Abstract
Syk is a 72-kDa protein-tyrosine kinase that regulates signaling through multiple cell surface receptors including those for antigens, immunoglobulins and proteins of the extracellular matrix. As part of its function, Syk binds a variety of downstream effectors through interactions that are often mediated by motifs that recognize phosphotyrosines. In a search for novel Syk-interacting proteins by yeast two-hybrid analysis, we identified tensin2 as a Syk-binding protein. Syk interacts with a fragment of tensin2 located near the C-terminus that contains SH2 and PTB domains. In epithelial cells, tensin2 localizes both to focal adhesions and to large cytoplasmic puncta. It is within these punctuate structures that Syk and tensin2 are co-localized. The clustering of Syk within these structures leads to its phosphorylation on tyrosine.
Keywords: Syk, tensin2, tyrosine phosphorylation, signal transduction
1. Introduction
Syk is a 72-kDa nonreceptor, protein-tyrosine kinase best characterized as a modulator of immune cell function through its associations with immune recognition receptors. In a variety of hematopoietic cell types, Syk couples receptors for antigens and immunoglobulins to multiple intracellular signaling pathways activated during host/pathogen interactions [1, 2]. In recent years Syk also has been identified in an increasingly wide variety of nonhematopoietic cells [3]. These include epithelial cells, endothelial cells, hepatocytes, melanocytes, nasal fibroblasts, vascular smooth muscle cells and neuronal cells. Syk has been described as having a number of functions in these cells including the regulation of mitosis, differentiation, cellular adhesion and motility. Syk functions both as a catalyst for the phosphorylation of protein substrates and as a scaffold for promoting protein-protein interactions, often involving proteins with SH2 or other phosphotyrosine interacting motifs that bind to specific phosphotyrosines on Syk [1, 2]. Since these associated proteins and substrates are critical effectors that mediate signaling through Syk-associated receptors, there is considerable interest in their identification and characterization. A few examples of Syk-binding proteins that have been reported and studied are Vav, Cbl, PLC-γ, PI3K, Fgr, TRIP, and USP25 [1, 2, 4].
As part of our analysis of Syk-interacting proteins, we carried out two yeast two-hybrid screens using libraries derived either from human bone marrow or mammary gland [5, 6]. In both screens we identified tensin2 as a Syk-interacting protein. Tensin2 is a member of a family of related cytoskeletal proteins that includes tensin1, tensin2/TENC1, tensin3 and tensin4/CTEN that modulate both cell motility and transformation [7]. Tensin2 was identified previously as a binding partner of a different kinase, the receptor tyrosine kinase Axl [8]. Like other tensin-family members, tensin2 possesses C-terminal Src-homology 2 (SH2) and phosphotyrosine binding [PTB) domains; and it was within this region that Axl binds. These domains also target tensin proteins to focal adhesions by binding to β integrins and mediate their interactions with DLC1 (deleted in liver cancer-1), a tumor suppressor and negative regulator of Rho-family GTPases [9–12]. We find that this region also mediates the interaction of tensin2 with Syk.
2. Materials and methods
2.1. Cells and antibodies
Syk-deficient MCF-7 cells and DT40 cells line stably expressing Myc-tagged Syk were described previously [13,14]. NIH 3T3 cells were obtained from American Type Culture Collection. HMEC-1 cells were obtained from the Centers for Disease Control. The monoclonal antibody against the Myc-epitope (9B11) was from Cell Signaling Technology. Antibodies against GST and Syk (N19) were purchased from Santa Cruz Biotechnology. The antibody against vinculin (hVIN-1) was obtained from Sigma. To generate an antibody against tensin2, the cDNA coding for amino acids 880–980 was PCR-amplified and inserted into the pGEX2T bacterial expression plasmid. The GST-tensin2 fusion protein was expressed in bacteria, purified by affinity chromatography on glutathione-Sepharose and used as an antigen for the generation of rabbit immune serum using a commercial service (Lampire Biological Laboratories).
2.2. Protein-protein interaction assays
Analyses by yeast two-hybrid screens of Syk-interacting proteins from human bone marrow and human mammary gland libraries were as described previously [5,6]. The plasmids pACT2-tensin2(PTB domain), pACT2-tensin2-tensin1, and pACT2-tensin1 were prepared by in-frame insertion of the desired cDNAs by standard PCR and DNA manipulation techniques. A cDNA encoding an HA-tagged C-terminal fragment of tensin2 (amino acids 936–1419)was PCR-amplified and inserted into the pFastBAC plasmid, which was then transformed into DH10bac cells (Gibco-BRL/Invitrogen) for generating the recombinant baculovirus. Baculoviruses for the expression of GST-Syk fusion proteins were described previously [15,16]. GST-fusion proteins were purified from cell lysates by adsorption onto glutathione-Sepharose. The vector for the expression of Syk tagged at the C-terminus with a Myc-epitope in DT40 B cells was as described [14]. HA-tagged proteins were isolated by adsorption onto protein A-Sepharose beads containing a bound HA antibody. Sf9 insect cells expressing GST-Syk fusion proteins or Syk-deficient DT40 B cells expressing Syk-Myc were lysed in buffer containing 20 mM HEPES, pH 7.5, 150 mM NaCl, 5 mM EDTA, 1% NP40, and 10 μg/ml each of aprotinin and leupeptin. Proteins in cell lysates were adsorbed to beads containing immobilized proteins. Bound proteins were separated by SDS-PAGE and detected by Western blotting using enzyme-linked chemiluminescence (ECL) reagents (Amersham Biosciences).
2.3. Fluorescence microscopy
MCF7 cells transiently transfected to express Syk-EGFP, EGFP-tensin2 or Myc-tensin2 were cultured on coverslips, fixed with 3.7% formaldehyde for 5 min, permeabilized with 1% Triton X-100 in PBS, blocked in PBS containing 1 mg/ml BSA, 0.05% Tween 20 and 10% goat serum and stained with antibodies against the Myc epitope or vinculin. Bound primary antibodies were detected using a Texas red-conjugated donkey anti-mouse antibody (Jackson Immunoresearch). Plasmids for the expression of Myc-tensin2 and EGFP-tensin2 were generously supplied by Drs. Sassan Hafizi (Lund University) and Su Hao Lo (University of California at Davis). The plasmid coding for Syk- EGFP was described previously [17].
3. Results
3.1. Syk interacts with tensin2 in a yeast two-hybrid assay
As part of our efforts to characterize the Syk interaction network, we carried out yeast two-hybrid protein interaction screens in search of proteins capable of interacting with Syk [5,6]. We used full-length Syk as bait and as prey, proteins coded by cDNA libraries representing human bone marrow or, in a second screen, human mammary gland. Both interaction screens identified tensin2 as a Syk-interacting protein. In these screens, tensin2 failed to interact with p53, which was used as a negative control.
Positive clones from each library coded for the C-terminus of tensin2. This region contains both an SH2 domain and a PTB domain (Fig. 1A), suggesting that an interaction with Syk might be mediated by phosphotyrosine. Syk, when expressed in yeast as a fusion protein with the GAL4 DNA-binding domain, is known to catalyze an autophosphorylation reaction, allowing it to interact with SH2 domain-containing proteins that bind to phosphotyrosines [5,6,18]. To examine the dependence of the Syk-tensin2 interaction on the status of Syk’s phosphorylation, we probed the interaction again using a catalytically inactive form of Syk (Syk(K396R)) incapable of catalyzing autophosphorylation. Fgr and p85 (the regulatory subunit of phosphoinositide 3-kinase), which have SH2 domains known to interact selectively with phosphorylated Syk (and hence with Syk, but not Syk(K396R)) [5,19], were used as positive controls. Interestingly, the ability of tensin2 to interact with catalytically active versus inactive Syk depended on the length of the C-terminal fragment. The longest version of the tensin2 C-terminus was able to interact with both Syk and Syk(K396R), indicating a mode of interaction independent of Syk’s phosphorylation on tyrosine. Neither Fgr nor p85 bound to the catalytically inactive Syk (Fig. 1B). However, a shorter C-terminal fragment of tensin2 lacking amino acid residues 917–1032, which lie just upstream of the SH2 domain, failed to interact with Syk(K396R), but still bound to catalytically active Syk (Fig. 1C). Thus, the C-terminal SH2 + PTB domains of tensin2 have the ability to bind to Syk, but likely do so only when the kinase is phosphorylated on one or more tyrosines. Removal of the SH2 domain from this construct abolished the interaction, suggesting that it is the tensin2 SH2 domain that interacts with one or more phosphotyrosines on Syk (Fig. 2A). Tensin2 was unable to interact in the two-hybrid screen with either a truncated form of Syk containing only the pair of N-terminal tandem SH2 domains or a construct containing only the catalytic domain (Fig. 1C and 2A).
Fig. 1.
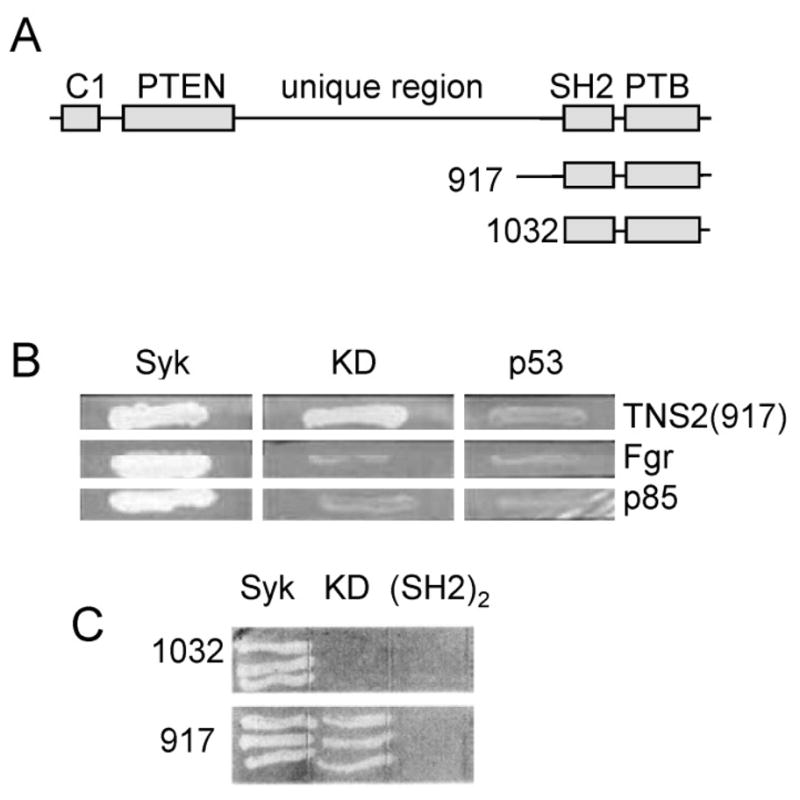
Syk interacts with tensin2 in a yeast two-hybrid screen. (A) Schematic diagram of full-length tensin2 and of the two C-terminal fragments identified in the two-hybrid screen. The number indicates the amino acid residue at the N-terminus of each fragment. (B) Gal4 DNA binding domain-fusion proteins containing Syk, the kinase dead Syk(K396R) mutant (KD), or the p53 protein were examined in a yeast two-hybrid assay for binding to the tensin2 fragment starting at residue 917 (TNS2), full-length Fgr or the C-terminal SH2 domain of p85. Growth of yeast colonies was measured on His and Ade double deficient plates. (C) Gal4 DNA binding domain-fusion proteins containing Syk, the kinase dead mutant (KD), or the tandem SH2 domains ((SH2)2) were examined in a yeast two-hybrid assay for binding to tensin2 fragments starting at residue 917 or 1032. Growth of yeast colonies was measured on His and Ade double deficient plates.
Fig. 2.

Syk interacts with tensin2, but not tensin1. (A) Gal4 DNA binding domain-fusion proteins containing Syk or only the Syk kinase domain (Syk K) were examined in a yeast two-hybrid assay for binding to the tensin2 C-terminal fragment starting at residue 917 (TNS2), the tensin2 PTB domain (PTB) or a tensin1 C-terminal fragment encompassing residues 1340–1736 (TNS1). (B) A Gal4 DNA binding domain-fusion protein containing Syk was examined in a yeast two-hybrid assay for binding to the tensin2 C-terminal fragment starting at residue 917 (TNS2), a tensin1 C-terminal fragment encompassing residues 1340–1736 (TNS1) the tensin1 fragment in which the N-terminal 115 amino acids were replaced by residues 917–1032 from tensin2 (TNS2-1). Growth of yeast colonies was measured on His and Ade double deficient plates.
3.2. Syk binds tensin2, but not tensin1
Tensin2 shares considerable sequence similarity with tensin1 (tensin) in both the N- and C-termini, including the SH2 and PTB domains, while the internal regions of each are unique [7]. To determine if tensin1 shared an ability with tensin2 to interact with Syk, we amplified the cDNA coding for amino acids 1340–1736 of tensin1 (corresponding to the largest fragment of tensin2 identified by the yeast two-hybrid screen) and used this for interaction assays in the yeast two-hybrid system. Interestingly, the tensin1 C-terminus failed to bind to Syk (Fig. 2A). The 115 amino acids located upstream of the tensin2 SH2 domain differs from those found in the tensin1 fragment. Replacement of the unique tensin1 sequence with the corresponding sequence from tensin2 (917–1032) failed to convert the tensin1 C-terminus into a Syk-binding protein (Fig. 2B). Thus, the interaction with Syk appears to be specific for tensin2 as opposed to tensin1.
3.3. Syk interacts with tensin2 in pull-down assays
To obtain further evidence for a direct interaction between tensin2 and Syk, we used biochemical approaches. We first generated a recombinant baculovirus for the expression in insect cells of an HA-tagged form of tensin2 consisting of the C-terminal region determined to bind Syk in the two-hybrid assay. HA-tensin2 was immunoprecipitated from lysates of infected insect cells with an HA antibody bound to protein A-Sepharose. The immobilized HA-tensin2 was then used in a pull-down assay to recover Syk from Syk-deficient chicken DT40 B cells transfected to express a Myc epitope-tagged form of murine Syk. The presence of Syk in the immune complex was detected by an anti-Syk antibody. The immobilized, tagged tensin2 protein was able to recover Myc-Syk from the cell lysate, further confirming the ability of these two proteins to interact (Fig. 3A). The anti-Syk antibody also detected the epitope-tagged protein in an anti-Myc immune complex.
Fig. 3.
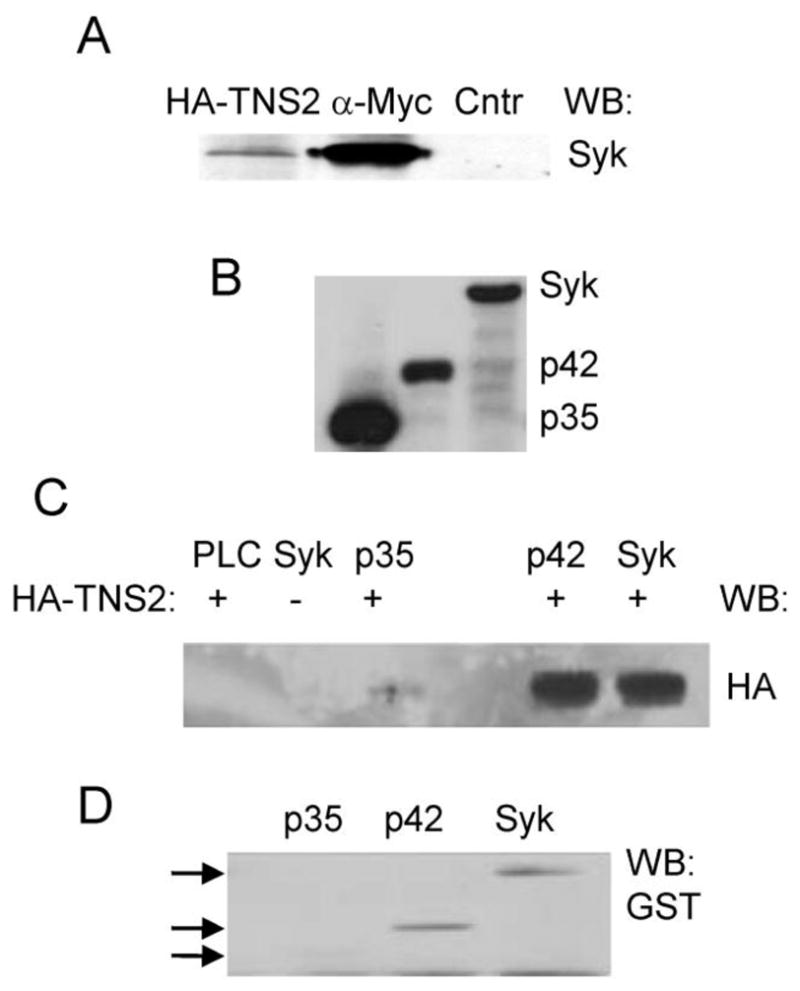
Syk interacts with tensin2 in pull-down assays. (A) Lysates from DT40 cells expressing Myc-tagged Syk were adsorbed to protein A-Sepharose containing either an HA-tagged C-terminal fragment of tensin2 bound to an antibody against HA (HA-TNS2), an antibody against the Myc-epitope (α-Myc) or no bound antibody (Ctrl). The presence of Syk bound to the beads was detected by Western blot (WB) using an antibody against Syk. (B) GST-Syk (Syk), GST-Sykp42.5 (p42), and GST-Sykp35 (p35) fusion proteins were isolated from insect cell lysates by adsorption to glutathione-agarose and detected by Western blotting with antibodies against GST. (C) Lysates from insect cells expressing (+) or not expressing (−) the HA-tensin2 C-terminal fragment were adsorbed to glutathione-agarose containing bound GST-Syk (Syk), GST-Sykp42.5 (p42), GST-Sykp35 or, as a control, GST linked to the SH2 domain of PLC-γ (PLC). Bound protein was detected by Western blot (WB) using an antibody against HA. (D) Purified GST-Syk (Syk), GST-Sykp42.5 (p42) or GST-Sykp35 (p35) were incubated with HA-tensin2 bound to an anti-HA antibody immobilized on protein A-Sepharose. The presence of the fusion protein in each HA-immune complex was detected by Western blotting (WB) using an anti-GST antibody.
In a related experiment, we adsorbed lysates from insect cells infected to express HA-tensin2 to glutathione-agarose beads to which were bound various forms of Syk: GST-Syk (full-length kinase), GST-Sykp42.5 (catalytic domain plus entire linker B region) or GST-Sykp35 (catalytic domain lacking most of linker B). We found that HA-tensin2 interacted with GST-Syk and GST-Sykp42.5, but not GST-Sykp35 (Fig. 3C). In a reciprocal experiment, these same GST fusion proteins were adsorbed to anti-HA immune complexes recovered from insect cells expressing the HA-tensin2 fragment. Bound proteins were recovered, separated by SDS-PAGE and detected by Western blotting with antibodies against GST. Again, GST-Syk and GST-Sykp42.5, but not GST-Sykp35 interacted with HA-tensin2 (Fig. 3D). These results indicate that the interaction of tensin2 with Syk is likely to require the linker B region of Syk including the 100 residues between amino acids 256 and 327.
3.4. Localization of tensin2 in MCF7 cells
To explore further a possible interaction between Syk and tensin2 and to examine where within a cell the two proteins might interact, we first examined the localization of the two proteins by expressing tagged versions of each. We used cultured MCF7 mammary epithelial cells since tensin2 was detected as a Syk-interacting protein using a mammary gland cDNA library and has been reported to be phosphorylated on tyrosine in mammary epithelial cells [20]. For these experiments we used a clone of MCF7 cells described previously that lacks endogenous Syk [13]. When expressed in confluent MCF7 cells, GFP-tensin2 appeared in large, brightly staining bodies within the cytoplasm (Fig. 4A). We found a similar distribution of tensin2 in cells transfected with a Myc-epitope tagged form of the protein and stained with an anti-Myc antibody (Fig. 4A). Thus, it was unlikely that the GFP tag alone had altered the subcellular distribution of the protein. To examine cells for the localization of endogenous tensin2, we generated polyclonal antibodies against a GST fusion protein containing the region of tensin2 between amino acids 880 and 980. As tensin2 is expressed at higher levels in endothelial cells, we fixed and stained HMEC-1 human microvascular endothelial cells with tensin2 antisera (Fig. 4A). Again tensin2 exhibited a punctate distribution throughout the cell. This distribution closely resembles the distribution of tensin2 reported previously in DU145 prostate adenocarcinoma cells [21].
Fig. 4.
Syk and tensin2 co-localize in cytoplasmic punctate bodies. (A) MCF7 cells were transiently transfected with plasmids expressing GFP-tensin2 (GFP-TNS2) or Myc-tensin2 (Myc-TNS2). Cells expressing GFP-TNS2 were fixed and examined by fluorescence microscopy. Cells expressing Myc-TNS2 were fixed, permeabilized and stained with a Myc antibody and a fluorescently tagged secondary antibody. HMC1 cells were fixed, permeabilized and stained with an antibody against tensin2 and a fluorescently tagged secondary antibody. (B) MCF7 cells transiently expressing GFP-tensin2 (GFP-TNS2) were fixed, permeabilized and stained with an antibody against vinculin and a fluorescently tagged secondary antibody. Examples of tensin2 and vinculin co-localized to focal adhesions are illustrated by arrows in the merged image. (C) MCF7 cells transiently expressing GFP-tensin2 were treated without (− nocod) or with (+ nocod) nocodazole, fixed and examined by fluorescence microscopy. The microscope is focused on the basolateral surface of the cells. (D) MCF7 cells were transiently transfected with plasmids coding for Syk-EGFP and Myc-tensin2. Cells were fixed, permeabilized and stained with anti-Myc and a fluorescently tagged secondary antibody. Merged images are shown in the third panel. (E) MCF7 cells expressing Syk-EGFP were examined by fluorescence microscopy. Bars represent 10 μm.
Previous studies on the localization of tensin2 in fibroblasts, however, described the protein as resident in focal adhesions [22,23]. To confirm this result, we transfected NIH 3T3 cells with the expression construct for GFP-tensin2 and examined the cells under a fluorescence microscope. Consistent with previous analyses, a fraction of the GFP-tensin2 was found in a punctate distribution at the cell periphery consistent with localization to focal adhesions (data not shown). To determine if tensin2 also could localize to focal adhesions in mammary epithelial cells, we plated MCF7 cells transfected to express GFP-tensin2 at a low density since confluent cells exhibit few peripheral focal adhesions. Under these growth conditions, a fraction of GFP-tensin2 could indeed be visualized in punctate structures localized at the cell periphery (Fig. 4B). The identification of these structures as focal adhesions was verified by co-staining transfected cells with antibodies against vinculin. Thus, tensin2 also localizes to focal adhesions in epithelial cells. However, much of the expressed GFP-tensin2 in subconfluent MCF7 cells was still present in what appeared to be large clusters in the cytoplasm.
We were concerned that these large clusters might represent insoluble aggregates of overexpressed protein, so we asked if the tensin2 within these bodies retained the ability to localize to focal adhesions. The number of stable focal adhesions can be increased by treating cells with agents that depolymerize microtubules [24,25]. This inhibits the microtubule-induced disassembly of focal adhesion complexes. In MCF7 cells treated with nocodazole, GFP-tensin2 relocalized from the cytoplasmic clusters to these stabilized focal adhesions (Fig. 4C). This result indicates that the tensin2 molecules present in the cytoplasmic clusters are not denatured and aggregated proteins as they were able to readily redistribute to focal adhesions.
3.5. Syk co-localizes with tensin2 in cytoplasmic puncta
When expressed in MCF7 breast epithelial cells, Syk-EGFP was present throughout the cell as described previously [13]. Some Syk-EGFP does redistribute to the cell periphery in breast epithelial cells spreading on surfaces coated with fibronectin, but does not obviously localize to focal adhesions [13]. The co-expression of Myc-tensin2 with Syk-EGFP caused a marked redistribution of Syk-EGFP from its normal, diffuse distribution (Fig. 4E) to punctuate structures in the cytoplasm where it co-localized with Myc-tensin2 (Fig. 4D). These data indicate that Syk and tensin2 retain the ability to interact when expressed in living cells. We did not, however, observe a localization of Syk with tensin2 in focal adhesions.
3.6. Binding of Syk to tensin2 leads to its phosphorylation on tyrosine
The binding of tensin2 to Syk could inhibit its activity. Conversely, the aggregation of Syk could lead to its activation via its enhanced phosphorylation on tyrosine. To examine the effects of tensin2 expression on the phosphorylation status of Syk, we expressed Syk-EGFP in the presence or absence of exogenous tensin2 in the Syk-deficient MCF7 cells. In these experiments, cells were transfected with vectors for the expression of tensin2 with either EGFP or Myc-epitope tags. Cell lysates were then separated by SDS-PAGE and analyzed by Western blotting using antibodies against Syk, tensin2 and phosphotyrosine. The extent of phosphorylation of Syk-EGFP on tyrosine was elevated in the cells in which tensin2 also was expressed (Fig. 5). These studies suggest that Syk is activated following tensin2 induced clustering of the kinase. We did not observe a phosphotyrosine-containing protein band migrating at the position of either Myc-tensin2 or GFP-tensin2. When we analyzed either anti-Myc or anti-GFP immune complexes isolated from cells also expressing Syk-EGFP, we could readily detect the tensin2 fusion proteins with anti-tensin2 antibodies by Western blotting, but were unable to detect the presence of phosphotyrosine on either protein using antibodies against phosphotyrosine (data not shown). Thus, it seems unlikely that tensin2 is robustly phosphorylated by Syk.
Fig. 5.
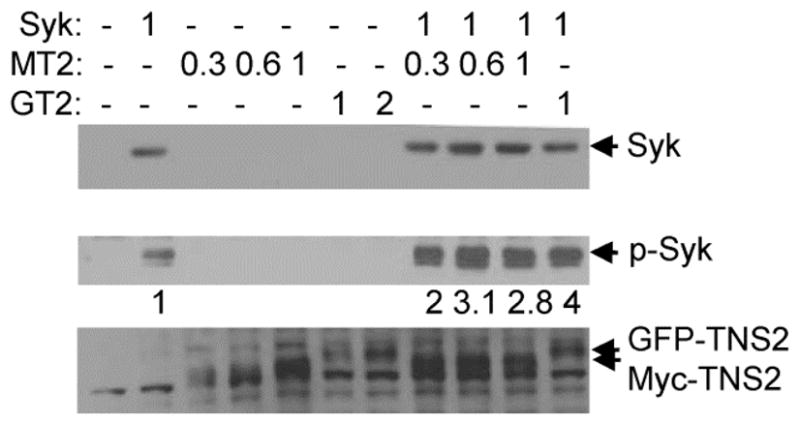
Expression of tensin2 enhances the phosphorylation of Syk on tyrosine. Syk-deficient MCF7 cells were transiently transfected with the indicated amounts of plasmid (in μg) coding for Syk-EGFP (Syk) and either GFP-tensin2 (GT2) or Myc-tensin2 (MT2). Protein expression and phosphorylation was measured by Western blotting using antibodies against Syk (N19) (upper panel), phosphotyrosine (4G10) (middle panel) or tensin2 (antibodies described in Materials and Methods) (lower panel). The migration positions of Syk (Syk), phospho-Syk (p-Syk), GFP-tensin2 (GFP-TNS2) or Myc-tensin2 (Myc-TNS2) are indicted by arrows. The relative extents of phosphorylation of Syk in the absence (normalized to a value of 1) or presence of tensin2 are indicated under the antiphosphotyrosine Western blot.
4. Discussion
In this study, we report for the first time an interaction between the tyrosine kinase Syk and the cytoskeletal protein tensin2. Most proteins that have been found to associate with Syk do so via interactions mediated by SH2 domains or related motifs (e.g., the SH2 domain of Vav1 or p85, or the TKB domain of Cbl [5,18,26,27]) that bind phosphotyrosines. Tensin2 also is capable of binding to Syk in this manner via a C-terminal region that contains both an SH2 and a PTB domain. The interaction with Syk of a short fragment of tensin2 consisting of the linked SH2 and PTB domains occurs only in screens in which the catalytically active kinase is used as bait and not when an inactive enzyme is used. In previous screens, this has been correlated with a phosphotyrosine-dependent interaction since only active Syk can catalyze an autophosphorylation reaction when expressed in yeast [5, 6]. Since removal of the SH2 domain of tensin2 blocks this interaction, it is probable that it is this domain that interacts with one or more phosphotyrosines on Syk.
Interestingly, a larger fragment of the tensin2 C-terminus that contains an addition 115 amino acids just upstream of the SH2 domain binds Syk independently of its state of phosphorylation as it interacts nearly equally well with the catalytically active and inactive kinases. This is an unusual mode of interaction for a Syk-binding protein. USP25, a ubiquitin C-terminal hydrolase, also binds to Syk in a phosphotyrosine-independent manner [4]. In this case, however, USP25 binds to one of the Syk SH2 domains. We were unable to detect an interaction of tensin2 with an N-terminal fragment of Syk containing only the tandem pair of SH2 domains in a yeast two-hybrid interaction analysis. However, we were able to observe an interaction of USP25 with this construct (data not shown). Thus, USP25 and tensin2 bind Syk by different mechanisms and do not share a common binding site. Pull-down assays suggest instead that tensin2 interacts with residues in linker B, which is the region of Syk that separates its pair of SH2 domains from the catalytic domain. It is within this region that most known Syk-interacting proteins bind. It is not yet known how the amino acids proximal to the SH2 domain of tensin2 confer on the C-terminal fragment the ability to bind an inactive form of Syk.
In addition to Syk, the tyrosine kinase Axl has been shown to interact with tensin2, also through its C-terminal SH2 and PTB domains [8]. The yeast two-hybrid interaction screen using Axl as bait identified not only tensin2 as a binding partner, but also the p85 subunit of PI3K, PLC-γ, Nck and Grb2. Interestingly, all of these also are known to interact with Syk or were observed in one or more of our two-hybrid screens. Although Syk and Axl are both tyrosine kinases, they are not members of closely related families as Axl is a receptor tyrosine kinase. Both, however, can associate with and phosphorylate the IL-15 receptor, the expression of both is associated with myeloid leukemia, and both are known regulators of cell motility and cell survival [2,13,28–33]. It is interesting to speculate that these similarities in function arise from their related complement of binding partners.
The tensin2 C-terminal region also interacts with the tumor suppressor DLC-1 [9–11]. Binding of DCL-1 independently to both the SH2 and PTB domains has been described [9–11]. This interaction is interesting as the phosphorylation of DCL-1 is not required for its binding to the tensin2 SH2 domain [10]. While DCL-1 binds multiple members of the tensin family including tensin1 [11], we were unable to demonstrate an interaction between Syk and tensin1, indicating differences in these two modes of binding. The binding of Syk with the C-terminal portion of tensin1 could not be detected despite the 65% sequence similarity between tensin1 and tensin2 within this fragment. Replacement of the unique region just proximal to the SH2 domain of tensin1 with the corresponding region of tensin2 failed to restore binding. These results indicate a degree of specificity in the Syk/tensin2 interaction and suggest that multiple tensin2 motifs participate in the phosphotyrosine-independent binding of Syk.
In cells, Syk and tensin2 interact within large cytoplasmic structures. This association alters the localization of Syk from its normally diffuse distribution and enhances the phosphorylation of the kinase on tyrosine. The increased phosphorylation of Syk is an event that normally accompanies the activation of the kinase as is seen in B cells where aggregation of the BCR leads to the co-clustering and activation of Syk [1,2]. Syk, when phosphorylated on tyrosine, serves both as a catalyst for further substrate phosphorylation and as a scaffold for the binding of proteins containing SH2 or related phosphotyrosine interaction motifs that include PLC-γ, PI3K and Vav1. We did not observe, however, the phosphorylation of tensin2 on tyrosine in these complexes. Our subsequent proteomic analyses of tyrosine phosphorylation in both B cells and breast cancer cells also failed to identify tensin2 as a substate for Syk [34].
The nature of these cytoplasmic puncta is, as yet, unclear. An examination by confocal microscopy of multiple Z-sections from GFP-tensin2 expressing cells indicated that these puncta were localized throughout the cell and were not confined to either the apical or basolateral surfaces. A previous attempt to identify these cytoplasmic spots as specific intracellular organelles such as endosomes or caveolae were unsuccessful [21] (although a localization of tensin2 to caveolae has been observed [10]). We also were unable to co-localize the tensin2-containing bodies with markers of early or late endosomes or lysosomes. These cytoplasmic structures do not seem to represent insoluble aggregates of protein, however, as the tensin2 within these bodies is readily available for recruitment to focal adhesions as indicated by its marked redistribution following the treatment of cells with nocodazole. Thus, these structures may represent a cytoplasmic pool of tensin2 available for recruitment to focal adhesions once integrins are activated and engaged by extracellular ligands. The binding of tensin-family proteins with the cytoplasmic tails of β integrins occurs via the C-terminal PTB domain, which binds independently of tyrosine-phosphorylation [35,36]. One consequence of this relocalization is likely to be a change in cell migration as the ectopic expression of tensin2 has been reported to alter motility. However, both positive and negative effects of tensin2 on motility have been described [22,37,38], perhaps depending on cellular context, the exact isoform that is expressed and the level of expression. It is interesting to note that Syk is a negative regulator of motility in epithelial cells [13,39]. Unlike DCL-1, which co-localizes with tensin2 at focal adhesions [10, 36], we have not observed a co-localization of Syk and tensin2 at these sites. However, it is certainly possible that the sequestration of Syk from the cell periphery could contribute to the ability of tensin2 to influence cell migration.
In addition to motility, tensin2 is also a modulator of cell survival, the regulation of PI3K lipid reaction products and the activation of Akt [9,11,37]. There are some indications that tensin2, due to its sequence similarity with PTEN, has protein-tyrosine phosphatase activity and that this underlies its inhibitory effects on cell survival [37,40]. However, a comparison of the amino acid sequence surrounding the putative active site cysteine of tensin2 (YCKGNKGKL) with that of the consensus sequence for tyrosine/lipid phosphatases (HCXXGXXRS/T) indicates that several key catalytic site residues are missing. Syk, in turn, is an upstream activator of PI3K in antigen receptor and integrin signaling [41,42], suggesting a possible connection between the sequestration of Syk or other tyrosine kinases and the inhibition of PI3K-mediated signaling by tensin2. Recently, tensin2 was identified as a critical factor required for connecting the thrombopoietin receptor (c-Mpl) to the activation of PI3K [43]. Thus, tensin2 may function as a scaffold to provide important connections between PI3K and tyrosine kinases such as Axl, Syk and Jak-2, with interactions made possible through the clustering and tyrosine phosphorylation of the associated kinases as shown for Syk in Fig. 5. Since the overexpression of scaffolding proteins can inhibit signaling by sequestering signaling components into separate complexes (e.g., [44]), this could potentially explain the negative effect of elevated levels of tensin2 on PI3K and Akt.
In conclusion, we have identified tensin2 as a novel Syk-interacting protein that exhibits a unique mode of binding involving interactions both dependent on and independent of tyrosine-phosphorylation. When expressed in epithelial cells, Syk and tensin2 co-localize in cytoplasmic clusters that lead to the aggregation and activation of the kinase.
Highlights.
Syk binds tensin2 in a yeast two-hybrid assay.
Syk binds the C-terminal SH2 and PTB domains of tensin2.
Syk does not bind tensin1.
Syk localizes in cells with tensin2 in cytoplasmic structures.
Syk clustered by binding tensin2 is activated.
Acknowledgments
This work was supported by United States Public Health Services grants CA115465 and CA037372 awarded by the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Geahlen RL. Syk and pTyr’d: Signaling through the B cell antigen receptor. Biochim Biophys Acta. 2009;1793:1115–1127. doi: 10.1016/j.bbamcr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mócsai A, Ruland J, Tybulewicz VLJ. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. 2010;10:387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yanagi S, Inatome R, Takano T, Yamamura H. Syk expression and novel function in a wide variety of tissues. Biochem Biophys Res Commun. 2001;288:495–498. doi: 10.1006/bbrc.2001.5788. [DOI] [PubMed] [Google Scholar]
- 4.Cholay M, Reverdy C, Benarous R, Colland F, Daviet L. Functional interaction between the ubiquitin-specific protease 25 and the SYK tyrosine kinase. Exp Cell Res. 2010;316:667–675. doi: 10.1016/j.yexcr.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 5.Moon KD, Post CB, Durden DL, Zhou Q, De P, Harrison ML, Geahlen RL. Molecular basis for a direct interaction between the Syk protein-tyrosine kinase and phosphoinositide 3-kinase. J Biol Chem. 2005;280:1543–1551. doi: 10.1074/jbc.M407805200. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Q, Geahlen RL. Interaction of the protein tyrosine kinase Syk and TRAF-interacting protein (TRIP) in the tumor necrosis factor (TNF)-mediated survival pathway in breast epithelial cells. Oncogene. 2009;28:1348–1356. doi: 10.1038/onc.2008.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lo SH. Molecules in focus: Tensin. Int J Biochem Cell Biol. 2004;36:31–34. doi: 10.1016/s1357-2725(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 8.Hafizi S, Alindri F, Karlsson R, Dahlbäck B. C1-TEN - Interaction of Axl receptor tyrosine kinase with C1-TEN, a novel C1 domain-containing protein with homology to tensin. Biochem Biophys Res Commun. 2002;299:793–800. doi: 10.1016/s0006-291x(02)02718-3. [DOI] [PubMed] [Google Scholar]
- 9.Yam JWP, Ko FCF, Chan CY, Jin DY, Ng IOL. Interaction of deleted in liver cancer 1 with tensin2 in caveolae and implications in tumor suppression. Cancer Res. 2006;66:8367–8372. doi: 10.1158/0008-5472.CAN-05-2850. [DOI] [PubMed] [Google Scholar]
- 10.Liao YC, Si L, deVere White RW, Lo SH. The phosphotyrosine-independent interaction of DLC-1 and the SH2 domain of cten regulates focal adhesion localization and growth suppression activity of DLC-1. J Cell Biol. 2007;176:43–49. doi: 10.1083/jcb.200608015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan LK, Ko FCF, Ng IOL, Yam JWP. Deleted in liver cancer 1 (DLC1) utilizes a novel binding site for tensin2 PTB domain interaction and is required for tumor-suppressive function. PLoS ONE. 2009;4:e5572. doi: 10.1371/journal.pone.0005572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian X, Li G, Asmussen HK, Asnaghi L, Vass WC, Braverman R, Yamada KM, Popescu NC, Papageorge AG, Lowy DR. Oncogenic inhibition by a deleted in liver cancer gene requires cooperation between tensin binding and Rho-specific GTPase-activating protein activities. Proc Natl Acad Sci USA. 2007;104:9012–9017. doi: 10.1073/pnas.0703033104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Shrikhande U, Zhou Q, Geahlen RL. A role for the protein-tyrosine kinase, Syk, in regulating cell-cell adhesion and motility in breast cancer cells. Mol Cancer Res. 2009;7:634–644. doi: 10.1158/1541-7786.MCR-08-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keshvara LM, Isaacson C, Yankee TM, Sarac R, Harrison ML, Geahlen RL. Syk- and Lyn-dependent phosphorylation of Syk on multiple tyrosines following B cell activation includes a site that negatively regulates signaling. J Immunol. 1998;161:5276–5283. [PubMed] [Google Scholar]
- 15.Peters JD, Furlong MT, Asai DJ, Harrison ML, Geahlen RL. Syk, activated by cross-linking the B-cell antigen receptor, localizes to the cytosol where it interacts with and phosphorylates α-tubulin on tyrosine. J Biol Chem. 1996;271:4755–4762. doi: 10.1074/jbc.271.9.4755. [DOI] [PubMed] [Google Scholar]
- 16.Furlong MT, Mahrenholz AM, Kim KH, Ashendel CL, Harrison ML, Geahlen RL. Identification of the major sites of autophosphorylation of the murine protein-tyrosine kinase Syk. Biochim Biophys Acta. 1997;1355:177–190. doi: 10.1016/s0167-4889(96)00131-0. [DOI] [PubMed] [Google Scholar]
- 17.Ma H, Yankee TM, Hu J, Asai DJ, Harrison ML, Geahlen RL. Visualization of Syk-antigen receptor interactions using green fluorescent protein: differential roles for Syk and Lyn in the regulation of receptor capping and internalization. J Immunol. 2001;166:1507–1516. doi: 10.4049/jimmunol.166.3.1507. [DOI] [PubMed] [Google Scholar]
- 18.Deckert M, Tartare-Deckert S, Couture C, Mustelin T, Altman A. Functional and physical interactions of Syk family kinases with the Vav proto-oncogene product. Immunity. 1996;5:591–604. doi: 10.1016/s1074-7613(00)80273-3. [DOI] [PubMed] [Google Scholar]
- 19.Vines CM, Potter JW, Xu Y, Geahlen RL, Costello PS, Tybulewicz VL, Lowell CA, Chang PW, Gresham HD, Willman CL. Inhibition of beta 2 integrin receptor and Syk kinase signaling in monocytes by the Src family kinase Fgr. Immunity. 2001;15:507–519. doi: 10.1016/s1074-7613(01)00221-7. [DOI] [PubMed] [Google Scholar]
- 20.Heibeck TH, Ding SJ, Opresko LK, Zhao R, Schepmoes AA, Yang F, Tolmachev AV, Monroe ME, Camp DG, Smith RD, Wiley HS, Qian WJ. An extensive survey of tyrosine phosphorylation revealing new sites in human mammary epithelial cells. J Proteome Res. 2009;8:3852–3861. doi: 10.1021/pr900044c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hafizia S, Sernstada E, Swinny JD, Gomezc MF, Dahlbäcka B. Individual domains of Tensin2 exhibit distinct subcellular localisations and migratory effects. Int J Biochem Cell Biol. 2010;42:52–61. doi: 10.1016/j.biocel.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Chen H, Duncan IC, Bozorgchami H, Lo SH. Tensin1 and a previously undocumented family member, tensin2, positively regulate cell migration. Proc Natl Acad Sci USA. 2002;99:733–738. doi: 10.1073/pnas.022518699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark K, Howe JD, Pullar CE, Green JA, Artym VV, Yamada KM, Critchley DR. Tensin 2 modulates cell contractility in 3D collagen gels through the RhoGAP DLC1. J Cell Biochem. 2010;109:808–817. doi: 10.1002/jcb.22460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaverina I, Krylyshkina O, Small JV. Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J Cell Biol. 1999;146:1033–1044. doi: 10.1083/jcb.146.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bershadsky A, Chausovsky A, Becker E, Lyubimova A, Geiger B. Involvement of microtubules in the control of adhesion dependent signal transduction. Curr Biol. 1996;6:1279–1289. doi: 10.1016/s0960-9822(02)70714-8. [DOI] [PubMed] [Google Scholar]
- 26.Lupher ML, Jr, Rao N, Lill NL, Andonioui CE, Miyake S, Clark EA, Druker B, Band H. Cbl-mediated negative regulation of the Syk tyrosine kinase. A critical role for Cbl phosphotyrosine-binding domain binding to Syk phosphotyrosine 323. J Biol Chem. 1998;273:35273–35281. doi: 10.1074/jbc.273.52.35273. [DOI] [PubMed] [Google Scholar]
- 27.Yankee TM, Keshvara LM, Sawasdikosol S, Harrison ML, Geahlen RL. Inhibition of signaling through the B cell antigen receptor by the proto-oncogene product, c-Cbl, requires Syk tyrosine 317 and the c-Cbl phosphotyrosine-binding domain. J Immunol. 1999;163:5827–5835. [PubMed] [Google Scholar]
- 28.Budagian V, Bulanova E, Orinska Z, Thon L, Mamat U, Bellosta P, Basilico C, Adam D, Paus R, Bulfone-Paus S. A promiscuous liaison between IL-15 receptor and Axl receptor tyrosine kinase in cell death control. EMBO J. 2005;24:4260–4270. doi: 10.1038/sj.emboj.7600874. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Bulanova E, Budagian V, Pohl T, Karuse H, Dürkop H, Paus R, Bulfone-Paus S. The IL-15R alpha chain signals through association with Syk in human B cells. J Immunol. 2001;167:6292–6302. doi: 10.4049/jimmunol.167.11.6292. [DOI] [PubMed] [Google Scholar]
- 30.O'Bryan JP, Frye RA, Cogswell PC, Neubauer A, Kitch B, Prokop C, Espinosa R, 3rd, Le Beau MM, Earp HS, Liu ET. Axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991;11:5016–5031. doi: 10.1128/mcb.11.10.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hahn CK, Berchuck JE, Ross KN, Kakoza RM, Clauser K, Schinzel AC, Ross L, Galinsky I, Davis TN, Silver SJ, Root DE, Stone RM, DeAngelo DJ, Carroll M, Hahn WC, Carr SA, Golub TR, Kung AL, Stegmaier K. Proteomic and genetic approaches identify Syk as an AML target. Cancer Cell. 2009;16:281–294. doi: 10.1016/j.ccr.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shankar SL, O'Guin K, Kim M, Varnum B, Lemke G, Brosnan CF, Shafit-Zagardo B. Gas6/Axl signaling activates the phosphatidylinositol 3-kinase/Akt1 survival pathway to protect oligodendrocytes from tumor necrosis factor alpha-induced apoptosis. J Neurosci. 2006;26:5638–5648. doi: 10.1523/JNEUROSCI.5063-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demarchi F, Verardo R, Varnum B, Brancolini C, Schneider C. Gas6 anti-apoptotic signaling requires NF-kappa B activation. J Biol Chem. 2001;276:31738–31744. doi: 10.1074/jbc.M104457200. [DOI] [PubMed] [Google Scholar]
- 34.Iliuk AB, Martin VA, Alicie BM, Geahlen RL, Tao WA. In-depth analyses of kinase-dependent tyrosine phosphoproteomes based on metal ion-functionalized soluble nanopolymers. Mol Cell Proteomics. 2010;9:2162–2172. doi: 10.1074/mcp.M110.000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calderwood DA, Fujioka Y, de Pereda JM, Garcia-Alvarez B, Nakamoto T, Margolis B, McGlade CJ, Liddington RC, Ginsberg MH. Integrin beta cytoplasmic domain interactions with phosphotyrosine-binding domains: a structural prototype for diversity in integrin signaling. Proc Natl Acad Sci USA. 2003;100:2272–2277. doi: 10.1073/pnas.262791999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCleverty CJ, Lin DC, Liddington RC. Structure of the PTB domain of tensin1 and a model for its recruitment to fibrillar adhesions. Protein Sci. 2007;16:1223–1229. doi: 10.1110/ps.072798707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hafizi S, Ibraimi F, Dahlbäck B. C1-TEN is a negative regulator of the Akt/PKB signal transduction pathway and inhibits cell survival, proliferation, and migration. FASEB J. 2005;19:971–973. doi: 10.1096/fj.04-2532fje. [DOI] [PubMed] [Google Scholar]
- 38.Yam JWP, Ko FCF, Chan CY, Yau TO, Tung EKK, Leung THY, Jin DY, Ng IOL. Tensin2 variant 3 is associated with aggressive tumor behavior in human hepatocellular carcinoma. Hepatology. 2006;44:881–890. doi: 10.1002/hep.21339. [DOI] [PubMed] [Google Scholar]
- 39.Coopman PJP, Do MTH, Barth M, Bowden ET, Hayes AJ, Basyuk E, Blancatok JK, Vezza PR, McLeskey SW, Mangeat PH, Mueller SC. The Syk tyrosine kinase suppresses malignant growth of human breast cancer cells. Nature. 2000;406:742–747. doi: 10.1038/35021086. [DOI] [PubMed] [Google Scholar]
- 40.Hafizi S, Gustafsson A, Oslakovic C, Idevall-Hagren O, Tengholm A, Sperandio O, Villoutreix BO, Dahlbäck B. Tensin2 reduces intracellular phosphatidylinositol 3,4,5-trisphosphate levels at the plasma membrane. Biochem Biophys Res Commun. 2010;399:396–401. doi: 10.1016/j.bbrc.2010.07.085. [DOI] [PubMed] [Google Scholar]
- 41.Beitz LO, Fruman DA, Kurosaki T, Cantley LC, Scharenberg AM. SYK is upstream of phosphoinositide 3-kinase in B cell receptor signaling. J Biol Chem. 1999;274:32662–32666. doi: 10.1074/jbc.274.46.32662. [DOI] [PubMed] [Google Scholar]
- 42.Schymeinsky J, Then C, Sindrilaru A, Gerstl R, Jakus Z, Tybulewicz VLJ, Scharffetter-Kochanek K, Walzog B. Syk-mediated translocation of PI3Kδ to the leading edge controls lamellipodium formation and migration of leukocytes. PLoS ONE. 2007;2:e1132. doi: 10.1371/journal.pone.0001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jung AS, Kaushansky A, Macbeth G, Kaushansky K. Tensin2 is a novel mediator in thrombopoietin (TPO)-induced cellular proliferation by promoting Akt signaling. Cell Cycle. 2011;10:1838–1844. doi: 10.4161/cc.10.11.15776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dickens M, Rogers JS, Cavanagh J, Raitano A, Xia Z, Halpern JR, Greenberg ME, Sawyers CL, Davis RJ. A cytoplasmic inhibitor of the JNK signal transduction pathway. Science. 1997;277:693–696. doi: 10.1126/science.277.5326.693. [DOI] [PubMed] [Google Scholar]



