
PDE9
The PDE9 family specifically hydrolyses cGMP with the highest affinity amongst all PDE families and is insensitive up to 100 µM to most reference PDE inhibitors, notably to IBMX. However, zaprinast inhibits PDE9 with an IC50 value of 35 µM (Fisher et al., 1998). Twenty-one N-terminal mRNA variants have been identified for the PDE9A gene (hcl: 21q22.3) (Rentero et al., 2003). There are no regulatory sequences identified on the N-terminal domain of PDE9 (Bender and Beavo, 2006). There is no characterization of PDE9A phosphorylation (Omori and Kotera, 2007). Only PDE9A1 and PDE9A6 (originally named PDE9A5 in the referenced paper) proteins have been expressed and characterized. They are predominant in some immune tissues. PDE9A6 is cytosolic, whereas PDE9A1 is associated to the nucleus (Wang et al., 2003). BAY 73-6691 inhibits PDE9A with a 25-fold greater selectivity compared with all other PDEs (Wunder et al., 2005). The use of BAY 73-6691 in rodents has demonstrated that PDE9 inhibition increases learning and memory (van der Staay et al., 2008). An orally bioavailable PDE9 inhibitor was synthetized for use as a potential hypoglycemic agent (DeNinno et al., 2009). Lastly, PF-04447943, a brain penetrant PDE9A inhibitor that enhances synaptic plasticity and cognitive function in rodents has been published (Hutson et al., 2011).
PDE10
The PDE10 family is a dual-substrate gene family encoded by the unique gene PDE10A. Human PDE10A maps to chromosome 6q26-27 (Fujishige et al., 1999; 2000). The deduced amino acid sequence contains 779 amino acids (88 kDa), including two GAF domains in the N-terminal region. In contrast to other PDEs, the GAF-A domain apparently binds only cAMP. Due to its kinetic properties for cAMP and cGMP hydrolysis, cGMP hydrolysis by PDE10 is potently inhibited by cAMP, which is notably the opposite compared with the PDE3 family (Bender and Beavo, 2006). The first known potent and specific PDE10A inhibitor is papaverine, with an EC50 value of 36 nM (Siuciak et al., 2006). There are 18 splice variants for PDE10A (PDE10A1–PDE10A18; for details, see Strick et al., 2006). PDE10A is expressed mainly in brain, particularly in striatal medium spiny neurons, in pineal gland and, to a lower extent, in testis. TP-10, a new potent and very specific PDE10 inhibitor, synthesized by Pfizer, was under investigation in preclinical study as a new therapeutic approach for the treatment of schizophrenia (Schmidt et al., 2008). Furthermore, papaverine and MP-10 treatments act on the negative symptoms of schizophrenia (Grauer et al., 2009). Recently, it was shown that this PDE10 inhibitor improves striatal and cortical pathology in the R6/2 mouse model of Huntington's disease (Giampàet al., 2010), and that PDE10A plays a key role in the pathophysiology of Parkinson's disease (Giorgi et al., 2011). A recent study demonstrates for the first time that PDE10A has a central role in progressive pulmonary vascular remodelling and suggests a novel therapeutic approach for the treatment of pulmonary hypertension (Tian et al., 2011).
PDE11
The PDE11 family represents a dual-substrate PDE family having a catalytic site more similar to PDE5 than to PDE10A. PDE11A1 (491 amino acids; predicted molecular mass 56 kDa) was cloned from human skeletal muscle and was shown to contain only one GAF domain. PDE11 is mainly present in prostate and to a lower amount in pituitary gland, liver and heart. Four N-terminal variants are encoded from the PDE11A gene mapped on human chromosome 2 (2q31.2): PDE11A1 to PDE11A4. PDE11A2 (65.8 kDa) and PDE11A3 (78 kDa) contain one complete and one incomplete GAF domain in the N-terminal region (Omori and Kotera, 2006). PDE11A4 (100 kDa) is the longest PDE11 protein, including two GAF domains and two phosphorylation sites for PKA and PKG (Yuasa et al., 2000). The role of the GAF domain in PDE11 is still unknown. PDE11A is inhibited by dipyridamole (IC50= 0.9–1.8 µM), zaprinast (IC50= 5–33 µM), IBMX (IC50= 25–81 µM) and is insensitive to EHNA, rolipram and milrinone. There are no specific inhibitors for PDE11A. However, amongst the newly launched PDE5 inhibitors, tadalafil was the most potent (IC50= 37–73 nM), inhibiting both cAMP and cGMP hydrolysis (Saenz de Tejada et al., 2002; for review, see Makhlouf et al., 2006). Although PDE11A is expressed in very restricted brain areas, such as ventral hippocampus, its deletion in a KO mouse model causes psychiatric disease-related phenotypes (Kelly et al., 2010). Carney complex patients possess PDE11A variants with a high frequency, suggesting that PDE11A is a genetic factor for the development of testicular and adrenal tumours (Libéet al., 2011).
The search for specific PDE family inhibitors might take advantage of yeast-based assays, which have been reported to be useful for detecting and characterising inhibitors of either cAMP- or cGMP-metabolizing PDEs (Demirbas et al., 2011).
Intracellular implication of PDEs in regulating signalling cascades
Intracellular cyclic nucleotide targets and PDE regulation
The multiplicity of intracellular cyclic nucleotide targets give rise to an expanding role for PDEs in controlling cellular events. For example, in addition to their very well known stimulating effect on PKA and PKG, binding of cyclic nucleotides to CNGCs results in activation of cation-permeable channels that have key cellular signalling roles (for review, see Kaupp and Seifert, 2002; Matulef and Zagotta, 2003; Biel and Michalakis, 2009). Amongst the direct cAMP targets, EPAC was newly discovered as a Rap1 guanine nucleotide exchange factor that is activated directly by cAMP (de Rooij et al., 1998; Gloerich and Bos, 2010). As described above, cyclic nucleotides regulate PDEs either by their binding to GAF domains or by competitive inhibition. The discovery of EPAC enriches the complexity of the network of cyclic nucleotide-mediated pathways in which PDEs play a critical function. EPAC proteins transduce diverse cellular actions of cAMP (for review, see: Borland et al., 2009). Studies of the molecular mechanisms of EPAC-related signalling have demonstrated that these novel cAMP sensors regulate many physiological processes either alone and/or in concert with PKA (Grandoch et al., 2010). Mechanisms for the anti-apoptotic response to cAMP likely involve EPAC. Therapeutic approaches that activate PKA-mediated pro-apoptosis or block EPAC-mediated anti-apoptosis may provide a means to enhance cell killing, such as in certain cancers. In contrast, efforts to block PKA or stimulate EPAC have the potential to be useful in disease settings (such as heart failure) associated with cAMP-promoted apoptosis (Insel et al., 2011).
PKA and PKG that are directly activated, respectively, by cAMP and cGMP control functional cellular responses including intracellular calcium signalling, cell proliferation, inflammation and transcription (for review, see Francis and Corbin, 1999). Since some of these kinases are attached to different cellular ultrastructures via specific A kinase anchoring proteins (AKAPs; Dell'Acqua and Scott, 1997; Moorthy et al., 2011) or G kinase anchoring proteins (GKAPs; Vo et al., 1998; Casteel et al., 2010; Manneville et al., 2010), they participate in intracellular signalling compartmentalization (Wong and Scott, 2004). Moreover, cAMP and cGMP were shown to cross-activate PKG and PKA, respectively (Forte et al., 1992; Eckly-Michel et al., 1997), thereby representing a crossroad in cyclic nucleotide pathways. GAF domains are present in the N-terminal region of PDE2, PDE5, PDE6, PDE10 and PDE11. PDE2, PDE5 and PDE6 contain both GAF-A and GAF-B domains, whereas PDE10 and PDE11 contain one complete GAF domain. The binding of cGMP to PDE2 GAF-A, PDE5 GAF-B and PDE6 GAF-B domains positively regulate cGMP hydrolysis. Furthermore, cAMP hydrolysis by PDE3 is inhibited by cGMP, whereas cGMP hydrolysis by PDE10 is potently inhibited by cAMP. It should be also emphasized that within cells, in the case of other dual-substrate PDEs (PDE1C, PDE2 and PDE11), the hydrolysis of one nucleotide might be competitively inhibited by the other cyclic nucleotide depending on their ratio at the catalytic site (Figure 1).
Figure 1.
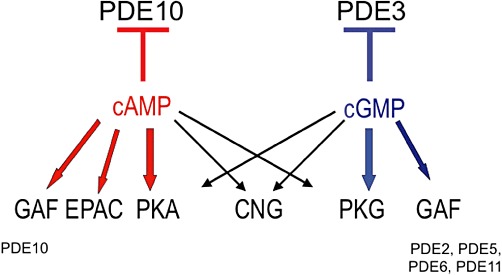
Intracellular targets for cAMP and cGMP.
Beyond cyclic nucleotide targets (PKA, PKG, GAF domains, EPAC, as well competitive substrates), it should be noticed that CaM, CaM-Kinase, ERK, PKC and phosphatidic acid also participate in short-term regulation of PDEs (Keravis and Lugnier, 2010).
Long-term regulation of PDEs is mediated by PKA-dependent phosphorylation of transcription factors such as CREB and CREM (Lamas and Sassone-Corsi, 1997) and ICER expression (Cho et al., 2005). Therefore, these molecular mechanisms will contribute to regulate physiological cellular events as well as dysfunctions associated with various pathologies.
Two examples of PDE regulation in altered signalling
Since the 1980s, the use of various PDE inhibitors in normal and diseased cells and tissues as well as in animal models has allowed to increase and more precisely define the possible implication of specific PDE families in various physiological systems and thereafter in associated pathologies, pointing out their roles in the CNS, in the cardiovascular system and in inflammation. Currently, biochemical and molecular biological tools as well as fluorescent imaging, allow specifying which PDE family and how a variant contribute to the studied dysfunction. In that way, hereafter, we present two examples where we studied the implications of different PDEs in diseases with altered cell cycle: angiogenesis and acute lymphoid leukaemia (ALL).
PDEs in angiogenesis. Angiogenesis, the development of new vessels from pre-existing ones, contributes mainly in tumour growth and metastasis that are dependent on VEGF secretion, which induces endothelial cell proliferation. Endothelial PDEs were first described as PDE2 and PDE4 in pig aortic endothelial cell (Souness et al., 1990) and in bovine aortic endothelial cells (Lugnier and Schini, 1990). On one hand, cAMP is anti-proliferative, and on the other hand, VEGF stimulates NOS that increases cGMP levels by stimulating soluble guanylyl cyclase. We wondered whether VEGF could modify PDE activity and expression in angiogenesis. This was investigated on primary HUVECs as a model of in vitro angiogenesis and the chicken embryo chorioallantoic membrane (CAM) as an in vivo angiogenesis model (Auerbach et al., 1974). HUVECs contain PDE2, PDE3, PDE4 and PDE5 families (Favot et al., 2003; Netherton and Maurice, 2005). Our results (Favot et al., 2003; 2004; Keravis et al., 2006) showed for the first time that VEGF specifically up-regulates PDE2 and PDE4 activity and mRNA expression of PDE2A, PDE4A, 4B and 4D without acting on PDE3 and other HUVEC PDEs except for a decrease in PDE5 activity. The treatment of HUVECs with the association of PDE2 and PDE4 inhibitors increases cAMP levels, decreases VEGF-induced HUVEC migration, proliferation and cell cycle progression in the transition phase S-G2/M for the PDE2 inhibitor and G0/G1-S for the PDE4 inhibitor. This is associated with decreases in cyclin A and cyclin D1 expression and with increases in the expression of repressors p21 and p27. The combination of PDE2 and PDE4 inhibitors decreases significantly VEGF-induced phosphorylation of p42/p44 MAPK (Figure 2A). Interestingly, this treatment inhibits in vivo angiogenesis, as shown by the dose-dependent reduction of total capillary surface compared with control untreated zones of CAM in the same embryo (Favot et al., 2003). PDE2 and PDE4 inhibitors, as well as PDE3 inhibitors, decrease cell migration in other vascular endothelial cells (Netherton and Maurice, 2005). Taken together, our data reveal that targeting specifically altered PDEs in angiogenesis might be an interesting alternative to the currently developed anti-angiogenic therapy with bevacizumab (a humanized monoclonal antibody that inhibits VEGF activity), that has been recently shown to increase mortality in cancer patients (Ranpura et al., 2011).
Figure 2.
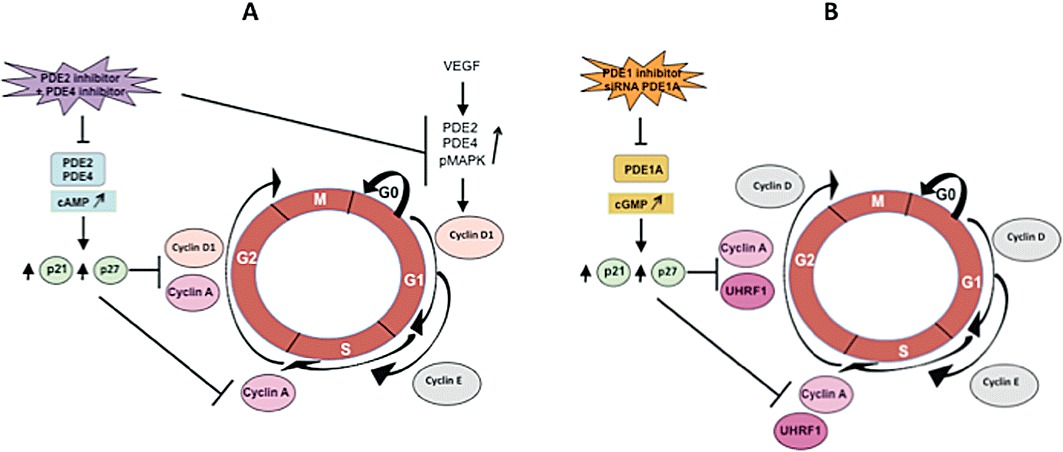
PDE participation in the control of cell cycle. (A) Primary cultured HUVECs. (B) Jurkat cell line.
PDEs in ALL. It has been reported that PDE4 inhibitors, which activate cAMP signalling by reducing cAMP catabolism, are known to induce apoptosis in B-lineage CLL cells (Lerner et al., 2000), but not in normal human T cells (Meyers et al., 2009) and T leukaemic cell lines (Tiwari et al., 2005). We recently showed in Jurkat cells (a human T-cell line, model of ALL) that the apoptotic effect of the natural compound thymoquinone is mediated by p73 up-regulation that induces down-regulation of the epigenetic integrator UHRF1 and G1 cell cycle arrest in a dose-dependent manner (Alhosin et al., 2010). Since thymoquinone has anti-oxidant and anti-inflammatory properties similarly to PDE4 inhibitors (Houslay et al., 2005), since some natural compounds are able to inhibit PDEs and since PDE4 inhibitors decrease cell proliferation in leukaemia (Kim and Lerner, 1998), we wondered whether a specific PDE isoform controlling apoptosis would be responsible for the thymoquinone effects. The determination of the PDE family profile contributing to total cAMP- and cGMP-hydrolysing activities reveals that PDE4 contributes mainly to cAMP hydrolysis, whereas cGMP-hydrolysing activity is mainly due to PDE5, PDE1 contributing only for 1%. Assessed as a PDE inhibitor, thymoquinone only inhibits cAMP hydrolysis by PDE4 with an IC50 value of 60 µM, in accordance with its previous described anti-oxidant and anti-inflammatory properties. However, and intriguingly, 24 h thymoquinone treatment dose-dependently decreases cAMP levels but increases cGMP levels, despite that thymoquinone does not act on cGMP hydrolysis by PDE1, PDE2, PDE3 and PDE5. This treatment inhibits Jurkat cell growth in a dose-dependent manner and induces apoptosis. In contrast to PDE3, PDE4 and PDE5 inhibitors, nimodipine, a PDE1 inhibitor, is able, at a concentration selectively active on PDE1, to inhibit the growth and apoptosis of Jurkat cells, similarly to thymoquinone. The effects of nimodipine and thymoquinone induce concomitant decreased expression of PDE1A and UHRF1, whereas p73 expression is upregulated. Knocking down PDE1A by RNA-interference leads to the same results. Conversely, re-expression of PDE1A induces UHRF1 expression and p73 down-regulation. Taken together, these data clearly and newly demonstrate that inhibition of PDE1A expression induces apoptosis of Jurkat cells by up-regulating p73 repressor and down-regulating the expression of the epigenetic integrator UHRF1 (Figure 2B). This also indicates for the first time that a PDE family, such as PDE1A, apparently participates in epigenetic regulation (Abusnina et al., 2011).
These two examples of alterations of PDEs participating in cell cycle regulation illustrate one aspect of how PDEs are implicated in cellular functions via critical partners (Figure 2). They point out that, although thymoquinone inhibits only PDE4 activity, its effect in Jurkat cells is not mediated by PDE4 but dependent on PDE1A regulation, illustrating the complexity of cyclic nucleotide pathways. This complexity could be linked to subcellular compartmentalization orchestrated by PDEs, AKAP and protein kinases (Houslay and Adams, 2003), together with subcellular changes in location of PDEs induced by some PDE inhibitors as recently shown for PDE4 inhibitors (Day et al., 2011). Furthermore, PDE/protein interactions as largely reviewed for the PDE4 interactome (Houslay, 2010) might well be extended to other ‘PDE interactomes’, as suggested for PDE1 by our work on PDE1A/UHRF1in Jurkat cells (Abusnina et al., 2011), for PDE3B in endothelial cells (Wilson et al., 2011). Other ways to disrupt this interactome include the design of specific peptides that would modify critical peptidic interaction during PDE/protein interaction as suggested recently (Keravis and Lugnier, 2010) and the stimulation of changes in microdomain location of a critical PDE variant for a particular cell function.
Perspectives for future therapeutic developments
After numerous disappointments in the development of PDE3 and PDE4 inhibitors due to their adverse effects (mortality and emesis, respectively), the success in PDE5 inhibitor development with Viagra®, associated with increasing knowledge in the PDE field, renews interest in developing selective PDE inhibitors as specific therapeutic agents with few adverse effects.
Since PDEs participate in the interactome that regulates cellular signalling as recently exemplified by PDE3B (Perino et al., 2011; Wilson et al., 2011), PDEs might be implicated in multiple dysfunctions and pathologies associated with deregulated signals. Recent studies demonstrate the interest of PDE inhibitors to treat and to reverse heart hypertrophy (Takimoto et al., 2005), to enhance cognition (Reneerkens et al., 2009) and to treat sleep deprivation (Vecsey et al., 2009). Concerning alterations of PDE expression, increase in PDE5 expression in human failing left ventricle heart tissue relates to oxidative stress and sildenafil treatment counteracts this alteration (Lu et al., 2010). Similarly, expression of PDE5 and PDE9 increases in aging and Alzheimer's disease (Domek-Łopacińska and Strosznajder, 2010). Lastly and interestingly, by using a global PDE8B KO mouse model and a new PDE8-selective inhibitor (PF-04957325), Beavo and colleagues reported that PDE8B controls steroidogenesis in mouse adrenal gland (Tsai et al., 2011).
To improve therapeutic index, dual PDE inhibitors inducing synergic potencies with lesser adverse effect have been envisionned. Indeed, dual PDE3/4 inhibitors are now designed as therapeutic agents for chronic obstructive pulmonary disease. By combining both inhibitions, these compounds have additive and synergistic bronchodilatory and anti-inflammatory effects (Banner and Press, 2009). Furthermore, PDE7/PDE4 dual inhibitors would represent a novel class of drugs that could regulate pro-inflammatory and immune T-cell function and be especially useful in treating a wide variety of immune and inflammatory disorders with less undesirable side effects (Castaño et al., 2009).
Another way to obtain an efficient therapeutic effect, without inducing adverse effects, may be to specifically target the altered PDE family and in particular the altered variant so that the other PDEs present are not modified. To do so, it is first of all necessary to (i) characterize the various PDE isozymes present in the studied model, (ii) identify the altered PDE isozyme(s) related to the given pathology (therapeutic target) and (iii) validate the therapeutic treatment by the induction of concomitant restoration of the altered PDE(s) and associated cellular function without changing the other non-altered PDEs. This approach might be useful in the case of diseases for which no specific therapy is available. It could also be worth considering in established treatments that induce too many adverse effects.
Throughout this review, many molecular mechanisms interacting with PDE regulation have been discussed (Figure 3), and they may well participate in the normal and pathophysiological regulation of the interactome. Beyond targeting PDEs by the synthesis of potent and very selective PDE family inhibitors, the development of inhibitors of specific PDE variants represents the next focus for therapeutic development.
Figure 3.
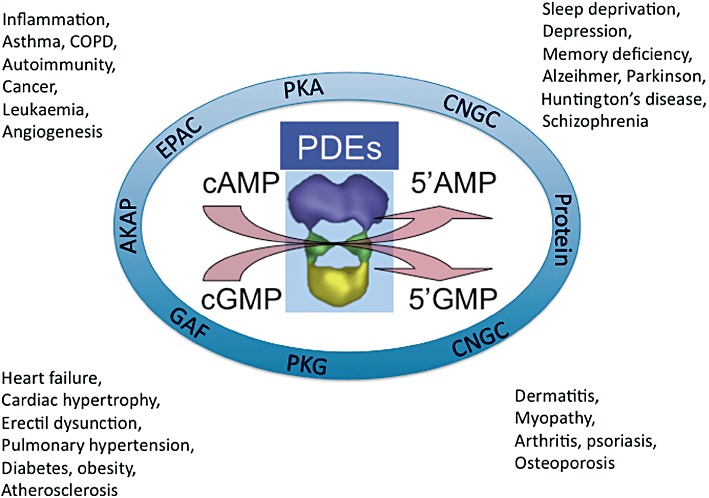
Participation of PDEs in intracellular signalling network: potential therapeutic implications. Local cAMP and cGMP levels controlled by PDEs activate PKA, PKG, CNGC and bind to GAF domains. PDE themselves interact with AKAP and other critical intracellular proteins. These two modes of interaction potentially participate in various diseases.
Furthermore, disrupting agents for PDE/protein interaction might be worth considering for controlling PDE-mediated signalling pathways, as recently demonstrated with the use of a peptide in the hypertrophic response of cardiac myocytes (Sin et al., 2011). Peptides or peptidomimetics acting on critical extra- or intra-PDE-binding sites and able to induce PDE activation could be of interest when the targeted PDE should be upregulated as a therapeutic goal (Keravis and Lugnier, 2010).
In conclusion, considering the participation of multiple PDE variants in the signalling network, their specific and various regulatory mechanisms together with their subcellular compartmentalization and association with diverse critical proteins for cellular function, there is no doubt that PDE super family should become a major target to treat various pathologies.
Acknowledgments
Not applicable.
Glossary
- AKAP
A-kinase anchoring protein
- ALL
acute lymphoblastic leukaemia
- ANP
atrial natriuretic peptide
- BRAF
B-RAF
- CaM-PDE
calmodulin-stimulated PDE
- CaM
calmodulin
- CAM
chorioallantoic membrane
- cAMP-GEFS
cAMP-regulated guanine nucleotide exchange factors
- cGI-PDE
cGMP-inhibited PDE
- cGS-PDE
cGMP-stimulated PDE
- CLL
chronic lymphocytic leukaemia
- CNGC
cyclic nucleotide gated channel
- COPD
chronic obstructive pulmonary disease
- CREB
cAMP response element-binding
- CREM
cAMP response element modulator
- EPAC
exchange protein activated by cAMP
- FDA
Food and Drug Administration
- GAF
domain, cGMP-activated PDE, adenylyl cyclase- and Fh1A-binding domain
- HUVEC
human umbilical vein endothelial cell
- ICER
inducible cAMP early repressor
- IKK
IκB kinase
- IPAH
idiopathic pulmonary arterial hypertension
- i.t
intratracheal
- KO
knock out
- NHRs
possess N-terminal hydrophobic membrane association regions
- PAS
Per–Arnt–Sim
- PASMC
pulmonary arterial smooth muscle cell
- PBMC
peripheral blood mononuclear cell
- PDE
cyclic nucleotide phosphodiesterase
- PHT
pulmonary hypertension
- PI3K
PI3 kinase
- Rap1
Ras-related protein 1
- REC
Response Regulator Receiver
- RT-PCR
real-time PCR
- SMC
smooth muscle cell
- SPH
secondary pulmonary hypertension
- TAPAS
tryptophan anchoring phosphatidic acid selective-binding domain
- UCR
upstream conserved region
- UHRF1
ubiquitin-like, containing PHD and RING finger domains 1
Conflict of interest
There is no conflict of interest on behalf both authors.
References
- Abusnina A, Alhosin M, Keravis T, Muller CD, Fuhrmann G, Bronner C, et al. Down-regulation of cyclic nucleotide phosphodiesterase PDE1A is the key event of p73 and UHRF1 deregulation in thymoquinone-induced acute lymphoblastic leukemia cell apoptosis. Cell Signal. 2011;23:152–160. doi: 10.1016/j.cellsig.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Ahmad F, Lindh R, Tang Y, Ruishalme I, Ost A, Sahachartsiri B, et al. Differential regulation of adipocyte PDE3B in distinct membrane compartments by insulin and the beta3-adrenergic receptor agonist CL316243: effects of caveolin-1 knockdown on formation/maintenance of macromolecular signalling complexes. Biochem J. 2009;424:399–410. doi: 10.1042/BJ20090842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn HS, Foster M, Foster C, Sybertz E, Wells JN. Effects of selective inhibitors on cyclic nucleotide phosphodiesterases of rabbit aorta. Biochem Pharmacol. 1989;38:3331–3339. doi: 10.1016/0006-2952(89)90631-x. [DOI] [PubMed] [Google Scholar]
- Ahn HS, Bercovici A, Boykow G, Bronnenkant A, Chackalamannil S, Chow J, et al. Potent tetracyclic guanine inhibitors of PDE1 and PDE5 cyclic guanosine monophosphate phosphodiesterases with oral antihypertensive activity. J Med Chem. 1997;40:2196–2210. doi: 10.1021/jm9608467. [DOI] [PubMed] [Google Scholar]
- Alhosin M, Abusnina A, Achour M, Sharif T, Muller C, Peluso J, et al. Induction of apoptosis by thymoquinone in lymphoblastic leukemia Jurkat cells is mediated by a p73-dependent pathway which targets the epigenetic integrator UHRF1. Biochem Pharmacol. 2010;79:1251–1260. doi: 10.1016/j.bcp.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Amer MS. Cyclic nucleotides as targets for drug design. Adv Drug Res. 1977;12:1–38. doi: 10.1016/b978-0-12-013312-3.50004-2. [DOI] [PubMed] [Google Scholar]
- Anant JS, Ong OC, Xie HY, Clarke S, O'Brien PJ, Fung BK. In vivo differential prenylation of retinal cyclic GMP phosphodiesterase catalytic subunits. J Biol Chem. 1992;267:687–690. [PubMed] [Google Scholar]
- Arozarena I, Sanchez-Laorden B, Packer L, Hidalgo-Carcedo C, Hayward R, Viros A, et al. Oncogenic BRAF induces melanoma cell invasion by downregulating the cGMP-specific phosphodiesterase PDE5A. Cancer Cell. 2011;19:45–57. doi: 10.1016/j.ccr.2010.10.029. [DOI] [PubMed] [Google Scholar]
- Artemyev NO, Surendran R, Lee JC, Hamm HE. Subunit structure of rod cGMP-phosphodiesterase. J Biol Chem. 1996;271:25382–25388. doi: 10.1074/jbc.271.41.25382. [DOI] [PubMed] [Google Scholar]
- Aspiotis R, Deschênes D, Dubé D, Girard Y, Huang Z, Laliberté F, et al. The discovery and synthesis of highly potent subtype selective phosphodiesterase 4D inhibitors. Bioorg Med Chem Lett. 2010;20:5502–5505. doi: 10.1016/j.bmcl.2010.07.076. [DOI] [PubMed] [Google Scholar]
- Assad-Kottner C, Chen D, Jahanyar J, Cordova F, Summers N, Loebe M, et al. The use of continuous milrinone therapy as bridge to transplant is safe in patients with short waiting times. J Card Fail. 2008;14:839–843. doi: 10.1016/j.cardfail.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Auerbach R, Kubai L, Knighton D, Folkman J. A simple procedure for the long-term cultivation of chicken embryos. Dev Biol. 1974;41:391–394. doi: 10.1016/0012-1606(74)90316-9. [DOI] [PubMed] [Google Scholar]
- Baillie GS, MacKenzie SJ, McPhee I, Houslay MD. Sub-family selective actions in the ability of Erk2 MAP kinase to phosphorylate and regulate the activity of PDE4 cyclic AMP-specific phosphodiesterases. Br J Pharmacol. 2000;131:811–819. doi: 10.1038/sj.bjp.0703636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillie GS, Huston E, Scotland G, Hodgkin M, Gall I, Peden AH, et al. TAPAS-1, a novel microdomain within the unique N-terminal region of the PDE4A1 cAMP-specific phosphodiesterase that allows rapid, Ca2+-triggered membrane association with selectivity for interaction with phosphatidic acid. J Biol Chem. 2002;277:28298–28309. doi: 10.1074/jbc.M108353200. [DOI] [PubMed] [Google Scholar]
- Banner KH, Press NJ. Dual PDE3/4 inhibitors as therapeutic agents for chronic obstructive pulmonary disease. Br J Pharmacol. 2009;157:892–906. doi: 10.1111/j.1476-5381.2009.00170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber R, Baillie GS, Bergmann R, Shepherd MC, Sepper R, Houslay MD, et al. Differential expression of PDE4 cAMP phosphodiesterase isoforms in inflammatory cells of smokers with COPD, smokers without COPD, and nonsmokers. Am J Physiol Lung Cell Mol Physiol. 2004;287:L332–L343. doi: 10.1152/ajplung.00384.2003. [DOI] [PubMed] [Google Scholar]
- Bates MG, Thompson AA, Baillie JK. Phosphodiesterase type 5 inhibitors in the treatment and prevention of high altitude pulmonary edema. Curr Opin Investig Drugs. 2007;8:226–231. [PubMed] [Google Scholar]
- Beard MB, Olsen AE, Jones RE, Erdogan S, Houslay MD, Bolger GB. UCR1 and UCR2 domains unique to the cAMP-specific phosphodiesterase family form a discrete module via electrostatic interactions. J Biol Chem. 2000;275:10349–10358. doi: 10.1074/jbc.275.14.10349. [DOI] [PubMed] [Google Scholar]
- Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- Beavo JA, Brunton LL. Cyclic nucleotide research – still expanding after half a century. Nat Rev Mol Cell Biol. 2002;3:710–718. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- Beavo JA, Francis SH, Houslay MD, editors. Cyclic Nucleotide Phosphodiesterases in Health and Disease. Boca Raton, USA: CRC Press; 2006. [Google Scholar]
- Bender AT. Calmodulin-stimulated cyclic nucleotide phosphodiesterases. In: Beavo JA, Francis SH, Houslay MD, editors. Cyclic Nucleotide Phosphodiesterases in Health and Disease. Boca Raton: CRC Press; 2006. pp. 35–54. [Google Scholar]
- Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- Berger K, Lindh R, Wierup N, Zmuda-Trzebiatowska E, Lindqvist A, Manganiello VC, et al. Phosphodiesterase 3B is localized in caveolae and smooth ER in mouse hepatocytes and is important in the regulation of glucose and lipid metabolism. PLoS ONE. 2009;4:e4671. doi: 10.1371/journal.pone.0004671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biel M, Michalakis S. Cyclic nucleotide-gated channels. Handb Exp Pharmacol. 2009;191:111–136. doi: 10.1007/978-3-540-68964-5_7. [DOI] [PubMed] [Google Scholar]
- Boess FG, Hendrix M, van der Staay FJ, Erb C, Schreiber R, van Staveren W, et al. Inhibition of phosphodiesterase 2 increases neuronal cGMP, synaptic plasticity and memory performance. Neuropharmacology. 2004;47:1081–1092. doi: 10.1016/j.neuropharm.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Boichot E, Wallace JL, Germain N, Corbel M, Lugnier C, Lagente V, et al. Anti-inflammatory activities of a new series of selective phosphodiesterase 4 inhibitors derived from 9-benzyladenine. J Pharmacol Exp Ther. 2000;292:647–653. [PubMed] [Google Scholar]
- Bolger GB. Phosphodiesterase isoforms – an annoted list. In: Beavo JA, Francis SH, Houslay MD, editors. Cyclic Nucleotide Phosphodiesterases in Health and Disease. Boca Raton: CRC Press; 2006. pp. 19–31. [Google Scholar]
- Bolger G, Michaeli T, Martins T, St John T, Steiner B, Rodgers L, et al. A family of human phosphodiesterases homologous to the dunce learning and memory gene product of Drosophila melanogaster are potential targets for antidepressant drugs. Mol Cell Biol. 1993;13:6558–6571. doi: 10.1128/mcb.13.10.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisy FF, Ronnett GV, Cunningham AM, Juilfs D, Beavo J, Synder SH. Calcium/calmodulin-activated phosphodiesterase expressed in olfactory receptor neurons. J Neurosci. 1992;12:915–923. doi: 10.1523/JNEUROSCI.12-03-00915.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland G, Smith BO, Yarwood SJ. EPAC proteins transduce diverse cellular actions of cAMP. Br J Pharmacol. 2009;158:70–86. doi: 10.1111/j.1476-5381.2008.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell-Smith V, Spina D, Page CP. Phosphodiesterase inhibitors. Br J Pharmacol. 2006;147:S252–S257. doi: 10.1038/sj.bjp.0706495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon JJ, Lugnier C, Abarghaz M, Lagouge Y, Wagner P, Mondadori C, et al. 2004. Cyclic nucleotide phosphodiesterase inhibitors, preparation and uses. Patent WO2004041258, published 21.05.2004. International Application N°: PCT/FR2003/003247 (30.10.2003)
- Burgin AB, Olafur T, Magnusson OT, Singh J, Witte P, Bart L, et al. Design of phosphodiesterase 4D (PDE4D) allosteric modulators for enhancing cognition with improved safety. Nat Biotechnol. 2010;28:63–70. doi: 10.1038/nbt.1598. [DOI] [PubMed] [Google Scholar]
- Campos-Toimil M, Keravis T, Orallo F, Takeda K, Lugnier C. Short-term or long-term treatments with a phosphodiesterase-4 (PDE4) inhibitor result in opposing agonist-induced Ca(2+) responses in endothelial cells. Br J Pharmacol. 2008;154:82–92. doi: 10.1038/bjp.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaño T, Wang H, Campillo NE, Ballester S, González-García C, Hernández J, et al. Synthesis, structural analysis, and biological evaluation of thioxoquinazoline derivatives as phosphodiesterase 7 inhibitors. ChemMedChem. 2009;4:866–876. doi: 10.1002/cmdc.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteel DE, Smith-Nguyen EV, Sankaran B, Roh SH, Pilz RB, Kim C. A crystal structure of the cyclic GMP-dependent protein kinase Iβ dimerization/docking domain reveals molecular details of isoform-specific anchoring. J Biol Chem. 2010;285:32684–32688. doi: 10.1074/jbc.C110.161430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RJ, Abrams K, Garceau NY, Kamath AV, Manley CM, Lilley SC, et al. A new chemical tool for exploring the physiological function of the PDE2 isozyme. Bioorg Med Chem Lett. 2006;16:307–310. doi: 10.1016/j.bmcl.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Cho ES, Yu JH, Kim MS, Yim M. Rolipram, a phosphodiesterase 4 inhibitor, stimulates inducible cAMP early repressor expression in osteoblasts. Yonsei Med J. 2005;46:149–154. doi: 10.3349/ymj.2005.46.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- Cote RH. Photoreceptor phosphodiesterase (PDE6): a G-protein-activated PDE regulating visual excitation in rod and cone photoreceptor cells. In: Beavo JA, Francis SH, Houslay MD, editors. Cyclic Nucleotide Phosphodiesterases in Health and Disease. Boca Raton: CRC Press; 2006. pp. 165–193. [Google Scholar]
- Croom KF, Curran MP. Sildenafil: a review of its use in pulmonary arterial hypertension. Drugs. 2008;68:383–397. doi: 10.2165/00003495-200868030-00009. [DOI] [PubMed] [Google Scholar]
- Cukkemane A, Seifert R, Kaupp UB. Cooperative and uncooperative cyclic-nucleotide-gated ion channels. Trends Biochem Sci. 2011;36:55–64. doi: 10.1016/j.tibs.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Daugan A, Grondin P, Ruault C, Le Monnier de Gouville AC, Coste H, Kirilovsky J, et al. The discovery of tadalafil: a novel and highly selective PDE5 inhibitor. 1: 5,6,11,11a-tetrahydro-1H-imidazo[1′,5′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione analogues. J Med Chem. 2003;46:4525–4532. doi: 10.1021/jm030056e. [DOI] [PubMed] [Google Scholar]
- Day JP, Lindsay B, Riddell T, Jiang Z, Allcock RW, Abraham A, et al. Elucidation of a structural basis for the inhibitor-driven, p62 (SQSTM1)-dependent intracellular redistribution of cAMP phosphodiesterase-4A4 (PDE4A4) J Med Chem. 2011;54:3331–3347. doi: 10.1021/jm200070e. [DOI] [PubMed] [Google Scholar]
- Degerman E, Manganiello V. Phosphodiesterase 3B: an important regulator of energy homeostasis. In: Beavo JA, Francis SH, Houslay MD, editors. Cyclic Nucleotide Phosphodiesterases in Health and Disease. Boca Raton: CRC Press; 2006. pp. 79–97. [Google Scholar]
- Dell'Acqua ML, Scott JD. Protein kinase A anchoring. J Biol Chem. 1997;272:12881–12884. doi: 10.1074/jbc.272.20.12881. [DOI] [PubMed] [Google Scholar]
- Demirbas D, Ceyhan O, Wyman AR, Ivey FD, Allain C, Wang L, et al. Use of a Schizosaccharomyces pombe PKA-repressible reporter to study cGMP metabolising phosphodiesterases. Cell Signal. 2011;23:594–601. doi: 10.1016/j.cellsig.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNinno MP, Andrews M, Bell AS, Chen Y, Eller-Zarbo C, Eshelby N. The discovery of potent, selective and orally bioavailable PDE9 inhibitors as potential hypoglycemic agents. Bioorg Med Chem Lett. 2009;19:2537–2541. doi: 10.1016/j.bmcl.2009.03.024. [DOI] [PubMed] [Google Scholar]
- Dodge KL, Khouangsathiene S, Kapiloff MS, Mouton R, Hill EV, Houslay MD, et al. mAKAP assembles a protein kinase A/PDE4 phosphodiesterase cAMP signaling module. EMBO J. 2001;20:1921–1930. doi: 10.1093/emboj/20.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domek-Łopacińska KU, Strosznajder JB. Cyclic GMP and nitric oxide synthase in aging and Alzheimer's disease. Mol Neurobiol. 2010;41:129–137. doi: 10.1007/s12035-010-8104-x. [DOI] [PubMed] [Google Scholar]
- Duncan GS, Wolberg G, Schmitges CJ, Deeprose RD, Zimmerman TP. Inhibition of lymphocyte-mediated cytolysis and cyclic AMP phosphodiesterase by erythro-9-(2-hydroxy-3-nonyl)adenine. J Immunopharmacol. 1982;4:79–100. doi: 10.3109/08923978209031077. [DOI] [PubMed] [Google Scholar]
- Eckly AE, Lugnier C. role of phosphodiesterase-III and phosphodiesterase-IV in the modulation of vascular cyclic-AMP content by the NO/cyclic-GMP pathway. Br J Pharmacol. 1994;113:445–450. doi: 10.1111/j.1476-5381.1994.tb17009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckly-Michel A, Martin V, Lugnier C. Involvement of cyclic nucleotide-dependent protein kinases in cyclic AMP-mediated vasorelaxation. Br J Pharmacol. 1997;122:158–164. doi: 10.1038/sj.bjp.0701339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favot L, Keravis T, Holl V, Le Bec A, Lugnier C. VEGF-induced HUVEC migration and proliferation are decreased by PDE2 and PDE4 inhibitors. Thromb Haemost. 2003;90:334–343. doi: 10.1160/TH03-02-0084. [DOI] [PubMed] [Google Scholar]
- Favot L, Keravis T, Lugnier C. Modulation of VEGF-induced endothelial cell cycle protein expression through cyclic AMP hydrolysis by PDE2 and PDE4. Thromb Haemost. 2004;92:634–645. doi: 10.1160/TH03-12-0768. [DOI] [PubMed] [Google Scholar]
- Fisher DA, Smith JF, Pillar JS, St. Denis SH, Cheng JB. Isolation and characterization of PDE9A, a novel human cGMP-specific phosphodiesterase. J Biol Chem. 1998;273:15559–15564. doi: 10.1074/jbc.273.25.15559. [DOI] [PubMed] [Google Scholar]
- Forte LR, Thorne PK, Eber SL, Krause WJ, Freeman RH, Francis SH, et al. Stimulation of intestinal Cl-transport by heat-stable enterotoxin: activation of cAMP-dependent protein kinase by cGMP. Am J Physiol. 1992;263:C607–C615. doi: 10.1152/ajpcell.1992.263.3.C607. [DOI] [PubMed] [Google Scholar]
- Francis SH, Corbin JD. Cyclic nucleotide-dependent protein kinases: intracellular receptors for cAMP and cGMP action. Crit Rev Clin Lab Sci. 1999;36:275–328. doi: 10.1080/10408369991239213. [DOI] [PubMed] [Google Scholar]
- Francis SH, Zoraghi R, Kotera J, Ke H, Bessay EP, Blount MA. Phosphodiesterase 5: molecular characteristics relating to structure function and regulation. In: Beavo JA, Francis SH, Houslay MD, et al., editors. Cyclic Nucleotide Phosphodiesterases in Health and Disease. Boca Raton: CRC Press; 2006. pp. 131–164. [Google Scholar]
- Francis SH, Morris GZ, Corbin JD. Molecular mechanisms that could contribute to prolonged effectiveness of PDE5 inhibitors to improve erectile function. Int J Impot Res. 2008;20:333–342. doi: 10.1038/ijir.2008.4. [DOI] [PubMed] [Google Scholar]
- Francis SH, Corbin JD, Bischoff E. Cyclic GMP-hydrolyzing phosphodiesterases. Handb Exp Pharmacol. 2009;191:367–408. doi: 10.1007/978-3-540-68964-5_16. [DOI] [PubMed] [Google Scholar]
- Francis SH, Blount MA, Corbin JD. Mammalian cyclic nucleotide phosphodiesterases: molecular mechanisms and physiological functions. Physiol Rev. 2011;91:651–690. doi: 10.1152/physrev.00030.2010. [DOI] [PubMed] [Google Scholar]
- Fujishige K, Kotera J, Michibata H, Yuasa K, Takebayashi S, Okumura K, et al. Cloning and characterization of a novel human phosphodiesterase that hydrolyzes both cAMP and cGMP (PDE10A) J Biol Chem. 1999;274:18438–18445. doi: 10.1074/jbc.274.26.18438. [DOI] [PubMed] [Google Scholar]
- Fujishige K, Kotera J, Yuasa K, Omori K. The human phosphodiesterase PDE10A gene genomic organization and evolutionary relatedness with other PDEs containing GAF domains. Eur J Biochem. 2000;267:5943–5951. doi: 10.1046/j.1432-1327.2000.01661.x. [DOI] [PubMed] [Google Scholar]
- Gamanuma M, Yuasa K, Sasaki T, Sakurai N, Kotera J, Omori K. Comparison of enzymatic characterization and gene organization of cyclic nucleotide phosphodiesterase 8 family in humans. Cell Signal. 2003;15:565–574. doi: 10.1016/s0898-6568(02)00146-8. [DOI] [PubMed] [Google Scholar]
- Gebska MA, Stevenson BK, Hemnes AR, Bivalacqua TJ, Haile A, Hesketh GG, et al. Phosphodiesterase-5A (PDE5A) is localized to the endothelial caveolae and modulates NOS3 activity. Cardiovasc Res. 2011;90:353–363. doi: 10.1093/cvr/cvq410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy V, Fouque F, Nivet V, Clot JP, Lugnier C, Desbuquois B, et al. Activation of a cGMP-stimulated cAMP phosphodiesterase by protein kinase C in a liver Golgi-endosomal fraction. Eur J Biochem. 1999;259:892–900. doi: 10.1046/j.1432-1327.1999.00123.x. [DOI] [PubMed] [Google Scholar]
- Geoffroy V, Fouque F, Lugnier C, Desbuquois B, Benelli C. Characterization of an in vivo hormonally regulated phosphodiesterase 3 (PDE3) associated with a liver Golgi-endosomal fraction. Arch Biochem Biophys. 2001;387:154–162. doi: 10.1006/abbi.2000.2252. [DOI] [PubMed] [Google Scholar]
- Gesellchen F, Zaccolo M. Phosphodiesterase 2A, cGMP stimulated. UCSD Nat Mol. 2011 DOI: 10.1038/mp.a001750.01. [Google Scholar]
- Ghofrani HA, Wiedemann R, Rose F, Schermuly RT, Olschewski H, Weissmann N, et al. Sildenafil for treatment of lung fibrosis and pulmonary hypertension: a randomised controlled trial. Lancet. 2002;360:895–900. doi: 10.1016/S0140-6736(02)11024-5. [DOI] [PubMed] [Google Scholar]
- Giampà C, Laurenti D, Anzilotti S, Bernardi G, Menniti FS, Fusco FR. Inhibition of the striatal specific phosphodiesterase PDE10A ameliorates striatal and cortical pathology in R6/2 mouse model of Huntington's disease. PLoS ONE. 2010;5:e13417. doi: 10.1371/journal.pone.0013417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi M, Melchiorri G, Nuccetelli V, D'Angelo V, Martorana A, Sorge R, et al. PDE10A and PDE10A-dependent cAMP catabolism are dysregulated oppositely in striatum and nucleus accumbens after lesion of midbrain dopamine neurons in rat: a key step in Parkinsonism physiopathology. Neurobiol Dis. 2011;43:293–303. doi: 10.1016/j.nbd.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Gloerich M, Bos JL. Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol. 2010;50:355–375. doi: 10.1146/annurev.pharmtox.010909.105714. [DOI] [PubMed] [Google Scholar]
- Gonçalves RL, Lugnier C, Keravis T, Lopes MJ, Fantini FA, Schmitt M, et al. The flavonoid dioclein is a selective inhibitor of cyclic nucleotide phosphodiesterase type 1 (PDE1) and a cGMP-dependent protein kinase (PKG) vasorelaxant in human vascular tissue. Eur J Pharmacol. 2009;620:78–83. doi: 10.1016/j.ejphar.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Grandoch M, Roscioni SS, Schmidt M. The role of Epac proteins, novel cAMP mediators, in the regulation of immune, lung and neuronal function. Br J Pharmacol. 2010;159:265–284. doi: 10.1111/j.1476-5381.2009.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grauer SM, Pulito VL, Navarra RL, Kelly MP, Kelley C, Graf R, et al. Phosphodiesterase 10A inhibitor activity in preclinical models of the positive, cognitive, and negative symptoms of schizophrenia. J Pharmacol Exp Ther. 2009;331:574–590. doi: 10.1124/jpet.109.155994. [DOI] [PubMed] [Google Scholar]
- Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo-controlled study. Circ Heart Fail. 2011;4:8–17. doi: 10.1161/CIRCHEARTFAILURE.110.944694. [DOI] [PubMed] [Google Scholar]
- Hanson MS, Stephenson AH, Bowles EA, Sprague RS. Insulin inhibits human erythrocyte cAMP accumulation and ATP release: role of phosphodiesterase 3 and phosphoinositide 3-kinase. Exp Biol Med. 2010;235:256–262. doi: 10.1258/ebm.2009.009206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzelmann A, Morcillo EJ, Lungarella G, Adnot S, Sanjar S, Beume R, et al. The preclinical pharmacology of roflumilast – a selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2010;23:235–256. doi: 10.1016/j.pupt.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Shimada Y, Nishimura Y, Hama T, Tanaka T. Genomic organization, chromosomal localization, and alternative splicing of the human phosphodiesterase 8B gene. Biochem Biophys Res Commun. 2002;297:1253–1258. doi: 10.1016/s0006-291x(02)02371-9. [DOI] [PubMed] [Google Scholar]
- Heikaus CC, Pandit J, Klevit RE. Cyclic nucleotide binding GAF domains from phosphodiesterases: structural and mechanistic insights. Structure. 2009;17:1551–1557. doi: 10.1016/j.str.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka H, Hayashi H, Kohri H, Kimura Y, Hosokawa T, Igawa T, et al. Selective inhibitor of platelet cyclic adenosine mono- phosphate phosphodiesterase, cilostamide, inhibits platelet aggregation. J Pharmacol Exp Ther. 1979;211:26–30. [PubMed] [Google Scholar]
- Houslay MD. Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends Biochem Sci. 2010;35:91–100. doi: 10.1016/j.tibs.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Houslay MD. Hard times for oncogenic BRAF-expressing melanoma cells. Cancer Cell. 2011;19:3–4. doi: 10.1016/j.ccr.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Houslay MD, Adams DR. PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem J. 2003;370:1–18. doi: 10.1042/BJ20021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay MD, Schafer P, Zhang KY. Keynote review: phosphodiesterase-4 as a therapeutic target. Drug Discov Today. 2005;10:1503–1519. doi: 10.1016/S1359-6446(05)03622-6. [DOI] [PubMed] [Google Scholar]
- Huston E, Gall I, Houslay TM, Houslay MD. Helix-1 of the cAMP-specific phosphodiesterase PDE4A1 regulates its phospholipase-D-dependent redistribution in response to release of Ca 2+ J Cell Sci. 2006;119:3799–3810. doi: 10.1242/jcs.03106. [DOI] [PubMed] [Google Scholar]
- Hutson PH, Finger EN, Magliaro BC, Smith SM, Converso A, Sanderson PE, et al. The selective phosphodiesterase 9 (PDE9) inhibitor PF-04447943 (6-[(3S,4S)-4-methyl-1-(pyrimidin-2-ylmethyl)pyrrolidin-3-yl]-1-(tetrahydro-2H-pyran-4-yl)-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one) enhances synaptic plasticity and cognitive function in rodents. Neuropharmacology. 2011;61:665–676. doi: 10.1016/j.neuropharm.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Insel PA, Zhang L, Murray F, Yokouchi H, Zambon AC. Cyclic AMP is both a pro-apoptotic and anti-apoptotic second messenger. Acta Physiol (Oxf) 2011 doi: 10.1111/j.1748-1716.2011.02273.x. DOI: 10.1111/j.1748-1716.2011.02273 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon KI, Xu X, Aizawa T, Lim JH, Jono H, Kwon DS, et al. Vinpocetine inhibits NF-kB-dependent inflammation via an IKK-dependent but PDE-independent mechanism. Proc Natl Acad Sci U S A. 2010;107:9795–9800. doi: 10.1073/pnas.0914414107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoshima-Yamaoka K, Murakawa M, Goto M, Tanaka Y, Inoue H, Murafuji H, et al. ASB16165, a novel inhibitor for phosphodiesterase 7A (PDE7A), suppresses IL-12-induced IFN-gamma production by mouse activated T lymphocytes. Immunol Lett. 2009;122:193–197. doi: 10.1016/j.imlet.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Kakkar R, Raju RV, Sharma RK. Calmodulin-dependent cyclic nucleotide phosphodiesterase (PDE1) Cell Mol Life Sci. 1999;55:1164–1186. doi: 10.1007/s000180050364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameni-Tcheudji JF, Lebeau L, Virmaux N, Maftei CG, Cote RH, Lugnier C, et al. Molecular organization of bovine rod cGMP- phosphodiesterase 6. J Mol Biol. 2001;310:781–791. doi: 10.1006/jmbi.2001.4813. [DOI] [PubMed] [Google Scholar]
- Kass DA, Champion HC, Beavo JA. Phosphodiesterase type 5: expanding roles in cardiovascular regulation. Circ Res. 2007;101:1084–1095. doi: 10.1161/CIRCRESAHA.107.162511. [DOI] [PubMed] [Google Scholar]
- Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiol Rev. 2002;82:769–824. doi: 10.1152/physrev.00008.2002. [DOI] [PubMed] [Google Scholar]
- Kelly MP, Logue SF, Brennan J, Day JP, Lakkaraju S, Jiang L, et al. Phosphodiesterase 11A in brain is enriched in ventral hippocampus and deletion causes psychiatric disease related phenotypes. Proc Natl Acad Sci USA. 2010;107:8457–8462. doi: 10.1073/pnas.1000730107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keravis T, Lugnier C. Cyclic nucleotide phosphodiesterases (PDE) and peptide motifs. Curr Pharm Des. 2010;16:1114–1125. doi: 10.2174/138161210790963760. [DOI] [PubMed] [Google Scholar]
- Keravis T, Silva AP, Favot L, Lugnier C. Role of PDEs in vascular health and disease: endothelial PDEs and angiogenesis. In: Beavo JA, Francis SH, Houslay MD, editors. Cyclic Nucleotide Phosphodiesterases in Health and Disease. Boca Raton: CRC Press; 2006. pp. 417–439. [Google Scholar]
- Keravis T, Vandecasteele G, Mokni W, Fischmeister R, Lugnier C. Identification of PDE1 and PDE5 in adult rat left ventricles cardiomyocytes. J Mol Cell Cardiol. 2007;42:S49–S50. [Google Scholar]
- Kim DH, Lerner A. Type 4 cyclic adenosine monophosphate phosphodiesterase as a therapeutic target in chronic lymphocytic leukemia. Blood. 1998;92:2484–2494. [PubMed] [Google Scholar]
- Komas N, Lugnier C, Le Bec A, Serradeil-Le Gal C, Barthelemy G, Stoclet JC. Differential sensitivity to cardiotonic drugs of cyclic AMP phosphodiesterases isolated from canine ventricular and sinoatrial-enriched tissues. J Cardiovasc Pharmacol. 1989;14:213–220. doi: 10.1097/00005344-198908000-00005. [DOI] [PubMed] [Google Scholar]
- Komas N, Lugnier C, Stoclet JC. Endothelium-dependent and independent relaxation of the rat aorta by cyclic-nucleotide phosphodiesterase inhibitors. Br J Pharmacol. 1991;104:495–503. doi: 10.1111/j.1476-5381.1991.tb12457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Francis GS, Tang WH. Phosphodiesterase 5 inhibition in heart failure: mechanisms and clinical implications. Nat Rev Cardiol. 2009;6:349–355. doi: 10.1038/nrcardio.2009.32. [DOI] [PubMed] [Google Scholar]
- Lamas M, Sassone-Corsi P. The dynamics of the transcriptional response to cyclic adenosine 3,5-monophosphate: recurrent inducibility and refractory phase. Mol Endocrinol. 1997;11:1415–1424. doi: 10.1210/mend.11.10.9988. [DOI] [PubMed] [Google Scholar]
- Lanfear DE, Hasan R, Gupta RC, Williams C, Czerska B, Tita C, et al. Short term effects of milrinone on biomarkers of necrosis, apoptosis, and inflammation in patients with severe heart failure. J Transl Med. 2009;7:67. doi: 10.1186/1479-5876-7-67. DOI: 10.1186/1479-5876-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Wolda S, Moon E, Esselstyn J, Hertel C, Lerner A. PDE7A is expressed in human B-lymphocytes and is up-regulated by elevation of intracellular cAMP. Cell Signal. 2002;14:277–284. doi: 10.1016/s0898-6568(01)00250-9. [DOI] [PubMed] [Google Scholar]
- Lerner A, Kim DH, Lee R. The cAMP signaling pathway as a therapeutic target in lymphoid malignancies. Leuk Lymphoma. 2000;37:39–51. doi: 10.3109/10428190009057627. [DOI] [PubMed] [Google Scholar]
- Libé R, Horvath A, Vezzosi D, Fratticci A, Coste J, Perlemoine K, et al. Frequent phosphodiesterase 11A gene (PDE11A) defects in patients with Carney complex (CNC) caused by PRKAR1A mutations: PDE11A may contribute to adrenal and testicular tumors in CNC as a modifier of the phenotype. J Clin Endocrinol Metab. 2011;96:E208–E214. doi: 10.1210/jc.2010-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Xu X, Hu X, Lee S, Traverse JH, Zhu G, et al. Oxidative stress regulates left ventricular PDE5 expression in the failing heart. Circulation. 2010;121:1474–1483. doi: 10.1161/CIRCULATIONAHA.109.906818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugnier C, editor. Phosphodiesterase Methods and Protocols, Methods in Molecular Biology 307. Totowa: Humana Press; 2005. [Google Scholar]
- Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther. 2006;109:366–398. doi: 10.1016/j.pharmthera.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Lugnier C, Schini VB. Characterization of cyclic nucleotide phosphodiesterases from cultured bovine aortic endothelial cells. Biochem Pharmacol. 1990;39:75–84. doi: 10.1016/0006-2952(90)90650-a. [DOI] [PubMed] [Google Scholar]
- Lugnier C, Stierlé A, Beretz A, Schoeffter P, Le Bec A, Wermuth CG, et al. Tissue and substrate specificity of inhibition by alkoxy-aryl-lactams of platelet and arterial smooth muscle cyclic nucleotide phosphodiesterases: relationship to pharmacological activity. Biochem Biophys Res Commun. 1983;113:954–959. doi: 10.1016/0006-291x(83)91091-4. [DOI] [PubMed] [Google Scholar]
- Lugnier C, Follénius A, Gérard D, Stoclet JC. Bepridil and flunarizine as calmodulin inhibitors. Eur J Pharmacol. 1984;98:157–158. doi: 10.1016/0014-2999(84)90128-6. [DOI] [PubMed] [Google Scholar]
- Lugnier C, Schoeffter P, Le Bec A, Strouthou E, Stoclet JC. Selective inhibition of cyclic nucleotide phosphodiesterases of human, bovine and rat aorta. Biochem Pharmacol. 1986;35:1743–1751. doi: 10.1016/0006-2952(86)90333-3. [DOI] [PubMed] [Google Scholar]
- Lugnier C, Muller B, Le Bec A, Beaudry C, Rousseau E. Characterization of indolidan- and rolipram-sensitive cyclic nucleotide phosphodiesterases in canine and human cardiac microsomal fractions. J Pharmacol Exp Ther. 1993;265:1142–1151. [PubMed] [Google Scholar]
- Lugnier C, Keravis T, Le Bec A, Pauvert O, Proteau S, Rousseau E. Characterization of cyclic nucleotide phosphodiesterase isoforms associated to isolated cardiac nuclei. Biochim Biophys Acta. 1999;1472:431–446. doi: 10.1016/s0304-4165(99)00145-2. [DOI] [PubMed] [Google Scholar]
- Makhlouf A, Kshirsagar A, Niederberger C. Phosphodiesterase 11: a brief review of structure, expression and function. Int J Impot Res. 2006;18:501–509. doi: 10.1038/sj.ijir.3901441. [DOI] [PubMed] [Google Scholar]
- Manneville JB, Jehanno M, Etienne-Manneville S. Dlg1 binds GKAP to control dynein association with microtubules, centrosome positioning, and cell polarity. J Cell Biol. 2010;191:585–598. doi: 10.1083/jcb.201002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning CD, Burman M, Christensen SB, Cieslinski LB, Essayan DM, Grous M, et al. Suppression of human inflammatory cell function by subtype-selective PDE4 inhibitors correlates with inhibition of PDE4A and PDE4B. Br J Pharmacol. 1999;128:1393–1398. doi: 10.1038/sj.bjp.0702911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marivet MC, Bourguignon JJ, Lugnier C, Mann A, Stoclet JC, Wermuth CG. Inhibition of cyclic adenosine-3′,5′-monophosphate phosphodiesterase from vascular smooth muscle by rolipram analogues. J Med Chem. 1989;32:1450–1457. doi: 10.1021/jm00127a009. [DOI] [PubMed] [Google Scholar]
- Martinez SE. PDE2 structure and functions. In: Beavo JA, Francis SH, Houslay MD, editors. Cyclic Nucleotide Phosphodiesterases in Health and Disease. Boca Raton: CRC Press; 2006. pp. 55–77. [Google Scholar]
- Martinez SE, Wu AY, Glavas NA, Tang XB, Turley S, Hol WG, et al. The two GAF domains in phosphodiesterase 2A have distinct roles in dimerization and in cGMP binding. Proc Natl Acad Sci USA. 2002;99:13260–13265. doi: 10.1073/pnas.192374899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masood A, Huang Y, Hajjhussein H, Xiao L, Li H, Wang W, et al. Anxiolytic effects of phosphodiesterase-2 inhibitors associated with increased cGMP signaling. J Pharmacol Exp Ther. 2009;331:690–699. doi: 10.1124/jpet.109.156729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matulef K, Zagotta WN. Cyclic nucleotide-gated ion channels. Annu Rev Cell Dev Biol. 2003;19:23–44. doi: 10.1146/annurev.cellbio.19.110701.154854. [DOI] [PubMed] [Google Scholar]
- Maurice DH, Haslam RJ. Molecular basis of the synergistic inhibition of platelet function by nitrovasodilators and activators of adenylate cyclase: inhibition of cyclic AMP breakdown by cyclic GMP. Mol Pharmacol. 1990;37:671–681. [PubMed] [Google Scholar]
- McCahill A, McSorley T, Huston E, Hill EV, Lynch MJ, Gall I, et al. In resting COS1 cells a dominant negative approach shows that specific, anchored PDE4 cAMP phosphodiesterase isoforms gate the activation, by basal cyclic AMP production, of AKAP-tethered protein kinase A type II located in the centrosomal region. Cell Signal. 2005;17:1158–1173. doi: 10.1016/j.cellsig.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Mentz F, Merle-Beral H, Ouaaz F, Binet JL. Theophylline, a new inducer of apoptosis in B-CLL: role of cyclic nucleotides. Br J Haematol. 1995;90:957–959. doi: 10.1111/j.1365-2141.1995.tb05225.x. [DOI] [PubMed] [Google Scholar]
- Meyers JA, Su DW, Lerner A. Chronic lymphocytic leukemia and B and T cells differ in their response to cyclic nucleotide phosphodiesterase inhibitors. J Immunol. 2009;182:5400–5411. doi: 10.4049/jimmunol.0804255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaeli T. PDE7. In: Beavo JA, Francis SH, Houslay MD, editors. Cyclic Nucleotide Phosphodiesterases in Health and Disease. Boca Raton: CRC Press; 2006. pp. 195–203. [Google Scholar]
- Michelakis E, Tymchak W, Lien D, Webster L, Hashimoto K, Archer S. Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension: comparison with inhaled nitric oxide. Circulation. 2002;105:2398–2403. doi: 10.1161/01.cir.0000016641.12984.dc. [DOI] [PubMed] [Google Scholar]
- Miki N, Baraban JM, Keirns JJ, Boyce JJ, Bitensky MW. Purification and properties of the light-activated cyclic nucleotide phosphodiesterase of rod outer segments. J Biol Chem. 1975;250:6320–6327. [PubMed] [Google Scholar]
- Miller CL, Oikawa M, Cai Y, Wojtovich AP, Nagel DJ, Xu X, et al. Role of Ca2+/calmodulin-stimulated cyclic nucleotide phosphodiesterase 1 in mediating cardiomyocyte hypertrophy. Circ Res. 2009;105:956–964. doi: 10.1161/CIRCRESAHA.109.198515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokni W, Keravis T, Etienne-Selloum N, Walter A, Kane MO, Schini-Kerth VB, et al. Concerted regulation of cGMP and cAMP phosphodiesterases in early cardiac hypertrophy induced by angiotensin II. PLoS ONE. 2010;5:e14227. doi: 10.1371/journal.pone.0014227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorthy BS, Gao Y, Anand GS. Phosphodiesterases catalyze hydrolysis of cAMP bound to regulatory subunit of protein kinase A and mediate signal termination. Mol Cell Proteomics. 2011;10:M110.002295. doi: 10.1074/mcp.M110.002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin F, Lugnier C, Kameni J, Voisin P. Expression and role of phosphodiesterase 6 in the chicken pineal gland. J Neurochem. 2001;78:88–99. doi: 10.1046/j.1471-4159.2001.00407.x. [DOI] [PubMed] [Google Scholar]
- Movsesian MA, Kukreja RC. Phosphodiesterase inhibition in heart failure. Handb Exp Pharmacol. 2011;204:237–249. doi: 10.1007/978-3-642-17969-3_10. [DOI] [PubMed] [Google Scholar]
- Movsesian MA, Smith CJ. Role of cyclic nucleotides phosphodiesterases in heart failure and hypertension. In: Beavo JA, Francis SH, Houslay MD, editors. Cyclic Nucleotide Phosphodiesterases in Health and Disease. Boca Raton: CRC Press; 2006. pp. 485–500. [Google Scholar]
- Murray F, MacLean MR, Pyne NJ. An assessment of the role of the inhibitory gamma subunit of the retinal cyclic GMP phosphodiesterase and its effect on the p42/p44 mitogen-activated protein kinase pathway in animal and cellular models of pulmonary hypertension. Br J Pharmacol. 2003;138:1313–1319. doi: 10.1038/sj.bjp.0705190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray F, Patel HH, Suda RY, Zhang S, Thistlethwaite PA, Yuan JX, et al. Expression and activity of cAMP phosphodiesterase isoforms in pulmonary artery smooth muscle cells from patients with pulmonary hypertension: role for PDE1. Am J Physiol Lung Cell Mol Physiol. 2007;292:L294–L303. doi: 10.1152/ajplung.00190.2006. [DOI] [PubMed] [Google Scholar]
- Nagel DJ, Aizawa T, Jeon KI, Liu W, Mohan A, Wei H, et al. Role of nuclear Ca2+/calmodulin-stimulated phosphodiesterase 1A in vascular smooth muscle cell growth and survival. Circ Res. 2006;98:777–784. doi: 10.1161/01.RES.0000215576.27615.fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netherton SJ, Maurice DH. Vascular endothelial cell cyclic nucleotide phosphodiesterases and regulated cell migration: implications in angiogenesis. Mol Pharmacol. 2005;67:263–272. doi: 10.1124/mol.104.004853. [DOI] [PubMed] [Google Scholar]
- Nikolova S, Guenther A, Savai R, Weissmann N, Ghofrani HA, Konigshoff M, et al. Phosphodiesterase 6 subunits are expressed and altered in idiopathic pulmonary fibrosis. Respir Res. 2010;11:146. doi: 10.1186/1465-9921-11-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson R, Ahmad F, Swärd K, Andersson U, Weston M, Manganiello V, et al. Plasma membrane cyclic nucleotide phosphodiesterase 3B (PDE3B) is associated with caveolae in primary adipocytes. Cell Signal. 2006;18:1713–1721. doi: 10.1016/j.cellsig.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Noguera MA, Ivorra MD, Lugnier C, D'Ocon P. Role of cyclic nucleotide phosphodiesterase isoenzymes in contractile responses of denuded rat aorta related to various Ca2+ sources. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:612–619. doi: 10.1007/s002100100397. [DOI] [PubMed] [Google Scholar]
- Omori K, Kotera J. PDE11. In: Beavo JA, Francis SH, Houslay MD, editors. Cyclic Nucleotide Phosphodiesterases in Health Disease. Boca Raton: CRC Press; 2006. pp. 255–274. [Google Scholar]
- Omori K, Kotera J. Overview of PDEs and their regulation. Circ Res. 2007;100:309–327. doi: 10.1161/01.RES.0000256354.95791.f1. [DOI] [PubMed] [Google Scholar]
- Pagès L, Gavaldà A, Lehner MD. PDE4 inhibitors: a review of current developments (2005-2009) Expert Opin Ther Pat. 2009;19:1501–1519. doi: 10.1517/13543770903313753. [DOI] [PubMed] [Google Scholar]
- Palmer D, Jimmo SL, Raymond DR, Wilson LS, Carter RL, Maurice DH. Protein kinase A phosphorylation of human phosphodiesterase 3B promotes 14-3-3 protein binding and inhibits phosphatase-catalyzed inactivation. J Biol Chem. 2007;282:9411–9419. doi: 10.1074/jbc.M606936200. [DOI] [PubMed] [Google Scholar]
- Pande RL, Hiatt WR, Zhang P, Hittel N, Creager MA, McDermott M. A pooled analysis of the durability and predictors of treatment response of cilostazol in patients with intermittent claudication. Vasc Med. 2010;15:181–188. doi: 10.1177/1358863X10361545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang DC. Cyclic AMP and cyclic GMP phosphodiesterases: target for drug development. Drug Dev Res. 1988;12:85–92. [Google Scholar]
- Patrucco E, Albergine MS, Santana LF, Beavo JA. Phosphodiesterase 8A (PDE8A) regulates excitation-contraction coupling in ventricular myocytes. J Mol Cell Cardiol. 2010;49:330–333. doi: 10.1016/j.yjmcc.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perino A, Ghigo A, Ferrero E, Morello F, Santulli G, Baillie GS, et al. Integrating cardiac PIP(3) and cAMP signaling through a PKA anchoring function of p110γ. Mol Cell. 2011;42:84–95. doi: 10.1016/j.molcel.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podzuweit T, Nennstiel P, Müller A. Isozyme selective inhibition of cGMP-stimulated cyclic nucleotide phosphodiesterases by erythro-9-(2-hydroxy-3-nonyl) adenine. Cell Signal. 1995;7:733–738. doi: 10.1016/0898-6568(95)00042-n. [DOI] [PubMed] [Google Scholar]
- Pozuelo Rubio M, Campbell DG, Morrice NA, Mackintosh C. Phosphodiesterase 3A binds to 14-3-3 proteins in response to PMA-induced phosphorylation of Ser428. Biochem J. 2005;392:163–172. doi: 10.1042/BJ20051103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzzo D, Sapienza S, Arancio O, Palmeri A. Role of phosphodiesterase 5 in synaptic plasticity and memory. Neuropsychiatr Dis Treat. 2008;4:371–387. doi: 10.2147/ndt.s2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampersad SN, Ovens JD, Huston E, Umana MB, Wilson LS, Netherton SJ, et al. Cyclic AMP phosphodiesterase 4D (PDE4D) Tethers EPAC1 in a vascular endothelial cadherin (VE-Cad)-based signaling complex and controls cAMP-mediated vascular permeability. J Biol Chem. 2010;285:33614–33622. doi: 10.1074/jbc.M110.140004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranpura V, Hapani S, Wu S. Treatment-related mortality with bevacizumab in cancer patients: a meta-analysis. JAMA. 2011;305:487–494. doi: 10.1001/jama.2011.51. [DOI] [PubMed] [Google Scholar]
- Reneerkens OA, Rutten K, Steinbusch HW, Blokland A, Prickaerts J. Selective phosphodiesterase inhibitors: a promising target for cognition enhancement. Psychopharmacology. 2009;202:419–443. doi: 10.1007/s00213-008-1273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentero C, Monfort A, Puigdomènech P. Identification and distribution of different mRNA variants produced by differential splicing in the human phosphodiesterase 9A gene. Biochem Biophys Res Commun. 2003;301:686–692. doi: 10.1016/s0006-291x(03)00021-4. [DOI] [PubMed] [Google Scholar]
- Rentsendorj O, Damarla M, Aggarwal NR, Choi JY, Johnston L, D'Alessio FR, et al. Knockdown of lung phosphodiesterase 2A attenuates alveolar inflammation and protein leak in a two-hit mouse model of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2011;301:L161–L170. doi: 10.1152/ajplung.00073.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter W, Conti M. The oligomerization state determines regulatory properties and inhibitor sensitivity of type 4 cAMP-specific phosphodiesterases. J Biol Chem. 2004;279:30338–30348. doi: 10.1074/jbc.M312687200. [DOI] [PubMed] [Google Scholar]
- Ridge KD, Abdulaev NG, Sousa M, Palczewski K. Phototransduction: crystal clear. Trends Biochem Sci. 2003;28:479–487. doi: 10.1016/S0968-0004(03)00172-5. [DOI] [PubMed] [Google Scholar]
- de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, et al. Epac is a Rap1 guanine- nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- Rybalkin SD, Rybalkina I, Beavo JA, Bornfeldt KE. Cyclic nucleotide phosphodiesterase 1C promotes human arterial smooth muscle cell proliferation. Circ Res. 2002;90:151–157. doi: 10.1161/hh0202.104108. [DOI] [PubMed] [Google Scholar]
- Saenz de Tejada I, Argulo Frutos J, Gadau M, Florio V. Comparative selectivity profiles of tadalafil, sildenafil and vardenafil using an in vitro phosphodiesterase activity assay. Int J Impot Res. 2002;14:S20. [Google Scholar]
- Sasaki T, Kotera J, Omori K. Transcriptional activation of phosphodiesterase 7B1 by dopamine D1 receptor stimulation through the cyclic AMP/cyclic AMP-dependent protein kinase/ cyclic AMP-response element binding protein pathway in primary striatal neurons. J Neurochem. 2004;89:474–483. doi: 10.1111/j.1471-4159.2004.02354.x. [DOI] [PubMed] [Google Scholar]
- Schaeffer HJ, Schwender CF. Enzyme inhibitors. 26. Bridging hydrophobic and hydrophilic regions on adenosine deaminase with some 9-(2-hydroxy-3-alkyl)adenines. J Med Chem. 1974;17:6–8. doi: 10.1021/jm00247a002. [DOI] [PubMed] [Google Scholar]
- Schermuly RT, Pullamsetti SS, Kwapiszewska G, Dumitrascu R, Tian X, Weissmann N, et al. Phosphodiesterase 1 upregulation in pulmonary arterial hypertension: target for reverse-remodeling therapy. Circulation. 2007;115:2331–2339. doi: 10.1161/CIRCULATIONAHA.106.676809. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Chapin DS, Cianfrogna J, Corman ML, Hajos M, Harms JF, et al. Preclinical characterization of selective phosphodiesterase 10A inhibitors: a new therapeutic approach to the treatment of schizophrenia. J Pharmacol Exp Ther. 2008;325:681–690. doi: 10.1124/jpet.107.132910. [DOI] [PubMed] [Google Scholar]
- Schneider HH, Schmiechen R, Brezinski M, Seidler J. Stereospecific binding of the antidepressant rolipram to brain protein structures. Eur J Pharmacol. 1986;127:105–115. doi: 10.1016/0014-2999(86)90210-4. [DOI] [PubMed] [Google Scholar]
- Schultz JE. Structural and biochemical aspects of tandem GAF domains. Handb Exp Pharmacol. 2009;191:93–109. doi: 10.1007/978-3-540-68964-5_6. [DOI] [PubMed] [Google Scholar]
- Schwabe U, Miyake M, Ohga Y, Daly JW. 4-(3-Cyclopentyloxy-4-methoxyphenyl)-2-pyrrolidone (ZK 62711): a potent inhibitor of adenosine cyclic 3′,5′-monophosphate phosphodiesterases in homogenates and tissue slices from rat brain. Mol Pharmacol. 1976;12:900–910. [PubMed] [Google Scholar]
- Scotland G, Houslay MD. Chimeric constructs show that the unique N-terminal domain of the cyclic AMP phosphodiesterase RD1 (RNPDE4A1A; rPDE-IVA1) can confer membrane association upon the normally cytosolic protein chloramphenicol acetyltransferase. Biochem J. 1995;308:673–681. doi: 10.1042/bj3080673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seybold J, Thomas D, Witzenrath M, Boral S, Hocke AC, Bürger A, et al. Tumor necrosis factor-alpha-dependent expression of phosphodiesterase 2: role in endothelial hyperpermeability. Blood. 2005;105:3569–3576. doi: 10.1182/blood-2004-07-2729. [DOI] [PubMed] [Google Scholar]
- Sharma RK, Das SB, Lakshmikuttyamma A, Selvakumar P, Shrivastav A. Regulation of calmodulin-stimulated cyclic nucleotide phosphodiesterase (PDE1): review. Int J Mol Med. 2006;18:95–105. [PubMed] [Google Scholar]
- Sin YY, Edwards HV, Li X, Day JP, Christian F, Dunlop AJ, et al. Disruption of the cyclic AMP phosphodiesterase-4 (PDE4)-HSP20 complex attenuates the β-agonist induced hypertrophic response in cardiac myocytes. J Mol Cell Cardiol. 2011;50:872–883. doi: 10.1016/j.yjmcc.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, Chapin DS, Harms JF, Lebel LA, McCarthy SA, Chambers L, et al. Inhibition of the striatum-enriched phosphodiesterase PDE10A: a novel approach to the treatment of psychosis. Neuropharmacology. 2006;51:386–396. doi: 10.1016/j.neuropharm.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Skroblin P, Grossmann S, Schäfer G, Rosenthal W, Klussmann E. Mechanisms of protein kinase A anchoring. Int Rev. Cell Mol Biol. 2010;283:235–330. doi: 10.1016/S1937-6448(10)83005-9. [DOI] [PubMed] [Google Scholar]
- Smith SJ, Cieslinski LB, Newton R, Donnelly LE, Fenwick PS, Nicholson AG, et al. Discovery of BRL 50481 [3-(N,N-dimethylsulfonamido)-4-methyl-nitrobenzene], a selective inhibitor of phosphodiesterase 7: in vitro studies in human monocytes, lung macrophages, and CD8+ T-lymphocytes. Mol Pharmacol. 2004;66:1679–1689. doi: 10.1124/mol.104.002246. [DOI] [PubMed] [Google Scholar]
- Snyder PB, Esselstyn JM, Loughney K, Wolda SL, Florio VA. The role of cyclic nucleotide phosphodiesterases in the regulation of adipocyte lipolysis. J Lipid Res. 2005;46:494–503. doi: 10.1194/jlr.M400362-JLR200. [DOI] [PubMed] [Google Scholar]
- Souness JE, Diocee BK, Martin W, Moodie SA. Pig aortic endothelial-cell cyclic nucleotide phosphodiesterases. Use of phosphodiesterase inhibitors to evaluate their roles in regulating cyclic nucleotide levels in intact cells. Biochem J. 1990;266:127–132. doi: 10.1042/bj2660127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina D. PDE4 inhibitors: current status. Br J Pharmacol. 2008;155:308–315. doi: 10.1038/bjp.2008.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Staay FJ, Rutten K, Bärfacker L, Devry J, Erb C, Heckroth H, et al. The novel selective PDE9 inhibitor BAY 73-6691 improves learning and memory in rodents. Neuropharmacology. 2008;55:908–918. doi: 10.1016/j.neuropharm.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Stoclet JC, Boulanger-Saunier C, Lassegue B, Lugnier C. Cyclic nucleotides and calcium regulation in heart and smooth muscle cells. Ann NY Acad Sci. 1988;522:106–115. doi: 10.1111/j.1749-6632.1988.tb33348.x. [DOI] [PubMed] [Google Scholar]
- Strick CA, Schmidt CJ, Menneti FS. PDE10A: a striatum-enriched, dual-substrate phosphodiesterase. In: Beavo JA, Francis SH, Houslay MD, editors. Cyclic Nucleotide Phosphodiesterases in Health and Disease. Boca Raton: CRC Press; 2006. pp. 237–254. [Google Scholar]
- Takimoto E, Champion HC, Belardi D, Moslehi J, Mongillo M, Mergia E, et al. cGMP catabolism by phosphodiesterase 5A regulates cardiac adrenergic stimulation by NOS3-dependent mechanism. Circ Res. 2005;96:100–109. doi: 10.1161/01.RES.0000152262.22968.72. [DOI] [PubMed] [Google Scholar]
- Tate RJ, Arshavsky VY, Pyne NJ. The identification of the inhibitory gamma-subunits of the type 6 retinal cyclic guanosine monophosphate phosphodiesterase in non-retinal tissues: differential processing of mRNA transcripts. Genomics. 2002;79:582–586. doi: 10.1006/geno.2002.6740. [DOI] [PubMed] [Google Scholar]
- Tian X, Vroom C, Ghofrani HA, Weissmann N, Bieniek E, Grimminger F, et al. Phosphodiesterase 10A upregulation contributes to pulmonary vascular remodeling. PLoS ONE. 2011;6:e18136. doi: 10.1371/journal.pone.0018136. DOI: 10.1371/journal.pone.0018136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley HN, Gary BD, Keeton AB, Zhang W, Abadi AH, Reynolds RC, et al. Sulindac sulfide selectively inhibits growth and induces apoptosis of human breast tumor cells by phosphodiesterase 5 inhibition, elevation of cyclic GMP, and activation of protein kinase G. Mol Cancer Ther. 2009;2009:3331–3340. doi: 10.1158/1535-7163.MCT-09-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari S, Dong H, Kim EJ, Weintraub L, Epstein PM, Lerner A. Type 4 cAMP phosphodiesterase (PDE4) inhibitors augment glucocorticoid-mediated apoptosis in B cell chronic lymphocytic leukemia (B-CLL) in the absence of exogenous adenylyl cyclase stimulation. Biochem Pharmacol. 2005;69:473–483. doi: 10.1016/j.bcp.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Tsai EJ, Kass DA. Cyclic GMP signaling in cardiovascular pathophysiology and therapeutics. Pharmacol Ther. 2009;122:216–238. doi: 10.1016/j.pharmthera.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai LC, Shimizu-Albergine M, Beavo JA. The high-affinity cAMP-specific phosphodiesterase 8B controls steroidogenesis in the mouse adrenal gland. Mol Pharmacol. 2011;79:639–648. doi: 10.1124/mol.110.069104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turko IV, Francis SH, Corbin JD. Binding of cGMP to both allosteric sites of cGMP-binding cGMP-specific phosphodiesterase (PDE5) is required for its phosphorylation. Biochem J. 1998;329:505–510. doi: 10.1042/bj3290505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeput F, Wolda SL, Krall J, Hambleton R, Uher L, McCaw KN, et al. Cyclic nucleotide phosphodiesterase PDE1C1 in human cardiac myocytes. J Biol Chem. 2007;282:32749–32757. doi: 10.1074/jbc.M703173200. [DOI] [PubMed] [Google Scholar]
- Vang AG, Ben-Sasson SZ, Dong H, Kream B, DeNinno MP, Claffey MM, et al. PDE8 regulates rapid Teff cell adhesion and proliferation independent of ICER. PLoS ONE. 2010;5:e12011. doi: 10.1371/journal.pone.0012011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasta V. cAMP-phosphodiesterase 8 family. In: Beavo JA, Francis SH, Houslay MD, editors. Cyclic Nucleotide Phosphodiesterases in Health and Disease. Boca Raton: CRC Press; 2006. pp. 205–219. [Google Scholar]
- Vecsey CG, Baillie GS, Jaganath D, Havekes R, Daniels A, Wimmer M, et al. Sleep deprivation impairs cAMP signaling in the hippocampus. Nature. 2009;461:1122–1125. doi: 10.1038/nature08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergne F, Bernardelli P, Lorthiois E, Pham N, Proust E, Oliveira C, et al. Discovery of thiadiazoles as a novel structural class of potent and selective PDE7 inhibitors: Part 2. Metabolism-directed optimization studies towards orally bioavailable derivatives. Bioorg Med Chem Lett. 2004;14:4615–4621. doi: 10.1016/j.bmcl.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Vo NK, Gettemy JM, Coghlan VM. Identification of cGMP-dependent protein kinase anchoring proteins (GKAPs) Biochem Biophys Res Commun. 1998;246:831–835. doi: 10.1006/bbrc.1998.8722. [DOI] [PubMed] [Google Scholar]
- Wallis RM, Corbin JD, Francis SH, Ellis P. Tissue distribution of phosphodiesterase families and the effects of sildenafil on tissue cyclic nucleotides, platelet function, and the contractile responses of trabeculae carneae and aortic rings in vitro. Am J Cardiol. 1999;83:3C–12C. doi: 10.1016/s0002-9149(99)00042-9. [DOI] [PubMed] [Google Scholar]
- Wan KF, Sambi BS, Frame M, Tate R, Pyne NJ. The inhibitory gamma subunit of the type 6 retinal cyclic guanosine monophosphate phosphodiesterase is a novel intermediate regulating p42/p44 mitogen-activated protein kinase signaling in human embryonic kidney 293 cells. J Biol Chem. 2001;276:37802–37808. doi: 10.1074/jbc.M105087200. [DOI] [PubMed] [Google Scholar]
- Wang H, Robinson H, Ke H. Conformation Changes, N-terminal Involvement, and cGMP Signal Relay in the Phosphodiesterase-5 GAF Domain. J Biol Chem. 2010;285:38149–38156. doi: 10.1074/jbc.M110.141614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Wu P, Egan RW, Billah MM. Human phosphodiesterase 8A splice variants: cloning, gene organization, and tissue distribution. Gene. 2001;280:183–194. doi: 10.1016/s0378-1119(01)00783-1. [DOI] [PubMed] [Google Scholar]
- Wang P, Wu P, Egan RW, Billah MM. Identification and characterization of a new human type 9 cGMP-specific phosphodiesterase splice variant (PDE9A5). Differential tissue distribution and subcellular localization of PDE9A variants. Gene. 2003;314:15–27. doi: 10.1016/s0378-1119(03)00733-9. [DOI] [PubMed] [Google Scholar]
- Wechsler J, Choi YH, Krall J, Ahmad F, Manganiello VC, Movsesian MA. Isoforms of cyclic nucleotide phosphodiesterase PDE3A in cardiac myocytes. J Biol Chem. 2002;277:38072–38078. doi: 10.1074/jbc.M203647200. [DOI] [PubMed] [Google Scholar]
- Weeks JL, Zoraghi R, Beasley A, Sekhar KR, Francis SH, Corbin JD. High biochemical selectivity of tadalafil, sildenafil and vardenafil for human phosphodiesterase 5A1 (PDE5) over PDE11A4 suggests the absence of PDE11A4 cross-reaction in patients. Int J Impot Res. 2005;17:5–9. doi: 10.1038/sj.ijir.3901283. [DOI] [PubMed] [Google Scholar]
- Weishaar RE, Kobylarz-Singer DC, Steffen RP, Kaplan HR. Subclasses of cyclic AMP-specific phosphodiesterase in left ventricular muscle and their involvement in regulating myocardial contractility. Circ Res. 1987;61:539–547. doi: 10.1161/01.res.61.4.539. [DOI] [PubMed] [Google Scholar]
- Welch EJ, Jones BW, Scott JD. Networking with AKAPs: context-dependent regulation of anchored enzymes. Mol Interv. 2010;10:86–97. doi: 10.1124/mi.10.2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensel TG. Signal transducing membrane complexes of photoreceptor outer segments. Vision Res. 2008;48:2052–2061. doi: 10.1016/j.visres.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkens H, Guth A, König J, Forestier N, Cremers B, Hennen B, et al. Effect of inhaled iloprost plus oral sildenafil in patients with primary pulmonary hypertension. Circulation. 2001;104:1218–1222. doi: 10.1161/hc3601.096826. [DOI] [PubMed] [Google Scholar]
- Wilson LS, Baillie GS, Pritchard LM, Umana B, Terrin A, Zaccolo M, et al. A phosphodiesterase 3B-based signaling complex integrates exchange protein activated by cAMP and phosphatidylinositol 3-kinase signals in human arterial endothelial cells. J Biol Chem. 2011;286:16285–16296. doi: 10.1074/jbc.M110.217026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nat Rev Mol Cell Biol. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- Wu SN, Li HF, Chiang HT. Vinpocetine-induced stimulation of calcium-activated potassium currents in rat pituitary GH3 cells. Biochem Pharmacol. 2001;61:877–892. doi: 10.1016/s0006-2952(01)00553-6. [DOI] [PubMed] [Google Scholar]
- Wunder F, Tersteegen A, Rebmann A, Erb C, Fahrig T, Hendrix M. Characterization of the first potent and selective PDE9 inhibitor using a cGMP reporter cell line. Mol Pharmacol. 2005;68:1775–1781. doi: 10.1124/mol.105.017608. [DOI] [PubMed] [Google Scholar]
- Wunder F, Gnoth MJ, Geerts A, Barufe D. A novel PDE2A reporter cell line: characterization of the cellular activity of PDE inhibitors. Mol Pharmacol. 2009;6:326–336. doi: 10.1021/mp800127n. [DOI] [PubMed] [Google Scholar]
- Yan C, Zhao AZ, Bentley JK, Beavo JA. The calmodulin-dependent phosphodiesterase gene PDE1C encodes several functionally different splice variants in a tissue-specific manner. J Biol Chem. 1996;271:25699–25706. doi: 10.1074/jbc.271.41.25699. [DOI] [PubMed] [Google Scholar]
- Yuasa K, Kotera J, Fujishige K, Michibata H, Sasaki T, Omori K. Isolation and characterization of two novel phosphodiesterase PDE11A variants showing unique structure and tissue-specific expression. J Biol Chem. 2000;275:31469–31479. doi: 10.1074/jbc.M003041200. [DOI] [PubMed] [Google Scholar]
- Zhang L, Murray F, Zahno A, Kanter JR, Chou D, Suda R, et al. Cyclic nucleotide phosphodiesterase profiling reveals increased expression of phosphodiesterase 7B in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2008;105:19532–19537. doi: 10.1073/pnas.0806152105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmuda-Trzebiatowska E, Oknianska A, Manganiello V, Degerman E. Role of PDE3B in insulin-induced glucose uptake, GLUT-4 translocation and lipogenesis in primary rat adipocytes. Cell Signal. 2006;18:382–390. doi: 10.1016/j.cellsig.2005.05.007. [DOI] [PubMed] [Google Scholar]


