Abstract
Circulating, cell-free microRNAs (miRNAs) hold great promise as a new class of cancer biomarkers due to their surprisingly high stability in plasma, association with disease states, and ease of sensitive measurement. Yet little is known about the origin of circulating miRNAs in either healthy or sick people, or what factors influence levels of circulating miRNA biomarkers. Of 79 solid tumor circulating miRNA biomarkers reported in the literature, we found that fifty-eight percent (47/79) are highly expressed in one or more blood cell type. Plasma levels of miRNA biomarkers expressed by myeloid (e.g., miR-223, miR-197, miR-574-3p, let-7a) and lymphoid (e.g., miR-150) blood cells tightly correlated with corresponding white blood cell counts. Plasma miRNA biomarkers expressed by red blood cells (e.g., miR-486-5p, miR-451, miR-92a, miR-16) could not be correlated to red cell counts due to limited variation in hematocrit in the cohort studied, but were significantly increased in hemolyzed specimens (20-30 fold plasma increase; p<0.0000001). Finally, in a patient undergoing autologous hematopoietic cell transplantation, plasma levels of myeloid- and lymphoid-expressed miRNAs (miR-223 and miR-150, respectively) tracked closely with changes in corresponding blood counts. We present evidence that blood cells are a major contributor to circulating miRNA, and that perturbations in blood cell counts and hemolysis can alter plasma miRNA biomarker levels by up to 50-fold. Given that a majority of reported circulating miRNA cancer biomarkers are highly expressed in blood cells, we suggest caution in interpretation of such results as they may reflect a blood cell-based phenomenon rather than a cancer-specific origin.
Keywords: miRNA, microRNA, plasma, serum, biomarker, cancer, flow cytometry
INTRODUCTION
Since the initial description of circulating miRNAs in 2008 (1-4), over 200 papers have reported circulating miRNAs as biomarkers for detection of a range of cancer types and other diseases (5, 6). At least 79 miRNAs have been reported as plasma or serum miRNA biomarkers of solid tumors (i.e., non-hematopoietic malignancies), including prostate, lung, breast, colon, ovarian, esophageal, melanoma, and gastric cancer (Table S1). However, little attention has been given to the cellular origin of circulating miRNAs and what impact this has on biomarker specificity. We hypothesized that blood cells may contribute significantly to circulating miRNA, and that this could have important implications for interpretation of results from circulating miRNA cancer biomarker studies. In this study we show that a majority of solid tumor-associated circulating miRNA biomarkers reported to date are highly expressed in blood cells, and that plasma levels of these biomarkers are correlated to blood cell counts. We discuss the implications of these findings on the interpretation of circulating miRNA tumor biomarker results reported to date.
MATERIALS AND METHODS
Clinical samples and plasma preparation
Healthy volunteers
Blood was collected from adult healthy volunteer donors by standard antecubital vein phlebotomy at least 2 hours following the last meal. Four 10 ml K2EDTA plasma tubes (BD Vacutainer 366643) were collected at each draw. All donors provided written informed consent.
Residual patient specimens
42 consecutive unique patient specimens (Study Code R1-R42) in which a complete blood count with differential was ordered were collected from the hematology laboratory at the University of Washington Medical Center, Seattle, WA. All specimens were collected in 3 ml K2EDTA plasma tubes (BD Vacutainer 367856) and complete differential blood counts were determined using a Sysmex XE 2100 instrument. Plasma was made on the same day of blood draw. Details on these patients specimens, including primary diagnosis, inpatient or outpatient status, age, gender, specimen characteristics (such as hemolysis determined by visual inspection), and complete blood count (CBC) data are given in Table S2. One patient (Study Code R46) had a total of 21 residual plasma specimens collected prospectively over a period of 33 days during the course of myeloablative chemotherapy and autologous hematopoietic stem cell transplant (Table S3). This patient had a history of diffuse large B-cell lymphoma (DLBCL), with normal blood counts and no evidence of residual disease at the beginning of conditioning chemotherapy. The conditioning regimen did not include irradiation. All specimens were obtained in accordance with the declaration of Helsinki guidelines and with ethics approval from the local institutional review board.
Plasma preparation
EDTA plasma was made by centrifugation of whole blood at 4200 RPM (3298 × g) for 10 minutes at room temperature in an Allegra X-22 centrifuge (Beckman Coulter) using a SX4250 swinging bucket rotor with high brake. Plasma supernatant was removed using a plastic transfer pipette, leaving at least 0.5 mL behind to avoid disturbing the buffy coat. All plasma processing was performed in the specimen processing area of a large clinical laboratory using the same standardized plasma processing procedure applied to clinical specimens.
Fluorescence activated cell sorting (FACS)
A total of 4 independent FACS experiments were performed on a Becton Dickinson ARIA cell sorter on separate days, using peripheral blood from the same healthy male volunteer donor (H1-1). Blood was drawn in K2EDTA tubes as described above and run within 1 hour of collection. Automated complete differential blood counts were measured prior to each experiment using a Sysmex XE 2100 instrument. A total of 10 cell types were sorted: red blood cells (RBC; 5 million), platelets (5 million), neutrophils (250,000), eosinophils (100,000), basophils (10,000), B-lymphocytes (100,000), T-lymphocytes (100,000), NK-cells (100,000), plasmacytoid dendritic cells (pDC; 10,000), and monocytes (100,000). Basophils and pDC were not used in additional experiments due to limited sample quantity. Detailed FACS methods are provided in supplemental material.
RNA isolation
RNA was isolated from all samples using the miRNeasy kit (Qiagen) according to the manufacturer's protocol with the following modifications. For cellular samples, 700μL of Qiazol were added. For liquid samples (plasma, whole blood), samples were mixed with 5 sample volumes of Qiazol reagent. All plasma RNA preparations were made from 200μL in duplicate to control for variation in the RNA preparation step. All plasma was processed fresh on the same day of blood draw (never frozen). Samples in Qiazol were incubated at room temperature for 5 min to inactivate RNases and stored at −80°C. After thawing samples on ice, we added 5 μl of synthetic C. elegans miRNA oligonucleotides prepared in Qiazol to each sample, vortexed, and then added 0.2 volumes of chloroform. At that point, the manufacturer's protocol was followed, with the entire aqueous phase from each sample loaded onto a single affinity column.
qRT-PCR
microRNA Profiling
Flow-sorted blood cells and corresponding plasma and whole blood samples were profiled for the relative abundance of 369 miRNAs using microRNA Ready-to-Use PCR, Human panel I, V1.M qRT-PCR arrays (Exiqon). A total of 15 experimental arrays were performed on blood cells, plasma, and whole blood (plasma: 2, RBC: 2, platelets: 2, neutrophils: 2, monocytes: 2, B-lymphocytes: 1, T-lymphocytes: 1, NK-cells: 1 whole blood: 1). An additional 12 control arrays were performed on serial 2-fold dilutions of 30 μl of plasma from a single healthy donor to determine the analytic measurement ranges for each qPCR assay on the Exiqon array as previously described (7). Detailed miRNA profiling methods and data analysis methods are provided in supplemental material.
Individual TaqMan Assays
Individual miRNAs were detected by qRT-PCR as previously described (8). TaqMan assays for human miRNAs hsa-miR-451, hsa-miR-16, hsa-miR-92a, has-miR-486-5p, hsa-let-7a, hsa-miR-223, hsa-miR-150, hsa-miR-574-3p, hsa-miR-197, and hsa-miR-122 and C. elegans miRNAs cel-miR-39, cel-miR-54, and cel-miR-238 were obtained from Applied Biosystems. Oligonucleotides corresponding to the mature sequence of each miRNA were synthesized (Integrated DNA Technologies) and diluted for standard curves. We performed absolute quantitation for each miRNA both in blood cells (Fig. S3) and in plasma.
RESULTS
We used qRT-PCR to examine expression of 79 miRNAs reported as circulating solid tumor biomarkers in purified sub-populations of blood cells and in matched healthy donor plasma (9-13) (Fig. S1). We found that 58% (46/79) of these literature-reported cancer biomarkers are highly expressed in one or more blood cell type. Most of the blood cell expressed biomarkers (42/46, 91%) were also present at considerable basal levels in healthy donor plasma (>50th percentile among all miRNAs detected), suggesting that blood cells could be a major source for these plasma miRNAs (Fig. 1, Fig. S1). Comparing expression profiles of miRNAs detectable in plasma to miRNA profiles of blood cells demonstrated that the distribution of miRNA expression values in plasma mirrors that of blood cells (Fig. 1).
Figure 1. Relationship between blood cell and plasma microRNA expression among published circulating cancer biomarkers.
(Left) The Venn diagram depicts the distribution of 79 miRNAs published as biomarkers of nonhematopoietic cancers in healthy donor plasma and matched blood cells. “Plasma high” (yellow circle) refers to expression above the 50th percentile among 292 reliably detectable miRNAs by qPCR; “Plasma low” (green circle) refers to miRNAs detected below the 50th percentile, or not detected. “Blood cell high” (red circle) refers to miRNAs detected in the top 50th percentile in whole blood and at least one blood cell class as described in detail in the supplemental methods. (Right) A heat map showing side-by-side comparisons of plasma and blood cell expression of the 79 reported biomarkers demonstrates the close correlation between plasma and blood cell miRNA expression. P1 and P2 represent two independent plasma specimens drawn on different days. For blood cells, the columns represent multiple replicates and/or cell types as detailed in the methods. Circulating miRNA biomarkers are sorted in descending order of expression in healthy donor plasma and are not clustered.
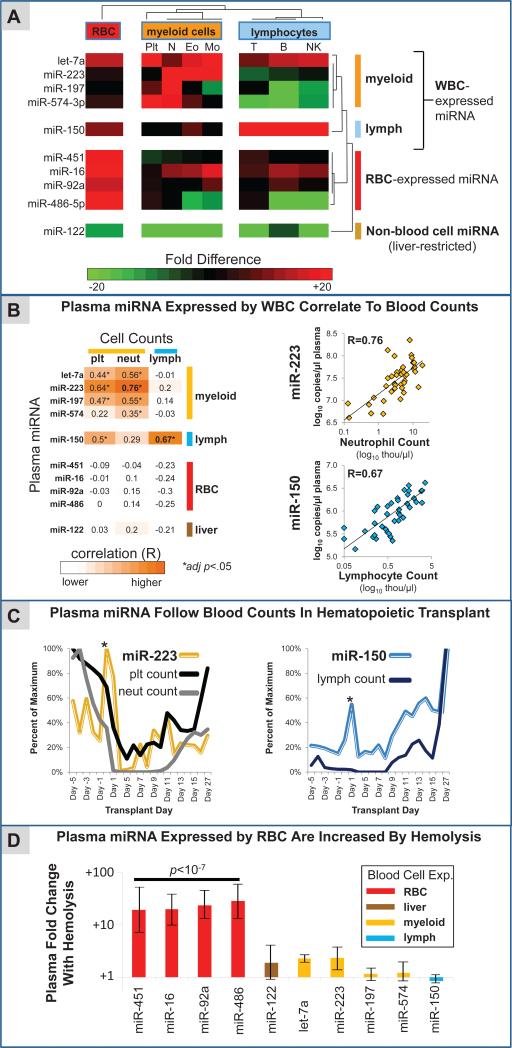
If circulating miRNA are derived from blood cells, we expected levels of these miRNAs to vary as a function of blood cell counts. In order to test this hypothesis, we collected a cohort of 42 plasma samples from a hospital clinical hematology lab, in which a complete differential blood count (CBC with differential) was performed prior to preparing plasma. The cohort consisted of consecutive residual patient plasma samples collected at a single academic medical center, including both inpatients and outpatients with a wide variety of underlying diseases (Table S2). We selected ten miRNAs to measure in plasma and correlate with blood cell counts. Eight of these were selected by virtue of being published circulating miRNA cancer biomarkers which we found in our blood cell miRNA profiling studies to be expressed in blood cells in a cell-type-enriched manner (for red blood cells: miR-451, miR-92a, miR-16, and miR-486-5p; for myeloid blood cells: miR-223, let-7a, miR-197, and miR-574-3p) (Fig. 2A). One additional miRNA, miR-150, was chosen that demonstrated strong lymphoid cell-enriched expression (Fig. 2A) but has not yet been reported as a circulating cancer biomarker. As a negative control, we selected the liver-specific miRNA, miR-122, that is not expressed in blood cells (14) (Fig. 2A).
Figure 2. Circulating microRNA biomarkers are influenced by blood cell counts and hemolysis.
(A) A heatmap depicts the relative expression in blood cells of the 10 selected plasma miRNAs, 8 of which are published circulating miRNA cancer biomarkers. Let-7a, miR-223, miR-197, and miR-574-3p had the highest expression in myeloid blood cells (plt= platelets, N= neutrophils, Eo= eosinophils, Mo= monocytes). miR-150 was most abundant in lymphocytes (T= T-cells, B= B-cells, NK= natural killer cells), while miR-451, miR-16, miR-92a, and miR-486-5p were enriched in RBC. A liver-specific miRNA selected as a negative control (miR-122) was not appreciably expressed in any of the blood cells. Blood cell expression levels were determined using Exiqon v1 qRT-PCR arrays and confirmed in independent samples with Taqman qRT-PCR assays as described in the supplemental methods. (B) (Left) Results of plasma miRNA correlation to blood cell counts in 42 consecutive plasma samples from an academic hospital clinical laboratory are shown. Blood cell expression levels of the 10 miRNAs were inferred based on qRT-PCR Ct values as described in the supplemental methods. Pearson correlation coefficients of plasma miRNA levels and blood cell counts in the 42 clinical samples are shown in the table. Statistically significant correlations after correcting for multiple comparisons (i.e., adjusted p-value < 0.05) are highlighted by black boxes. (Right) Data corresponding to correlations for miR-223 with neutrophil count and miR-150 with lymphocyte count are plotted. (C) In a patient undergoing myeloablative chemotherapy and autologous hematopoietic stem cell transplant, plasma miR-223 tracked with changes in myeloid blood counts (neutrophils and platelets), and plasma miR-150 correlated with changes in lymphocyte counts. Notably, spikes in plasma miR-223 and miR-150 were observed following infusion of hematopoietic stem cells (asterisks). (D) Shown are the differences in mean expression of plasma miRNAs in hemolyzed specimens (n=3) compared to non-hemolyzed specimens (n=39). Error bars represent the standard error of the difference of means. The biomarkers that were most highly expressed in RBC (miR-451, miR-16, miR-92a, miR-486-5p) were 20 to 30-fold higher in hemolyzed specimens, which was highly statistically significant (p<10−7, two-tailed t-test).
Plasma levels of the myeloid-expressed miRNAs let-7a, miR-223, miR-197, and miR-574-3p showed significant positive correlations with myeloid blood cell counts (granulocytes and platelets) (Fig. 2B – left), with greater than 50-fold differences in plasma miRNA biomarker levels between patients with the highest and lowest overall cell counts (Fig. 2B – right). Also consistent with our hypothesis, levels of the lymphoid-enriched miRNA, miR-150, were most highly correlated with lymphocyte count (Fig. 2B). Importantly, plasma levels of a non-blood cell expressed miRNA (miR-122) did not strongly correlate with blood cell counts, and RBC-expressed miRNAs we had selected did not show correlations with white blood cell or platelet counts (Fig. 2B - left). Furthermore, when these ten miRNAs were subjected to unsupervised hierarchical clustering based on their expression in plasma across the 42 patient samples, they clustered into separate groups corresponding to the four RBC-expressed miRNAs, the five white blood cell-expressed miRNAs, and the non-blood cell expressed miRNA (Fig. S2A), consistent with the notion that plasma expression patterns for many miRNAs are reflective of their blood cell origin.
To test whether there is a causal relationship underlying correlations observed between blood cell count and plasma miRNA biomarkers, we examined miR-223 (myeloid cell-expressed) and miR-150 (lymphoid cell-expressed) miRNAs in the plasma of a patient over a time-course of myeloablative chemotherapy and hematopoietic stem cell transplant engraftment (Table S3). We collected serial plasma samples and corresponding blood count data at 25 timepoints over a 32-day period (Fig. 2C). We found that plasma levels of the myeloid-enriched miRNA, miR-223, closely tracked with changes in myeloid blood counts (platelets and neutrophils), while plasma levels of the lymphoid-enriched miRNA, miR-150, mirrored lymphoid counts (Fig. 2C), demonstrating that white blood cell counts can significantly influence plasma levels of blood cell-expressed miRNAs.
We could not assess correlations between RBC-expressed miRNAs and RBC counts because there was limited variation in RBC count among individuals in the cohort we analyzed. However, 3 of the 42 plasma specimens were noted to be hemolyzed. Comparing expression of the ten miRNAs above in hemolyzed vs. non-hemolyzed specimens, we found that all four RBC-associated miRNAs were increased by 20-30 fold in hemolyzed plasma, including the published colon cancer plasma biomarker miR-92a (15, 16). In contrast, none of the six non-RBC associated miRNAs were significantly increased with hemolysis (Fig. 2D). We also found that plasma levels of RBC-expressed miRNAs were tightly inter-correlated even among non-hemolyzed specimens, but not substantially correlated to six non-RBC expressed miRNAs we examined (Figs. S2B and S2C). The results suggest that RBC can contribute significant levels of reported cancer biomarkers into plasma and have important implications for biomarker interpretation. For example, a greater propensity for RBC hemolysis in colon cancer patients (17) could explain the relatively modest (<5 fold) increase in plasma miR-92a that has been reported in colon cancer patients (15, 16).
DISCUSSION
Taken together, our results indicate that a majority of miRNAs reported in the literature as circulating cancer biomarkers may originate in large part from blood cells. This finding is supported by two recent studies in this area (18, 19). Importantly, our studies in the setting of myeloablative chemotherapy and hematopoietic stem cell transplant engraftment provide the first direct, in vivo evidence that blood cell abundance can influence circulating miRNA levels. Among the miRNA biomarkers we examined, we observed variation attributable to blood cell effects that was greater in magnitude than many of the differences reported between cancer patients and controls (13, 15, 20). This raises the concern that many miRNAs reported as circulating cancer biomarkers reflect a secondary effect on blood cells rather than a tumor cell-specific origin. For example, elevated neutrophil counts are associated with shortened progression-free and overall survival in several cancers including non-small cell lung cancer and breast cancer (21, 22). A recent study published in PNAS found that increased ratios of neutrophil-expressed plasma miRNAs (miR-197, miR-142-3p, miR-140-5p, miR-17, miR-21) compared to RBC-expressed miRNAs (miR-92a, miR-486-5p, miR-16, and miR-451) were associated with poorer outcomes in non-small cell lung cancer patients (13). Although blood cell counts were not available in that report, our findings suggest the possibility that the reported plasma microRNA ratios primarily reflect differences in blood counts that could be more readily measured with a routine CBC. A similar concern may be raised for many other studies where circulating miRNAs that are expressed highly in blood cells have been reported as cancer biomarkers (9-11, 15, 16, 20, 23-32).
In contrast to blood cell-expressed miRNAs, plasma levels of liver-restricted miR-122 that we selected as a negative control were not significantly correlated to blood cell counts or influenced by red blood cell hemolysis. This suggests that organ-restricted miRNAs may escape the problem of blood cell interference.
Our blood cell findings should be interpreted in the context of other pre-analytical and analytical sources of variation that may influence plasma miRNA levels. For example, differences in collection procedures and specimen processing conditions have been shown to contribute to plasma miRNA variability (18, 19). Imprecision attributable to specific methods of RNA extraction, miRNA measurement, data acquisition, and data normalization is also likely to a have meaningful impact on plasma miRNA biomarker assessments, especially when fixed biases exist.
This study does not directly assess the relative contribution of different blood cell types to plasma miRNA, or examine mechanisms of miRNA release into plasma. Here, we provide a framework and rationale for future investigations into these questions. A more detailed understanding of the cellular origin of circulating miRNA will inform the appropriate use of this exciting new class of analyte as a cancer biomarker.
In conclusion, we demonstrate for the first time that blood cell counts can substantially influence plasma miRNA biomarker levels. For studies of circulating miRNA biomarkers that are expressed in blood cells, we propose that CBC data be collected and that miRNA expression levels be interpreted in light of blood cell counts. Acceptable ranges for blood cell counts might be established for specific miRNA biomarkers that are particularly vulnerable to blood cell effects. In the future, deeper quantitative understanding of the contributions of specific blood cell types to circulating miRNAs may enable correction for variation in blood cell number in some cases. That said, it is important to note that a significant minority of literature-reported solid tumor biomarkers were not highly expressed in blood cells. In clinical contexts where highly specific circulating miRNA biomarkers of cancer are sought, efforts may be most effective if focused on such miRNAs that are not blood cell-expressed.
Supplementary Material
Acknowledgements
We thank Brian Reid, Beatrice Knudson, Karen Stephens, Martin McIntosh, Peter Nelson, and Charles Drescher for help reviewing the manuscript. We thank Kavita Garg for help with data analysis. We thank Anne Gerard, Linh Ngo, and Vivian McCulloch for help with flow-sorting experiments.
Grant Support: M.T. acknowledges support from the NIH (R01DK085714 from the National Institute of Diabetes and Digestive and Kidney Diseases; P50 CA83636 from the National Cancer Institute - Pacific Ovarian Cancer Research Consortium Specialized Program of Research Excellence (SPORE) in Ovarian Cancer; 5 P30 CA015704 from the National Cancer Institute - Cancer Center Support Grant; and P50 CA97186 from the National Cancer Institute - Pacific Northwest Prostate Cancer SPORE), Prostate Cancer Foundation (Creativity Award), Stand Up To Cancer-American Association for Cancer Research (Innovative Research Grant SU2C-AACR-IRG1109), Department of Defense (PC074012 Prostate Cancer New Investigator Award; OC080159 Ovarian Cancer Career Development Award), Canary Foundation and Damon Runyon Cancer Research Foundation (Damon Runyon-Rachleff Innovation Award). JDA was supported by an American Cancer Society Postdoctoral Fellowship. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders. The funders had no role in study design, in data collection, analysis, or interpretation, in writing of the manuscript, nor in the decision to submit the paper for publication.
Footnotes
Conflict of Interest: Dr. Tewari is an inventor on patent applications related to circulating microRNA.
Methods, Supplemental Tables, and Supplemental Figures: Please see supplemental material for detailed materials and methods, Tables S1, S2, and S3, and Figures S1, S2.
Ethics Approval: All specimens were obtained in accordance with the declaration of Helsinki guidelines and with ethics approval from the local institutional review board. All healthy donors provided written informed consent.
REFERENCES
- 1.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–8. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chim SS, Shing TK, Hung EC, Leung TY, Lau TK, Chiu RW, et al. Detection and characterization of placental microRNAs in maternal plasma. Clin Chem. 2008;54:482–90. doi: 10.1373/clinchem.2007.097972. [DOI] [PubMed] [Google Scholar]
- 3.Lawrie CH, Gal S, Dunlop HM, Pushkaran B, Liggins AP, Pulford K, et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol. 2008;141:672–5. doi: 10.1111/j.1365-2141.2008.07077.x. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 5.Kosaka N, Iguchi H, Ochiya T. Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010;101:2087–92. doi: 10.1111/j.1349-7006.2010.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wittmann J, Jack HM. Serum microRNAs as powerful cancer biomarkers. Biochim Biophys Acta. 2010;1806:200–7. doi: 10.1016/j.bbcan.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003–8. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kroh EM, Parkin RK, Mitchell PS, Tewari M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR). Methods. 2010;50:298–301. doi: 10.1016/j.ymeth.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brase JC, Johannes M, Schlomm T, Falth M, Haese A, Steuber T, et al. Circulating miRNAs are correlated with tumor progression in prostate cancer. Int J Cancer. 2011;128:608–16. doi: 10.1002/ijc.25376. [DOI] [PubMed] [Google Scholar]
- 10.Heneghan HM, Miller N, Lowery AJ, Sweeney KJ, Newell J, Kerin MJ. Circulating microRNAs as novel minimally invasive biomarkers for breast cancer. Ann Surg. 2010;251:499–505. doi: 10.1097/SLA.0b013e3181cc939f. [DOI] [PubMed] [Google Scholar]
- 11.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 12.Zhang C, Wang C, Chen X, Yang C, Li K, Wang J, et al. Expression profile of microRNAs in serum: a fingerprint for esophageal squamous cell carcinoma. Clin Chem. 2010;56:1871–9. doi: 10.1373/clinchem.2010.147553. [DOI] [PubMed] [Google Scholar]
- 13.Boeri M, Verri C, Conte D, Roz L, Modena P, Facchinetti F, et al. MicroRNA signatures in tissues and plasma predict development and prognosis of computed tomography detected lung cancer. Proc Natl Acad Sci U S A. 2011;108:3713–8. doi: 10.1073/pnas.1100048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Jia Y, Zheng R, Guo Y, Wang Y, Guo H, et al. Plasma microRNA-122 as a biomarker for viral-, alcohol-, and chemical-related hepatic diseases. Clin Chem. 2010;56:1830–8. doi: 10.1373/clinchem.2010.147850. [DOI] [PubMed] [Google Scholar]
- 15.Ng EK, Chong WW, Jin H, Lam EK, Shin VY, Yu J, et al. Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut. 2009;58:1375–81. doi: 10.1136/gut.2008.167817. [DOI] [PubMed] [Google Scholar]
- 16.Huang Z, Huang D, Ni S, Peng Z, Sheng W, Du X. Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int J Cancer. 2010;127:118–26. doi: 10.1002/ijc.25007. [DOI] [PubMed] [Google Scholar]
- 17.Avinash SS, Anitha M, Vinodchandran Rao GM, Sudha K, Shetty BV. Advanced oxidation protein products and total antioxidant activity in colorectal carcinoma. Indian J Physiol Pharmacol. 2009;53:370–4. [PubMed] [Google Scholar]
- 18.Duttagupta R, Jiang R, Gollub J, Getts RC, Jones KW. Impact of Cellular miRNAs on Circulating miRNA Biomarker Signatures. PLoS One. 2011;6:e20769. doi: 10.1371/journal.pone.0020769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonald JS, Milosevic D, Reddi HV, Grebe SK, Algeciras-Schimnich A. Analysis of Circulating MicroRNA: Preanalytical and Analytical Challenges. Clinical chemistry. 2011;57:833–40. doi: 10.1373/clinchem.2010.157198. [DOI] [PubMed] [Google Scholar]
- 20.Hu Z, Chen X, Zhao Y, Tian T, Jin G, Shu Y, et al. Serum microRNA signatures identified in a genome-wide serum microRNA expression profiling predict survival of non-small-cell lung cancer. J Clin Oncol. 2010;28:1721–6. doi: 10.1200/JCO.2009.24.9342. [DOI] [PubMed] [Google Scholar]
- 21.Azab B, Bhatt VR, Phookan J, Murukutla S, Kohn N, Terjanian T, et al. Usefulness of the Neutrophil-to-Lymphocyte Ratio in Predicting Short- and Long-Term Mortality in Breast Cancer Patients. Annals of surgical oncology. 2011 doi: 10.1245/s10434-011-1814-0. [DOI] [PubMed] [Google Scholar]
- 22.Teramukai S, Kitano T, Kishida Y, Kawahara M, Kubota K, Komuta K, et al. Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: an analysis of Japan Multinational Trial Organisation LC00-03. Eur J Cancer. 2009;45:1950–8. doi: 10.1016/j.ejca.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 23.Heegaard NH, Schetter AJ, Welsh JA, Yoneda M, Bowman ED, Harris CC. Circulating microRNA expression profiles in early stage non-small cell lung cancer. Int J Cancer. 2011 doi: 10.1002/ijc.26153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heneghan HM, Miller N, Kerin MJ. Circulating miRNA signatures: promising prognostic tools for cancer. J Clin Oncol. 2010;28:e573–4. doi: 10.1200/JCO.2010.29.8901. author reply e5-6. [DOI] [PubMed] [Google Scholar]
- 25.Lodes MJ, Caraballo M, Suciu D, Munro S, Kumar A, Anderson B. Detection of cancer with serum miRNAs on an oligonucleotide microarray. PLoS One. 2009;4:e6229. doi: 10.1371/journal.pone.0006229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsujiura M, Ichikawa D, Komatsu S, Shiozaki A, Takeshita H, Kosuga T, et al. Circulating microRNAs in plasma of patients with gastric cancers. Br J Cancer. 2010;102:1174–9. doi: 10.1038/sj.bjc.6605608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Chen J, Chang P, LeBlanc A, Li D, Abbruzzesse JL, et al. MicroRNAs in plasma of pancreatic ductal adenocarcinoma patients as novel blood-based biomarkers of disease. Cancer Prev Res (Phila) 2009;2:807–13. doi: 10.1158/1940-6207.CAPR-09-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu J, Wu C, Che X, Wang L, Yu D, Zhang T, et al. Circulating MicroRNAs, miR-21, miR-122, and miR-223, in patients with hepatocellular carcinoma or chronic hepatitis. Mol Carcinog. 2011;50:136–42. doi: 10.1002/mc.20712. [DOI] [PubMed] [Google Scholar]
- 29.Zhu W, Qin W, Atasoy U, Sauter ER. Circulating microRNAs in breast cancer and healthy subjects. BMC Res Notes. 2009;2:89. doi: 10.1186/1756-0500-2-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ali S, Almhanna K, Chen W, Philip PA, Sarkar FH. Differentially expressed miRNAs in the plasma may provide a molecular signature for aggressive pancreatic cancer. Am J Transl Res. 2010;3:28–47. [PMC free article] [PubMed] [Google Scholar]
- 31.Moltzahn F, Olshen AB, Baehner L, Peek A, Fong L, Stoppler H, et al. Microfluidic-based multiplex qRT-PCR identifies diagnostic and prognostic microRNA signatures in the sera of prostate cancer patients. Cancer Res. 2011;71:550–60. doi: 10.1158/0008-5472.CAN-10-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao H, Shen J, Medico L, Wang D, Ambrosone CB, Liu S. A Pilot Study of Circulating miRNAs as Potential Biomarkers of Early Stage Breast Cancer. PLoS One. 2010;5:e13735. doi: 10.1371/journal.pone.0013735. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.