Abstract
The concomitant presence of a complete fsr quorum-sensing system and gelE–sprE operons in Enterococcus faecalis is known to be essential for the detection of gelatinase activity. However, there are reports of the absence of gelatinase activity despite the presence of complete fsr and gelE loci. In order to understand this incongruence between genotype and phenotype we sequenced fsr and gelE loci of the E. faecalis LN68 strain, which was previously found to carry both operons but to lack gelatinase activity. Of the 59 nucleotide differences detected compared with the gelatinase-positive V583 strain, we found a nonsense mutation (a premature STOP codon) predicted to truncate the ATPase sensor domain of the FsrC protein, responsible for sensing and transducing the signal from the quorum-sensing molecule. Strain LN68 was highly affected in the expression of the gelE and sprE genes, further supporting the lack of Fsr-dependent gelE induction. When we constructed a V583 mutant with the same premature stop mutation in the fsrC gene the resulting strain was no longer able to degrade gelatin. We conclude that the reduced ability to transduce the quorum-sensing signal of the prematurely truncated FsrC protein is sufficient to explain the negative gelatinase phenotype. As the incongruent genotype and phenotype is detected in natural isolates, we believe that the silencing of the quorum-sensing system Fsr may be beneficial for some E. faecalis strains.
Introduction
Enterococci are natural inhabitants of the oral cavity, intestinal tract and female genital tract of both humans and animals, and are also among the predominant microbiota of traditionally fermented dairy products (Lopes et al., 1999). In contrast to their beneficial role in intestinal homeostasis, these micro-organisms are becoming increasingly important to human health as leading causes of nosocomial infections, namely of the urinary tract, bloodstream, intra-abdominal and pelvic regions, and surgical sites. To do so, they rely on several mechanisms, including the fsr operon of Enterococcus faecalis, the species most frequently associated with nosocomial infections (Gilmore et al., 2002; Mundt, 1986; Ogier & Serror, 2007). The fsr (Enterococcus faecalis regulator) two-component system, a homologue of the agr system in Staphylococcus aureus, is a quorum sensing-dependent regulatory system. The fsr operon comprises four genes: fsrA, fsrB, fsrC and fsrD. The last encodes an auto-inducing cyclic peptide named gelatinase biosynthesis-activating pheromone (GBAP) that is processed and exported out of the cell by the FsrB protein. Accumulation of GBAP outside cells is sensed by the FsrC histidine kinase, leading to the activation of the response regulator FsrA. Activated FsrA induces expression of the fsrBDC genes. These genes are involved in an autoregulatory circuit that results in a boost of GBAP signalling and in induction of the Fsr regulon, among which the gelE–sprE operon is the most induced (Bourgogne et al., 2006). This operon encodes gelatinase (GelE), an extracellular zinc metalloprotease (Mäkinen et al., 1989; Su et al., 1991), and SprE, a serine protease (Nakayama et al., 2001a, b; Qin et al., 2000).
Although the secreted protease SprE has been implicated in disease in animal models, the role of SprE is still unknown. GelE is known to contribute to biofilm formation, and contributes also to virulence through degradation of a broad range of host proteinaceous substrates (Hancock & Perego, 2004; Park et al., 2007, 2008; Steck et al., 2011). The role of the gelE and fsr loci in E. faecalis virulence has been demonstrated in different mammalian infection models (Mohamed & Murray, 2006), in the Caenorhabditis elegans nematode model (Gaspar et al., 2009; Sifri et al., 2002) and in the Arabidopsis thaliana plant model (Jha et al., 2005). Gelatinase has also been implicated in evasion of the immune system of the insect Galleria mellonella (Park et al., 2007). Recent transcription studies have also shown that fsr and gelE–sprE expression is modulated during some stress conditions, namely in blood (Vebø et al., 2009) and urine (Vebø et al., 2010). Despite their evident importance for E. faecalis virulence and stress responses inside the host, many reports have shown that both loci are present in enterococcal isolates from different environments. This suggests that both the Fsr system and the GelE and SprE proteins may play a role, not associated with virulence, in the biology of E. faecalis (Thomas et al., 2009).
The presence of the gelE genetic locus has often been complemented by the search for the phenotype, i.e. detection of gelatinase activity on plates containing gelatin. Qin et al. (2000) reported that the gelatinase phenotype requires the concomitant presence of the fsr and gelE genes. Soon after, Eaton & Gasson (2001) reported the loss by subculturing of the gelatinase phenotype in 20 % of the analysed strains. Later, Nakayama et al. (2002) suggested that the loss of the gelatinase phenotype after subculturing might be due to a 23.9 kb chromosomal deletion, which was found in urine isolates with silent gelE genes. This deletion included the fsrA, fsrB and partially the fsrC gene. However, it has recently been shown that the 23.9 kb chromosomal deletion does not occur spontaneously by subculturing strains in the laboratory, but likely results from horizontal transfer and recombination (Galloway-Peña et al., 2011). We and others have reported the loss of the gelatinase phenotype by subculturing, but also the existence of natural gelatinase-negative strains carrying gelE and an apparently complete fsr operon (Lopes et al., 2006; Nakayama et al., 2002). Altogether, these reports indicate that silent gelE genes, in the presence of an apparently complete fsr operon, occur both in natural and laboratory-subcultured E. faecalis isolates. However, no further demonstration of the involvement of mutations in either the fsr or the gelE–sprE operon in the absence of gelatinase activity has been reported or demonstrated so far.
In the present study we investigated the reason for the incongruence between the fsr and gelE genotype and the gelatinase phenotype in E. faecalis strain LN68 (Lopes et al., 2006). We demonstrate that this incongruence, in our strains with apparently complete fsr and gelE loci, is correlated with a specific nonsense codon in the FsrC protein. We further demonstrate that this codon change originates a truncated FsrC protein, preventing E. faecalis from sensing GBAP.
Methods
Bacterial strains and plasmids.
Strains and plasmids used in this study are listed in Table 1. Enterococcal strains were grown in BHI (brain heart infusion) medium at 37 °C, and Escherichia coli strains were grown in LB medium at 37 °C with agitation.
Table 1. Strains, plasmids and primers used in this study.
Restriction sites are underlined.
| Strain, plasmid or primer | Relevant characteristics or sequence | Reference or source |
| Strains | ||
| Escherichia coli DH5α | F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK− mK−) phoA supE44 λ− thi-1 gyrA96 relA1 | Grant et al. (1990) |
| Escherichia coli TG1 RepA | supE hsdD5 thi (Δlac-proAB) F− (traD36 proAB-lacZΔM15) glgB : : repA | Law et al. (1995) |
| Escherichia coli EC1000 | F~ araD139(ara ABC-leu)7679 gal° galK lacX74 rsp thi repA | Leenhouts et al. (1996) |
| E. faecalis V583 | Clinical isolate, TIGR sequence strain; VanR | Sahm et al. (1989) |
| V583 ErmS | Derivative of E. faecalis V583, susceptible to erythromycin due to deletion of the erm(B) | Yan et al. (2009) |
| LN68 | Wild-type E. faecalis; isolated from milk, GelE− | Lopes et al. (2006) |
| LN66 | Wild-type E. faecalis; isolated from milk, GelE− | This study |
| QSE15 | Wild-type E. faecalis; isolated from cheese, GelE− | This study |
| QN8 | Wild-type E. durans; isolated from cheese, GelE− | This study |
| EF_SAVE3 | E. faecalis V583ermS W403STOP, GelE− | This study |
| EF_SAVE4 | E. faecalis LN68 STOP403W, GelE+ | This study |
| EF_SAVE5 | E. faecalis LN68 carrying pSAVE7, EryR, GelE+ | This study |
| VT01 | E. faecalis V583 ΔgelE, GelE−, GBAP+ | Thomas et al. (2008) |
| VT03 | E. faecalis V583 ΔgelEΔsprE, GelE−, SprE−, GBAP+ | Thomas et al. (2008) |
| VI13 | E. faecalis V583 ΔfsrB, GelE−, GBAP− | This study |
| Plasmids | ||
| pORI23 | Escherichia coli–E. faecalis shuttle plasmid, EryR | Que et al. (2000) |
| pGEM-T | Escherichia coli replicating plasmid, AmpR | Promega |
| pG+host9 | E. faecalis thermosensitive plasmid, EryR | Maguin et al. (1996) |
| pSAVE3 | pGEM-T derivative carrying fsrC_W403STOP from V583 | This study |
| pSAVE4 | pGEM-T derivative carrying fsrC_W403STOP from LN68 | This study |
| pSAVE5 | pGhost9 derivative carrying fsrC_W403STOP from V583 | This study |
| pSAVE6 | pGhost9 derivative carrying fsrC_STOP403W from LN68 | This study |
| pSAVE7 | pORI23 derivative carrying a 1595 pb BamHI/PstI fsrC fragment from V583 | This study |
| pLT06 | Temperature-sensitive cloning vector | Thurlow et al. (2009) |
| pVI02 | pLT06 containing engineered fsrB deletion | This study |
| Primers | ||
| sprE_4 | TTTCCTGTTTGCTTAATGCCGC | |
| msrpE_4 | GGCTGAAAGTTTTAAGAATGCCAAA | |
| LN68_fsrC-PGMT5 | TTATAAACCAATGATACGGG | |
| LN68_fsrC_PGMT3 | AAAATAAATTATTATGGATTGCC | |
| LN68_mfsrC_M | CCCAGAAGAGCACGGTTGGGGATTGTTATATGTAAAAG | |
| LN68_fsrC_M | CTTTTACATATAACAATCCCCAACCGTGCTCTTCTGGG | |
| V583_mfsrC_M | CCAGAAGAGCACGGTTGAGGATTGTTATATGTAAAAG | |
| V583_fsrC_M | CTTTTACAATAACAATCCTCAACCGTGCTCTTCTGG | |
| fsrC3′ PstI | GAATCCTGCAGTTGCTTTATCCTCCC | |
| fsrC5′ BamHI | GAATCGGATCCGACAATGGATGGGAC | |
| RNA23S_fw_V583 | AAAGAAATCAACCGAGATTCCCC | |
| RNA23S_rv_V583 | AAACAGTGCTCTACCCCCGG | |
| FsrBP1 | GAGAGAATTCCAAGAACAGTTTGGCGGTTG | |
| FsrBP2 | CTCTGGATCCATCGATTAGCATATCGCC | |
| FsrBP3 | GAGAGGATCCGGTCTGATGATACTCCCT | |
| FsrBP4 | CTCTCTGCAGGGTTGGTGCAATCGTTTC | |
| FsrAP1 | GGGATTGACGGAAATCAAGA | |
| GelEP2 | AAAGATGCCTGTACCTAAAATG |
General DNA techniques.
General molecular biology techniques were performed by standard methods (Sambrook et al., 1989). Restriction enzymes, polymerases and T4 DNA ligase were used according to manufacturers’ instructions. PCR amplification was performed using a Biometra thermocycler. When necessary, PCR products and DNA restriction fragments were purified with purification kits (Macherey-Nagel). Plasmids were purified using the Miniprep kit (Macherey-Nagel). Electrotransformation of Escherichia coli and E. faecalis was carried out as described by Dower et al. (1988) and Dunny et al. (1991), using a Gene Pulser apparatus (Bio-Rad) (Dower et al., 1988; Dunny et al., 1991). Plasmid inserts were sequenced at BaseClear (The Netherlands).
Sequence analysis of the fsr and gelE–sprE regions of E. faecalis LN68.
PCR amplification of overlapping fragments of the fsr and gelE–sprE regions was carried out using Expand High FidelityPLUS DNA polymerase (Roche) and primers from Gaspar et al. (2009). Amplicons were sequenced by BaseClear and DNA sequence analysis was accomplished using the Vector NTI 10.3.0 program (Invitrogen). The results were compared with the V583 genomic DNA sequence available at the J. Craig Venter Institute website (http://www.jcvi.org/).
Mutant construction.
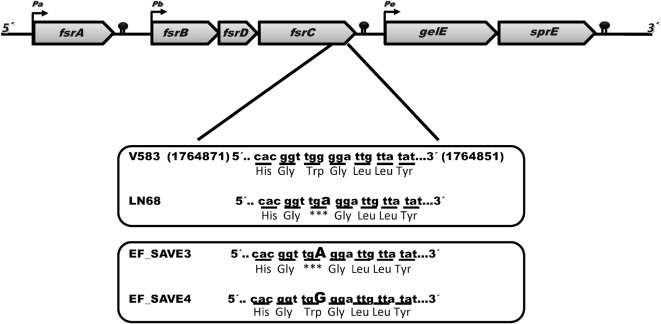
Markerless fsrC mutants of E. faecalis LN68 and of a derivative erythromycin-susceptible V583 strain (kindly provided by Axel Hartke, Université de Caen, France) were constructed essentially as described by Brinster et al. (2007) (Fig. 1). In this procedure we changed the nucleotide guanine for adenine (position 1764864 in the V583 genome), which leads to the substitution of a tryptophan by a STOP codon (in V583). In LN68, we did the opposite, i.e. we substituted the STOP codon by a tryptophan at position 403. Briefly, flanking regions of fsrC were amplified from chromosomal DNA by PCR with primers LN68_fsrC-PGMT5 and LN68_mfsrC_M, LN68_fsrC_PGMT3 and LN68_fsrC_M, for strain LN68; and primers V583_mfsrC_M and LN68_fsrC-PGMT5, LN68_fsrC_PGMT3 and V583_fsrC_M, for strain V583 (Table 1). The two cognate PCR fragments were fused by PCR using the external primers LN68_fsrC-PGMT5 and LN68_fsrC_PGMT3, for both strains, and the resulting products were cloned into pGEM-T (Promega). After being checked by sequencing, the inserted PCR fragment was removed from its cloning vector by restriction enzymes and subsequently cloned into plasmid pG+host9 (Maguin et al., 1996), which was then electroporated into the respective E. faecalis strain. The fsrC single- and double-crossover mutants were selected as described by Brinster et al. (2007). Successful targeted mutations of fsrC in strains LN68 and V583 were first identified by PCR screening and then confirmed by sequencing. The cognate phenotypes were confirmed by gelatinase activity assay.
Fig. 1.
Schematic representation of the fsr and gelE loci in V583 and LN68, and the mutants derived from point mutations, EF_SAVE3 and EF_SAVE4, respectively. The nonsense codon W403STOP is also indicated.
An isogenic in-frame deletion of fsrB in E. faecalis V583 was generated using an Escherichia coli–enterococcal temperature-sensitive cloning vector, pLT06 (Thurlow et al., 2009). Upstream regions flanking fsrB (ef1821) in the V583 genome were PCR-amplified using the primer <1?tlsb=-.005w?>pair FsrBP1 and FsrBP2, and the downstream region of ef1821 was amplified using primers FsrBP3 and FsrBP4 (Table 1). The resultant PCR products were digested with BamHI followed by ligation, and the resulting product was reamplified with primers FsrBP1 and FsrBP4. For the construction of the fsrB deletion vector pVI02, the amplified product was digested with EcoRI and PstI, followed by ligation to similarly digested plasmid vector pLT06. The ligated product was electroporated into Escherichia coli EC1000 for propagation, and blue colonies were selected for by culture at 37 °C on LB agar containing chloramphenicol (10 µg ml−1) and X-Gal (80 µg ml−1). Positive clones were identified by PCR using primers OriF and SeqR (Thurlow et al., 2009). Plasmid pVI02 was confirmed by restriction mapping and electroporated into E. faecalis V583 cells. To generate E. faecalis strain VI13 possessing an in-frame deletion of fsrB, a published protocol was used (Thurlow et al., 2009). Primers FsrAP1 and GelEP2 were employed to confirm the presence of the deletion.
E. faecalis LN68 complementation with the V583 fsrC gene.
To complement strain LN68 with the fsrC gene from V583, a 1595 bp BamHI–PstI fragment from V583 was recovered from the PCR amplicon obtained by using primers fsrC3′PstI and fsrC5′BamHI (Gaspar et al., 2009). The fragment was cloned into the vector pOri23 (Que et al., 2000) cut with BamHI and PstI, resulting in plasmid pOri23–fsrC. Primers pOri23_fw and pOri23_rv (Braga et al., 2011) were used to confirm cloning. pOri23–fsrC was then introduced into LN68 cells by electroporation. Gelatinase activity was evaluated as described below, on gelatin plates supplemented with 500 µg erythromycin ml−1.
Gelatinase activity.
The phenotypic assay for detection of gelatinase activity was performed as described by Lopes et al. (2006). Briefly, enterococcal strains were grown on agar plates containing 3 % (w/v) gelatin (Oxoid) and flooded with a saturated solution of ammonium sulfate (Merck). A transparent halo around cells indicated gelatinase activity. Assays to study the ability of cells to sense and produce GBAP were performed on plates containing 1.5 % skimmed milk. A transparent halo around cells detected after 24 h incubation at 37 °C indicated gelatinase production and activity. When indicated, GBAP was added to cells at a final concentration of 10 nM prior to plating 10 µl of culture onto the skimmed milk plates.
RNA extraction and semiquantitative RT-PCR.
RNA was extracted from cells grown in BHI broth at 37 °C. Briefly, overnight cultured cells were diluted 1 : 100 and growth was monitored by following OD600. Following exponential, early stationary and late stationary growth phases, cells were collected for RNA isolation. Total RNA was extracted and purified with an RNeasy Mini kit (Qiagen). RNA integrity was checked by electrophoresis on a 1 % agarose gel (RNase free), and DNA contamination was checked using primers for 23S (Supplementary Table S1). The cDNA was synthesized using random primers (Roche Diagnostics), 3 µg total RNA and a Transcriptor High Fidelity cDNA Synthesis kit (Roche Diagnostics). Two serial dilutions (1 : 10 and 1 : 100) of cDNA were used for PCR in order to amplify cDNA of fsrA (primers: mfsrA, fsrA), fsrB (primers: fsrB, mfsrB), fsrC (primers: fsrC_2, mfsrC), gelE (primers: mgelE_2, gelE) and sprE (primers: sprE_4, msrpE_4) transcripts (Supplementary Table S1). 23S rRNA was used as a control.
Results and Discussion
The importance of E. faecalis gelatinase to virulence and biofilm-forming ability has promoted the detection of the fsr and gelE–sprE operons as a presumption of pathogenicity. However, an incongruent genotype and phenotype may lead to false-positive assumptions on the virulence potential of strains, since the detection of the gene does not necessarily equate to gelatinase activity (Eaton & Gasson, 2001). In a previous study, the E. faecalis LN68 dairy strain was found to lack gelatinase activity on plates, despite apparently carrying the entire fsr and gelE–sprE operons, suggesting a defect in the functionality of the fsr and gelE–sprE operons or in their expression (Lopes et al., 2006).
We first sequenced 6106 bp, including the promoter and terminator regions, of both the fsr and gelE–sprE operons of LN68 strain. Comparison of the LN68 sequence with that of E. faecalis V583 strain revealed 59 differences. Thirty-one, 18 and one corresponded to silent, missense and nonsense mutations, respectively. We found also nine differences located in the intergenic regions (Table 2). Even if all of these differences could potentially influence the gelatinase phenotype, either by disturbing the regulation of gene expression through changed transcript stability for silent mutations, or by promoting a change in protein structure for missense mutations, the most drastic effect was predicted to result from the nonsense codon in the fsrC gene responsible for the substitution of tryptophan 403 by a STOP codon (Fig. 1). This leads to FsrC402, a truncated histidine kinase shortened by the last 45 aa compared with the FsrC of V583 strain (447 aa).
Table 2. Nucleotide differences identified in E. faecalis LN68.
| Mutation type | Gene/IR* | ||||||||||
| IR | fsrA | IR | fsrB | IR | fsrC | IR | gelE | IR | sprE | IR | |
| Silence | − | 2 | − | 4 | − | 5 | − | 12 | − | 8 | − |
| Missense | − | 2 | − | 3 | − | 5 | − | 6 | − | 3 | − |
| Nonsense | 3 | − | 4 | − | − | 1 | − | − | − | − | 1 |
| Deletion | − | − | − | − | − | − | − | − | − | − | − |
| Insertion | − | − | − | − | − | − | − | − | − | − | − |
| Other† | 3 | − | 5 | − | − | − | − | − | − | − | 1 |
IR, intergenic region.
Other: differences found in the intergenic regions.
To further investigate how the detected differences in the LN68 sequence would affect the structure of FsrC we used TopPred software (http://mobyle.pasteur.fr/cgi-bin/portal.py?#forms::toppred). FsrC proteins of both the V583 and the LN68 strain are predicted to have six transmembrane segments and three extracellular loops (Fig. 2). The major difference in LN68 FsrC is a truncated kinase domain due to the nonsense mutation in the C-terminal region. These results support our prediction that substitution of tryptophan 403 by a STOP codon promotes a structural change in the FsrC protein and most likely has an impact on its activity. Noticeably, the GXG triplet highly conserved in the ATPase domain of many histidine kinases is interrupted in LN68 FsrC (Fig. 2). This triplet is important for nucleotide binding and histidine phosphorylation of the cognate response regulators (Parkinson & Kofoid, 1992; Zhu & Inouye, 2002). In response to GBAP, the ATPase domain of FsrC phosphorylates the FsrA response regulator, which activates transcription from PfsrB and PgelE promoters, increasing expression of the fsrBDC, gelE and sprE genes. If phosphorylation of FsrA does not occur, the response regulator will not activate transcription, and expression of the proteases will not increase. Thus, we hypothesize that a truncated ATPase domain of FsrC impairs its ability to transduce GBAP signalling and consequently it cannot induce the expression of the GelE and SprE proteases. The low transcript levels of the gelE and sprE genes in LN68 (Supplementary Fig. S1) are in accordance with the absence of gelatinase activity on gelatin plates.
Fig. 2.

Intramembrane structures of FsrC proteins from V583 and LN68, predicted using TopPred software. Mutations found in the fsrC gene of LN68 are shown: tyrosine (Y) 54 to cysteine (C); leucine (L) 124 to isoleucine (I); tyrosine 252 to asparagine (N); lysine (K) 385 to arginine (R); tryptophan (W) 403 to STOP. ES, extracellular space; IS, intracellular space; ECL, extracellular loop.
In order to prove that LN68 has a negative gelatinase phenotype because it is a GBAP non-responder (derived from its functionally impaired FsrC) and not because of any other mutation detected in the fsrA, fsrB and gelE genes (Table 2), we constructed two mutants: EF_SAVE3 in V583, where tryptophan 403 was substituted by a STOP codon, and EF_SAVE4 in LN68, where the 403STOP codon was substituted by a tryptophan (Fig. 1), and we checked for induction of gelatinase activity of LN68, V583 and their derived mutants by exogenous GBAP. The activity of FsrC was indirectly detected by testing gelatinase activity on plates. Compared with the V583 wild-type strain, EF_SAVE3 showed no gelatinase activity, indicating that the nonsense mutation is sufficient to explain loss of FsrC activity (Fig. 3). Unexpectedly, we observed that the E. faecalis EF_SAVE4 mutant was unstable, as it reverted easily to the wild-type sequence of the LN68 fsrC gene. In fact, if gelatinase activity was tested right after constructing and confirming strain EF_SAVE4, the strain behaved as a gelatinase producer. However, if we repeated the test with EF_SAVE4 subcultures, it behaved as a gelatinase non-producer, and sequencing of fsrC showed that the strain reverted to the STOP codon of the wild-type strain. We thus decided to complement strain LN68 with fsrC from strain V583, using plasmid pORI23. The resulting strain, E. faecalis EF_SAVE5, exhibited gelatinase activity, indicating that expression of fsrC from V583 was sufficient to restore gelatinase expression in strain LN68 (Fig. 3).
Fig. 3.

Images from gelatin agar plates inoculated with the V583, EF_SAVE3, LN68 and EF_SAVE5 strains. A transparent halo, indicative of gelatin degradation, is clearly seen around V583 and EF_SAVE5 growth, demonstrating the presence of gelatinase activity in these two strains.
Then, to investigate whether LN68 and EF_SAVE3 were GBAP non-responders and GBAP producers, we performed two experiments, whose results are presented in Fig. 4. We observed no gelatinase activity when purified GBAP was added to LN68 and EF_SAVE3 cells (Fig. 4a). These results indicate that neither LN68 nor EF_SAVE3 is a GBAP responder, showing that the W403STOP mutation in the FsrC histidine kinase is enough to turn E. faecalis into a GBAP non-responder. We then wondered whether these two strains were still producing sufficient amounts of GBAP to induce detectable gelatinase. The results are presented in Fig. 4(b). VI13 is an fsrB mutant of V583, which is not able to produce GBAP, but responds to it. In the presence of another GBAP producer strain, it is possible to see a transparent halo around VI13 cells, indicative of gelatinase activity and thus of Fsr induction by GABP. When VI13 was allowed to grow in the proximity of either VT01 or VT03, GBAP producer strains but with a negative gelatinase phenotype, the GBAP produced by VT01 and VT03 was able to induce gelatinase activity in VI13. Similarly, both LN68 and EF_SAVE3 were able to induce gelatinase activity in VI13, although to different extents, and at much lower levels then those seen with VT01 or VT03. This is because both LN68 and EF_SAVE3 have a truncated FsrC, which allows these strains to produce only basal levels of GBAP to which VI13 responded. Our results thus demonstrate that both LN68 and EF_SAVE3 are GBAP producers.
Fig. 4.
LN68 and EF_SAVE3 gelatinase activity on skimmed milk plates. (a) Demonstration of the inability of LN68 and EF_SAVE3 to induce gelatinase upon addition of exogenous GBAP. Strain VI13 was used as a proof of concept of this assay. This strain is able to sense GBAP, and induce gelatinase activity upon GBAP addition, but it is not able to produce GBAP. (b) Demonstration of the ability of both LN68 and EF_SAVE3 to produce GBAP. Proof came from the observation of a transparent halo around growth of the VI13 strain, which is not able to produce GBAP and depends on exogenous GBAP to produce visible gelatinase activity.
Recently, Thomas et al. (2009) have reported the existence, among planktonic E. faecalis cells, of GBAP non-responders, i.e. cheaters, which, at the end of stationary phase, reach approximately 12 % of the population. In a population where individuals work cooperatively, cheaters are individual cells that benefit from the cooperation with others, but do not cooperate. In light of our data, it is possible that E. faecalis strains accumulate mutations in the fsr locus, in particular in fsrC, and upon selection, due to still unknown factors, the loss of the ability to transduce the GBAP signal is selected, as if the strains no longer need high levels of GelE and SprE for survival, and therefore do not require a fully efficient quorum-sensing Fsr system. As fsr has also been suggested to regulate other genes in the genome of E. faecalis (Bourgogne et al., 2006), it is also plausible that the advantage of the loss of quorum sensing is selected by other functions in the cell which are shut down. This raises interesting future prospects for further investigating the role of Fsr, GelE and SprE in the biology of E. faecalis.
In order to establish whether the GBAP non-responder behaviour in other E. faecalis isolates was due to the same W403STOP mutation or to other codon variations, we searched for this nonsense codon in seven genetically unrelated enterococcal isolates from our culture collection, which also carried the apparently complete fsr and gelE loci, but lacked gelatinase activity (Fig. 5). The same nonsense codon was detected in only two E. faecalis tested strains, suggesting that it is likely that other variations in the FsrC sequence lead to the same GBAP non-responder behaviour. The detection of this nonsense codon in Enterococcus durans suggests either an ancient event that was disseminated in the genus or a mutation which frequently occurs due to unknown specific conditions. We have also detected other codon variations in the fsrC gene of strain LN68. It is possible that these, though not responsible for the loss of FsrC activity, are needed for stabilization of the STOP403W substitution that seems to be deleterious in the LN68 genetic background. It is thus likely that different sets of fsrC codon variations will be responsible for negative gelatinase phenotypes in different genomes. In fact, when we searched the E. faecalis sequenced genomes (http://www.broadinstitute.org/annotation/genome/enterococcus_faecalis/MultiHome.html), we found different sets of codon variations in fsrC among strains with a negative gelatinase phenotype and positive genotype (Galloway-Peña et al., 2011). This fact, together with the inability of LN68 to displace the W403STOP mutation, suggests that each genome may accommodate different sets of codon variations in fsrC in order to achieve a gelatinase-negative phenotype.
Fig. 5.

Enterococcal strains with an incongruent gelatinase genotype and phenotype. The fsrC gene of these strains was sequenced and was found to carry the W403STOP mutation, indicated by a star.
In summary, previous work suggested that, besides deletion, mutations in any of the fsr or gelE genes could explain the negative gelatinase phenotype observed in natural E. faecalis isolates. However, no proof was available. In the present work we demonstrate that a single nonsense codon in fsrC is enough to explain the incongruence between a gelE genotype and a gelatinase-negative phenotype. The substitution of tryptophan 403 by a STOP codon in strain LN68 was shown to generate a truncated FsrC that was most likely impaired in the ATPase activity needed for GBAP signal transduction. By demonstrating the hypothesized cause–effect relationship between codon variations in the fsr–gelE loci and the gelatinase-negative phenotype, we came across interesting findings which may prove very important for future research on E. faecalis biology and host–pathogen relationships. First, it is clear that the missense mutations that we detected in other fsr genes and in gelE were not important for the negative gelatinase phenotype, as strain EF_SAVE5, despite still carrying those mutations, was able to produce gelatinase. Therefore, E. faecalis achieves the gelatinase-negative phenotype by accumulating mutations in the fsrC gene. Accordingly, and despite the fact that each isolate may accumulate different sets of codon variations in fsrC, the important fact here is that some E. faecalis isolates appear to stabilize a quorum-sensing non-responder behaviour. This is worth studying in future research on E. faecalis, as it could shed some light on the role of Fsr and gelatinase in the biology and pathogenicity of this important nosocomial pathogen.
Acknowledgements
The authors are grateful to Axel Hartke (Université de Caen, France) for kindly providing the V583 ErmS strain. This work was supported by Fundação para a Ciência e a Tecnologia (FCT) through project grant PDC/CVT/67270/2006, co-financed through FEDER, and grant PEst-OE/EQB/LA0004/2011, and by the bilateral cooperation project Portugal/France (GRICES/EGIDE, Pessoa program, 2006/07). N. T. is grateful to FCT for grant SFRH/BD/65750/2009. P. M. is grateful to FCT for grant SFRH/BPD/14595/2003.
Abbreviations:
- GBAP
gelatinase biosynthesis-activating pheromone
A supplementary figure and a supplementary table are available with the online version of this paper.
Edited by:
References
- Bourgogne A., Hilsenbeck S. G., Dunny G. M., Murray B. E. (2006). Comparison of OG1RF and an isogenic fsrB deletion mutant by transcriptional analysis: the Fsr system of Enterococcus faecalis is more than the activator of gelatinase and serine protease. J Bacteriol 188, 2875–2884. 10.1128/JB.188.8.2875-2884.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga T. M., Marujo P. E., Pomba C., Lopes M. F. (2011). Involvement, and dissemination, of the enterococcal small multidrug resistance transporter QacZ in resistance to quaternary ammonium compounds. J Antimicrob Chemother 66, 283–286. 10.1093/jac/dkq460 [DOI] [PubMed] [Google Scholar]
- Brinster S., Furlan S., Serror P. (2007). C-terminal WxL domain mediates cell wall binding in Enterococcus faecalis and other Gram-positive bacteria. J Bacteriol 189, 1244–1253. 10.1128/JB.00773-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. (1988). High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res 16, 6127–6145. 10.1093/nar/16.13.6127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Lee L. N., LeBlanc D. J. (1991). Improved electroporation and cloning vector system for Gram-positive bacteria. Appl Environ Microbiol 57, 1194–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton T. J., Gasson M. J. (2001). Molecular screening of Enterococcus virulence determinants and potential for genetic exchange between food and medical isolates. Appl Environ Microbiol 67, 1628–1635. 10.1128/AEM.67.4.1628-1635.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway-Peña J. R., Bourgogne A., Qin X., Murray B. E. (2011). Diversity of the fsr-gelE region of the Enterococcus faecalis genome but conservation in strains with partial deletions of the fsr operon. Appl Environ Microbiol 77, 442–451. 10.1128/AEM.00756-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar F., Teixeira N., Rigottier-Gois L., Marujo P., Nielsen-LeRoux C., Crespo M. T., Lopes M. F., Serror P. (2009). Virulence of Enterococcus faecalis dairy strains in an insect model: the role of fsrB and gelE. Microbiology 155, 3564–3571. 10.1099/mic.0.030775-0 [DOI] [PubMed] [Google Scholar]
- Gilmore M. S., Coburn P. S., Nallapareddy S. R., Murray B. E. (2002). Enterococcal virulence. In The Enterococci – Pathogenesis, Molecular Biology, and Antibiotic Resistance, pp. 301–354. Edited by Gilmore M. S. Washington, DC: American Society for Microbiology. [Google Scholar]
- Grant S. G., Jessee J., Bloom F. R., Hanahan D. (1990). Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc Natl Acad Sci U S A 87, 4645–4649. 10.1073/pnas.87.12.4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock L. E., Perego M. (2004). The Enterococcus faecalis fsr two-component system controls biofilm development through production of gelatinase. J Bacteriol 186, 5629–5639. 10.1128/JB.186.17.5629-5639.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha A. K., Bais H. P., Vivanco J. M. (2005). Enterococcus faecalis mammalian virulence-related factors exhibit potent pathogenicity in the Arabidopsis thaliana plant model. Infect Immun 73, 464–475. 10.1128/IAI.73.1.464-475.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law J., Buist G., Haandrikman A., Kok J., Venema G., Leenhouts K. (1995). A system to generate chromosomal mutations in Lactococcus lactis which allows fast analysis of targeted genes. J Bacteriol 177, 7011–7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenhouts K., Buist G., Bolhuis A., ten Berge A., Kiel J., Mierau I., Dabrowska M., Venema G., Kok J. (1996). A general system for generating unlabelled gene replacements in bacterial chromosomes. Mol Gen Genet 253, 217–224. 10.1007/s004380050315 [DOI] [PubMed] [Google Scholar]
- Lopes M. F., Pereira C. I., Rodrigues F. M., Martins M. P., Mimoso M. C., Barros T. C., Figueiredo Marques J. J., Tenreiro R. P., Almeida J. S., Barreto Crespo M. T. (1999). Registered designation of origin areas of fermented food products defined by microbial phenotypes and artificial neural networks. Appl Environ Microbiol 65, 4484–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes M. F., Simões A. P., Tenreiro R., Marques J. J., Crespo M. T. (2006). Activity and expression of a virulence factor, gelatinase, in dairy enterococci. Int J Food Microbiol 112, 208–214. 10.1016/j.ijfoodmicro.2006.09.004 [DOI] [PubMed] [Google Scholar]
- Maguin E., Prévost H., Ehrlich S. D., Gruss A. (1996). Efficient insertional mutagenesis in lactococci and other Gram-positive bacteria. J Bacteriol 178, 931–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkinen P. L., Clewell D. B., An F., Mäkinen K. K. (1989). Purification and substrate specificity of a strongly hydrophobic extracellular metalloendopeptidase (“gelatinase”) from Streptococcus faecalis (strain 0G1-10). J Biol Chem 264, 3325–3334. [PubMed] [Google Scholar]
- Mohamed J. A., Murray B. E. (2006). Influence of the fsr locus on biofilm formation by Enterococcus faecalis lacking gelE. J Med Microbiol 55, 1747–1750. 10.1099/jmm.0.46729-0 [DOI] [PubMed] [Google Scholar]
- Mundt J. O. (1986). Enterococci. In Bergey’s Manual of Systematic Bacteriology, pp. 1063–1065. Edited by Sneath P. H. A., Mair N. S., Sharpe M. E., Holt J. G. Baltimore: Williams & Wilkins. [Google Scholar]
- Nakayama J., Cao Y., Horii T., Sakuda S., Akkermans A. D., de Vos W. M., Nagasawa H. (2001a). Gelatinase biosynthesis-activating pheromone: a peptide lactone that mediates a quorum sensing in Enterococcus faecalis. Mol Microbiol 41, 145–154. 10.1046/j.1365-2958.2001.02486.x [DOI] [PubMed] [Google Scholar]
- Nakayama J., Cao Y., Horii T., Sakuda S., Nagasawa H. (2001b). Chemical synthesis and biological activity of the gelatinase biosynthesis-activating pheromone of Enterococcus faecalis and its analogs. Biosci Biotechnol Biochem 65, 2322–2325. 10.1271/bbb.65.2322 [DOI] [PubMed] [Google Scholar]
- Nakayama J., Kariyama R., Kumon H. (2002). Description of a 23.9-kilobase chromosomal deletion containing a region encoding fsr genes which mainly determines the gelatinase-negative phenotype of clinical isolates of Enterococcus faecalis in urine. Appl Environ Microbiol 68, 3152–3155. 10.1128/AEM.68.6.3152-3155.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogier J. C., Serror P. (2007). Safety assessment of dairy microorganisms: the Enterococcus genus. Int J Food Microbiol 126, 291–301. 10.1016/j.ijfoodmicro.2007.08.017 [DOI] [PubMed] [Google Scholar]
- Park S. Y., Kim K. M., Lee J. H., Seo S. J., Lee I. H. (2007). Extracellular gelatinase of Enterococcus faecalis destroys a defense system in insect hemolymph and human serum. Infect Immun 75, 1861–1869. 10.1128/IAI.01473-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. Y., Shin Y. P., Kim C. H., Park H. J., Seong Y. S., Kim B. S., Seo S. J., Lee I. H. (2008). Immune evasion of Enterococcus faecalis by an extracellular gelatinase that cleaves C3 and iC3b. J Immunol 181, 6328–6336. [DOI] [PubMed] [Google Scholar]
- Parkinson J. S., Kofoid E. C. (1992). Communication modules in bacterial signaling proteins. Annu Rev Genet 26, 71–112. 10.1146/annurev.ge.26.120192.000443 [DOI] [PubMed] [Google Scholar]
- Qin X., Singh K. V., Weinstock G. M., Murray B. E. (2000). Effects of Enterococcus faecalis fsr genes on production of gelatinase and a serine protease and virulence. Infect Immun 68, 2579–2586. 10.1128/IAI.68.5.2579-2586.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que Y. A., Haefliger J. A., Francioli P., Moreillon P. (2000). Expression of Staphylococcus aureus clumping factor A in Lactococcus lactis subsp. cremoris using a new shuttle vector. Infect Immun 68, 3516–3522. 10.1128/IAI.68.6.3516-3522.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahm D. F., Kissinger J., Gilmore M. S., Murray P. R., Mulder R., Solliday J., Clarke B. (1989). In vitro susceptibility studies of vancomycin-resistant Enterococcus faecalis. Antimicrob Agents Chemother 33, 1588–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E. F., Maniatis T. (1989). Molecular Cloning: a Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory. [Google Scholar]
- Sifri C. D., Mylonakis E., Singh K. V., Qin X., Garsin D. A., Murray B. E., Ausubel F. M., Calderwood S. B. (2002). Virulence effect of Enterococcus faecalis protease genes and the quorum-sensing locus fsr in Caenorhabditis elegans and mice. Infect Immun 70, 5647–5650. 10.1128/IAI.70.10.5647-5650.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck N., Hoffmann M., Sava I. G., Kim S. C., Hahne H., Tonkonogy S. L., Mair K., Krueger D., Pruteanu M. & other authors (2011). Enterococcus faecalis metalloprotease compromises epithelial barrier and contributes to intestinal inflammation. Gastroenterology 141, 959–971. 10.1053/j.gastro.2011.05.035 [DOI] [PubMed] [Google Scholar]
- Su Y. A., Sulavik M. C., He P., Makinen K. K., Makinen P. L., Fiedler S., Wirth R., Clewell D. B. (1991). Nucleotide sequence of the gelatinase gene (gelE) from Enterococcus faecalis subsp. liquefaciens. Infect Immun 59, 415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas V. C., Thurlow L. R., Boyle D., Hancock L. E. (2008). Regulation of autolysis-dependent extracellular DNA release by Enterococcus faecalis extracellular proteases influences biofilm development. J Bacteriol 190, 5690–5698. 10.1128/JB.00314-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas V. C., Hiromasa Y., Harms N., Thurlow L., Tomich J., Hancock L. E. (2009). A fratricidal mechanism is responsible for eDNA release and contributes to biofilm development of Enterococcus faecalis. Mol Microbiol 72, 1022–1036. 10.1111/j.1365-2958.2009.06703.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurlow L. R., Thomas V. C., Hancock L. E. (2009). Capsular polysaccharide production in Enterococcus faecalis and contribution of CpsF to capsule serospecificity. J Bacteriol 191, 6203–6210. 10.1128/JB.00592-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vebø H. C., Snipen L., Nes I. F., Brede D. A. (2009). The transcriptome of the nosocomial pathogen Enterococcus faecalis V583 reveals adaptive responses to growth in blood. PLoS ONE 4, e7660. 10.1371/journal.pone.0007660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vebø H. C., Solheim M., Snipen L., Nes I. F., Brede D. A. (2010). Comparative genomic analysis of pathogenic and probiotic Enterococcus faecalis isolates, and their transcriptional responses to growth in human urine. PLoS ONE 5, e12489. 10.1371/journal.pone.0012489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X., Zhao C., Budin-Verneuil A., Hartke A., Rincé A., Gilmore M. S., Auffray Y., Pichereau V. (2009). The (p)ppGpp synthetase RelA contributes to stress adaptation and virulence in Enterococcus faecalis V583. Microbiology 155, 3226–3237. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Inouye M. (2002). The role of the G2 box, a conserved motif in the histidine kinase superfamily, in modulating the function of EnvZ. Mol Microbiol 45, 653–663. 10.1046/j.1365-2958.2002.03061.x [DOI] [PubMed] [Google Scholar]