Abstract
Titanium (Ti) has been widely used as an implant material due to the excellent biocompatibility and corrosion resistance of its oxide surface. Biomaterials must be sterile before implantation, but the effects of sterilization on their surface properties have been less well studied. The effects of cleaning and sterilization on surface characteristics were bio-determined using contaminated and pure Ti substrata first manufactured to present two different surface structures: pretreated titanium (PT, Ra = 0.4 μm) (i.e. surfaces that were not modified by sandblasting and/or acid etching); (SLA, Ra = 3.4 μm). Previously cultured cells and associated extracellular matrix were removed from all bio-contaminated specimens by cleaning in a sonicator bath with a sequential acetone–isopropanol–ethanol–distilled water protocol. Cleaned specimens were sterilized with autoclave, gamma irradiation, oxygen plasma, or ultraviolet light. X-ray photoelectron spectroscopy (XPS), contact angle measurements, profilometry, and scanning electron microscopy were used to examine surface chemical components, hydrophilicity, roughness, and morphology, respectively. Small organic molecules present on contaminated Ti surfaces were removed with cleaning. XPS analysis confirmed that surface chemistry was altered by both cleaning and sterilization. Cleaning and sterilization affected hydrophobicity and roughness. These modified surface properties affected osteogenic differentiation of human MG63 osteoblast-like cells. Specifically, autoclaved SLA surfaces lost the characteristic increase in osteoblast differentiation seen on starting SLA surfaces, which was correlated with altered surface wettability and roughness. These data indicated that recleaned and resterilized Ti implant surfaces cannot be considered the same as the first surfaces in terms of surface properties and cell responses. Therefore, the reuse of Ti implants after resterilization may not result in the same tissue responses as found with never-before-implanted specimens.
Keywords: Titanium, Sterilization, Roughness, Hydrophilicity, MG63 cells
1. Introduction
Titanium (Ti) has been widely used as an implant material due to the biocompatibility and excellent corrosion resistance of its thin titanium oxide layer [1]. In order to enhance osseointegration of dental and orthopaedic implants, many surface modification strategies have been pursued, focusing on the important role of the biomaterial surface properties [1–3]. Since the biomaterial device has to be sterile before implanting in the body, the sterilization process is considered as the last step of the surface modification [4]. Depending on the biomedical device or material, secondary cleaning or sterilization may be done in a clinical setting. Therefore, it is possible for biomaterials to undergo several uncounted cleaning and sterilization steps in an uncontrolled manner. In situations where implant devices are approved for reuse, cleaning and sterilization are key steps in the reconditioning of the implant to its initial state, but may also contribute to modification from initial surface properties [5].
Cleaning and sterilization can be distinguished in terms of function. The purpose of cleaning is to remove or reduce visible soils including blood, protein and debris on the surface of substrata. Sterilization serves to eliminate or stop reproduction of microorganisms including bacteria, spores, and fungi [6]. Usually, cleaning is done first, followed by sterilization. Fig. 1A illustrates the processing of biomaterials from cleaning to implantation.
Fig. 1.
(A) Illustration of events at the Ti surface during cleaning, sterilization, and implantation. (B) Cleaning procedures (CP) and sterilization methods used in this study.
There are many different sterilization methods available, each with different modes of action. Depending on material properties, the methods used for a particular device have to be carefully selected. In this study, autoclaving (AC), gamma irradiation (GI), oxygen plasma (OP), and ultraviolet (UV) were used to sterilize titanium. Autoclaving (AC) is a physical method of sterilization, which exposes the living organisms to unsustainable conditions of temperature, pressure, and time (121 °C, 18 psi, 20 min). However, depending on the material density, volume, and size, the effectiveness of the autoclave may vary [7]. Chemical methods such as oxygen plasma (OP) can also be used. In this method, an ionized gas bombards the substratum surface and promotes the formation of free radicals under vacuum. Active species like polar groups break down and strip the surface layer. The thickness of the removed layer and the new surface properties can be modified by the type of gas, supplied gas purity, applied gas pressure, and sample position [8–10]. Ultraviolet light is also used for surface sterilization. The shortest wavelength region with the highest energy intensity, 280–100 nm, is used to eliminate bacteria by inducing formation of thymine dimers, inhibiting DNA replication [7,11]. Gamma irradiation is used to sterilize biomaterials and medical equipment because ionized high energy is strong enough to promote DNA damage without releasing toxic residues. Due to good penetration depth, GI is useful for densely packed products and pre-packed materials.
GI is an appropriate sterilization method for heat-sensitive devices or materials because the temperature of the materials does not noticeably increase during or after the process. In AC, the material temperature increases due to applied steam heat. Also, the AC chamber must remain closed until the heat cools to room temperature and any moisture dries. Therefore, AC is not recommended for heat-sensitive biomaterials. [12,13]. Although the ethylene oxide (EO) sterilization method is often used for cellulose-based biomaterials and plastic products, we did not use it in this study because it can leave toxic residues on the device surface [14] which could negatively impact the cells growing on the titanium surface. Dry heat sterilization has a long history compared to other methods. However, steam heat sterilization is more efficient in terms of heat transfer. Therefore, in this study, autoclaving was used instead of using both dry and steam heat sterilization methods.
It is well understood that the biological, chemical and mechanical properties of biomaterials are susceptible to change by diverse sterilization methods [12,15–18]. However, the contribution of the outermost molecular layer of an implant to its in vivo function is less well established. This layer plays an important role in determining the surface properties including surface energy, chemistry, and wettability. These properties directly affect the interactions with surrounding host tissue in vitro and in vivo [2,3,19–22]. Cleaning and sterilization can cause changes in the surface properties of biomaterials [5,6,13,16,23], but how these changes alter biological response is less well understood.
Because physical and chemical properties of materials are sensitive to small variations in their surface chemical composition and morphology [24,25], two contrasting types of Ti implant surfaces were used: (1) PT, consisting of Ti surfaces that were pretreated with a prepickling step, and (2) SLA, consisting of Ti surfaces that were sandblasted followed by acid etching. Prior to exposure to cell culture, these two types of surfaces differ in their micron and submicron scale combined surface roughness, a property that has been recognized as important in the enhancement of peri-implant bone formation [26]. The purpose of the present study was to assess the effects of commonly used sterilization methods on the surface properties of Ti and to correlate these changes with cell responses associated with osteoblastic differentiation. We also examined how cleaning contaminated Ti substrata prepared by exposing to biological cell growth and extracellular matrix production alters the surface and cell response following resterilization.
2. Materials and methods
2.1. Materials
Gamma-irradiation-sterilized Ti disks 1 mm thick and 15 mm in diameter were donated by Institut Straumann AG (Basel, Switzerland). Prior to gamma irradiation, the disks had been treated so as to exhibit two different surface structures: pretreated surface (PT, Ra = 0.4 μm) and sandblasted/acid-etched surface (SLA, Ra = 3.4 μm). Pretreated surfaces are prepared by washing in acetone and then processing through 2% ammonium fluoride/2% hydrofluoric acid/10% nitric acid solution at 55 °C for seconds, they but have not yet been modified by the sand blasting and acid etching process used to generate the SLA surface. PT and SLA surface characterization prior to cell culture has been described previously [27]. Reagent grade acetone, isopropanol, and ethanol were purchased from Sigma–Aldrich (St. Louis, MO).
2.2. Disk cleaning and sterilization
In order to demonstrate whether cleaning protocols used in this study effectively remove organic contaminants on Ti surfaces, bio-contaminated PT and SLA surfaces were prepared as follows: control Ti disks were placed in full media with cells and cultured for 5–7 days as described previously [21]. Confluent cell monolayers were removed by two sequential trypsin digestions based on previous observations showing this removes all cells on the surface, but it does not remove extracellular matrix adhered to the disk. Contaminated Ti disks were cleaned by sonication in 2% Micro-90® detergent (International Product Corporation, Burlington, NJ) in distilled, ultrapure water for 2 × 15 min followed by two 15 min rinses in ultrapure water (Fig. 1B). Samples were rinsed in sequential ultrasonic baths of reagent grade acetone, isopropanol, and ethanol. Two different cleaning regimens were performed, varying the rinse time in each solvent: two 15 min rinses (CP2); or three 30 min rinses (CP3). After cleaning, disks were rinsed with ultrapure water three times for 10 min in a sonicated bath.
Cleaned disks were packed in self-sealed sterilization pouches (Cardinal Health, Dublin, OH) and sterilized by gamma irradiation or autoclaving. For ultraviolet light sterilization the disks were exposed to UV overnight in a chamber and then packaged in sterile pouches. Cleaned individual Ti disks were treated with oxygen plasma under a sterile hood and packed in sterile pouches for further experiments. The detailed conditions of each sterilization method were as follows: autoclaving for 20 min at 121 °C and 18 psi (2540E, Tuttnauer Autoclave, NY, USA); gamma irradiation at 25 kGy overnight; oxygen plasma for 2 min per side (PDC-32G, Harrick Plasma, NY, USA); or ultraviolet light for 90 min at 254 nm (CL-1000 Ultraviolet Crosslinker, VUP). Gamma irradiated PT and SLA disks as supplied from the manufacturer were used as the primarily cleaned and sterilized controls. Surfaces cleaned and sterilized as described above are denoted as follows with respect to their substratum-cleaning protocol-sterilization method. For example, the PT-CP2-GI represents a bio-contaminated used PT surface that was cleaned with cleaning protocol (CP2, two sequential 15 min rinses) and then sterilized with gamma irradiation.
2.3. Surface characterization of secondarily cleaned and/or sterilized PT and SLA surfaces
2.3.1. X-ray photoelectron spectroscopy
The surface chemical composition of Ti disks before and after cleaning and sterilization was determined by X-ray photoelectron spectroscopy (XPS) (ESCA-SSX 100, Service Physics, Bend, OR). The XPS analysis chamber was evacuated to a pressure of 10−8 Torr or lower before collecting XPS spectra. This system was equipped with a monochromatic Al Kα X-ray source (hv = 1486.6 eV photons) at a 55° takeoff angle. General survey spectra were obtained using an X-ray spot size of 800 μm. The C1s binding energy of the aliphatic C–C bond at 284.6 eV was used as an internal reference. XPS results were evaluated using the ESCA 2005G software package provided by Service Physics, Inc. Three measurements were made on each of two separate surfaces per group.
2.3.2. Contact angle measurement
Oxygen plasma can make surfaces very active. OP-sterilized surfaces have a high affinity for the adsorption of molecules onto the surface, including contaminants [28]. Therefore, precise control of the time between OP sterilization and implantation is critical in minimizing recontamination of the surface. To demonstrate this, the contact angle was measured as a function of time after OP sterilization. The contact angle of Ti disks before and after secondary cleaning and sterilization was determined by a Ramé-Hart goniometer (Model 250-F1, Mountain Lakes, NJ). Images were analyzed with the DROPimage CA software package (Ramé-Hart Instrument Co.). The ultrapure water droplet volume (2 μl) was precisely controlled with a syringe scale. All measurements were performed in a cleanroom. Three measurements were made on two separate surfaces per group (i.e. a group being a particular cleaning and sterilization protocol).
2.3.3. Profilometry
The two-dimensional surface roughness of Ti disks before and after secondary cleaning and sterilization was measured using a KLA-Tencor P-15 contact profilometer (KLA Tencor, CA, USA) equipped with a 2 μm diamond-tracing stylus tip and 90° point angle. Twelve random areas per specimen (n = 6) were measured over a scan length of 500 μm to obtain the arithmetic mean roughness (Ra, μm). All analyses were performed in a cleanroom.
2.3.4. Scanning electron microscopy
The surface morphology of Ti disks before and after secondary cleaning and sterilization was examined by scanning electron microscopy (SEM) using an Ultra 60 field emission (FE) microscope (Carl Zeiss SMT Ltd, Cambridge, UK). Samples were not over-coated; images were recorded using a 5 kV accelerating voltage. Two separate surfaces per group were examined (n = 2).
2.4. Cell response
Cell responses to primary and secondarily cleaned and sterilized surfaces were performed using human osteoblast-like MG63 cells (American Type Culture Collection, Manassas, VA). Cells were plated at a density of 10,000 cells cm−2 on either tissue culture polystyrene (TCPS) or Ti surfaces and cultured in Dulbecco's modification of Eagle's medium (Cellgro®, Mediatech Inc., Manassas, VA) supplemented with 10% fetal bovine serum (Hyclone, Waltham, MA) and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA) at 37 °C, 5% CO2, and 100% humidity. Cells were grown until confluence was achieved on TCPS. At confluence, fresh media were added to all cells and the cells were further incubated for 24 h. After incubation, cell number was counted in all cultures and conditioned media were collected. To collect all cells on the rough Ti surfaces, cells were released by two sequential 10 min incubations in 0.25% trypsin-EDTA. Cells were counted using a Z1 Particle Counter (Beckman Coulter, Fullerton, CA). Cellular alkaline phosphatase specific activity was measured by determining p-nitrophenol (pNP) release from p-nitrophenylphosphate (pNPP) at pH 10.2 in the cell lysate and normalized to total protein content (Macro BCA Protein Assay kit, Pierce). Osteocalcin (OCN) levels in the conditioned media were determined by radioimmunoassay (Human Osteocalcin RIA Kit, Biomedical Technologies, Stoughton, MA). Osteoprotegerin (OPG) was determined by enzyme-linked immunosorbent assay (ELISA) kit (DY805 Osteoprotegerin DuoSet, R&D System, Minneapolis, MN). Immunoassay results were normalized to total cell number.
2.5. Statistical analysis
Data were analyzed using a one-way analysis of variance (ANO-VA) for all surfaces. If there was a statistical difference noted, Bonferroni's modification of Student's t-test for multiple comparisons was used. P < 0.05 was considered significant. The presented data are from one of two repeated experiments, both with comparable results.In each experiment, there were six individual samples or cultures per variable, which provided sufficient power (n = 6) to allow statistically significant differences to be detected. Thus, we did not have to combine samples from multiple experiments and can present actual values rather than treated/control ratios.
3. Results
3.1. Effect of secondary cleaning on Ti surfaces
Both CP2 and CP3 protocols effectively cleaned and removed small organic molecules (C and N) from the contaminated Ti surfaces. SEM images of contaminated PT and SLA surfaces prior to cleaning showed unidentified materials (Fig. 2A). However, visible surface deposits were removed after cleaning with both CP2 (Fig. 2A) and CP3 (data not shown). XPS analysis confirmed the presence of carbon (C), oxygen (O), titanium (Ti), and nitrogen (N) on both contaminated and secondarily cleaned Ti surfaces (Fig. 2B and C). Traces of sulfur (0.8%) were detected on contaminated PT surfaces, but not on contaminated SLA surfaces (Table 1). XPS analysis indicated that the outermost surface of contaminated PT and SLA substrata was composed mainly of molecules C, O, N, and Ti. However, after cleaning the amounts of C and N were markedly reduced and the percentages of Ti and O on the surface were increased.
Fig. 2.
Effect of cleaning procedures on surface properties of used PT and SLA disks. Surface properties of PT and SLA surfaces before and after cleaning with CP2 were examined using SEM (A), XPS low resolution spectra (B, C), water contact angle (n = 2) (D) and surface roughness (n = 6) (E). *, p < 0.05, PT vs. SLA; #, p < 0.05, vs. before cleaning.
Table 1.
XPS chemical component analysis on used PT and SLA before and after cleaning. B.C is before cleaning (contaminated Ti disks), CP2 is two-time cleaning protocol, and CP3 is three-time cleaning protocol.
| Atom | Cleaning (atomic %, mean ± SEM) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| PT | SLA | |||||
|
|
|
|||||
| B.C. | CP2 | CP3 | B.C. | CP2 | CP3 | |
| C | 44.6 ± 3.2 | 16.3 ± 1.8 | 20.5 ± 0.9 | 69.9 ± 5.8 | 13.6 ± 2.8 | 19.3 ± 1.2 |
| O | 31.1 ± 2.9 | 38.7 ± 2.2 | 39.7 ± 3.6 | 22.3 ± 2.5 | 39.6 ± 3.9 | 37.9 ± 6.7 |
| Ti | 18.4 ± 1.5 | 43.9 ± 5.2 | 38.8 ± 3.4 | 1.1 ± 0.4 | 45.5 ± 7.2 | 42.4 ± 2.2 |
| N | 5.1 ± 0.9 | 1.0 ± 0.2 | 0.9 ± 0.1 | 6.7 ± 0.1 | 1.3 ± 0.3 | 0.8 ± 0.1 |
| S | 0.8 ± 0.2 | |||||
The water contact angle of the PT-CP2 and PT-CP3 surfaces increased compared to the contact angle of the contaminated PT surfaces before cleaning (Fig. 2D). However, only the SLA-CP3 surface exhibited an increase in water contact angle as compared to the contaminated SLA surfaces prior to cleaning. Contaminated (before cleaning) PT and SLA surfaces had a roughness of 0.4 μm and 3.9 μm, respectively. After cleaning, there was no difference in roughness, regardless of the cleaning protocol used (Fig. 2E).
3.2. Effect of sterilization on surface properties
3.2.1. Surface chemistry
Low resolution XPS spectra revealed the presence of C, O, Ti, and N atoms on control PT and SLA surfaces and surface chemical composition fluctuated depending on the different cleaning and sterilization methods used (Fig. 3). The chemical composition of the PT-CP(2 or 3)-GI and SLA-CP(2 or 3)-GI surfaces showed a similar distribution of elemental concentration as control PT and SLA (GI sterilized Ti disks) surfaces. A higher percentage of C was detected on the AC sterilized PT and SLA surfaces and SLA-CP(2 or 3)-UV surfaces compared to control substrates and other methods used. Increased O concentration with decreased C concentration on the PT-CP(2 or 3)-OP and SLA-CP2-OP surfaces was detected by XPS analysis. Silicon (Si) was detected only on the SLA-CP3-OP surface. The percentage of N was increased on the SLA-CP(2 or 3)-UV surface. However, N was not detected on the PT-CP2-UV surface.
Fig. 3.
Effect of sterilization method on surface chemical composition of cleaned PT and SLA surfaces (n = 2). Atomic percentages (%) of components on the surface after sterilization were obtained by XPS for the PT-CP2 surfaces (A), the PT-CP3 surfaces (B), the SLA-CP2 surfaces (C), the SLA-CP3 surfaces (D). PT and SLA are new, unused and sterilized with gamma irradiation surfaces. Before cleaning (BC); autoclave (AC); gamma irradiation (GI); oxygen plasma (OP); and ultraviolet (UV).
3.2.2. Surface wettability
The PT-CP(2 or 3)-AC and SLA-CP(2 or 3)-AC surfaces exhibited an increase in water contact angle in comparison to control PT and SLA surfaces (Fig. 4A and B). Other sterilization methods including OP, GI, or UV resulted in a decrease in contact angle on both PT and SLA surfaces in comparison to control and AC-sterilized surfaces. The PT-CP(2 or 3)-OP and SLA-CP(2 or 3)-OP surfaces had the best water wettability among all surfaces. Surface wettability was sensitive to storage time after OP (Fig. 4C). There was a 63% increase in water contact angle within 30 min after processing. There was a further increase in contact angle at 6 h, 8 h, and 24 h.
Fig. 4.
Effect of sterilization method on water contact angle of cleaned PT and SLA surfaces. Contact angle was measured on PT and SLA disks after cleaning with CP2 (A) and CP3 (B). Control surfaces are new, unused PT and SLA surfaces. (C) PT-CP3 surfaces were sterilized with oxygen plasma and contact angle was measured as a function of hours after oxygen plasma. *, p < 0.05, PT vs. SLA control surface; #, p < 0.05, vs. control, ■, p < 0.05, vs. CP, $, p < 0.05, vs. GI, ^, p < 0.05, vs. AC, &, p < 0.05 vs. OP. Cleaning protocol (CP); autoclave (AC); gamma irradiation (GI); oxygen plasma (OP); and ultraviolet (UV).
3.2.3. Surface roughness
Contact profilometry showed that unused PT surfaces (Ra = 0.4 μm) were relatively smoother than SLA surfaces (Ra = 3.4 μm) (Fig. 5A and B). None of the sterilization methods altered the roughness of the PT surface. SLA surface roughness was modified with AC, OP, and UV sterilization. Autoclaved SLA surfaces had lower Ra values than any of the other treated SLA surfaces, while OP-treated SLA surface roughness was much rougher than that of the unused control SLA surface. The SLA surface roughness increased after UV sterilization. This suggests surface deposition of steam-borne contaminants during autoclaving, vs. oxidative stripping away of such a priori contaminants in the plasma device.
Fig. 5.
Effect of sterilization method on roughness of cleaned PT and SLA surfaces. Surface roughness of PT and SLA surfaces was measured after cleaning with CP2 (A) or CP3 (B). Control surfaces are new, unused PT and SLA surfaces. *, p < 0.05, PT vs. SLA control surface; #, p < 0.05, vs. control, ■, p < 0.05, vs. CP, $, p < 0.05, vs. GI, ^, p < 0.05, vs. AC. Cleaning protocol (CP); autoclave (AC); gamma irradiation (GI); oxygen plasma (OP); and ultraviolet (UV).
3.2.4. Surface morphology
SEM confirmed that control PT and SLA surfaces had different surface morphologies (Fig. 6A). Whereas control PT was relatively smooth, SLA had micrometer scale craters and submicrometer scale pits. After secondary cleaning and sterilization, the surface morphology of PT surfaces (Fig. 6B) was similar to that of the control surfaces. However, irregular scratch marks were observed on all secondarily cleaned and sterilized PT surfaces. Similarly, a larger number and bigger size cracks were observed on secondarily cleaned and sterilized SLA surfaces. No differences were detected as a function of sterilization method.
Fig. 6.
Surface morphology of PT and SLA surfaces. (A) SEM images of new PT (left) and SLA (right) surfaces. (B) SEM images of PT and SLA surfaces after cleaning (CP2 or CP3) and sterilization. Autoclave (AC); gamma irradiation (GI); oxygen plasma (OP); ultraviolet (UV).
3.3. Effect of sterilization on MG63 cell response
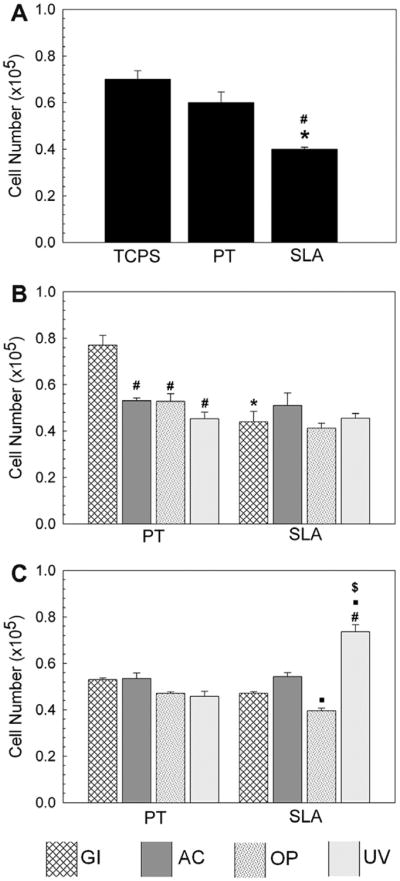
Responses of the MG63 cells were sensitive to the secondary cleaning and sterilization protocols. Cell number was lower on unused SLA than on TCPS or PT surfaces (Fig. 7A). Similarly, cell numbers on SLA-CP2-GI were lower than on PT-CP2-GI (Fig. 7B). However, cell number on SLA-CP3-GI and PT-CP3-GI surfaces was not different (Fig. 7C). Cell number on PT-CP2-GI was higher than on PT secondarily sterilized with other methods (Fig. 7B). The SLA-CP3-UV surfaces had more cells than control and SLA resterilized with GI, AC, and OP (Fig. 7C).
Fig. 7.
Effect of sterilization method of PT and SLA on MG63 cell number. MG63 cells were cultured on TCPS, PT and SLA surfaces and grown to confluence. At confluence, cell number on (A) new, unused, and sterilized with gamma irradiation PT and SLA surfaces,*, p < 0.05 Ti vs. TCPS; #, p < 0.05 SLA vs. PT, (B) cleaned with CP2 and then sterilized, and (C) cleaned with CP3 and sterilized. *, p < 0.05, PT vs. SLA; #, p < 0.05, vs. GI, ■, p < 0.05, vs. AC; $, p < 0.05, vs. OP.
Alkaline phosphatase specific activity, osteocalcin, and osteoprotegerin levels were higher on control SLA than on TCPS or control PT surfaces as shown in Fig. 8A, D, and G, respectively. Similarly, alkaline phosphatase specific activity on the PT-CP2-GI surface was lower than on the SLA-CP2-GI surface (Fig. 8B). In contrast, enzyme activity was higher on the PT-CP3-GI surface than on the SLA-CP3-GI surface (Fig. 8C). Enzyme activity on PT surfaces secondarily sterilized with AC, OP, or UV was comparable to activity on the SLA surface cleaned and sterilized using the same protocols.
Fig. 8.
Effect of PT and SLA surface treated with different sterilization methods on MG63 cells. MG63 cells were plated on TCPS, PT and SLA surfaces and grown to confluence. At confluence, alkaline phosphatase (ALP) specific activity, osteocalcin (OCN), and osteoprotegerin (OPG) for new surface (A), (D), and (G), respectively. *, p < 0.05 Ti vs. TCPS; #, p < 0.05 SLA vs. PT. Alkaline phosphatase specific activity with CP2 and CP3 is (B) and (C), respectively. Osteocalcin with CP2 and CP3 is (E) and (F), respectively. Osteoprotegerin with CP2 and CP3 is (H) and (I), respectively. *, p < 0.05, PT vs. SLA; #, p < 0.05, vs. GI, ■, p < 0.05, vs. AC; $, p < 0.05, vs. OP. Inserted red and blue dotted lines indicate PT and SLA control surfaces, respectively.
Osteocalcin was greater in the conditioned media of cells grown on control SLA compared to control PT or TCPS (Fig. 8D). Sterilization methods altered osteocalcin levels in cultures grown on cleaned PT and SLA surfaces. Osteoblasts grown on SLA-CP2-GI had higher levels of osteocalcin in their media than were seen when the cells were cultured on PT-CP2-GI surface (Fig. 8E). No difference was observed between PT-CP3-GI and SLA-CP3-GI surfaces. Osteocalcin production on PT-CP2-AC was greater than on PT-CP2-GI, whereas no differences were observed among PT-CP2-OP, PT-CP2-UV, control and GI treated surfaces. Osteocalcin levels were lower on SLA-CP3-UV compared to SLA-CP3-OP (Fig. 8E and F).
Osteoblasts grown on PT-CP2-AC, OP, or UV surfaces had higher levels of osteoprotegerin in their conditioned media by sterilization methods on PT-CP3 surfaces (Fig. 8I). Significantly more osteoprotegerin was produced on SLA-CP3-OP when compared to SLA-CP3-GI, SLA-CP3-AC, and SLA-CP3-UV (Fig. 8I). The lowest osteoprotegerin production levels were found in cultures grown on SLA-CP3-UV.
4. Discussion
Ti implant surface properties such as surface chemistry, wettability, and morphology have been shown to affect osteoblast proliferation, extracellular matrix production, local factor production, and stimulation of an osteogenic microenvironment [2,7,29]. Therefore, understanding how the sterilization process affects these surface properties is important for clinical outcome. There is no general agreement, yet, on what the most desirable starting surface properties should be for clinically placed dental implants or for orthopaedic endoprostheses. The effect of sterilization on surface properties has not been well studied, but may affect responses of implants both in vitro and in vivo.
Although implants are intended for single use clinically, they may be reused under some circumstances. Moreover, investigators developing new surfaces or investigating biological responses to biomaterials may consider their reuse to conserve resources. Thus, an understanding of how cleaning can alter surface properties and how this might affect changes due to sterilization is important.
In the present study, we developed two cleaning protocols, CP2 and CP3, to clean bio-contaminated PT and SLA surfaces. Surface roughness was not changed by the two protocols. However, increased water contact angle values showed that increased surface hydrophobicity of secondarily cleaned surfaces was due to chemical differences in the outermost surface layer. XPS analysis also indicated that the chemical composition of the secondarily cleaned surfaces was altered after cleaning, even though both cleaning protocols were effective at removing organic contaminants based on XPS analysis. However, the CP3 protocol resulted in a greater contact angle on the rougher SLA surfaces, indicating that it was less effective on the more complex topography.
Surface preparation outcomes were also affected not only by cleaning but also by the sterilization method used. Others have shown that autoclaved Ti surfaces might be coated with N, F, Mg, Si, and Cl [30], although these contaminants were not observed on the autoclaved PT and SLA surfaces in this study.
All of the secondarily sterilized surfaces had adventitious carbon-based contaminants, but the amounts differed as a function of cleaning protocol and sterilization method. The presumed source of the most abundant carbon species on the surfaces was molecules that were adsorbed at the surface/air interface [24]. PT and SLA surfaces were directly treated with OP in a glass chamber. OP was under vacuum conditions, and AC was performed under pressure (18 psi). The other two sterilization methods were done at atmospheric pressure (14.7 psi). Molecule adsorption can be influenced by these factors. Moreover, molecules can be re-deposited or bounced from the surface during sterilization. This was particularly the case for the autoclaved surfaces [17,23,31], and may have contributed to the increase in hydrophobicity.
Detecting Si only on the SLA-CP3-OP surfaces is an interesting finding in this study. It is hard to explain this phenomenon other than the possibility of Si coming from the glass chamber, as noted previously [4,32,33]. Operational conditions such as plasma treatment time, gas pressure, and the purity of gas do affect the rate and amount of deposition of SiO2 on the surfaces. Beneficially, OP sterilization best decreased surface carbon content and hydrophobicity of the PT and SLA surfaces.
Differences in surface wettability may also be due to the reduction of titanium in the oxide layer, i.e. the transition from the more stable and less polar Ti4+ state to the Ti3+ state [7,34]. Machnee et al. reported that the oxide layer thickness was not modified significantly by surface treatments such as cleaning, sterilization in an autoclave, or radiofrequency glow discharge [35]. Although there was no statistical difference in oxide layer thickness observed, the surfaces do not necessarily have similar oxidation states. In contrast, other researchers have found that surface oxide layer thickness and composition vary with the sterilization processes used, including sterilization in an autoclave [23,30,36]. The temperature and pressure during AC can affect the oxidation states and the oxide layer thickness on the surface. Not only the processing conditions but also defects in the grain boundaries of the metal structure may contribute to modification of the oxide layer [30,34]. In addition, sterilization methods have been shown to alter mechanical properties and cellular response [36,37]. OP treatment can increase the Ti3+ concentration on the surface and improve surface hydrophilicity on PT and SLA, as exhibited by a lower water contact angle.
The results assessing the effects of GI are particularly important. We were able to directly compare control surfaces (i.e. PT or SLA sterilized with GI) with contaminated PT and SLA surfaces that were cleaned with either CP2 or CP3 and then again sterilized with GI. Even though the final step in both cases was sterilization with GI, the cleaning history of the underlying surface played a role in determining the final surface properties of the sterile material. Control surfaces exhibited a higher degree of hydrophobicity than contaminated surfaces that were both secondarily cleaned and sterilized with GI. This discrepancy validates our initial hypothesis that surface properties are sensitive to the history of the surface treatment.
Interestingly, following sterilization by the AC method, surface roughness of SLA decreased significantly, regardless of the cleaning procedure used, supporting previous observations of Smith et al. in which AC altered the surface structure of Ti alloy and Co–Cr–Mo substrates [32]. The mechanism is still not clear. It is likely that autoclaving leaves behind stream-borne surface deposits. In contrast, the SLA-CP(2 or 3)-OP surface roughness increased. This suggests that superficial material removal and sterilization occurs at the same time, contributing to the increased surface roughness. SLA surface roughness increased after UV sterilization. Ponter et al. demonstrated how polymer surface roughness gradually increased after UV sterilization in combination with O3. It is likely that the terminal groups exposed on SLA surfaces were modified by UV [38]. The fact that UV did not alter roughness of the PT surface suggests that chemical groups exposed on the SLA surface were more sensitive to UV.
At least some of the differences in surface morphology were due to specimen handling. Irregular traces on control and secondarily sterilized PT surfaces seen on SEM images were most probably a result of the initial PT preparation process. In addition, these irregular marks may have been due to the cell harvesting process, as contaminated PT and SLA were prepared to add full media with cells and then cells were collected. Additional cracks were observed on SLA surfaces after secondary sterilization. The long sonication time used to clean these surfaces may have contributed to the increased number of cracks on these substrata.
Cell responses were sensitive to different cleaning and sterilization methods. Changes in surface morphology may have accounted for some of this variation, but certainly chemistry played a role as well [26]. Alkaline phosphatase specific activity as an early differentiation marker, osteocalcin as a later differentiation marker, and osteoprotegerin, which inhibits osteoclast differentiation, on the SLA-CP(2 or 3)-AC surfaces, did not significantly increase as compared to the PT-CP(2 or 3)-AC surfaces, although these outcomes were increased when MG63 cells were grown on unused SLA vs. PT [26]. These results suggest that MG63 cells are capable of recognizing changes in surface wettability and roughness that are due to the secondary cleaning and sterilization procedures performed on contaminated surfaces. Cell responses observed with the PT-CP2-GI and the SLA-CP2-GI surfaces resembled those obtained with control PT and SLA surfaces. In contrast, the PT or SLA-CP3-GI surfaces supported an opposite cellular response compared to control surfaces. This difference can be induced with different cleaning protocols such as CP2 and CP3. Surface roughness was stable with cleaning and GI sterilization on PT and SLA surface. However, surface wettability was dominantly influenced with cleaning protocols. PT or SLA-CP(2 or 3)-OP, characterized by enhanced wettability and decreased roughness, showed no differences between PT and SLA and control surfaces, except for osteoprotegerin production levels.. While resterilization may be sufficient to eliminate viable microorganisms, original implant properties cannot be reproduced.
5. Conclusions
In conclusion, bio-contaminated PT and SLA surfaces can be cleaned with CP2 and CP3 without modifying surface roughness. However, the combination of cleaning followed by sterilization can result in altered micrometer scale or submicrometer scale surface morphology, as well as chemistry. AC deposited carbon-rich matter on the surface, increasing hydrophobicity, whereas OP sterilized surfaces showed increased hydrophilicity and roughness due to the concurrent superficial material removal during plasma sterilization. Surface wettability of GI sterilized PT and SLA surfaces was enhanced without changing surface roughness. Modified surface properties regulated osteoblast proliferation and differentiation in a different manner compared to control substrata. Taken together, this study indicates that cleaned and sterilized contaminated Ti disks cannot be considered equivalent to unused status. Furthermore, in vitro study results suggest that different cellular responses were due to the modified surface. Although in vivo and clinical studies need to be performed before making conclusions about performance, the reuse of Ti implants after resterilization may not be an option if the same clinical responses as were achieved using unused Ti implants are sought.
Acknowledgments
This work was supported by an USPHS Grant AR052102. A graduate fellowship (to J.H.P.) was awarded by the Paper Science and Engineering (PSE) program and funded through the Institute of Paper Science and Technology (IPST) at Georgia Tech. The PT and SLA disks were provided by Institut Straumann AG.
Appendix A. Figures with essential colour discrimination
Certain figures in this article, particularly Figures 1–3, and 8, are difficult to interpret in black and white. The full colour images can be found in the on-line version, at doi:10.1016/j.actbio. 2011.11.026.
References
- 1.Mavrogenis AF, Dimitriou R, Parvizi J, Babis GC. Biology of implant osseointegration. J Musculoskeletal Neuronal Interact. 2009;9(2):61–71. [PubMed] [Google Scholar]
- 2.Schwarz F, Wieland M, Schwartz Z, Zhao G, Rupp F, Geis-Gerstorfer J, et al. Potential of chemically modified hydrophilic surface characteristics to support tissue integration of titanium dental implants. J Biomed Mater Res B. 2009;88B(2):544–57. doi: 10.1002/jbm.b.31233. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz Z, Boyan BD. Underlying mechanisms at the bone–biomaterial interface. J Cell Biochem. 1994;56(3):340–7. doi: 10.1002/jcb.240560310. [DOI] [PubMed] [Google Scholar]
- 4.Kasemo B, Lausmaa J. Biomaterial and implant surface – on the role of cleanliness, contamination, and preparation procedures. J Biomed Mater Res-A. 1988;22(A2):145–58. doi: 10.1002/jbm.820221307. [DOI] [PubMed] [Google Scholar]
- 5.Martin JY, Dean DD, Chran DL, Simpson J, Boyan BD, Schwartz Z. Proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63) cultured on previously used titanium surfaces. Clin Oral Implan Res. 1996;7(1):27–37. doi: 10.1034/j.1600-0501.1996.070104.x. [DOI] [PubMed] [Google Scholar]
- 6.Kilpadi DV, Weimer JJ, Lemons JE. Effect of passivation and dry heat-sterilization on surface energy and topography of unalloyed titanium implants. Colloid Surf A. 1998;135(1-3):89–101. [Google Scholar]
- 7.Doundoulakis JH. Surface-analysis of titanium after sterilization – role in implant – tissue interface and bioadhesion. J Prosthet Dent. 1987;58(4):471–8. doi: 10.1016/0022-3913(87)90279-4. [DOI] [PubMed] [Google Scholar]
- 8.Roth S, Feichtinger J, Hertel C. Characterization of Bacillus subtilis spore inactivation in low-pressure, low-temperature gas plasma sterilization processes. J Appl Microbiol. 2010;108(2):521–31. doi: 10.1111/j.1365-2672.2009.04453.x. [DOI] [PubMed] [Google Scholar]
- 9.Rossi F, Kylian O, Rauscher H, Hasiwa M, Gilliland D. Low pressure plasma discharges for the sterilization and decontamination of surfaces. New J Phys. 2009;11:115017–50. [Google Scholar]
- 10.Yang LQ, Chen JR, Gao JL, Guo YF. Plasma sterilization using the RF glow discharge. Appl Surf Sci. 2009;255(22):8960–4. [Google Scholar]
- 11.Singh S, Schaaf NG. Dynamic sterilization of titanium implants with ultraviolet light. Int J Oral Maxillofac Implants. 1989;4(2):139–46. [PubMed] [Google Scholar]
- 12.Premnath V, Harris WH, Jasty M, Merrill EW. Gamma sterilization of UHMWPE articular implants: an analysis of the oxidation problem. Biomaterials. 1996;17(18):1741–53. doi: 10.1016/0142-9612(95)00349-5. [DOI] [PubMed] [Google Scholar]
- 13.Lausmaa J, Kasemo B, Hansson S. Accelerated oxide growth on titanium implants during autoclaving caused by fluorine contamination. Biomaterials. 1985;6(1):23–7. doi: 10.1016/0142-9612(85)90033-x. [DOI] [PubMed] [Google Scholar]
- 14.Holyoak GR, Wang S, Liu Y. Toxic effects of ethylene oxide residues on in vitro production of bovine embryos. Theriogenology. 1995;43(1):237. [Google Scholar]
- 15.Qiu QQ, Connor J. Effects of gamma-irradiation, storage and hydration on osteoinductivity of DBM and DBM/AM composite. J Biomed Mater Res A. 2008;87A(2):373–9. doi: 10.1002/jbm.a.31790. [DOI] [PubMed] [Google Scholar]
- 16.Serro AP, Saramago B. Influence of sterilization on the mineralization of titanium implants induced by incubation in various biological model fluids. Biomaterials. 2003;24(26):4749–60. doi: 10.1016/s0142-9612(03)00372-7. [DOI] [PubMed] [Google Scholar]
- 17.Fleith S, Ponche A, Bareille R, Amedee J, Nardin M. Effect of several sterilisation techniques on homogeneous self assembled monolayers. Colloid Surf B. 2005;44(1):15–24. doi: 10.1016/j.colsurfb.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Pandiyaraj KN, Selvarajan V, Pavese M, Falaras P, Tsousleris D. Investigation on surface properties of TiO2 films modified by DC glow discharge plasma. Curr Appl Phys. 2009;9(5):1032–7. [Google Scholar]
- 19.Boyan BD, Hummert TW, Dean DD, Schwartz Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials. 1996;17(2):137–46. doi: 10.1016/0142-9612(96)85758-9. [DOI] [PubMed] [Google Scholar]
- 20.Boyan BD, Lossdoerfer S, Wang L, Zhao G, Lohmann CH, Cochran DL, et al. Osteoblasts generate an osteogenic microenvironment when grown on surfaces with rough microtopographies. Eur Cells Mater. 2003;6:22–7. doi: 10.22203/ecm.v006a03. [DOI] [PubMed] [Google Scholar]
- 21.Kieswetter K, Schwartz Z, Hummert TW, Cochran DL, Simpso J, Dean DD, et al. Surface roughness modulates the local production of growth factors and cytokines by osteoblast-like MG-63 cells. J Biomed Mater Res. 1996;32(1):55–63. doi: 10.1002/(SICI)1097-4636(199609)32:1<55::AID-JBM7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 22.Olivares-Navarrete R, Hyzy SL, Hutton DL, Erdman CP, Wieland M, Boyan BD, et al. Direct and indirect effects of microstructured titanium substrates on the induction of mesenchymal stem cell differentiation towards the osteoblast lineage. Biomaterials. 2010;31(10):2728–35. doi: 10.1016/j.biomaterials.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pegueroles M, Gil FJ, Planell JA, Aparicio C. The influence of blasting and sterilization on static and time-related wettability and surface-energy properties of titanium surfaces. Surf Coat Tech. 2008;202(15):3470–9. [Google Scholar]
- 24.Lausmaa J, Kasemo B, Mattsson H. Surface spectroscopic characterization of titanium implant materials. Appl Surf Sci. 1990;44(2):133–46. [Google Scholar]
- 25.Esposito M, Lausmaa J, Hirsch JM, Thomsen P. Surface analysis of failed oval titanium implants. J Biomed Mater Res. 1999;48(4):559–68. doi: 10.1002/(sici)1097-4636(1999)48:4<559::aid-jbm23>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 26.Zhao G, Raines AL, Wieland M, Schwartz Z, Boyan BD. Requirement for both micron- and submicron scale structure for synergistic responses of osteoblasts to substrate surface energy and topography. Biomaterials. 2007;28(18):2821–9. doi: 10.1016/j.biomaterials.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rupp F, Scheideler L, Olshanska N, de Wild M, Wieldan M, Geis-Gerstorfer J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J Biomed Mater Res A. 2006;76A(2):323–34. doi: 10.1002/jbm.a.30518. [DOI] [PubMed] [Google Scholar]
- 28.Gengenbach TR, Vasic ZR, Chatelier RC, Griesser HJ. A multitechnique study of the spontaneous oxidation of n-hexane plasma polymers. J Polym Sci Polym Chem. 1994;2(8):1399–414. [Google Scholar]
- 29.Zhao G, Schwartz Z, Wieland M, Rupp F, Geis-Gerstorfer J, Cochran DL, et al. High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res A. 2005;74A(1):49–58. doi: 10.1002/jbm.a.30320. [DOI] [PubMed] [Google Scholar]
- 30.Lausmaa J. Surface spectroscopic characterization of titanium implant materials. J Electron Spectrosc. 1996;81(3):343–61. [Google Scholar]
- 31.Baier RE, Meyer AE, Akers CK, Natiella JR, Meenaghan M, Carter JM. Degradative effects of conventional steam sterilization on biomaterial surfaces. Biomaterials. 1982;3(4):241–5. doi: 10.1016/0142-9612(82)90027-8. [DOI] [PubMed] [Google Scholar]
- 32.Smith DC, Pilliar RM, Chernecky R. Dental implant materials. I. Some effects of preparative procedures on surface topography. J Biomed Mater Res. 1991;25(9):1045–68. doi: 10.1002/jbm.820250902. [DOI] [PubMed] [Google Scholar]
- 33.Smith DC, Pilliar RM, Metson JB, Mcintyre NS. Dental implant materials. 2. Preparative procedures and surface spectroscopic studies. J Biomed Mater Res. 1991;25(9):1069–84. doi: 10.1002/jbm.820250903. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki T, Hori N, Att W, Kubo K, Iwasa F, Ueno T, et al. Ultraviolet treatment overcomes time-related degrading bioactivity of titanium. Tissue Eng PT A. 2009;15(12):3679–88. doi: 10.1089/ten.TEA.2008.0568. [DOI] [PubMed] [Google Scholar]
- 35.Machnee CH, Wagner WC, Jaard MJ, Lang BR. Identification of oxide layers of commercially pure titanium in response to cleaning procedures. Int J Oral Maxillofac Implants. 1993;8(5):529–33. [PubMed] [Google Scholar]
- 36.Keller JC, Draughn RA, Wightman JP, Dougherty WJ, Meletiou SD. Characterization of sterilized cp titanium implant surfaces. Int J Oral Maxillofac Implants. 1990;5(4):360–8. [PubMed] [Google Scholar]
- 37.Stanford CM, Keller JC, Solursh M. Bone cell expression on titanium surfaces is altered by sterilization treatments. J Dent Res. 1994;73(5):1061–71. doi: 10.1177/00220345940730050801. [DOI] [PubMed] [Google Scholar]
- 38.Ponter AB, Jinna KR, Asapu M, Jones WR. Surface energy and surface roughness changes produced by irradiating polymers with ultraviolet-ozone. Contact Angle, Wettability Adhes. 2002;2:331–44. [Google Scholar]