Background: HMG-CoA reductase inhibitors (statins) affect not only cholesterol synthesis but also complex lipids that modify signaling proteins, including G proteins.
Results: Simvastatin decreases endothelial cell Gαs abundance by inhibiting protein synthesis initiation.
Conclusion: Simvastatin modulates β-adrenergic signaling in vascular endothelium by attenuating Gαs subunit abundance.
Significance: These findings may help explain some of the therapeutic effects of statins independent of lipid lowering.
Keywords: Adrenergic Receptor, G Protein-coupled Receptors (GPCR), Nitric-oxide Synthase, Signal Transduction, Vascular Biology
Abstract
These studies explore the effects of statins on cyclic AMP-modulated signaling pathways in vascular endothelial cells. We previously observed (Kou, R., Sartoretto, J., and Michel, T. (2009) J. Biol. Chem. 284, 14734–14743) that simvastatin treatment of endothelial cells leads to a marked decrease in PKA-modulated phosphorylation of the protein VASP. Here we show that long-term treatment of mice with simvastatin attenuates the vasorelaxation response to the β-adrenergic agonist isoproterenol, without affecting endothelin-induced vasoconstriction or carbachol-induced vasorelaxation. We found that statin treatment of endothelial cells dose-dependently inhibits PKA activation as assessed by analyses of serine 157 VASP phosphorylation as well as Epac-mediated Rap1 activation. These effects of simvastatin are completely reversed by mevalonate and by geranylgeranyl pyrophosphate, implicating geranylgeranylation as a critical determinant of the stain response. We used biochemical approaches as well as fluorescence resonance energy transfer (FRET) methods with a cAMP biosensor to show that simvastatin treatment of endothelial cells markedly inhibits cAMP accumulation in response to epinephrine. Importantly, simvastatin treatment significantly decreases Gαs abundance, without affecting other Gα subunits. Simvastatin treatment does not influence Gαs protein stability, and paradoxically increases the abundance of Gαs mRNA. Finally, we found that simvastatin treatment inhibits Gαs translation mediated by Akt/mTOR/eIF4/4EBP. Taken together, these findings establish a novel mechanism by which simvastatin modulates β-adrenergic signaling in vascular wall, and may have implications for cardiovascular therapeutics.
Introduction
Statins are 3-hydroxy-3-methyl glutaryl coenzyme A (HMG-CoA)2 reductase inhibitors that are widely used to treat hypercholesterolemia and other cardiovascular diseases. It is clear that many of the salutary clinical effects of statins are independent of their effects on lowering serum lipoproteins, and appear to be mediated by other molecular mechanisms (1, 2). In addition to attenuating cholesterol formation, inhibition of HMG-CoA reductase suppresses cellular synthesis of isoprenoid compounds such as farnesyl pyrophosphate (Fpp) and geranylgeranyl pyrophosphate (GGpp), which are required for covalent modifications of many key signaling proteins, including members of small G protein Rho GTPase family. Statins have been found to attenuate β-adrenergic signaling via effects on heterotrimeric G proteins in cardiac myocytes (3). However, the mechanisms whereby statins modulate G protein-mediated responses in vascular endothelium remain incompletely understood.
β-Adrenergic receptors are G protein-coupled receptors that activate adenylyl cyclase in many cell types in response to adrenergic agonists such as epinephrine and isoproterenol. β-adrenergic antagonist drugs (beta blockers) are commonly used to treat patients suffering from hypertension, congestive heart failure, arrhythmias, and angina. Many patients being treated with beta blockers also take statins, yet the effects of statins on adrenergic signaling have not been explored in detail. We have previously shown that simvastatin treatment of endothelial cells significantly decreases epinephrine-stimulated phosphorylation of VASP at Ser157, the VASP residue that is preferentially phosphorylated by PKA (4). This observation implicated simvastatin in the modulation of cAMP-dependent β-adrenergic responses in these cells. The present studies explore the mechanisms whereby statins attenuate cAMP-dependent signaling pathways in the vascular endothelium.
EXPERIMENTAL PROCEDURES
Materials
Fetal bovine serum (FBS) was from Hyclone (Logan, UT). All other cell culture reagents, media, and TRIzol were from Invitrogen (Carlsbad, CA). FuGENE®6 was from Roche (Indianapolis, IN). Anti-Gαs monoclonal antibody and polyclonal antibody were from BD Pharmingen (Lexington, KY) and Millipore (Billerica, MA), respectively. Super Signal substrate for chemiluminescence detection and secondary antibodies conjugated with horseradish peroxidase were from Pierce. All other antibodies were from Cell Signaling (Beverley, MA). Protein A/G-agarose beads were from Santa Cruz Biotechnology (Santa Cruz, CA). Cycloheximide and all other reagents were from Sigma. cAMP sensor CFP-Epac(ΔDEP)-YFP was kindly provided by Professor Kees Jalink; this cAMP biosensor has been extensively validated previously (1).
Cell Culture and Transfection
Bovine aortic endothelial cells (BAEC) were obtained from Cell Applications, Inc. (San Diego, CA) and maintained in culture in Dulbecco's-modified Eagle's medium supplemented with FBS (10% v/v) as described previously (5, 6). BAEC were plated onto gelatin-coated culture dishes and studied prior to cell confluence between passage 5 and 9. Human aortic smooth muscle cells were from Lonza, Inc (Walkersville, MD), maintained in SmGM-2 media supplemented with growth factors as recommended by the manufacturer, and were studied in passage 5 or 6. Cells were passaged at 80% confluency at a 1:4 ratio. Control siRNA and Akt siRNA sequences and transfection conditions have been described previously (4).
Cell Treatments and Immunoblot Analysis
BAEC were treated with 10 μm simvastatin for 22 h unless otherwise indicated. Treated cells were washed with PBS, and cell lysates were collected in Nonidet P-40 buffer (50 mm Tris-HCl, pH7.4, 150 mm NaCl, 1% Nonidet P-40, 0.025% sodium deoxycholate, 1 mm EDTA, 2 mm Na3VO4, 1 mm NaF, 2 μg/ml leupeptin, 2 μg/ml antipain, 2 μg/ml soybean trypsin inhibitor, and 2 μg/ml lima trypsin inhibitor). Protein determinations were made with a Bio-Rad protein assay kit. 30 μg protein of each sample were resolved by SDS-PAGE and transferred onto nitrocellulose membranes. Immunoblots were probed with specific antibodies; analyses of protein expression and phosphorylation were assessed as previously described in detail (7, 8). Quantitative analyses of immunoblots were determined using a ChemiImager HD4000 (Alpha-Innotech, San Leandro, CA).
Protein Stability Analysis
Protein half-life was determined by analysis of immunoblots after treatment of cells with the protein synthesis inhibitor cycloheximide, as well as by pulse-chase analyses. For experiments with cycloheximide, confluent BAEC were treated with 10 μm simvastatin for 22 h plus 10 ng/ml cycloheximide for indicated times, followed by cell lysis. Cell lysates were resolved by SDS-PAGE, transferred onto nitrocellulose membrane and analyzed in immunoblots. For pulse-chase experiments, BAEC were cultured to 90% confluency, incubated for 2 h with Cys/Met-free DMEM plus 10% dialyzed FBS, and the “pulse” was initiated by replacing media with Cys−/Met− DMEM supplemented with 10% dialyzed FBS plus 100 μCi/ml [35S] methionine/cysteine (TRAN35S-LABEL) for 2 h. The “chase” was initiated by washing the cells three times with DMEM, followed by incubation with DMEM/10% FBS containing 4 mm methionine and 4 mm cysteine; cells were treated with PBS or 10 μm simvastatin for indicated times, followed by lysis in 600 μl of Nonidet P-40 lysis buffer. The lysates were immunoprecipitated by incubation 1 h to overnight with 4 μg/ml polyclonal Gαs antibody followed by 1 h with 60 μl/ml protein A/G-agarose beads at 4 °C. After washing three times with lysis buffer and boiling for 5 min, immunoprecipitates were subjected to SDS-PAGE. Gels were then air-dried and analyzed with a Cyclone PhosphorImaging System (Packard Instrument Company, Meriden, CT). Labeled proteins were quantified using software provided by the manufacturer. A portion of the cell lysates was used for immunoblot analysis for β-actin.
RT-PCR
To explore effects of simvastatin on Gαs mRNA abundance, mRNA from treated cells was isolated using Trizol (Invitrogen), and reverse transcribed into cDNA using an AMV cDNA synthesis kit (Invitrogen) according to the protocol provided by the manufacturer. 2 μl of cDNA was subjected to real-time PCR using 2× Premix (AB Applied Biosystems, Carlsbad, CA) and primer pairs 5′-GCTGGCTGAGAAAGTCCTTG-3′ and 5′-GCAGGTGAAGTGAGGGTAGC-3′ for Gαs and 5′-CTGGGCAAGTTAGGTTTTGTC-3′ and 5′-CAAGTACTCCGTGTGGATTGG-3′ for control β-actin under the following conditions: 95 °C for 5 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 30 s and final elongation at 60 °C for 5 min using 7300 Real Time PCR System (Applied Biosystems). Authenticity of the reaction was confirmed by melting analysis, using the manufacturer's protocol. Relative Gαs to β-actin mRNA level was calculated by 2−ΔΔCt (9).
Rap1 Activity Assay
Rap1 activity was measured in cultured endothelial cells using a pull-down assay following the manufacturer's protocols (Millipore, Billerica, MA). In brief, endothelial cells in 100-mm dishes were incubated with either control or 10 μm simvastatin for 22 h, and then treated with 1 μm epinephrine for 5 min. Cells were washed twice with ice-cold PBS, and lysed in lysis buffer supplemented with 2 mm Na3VO4, 1 mm NaF, 2 μg/ml leupeptin, 2 μg/ml antipain, 2 μg/ml soybean trypsin inhibitor, and 2 μg/ml lima trypsin inhibitor according to the manufacturer's protocol. The GTP-bound active form of Rap1 was pulled down by incubating cell lysates with Ral-GDS RBD-agarose beads. The beads were washed three times with washing buffer, eluted with SDS-PAGE sample buffer, and analyzed in immunoblots probed with a Rap1 antibody provided by the manufacturer.
Biochemical Assay for cAMP
Cellular cAMP content was measured using a cyclic AMP EIA Kit from Cayman (Ann Arbor, MI). Treated BAEC in P60 dishes were washed once with PBS, extracted twice with 65% ethanol, and dried in a vacuum centrifuge. Pellets were dissolved in 120 μl assay buffer provided by the manufacturer, and supernatants were collected by centrifugation and assayed according to the protocols provided. Cells in one separate plate were lysed with Nonidet P-40 buffer and used to measure protein concentration using the Bio-Rad protein assay kit.
FRET-based Biosensor Measurement of Intracellular cAMP
BAEC were split in glass-bottom microwell dishes (MatTeK Co., Ashland, MA) at ratio of 1:8, and transfected with 1 μg per dish CFP-Epac(ΔDEP)-YFP (1) with FUGENE according to the protocol provided by the manufacturer (Roche, Indianapolis, IN). 24–48 h after transfection, the culture media was replaced with Hank's balanced salt solution. The transfected cells were excited at 425 nm; emission of CFP and YFP was detected simultaneously using a 475 ± 10 and 540 ± 10 nm Semrock FRET-CFP/YFP-B four-filter, single band set, respectively. Data were digitized using Metamorph software and FRET was expressed as ratio of YFP to CFP signals, which was set to 1.0 at the onset of the experiments. FRET values between treatments were normalized as the ratio of the absolute values of FRET slopes measured in vehicle- and agonist-treated cells. A decrease in CFP-Epac(ΔDEP)-YFP FRET indicates an increase in the level of cAMP.
In Vivo Treatment and ex Vivo Vasorelaxation
Male wild-type C57BL/6J mice from Jackson Laboratories (Bar Harbor, ME) were studied at age 8–9 weeks. All procedures were performed according to protocols approved by the Harvard Medical School Standing Committee for Animal Experimentation. For these studies, mice received simvastatin (10 mg/kg) or PBS by intraperitoneal (ip) injection daily for 2 weeks, during which time the animals were provided standard chow and water ad libitum. Mice were euthanized with a lethal dose of pentobarbital sodium (120 mg/kg intraperitoneal). After euthanasia, the aorta was removed, homogenized, and analyzed in immunoblots probed with antibodies as described previously (4), while the femoral vascular bed was removed and placed in a dish containing ice-cold PBS. Intact segments of dissected femoral arteries (300–400 μm) were mounted between glass cannulae in an arteriograph (Living Systems Instrumentation; Burlington, VT) at room temperature (∼25 °C) and pressurized to 60 mm Hg under conditions of no luminal flow. Constant pressure was maintained via a pressure servo-control system (model PS200; Living Systems Instrumentation). Pressure transducers at both ends of the artery allowed continual monitoring of intraluminal pressure. Vessel diameter was quantitated by video microscopy on a Nikon inverted microscope; a measurement of the external diameter was determined by using the Ionoptix SoftEdge system (Ionoptix; Milton, MA). The preparation was continually superfused with modified Krebs-Henseleit solution (in mm: 113 NaCl, 4.7 KCl, 2.5 CaCl2, 25 NaHCO3, 1.1 MgSO4, 1.1 KH2PO4, 0.03 EDTA, 0.01 l-arginine, and 11 glucose, pH 7.4) and oxygenated with a mixture of 95% O2-5% CO2 at a rate of 10 ml/min. The superfusing solution was warmed to 37 °C by passage through a heat-exchange coil before entering the monitoring apparatus. After a 60 min stabilization period, vessels were pre-constricted with 100 nm endothelin-1 in Krebs buffer containing 50 μm phentolamine prior to treatments with isoproterenol or carbachol at indicated dosages. Endothelial integrity was confirmed by verifying the vasorelaxation response to 100 μm carbachol. Vessel relaxation was normalized as the fractional reversal of endothelin-induced vessel contraction.
Other Methods
All experiments were performed at least three times. EC50 values were determined by analysis of Eadie-Hofstee plots. Mean values for individual experiments were expressed as mean ± S.D. Statistical differences were assessed by ANOVA or t test, as appropriate. A p value less than 0.05 was considered statistically significant.
RESULTS
To extend our prior observations on the effects of simvastatin on signaling responses in cultured endothelial cells, we investigated β-adrenergic signaling in arterial preparations that we isolated following statin treatment in vivo. Mice were injected with simvastatin (10 mg/kg) or vehicle daily for 2 weeks, and the vasomotor responses to isoproterenol and other agonists were analyzed in femoral artery preparations. As shown in Fig. 1A, in both control and statin-injected mice, there was a clear dose-dependent vasorelaxation response to the β-adrenergic agonist isoproterenol. However, the isoproterenol dose curve was significantly shifted to the right in arteries from statin-injected mice (p < 0.001), as determined from analysis of Eadie-Hofstee plots. In addition, the maximal relaxation in statin-treated mice was significantly decreased, from 36.3 ± 3.7% in PBS-injected mice to 24.1 ± 6.2% (p < 0.001, n = 9). In contrast, we found that the vasorelaxation response to the muscarinic cholinergic carbachol was unaffected by statin treatment, as was endothelin-dependent vasoconstriction (Fig. 1, B and C). Immunoblot analysis of aorta preparations showed that phosphorylation of VASP is decreased following in vivo simvastatin treatment (4), while Gαs abundance is unchanged (Fig. 1D).
FIGURE 1.
Effects of in vivo simvastatin treatment on ex vivo vascular responses. This figure shows vascular responses of arterial preparations isolated from mice treated with simvastatin (10 mg/kg) or PBS by intraperitoneal injection daily for 2 weeks. Intact femoral arteries were constricted with 100 nm endothelin-1 plus 50 μm phentolamine (to block α1 adrenergic vasoconstriction) before treatments with isoproterenol (panel A) or carbachol (panel B) at the indicated dosages. Vessel relaxation was normalized as the fractional reversal of endothelin-induced vessel contraction. The data shown were pooled from nine separate experiments, and are expressed as mean ± S.D. Statistical difference was assessed by ANOVA; the asterisk indicates p < 0.001 between the control and simvastatin-treated mice. Panel A shows the responses of arterial preparations isolated from mice treated with simvastatin or PBS to isoproterenol at indicated dosages. Panel B shows the responses of arterial preparations isolated from mice treated with simvastatin or vehicle to carbachol at indicated dosages; please note the difference in the absolute value on the ordinate compared with panel A. Panel C shows pooled data showing the maximal vascular responses of arterial preparations from mice treated with simvastatin or PBS, then exposed to isoproterenol and carbachol (leading to vasorelaxation) or to endothelin-1 (leading to vasoconstriction). Panel D shows representative immunoblots analyzed in aorta preparations of mice treated daily with PBS or simvastatin for 2 weeks. Panel E shows the quantitative analysis of pooled data from four independent experiments identical in design to panel D; the asterisk indicates p < 0.05 (ANOVA).
To further explore the mechanisms underlying the attenuated vasorelaxation response to β-adrenergic agonists following in vivo statin treatment, we studied β-adrenergic signaling pathways in cultured endothelial cells. Previous work has established that β3-adrenergic receptors in vascular endothelial cells are coupled to adenylate cyclase via Gαs (10). As shown in Fig. 2, treatment of endothelial cells with simvastatin (10 μm, 22 h) significantly attenuated epinephrine-stimulated VASP phosphorylation at Ser157, with the EC50 for epinephrine-stimulated VASP phosphorylation increasing from 0.9 ± 0.1 nm to 9.0 ± 0.2 nm (p < 0.05, n = 3). The decrease in total VASP abundance following epinephrine treatment is more apparent than real: VASP phosphorylation leads to a change in Mr and to decreased antigenicity for the VASP antibody (5). While these findings implicate the cyclic AMP-dependent protein kinase (PKA), yet not all cyclic AMP-dependent signaling responses involve PKA activation (11). Perhaps the best-characterized cAMP-modulated signaling response that is independent of PKA is the cAMP-dependent but PKA-independent activation of the small G protein Rap1. As shown in Fig. 3, pretreatment of endothelial cells with simvastatin completely blocked epinephrine-induced Rap1 activation, indicating that simvastatin treatment appears to affect cAMP responses upstream of PKA.
FIGURE 2.
Epinephrine-stimulated VASP phosphorylation following simvastatin treatment of endothelial cells. Endothelial cells were treated with simvastatin (10 μm) for 22 h, and epinephrine was added at the indicated doses for 5 min, followed by cell harvest, and lysis. Cell lysates were analyzed in immunoblots probed with antibodies as shown. Panel A shows a series of immunoblots from a representative experiment, and panel B shows quantitative analysis of pooled data from three independent experiments; the asterisk indicates p < 0.05 (ANOVA).
FIGURE 3.

Simvastatin inhibits epinephrine-stimulated Rap1 activation in endothelial cells. Panel A shows the results of Rap1 activity assays in BAEC treated with simvastatin (10 μm) for 22 h; epinephrine was added for 5 min, and then Rap1 activation was measured using a pull-down assay. Active Rap1 and total Rap1 as well as phosphorylated VASP were detected in immunoblots probed with Rap1 and pVASP157 antibodies, respectively. This experiment was repeated three times with equivalent results. Panel B shows pooled data from three experiments identical in design to the experiment shown in panel A, normalizing the signals of active Rap1 to total Rap1; the normalized Rap1 activity in vehicle-treated cells was defined as 1.0; the asterisk indicates p < 0.05 (ANOVA).
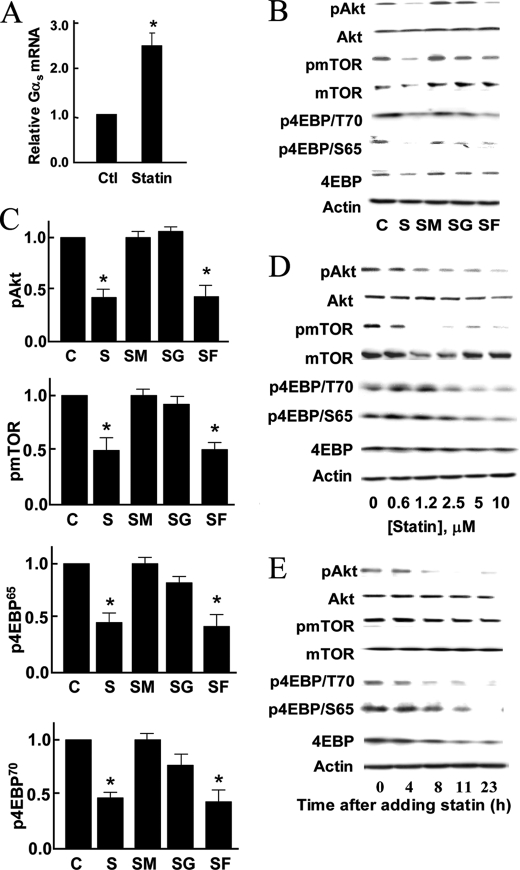
We next pursued cellular imaging approaches using the CFP-Epac(ΔDEP)-YFP FRET biosensor, which specifically detects intracellular cAMP (1); this biosensor yields a decrease in FRET following cAMP binding. We transfected endothelial cells with the biosensor for cAMP, and then analyzed FRET signals in real time following various drug treatments. As shown in Fig. 4, A and B, when endothelial cells transfected with the cAMP biosensor are treated with epinephrine, there is a striking decrease in FRET, indicating an increase in intracellular cAMP; a similar FRET decrease is seen following treatments with the G protein-sensitizing drug forskolin or with the phosphodiesterase inhibitor IBMX, indicating a ∼2-fold increase in cAMP abundance, consistent with previous reports (12); these agonists yielded similar increases in cellular cAMP levels. We used cAMP biosensor-transfected endothelial cells to determine the effect of simvastatin on epinephrine-induced cAMP accumulation. As shown in Fig. 4, C and D, simvastatin treatment significantly attenuated cAMP accumulation by epinephrine (p < 0.05, n = 6). We complemented these FRET-based cellular imaging approaches with standard biochemical assays of cAMP abundance. As shown in Fig. 4E, treatment of endothelial cells with simvastatin significantly attenuated the epinephrine-promoted increase in cAMP accumulation by ∼2-fold (p < 0.05, n = 4). Importantly, the statin-promoted decrease in intracellular cAMP is reversed by addition of mevalonate, which is the immediate substrate of HMG CoA reductase; the isoprenoid compound GGpp also blocked the effects of simvastatin (Fig. 4F). Taken together, these findings provide evidence that implicates upstream statin-sensitive geranylgeranylation pathways in modulating the suppression of cAMP responses seen after simvastatin treatment of endothelial cells. We therefore turned our attention to the analysis of G proteins that influence intracellular cAMP metabolism. We performed immunoblot analyses to investigate the effects of simvastatin on G protein abundance in endothelial cells. Fig. 5 shows that simvastatin treatment of endothelial cells leads to a marked decrease in the abundance of Gαs (80 ± 10% decrease compared with untreated cells, p < 0.05, n = 3). Importantly, the simvastatin-promoted decrease in Gαs was entirely reversed by treatment with mevalonate or GGpp, but not by Fpp (Fig. 5). When added alone, mevalonate had no effect on these responses (supplemental Fig. S1). Simvastatin treatment led to a more subtle ∼40% decrease in Gβ1, and Gγ2, (not shown), while the abundance of Gαi and Gαo were not significantly affected (Fig. 5, A and B). The abundance of Gαs was not affected by simvastatin treatment of human aortic smooth muscle cells (Fig. 5, C and D). As shown in Fig. 6, simvastatin time- and dose-dependently attenuated Gαs abundance. The EC50 for the statin-promoted decrease in abundance is ∼8 μm, which is within the therapeutic concentration range for patients taking standard doses of simvastatin (13).
FIGURE 4.
Simvastatin treatment suppresses cAMP levels in cultured endothelial cells. Panel A shows a representative time course of FRET responses in endothelial cells transfected with the cAMP biosensor CFP-Epac(ΔDEP)-YFP, which were treated either with PBS (Ctl), 1 μm epinephrine (Epi), 1 μm forskolin (FSK), or 100 nm 3-isobutyl-1-methylxanthine (IBMX). Panel B shows cAMP levels from pooled experiments, calculated based on the slopes of the linear region of the time course of YFP/CFP responses from six independent experiments identical in design to the experiment shown in panel A. Panel C shows a representative time course of YFP/CFP responses in endothelial cells transfected with the cAMP biosensor for 48 h, and then incubated in the presence or absence of 10 μm simvastatin (Statin) for 22 h, and then treated either with PBS (Ctl) or 1 μm epinephrine (Epi). Panel D shows pooled data analyzing cAMP content (calculated as the YFP/CFP slope following drug addition) from six independent experiments identical in design to the experiment shown in panel C. Panel E shows the results of cAMP measurements quantitated using an EIA kit; endothelial cells were treated with 10 μm simvastatin (statin) or with vehicle (Ctl) for 22 h, and then treated for 5 min with varying doses of epinephrine (Epi) as indicated. Panel F shows pooled data from four experiments analyzing cAMP levels using an EIA kit in endothelial cells treated for 22 h with vehicle (Ctl) or 10 μm simvastatin (S), either alone or in the presence of mevalonate (400 μm), or GGpp (10 μm), as indicated. The asterisk indicates p < 0.05 (ANOVA) compared with cells incubated with vehicle.
FIGURE 5.
Simvastatin treatment decreases Gαs abundance in cultured endothelial cells. Shown are immunoblot results obtained following statin treatments of endothelial cells (panels A and B) or vascular smooth muscle cells (panels C and D). Cells were treated for 22 h with simvastatin (10 μm) alone, or in the presence of mevalonate (400 μm), or GGpp (10 μm) or Fpp (10 μm) followed by cell lysis. Cell lysates were analyzed in immunoblot probed with antibodies as shown. Panels A and C show representative immunoblots, and panels B and D show quantitative analysis of pooled data from three independent experiments identical in design to the representative experiments shown above; the asterisk indicates p < 0.05 (ANOVA).
FIGURE 6.

Simvastatin treatment decreases Gαs abundance. Endothelial cells were treated with varying doses of simvastatin for 22 h or with 10 μm simvastatin for varying times as indicated. Cells were harvested and cell lysates analyzed in immunoblots probed with antibodies directed against Gαs and β-actin, as indicated. Panels A and C show representative immunoblot experiments, which were repeated five times with equivalent results; Panels B and D present pooled data, quantitating the immunoblot signals using digital chemiluminescence imaging. The basal phosphorylation in vehicle-treated cells was 1.0. The asterisk indicates p < 0.05 (ANOVA).
We next used two complementary experimental approaches to explore the hypothesis that simvastatin changes the stability of Gαs protein. We first determined the effects of simvastatin on Gαs protein half-life following treatment of endothelial cells with the protein synthesis inhibitor cycloheximide (Fig. 7, A and B); these experiments showed no change in the stability of the Gαs protein following simvastatin treatment (t1/2 7.8 versus 8.2 h, p = NS). We then performed pulse-chase experiments to measure the half-life of Gαs protein with and without simvastatin treatment, and again found no substantive change in Gαs protein stability (Fig. 7, C and D). To determine whether the level of Gαs mRNA is affected by simvastatin treatment, we performed quantitative PCR analyses, and found to our surprise that simvastatin treatment significantly increased Gαs mRNA by 2.5-fold (Fig. 8A). Taken together, these results indicate that the marked decrease in Gαs protein seen following simvastatin treatment was accompanied neither by a decrease in protein stability nor by a decrease in transcript abundance, and suggest that simvastatin might influence the translational regulation of Gαs.
FIGURE 7.
Simvastatin and Gαs protein stability in cultured endothelial cells. Panel A shown a representative immunoblot experiment in endothelial cells treated with 10 μm simvastatin for 22 h plus 10 ng/ml cycloheximide for the indicated times. Cells were harvested and cell lysates analyzed in immunoblots probed with antibodies directed against Gαs and β-actin, as indicated. Panel B shows quantitative analysis of pooled data from three independent experiments. Panel C shows the representative result of a pulse-chase experiment, in which newly synthesized proteins were labeled with 35S for 2 h and chased while cells were treated with PBS or 10 μm simvastatin for the indicated times followed by cell lysis. The abundance of 35S-labeled Gαs in cell lysates was analyzed by immunoprecipitation with a Gαs antibody followed with SDS-PAGE, and then quantified with a Cyclone PhosphorImager. Shown below are immunoblots of β-actin in cell lysates as a loading control. Panel D shows quantitative analyses of three experiments identical in design to the experiment shown in panel C.
FIGURE 8.
Effects of simvastatin on phosphorylation of proteins involved in translational regulation. Panel A shows pooled data from three experiments of real-time PCR for Gαs in endothelial cells treated with PBS or 10 μm simvastatin for 22 h; the asterisk indicates p < 0.05. Panel B shows representative results of immunoblots analyzed in endothelial cells treated with simvastatin (10 μm) alone, or in the presence of mevalonate (400 μm), GGpp (10 μm), or Fpp (10 μm) for 22 h followed by cell lysis. Cell lysates were analyzed in immunoblots probed with antibodies as shown. Panel C shows pooled data from three experiments identical in design to the experiment shown in panel B, quantitating the immunoblot signals using digital chemiluminescence imaging, setting the basal phosphorylation in vehicle-treated cells at 1.0. The asterisk indicates p < 0.05 compared with the basal level of phosphorylation. Panel D shows immunoblots analyzed in endothelial cells treated for 22 h with varying doses of simvastatin; cell lysates were analyzed in immunoblots probed with specific antibodies as indicated. Panel E shows the results of immunoblot analyzed in endothelial cells treated with 10 μm simvastatin for varying times. Cells were harvested and cell lysates analyzed in immunoblots probed with antibodies as shown. All experiments were performed at least three times with similar results.
The Akt-mTOR pathway plays a key role in cap-dependent initiation of mRNA translation by regulating phosphorylation of the initial factor eIF4E-binding protein (4EBP) in endothelial cells and in other cell types (14–19). We therefore explored the effects of simvastatin on phosphorylation of Akt, mTOR, and 4EBP. As shown in Fig. 8, B–E, simvastatin treatment of endothelial cells led to a significant decrease in the levels of phosphorylated Akt, mTOR, and 4EBP in a time- and dose-dependent manner. Importantly, these simvastatin-promoted decreases in phosphorylation were completely reversed by addition of mevalonate or GGpp, but not by Fpp. Because these findings implicate Akt in simvastatin-modulated Gαs regulation, we explored the effects of siRNA-mediated Akt knockdown on the abundance of Gαs as well as on the levels of mTOR, VASP, and 4EBP phosphorylation. As shown in Fig. 9, knockdown of Akt led to a significant reduction in Gαs abundance, and also markedly attenuated the phosphorylation of VASP, mTOR, and 4EBP. These effects of Akt knockdown were not recovered by adding mevalonate. We next examined whether these effects of statin on signaling pathways in cultured endothelial cells also occur in vivo in mice treated with simvastatin. We treated mice with daily administration of simvastatin for 2 weeks, and then harvested aortas, which were analyzed in immunoblots probed with a panel of phosphospecific antibodies. As shown in Fig. 10, A–D, in vivo simvastatin treatment of mice led to marked reductions in the phosphorylation of Akt, mTOR, and 4EBP in aorta, as compared with vehicle-treated mice.
FIGURE 9.
siRNA-mediated Akt knockdown modulates Gαs abundance and phosphorylation of proteins involved in translational regulation. Panel A shows representative immunoblots analyzed in endothelial cells transfected with control siRNA or Akt siRNA as noted, then incubated with or without the mevalonate (400 μm) for 22 h. Cell lysates were analyzed in immunoblots probed with antibodies as shown. Panels B–G show pooled data from three experiments identical in design to the experiment shown in panel A, quantitating the immunoblot signals using digital chemiluminescence imaging and normalizing the basal signals in vehicle-treated cells at 1.0. The asterisk indicates p < 0.05 (by ANOVA) compared with the basal level of phosphorylation. All experiments were performed at least three times with similar results.
FIGURE 10.
In vivo simvastatin treatment and phosphorylation of aortic proteins involved in translational regulation. Panel A shows representative immunoblots analyzed in aorta preparations isolated from mice that had been treated by intraperitoneal injection daily for 2 weeks with PBS (left lane) or simvastatin (right lane). Panels B–D show quantitative analyses of pooled data from four independent experiments identical in design to panel A; the asterisk indicates p < 0.05 (ANOVA).
DISCUSSION
These studies have revealed that simvastatin treatment of mice results in attenuated β-adrenergic vasorelaxation in isolated arterial preparations (Fig. 1). Our findings indicate that the statin effect on receptor-modulated vasorelaxation is selective: simvastatin treatment of mice leads to an attenuation of β-adrenergic but not muscarinic cholinergic responses analyzed in isolated arterial preparations. We had previously observed that in vivo simvastatin treatment decreases VASP phosphorylation in vascular preparations at the residue preferentially phosphorylated by PKA (VASP Ser157) (4, 20). The present studies show that simvastatin treatment of cultured endothelial cells significantly attenuates PKA-dependent as well as PKA-independent cAMP responses: both PKA-mediated VASP Ser157 phosphorylation (Fig. 2) as well as PKA-independent Rap1 activation (Fig. 3) are markedly suppressed by simvastatin. Moreover, statin treatment leads to a marked decrease in epinephrine-stimulated cAMP levels, as assessed using both biochemical assays for cAMP (Fig. 5) as well as FRET-based biosensor methods (Fig. 4). Taken together, these findings suggest that statins inhibit upstream β-adrenergic receptor-modulated pathways leading to cAMP formation.
We found that the effects of simvastatin on cAMP abundance are completely inhibited by the addition of mevalonate, the metabolite synthesized by HMG-CoA reductase, providing strong evidence that statin induced decrease of cAMP abundance is a direct consequence of HMG-CoA reductase inhibition. There are many biologically active metabolites downstream of mevalonate, and the present studies provide several lines of evidence that the effect of simvastatin on cAMP abundance involves enzymatic geranylgeranylation. Addition of the geranylgeranyl transferase product GGpp reverses statin-attenuated cAMP abundance (Fig. 4F). However, Fpp does not affect statin-attenuated cAMP and protein abundance (Fig. 5). Taken together, these observations implicate protein geranylgeranylation as the key molecular event that is inhibited by statin treatment and leads to attenuated β-adrenergic responses. It seems plausible that at least some of the effects of statins on Gαs may involve alterations in signaling pathways that are regulated by members of the Rho GTPase family, which undergo geranylgeranylation and modulate a broad range of cellular responses in endothelial cells.
Several lines of evidence in these studies indicate that the inhibitory effects of statin on Gαs abundance appear to be a consequence of translational regulation. Using two independent methods (Fig. 7), we found that simvastatin has no effect on Gαs protein stability. Moreover, the abundance of Gαs mRNA is paradoxically increased following statin treatment (Fig. 7), making transcriptional regulation an unlikely mechanism for the statin-promoted decrease in Gαs protein abundance. We were intrigued to note that simvastatin treatment significantly attenuates phosphorylation of Akt/mTOR and of eIF4/4EBP both in cultured endothelial cells (Figs. 8 and 9) and following in vivo statin treatment (Fig. 10). siRNA-mediated knockdown of Akt had a similar inhibitory effect on VASP phosphorylation and Gαs abundance (Fig. 9), implicating Akt as a determinant of the effect of simvastatin on Gαs protein synthesis. However, it remains unclear why the statin-dependent modulation of these phosphoproteins, which have such a broad role in translational regulation, lead to a more circumscribed effect on Gαs abundance. The molecular mechanism for the differential modulation of protein translation by simvastatin remains to be fully defined.
The effects of statins on β-adrenergic/cAMP pathways have been explored in a handful of prior studies in cardiac myocytes, but we are aware of no previous reports that have characterized statin effects these pathways in endothelial cells. In cultured neonatal cardiac myocytes, statin treatment has been shown to lead to a decrease in Gαs and in cAMP generation without affecting β-adrenergic receptor density (3). These authors found that statins lead to decreased Gγ isoprenylation and enhanced Gαs degradation. However, our results indicate that simvastatin treatment of endothelial cells does not affect Gαs protein stability (Fig. 7), and simvastatin has nominal effects on other G protein subunits in these cells (Fig. 5). An in vivo study showed that administration of statins partially protects the rat heart from catecholamine-induced hypertrophy (21), but the molecular mechanisms for this effect are incompletely understood. These effects of statins appear to be cell type-specific: statin treatment of vascular smooth muscle cells did not modulate cAMP pathways (Fig. 5), in contrast to the striking effects that we observed in endothelial cells.
These studies add to the known pleiotropic effects of statins clear evidence for attenuated β-adrenergic/cAMP responses in the vascular endothelium following simvastatin treatment in vivo or in vitro. It is intriguing to note that both statins and β-adrenergic blocking drugs lead to decreased mortality when administered in the early time period following myocardial infarction (22). These salutary effects of statins are observed well before any change in serum lipids can be detected, an observation that indeed formed the basis for investigations into the so-called “lipid-independent” effects of statins. We speculate that statins may have some “β blocker-like” properties when acutely administered: the present studies present clear evidence that β-adrenergic signaling to cAMP formation is attenuated following statin treatment, with the attenuation of Gαs abundance yielding similar biochemical consequences as β-antagonist treatment, albeit by a very different mechanism. We are unaware of any clinical study that has revealed a significant drug interaction between statins and beta blockers, and it seems likely that the interaction may be subtle, yet possibly important. Although there are many trials of statins in which beta blocker use was not uniform among all study subjects, it is not clear that informative clinical data that might reveal a drug interaction were either not obtained (e.g. urinary cAMP levels) or not analyzed (blood pressure, heart rate) based on drug use. Despite abundant clinical studies, it is quite possible that we simply have missed a subtle interaction between these widely used drugs. We hope that this present study may stimulate such an analysis.
Supplementary Material
Acknowledgments
We thank Professor Jalink for kindly providing the CFP-Epac(ΔDEP)-YFP cAMP biosensor and Dr. Hermann Kalwa for his help in the FRET-based cAMP measurements.
This work was supported, in whole or in part, by National Institutes of Health Grants GM36259, HL46457, and HL48743 (to T. M.), and by funding from the Japan Heart Foundation and the Uehara Memorial Foundation (to T. S.).

This article contains supplemental Fig. S1.
- HMG-CoA
- 3-hydroxy-3-methylglutaryl coenzyme A
- PKA
- protein kinase A
- VASP
- vasodilator-stimulated phosphoprotein
- Fpp
- farnesyl pyrophosphate
- GGpp
- geranylgeranyl pyrophosphate
- cAMP
- cyclic adenosine monophosphate
- FRET
- fluorescence resonance energy transfer.
REFERENCES
- 1. Ponsioen B., Zhao J., Riedl J., Zwartkruis F., van der Krogt G., Zaccolo M., Moolenaar W. H., Bos J. L., Jalink K. (2004) Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator. EMBO Rep. 5, 1176–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jain M. K., Ridker P. M. (2005) Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat. Rev. Drug Discov. 4, 977–987 [DOI] [PubMed] [Google Scholar]
- 3. Mühlhäuser U., Zolk O., Rau T., Münzel F., Wieland T., Eschenhagen T. (2006) Atorvastatin desensitizes β-adrenergic signaling in cardiac myocytes via reduced isoprenylation of G-protein γ-subunits. FASEB J. 20, 785–787 [DOI] [PubMed] [Google Scholar]
- 4. Kou R., Sartoretto J., Michel T. (2009) Regulation of Rac1 by simvastatin in endothelial cells: differential roles of AMP-activated protein kinase and calmodulin-dependent kinase kinase-β. J. Biol. Chem. 284, 14734–14743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Igarashi J., Bernier S. G., Michel T. (2001) Sphingosine 1-phosphate and activation of endothelial nitric-oxide synthase. differential regulation of Akt and MAP kinase pathways by EDG and bradykinin receptors in vascular endothelial cells. J. Biol. Chem. 276, 12420–12426 [DOI] [PubMed] [Google Scholar]
- 6. Michel T., Li G. K., Busconi L. (1993) Phosphorylation and subcellular translocation of endothelial nitric-oxide synthase. Proc. Natl. Acad. Sci. U.S.A. 90, 6252–6256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kou R., Greif D., Michel T. (2002) Dephosphorylation of endothelial nitric-oxide synthase by vascular endothelial growth factor. Implications for the vascular responses to cyclosporin A. J. Biol. Chem. 277, 29669–29673 [DOI] [PubMed] [Google Scholar]
- 8. Kou R., Igarashi J., Michel T. (2002) Lysophosphatidic acid and receptor-mediated activation of endothelial nitric-oxide synthase. Biochemistry 41, 4982–4988 [DOI] [PubMed] [Google Scholar]
- 9. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔC(T)) Method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 10. Moens A. L., Yang R., Watts V. L., Barouch L. A. (2010) β3-adrenoreceptor regulation of nitric oxide in the cardiovascular system. J. Mol. Cell Cardiol. 48, 1088–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gloerich M., Bos J. L. (2010) Epac: defining a new mechanism for cAMP action. Annu. Rev. Pharmacol. Toxicol. 50, 355–375 [DOI] [PubMed] [Google Scholar]
- 12. Namiecinska M., Wiktorowska-Owczarek A., Loboda A., Dulak J., Nowak J. Z. (2006) Cyclic AMP generating system in human microvascular endothelium is highly responsive to adrenaline. Pharmacol. Rep. 58, 884–889 [PubMed] [Google Scholar]
- 13. Lilja J. J., Kivistö K. T., Neuvonen P. J. (1998) Grapefruit juice-simvastatin interaction: effect on serum concentrations of simvastatin, simvastatin acid, and HMG-CoA reductase inhibitors. Clin. Pharmacol. Ther. 64, 477–483 [DOI] [PubMed] [Google Scholar]
- 14. Gingras A. C., Raught B., Sonenberg N. (1999) eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 68, 913–963 [DOI] [PubMed] [Google Scholar]
- 15. Bhandari B. K., Feliers D., Duraisamy S., Stewart J. L., Gingras A. C., Abboud H. E., Choudhury G. G., Sonenberg N., Kasinath B. S. (2001) Insulin regulation of protein translation repressor 4E-BP1, an eIF4E-binding protein, in renal epithelial cells. Kidney Int. 59, 866–875 [DOI] [PubMed] [Google Scholar]
- 16. Senthil D., Faulkner J. L., Choudhury G. G., Abboud H. E., Kasinath B. S. (2001) Angiotensin II inhibits insulin-stimulated phosphorylation of eukaryotic initiation factor 4E-binding protein-1 in proximal tubular epithelial cells. Biochem. J. 360, 87–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brunn G. J., Hudson C. C., Sekuli A., Williams J. M., Hosoi H., Houghton P. J., Lawrence J. C., Jr., Abraham R. T. (1997) Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science 277, 99–101 [DOI] [PubMed] [Google Scholar]
- 18. Beretta L., Gingras A. C., Svitkin Y. V., Hall M. N., Sonenberg N. (1996) Rapamycin blocks the phosphorylation of 4E-BP1 and inhibits cap-dependent initiation of translation. EMBO J. 15, 658–664 [PMC free article] [PubMed] [Google Scholar]
- 19. Pause A., Belsham G. J., Gingras A. C., Donzé O., Lin T. A., Lawrence J. C., Jr., Sonenberg N. (1994) Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5'-cap function. Nature 371, 762–767 [DOI] [PubMed] [Google Scholar]
- 20. Comerford K. M., Lawrence D. W., Synnestvedt K., Levi B. P., Colgan S. P. (2002) Role of vasodilator-stimulated phosphoprotein in PKA-induced changes in endothelial junctional permeability. FASEB J. 16, 583–585 [DOI] [PubMed] [Google Scholar]
- 21. Schmechel A., Grimm M., El-Armouche A., Höppner G., Schwoerer A. P., Ehmke H., Eschenhagen T. (2009) Treatment with atorvastatin partially protects the rat heart from harmful catecholamine effects. Cardiovasc Res. 82, 100–106 [DOI] [PubMed] [Google Scholar]
- 22. Stenestrand U., Wallentin L. (2001) Early statin treatment following acute myocardial infarction and 1-year survival. JAMA 285, 430–436 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.