Abstract
The lipid transporter Arv1 regulates sterol trafficking, and glycosylphosphatidylinositol and sphingolipid biosyntheses in Saccharomyces cerevisiae. ScArv1 contains an Arv1 homology domain (AHD) that is conserved at the amino acid level in the pathogenic fungal species, Candida albicans and Candida glabrata. Here we show S. cerevisiae cells lacking Arv1 are highly susceptible to antifungal drugs. In the presence of drug, Scarv1 cells are unable to induce ERG gene expression, have an altered pleiotrophic drug response, and are defective in multi-drug resistance efflux pump expression. All phenotypes are remediated by ectopic expression of CaARV1 or CgARV1. The AHDs of these pathogenic fungi are required for specific drug tolerance, demonstrating conservation of function. In order to understand how Arv1 regulates antifungal susceptibility, we examined sterol trafficking. CaARV1/CgARV1 expression suppressed the sterol trafficking defect of Scarv1 cells. Finally, we show that C. albicans arv1/arv1 cells are avirulent using a BALB/c disseminated mouse model. We suggest that overall cell survival in response to antifungal treatment requires the lipid transporter function of Arv1.
Keywords: antifungal, Candida, virulence, sterol, Arv1, transcription, mycology, hyphae
1. Introduction
Candida species are opportunistic fungal pathogens responsible for a variety of mucosal and systemic infections in humans, especially immunocompromised individuals (Odds et al. 1988; Pfaller et al. 2000; Vazquez et al. 1999). C. albicans is considered the major pathogenic species in both mucosal and bloodstream infections; however, over the last two decades, a shift in prevalence of species has occurred. There is now an increase in non-albicans Candida, particularly C. glabrata (Krcmery and Barnes 2002; Odds et al. 1988; Pfaller et al. 2000; Pfaller et al. 1996; Sionov et al. 2005; Vazquez et al. 1999). C. glabrata is second only to C. albicans as the cause of systemic candidiasis in the United States (Pfaller et al. 1999). The shift is a result of the extensive use of azole drugs targeting the sterol pathway, which has led to the emergence of C. glabrata with inherently low susceptibility to azoles. The azole class of antifungal drugs includes the triazoles such as fluconazole and itraconazole, and imidazoles such as miconazole. (Ostrosky-Zeichner et al. 2010). Azole drugs inhibit the Erg11 lanosterol 14-α demethylase through binding to its cytochrome P450 element, which decreases Δ22 desaturation of the sterol moiety and end product ergosterol biosynthesis (Sanglard et al. 1998; Venkateswarlu 1997).
Candida sp. can become azole drug resistant by harboring mutations that (i) are within Erg11 and reduce its affinity for azole binding, (ii) increase ERG11 expression, (iii) result in loss of function of the Erg3 C-5 sterol desaturase, or (iv) increase the efflux of drug by overexpression of multi-drug transporters., (Morschhauser 2002). To date, very few azole resistant Candida glabrata strains have elevated ERG11 expression or Erg11 gain-of-function mutations. Rather, CgPDR1 transcription factor mutations are presently seen (Vermitsky and Edlind 2004).
S. cerevisiae has been used extensively as a model to study multi-drug resistance (Cannon et al. 2009; Gulshan and Moye-Rowley 2007; Morschhauser 2002; Moye-Rowley 2005; Sanglard et al. 2009; Shahi and Moye-Rowley 2009). Azole tolerance in S. cerevisiae is induced by activation of the pleiotrophic drug resistance pathway (PDR) (Balzi and Goffeau 1995; Moye-Rowley 2003; Prasad et al. 1995). Elevated expression of multiple ATP-binding cassette (ABC) and major facilitator superfamily transporters (MFS) is a hallmark of PDR induction (Bolhuis et al. 1997; Gaur et al. 2008). The S. cerevisiae ScPdr1/ScPdr3 zinc cluster transcription factors induce expression of the ScPdr5, ScSnq2, and ScYor1 ABC transporters necessary for drug resistance (Balzi et al. 1987; Mahe et al. 1996). ScPdr1 binds to PDRE promoter sites to activate transcription (Katzmann et al. 1994). Strains carrying dominant alleles of ScPDR1 (PDR1-3) or ScPDR3 (PDR3-7) constitutively express multiple ABC transporters and are azole resistant (Carvajal et al. 1997).
C. albicans contains the CaTAC1 gene encoding a transcription factor involved in regulating multi-drug efflux pump gene expression (Coste et al. 2004). Azole resistant clinical isolates carrying a hyperactive allele of CaTAC1 (TAC1-2) have constitutive expression of the CaCdr1/Cdr2 ABC transporters (Coste et al. 2004). The reintroduction of this allele into a tac1/tac1 null strain confers antifungal drug resistance to susceptible strains. Thus, it is well established that pathogenic and nonpathogenic fungi can harbor mutations in ScPDR1/3/CgPDR1/CaTAC1 that cause constitutive expression of multi-drug transporters in order to induce antifungal drug resistance.
The lipid transporter Arv1 is conserved from yeast to human (Swain 2002; Tinkelenberg 2000). In S. cerevisiae, ScArv1 is a 321-amino acid protein with three transmembrane domains (Villasmil and Nickels 2011). ScArv1 function is essential at high temperature and in mutants unable to esterify sterols (Tinkelenberg et al. 2000). Loss of Arv1 function results in defects in sphingolipid (Swain 2002) and glycosylphosphatidylinositol biosyntheses (Kajiwara et al. 2008), defects in sterol trafficking (Beh and Rine 2004; Fei et al. 2008; Tinkelenberg et al. 2000), and hypersusceptibility to the antifungal drugs nystatin and miconazole (Tinkelenberg et al. 2000). ScArv1 contains a conserved Arv1 homology domain (AHD) (Fig. 1) (Fores et al. 2006). Within the AHD is a zinc-binding motif characterized by two CXXC motifs separated by 20 amino acids. The S. cerevisiae AHD shares 49% and 67% identities with AHDs from C. albicans and C. glabrata, respectively.
Figure 1.
A sequence alignment of various eukaryotic AHDs. The zinc-binding motif is underlined. Identical and conserved amino acids are indicated.
Here we show that Scarv1 cells have increased susceptibility to several antifungal drugs, and that the ScAHD is required for normal drug susceptibility. Scarv1 cells are defective in inducing ScERG expression and increasing protein levels in the presence of fluconazole or lovastatin. They are also unable to activate PDR expression. Ectopic expression of CgARV1 or CaARV1 restores normal antifungal drug susceptibility, and proper gene and protein expression. Moreover, CaARV1/CgARV1 expression suppresses the sterol trafficking defect of Scarv1 cells (Fei et al. 2008). C. albicans strains lacking CaArv1 function harbor similar phenotypes. Finally, we show that loss of CaArv1 renders cells avirulent using a BALB/c disseminated mouse model. We suggest that Arv1 plays a central role in regulating multiple pathways required for normal antifungal susceptibility in pathogenic and nonpathogenic fungi, most likely through regulating the sterol trafficking necessary for antifungal-dependent signaling.
2. Materials and methods
2.1. Abbreviations
The following abbreviations are used throughout the text and figures: PDR, pleiotrophic drug response; ERG, ergosterol; Ca, Candida albicans; Cg, Candida glabrata; Sc, Saccharomyces cerevisiae; GPI, glycosylphosphoinositide.
2.2. Media, and Miscellaneous Microbial Techniques
Yeast strains were grown in YEPD (1% yeast extract, 2% bacto-peptone, 2% glucose) or in synthetic minimal media (0.67% Yeast Nitrogen Base (Difco) supplemented with the appropriate amino acids and adenine. All antifungal drugs were purchased from Sigma Chemicals. Antifungal drugs were added directly to liquid YEPD or synthetic media. For qRT-PCR analysis, cells were grown in the absence and presence of lovastatin (50 µg/ml) for 16 hr or fluconazole (50 µg/ml) for 6 hr. The concentrations and length of treatment for these compounds is based on literature (Davies 2005; Dimster-Denk 1996), as well as our own validation using MIC50 assays and qT-PCR analysis over time courses to demonstrate comparability between fluconazole and lovastatin treatments. Yeast transformation was performed using the procedure described by Ito et al., (Ito 1983). For routine propagation of plasmids, E. coli XL1Blue cells were used and grown in LB medium supplemented with ampicillin (150 µg/ml). RPMI 1640 media without bicarbonate, and containing glutamine and 2% glucose (pH 7.0) was used for susceptibility assays (Invitrogen).
2.3. Strain and Plasmid Construction
The S. cerevisiae strains used are derived from W303 (MATa ura3-52 leu2 his3 lys2 ade2) or BY4741 (MATa his3 leu2 met15 ura3). The C. albicans strain BWP17 (ura3/ura3 arg4/arg4 his1/his1) was used for all studies. Plasmids containing the S. cerevisiae, C. glabrata, and C. albicans full-length or AHD are derivatives of pRS416-URA3-CEN. The expression of various ARV1 alleles is driven from their endogenous promoters (−500 to 0). The Erg1, Pdr5, and Snq2 expressing plasmids are derivatives of pRS416-URA3-CEN, where a 13-Myc tag has been inserted at the carboxyl terminus. In each case, the endogenous promoter drives protein expression.
For disruption of ARV1 in C. albicans, the PCR-based gene disruption method (Wilson et al 1999) was utilized with primers that contained 20 bp homologous to the disruption plasmids, pGEM-URA3 and pRS-ARG4ΔSpeI, flanked with 70 bp of CaARV1 sequence to replace the CaARV1 coding sequence with URA3 or ARG4, respectively (CaARV1-5DR 5’CTGCTCTGATACTAGAGGCATTCAACGCCAGCATGTTTACATTGGGGAAGATACCG GATGTACCACCACCACTTTCCCAGTCACGACGTT 3’ and CaARV1-3DR 5’AATTGAACACTAAATACGAATACCCCAATCTAGTTAATGATTTAGACGGGCCAAT GACATTGGATGGTGTGGAATTGTGAGCGGATA 3’). To restore CaARV1 in deletion strains, the disruption plasmid, pDDB78-HIS, was used and was linearized with NruI to integrate it into the host strain. The C. albicans CaARV1 allele was amplified from genomic DNA and it contained NotI sites on each end (hARV1-CalbicanComp5F 5’ CAATCTGCATTTGGAACG 3’and hARV1-3RCalbicanComp 5’ TGAGCAAATACCAATTGC 3’). pDDB78-HIS1 was linearized with NotI and the CaARV1 allele was subcloned into the linearized vector. The vector generated was pDDB78-HIS –CaARV1, and was linearized with NruI and integrated into the CaARV1/CaARV1 host strain to generate the CaARV1/CaARV1 (his1::ARV1::HIS3). Yeast transformations were performed using the Frozen EZ Yeast Transformation II Kit as per the manufacturers’ instructions (Zymo Research). Recombinant heterozygote transformants were selected on synthetic minimal medium lacking uracil, arginine or histidine to obtain URA3, ARG4, and HIS3 transformants, respectively. Integration was verified by PCR. All strains were integrated with each marker (URA3, ARG4, and HIS3) to alleviate any effects due these alleles. Primer sequences used for all methods are available upon request.
2.4. S. cerevisiae relative fold susceptibility assays
For relative fold susceptibility assays using S. cerevisiae strains, cells were grown to early exponential phase (1 X 106) in Ura− synthetic media to maintain plasmid selection. Wild type and arv1 strains carrying the vector pRS424-URA3-CEN alone were used as controls. Strains were inoculated into 96-well plates with each well containing 1×103 cells. Serial two-fold dilutions of antifungal drug were tested for each strain. Amphotericin B and itraconazole drug concentration ranges were 0.016 to 16 µg/ml, and terbinafine and fluconazole drug concentration ranges were 0.063 to 64 µg/ml. Relative fold sensitivities were determined after 24hr of growth at 30°C for S. cerevisiae. Cell concentrations were measured at 630 nm (OD630) using a Biotek PowerWave HT340 plate reader. Relative fold susceptibility values were determined as the lowest concentration causing ~50% inhibition of growth at 24 hr. The values are the average of five independent experiments.
2.5. C. albicans relative fold susceptibility assays
C. albicans strains grown in the appropriate medium were serially 5-fold diluted, at a starting concentration of 1×106 cells/ml, and spotted onto agar plates with or without 0.5 µg/ml nystatin and incubated for 3 days at 35°C. Liquid relative fold susceptibility values were determined by serial diluting cultures to 1 X 104 cells/ml in RPMI. The starting concentration of drug used for C. albicans cells was 100 µg/ml terbinafine, 1 µg/ml itraconazole, 8 µg/ml fluconazole and 2 µg/ml amphotericin B. Absorbance was read at 630 nm at 24 hr.
2.6. Total RNA Isolation
All solutions were prepared with DEPC-treated water. Cells were harvested, centrifuged and pelleted for 30 sec. Cell pellets were resuspended in 200 µl of YRL buffer (200 mM Tris pH 7.5, containing 500 mM NaCl, 10 mM EDTA, 1% SDS) and 200 µl PCIAA (phenol, chloroform, isoamyl alcohol). 200 µl of nitric acid-washed beads were added and cells were vortexed for 2.5 min. 300 µl of YRL buffer and 200 µl PCIAA were added and cells were vortexed for 2.5 min. Cells were centrifuged for 5 min, and the resulting clear lysate was removed and added to 400 ml PCIAA, vortexed for 2.5 min, and centrifuged for 5 min. The resulting aqueous layer was added to 500 µl of 100% EtOH and total RNA was precipitated overnight at −20°C. A total RNA pellet was obtained by centrifuging at 13,000 rpm for 15 min. at 4°C, washed twice with 70% EtOH, vacuum-dried, and resuspended in water containing 1ug/ml DNase for removal of genomic material. RNA was stable at −20°C for several weeks.
2.7. qRT-PCR analysis
Cells were grown to exponential log phase at 30°C in YEPD media. Total RNA was extracted from 1 X 107 cells using acid-washed glass beads, SDS-YRL buffer (200 mM Tris pH 7.5, containing 500 mM NaCl, 10 mM EDTA, 1% SDS) and 200 µl PCIAA (phenol, chloroform, isoamyl alcohol). 1µl of 50 ng/ul RNA from samples and standards were assayed in triplicate. The reaction mix contained 11.5 µl of the SYBR Green master mix (Quanta) and 5µM primer sets and was loaded into 96-well plates. The plates were centrifuged at 1000 RPM for 30 seconds. qRT-PCR amplification was performed using a Stratagene MX3005 qRT-PCR system and data analysis was performed using the Stratagene Max-Pro (Mx3005) software version 4.0.
2.8. Western analysis
Erg1-myc, Erg11-myc and Erg25 containing strains were grown at 30°C in Ura− medium to exponential log phase. Cells were disrupted with cold glass beads and lysis buffer (Tris base, pH 7.9 containing (NH4)2SO4, MgSO4, Glycerol, and EDTA). Lysed cells were centrifuged at 3,300 rpm for 5 min at 4°C to obtain a total cell-free extract. 30ug of total cell extract was resolved by SDS-PAGE and transferred onto a nitrocellulose membrane.
Pdr5-myc and Snq2-myc containing strains were grown at 30°C in Ura− medium to exponential log phase. Cells were disrupted with cold glass beads and lysis buffer 1 (Tris base, pH 7.6 containing EDTA, AEBSF, and N-ethylmaleimide). Cells were then lysed for a second time with cold glass beads and lysis buffer 1 + 2% SDS. Lysed cells were centrifuged at 13,000g for 5 minutes at 4°C to obtain a total cell-free extract. Protein was resolved by SDS-PAGE in a 2X sample buffer and transferred onto a nitrocellulose membrane.
Western blot analysis was performed using anti-myc monoclonal antibodies: 1:1,000 dilutions (Erg1 and Erg11) and 1:500 (Pdr5 and Snq2); for Erg25, anti-Erg25 rabbit polyclonal antibodies were used: 1:1,000 dilutions; for Pgk1, anti-Pgk1 polyclonal antibodies were used at 1:10,000. For Arv1 and AHD, anti-Arv1 polyclonal antibodies were used: 1:10,000. Secondary antibodies were horseradish peroxidase-conjugated IgG secondary antibodies. For Myc epitope tag, a 1:2,000 dilution was used. For Erg25, a 1:2,000 dilution, and for Pgk1 and Arv1 a 1:20,000. Erg1-myc migrates at 55 kDa, Erg11-myc migrates at 60 kDa, Erg25 migrates at 36 kDa, Pdr5-myc and Snq2-myc migrate at approximately 140 kDA, Arv1 migrates at ~37 kDa and AHD at 12 kDa, and Pgk1 (loading standard) migrates at 44 kDa. All antibodies were dissolved in TBS + 1% non-fat milk and 1% goat serum.
2.9. Arv1 immunoprecipitation and western analysis
Cell extracts were obtained as described in the methods. 500 µg of cell free extract and 1 µg affinity-purified anti-Arv1 rabbit polyclonal antibodies were used to immunoprecipitate various forms of Arv1. Samples were incubated for 2 hr at 4°C, with agitation. A slurry of protein G Sepharose beads was mixed with samples and incubated at 4°C under rotary agitation for 4 hr. Samples were then centrifuged at 3,000 X g for 5 min. Supernatants were removed and beads were washed with RIPA buffer 2 times (each time centrifuging at 4°C and removing the supernatant). 25–50 µl of 2× loading buffer was added to the final pellet. Samples were boiled for 5 minutes, resolved using SDS-PAGE, and blotted onto PVDF paper.
The anti-Arv1 rabbit polyclonal antibodies were made by Lampire Biological Laboratories (Pipersville, PA) using a10 amino acid peptide of the ScAHD. Polyclonal antibodies were affinity purified using a peptide-bound resin. The amino acid sequence chosen was optimized so that the antibodies recognize ScArv1, CaArv1, and CgArv1 and their respective AHDs.
Western analysis was performed using affinity-purified anti-Arv1 rabbit polyclonal antibodies. The PVDF membrane was blocked with 5% Milk in Buffer A (10 mM Tris-HCl, pH 7.4, containing 125 mM NaCl) + 0.05% Tween overnight at 4°C. The membrane was washed four times with Buffer A + 0.05% Tween, and subsequently incubated with primary antibodies dissolved in buffer A + 1% goat serum for 2 hr. The membrane was washed 4 times in buffer A + 0.05% Tween, and incubated with secondary mouse anti-rabbit IgG HRP-conjugated antibodies dissolved in buffer A + 1% goat serum. The membrane was washed six times with buffer A + 0.05% Tween and developed using ECL Chemiluminescence reagents (ECL, Amersham). Bands were visualized using Kodak Xomat film. A 1:1,000 dilution of primary and 1:5,000 dilution of secondary antibodies were used.
2.10. rabbit anti-Arv1 polyclonal antibodies production
Rabbit anti-Arv1 polyclonal antibodies were generated by Lampire Biological Laboratories (Pipersville, PA). Antibodies were generated against the amino acid sequence YVEIDNVLLFIDLC. The antibodies were affinity purified using an amino acid sequence linked-resin by Lampire. The antibodies recognize ScArv1, ScAHD, CaArv1, CaAHD, CgArv1, and CgAHD (Fig. S1).
2.11. S. cerevisiae and Candida albicans filipin staining
Sterol lipid distribution was examined using filipin staining. Briefly, S.cerevisiae cells were grown to 2 X107 cells/ml in synthetic medium and C albicans cells were grown to 3 X107 cells/ml in YEPD. They were fixed with 3.7% EM-grade formaldehyde for 10 min at 23°C under constant mixing. Fixed cells were centrifuged and washed with distilled water. Cells were incubated with 10 µg/ml filipin complex for S.cerevisiae and 5 µg/ml filipin complex (Sigma Chemicals) for C. albicans (Sigma Chemicals) in the dark for 15 min at 23°C. Filipin fluorescence was observed with UV optics using a Leica DRBME fluorescence microscope. 500 cells were analyzed for staining. All pictures were exposed to fluorescence for 2 seconds. Values are the average of three independent experiments.
2.12 Morphology studies
To induce hyphal growth in liquid culture, overnight cultures of C. albicans wild-type and arv1 mutant cells grown in YEPD were diluted to 5 × 106 cells/ml into YEPD, pH 7 + 10% fetal bovine serum and incubated at 37°C. Cells were visualized by light microscopy using a Leica DRBME microscope and PlanAPO 40X and 100X objectives. Images were obtained using Open Labs software (version 2.1)
2.13 Disseminated mouse model studies
For virulence assays, the disseminated candidiasis mouse model was employed. Female BALB/c mice (Jackson Labs) ages 6–8 weeks, weighing approximately 18–22g, housed in groups of as many as four animals, were supplied food and water ad libitum. C. albicans strains CaJN5 (ARV1/ARV1), CaJN3 (ARV1/arv1::URA3), and CaJN7 (arv1::URA3/arv1::ARG4) are in the BWP17 strain background. They were grown overnight in YEPD medium (1% yeast extract, 2% bactopeptone and 2% dextrose) at 30°C, harvested by centrifugation, washed twice with 1X phosphate-buffered saline (PBS), counted by hemocytometry, and resuspended in 1X PBS at the required density. For survival experiments, mice were injected via the tail vein with 200 µl of 1X106 cells/ml of C. albicans in 1X PBS. Infected animals were monitored daily for up to 30 days post-infection and were considered moribund when they had a ruffled coat, were hypothermic, and could no longer reach food or water. Moribund animals, along with mice surviving to the end of the experiment, were euthanized by CO2 asphyxiation and survival times were recorded. Kaleidagraph 4.0 was used for statistical significance determination. All experimental procedures were carried out according to the NIH guidelines for the ethical treatment of animals.
3. Results
3.1. Scarv1 cells are hypersusceptible to multiple antifungal drugs
Scarv1 cells are susceptible to nystatin, miconazole and fluconazole (Thevissen et al. 2007; Tinkelenberg et al. 2000). We were interested in determining if they were susceptible to other commonly used antifungal drugs. We also wanted to determine whether the conserved AHD was required for maintaining normal drug susceptibility. Western analysis has already demonstrated that the ScAHD is expressed in mutant cells to a level that is similar to full-length ScArv1 (Fig. S1A & B) (Villasmil and Nickels. 2011).
We extended the drug susceptibility profile, and found that mutant cells were also highly susceptible to terbinafine (squalene epoxidase inhibitor, Erg1) (28-fold), itraconazole (lanosterol 14-α demethylase inhibitor, Erg11) (5-fold), lovastatin (HMG CoA reductase inhibitor) (32-fold), and amphotericin B (ergosterol binding agent) (3-fold) when compared to wild type cells (Table 1). Expressing full-length ScARV1 in Scarv1 cells restored normal antifungal drug susceptibility for all drugs tested (Table 1). While ScAHD expression in Scarv1 cells restored normal susceptibility to the polyene drugs, amphotericin B (Table 1) and nystatin (Fig. 2A), mutant cells remained susceptible to itraconazole (4.3-fold), terbinafine (24-fold), and lovastatin (27-fold). These results indicate, that although the AHD is important for maintaining normal polyene antifungal drug susceptibility, domains outside of the AHD are required for normal susceptibility to multiple antifungal drugs with different modes of action.
Table 1.
Summary of MIC50 values
| MIC50 valuesa | |||||
|---|---|---|---|---|---|
| Strain | Plasmid | Amphotericin | Itraconazole | Terbinafine | Lovastatin |
| WT | pRS414 | 0.42 ± 0.08 | 0.17 ± 0.04 | 8.0 ± 0.01 | 50 ± 0.74 |
| arv1 | pRS414 | 0.13 ± 0.01 | 0.03 ± 0.01 | 0.31 ± 0.04 | 1.6 ± 0.3 |
| arv1 | pRS414-ScARV1 | 0.40 ± 0.11 | 0.19 ± 0.01 | 7.8 ± 0.03 | 52 ± 0.5 |
| arv1 | pRS414-ScAHD | 0.37 ± 0.03 | 0.04 ± 0.07 | 0.33 ± 0.04 | 1.9 ± 0.7 |
values are µg/ml
Figure 2.
The effects of expressing CaARV1 or CgARV1 on growth in the presence of nystatin. Cells were dropped onto the appropriate drop out minimal medium plates at 10-fold serial dilutions (1 X 106 cells in the first well). Cells were grown in the absence or presence of 0.5 µg/ml nystatin at 30°C for 48 hr. (A) The expression of various forms of ScArv1 and their effect on growth. (B) The expression of various forms of CaArv1 and their effect on growth. (C) The expression of various forms of CgArv1 and their effect on growth. (D) The growth of various CaARV1 strains at 35°C for 48 hr.
3.2. The expression of CaARV1 or CgARV1 in Scarv1 cells can compensate for ScArv1 function
The pathogenic fungi C. albicans and C. glabrata each contain a single Arv1 ortholog. We asked if their functions were conserved with ScArv1. We expressed CaARV1 or CgARV1 from their endogenous promoters in Scarv1 cells, and determined whether their expression suppressed antifungal susceptibilities. Western analysis showed that CaArv1 and CgArv1 were expressed in Scarv1 cells to levels seen for ScArv1 (Fig. S1A). Expressing full-length CaARV1 or CgARV1 in Scarv1 cells suppressed the nystatin growth defect (Fig. 2B and 2C) and the multiple antifungal susceptibilities listed in Table 2. The susceptibility values observed were similar to those seen for wild type cells. Thus, CaArv1 and CgArv1 share function with ScArv1 with regards to maintaining normal susceptibility to multiple antifungal drugs.
Table 2.
Summary of MIC50 values in strains expressing CaARV1/CgARV1 alleles
| MIC50 valuesa | |||||
|---|---|---|---|---|---|
| Strain | Plasmid | Amphotericin | Itraconazole | Terbinafine | Lovastatin |
| WT | pRS414 | 0.41 ± 0.04 | 0.15 ± 0.02 | 8.4 ± 0.0 | 47 ± 0.6 |
| arv1 | pRS414 | 0.11 ± 0.02 | 0.01 ± 0.01 | 0.27 ± 0.02 | 1.5 ± 0.1 |
| arv1 | pRS414-CaARV1 | 0.44 ± 0.02 | 0.14 ± 0.05 | 8.2 ± 0.07 | 45 ± 0.3 |
| arv1 | pRS414-CaAHD | 0.38 ± 0.02 | 0.02 ± 0.01 | 0.30 ± 0.02 | 1.5 ± 0.1 |
| arv1 | pRS414-CgARV1 | 0.37 ± 0.06 | 0.14 ± 0.02 | 7.6 ± 0.03 | 45 ± 0.3 |
| arv1 | pRS414-CgAHD | 0.38 ± 0.06 | 0.02 ± 0.01 | 0.24 ± 0.05 | 1.8 ± 0.04 |
Values are µg/ml.
We next asked whether the AHDs of C. albicans and C. glabrata were also conserved in function. Again, we expressed CaAHD and CgAHD from their endogenous promoters in Scarv1 cells. Western analysis indicated that CaAHD and CgAHD were expressed in Scarv1 to levels similar for ScAHD (Fig. S1B). Expression of the C. albicans AHD in Scarv1 cells partially remediated the nystatin (Fig. 2B) and amphotericin growth defects of Scarv1 cells, but was unable to remediate the remaining antifungal susceptibilities (Table 2). The C. glabrata AHD also suppressed only nystatin (Fig. 2C) and amphotericin B susceptibilities (Table 2). These results were similar to those observed for the ScAHD. Based on these results, we conclude that the C. glabrata and C. albicans AHDs share functional redundancy with ScAHD with regards to antifungal susceptibility.
3.3. arv1 cells are defective in stimulating ERG and PDR expression in the presence of multiple antifungal drugs
What is the reason for the antifungal drug susceptibilities of Scarv1 cells? As induced ERG/PDR gene expression is a prerequisite for drug tolerance, we tested if Scarv1 cells had defects in this process. We determined the expression levels of several ERG and PDR genes in Scarv1 cells treated with 50µg/ml lovastatin for 16 hr. We observed that wild type cells induced expression to varying degrees in response to drug treatment (Fig. 3A–3H). While Scarv1 cells treated with lovastatin induced the expression of ScERG3 to nearly wild type levels (~2.5) and ScERG25 to greater than wild type (~4.5 fold), they did not induce ScERG1 or ScERG11 (Fig. 3A–3D). In addition, Scarv1 cells had higher basal expression levels of ScPDR1 and ScPDR3 than wild type cells (~1.5 and 2.5 fold, respectively) (Fig. 3E & 3F). Strikingly, elevated levels of all genes returned to wild type basal levels in the presence of drug (Fig. 3E and 3F). Thus, Scarv1 cells are unable to properly induce expression of the ERG and PDR pathways in the presence of antifungal drug treatment.
Figure 3.
The effects on mRNA expression levels due to loss of ScArv1. Cells were grown to exponential phase (1 X 107 cells) at 30°C in YEPD liquid medium. Wild type and Scarv1 cells were then grown in the absence and presence of 50 µg/ml lovastatin for 16 hr, and mRNA levels of various genes were determined using qRT-PCR. The relative expression values are the average of three independent experiments.
We also examined the expression levels of the ABC transporter ScSNQ2 and the multidrug transporter ScFLR1 (Alarco et al. 1997; Decottignies et al. 1995), which are induced in the presence of antifungal drugs through PDR activation. We reasoned, that if PDR1/3 levels were elevated in Scarv1 in the absence of drug, but reduced in its presence, genes activated by this pathway should show the same trend. ScFLR1 and ScSNQ2 were induced to varying degrees in wild type cells in the presence of drug (Fig. 3G & 3H). Once again elevated basal levels of ScSNQ2 and ScFLR1 were seen in Scarv1 cells compared to wild type (~2.5- and 50-fold), and their expression levels were reduced rather then induced in the presence of lovastatin. Thus, SNQ2/FLR1 expression levels in the absence and presence of lovastatin parallels those levels observed for ScPDR1/3.
We next asked whether Scarv1 cells show the same expression defects in the presence of the azole drug, fluconazole. We treated cells in the absence and presence of 50 µg/ml fluconazole for 6 hr, and then determined expression levels. Wild type cells responded as expected, we observed a drug-dependent increase in the expression of all genes tested (Fig. 4A–4H). While Scarv1 cells had a moderate defect in upregulating ScERG3 in the presence of drug compared to wild type (~12- vs. 24-fold), they were unable to induce ScERG1 and ScERG11 expression (Fig. 4A–4C). ERG25 expression was induced in wild type and Scarv1 cells in the presence of fluconazole (Fig. 4D).
Figure 4.
The effect of loss of ScArv1 on mRNA expression levels in fluconazole-treated cells. Cells were grown to exponential phase at 30°C in liquid YEPD medium. Wild type and Scarv1 cells were grown at 30°C in the absence or presence of 50 µg/ml fluconazole for 6 hr, and mRNA levels of various genes were determined using qRT-PCR. The relative expression values are the average of three independent experiments.
A similar trend was seen for ScPDR1 and ScPDR3 expression (Fig. 4E & 4F). Wild type cells induced the expression of ScPDR1 or ScPDR3 in the presence of fluconazole, while Scarv1 cells were unable to stimulate drug-dependent gene induction. Again elevated levels of ScPDR1 and ScPDR3 were observed in mutant cells. Although we did not see elevated basal levels of ScSNQ2/ScFLR1 in mutant cells, we did observe the same trend of lacking the ability to stimulate expression in the presence of drug (Fig. 4G & 4H). Based on these results, we conclude that Scarv1 cells have defects in ERG/PDR signaling pathways required for normal antifungal drug susceptibility. We must point out that arv1 mutants have elevated basal gene expression and retain basal levels of pump expression in the presence of drug. Thus, we cannot rule out that there are sufficient pump protein levels for drug tolerance, but susceptibility occurs because they are defective in function due to loss of Arv1.
3.4. ScArv1 is required for antifungal drug-induced increased protein levels
We next wanted to know whether the mRNA expression defects we observed caused a concomitant decrease in protein levels. ScErg1, ScErg11, and ScErg25 protein levels were analyzed by western analysis. Wild type cells responded to lovastatin or fluconazole treatment by increasing ScErg1, ScErg11, and ScErg25 levels (Fig. 5A), whereas only ScErg25 levels increased in Scarv1 cells in the presence of either drug; the exception being the induction of ScErg11 in the presence of fluconazole (Fig. 5A). The reason for this is unknown, but may be due to fluconazole stabilizing Erg11. The lower band seen for Erg1 in the absence of fluconazole is most likely a degradative product, possibly due to some variation in its stability during extraction (double asterisk).
Figure 5.
The effects on protein levels due loss of ScArv1. Cells were grown to exponential phase at 30°C in liquid YEPD medium. Wild type and Scarv1 cells were then grown at 30°C in the absence or presence of 50 µg/ml of lovastatin and 50 µg/ml fluconazole for 16 hr and 6 hr, respectively. Protein expression levels were determined by western analysis. Epitope tagged Erg1 and epitope-tagged Erg11 were detected using monoclonal anti-Myc antibodies; Erg25 was detected using anti-Erg25 polyclonal antibodies (A) WT and Scarv1 cells harboring an empty vector control, (B) Scarv1 cells harboring a full-length CaARV1, (C) Scarv1 cells harboring a full-length CgARV1. The western blots are typical representations of three independent experiments. Left panels represent protein levels in the absence and presence of lovastatin; right panels represent protein levels in the absence and presence of fluconazole. Asterisk, possible Erg1 degradative product.
We next examined ScPdr5 and ScSnq2 levels and quantitated protein using densitometry; levels were quantitated to eliminate any ambiguity in our results due to the low levels of protein observed (Fig. 6A–6D). In our hands, Pdr5 and Snq2 migrated as 140 kDa proteins, possibly because of migration defects due to the epitope tag. These bands were not present in wild type or Scarv1 cells harboring vector control.
Figure 6.
The effect of loss of ScArv1 on ScPdr5 and ScSnq2 protein levels. Cells were grown to exponential phase at 35°C in YEPD medium. Wild type and Scarv1 cells were then grown at 30°C in the absence or presence of 50 µg/ml of lovastatin or 50 µg/ml fluconazole for 16 hr and 6 hr, respectively. Protein expression levels were determined by western analysis. Epitope-tagged Pdr5 and Snq2 were detected using monoclonal anti-Myc antibodies. (A) Pdr5; (B) Snq2. The western blots are typical representations of three independent experiments. Left panels represent protein levels in the absence and presence of lovastatin; right panels represent protein levels in the absence and presence of fluconazole.
Wild type cells had a slight increase in ScPdr5 in the presence of lovastatin (1.3-fold) or fluconazole (1.8-fold) (Fig. 6A & B), while Scarv1 cells had higher basal levels in the absence of each drug (1.5-fold and 3-fold), respectively (Fig. 6A & 6B). Concomitant with the decrease in expression levels we observed for PDR5, there was a decrease in protein level in mutant cells in the presence of drug (Fig. 6A & 6B). As with Pdr5, the levels of ScSnq2 in drug treated wild type cells increased in the presence of lovastatin (2.0-fold) or fluconazole (2.5-fold), but Scarv1 cells had higher basal levels (5.0- and 1.2-fold) that slightly decreased upon drug treatment (Fig. 6C & 6D).
3.5. CaARV1 and CgARV1 suppress the mRNA and protein expression defects of Scarv1 cells
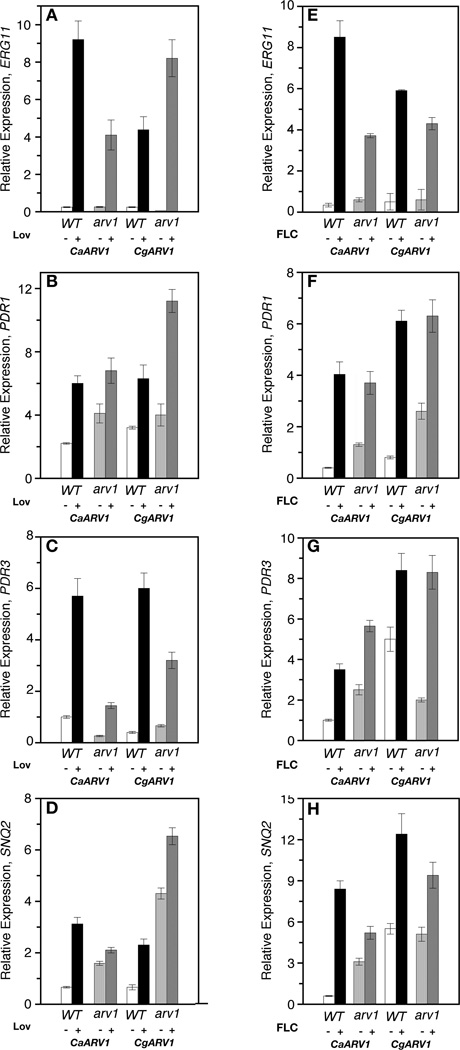
We next tested if CaARV1 or CgARV1 expression suppressed the gene expression and/or the protein level defect of mutant cells. Scarv1 cells expressing CaARV1 or CgARV1 were treated with lovastatin or fluconazole. Gene expression was detected by qRT-PCR (Fig. 7), and protein levels were determined by western analysis (Fig. 6). The expression of either gene suppressed the mRNA defects to varying degrees in the presence of either lovastatin (Fig. 7A–D) or fluconazole (Fig. 7E–7H).
Figure 7.
mRNA levels in antifungal-treated Scarv1 cells expressing CaARV1 or CgARV1. Cells were grown to exponential phase at 30°C in the appropriate drop out minimal medium. Wild type or Scarv1 cells expressing CaARV1 or CgARV1 were then grown at 30°C in the presence of 50 µg/ml lovastatin (A–D) and fluconazole (E–H) for 16 hr and 6 hr, respectively, and the mRNA levels of various genes were subsequently determined using qRT-PCR. The relative values are the average of three independent experiments.
Some differences, however, were observed when compared to mutant cells expressing ScARV1. In lovastatin-treated mutant cells, CaARV1 expression was not as efficient in inducing the expression of PDR3 and SNQ2 (Fig. 7C & 7D), and in the presence of fluconazole, ERG11 and SNQ2 expression was reduced (Fig. 7E & 7H). CgARV1 expression in lovastatin treated mutant cells resulted in a lower drug-induced level of PDR3 (Fig. 7C), while Scarv1 cells expressing the same allele had nearly wild type expression patterns in cells treated with fluconazole (Fig. 7E–7H). Although the mRNA expression patterns were not identical when compared to wild type cells, protein levels were induced. ScErg1, ScErg11, Pdr5 and Snq2 levels increased in the presence of either drug in Scarv1 cells expressing CaARV1 or CgARV1 (Fig. 5B & 5C; 6I & 6J).
3.6. Expression of CaARV1 or CgARV1 suppress the sterol trafficking defects of Scarv1 cells
Several lipid microdomains having distinct subcellular structures have been visualized in S. cerevisiae by filipin staining (Villasmil et al. 2011) (Fig. 8). Using this method, it was shown that Scarv1 cells harbor defects in sterol localization and trafficking (Beh and Rine 2004; Fei et al. 2008; Tinkelenberg 2000). The sterol trafficking defects of Scarv1 cells have been suggested to contribute to, or be responsible for, some or all Scarv1 phenotypes (Kajiwara et al. 2008; Swain et al. 2002; Tinkelenberg et al. 2000; Villasmil et al. 2011). Based on this hypothesis, we asked if CaARV1 or CgARV1 expression remediated the sterol trafficking defects of Scarv1 cells. Using filipin staining. we found that wild type cells localized their sterol content to several domains, including the plasma membrane rim (~71%), within membrane strands that are thought to be ER-associated (10%), and to punctate structures (18%) (Fig. 8). Scarv1 cells localized a greater proportion of their sterol in membrane strand lipid domains (41% (arv1) vs. 10% (wt)), while they also harbored defects in localizing lipid to punctate structures and the plasma membrane surface (rim). The sterol domain localization percentages we observed were similar to what has been previously reported (Fei et al. 2008; Villasmil and Nickels 2011). Importantly, CaARV1 or CgARV1 expression restored normal sterol trafficking and localization to Scarv1 cells (Table 3). Thus, remediation of the sterol trafficking defects of Scarv1 cells by CaArv1 and CgArv1 correlates well with the restoration of ERG/PDR signaling, and the re-establishment of normal susceptibility to antifungal drug treatment.
Figure 8.
Percentage of various lipid microdomains in various ScARV1 cells. Cells were grown to exponential phase at 30°C in the appropriate drop out minimal medium. Cells were fixed with EM-grade formaldehyde for 10 min at 23°C. Fixed cells were incubated with 10 µg/ml filipin complex in the dark for 15 min at 23°C. Unesterified microdomains were visualized using fluorescence microscopy. Values are expressed as percentages of total cells.
3.7. C. albicans arv1/arv1 are susceptible to antifungal drugs and harbor defects in antifungaldrug induced gene expression
All our conservation experiments have been performed by ectopic expression of either CaARV1 or CgARV1 in S. cerevisiae. To further confirm conservation, we asked if C. albicans strains lacking Arv1 function had similar phenotypes. We first examined antifungal susceptibilities and found that C. albicans arv1/arv1 cells were 50- and 3-fold more susceptible to itraconazole and terbinafine, respectively, when compared to wild type cells. CaARV1/arv1 MIC50 values were 22.5 ug/ml for itraconazole and 0.04 µg/ml for terbinafine. They were also more susceptible then wild type cells to nystatin using solid agar growth assays (Fig. 2D), but were not susceptible to fluconazole or amphotericin B treatment. The reintroduction of the wild CaARV1 allele restored normal drug susceptibilities. The growth rates of all strains tested were similar, thus the differences in susceptibilities were not due to defects in growth rate.
We next asked if CaARV1/arv1 cells were defective in drug-induced gene expression. CaARV1/ARV1 cells were able to induce the expression of CaTAC1, CaERG11, CaERG25, the efflux pump gene, CaCDR1, and the CaFCR1 transcription factor in the presence of fluconazole (Fig. 9A–9E). While CaARV1/arv1 cells induced CaERG25 expression in the presence of drug (Fig. 9C), they were unable to induce any other genes. Moreover, TAC1 (~4-fold) and ERG11 (~3-fold) expression levels were elevated in the absence of fluconazole (Fig. 9A & B). While the expression of ERG11 returned to basal level (Fig. 9B) in the presence of drug, TAC1 levels remained high (Fig. 9A). Thus, as observed for Scarv1 cells, C. albicans arv1/arv1 cells are also unable to maintain normal antifungal drug susceptibility, and lack the ability to properly induce antifungal-responsive gene expression.
Figure 9.
The effects on mRNA expression levels due to loss of CaArv1. Cells were grown to exponential phase at 30°C in YEPD liquid medium. Wild type and arv1 cells were then grown in the absence and presence of fluconazole for 6 hr, and mRNA levels of various genes were determined using qRT-PCR. The relative expression values are the average of three independent experiments.
As the sterol trafficking defects of Scarv1 cells are thought to contribute to Scarv1 phenotypes, we examined sterol localization in CaARV1/ARV1, CaARV1/arv1, and CaARV1/arv1 cells using filipin staining. We found that CaARV1/ARV1 cells localized a large percentage of their sterol to the plasma membrane (93%), while the majority of CaARV1/arv1 cells contained intracellular punctate structures (85%) (Fig. 10). Upon transfer to hyphal medium, CaARV1/ARV1 cells began to form hyphae by 1 hr, and their sterol content localized to the hyphal tip (88%) (Fig. 10). CaARV1/arv1 cells were delayed in hyphae formation, but also localized their sterol to the hyphal tip (85%) (Fig. 10). At 1 hr, wild type cells began to form striated lipid structures along the hyphal projection (Fig. 10, arrow) (Martin and Konopka 2004). CaARV1/arv1 mutants were delayed in the formation of these structures by approximately 1 hr. This may be the result of differences in CaARV1 gene dosage. CaARV1/arv1 strains were even more delayed in the formation of these structures, and the percentage of cells containing these structures at 3 hr was only 20% compared to ~90% for wild type cells. Thus, CaARV1/arv1 cells, like Scarv1 cells, harbor defects in lipid trafficking. We point out that we do not know what sterol intermediates are being labeled by filipin, only that they are unesterified. In any case, the antifungal drug response defects and sterol trafficking defects due to loss of CaArv1 are highly similar to those seen for loss of ScArv1, strongly suggesting that Arv1 function is conserved between pathogenic and nonpathogenic fungi.
Figure 10.
Filipin staining of various C. albicans strains. All strains were grown to exponential phase in YEPD at 30°C. Cells were then transferred to serum containing medium and grown at 37°C. After shift to serum, cells were fixed at the indicated times with 3.7% EM grade formaldehyde, and subsequently filipin-stained in order to visualize unesterified sterol localization. The arrows point out striated sterol containing structures. Insets are enlargements of the indicated picture; arrows with asterisks indicate where the picture has been enlarged. Pictures were taken using a 2 second exposure time.
3.8. C. albicans arv1/arv1 cells lack virulence using a disseminated mouse model
What physiological consequences to virulence result from CaARV1/arv1 phenotypes? We tried to answer this question by using a BALB/c disseminated candidiasis mouse model and determining whether C. albicans cells lacking CaArv1 function retained virulence. 5 X 105 cells of each strain were injected into the tail veins of BALB/c mice and viability was monitored for 30 days (Fig. 11). All mice injected with CaARV1/ARV1 or CaARV1/arv1 cells died within the first 8 days (Fig. 11), with ~50% death observed by 4 days. Mice injected with CaARV1/arv1 cells were all viable at 30 days post injection at which time the study was terminated (Fig. 11).
Figure 11.
Disseminated candidiasis mouse model studies. For disseminated candidiasis studies, BALB/c mice were tail vein injected with 1 X 105 cells of CaARV1/ARV1, CaARV1/arv1, and CaARV1/arv1 strains that were grown to exponential phase at 30°C. Mice were checked every day for signs of disseminated candidiasis, such as hypothermia and coat ruffling. The mice were sacrificed at this point and counted as deceased on that day. The average days survived; solid line, ARV1/ARV1; segmented line, ARV1/arv1; dotted line, arv1/arv1.
4. Discussion
C. albicans and C. glabrata cause fungal infections, which are an increasing problem in immunocompromised individuals (Odds et al. 1988; Pfaller et al. 2000; Vazquez et al. 1999). Gain-of-function mutations, loss of heterozygosity, and gene amplification all contribute to clinical resistance (Morschhauser 2002; Morschhauser 2010). Drug-induced transcription of ERG and PDR genes facilitates drug resistance by inducing a dramatic increase in factors required for tolerance (Gulshan and Moye-Rowley 2007; Sanglard et al. 2009). Scarv1 cells harbor defects in sterol, sphingolipid, and GPI biosyntheses and they have lipid trafficking defects (Fei et al. 2008; Swain 2002; Tinkelenberg 2000). Based on these data, we hypothesized arv1 defects lead to hypersusceptibility to antifungal drugs. We further hypothesized, based on amino acid sequence similarities between the C. albicans and C. glabrata AHDs with their ScAHD ortholog, that Arv1 function was conserved. Here, we have demonstrated a functional conservation between the pathogenic and nonpathogenic yeast Arv1 lipid transporter, particularly, the AHD. Moreover, we have discovered an important role for CaArv1 in hyphal formation, sterol trafficking, proper antifungal-induced gene expression, and being involved either directly or indirectly in virulence.
We recently elucidated the membrane topology of ScArv1 (Villasmil and Nickels 2011). ScArv1 has a large ER luminal loop that is important for growth at high temperature, sterol trafficking, and maintaining antifungal tolerance. Mutations within this loop result in susceptibility to multiple antifungal agents (M. Villasmil and J.T. Nickels, manuscript submitted). These results will help us to elucidate why Arv1 lipid transporter function is required for the proper response to antifungal drug-induced stress, while uncovering domains outside the AHD required for maintaining normal antifungal susceptibility.
CaARV1 and CgARV1 functions are conserved with ScArv1
Arv1 function is required for antifungal drug-induced transcription, as ERG and PDR/CDR1 expression profiles are perturbed in S. cerevisiae and C. albicans arv1 mutants. The expression of full-length CaARV1 or CgARV1 in Scarv1 cells can compensate for loss of ScArv1. Thus, there is a strong correlation between heterologous expression of CaARV1 and CgARV1 and suppression of antifungal susceptibility, transcriptional phenotypes, and sterol trafficking defects, further suggesting conservation of function. It must be noted that CaARV1 uses CTG for Leu rather than Ser and consequently CaArv1 L260 is translated as S260 in S.cerevisiae. This could have an effect on expression and possibly function.
How might Arv1 function to regulate these processes? We suggest that Arv1 may be required to properly localize cell factors, and/or lipid constituents to the plasma membrane in order to maintain stress signaling and proper sterol trafficking in the presence of antifungal drugs. Alternatively, loss of function of arv1 may result in the constitutive upregulation of ERG and PDR expression as a compensating response. It is possible that the altered membrane lipids associated with arv1 mutation result in reduced function of the ERG and PDR membrane-associated proteins. This issue could be resolved by performing enzymatic and efflux pump assays in wild type and arv1 mutants.
CaARV1 or CgARV1 expression in Scarv1 cells remediates the sterol trafficking defects of mutant cells, suggesting that CaArv1 and CgArv1 can properly interact with the S. cerevisiae sterol trafficking machinery that is necessary for lipid transport and localization. What might be the function of fungal Arv1 during trafficking? It has been suggested that ScArv1 may be a GPI flippase that is required for the transport of a specific GPI intermediate (Kajiwara et al. 2008). Thus, it is possible that the sterol trafficking defects we observe are secondary to loss of GPI synthesis. It is just as conceivable that the GPI defects observed in that study may be secondary to the sterol trafficking. ScArv1 could be a general lipid flippase, which has the ability to traffic different lipids. It has been suggested that Pdr5 is a PE/PC flippase (Kean et al. 1997; Pomorski et al. 2003). Arv1 may function in a similar manner. Finally, it is possible that ScArv1 may be a sterol binding protein that directly traffics sterol from the ER to the golgi. We have shown that ScArv1 is localized to both the ER and golgi in S. cerevisiae (Swain 2002). In mammalian cells, the loss of hArv1 by siRNA administration causes a defect in the trafficking of cholesterol from the ER to the golgi and ultimately to the plasma membrane (Tong et al. 2010), and it causes the constitutive activation of the unfolded protein response (Shechtman et al. 2011). Interestingly, loss of ScArv1 also causes constitutive activation of the unfolded protein response (Shechtman et al. 2011). Thus hArv1, like its yeast counterpart, may be a sterol trafficking protein, which regulates stress pathways in response to changes in sterol levels and/or trafficking.
CaARV1/arv1 cells were delayed in hyphae formation. They did properly localize their sterol at the hyphal tip during the initial stages of hyphae formation. At later time points they were defective in forming striated structures along the growing hyphae. These striated structures are thought to be ergosterol microdomains and/or lipid rafts that localize to septation sites (Martin and Konopka 2004). It has been shown that the septum protein, CaCdc10, co-localizes with sterol to these sites (Martin and Konopka 2004). However, only a small amount of CaCdc10 co-fractionates with lipid microdomains, so it is not known if CaCdc10 is directly trafficked to the septum by these microdomains. We have found that ScArv1 is required for the proper trafficking of the pheromone-specific Ste5 MAP kinase scaffold to the plasma membrane during mating (Villasmil et al. 2011). Thus, the co-localization of CaCdc10 with sterol migration sites during hyphal formation in Candida albicans, the aberrant trafficking of ScSte5 and ergosterol during mating in Scarv1 cells, and the general sterol localization and trafficking defects seen in both fungi due to loss of Arv1, overall lends support for the idea that one function for fungal ergosterol, and possibly lipid rafts containing sterol, is to transport cell proteins to polarized membrane contact sites.
There is evidence in S. cerevisiae to support this hypothesis. In S. cerevisiae, lipid rafts made up of sterol/sphingolipid are required to traffic specific proteins to the plasma membrane (Bagnat 2000; Bagnat et al. 2001), and to polarize sterol to the shmoo tip during haploid mating in response to pheromone (Jin et al. 2008; Proszynski et al. 2006). Interestingly, we have shown that Scarv1 mutants are mating defective, and that ScArv1 is required for ergosterol polarization to the growing shmoo tip (Villasmil et al. 2011). We have also shown that its function is required to polarize PIP2 during mating (Villasmil et al. 2011). Thus, ScArv1 has a critical role during mating that encompasses trafficking lipids and critical signaling proteins (Ste5) to the polarized shmoo tip. Overall, our Arv1 data, and work from others (Bagnat et al. 2001; Francois et al. 2009; Fullekrug and Simons 2004; Gaigg et al. 2005; Germann 2005; Jin et al. 2008; Klemm et al. 2009; Simons and Toomre 2000), strongly supports the hypothesis that yeast sterol microdomains and/or sterol/sphingolipid rafts either directly or indirectly traffic critical factors/lipids to specific membrane contact sites during many different cell events. Based on the results presented here, we suggest that Arv1-mediated sterol/lipid transport is critical in maintaining the proper signaling and/or function of several cascades or cell factors required for virulence, and for tolerance to a variety of antifungal drugs.
Highlights.
We examine the degree of homology between pathogenic and nonpathogenic Arv1 > The Arv1 homology domains in all species tested are conserved > The Arv1 homology domain is required for antifungal drug tolerance > C. albicans cells lacking CaArv1 are avirulent
Supplementary Material
Acknowledgements
We thank Drs. Martin Adelson and Eli Mordechai for helpful discussions. This work was supported in part by the National Institutes of Health Grant HL67401. We acknowledge and greatly appreciate the financial support of the Genesis Biotechnology Group.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alarco AM, et al. AP1-mediated multidrug resistance in Saccharomyces cerevisiae requires FLR1 encoding a transporter of the major facilitator superfamily. J Biol Chem. 1997;272:19304–19313. doi: 10.1074/jbc.272.31.19304. [DOI] [PubMed] [Google Scholar]
- Bagnat A, Keranen S, Shevchenko A, Simons K. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc. Natl. Acad. Sci. USA. 2000;97:3254–3259. doi: 10.1073/pnas.060034697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnat M, et al. Plasma Membrane Proton ATPase Pma1p Requires Raft Association for Surface Delivery in Yeast. Mol Biol Cell. 2001;12:4129–4138. doi: 10.1091/mbc.12.12.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzi E, et al. The multidrug resistance gene PDR1 from Saccharomyces cerevisiae. J Biol Chem. 1987;262:16871–16879. [PubMed] [Google Scholar]
- Balzi E, Goffeau A. Yeast multidrug resistance: the PDR network. J Bioenerg Biomembr. 1995;27:71–76. doi: 10.1007/BF02110333. [DOI] [PubMed] [Google Scholar]
- Beh CT, Rine J. A role for yeast oxysterol-binding protein homologs in endocytosis and in the maintenance of intracellular sterol-lipid distribution. J Cell Sci. 2004;117:2983–2996. doi: 10.1242/jcs.01157. [DOI] [PubMed] [Google Scholar]
- Bolhuis H, et al. Mechanisms of multidrug transporters. FEMS Microbiol Rev. 1997;21:55–84. doi: 10.1111/j.1574-6976.1997.tb00345.x. [DOI] [PubMed] [Google Scholar]
- Cannon RD, et al. Efflux-mediated antifungal drug resistance. Clin Microbiol Rev. 2009;22:291–321. doi: 10.1128/CMR.00051-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal E, et al. Molecular and phenotypic characterization of yeast PDR1 mutants that show hyperactive transcription of various ABC multidrug transporter genes. Mol Gen Genet. 1997;256:406–415. doi: 10.1007/s004380050584. [DOI] [PubMed] [Google Scholar]
- Coste AT, et al. TAC1, transcriptional activator of CDR genes, is a new transcription factor involved in the regulation of Candida albicans ABC transporters CDR1 and CDR2. Eukaryot Cell. 2004;3:1639–1652. doi: 10.1128/EC.3.6.1639-1652.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies BSJ, Wang HS, Rine J. Dual Activators of the Sterol Biosynthetic Pathway of Saccharomyces cerevisiae: Similiar Activation/Regulatory Domains but Different Response Mechanisms. Mol. Cell Biol. 2005;25:7375–7385. doi: 10.1128/MCB.25.16.7375-7385.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decottignies A, et al. Identification and characterization of SNQ2, a new multidrug ATP binding cassette transporter of the yeast plasma membrane. J Biol Chem. 1995;270:18150–18157. doi: 10.1074/jbc.270.30.18150. [DOI] [PubMed] [Google Scholar]
- Dimster-Denk D, Rine Jasper. Transcriptional regulation of a sterol-biosynthetic enzyme by sterol levels in Saccharomyces cerevisiae. Molecular and Cellular Biology. 1996;16:3981–3989. doi: 10.1128/mcb.16.8.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei W, et al. Genome-Wide Analysis of Sterol-Lipid Storage and Trafficking in Saccharomyces cerevisiae. Eukaryot Cell. 2008;7:401–414. doi: 10.1128/EC.00386-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fores O, et al. Arabidopsis thaliana expresses two functional isoforms of Arvp, a protein involved in the regulation of cellular lipid homeostasis. Biochim Biophys Acta. 2006;1761:725–735. doi: 10.1016/j.bbalip.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Francois IE, et al. Membrane rafts are involved in intracellular miconazole accumulation in yeast cells. J Biol Chem. 2009;284:32680–32685. doi: 10.1074/jbc.M109.014571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullekrug J, Simons KAI. Lipid Rafts and Apical Membrane Traffic. Ann NY Acad Sci. 2004;1014:164–169. doi: 10.1196/annals.1294.017. [DOI] [PubMed] [Google Scholar]
- Gaigg B, et al. Synthesis of sphingolipids with very long chain fatty acids but not ergosterol is required for routing of newly synthesized plasma membrane ATPase to the cell surface of yeast. J Biol Chem. 2005;280:22515–22522. doi: 10.1074/jbc.M413472200. [DOI] [PubMed] [Google Scholar]
- Gaur M, et al. MFS transportome of the human pathogenic yeast Candida albicans. BMC Genomics. 2008;9:579. doi: 10.1186/1471-2164-9-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germann M, Gallo Christina, Doanhue Tomithy, Shirzadi Reza, Stukey Joseph, Lang Silvia, Ruckenstuhl Christoph, Oliaro-Bosso Simonetta, McDonough Virginia, Turnowsky Friederike, Baliiano Gianni, Nickels Joseph T., Jr Characterizing sterol defect suppressors uncovers a novel transcriptional signaling pathway regulating zymosterol biosynthesis. J. Biol. Chem. 2005;280:35904–35913. doi: 10.1074/jbc.M504978200. [DOI] [PubMed] [Google Scholar]
- Gulshan K, Moye-Rowley WS. Multidrug resistance in fungi. Eukaryot Cell. 2007;6:1933–1942. doi: 10.1128/EC.00254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J.Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, et al. Ergosterol promotes pheromone signaling and plasma membrane fusion in mating yeast. J Cell Biol. 2008;180:813–826. doi: 10.1083/jcb.200705076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara K, et al. Yeast ARV1 Is Required for Efficient Delivery of an Early GPI Intermediate to the First Mannosyltransferase during GPI Assembly and Controls Lipid Flow from the Endoplasmic Reticulum. Mol Biol Cell. 2008;19:2069–2082. doi: 10.1091/mbc.E07-08-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmann DJ, et al. Transcriptional control of the yeast PDR5 gene by the PDR3 gene product. Mol Cell Biol. 1994;14:4653–4661. doi: 10.1128/mcb.14.7.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean LS, et al. Plasma membrane translocation of fluorescent-labeled phosphatidylethanolamine is controlled by transcription regulators, PDR1 and PDR3. J Cell Biol. 1997;138:255–270. doi: 10.1083/jcb.138.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm RW, et al. Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network. J Cell Biol. 2009;185:601–612. doi: 10.1083/jcb.200901145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krcmery V, Barnes AJ. Non-albicans Candida spp. causing fungaemia: pathogenicity and antifungal resistance. J Hosp Infect. 2002;50:243–260. doi: 10.1053/jhin.2001.1151. [DOI] [PubMed] [Google Scholar]
- Mahe Y, et al. The ATP-binding cassette multidrug transporter Snq2 of Saccharomyces cerevisiae: a novel target for the transcription factors Pdr1 and Pdr3. Mol Microbiol. 1996;20:109–117. doi: 10.1111/j.1365-2958.1996.tb02493.x. [DOI] [PubMed] [Google Scholar]
- Martin SW, Konopka JB. Lipid raft polarization contributes to hyphal growth in Candida albicans. Eukaryot Cell. 2004;3:675–684. doi: 10.1128/EC.3.3.675-684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morschhauser J. The genetic basis of fluconazole resistance development in Candida albicans. Biochim Biophys Acta. 2002;1587:240–248. doi: 10.1016/s0925-4439(02)00087-x. [DOI] [PubMed] [Google Scholar]
- Morschhauser J. Regulation of multidrug resistance in pathogenic fungi. Fungal Genet. Biol. 2010;47:94–106. doi: 10.1016/j.fgb.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Moye-Rowley WS. Transcriptional control of multidrug resistance in the yeast Saccharomyces. Prog Nucleic Acid Res Mol Biol. 2003;73:251–279. doi: 10.1016/s0079-6603(03)01008-0. [DOI] [PubMed] [Google Scholar]
- Moye-Rowley WS. Retrograde regulation of multidrug resistance in Saccharomyces cerevisiae. Gene. 2005;354:15–21. doi: 10.1016/j.gene.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Odds FC, et al. Candida concentrations in the vagina and their association with signs and symptoms of vaginal candidosis. J Med Vet Mycol. 1988;26:277–283. doi: 10.1080/02681218880000391. [DOI] [PubMed] [Google Scholar]
- Ostrosky-Zeichner L, et al. An insight into the antifungal pipeline: selected new molecules and beyond. Nat Rev Drug Discov. 2010 doi: 10.1038/nrd3074. [DOI] [PubMed] [Google Scholar]
- Pfaller MA, et al. Bloodstream infections due to Candida species: SENTRY antimicrobial surveillance program in North America and Latin America, 1997–1998. Antimicrob Agents Chemother. 2000;44:747–751. doi: 10.1128/aac.44.3.747-751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller MA, et al. Multisite reproducibility of the Etest MIC method for antifungal susceptibility testing of yeast isolates. J. Clin. Microbiol. 1996;34:1691–1693. doi: 10.1128/jcm.34.7.1691-1693.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller MA, et al. Trends in species distribution and susceptibility to fluconazole among blood stream isolates of Candida species in the United States. Diagn Microbiol Infect Dis. 1999;33:217–222. doi: 10.1016/s0732-8893(98)00160-6. [DOI] [PubMed] [Google Scholar]
- Pomorski T, et al. Drs2p-related P-type ATPases Dnf1p and Dnf2p are required for phospholipid translocation across the yeast plasma membrane and serve a role in endocytosis. Mol Biol Cell. 2003;14:1240–1254. doi: 10.1091/mbc.E02-08-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad R, et al. Multiple drug resistance in Candida albicans. Acta Biochim Pol. 1995;42:497–504. [PubMed] [Google Scholar]
- Proszynski TJ, et al. Plasma membrane polarization during mating in yeast cells. J. Cell Biol. 2006;173:861–866. doi: 10.1083/jcb.200602007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanglard D, et al. Antifungal drug resistance mechanisms in fungal pathogens from the perspective of transcriptional gene regulation. FEMS Yeast Res. 2009;9:1029–1050. doi: 10.1111/j.1567-1364.2009.00578.x. [DOI] [PubMed] [Google Scholar]
- Sanglard D, et al. Amino acid substitutions in the cytochrome P-450 lanosterol 14alpha-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob Agents Chemother. 1998;42:241–253. doi: 10.1128/aac.42.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahi P, Moye-Rowley WS. Coordinate control of lipid composition and drug transport activities is required for normal multidrug resistance in fungi. Biochim Biophys Acta. 2009;1794:852–859. doi: 10.1016/j.bbapap.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechtman CF, et al. Loss of subcellular lipid transport due to ARV1 deficiency disrupts organelle homeostasis and activates the unfolded protein response. J Biol Chem. 2011;286:11951–11959. doi: 10.1074/jbc.M110.215038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Sionov E, et al. Antifungal effect and possible mode of activity of a compound from the marine sponge Dysidea herbacea. Journal of Infection. 2005;50:453–460. doi: 10.1016/j.jinf.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Swain E, Stukey J, McDonough V, Germann M, Liu Y, Sturley S, Nickels JT. Yeast cells lacking the ARV1 gene harbor defects in sphingolipid metabolism. Complementation by human ARV1. J Biol. Chem. 2002;277:36152–36160. doi: 10.1074/jbc.M206624200. [DOI] [PubMed] [Google Scholar]
- Swain E, et al. Yeast cells lacking the ARV1 gene harbor defects in sphingolipid metabolism. Complementation by human ARV1. J Biol Chem. 2002;277:36152–36160. doi: 10.1074/jbc.M206624200. [DOI] [PubMed] [Google Scholar]
- Thevissen K, et al. Miconazole induces changes in actin cytoskeleton prior to reactive oxygen species induction in yeast. J Biol Chem. 2007;282:21592–21597. doi: 10.1074/jbc.M608505200. [DOI] [PubMed] [Google Scholar]
- Tinkelenberg AH, et al. Mutations in yeast ARV1 alter intracellular sterol distribution and are complemented by human ARV1. J Biol Chem. 2000;275:40667–40670. doi: 10.1074/jbc.C000710200. [DOI] [PubMed] [Google Scholar]
- Tinkelenberg AH, Liu Y, Alcantara F, Khan S, Guo Z, Bard M, Sturley SL. Mutations in yeast ARV1 alter intracellular sterol distribution and are complemented by human ARV1. J Biol. Chem. 2000;275:40667–44070. doi: 10.1074/jbc.C000710200. [DOI] [PubMed] [Google Scholar]
- Tong F, et al. Decreased expression of ARV1 results in cholesterol retention in the endoplasmic reticulum and abnormal bile acid metabolism. J Biol Chem. 2010;285:33632–33641. doi: 10.1074/jbc.M110.165761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez JA, et al. Evolution of vaginal Candida species recovered from human immunodeficiency virus-infected women receiving fluconazole prophylaxis: the emergence of Candida glabrata? Terry Beirn Community Programs for Clinical Research in AIDS (CPCRA) Clin Infect Dis. 1999;28:1025–1031. doi: 10.1086/514746. [DOI] [PubMed] [Google Scholar]
- Venkateswarlu K, Denning DW, Kelly SL. Inhibition and interaction of cytochrome P450 of Candida krusei with azole antifungal drugs. J Med Vet Mycol. 1997;35:19–25. [PubMed] [Google Scholar]
- Vermitsky JP, Edlind TD. Azole resistance in Candida glabrata: coordinate upregulation of multidrug transporters and evidence for a Pdr1-like transcription factor. Antimicrob Agents Chemother. 2004;48:3773–3781. doi: 10.1128/AAC.48.10.3773-3781.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villasmil ML, et al. The putative lipid transporter, Arv1, is required for activating pheromone-induced MAP kinase signaling in Saccharomyces cerevisiae. Genetics. 2011;187:455–465. doi: 10.1534/genetics.110.120725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villasmil ML, Nickels JT., Jr Determination of the membrane topology of Arv1 and the requirement of the ER luminal region for Arv1 function in Saccharomyces cerevisiae. FEMS Yeast Res. 2011 doi: 10.1111/j.1567-1364.2011.00737.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.