Abstract
With the paradigm shift from the reduction of morbidity and mortality to the interruption of transmission, the focus of malaria control broadens from symptomatic infections in children ≤ 5 years of age to include asymptomatic infections in older children and adults. In addition, as control efforts intensify and the number of interventions increases, there will be decreases in prevalence, incidence and transmission with additional decreases in morbidity and mortality. Expected secondary consequences of these changes include upward shifts in the peak ages for infection (parasitemia) and disease, increases in the ages for acquisition of antiparasite humoral and cellular immune responses and increases in false-negative blood smears and rapid diagnostic tests. Strategies to monitor these changes must include: 1] studies of the entire population (that are not restricted to children ≤ 5 or ≤ 10 years of age), 2] study sites in both cities and rural areas (because of increasing urbanization across sub-Saharan Africa) and 3] innovative strategies for surveillance as the prevalence of infection decreases and the frequency of false-negative smears and rapid diagnostic tests increases.
Keywords: Malaria control, paradigm shift, Plasmodium falciparum, population-based data
1. Introduction
1.1 Malaria in sub-Saharan Africa: an overwhelming Unresolved Problem
Malaria is one of the most important public health problems in the world. In sub-Saharan Africa alone, there are 400–900 million cases each year with an annual mortality of 1–2 million (Águas, et al., 2008; Hay et al., 2008; Breman, et al., 2004). The previous effort at global malaria elimination failed because of selection for resistance to the antimalarial chloroquine and the insecticide DDT and because it ignored sub-Saharan Africa, although it did eliminate malaria from Europe, North America, the Caribbean and parts of Asia and South America (Cohen, et al., 2010; Tanner & de Savigny, 2008; Carter & Mendis, 2002; Pampana, 1969).
1.2 The Need for Baseline Epidemiologic and Transmission Data
Planning to identify and resolve obstacles to malaria control in the future presents an extraordinary challenge because the factors that will be major obstacles 2–7 years from now are unknown, although they are likely to include increased resistance to insecticides and antimalarial drugs. In addition, although there have been major reductions in the numbers of malaria cases and deaths based on the three strategies recently adopted by National Malaria Control Programmes (NMCPs) and Ministries of Health (MOHs) across West Africa (Feachem, et al., 2010; O’Meara, et al., 2010; Greenwood, et al., 2009; Mendis, et al., 2009; Águas, et al., 2008; Ceesay, et al., 2008; Nahlen & Low-Beer, 2007) (Table 1), the data available thus far are often of limited value because: 1] they are primarily from health centers and are thus not population- (or community-) based, and 2] malaria control strategies have often not been examined consistently or studied systematically. As a result, it is difficult to attribute changes in human infection and disease to any one intervention or combination of interventions. Therefore, the most important priority for the initial years of support is to obtain baseline epidemiologic, entomologic, immunologic and drug efficacy data that can serve as reference comparators in subsequent years for the evaluation of new candidate interventions and combinations of interventions as they are introduced.
Table 1.
Common Malaria Control Policies Today (in 2011) across West Africa: Policies Shared by National Malaria Control Programmes *
|
The first three interventions are policy in all countries with National Malaria Control Programmes in West Africa (ACTs, LLINs, and IPTp). However, the target population for bed nets varies among countries and programmes from pregnant women and their infant children to all individuals (universal coverage). The fourth intervention (IRS) varies from country to country and within individual countries.
1.3 Development of Infrastructure at Laboratories and Field Sites, including Molecular Reagents and Equipment, Internet Access and Stable Electrical Power
Advantages
Molecular reagents and the equipment and training to use them effectively are major limitations for most endemic area laboratories and field sites (e.g., lack of access to thermocyclers, agarose gel electophoresis, nucleotide sequencing). However, the long-term mentoring relationships and institutional ties on which this collaboration is based have provided unique opportunities for the acquisition of molecular skills and understanding relevant to the epidemiology and control of malaria (Table 2). These diverse resources and experiences have supported epidemiologic studies (Bojang, et al., 2009; Sogoba, et al., 2008; Colborn, et al., 2005); publications on host, parasite and vector genes related to transmission and disease (Walther, et al., 2009; Coulibaly, et al., 2007; Volkman, et al., 2007); sequencing of parasite isolates from humans (Dharia, et al., 2009) and the development of screening strategies for genetic loci related to antimalarial drug resistance (Daniels, et al., 2008).
Table 2.
Assays and Skills available within the West African ICEMR
| Endemic Country Institution | Medical Research Council | Univ Cheikh Anta Diop | University of Bamako, Mali |
|---|---|---|---|
| Field Site(s) | Gambissara, The Gambia | Thies, Senegal | Kenieroba Dioro |
| Partner Institution(s) | Medical Research Council | Harvard Sch Publ Hlth | LMVR (NIAID), Tulane SPHTM |
| PCR-based Molecular Markers | msp1, msp2 | msp1, msp2, | msp1, msp2 msp1, pfcrt, dhfr |
| Entomologic PCR-based Markers Insecticide-related PCR Markers | An. gambiae sl, - arabiensis kdr, AChE | An. gambiae sl,- arabiensis kdr | An. gambiae sl, An. arabiensis kdr |
| PCR-RFLP for M, S Mol Forms | M and S molecular forms | M and S molecular forms | M and S molecular forms |
| Chromosomal Inversion Polymorphisms | |||
| Polytene chromosomes | Not available | Not available | Cytogenetic studies available |
| PCR Assays for Inversions | Not available | Not available | Currently available |
| ELISA Assays for Fluorescence Activated Cell Sorter | Blood meals, Sporozoites Cell Typing | Blood meals, Sporozoites Cell Typing | Blood meals, Sporozoites Cell Typing Cell Typing |
| High-Resolution Melting | Initial Training | Initial Training | Initial Training Initial Training |
| Sequence Data/Genomics | Training | Training | Training Training |
Limitations
Although most laboratories in malaria-endemic areas have the supplies and equipment necessary to perform conventional malaria field studies (microscopes, slides, stain, skilled microscopists), they have often been limited by logistic problems difficult to resolve, such as: 1] obtaining effective access to the Internet (band widths ≥ 300–500 kbps) and 2] stabilizing electrical power for the laboratory equipment and freezers used to study and store field specimens. Internet connections in West Africa often have band widths of only 20–30 kb during working hours and are frequently expensive ($500 to $4,000 per month for 20–100 kbps=$6,000 to $48,000 per year vs. $20 per month=$240 per year for band widths of 1–10 MBps in the U.S.). In addition, variations in electrical power with the potential to destroy laboratory equipment and freezers are common. Therefore, voltage regulators, generators and supplies of gasoline protected from theft and fire are essential on-site investments that require regular maintenance in order to ensure that the generators are in working condition and sufficient amounts of gasoline are available. (Note that heavy-duty voltage regulators are necessary to protect against sudden power surges and brownouts in many developing countries. Conversely, uninterruptible power supplies [UPS systems] are usually sufficient in western countries, where voltage fluctuations are infrequent and less severe.)
1.4 Development and Review of Human Studies Protocols across Field Sites
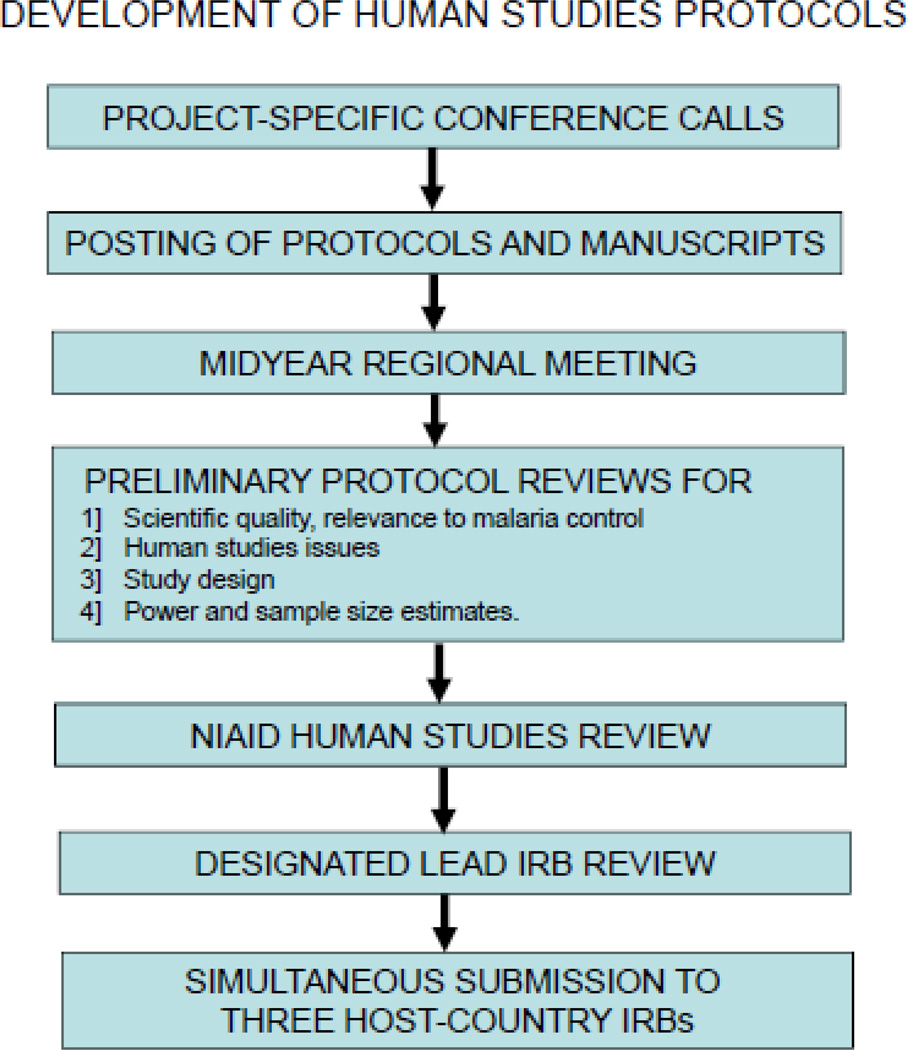
The rationale for selecting field sites that differ in the epidemiology and transmission of malaria is to permit comparisons across sites in order to identify obstacles to malaria control. However, for those comparisons to be valid, the protocols must be the same – so differences in the results reflect differences in the endemicity, transmission or control of malaria, not differences between study protocols. With a complex consortium that has 4 field sites in three countries and a substantial number of investigators (n=53), the development and review of study protocols is also more challenging. Steps which have been taken in an effort to streamline this process include: 1] weekly conference calls during protocol development linked to 2] iterative posting of protocol revisions on an ICEMR WEB Portal (Google Apps), followed by 3] face-to-face discussions of those protocols at regional meetings in the endemic area (first meeting in Dakar during February 2011), 4] preliminary reviews of draft protocols (with 2–3 day turn-around times) for a] scientific and disease control value, b] human studies issues, c] study design and data analysis and d] power and sample size estimates before submission for NIAID and IRB reviews, 5] designation of one non-endemic country IRB as the lead for protocols with no more than minimal risk, and 6] simultaneous submission to the three host-country IRBs in the malaria-endemic area after review by the lead non-endemic country IRB (Figure 1).
Figure 1.
1.5 Development and Implementation of Uniform Laboratory Protocols
To ensure that differences in study results between sites reflect differences in the endemicity or control of malaria, it is likewise necessary to ensure that laboratory protocols which appear to be identical when they are discussed or read yield similar results for the same specimens in different laboratories. This means that the development of standard operating procedures (SOPs) for laboratory testing is an essential first step, which must then be reinforced by collaborative laboratory testing involving investigators from all participating sites, and by ongoing Quality Control testing (Table 3).
Table 3.
Development and Implementation of Uniform Laboratory Protocols
|
1.6 Telecommunications and the Use of Servers at Distant Sites
Global telecommunications have developed rapidly during the past 10–15 years, and Asia and Latin America have benefited enormously from those advances. In contrast, West Africa has lagged behind in the development of telecommunications and Internet access (Ajuwon & Rhine, 2008; Smith, et al., 2007). This delay may reflect less intense economic activity and thus less opportunity for profit. However, whatever its cause, it has had a profound effect on the conference calls and Internet access essential for the successful development and implementation of multi-center protocols. For conference calls, it has been necessary to use systems based in the U.S., which vary in their ability to control echoes and the other interferences that affect overseas calls. For Internet access, it has been necessary to use microwave relays and underground placement of fiber optic cables – in order to prevent the theft of marketable fiber optic cables from more accessible sites such as utility poles.
Because of limited Internet availability, the need to provide uninterrupted access to study data for as many sites as possible and the costs and delays that result from service calls and repairs, the central server for the data management system has been placed in the U.S. to ensure it will be accessible to sites with continued Internet access (in West Africa, Europe or the U.S.) regardless of how many sites are down in the endemic area. This approach now also includes a shared WEB Portal (Google Apps) which permits simultaneous access for up to 100 investigators, and allows those investigators to view and edit protocols, SOPs, milestones, timelines and manuscripts on-line with a User ID and password.
1.7 Initial Priorities and Challenges
Because the amount of population-based data on malaria in West Africa is limited, the initial priority for the West African ICEMR is the development of population (community)-based data on the prevalence, incidence, transmission and human behaviors related to malaria prevention and control. A second priority is to evaluate the effectiveness of essential malaria control interventions such as ACTs, LLINs and IPTp.
2. Administrative Issues
2.1 Basic Organizational Issues, Financial Management and Record-Keeping
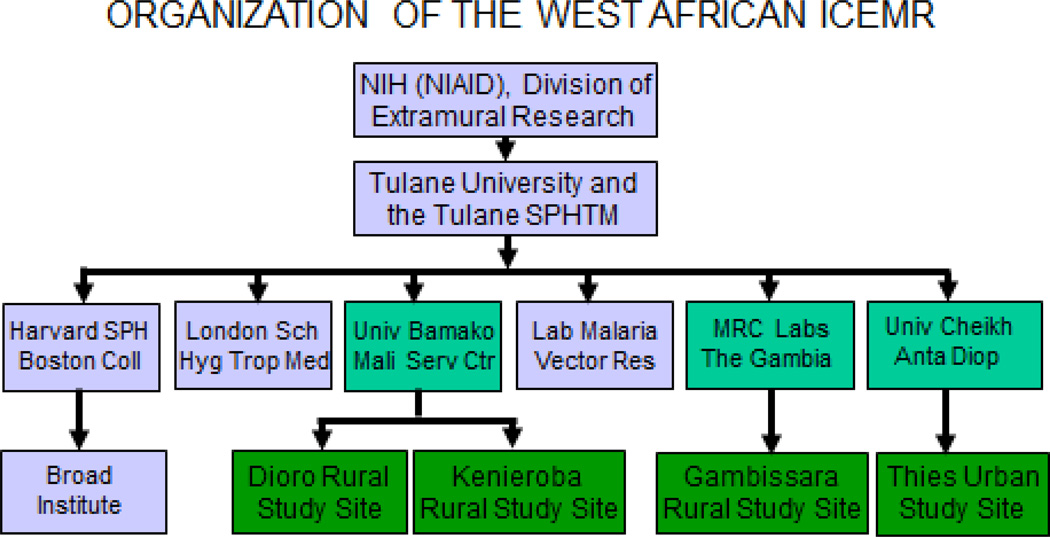
The complexity of a project inevitably increases with greater numbers of sites (Figure 3) and investigators. In addition, organizational issues such as administrative responsibilities, financial procedures and record-keeping vary between U.S. and overseas institutions. For example, administrative responsibilities and financial management and record-keeping in The Gambia are based on those of the MRC in the U.K. In contrast, financial procedures and record-keeping in Senegal and Mali are based on the French system. Although those differences are also reflected in the languages of education and business (English in The Gambia, French in Senegal and Mali), their major impact has been the centralization of financial procedures and record-keeping at the MRC Laboratories in The Gambia. In order to provide accounting results from Senegal that are acceptable to NIH and can be understood in the U.S., we have identified and are using a commercially-available software package that toggles between French and English as desired (Simply Accounting – Sage, McLean, VA). At the University of Bamako in Mali these functions were centralized 10 years ago by the NIH (NIAID)-supported development of a financial services center adjacent to the medical campus of the University of Bamako.
Figure 3.
2.2 Tracking and Coordinating Studies across Time Zones and Study Sites
Likewise, tracking and coordination of field studies across 5 time zones and ≥ 5,000 miles presents major logistical challenges. These challenges are being addressed by using strategies similar to those used to develop laboratory protocols, such as: 1] weekly or bi-weekly conference calls (with schedules adjusted to match the varying needs of different projects), 2] posting and revision of study protocols, data entry and analysis plans, SOPs and CRFs on the WEB Portal, 3] domestic and international travel for face-to-face discussions as needed and 4] face-to-face meetings of smaller working groups to finalize laboratory protocols and study plans (e.g., standardization of surveys, smears, filter paper blots, vector identification, pyrethrum spray catches, PCR and invasion assays, ELISA testing and ex vivo testing).
2.3 A Paradigm Shift: Changing the Priority from the Reduction of Morbidity and Mortality to the Interruption of Transmission
The purpose of the ICEMR program is to identify and resolve obstacles to malaria control. However, its starting point is the collection of baseline epidemiologic and entomologic data on human behavior and the transmission of malaria. Because the most striking gap in our current knowledge is the paucity of population (community)-based biological data in areas where there has been substantial recent progress in malaria control, the initial priority is to obtain population-based data from the different study sites in order to provide reference comparators for the introduction and evaluation of candidate interventions in subsequent years.
Please note also that the priority change from reducing morbidity and mortality to the interruption of transmission requires a programmatic change from an emphasis on symptomatic infections in children ≤ 5 years of age to a broader focus that includes asymptomatic infections in persons > 5 years of age – especially those who have acquired the semi-immune state (Bountogo, et al., 2010; Traoré, et al., 2009) (Table 3). This paradigm shift increases the need for studies of semi-immune adults with asymptomatic P. falciparum infection because they are likely to serve as reservoirs of gametocytes. In addition, as the intensity of transmission decreases, gametocyte-positive adults who travel may re-initiate transmission in communities that were previously malaria-free (Griffing, et al., 2011).
2.4 Potential Practical Consequences of Decreasing the Intensity of Transmission
The potential consequences of less intense transmission include: 1] lower parasitemias and 2] lower multiplicities of infection (MOIs). In terms of diagnosis based on microscopy or rapid diagnostic tests (RDTs), lower parasitemias are associated with decreased sensitivity (more false-negative results) (Bisoffi, et al., 2010; Satoguina, et al., 2009) (Table 4). In terms of MOIs, the most common RDTs are based on detecting the histidine-rich protein 2 product of a gene (hrp2) that may be deleted spontaneously (Pologe & Ravetch, 1986; Kemp, et al, 1987; Biggs, et al., 1989; Gamboa, et al., 2010). For this reason, false-negative RDTs are more likely in subjects with lower MOIs (populations with less intense transmission) (Koita, et al., Personal Communication).
Table 4.
Effects of the Paradigm Shift from Reducing Morbidity and Mortality to the Interruption of Transmission
| Initial Paradigm: Prevent Morbidity, Mortality | New Paradigm: Interruption of Transmission | |
|---|---|---|
| Target Populations | Children ≤ 5 years of age and pregnant women Increased risk of severe disease and death | Include older subjects with asymptomatic infections who are protected from severe disease and death by the semi-immune state |
| Asexual parasite counts | Elevated, especially in children ≤ 5 years of age | Lower parasite counts across the population |
| Multiplicity of infection (MOI) | MOIs often 2–3, may be ≥ 4–5 | MOIs approach 1.0 |
| False Negative Smears | Less frequent because of higher parasite densities | More frequent because of lower parasite densities |
| False Negative RDTs | Less frequent with higher MOIs (more genotypes) | More frequent with lower MOIs (fewer genotypes) |
| Timing of Maximal Intervention(s) | Treatment of children with malarial disease in the transmission season (September–November) | Potential treatment of asymptomatic infection in the dry season (May–July) |
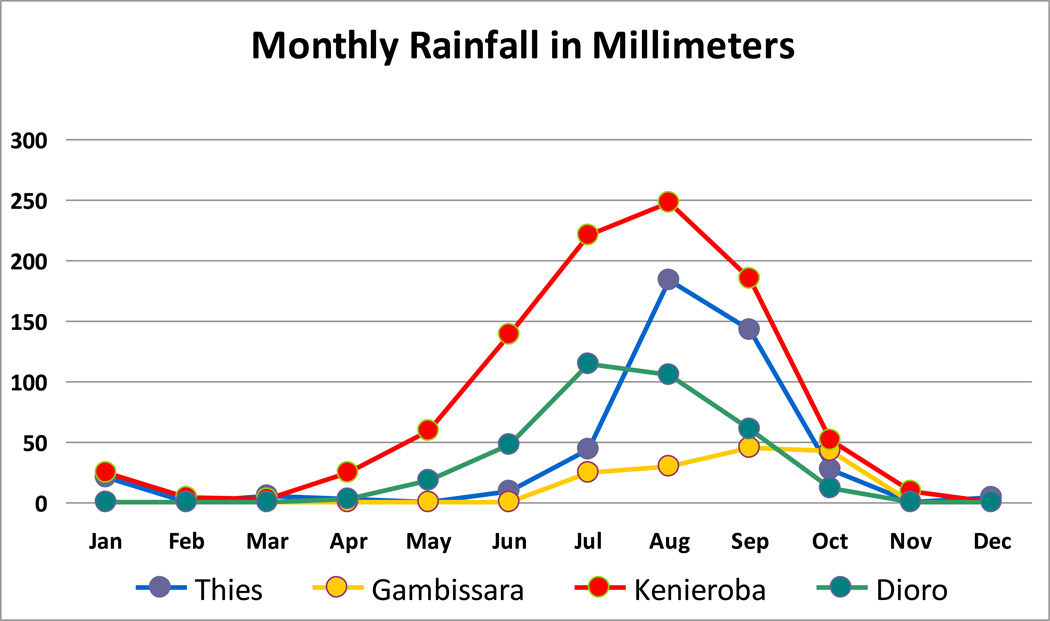
This change in focus will also require greater understanding of the differences between infected human subjects in areas with minimal malaria control (e.g., Koila Bamana, Tiby and Farako in Dioro) and those in areas with more effective malaria control (Gambissara in the Gambia) or lower levels of transmission (Thies in Senegal).
3. Data Management and Biostatistics
3.1 Clinical Data Management Systems
Each of the host (endemic area) institutions in this ICEMR has performed human studies requiring Good Clinical Practice (GCP) previously and has an IRB with a valid Federal-Wide Assurance (FWA) Number. However, in their previous studies, these institutions used data management systems that did not comply with the Code of Federal Regulations (CFR21 Part 11) requirements for security (controlled access to study data) because they did not provide tracking of data forms, activities and samples; date and time stamping of records with electronic signatures; audit trails to track changes in data records; error reports or summary reports. After review of the available software, two systems have been identified which are compliant with the CFR21 Part II requirements and will therefore be used in these studies: OpenClinica (Akaza Research, LLC – Waltham, MA) and StudyTRAX (ScienceTRAX – Macon, GA). OpenClinica is open source software, although it requires Internet access that is often unavailable in Mali and Senegal. Although StudyTRAX requires both initial and annual investments, it does not require Internet access if the software has been downloaded to the investigator’s desktop or laptop.
3.2 Sample Tracking and Management Systems
Previous studies in West Africa have often used EXCEL, ACCESS or other spread sheets to track and manage the flow of subject samples for laboratory testing. However, those systems do not meet the CFR21 Part 11 requirements for tracking the acquisition, storage, processing and distribution of specimens, nor do they provide a full history for each sample with repository- and study-specific data. Therefore, a different software package (Freezerworks – Seattle, WA) will be used for these studies. This software package will be installed on computers at the three participating central laboratories, and the collaborating institutions.
3.3 Strengthening of Information Technology Infrastructure
Central Laboratories
Internet access with band widths ≥ 200 kb has been available at the University Cheikh Anta Diop in Dakar, the MRC Laboratories in Banjul, and the Faculty of Medicine at the University of Bamako in Mali. In contrast, Internet access at the Faculty of Science in Bamako has been inadequate – with band widths often as low as 20–30 kb during working hours. Recent steps to improve access from the Faculty of Science (site of the Administrative and Data Management and Biostatistics Cores) have increased band width to 400–500 kb by installing a new fiber optic cable to create a new local area network with a dedicated separate server and router in a room with sealed windows and doors to protect against external contamination and failure during dust storms (harmattans) in the dry season.
Field Sites
Internet access from the Gambissara and Kenieroba field sites in The Gambia and Mali to the central laboratories in Fajara and Bamako will be provided using cell phone (air) cards via USB connections with laptop microcomputers. Please note that it is often necessary to travel 10–20 km in Mali and Senegal to reach a “hot spot” served by cell phone towers in order to access the Internet. In contrast, the laboratory in Segou which is the base for the Dioro field site is hard-wired to the Internet, and fiber optic cable is now being extended from the University of Thies to the Thies urban site in Senegal.
3.4 Biostatistical Issues: Power and Sample Size Estimates, Data Analysis
As noted above, the presubmission process for protocols involving human studies now includes biostatistical review within the ICEMR to ensure that: a] statistical power and sample size are sufficient to test the hypothesis(es) proposed, and b] the data analyses are appropriate for the data, the hypotheses being tested and the study design. In addition, biostatistical support is provided to investigators as needed through the director of the data management and biostatistics core facility in the Faculty of Science at the University of Bamako, in conjunction with the statistician in Bamako and the Leader of the Data Management Core in the U.S.
4. Epidemiology of Malaria and its Vectors
4.1 The Need for Population-based Data on Prevalence and Incidence of Malaria
There are two important differences between the approach proposed here and previous approaches to the epidemiology of malaria, which have focused on health centers (because the primary goal of malaria control programs for 50 years has been to reduce morbidity and mortality) in children ≤ 5 years of age - because they have the greatest risk of severe disease and death. In contrast, the priorities of the studies proposed here are to interrupt the transmission of malaria and evaluate the feasibility of malaria elimination. For these reasons, the major epidemiologic objectives are to: 1] obtain population (community)-based data (rather than health center-based data) on the prevalence and incidence of P. falciparum infection (parasitemia) at the four study sites, and 2] ensure that those data represent the entire population (are not restricted to children ≤ 5 or ≤ 10 years of age or to symptomatic individuals). These objectives are based on two rationales: 1] the approach to malaria control must be based on its prevalence across the entire community (not restricted to individuals with symptomatic infections or children ≤ 5 or ≤ 10 years of age) and 2] the majority of infected persons capable of transmitting gametocytes to the mosquito vector and thus to other people in the community are likely to be asymptomatic (and are therefore unlikely to be detected using health center-based surveillance [passive case detection]). Different diagnostic techniques including antigen detection tests (HRP-2, pLDH), microscopy and PCR will be used to estimate prevalence and incidence and will be standardized across the study sites.
4.2 Sampling Strategies to estimate Prevalence and Incidence of Human Infection
However, these changes (obtaining prevalence and incidence data from persons of all ages across the entire community) inevitably increase the difficulty of estimating the necessary sample sizes. This is because most of the recent data available from The Gambia and Senegal are health center-based (they do not include persons with asymptomatic infections) and are restricted to children ≤ 10 years of age, with few individuals from 10–15 years of age and virtually none ≥ 25 years of age.
As a result, there is substantial uncertainty about the baseline parameters required to perform sample size calculations (e.g., the current prevalence and incidence of P. falciparum infection in persons ≥ 10 years of age are unknown at all four sites, as are the prevalence and incidence of asymptomatic P. falciparum infection across age groups in The Gambia and Senegal). In addition, because the changes that occur with age are unknown, it is not clear how many age ranges will be necessary or appropriate. These unknowns place additional demands on the estimation of sample sizes which will need to be re-examined after the differences across age groups have been clarified in the initial pilot studies.
Because of these uncertainties, estimates of sample size based on a 5% increment in prevalence during the transmission season are 1,803 to 2,268 for initial prevalences of 20% to 30% in areas intense transmission (e.g., Dioro and Kenieroba). Conversely, sample size estimates are lower (716 to 1,130) for initial prevalences of 5% and 10% in areas with less intense transmission (e.g., Gambissara in The Gambia and Thies in Senegal).
In addition, each of these parameters is a moving target. For example, the National Malaria Control Programme (NMCP) in The Gambia expects that the prevalence and incidence of P. falciparum infection in humans and the intensity of transmission will decrease during the next 6–7 years with more intense application of existing interventions and the potential introduction of new interventions such as IRS. Likewise, we expect that there will be differences between sites such as Koila Bamana or Tiby in Dioro (which receive support from the Millennium Village Project) and comparator sites which do not; and between Kenieroba which receives support from the Laboratory of Malaria and Vector Research at NIH and neighboring villages which do not. If this is the case, the important follow-up question will be what are the key differences in the practice of malaria control between these villages which have the same official malaria control policies?
4.3 Epidemiology of the Anopheline Vector
Selection of the optimal mix of interventions for individual malaria-endemic areas has rarely been evidence-based and remains challenging. Major obstacles include weak health systems and insufficient data (Rowe, 2009). We propose to examine the combinations of malaria control strategies currently in use in each country as integrated intervention packages in order to identify the most cost-effective combinations of interventions for use at each study site (WHO, 2006).
The objectives of these studies are to examine: 1] variations in vector populations and epidemiology over time (with seasons and from year-to-year) and 2] vector-human interactions. The results of these studies should clarify the vector epidemiology at each of the four study sites and should therefore complement the previous data from the field sites. In addition, the identification of species and molecular forms in relation to biting behavior and infection status (sporozoite rate) should permit us to compare vector competence for the different vector species and for the molecular and the M and S chromosomal forms within An. gambiae s.s.
The measurement of entomological inoculation rates (EIRs) will allow us to assess spatial and seasonal variations in transmission (Kelly-Hope, 2009). In addition, because biting rates are underestimated by pyrethrum spray catch (PSC) collections and overestimated by human landing catch (HLC) collections, we should benefit by being able to compare both types of data from each of the field sites. Partitioning of EIRs by vector species will allow us to identify the respective contributions of each vector species to malaria transmission and the variation in biting rates across species. In addition, the results of these studies should permit the examination of commonly held assumptions about malaria transmission. For example, it should be possible to test the hypothesis that sporozoite infection rates increase late in the transmission season shortly before the peak incidence of severe disease.
5. Immunology and Pathogenesis of Malaria
5.1 Prevalence and Titers of Antibodies to Selected (Candidate) Parasite Antigens
There is a consensus among investigators that the elimination and ultimate eradication of malaria will require an effective and safe vaccine which does not yet exist and there are as yet no laboratory tests that could serve as proxies (biomarkers) for immune protection against P. falciparum infection or disease. However, the generation of antibodies to candidate vaccine antigens provides presumptive evidence that the antigens being tested are in the appropriate conformation - if they elicit antibodies similar to those found in persons from endemic areas.
For this reason, the initial studies of the host immune response will use carefully-standardized ELISA testing procedures (Miura, et al., 2008) to compare antibody titers to candidate vaccine antigens across study sites at times of high and low transmission (at the end and the beginning of the rainy season, respectively). These studies will begin with two well characterized parasite antigens (MSP1-42, AMA-1) which have the advantage that they should continue to be available in substantial quantities during the next 6–7 years. In addition, as the number of candidate vaccine antigens increases, other promising vaccine candidates will be made available for testing (e.g., EBA175, SSP2).
Although ELISA testing has been performed previously at each of the participating laboratories, these studies will begin with a workshop for immunologic investigators to ensure that the same protocol is used at all sites. The workshop will then be followed by sharing of control specimens to ensure that the results obtained across laboratories are truly comparable.
5.2 Studies of Cell-mediated Immune Responses
Studies by a number of investigators suggest that cell-mediated responses to vaccine antigens will likely be necessary for an effective vaccine, and therefore that cell-mediated responses should be examined in studies related to malaria control. However, studies of cell-mediated responses are more demanding in terms of laboratory facilities and investigator experience than studies of humoral responses and have not been performed previously at all of the participating sites. For those reasons, only pilot studies of cell-mediated responses will be performed initially (laboratory examination of peripheral blood mononuclear cells [PBMCs] based on fluorescence staining for selected surface antigens) to ensure that each site has the capability to identify antigens (markers) expressed on the surface of PBMCs.
5.3 Invasion Assays for the Study of Pathogenesis
Laboratory assays to examine the effects of host factors (such as red cell sialic acid) on the parasite’s invasion of the erythrocyte are relevant to the pathogenesis of malaria, but have been performed previously at only two study sites: the MRC Laboratories in The Gambia and the University Cheikh Anta Diop in Senegal, but not at the University of Bamako or its Dioro or Kenieroba field sites (Bates, et al., 2010). Therefore, the initial step in accomplishing this objective will be a workshop on the performance of these assays for immunologic investigators from all sites in preparation for field studies beginning in Year Two.
5.4 Hemoglobin Typing
Previous studies by Diakité, Fairhurst and their colleagues in Kenieroba suggest that 60% of individuals in the highly endemic area of Kenieroba have one or more red cell polymorphisms (variant hemoglobins or red cell membrane alterations) that interfere with invasion of the red cell by the parasite or its subsequent replication in the red blood cell. If this association reflects a cause and effect relationship between the intensity of transmission and the prevalence of (selection for) red cell polymorphisms, the prevalence of these polymorphisms should be similarly high (or higher) in areas with more intense transmission such as Dioro and should decrease over time in areas with less intense transmission such as Gambissara in The Gambia (Wellems, et al., 2010; Cholera, et al., 2008). This hypothesis will be examined in subsequent years, beginning with studies in highly endemic villages such as Koila Bamana and Tiby in Dioro.
6. Antimalarial Resistance
6.1 Ex vivo Testing for Antimalarial Resistance and Susceptibility
Conventional in vitro testing for antimalarial resistance and susceptibility has required parasites that grow well in the in vitro culture system (which is not the case with all field isolates) and may require the use of radioisotopes which is more difficult in malaria-endemic areas than in the non-endemic areas of North America, Europe or Australia. To address this problem, Ndiaye and his colleagues in Senegal have developed an ex vivo assay which uses a non-radioactive fluorescent dye (4,6-diamidino-2-phenylindole = DAPI) rather than radioisotopes to measure parasite growth and the inhibition of parasite growth by antimalarial drugs and does not require long-term growth in the in vitro culture system (Ndiaye, et al., 2010).
6.2 Clinical Follow-Up to relate Ex vivo Test Results to In Vivo Efficacy
The ex vivo assay described above has the potential to provide epidemiologically and clinically relevant information within a short period of time (72–80 hours). However, its major limitation is that there have been no studies with clinical follow-up to relate the IC50s obtained in this ex vivo assay to the (in vivo) clinical outcome. This limitation will be addressed in the upcoming transmission season by obtaining follow-up clinical and parasitologic examinations for 42 days after the treatment of uncomplicated P. falciparum malaria with ACTs. The ACTs used will be those recommended by the NMCPs in Mali, Senegal and The Gambia for uncomplicated P. falciparum malaria such as artemether + lumefantrine (Coartem - Novartis) or artesunate + amodiaquine (Arsucam − Sanofi Aventis). Please note that dihydroartemisinin + piperaquine (Duo-Cotecxin − Beijing-Holley-Cotec) has also been used in Senegal. These follow-up studies will be performed in conjunction with the ex vivo test for antimalarial drug susceptibility and resistance. Parasitologic information obtained at the times of follow-up will include thick smears (for asexual stage parasites) and filter paper blots to distinguish new infections (which are not treatment failures) from recurrences of the initial parasites (which do represent treatment failures) by testing for molecular markers which were not present at the time of diagnosis but are present at the time of recurrence (Tshefu, et al., 2010; Ndiaye et al., 2009; FDA Guidance, 2007).
7. Discussion and Conclusions
7.1 Short-Term (Current Year) Expectations
The central unresolved paradox across the four field sites in this ICEMR is the remarkable variation among them in the intensity of transmission and the prevalence of human infection despite their essentially identical malaria control policies (Table 1). Potential explanations for these differences which will be examined include: 1] differences in ecology, rainfall and other factors related to transmission (which do exist), 2] differences among countries and sites in the resources (people and funds) available to support malaria control (which may exist, but are presently unclear), 3] differences between policy and practice (which are likely and could be random or driven by disparities in resources for interventions such as ACTs and LLINs), 4] LLINs that do not remain effective after multiple washes (Atieli, et al., 2010), 5] emergence of resistance to ACTs or the insecticides used in LLINs, ITNs and IRS and 6] subsets of the anopheline vector population that preferentially bite outside houses (Riehle, et al., 2011) which are therefore less susceptible to strategies such as LLINs and IRS to interrupt transmission.
7.2 Programmatic Changes potentially related to the Interruption of Transmission
As noted in Table 4, the optimal time to interrupt transmission is likely to be during the dry season when the prevalence of infection is lowest. The necessary first step in considering such a strategy is to define the low point of infection by collecting prevalence data in the dry season (April–June) during the current grant year. Based on those data, we expect to propose the treatment of individual subjects with asymptomatic P. falciparum infection (positive blood smears for asexual parasites or gametocytes) during the dry season in the subsequent year in order to compare the incidence of infection and disease in treated vs. control communities during the subsequent transmission season. Studies such as these have the potential to address an important question related to the interruption of transmission: What is the impact of dry season treatment of persons with asymptomatic parasitemia on the transmission of infection and the incidence of disease in the subsequent transmission season?
Figure 2.
Table 5.
Malaria Field and Laboratory Studies planned during the Current Grant Year
| Baseline Epidemiologic Studies of P. falciparum Infection | Random sample of 1400 individuals from each of the four study sites transbased on 200–300 households | Prevalence of infection with asexual or gametocyte stage parasites at the conclusion of the transmission season and the dry season |
| Baseline Studies of Malarial Disease | Monitoring of community health centers associated with the four study sites | Community-based incidence of uncomplicated malaria in the transmission season |
| Baseline Immunologic Studies | ELISA antibody titers to candidate vaccine antigens | Population-based responses to candidate malaria vaccine antigens |
| Baseline Entomologic Studies in order to estimate the Entomologic Inoculation Rate | Pyrethrum spray catches (30/site) Human Landing Catches (6×3 nights/site) CSP ELISA for Sporozoite Rates | Number recently blood-fed mosquitoes per house Biting rate per person per night Sporozoite rate (% of infected mosquitoes) |
| Coartem (ACT) Treatment of Uncomplicated Malaria | Up to 120 subjects with uncomplicated malaria per study site | Clinical response and parasite clearance based on Coartem, 42 day follow-up for recurrence |
| Testing for ACT Resistance | Comparison of in vivo and in vitro test results |
Acknowledgments
The studies described and proposed here are supported by a cooperative agreement from the National Institutes of Allergy and Infectious Diseases (NIAID U19 AI 089696) for an International Center of Excellence in Malaria Research (ICEMR award) to a consortium involving the University of Bamako in Mali, the University Cheikh Anta Diop in Senegal, the Medical Research Council (MRC) Laboratories in The Gambia, Harvard School of Public Health, Boston College, London School of Hygiene and Tropical Medicine, the Laboratory of Malaria and Vector Research (NIAID) and the Tulane School of Public Health and Tropical Medicine. We thank our many colleagues for administrative and logistic support (Abdoulie Barry, Ron Cail, Salif Camara, Rosie Chavez, Abou Alassane Diallo, Khady Touré Diop, Ramona Gonski, Shannon Joyce, Dembo Kanteh, Denise Majnerick, Glen McGugan, Carmen Mejia, Papa Alioune Ndao, Seybatou Magatte Ndaw, Peter Noble, Malla Rao, Brenda Rodriguez, Mamkumbah Sanneh, Moussa Dieng Sarr, IP Singh, Paula Strickland, Don Van Noy, Joan Vivestomas, Tonu Wali, James Clint Welty, Kijuana Yarls), advice and mentoring (Tumani Corrah, Abdarahmane Dia, Amadou Diallo, Souleymanne Mboup, Saliou Nidaye, Omar Ndir), public health and malaria control partnerships (Moussa Thior, Mrs. Adam Jagne Sonko, Claude-Emile Rwagacondo) and for thoughtful discussions and access to their personal expertise (Jennifer Anderson, Thomas P. Eisele, Ivo Foppa, Nicholas Manoukis, Meredith McMorrow, David Parker, Robert Perry, Margaret Pinder, Jon Eric Tongren, Sixte Zigirumugabe).
Abbreviations
- ACTs
Artemisinin Combination Therapies
- CFR21 Part 11
Section 21 Part 11 in the Code of Federal Regulations is related to electronic record-keeping
- CRF
Case Report Form
- DDT
Dichloro Diphenyl Trichloroethane
- EBA175
Erythrocyte Binding Antigen 175 kDa
- ELISA
Enzyme-Linked Immunosorbent Assay
- FWA
Federal-Wide Assurance
- GCP
Good Clinical Practice
- HLC
Human Landing Catch
- HRP2
histidine-rich protein 2 (parasite antigen test, rapid diagnostic test = RDT)
- ICEMR
International Center for Excellence in Malaria Research
- IPTp
Intermittent Preventive Treatment of malaria during Pregnancy with sulfadoxine-pyrimethamine during the second and third trimesters
- IRB
Institutional Review Board
- IRS
Indoor Residual Spraying with residual insecticides such as DDT
- kb (kbps)
kilobit (per second) = 1000 bits per second
- LLINs
Long-Lasting Insecticide treated bed Nets
- MOH
Ministry of Health
- MOI
Multiplicity Of Infection
- MRC
Medical Research Council
- NMCP
National Malaria Control Programme
- PBMCs
Peripheral Blood Mononuclear Cells
- pLDH
parasite lactate dehydrogenase (parasite antigen test = rapid diagnostic test = RDT)
- PSC
Pyrethrum Spray Catch
- RDT
Rapid Diagnostic Test
- SOPs
Standard Operating Procedures
- SSP2
Sporozoite Surface Protein 2 (same as TRAP)
- TRAP
thrombospondin-related adhesive protein
- UPS
Uninterruptible Power Supply
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Águas R, White LJ, Snow RW, Gomes MGM. Prospects for malaria eradication in sub-Saharan Africa. PLoS One. 2008;3(3):e1767. doi: 10.1371/journal.pone.0001767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajuwon GA, Rhine L. The level of Internet access and ICT training for health professionals in sub-Saharan Africa. Health Info. Libr. J. 2008;25(3):174–185. doi: 10.1111/j.1471-1842.2007.00758.x. [DOI] [PubMed] [Google Scholar]
- Atieli FK, Munga SO, Ofulla AV, Vulule JM. The effect of repeated washing of long-lasting insecticide-treated nets (LLINs) on the feeding success and survival rates of Anopheles gambiae. Malar. J. 2010;9:304. doi: 10.1186/1475-2875-9-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates AH, Jiang H, Fairhurst RM, Su X-Z. Use of magnetically purified Plasmodium falciparum parasites improves the accuracy of erythrocyte invasion assays. Exp. Parasitol. 2010;126(2):278–280. doi: 10.1016/j.exppara.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs BA, Kemp DJ, Brown GV. Subtelomeric chromosome deletions in field isolates of Plasmodium falciparum and their relationship to loss of cytoadherence in vitro. Proc. Natl. Acad. Sci. U.S.A. 1989;86(7):2428–2432. doi: 10.1073/pnas.86.7.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisoffi Z, Sirima SB, Menten J, Pattaro C, Angheben A, Gobbi F, Tinto H, Lodesani C, Neya B, Gobo M, Van den Ende J. Accuracy of a rapid diagnostic test on the diagnosis of malaria infection and of malaria-attributable fever during low and high transmission season in Burkina Faso. Malar. J. 2010;9:192. doi: 10.1186/1475-2875-9-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojang K, Milligan P, Pinder M, Doherty T, Leach A, Ofori-Anyinam O, Lievens M, Kester K, Schaecher K, Ballou WR, Cohen J. Five year safety and immunogenicity of GlaxoSmithKline's candidate malaria vaccine RTS,S/AS02 following administration to semi-immune adult men living in a malaria-endemic region of The Gambia. Hum Vaccin. 2009;2009:5. doi: 10.4161/hv.5.4.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breman JG, Alilio MS, Mills A. Conquering the intolerable burden of malaria: what’s new, what’s needed: a summary. Am. J. Trop. Med. Hyg. 2004;71(2 Suppl):1–15. [PubMed] [Google Scholar]
- Bountogo M, Zoungrana A, Coulibaly B, Kose C, Mansmann U, Mockenhaupt FP, Burhenne J, Mikus G, Walter-Sack I, Schirmer RH, Sie A, Meissner P, Muller O. Efficacy of methylene blue monotherapy in semi-immune adults with uncomplicated Plasmodium falciparum malaria: a controlled trial in Burkina Faso. Trop. Med. Int. Health. 2010;15(6):713–717. doi: 10.1111/j.1365-3156.2010.02526.x. [DOI] [PubMed] [Google Scholar]
- Carter R, Mendis KN. Evolutionary and historical aspects of the burden of malaria. Clin. Microbiol. Rev. 2002;15:564–594. doi: 10.1128/CMR.15.4.564-594.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceesay SJ, Casals-Pascual C, Erskine J, Anya SE, Duah NO, Fulford AJC, Sesay SSS, Abubakar I, Dunyo S, Sey O, Palmer A, Fofana M, Corrah T, Bojang KA, Whittle HC, Greenwood BM, Conway DJ. Changes in malaria indices between 1999 and 2007 in The Gambia: a retrospective analysis. Lancet. 2008;372(9649):1545–1554. doi: 10.1016/S0140-6736(08)61654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholera R, Brittain NJ, Gillrie MR, Lopera-Mesa TM, Diakite SA, Arie T, Krause MA, Guindo A, Tubman A, Fujioka H, Diallo DA, Doumbo OK, Ho M, Wellems TE, Fairhurst RM. Impaired cytoadherence of Plasmodium falciparum-infected erythrocytes containing sickle hemoglobin. Proc. Natl. Acad. Sci. U.S.A. 2008;105(3):991–996. doi: 10.1073/pnas.0711401105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JM, Moonen B, Snow RW, Smith DL. How absolute is zero? an evaluation of historical and current definitions of malaria elimination. Malar. J. 2010;9:213. doi: 10.1186/1475-2875-9-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colborn JM, Koita OA, Cissé OH, Bagayoko MW, Guthrie EJ, Krogstad DJ. Identifying and quantifying genotypes in polyclonal infections due to single species. Emerg. Infect. Dis. 2005;12(3):475–482. doi: 10.3201/eid1203.05057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulibaly MB, Pombi M, Caputo B, Nwakanma D, Jawara M, Konaté L, Dia I, Fofana A, Kern M, Simard F, Conway DJ, Petrarca V, della Torre A, Traoré SF, Besansky NJ. PCR-based karyotyping of Anopheles gambiae inversion 2Rj identifies the BAMAKO chromosomal form. Malar. J. 2007;6:133. doi: 10.1186/1475-2875-6-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels R, Volkman SK, Milner DA, Jr, Mahesh N, Neafsey DE, Park DJ, Rosen DR, Angelino E, Sabeti PC, Wirth DF, Wiegand RC. A general SNP-based molecular barcode for P. falciparum identification and tracking. Malar. J. 2008;7:223. doi: 10.1186/1475-2875-7-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharia NV, Sidhu AB, Cassera MB, Westenberger SJ, Bopp SE, Eastman RT, Plouffe D, Batalov S, Park DJ, Volkman SK, Wirth DF, Zhou Y, Fidock DA, Winzeler EA. Use of high-density tiling microarrays to identify mutations globally and elucidate mechanisms of drug resistance in Plasmodium falciparum. Genome Biol. 2009;10(2):R21. doi: 10.1186/gb-2009-10-2-r21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feachem RGA, Phillips AA, Hwang J, Cotter C, Wielgosz B, Greenwood BM, Sabot O, Rodriguez MH, Abeyasinghe RR, Ghebreyesus TA, Snow RW. Malaria Elimination 1: Shrinking the malaria map: progress and prospects. Lancet. 2010;376(9752):1566–1578. doi: 10.1016/S0140-6736(10)61270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration. Guidance for Industry, Malaria: Developing Drug and Nonvaccine Biological Products for Treatment and Prophylaxis. [Accessed October 16th, 2011];2007 http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071951.pdf.
- Gamboa D, Ho M-F, Bendezu J, Torres K, Chiodine PL, Barnwell JW, Incardona S, Perkins M, Bell D, McCarthy J, Cheng Q. A large proportion of P. falciparum isolates in the American region of Peru lack pfhrp2 and pfhrp3: implications for malaria rapid diagnostic tests. PLoS One. 2010;5(1):e8091. doi: 10.1371/journal.pone.0008091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Google Apps for Higher Education (Universities and Colleges) [accessed April 5, 2011]; http://www.google.com/a/help/intl/ened/university.html.
- Greenwood BM. Can malaria be eliminated? Trans. R. Soc. Trop. Med. Hyg. 2009;103 Suppl. 1:S2–S5. doi: 10.1016/j.trstmh.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Griffing SM, Mixson-Hayden T, Sridaran S, Alam MT, McCollum AM, Cabezas C, Quezeda WM, Barnwell JW, De Oliveira AM, Lucas C, Arrospide N, Escalante AA, Bacon DJ, Udhayakumar V. South American Plasmodium falciparum after the malaria eradication era: clonal population expansion and survival of the fittest hybrids. PLoS One. 2011;6(9):e23486. doi: 10.1371/journal.pone.0023486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay SI, Smith DL, Snow RW. Measuring malaria endemicity from intense to interrupted transmission. Lancet Infect. Dis. 2008;8(6):369–378. doi: 10.1016/S1473-3099(08)70069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Hope LA, McKenzie FE. The multiplicity of malaria transmission: a review of entomological inoculation rate measurements and methods across sub-Saharan Africa. Malaria J. 2009;8:19. doi: 10.1186/1475-2875-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp DJ, Thompson JK, Walliker D, Corcoran LM. Molecular karyotype of Plasmodium falciparum: conserved linkage groups and expendable histidine-rich protein genes. Proc. Natl. Acad. Sci. U.S.A. 1987;84(21):7672–7676. doi: 10.1073/pnas.84.21.7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendis K, Rietveld A, Warsame M, Bosman A, Greenwood BM, Wernsdorfer WH. From malaria control to eradication: the WHO perspective. Trop. Med. Int. Health. 2009;14(7):802–809. doi: 10.1111/j.1365-3156.2009.02287.x. [DOI] [PubMed] [Google Scholar]
- Miura K, Orcutt AC, Muratova OV, Miller LH, Saul A, Long CA. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine. 2008;26(2):193–200. doi: 10.1016/j.vaccine.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahlen BL, Low-Beer D. Building to collective impact: the Global Fund support for measuring reduction in the burden of malaria. Am. J. Trop. Med. Hyg. 2007;77 Suppl. 6:321–327. [PubMed] [Google Scholar]
- Ndiaye D, Patel V, Demas A, LeRoux M, Ndir O, Mboup S, Clardy J, Lakshmanan V, Daily JP, Wirth DF. A non-radioactive DAPI-based high-throughput in vitro assay to assess Plasmodium falciparum responsiveness to antimalarials – increased sensitivity of P. falciparum to chloroquine in Senegal. Am. J. Trop. Med. Hyg. 2010;82(2):228–230. doi: 10.4269/ajtmh.2010.09-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndiaye J-L, Randrianarivelojosia M, Sagara I, Brasseur P, Ndiaye I, Faye B, Randrianasolo L, Ratsimbasoa A, Forlemu D, Moor VA, Traoré A, Dicko Y, Dara N, Lameyre V, Diallo M, Djimde A, Same-Ekobo A, Gaye O. Randomized, multicenter assessment of the efficacy and safety of ASAQ – a fixed dose artesunate-amodiaquine combination therapy in the treatment of uncomplicated Plasmodium falciparum malaria. Malar. J. 2009;8:125. doi: 10.1186/1475-2875-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Meara WP, Mangeni JN, Steketee RW, Greenwood BM. Changes in the burden of malaria in sub-Saharan Africa. Lancet Infect. Dis. 2010;10(8):545–555. doi: 10.1016/S1473-3099(10)70096-7. [DOI] [PubMed] [Google Scholar]
- Pampana EJ. A Textbook of Malaria Eradication. second edition. Oxford, London: 1969. pp. 1–510. [Google Scholar]
- Pologe LG, Ravetch JV. A chromosomal rearrangement in a P. falciparum histidine-rich protein gene is associated with the knobless phenotype. Nature. 1986;322(6078):433–477. doi: 10.1038/322474a0. [DOI] [PubMed] [Google Scholar]
- Riehle MM, Guelbeogo WM, Gneme A, Eiglmeier K, Holm I, Bischoff E, Garnier T, Snyder GM, Li X, Markianos K, Sagnon N-F, Vernick KD. A cryptic subgroup of Anopheles gambiae is highly susceptible to human malaria parasites. Science. 2011;331(6017):596–598. doi: 10.1126/science.1196759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe AK. Potential of integrated continuous surveys and quality management to support monitoring, evaluation, and the scale-up of health interventions in developing countries. Am. J. Trop. Med. Hyg. 2009;80(6):971–979. [PubMed] [Google Scholar]
- Satoguina J, Walther B, Drakeley C, Nwakanma D, Oriero EC, Correa S, Corran P, Conway DJ, Walther M. Comparison of surveillance methods applied to a situation of low malaria prevalence at rural sites in The Gambia. Malar. J. 2009;8:274. doi: 10.1186/1475-2875-8-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H, Bukirwa H, Mukasa O, Snell P, Adeh-Nsoh S, Mbuyita S, Honorati M, Orji B, Garner P. Access to electronic health knowledge in five countries in Africa: a descriptive study. BMC Health Serv. Res. 2007;7:72. doi: 10.1186/1472-6963-7-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogoba N, Vounatsou P, Bagayoko MM, Doumbia SO, Dolo G, Gosoniu L, Traoré SF, Smith TA, Touré YT. Spatial distribution of the chromosomal forms of Anopheles gambiae in Mali. Malar. J. 2008;7:205. doi: 10.1186/1475-2875-7-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner M, de Savigny D. Malaria eradication back on the table. Bull. W.H.O. 2008;86(2):82–83. doi: 10.2471/BLT.07.050633. [DOI] [PMC free article] [PubMed]
- Traoré B, Kone Y, Doumbo S, Doumtabe D, Traoré A, Crompton PD, Mircetic M, Huang CY, Kayentao K, Dicko A, Ellis RD, Miura K, Guindo A, Miller LH, Doumbo OK, Pierce SK. The TLR9 agonist CpG fails to enhance the acquisition of Plasmodium falciparum-specific memory B cells in semi-immune adults in Mali. Vaccine. 2009;27(52):7299–7303. doi: 10.1016/j.vaccine.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tshefu AK, Gaye O, Kayentao K, Thompson R, Bhatt KM, Sesay SSS, Bustos DG, Tjitra E, Bedu-Addo G, Borghini-Fuhrer I, Duparc S, Shin CK, Fleckenstein L the Pyronaridine-artesunate Study Team. Efficacy and safety of a fixed-dose oral combination of pyronaridine-artesunate compared with artemether-lumefantrine in children and adults with uncomplicated Plasmodium falciparum malaria: a ran domized non-inferiority trial. Lancet. 2010;375(9724):1457–1467. doi: 10.1016/S0140-6736(10)60322-4. [DOI] [PubMed] [Google Scholar]
- Volkman SK, Sabeti PC, DeCaprio D, Neafsey DW, Schaffner SF, Milner DA, Jr, Daily JP, Sarr O, Ndiaye D, Ndir O, Mboup S, Duraisingh MT, Lukens A, Derr A, Stange-Thomann N, Waggoner S, Onofrio R, Mauceli E, Gnerre S, Jaffe DB, Zainoun J, Wiegand RC, Birren BW, Hartl DL, Galagan JE, Lander ES, Wirth DF. A genome-wide map of diversity in Plasmodium falciparum. Nat. Gen. 2007;39(1):113–11.9. doi: 10.1038/ng1930. [DOI] [PubMed] [Google Scholar]
- Walther M, Jeffries D, Finney OC, Njie M, Ebonyi A, Deininger S, Lawrence E, Ngwa-Amambua A, Jayasooriya S, Cheeseman I, Gomez-Escobar N, Okebe J, Conway DJ, Riley EM. Distinct roles for FOXP3+ and FOXP3− CD4+ T cells in regulating cellular immunity to uncomplicated and severe Plasmodium falciparum malaria. PLOS Pathogens. 2009;3 doi: 10.1371/journal.ppat.1000364. e1000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellems TE, Hayton K, Fairhurst RM. The impact of malaria parasitism: from corpuscles to communities. J. Clin. Invest. 2009;119(9):2496–2505. doi: 10.1172/JCI38307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Global Malaria Programme Indoor Residual Spraying. [Accessed August 4, 2008];WHO position statement. 2006 Available at: http://www.who.int/malaria/docs/IRS/IRS-position.pdf.