Abstract
Using zinc naphthenate dissolved in xylene as a precursor undoped ZnO nanopowders were synthesized by the flame spray pyrolysis technique. The average diameter and length of ZnO spherical and hexagonal particles were in the range of 5 to 20 nm, while ZnO nanorods were found to be 5–20 nm wide and 20–40 nm long, under 5/5 (precursor/oxygen) flame conditions. The gas sensitivity of the undoped ZnO nanopowders towards 50 ppm of NO2, C2H5OH and SO2 were found to be 33, 7 and 3, respectively. The sensors showed a great selectivity towards NO2 at high working temperature (at 300 °C), while small resistance variations were observed for C2H5OH and SO2, respectively.
Keywords: undoped ZnO, flame spray pyrolysis, NO2, C2H5OH, SO2, gas sensor
1. Introduction
Zinc oxide has attracted increased attention during the last few years due to the possibility of its relatively simple transformation into various nanoscale structures. Nanostructures like rods and particles have become the most promising research materials because of their wide range of applications. Different techniques, namely sol-gel [1], spray pyrolysis [2], hydrothermal method [3,4], electrospinning [5], thermal evaporation [6,7], etc. [8–13] are prevalent for the synthesis of zinc oxide nanoparticles and nanorods. In the present work, nanorods and nanoparticles have been prepared by flame spray pyrolysis (FSP), a promising technique for the synthesis of high purity nano-sized materials with controlled size and crystallinity in a single step. This was systematically investigated by using an external-mixing gas-assisted atomizer supported by six premixed methane-oxygen flamelets [14].
Semiconducting metal oxide sensors have been extensively studied due to their simple preparation and high sensitivity under ambient conditions [15–20]. Zinc oxide (ZnO), an n-type metal oxide semiconductor sensing material with a wide band gap (Eg = 3.37 eV at 300 K), has attracted much attention due to its high chemical stability, low cost, and good flexibility in fabrication. It was found that ZnO exhibits pronounced gas sensing properties towards many toxic/non-toxic gases such as NO2, SO2, ethanol, etc. [21–31]. A summary on the sensing properties toward NO2, ethanol (C2H5OH) and SO2 gases of the undoped ZnO prepared by several synthetic methods is shown in Table 1.
Table 1.
A summary on the gas sensing properties of differently-prepared undoped ZnO for NO2, ethanol (C2H5OH) and SO2 gases.
| Authors [ref.] | Method | Nanoparticles | Gas concentration | Sensitivity |
|---|---|---|---|---|
| Ghimbeu et al. [21] | Electrostatic spray deposition (ESD) technique | Undoped ZnO | 1 ppm of NO2, at 300 °C | ∼1.84 |
| Cho et al. [22] | Hydrothermal | Undoped ZnO | 1 ppm of NO2, at 300 °C | ∼1.8 |
| Sadek et al. [23] | Conventional solid-state method | Undoped ZnO | 10 ppm of NO2, at 350 °C | ∼1.81 |
| Lupan et al. [26] | A solution method | Undoped ZnO | 100 ppm of SO2 | <0.5 |
| Singh et al. [28] | A simple chemical route | Undoped ZnO | 250 ppm of ethanol, at 400, 600 and 800 °C | ∼6.5, 5.6 and 4, |
| Hieu et al. [31] | Thermal evaporation | Undoped ZnO | 500 ppm of ethanol, at 300 °C | 5.3 |
| Present work | Flame spray pyrolysis (FSP) | Undoped ZnO | 1, 5 and 10 ppm of NO2, at 300 °C | ∼2.7, 6.2 and 11.8 |
| 100 ppm of SO2, at 300 °C | ∼2.8 | |||
| 200, 300 and 500 ppm of ethanol, at 300 °C | ∼18.2, 22.4 and 27.5 |
Great interest in improving the gas sensitivity as well as selectivity and in decreasing the working temperature has been witnessed. Nitrogen dioxide (NO2) is considered a common air pollutant produced during combustion in automotive engines, industrial factories, and power plants. Therefore, the development of stable NO2 gas sensors that can detect extremely low concentrations of NO2 with high sensitivity is highly desirable [32]. In this study, undoped ZnO nanopowders have been prepared by the flame spray pyrolysis process and their gas sensing responses towards different gases have been comparatively examined. In particular, three types of sensors were tested under oxidizing and reducing gases, like nitrogen dioxide, ethanol and sulfur dioxide.
2. Experimental
2.1. Particle synthesis and characterization
Zinc naphthenate (Aldrich, 8 wt% Zn) was used as a precursor. The precursor was dissolved in xylene (Carlo Erba, 98.5%) to obtain a 0.5 mol/L precursor solution. In a typical run, the precursor was fed into a FSP reactor by a syringe pump with a rate of 5 mL/min while 5 L/min O2 was being dispersed (5/5 flame). The gas flow rates of methane and O2 supporting flamelets were 1.19 and 2.46 L/min, respectively. The pressure drop at the capillary tip was kept constant at 1.5 bars by adjusting the orifice gap area at the nozzle.
The flame height was observed to be approximately 10–12 cm. The sample showed a yellowish-orange flame. The liquid precursor mixture was rapidly dispersed by a gas stream and ignited by a premixed methane/oxygen flame. After evaporation and combustion of precursor droplets, particles are formed by nucleation, condensation, coagulation and coalescence. Finally, the nanoparticles were collected on glass microfiber filters with the aid of a vacuum pump. The undoped ZnO nanopowders were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Specific surface area (SSABET) of the nanoparticles was also investigated by nitrogen adsorption (BET analysis).
2.2. Sensing film preparation and characterization of the gas sensing properties
The undoped ZnO sensing film was prepared by mixing the nanoparticles into an organic paste composed of ethyl cellulose and terpineol, which acted as a vehicle binder and solvent, respectively. The resulting paste was spin-coated on Al2O3 substrates with predeposited interdigitated Au electrodes. The films were then annealed at 400 °C for 2 h (with heating rate of 2 °C/min) for binder removal. The morphology and the cross section of sensing films were analyzed by SEM.
The gas-sensing characteristics of the undoped ZnO nanoparticles towards NO2, C2H5OH and SO2 were characterized. The flow through technique was used to test the gas-sensing properties of thin films. A constant flux of synthetic air of 2 L/min was mixed with desired concentrations of pollutants. All measurements were conducted in a temperature-stabilized sealed chamber at 20 °C under controlled humidity. The external NiCr heater was heated by a regulated dc power supply to different operating temperatures. The operating temperature was varied from 200 °C to 350 °C. The resistances of various sensors were continuously monitored with a computer-controlled system by voltage-amperometric technique with 5 V dc bias and current measurement through a picoammeter. The sensor was exposed to a gas sample for ∼5 minutes for each gas concentration testing and then the air flux was restored for 15 minutes. The concentration of NO2, C2H5OH and SO2 were varied from 1 to 50 ppm, 50 to 100 ppm and 10 to 500 ppm, respectively.
3. Results and Discussion
3.1. Particle properties
Figure 1 shows the XRD patterns of the undoped ZnO sample. All peaks can be confirmed to correspond to the hexagonal structure of ZnO (JCPDS No. 79-205).
Figure 1.
The XRD patterns of flame-spray-made (5/5) undoped ZnO nanopowders.
An average BET equivalent particle diameter (dBET) was calculated using the average density of ZnO as shown in Table 2. The accurate particle size and morphology of undoped ZnO dispersion were confirmed by SEM and TEM images.
Table 2.
The specific surface area (SSABET) and dBET of undoped ZnO nanoparticles.
| Sample | Specific surface area (SSABET), (m2/g) | dBET (nm) |
|---|---|---|
| Undoped ZnO | 78.8 | 13.6 |
Figure 2 shows the morphology of highly crystalline flame-made (5/5) undoped ZnO nanoparticles from SEM analysis. The SEM micrograph clearly showed nanostructural homogeneities and remarkably different morphologies of the undoped ZnO nanoparticles synthesized by the FSP technique. The SEM result showed the presence of agglomerated nanospheres with an average diameter of 10–20 nm. Therefore, from this observation only the rough morphology was found. Nevertheless, the accurate sizes and morphology of the nanoparticles can be estimated from the TEM analysis. While the SEM images provide 3-D morphology and estimated particle sizes, TEM images can reveal internal structure and a more accurate measurement of particle size and morphology.
Figure 2.
The SEM micrograph of highly crystalline flame-made (5/5) undoped ZnO nanoparticles.
Figure 3 shows the TEM-bright-field images of undoped ZnO nanoparticles. The ZnO morphologies were revealed to be spherical, hexagonal and rod-like. The presence of ZnO spherical nanoparticles along with a few nanorods was observed as shown in Figure 3(a). The crystallite sizes of spherical particles were found to be in the range of 5–20 nm whereas the nanorods were found to be ranging from 5–20 nm in width and 20–40 nm in length. Hexagonal ZnO nanoparticles with the size of 5–20 nm were also observed, as shown in Figure 3 (b).
Figure 3.
The TEM images of undoped ZnO morphologies showing the spherical, hexagonal and rod-like shapes.
3.2. SEM sensing layer
The cross-section, film thickness, and surface morphology of the undoped ZnO sensing film layer after annealing and sensing test at 300 °C were observed using SEM analysis, as shown in Figure 4. The thickness of sensing film was approximately 10 μm (side view) which benefited tremendously the NO2, C2H5OH and SO2 gas sensing properties. Irregularities in the film thickness (top view) stem from the spin coating technique. The high density Al2O3 substrate interdigitated with Au electrodes was also visible. After the annealing process, a denser film layer was formed.
Figure 4.
The SEM micrographs of flame-made undoped ZnO thick films sensor on an Al2O3 substrate interdigitated with Au electrodes after annealing and sensing test at 300 °C in dry air. The film thickness was approximately 10 μm.
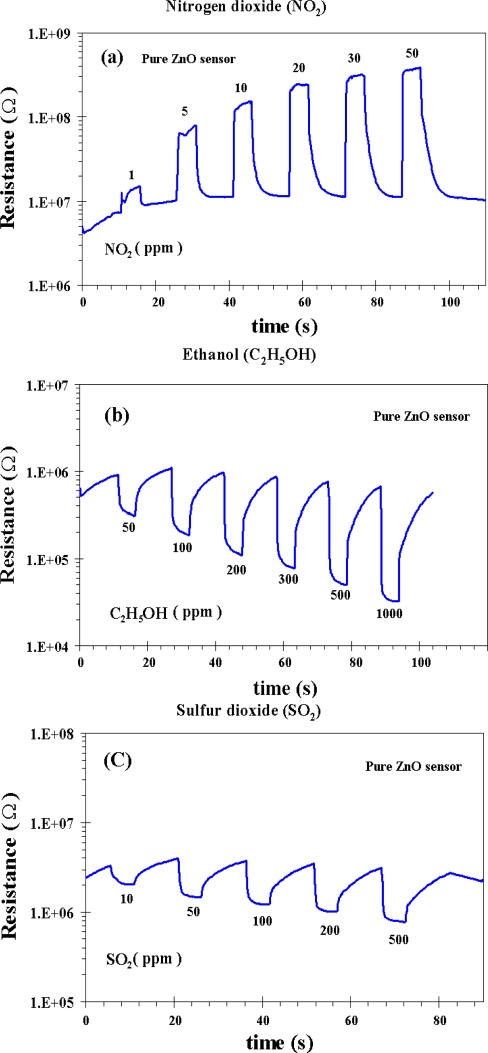
The sensitivity and response time of the thick films of the undoped ZnO nanoparticles as a function of NO2, C2H5OH and SO2 concentrations at 300 °C are shown in Figure 5. In Figure 5(a), it can be seen that the sensitivity toward NO2 is increased considerably at 50 ppm NO2 concentration. The sensitivity and response time for the undoped ZnO nanoparticles at 50 ppm NO2 concentration were found to be 33 and 7 s, respectively. The sensitivity, however, are decreased considerably by testing the undoped ZnO sensor with C2H5OH and SO2 at 50 ppm concentration of each gas. The sensitivity of 7 and 3 with the response time of 94 and 17 s are obtained at 50 ppm of C2H5OH and 50 ppm of SO2, respectively. It is important to note that the undoped ZnO nanoparticles behave as an n-type semiconductor with decreased resistance during NO2, C2H5OH and SO2 gas exposure, which is a typical behavior of ZnO material [33]. The gas-sensing sensitivity, S, is defined as the ratio of Ra/Rg where Ra is the resistance in dry air, and Rg is the resistance in test gas. The response time, Tres, is defined as the time required until 90% of the response signal is reached. The recovery time, Trec, denotes the time needed until 90% of the original baseline signal is recovered. The sensor behaviors under the operating temperature of 300 °C versus the NO2 concentrations ranging from 1–50 ppm for the flame-made undoped ZnO nanoparticles were plotted as shown in Figure 5(a). The changes in resistance of the undoped ZnO sensor for C2H5OH and SO2 gases under exposure to 50–1,000 ppm of C2H5OH and 10–500 ppm of SO2 during forward cycle at 300 °C are shown in Figures 5(b,c), respectively.
Figure 5.
The change in resistance of undoped ZnO sensor for NO2 (a) C2H5OH (b) and SO2 (c) gases under exposure to oxidizing gas of NO2 and reducing gases of C2H5OH and SO2 during forward cycle at 300 °C, respectively.
It is well known that the sensitivity of a semiconductor gas sensor is highly influenced by its operating temperature [34,35]. In order to determine the optimum operating temperatures, the response of the undoped ZnO gas sensor to 50 ppm concentration of nitrogen dioxide, ethanol and sulfur dioxide in air was tested as a function of operating temperature, as shown in Figure 6. It is clear that the responses of three gases tested varied with operating temperature. The sensitivity to NO2 first increased with temperature, up to 300 °C, and then gradually decreased. The maximum sensitivity towards NO2 was 33, at 300 °C. For ethanol and SO2, the sensitivity continuously increased when operating temperatures varied from 200 to 300 °C, and then decreased. The maximum sensitivities obtained were 7 and 3, at 300 °C. Therefore, optimal operating temperatures of 300 °C were chosen for NO2, ethanol and SO2 respectively, to further examine the characteristics of the gas sensor. Results suggest that the undoped ZnO sensor can act as a multifunctional selective gas sensor, detecting NO2, ethanol and SO2 gases. In other words, the above mentioned sensor can be used as an excellent NO2 sensor at an operating temperature of 300 °C.
Figure 6.
The sensitivity versus variation of the operating temperature of NO2, C2H5OH and SO2 (at 50 ppm of concentration) for the undoped ZnO sensor.
The gas sensing selectivity of the undoped ZnO gas sensor has been characterized towards one common oxidizing gas, nitrogen dioxide (NO2), and two other common reducing gases, ethanol (C2H5OH) and sulfur dioxide (SO2) as shown in Figure 7. In Figure 7, the sensitivities towards NO2, C2H5OH and SO2 under the operating temperature of 300 °C were found to be 33, 7 and 3, respectively. This indicates an excellent NO2 selectivity of our undoped ZnO gas sensor.
Figure 7.
The sensitivity variation of the undoped ZnO sensor testing with 50 ppm concentration of NO2, C2H5OH and SO2 under the operating temperature of 300 °C.
The sensitivities of the flame-made ZnO sensor towards different concentrations of NO2, C2H5OH and SO2 gases obtained from our studies are summarized in Table 3. In comparison with the previously reported sensitivities of other ZnO sensors given in Table 1 [21–24], the flame-made ZnO films showed higher sensitivity towards the same NO2 concentration in all cases. Sensitivity of flame-made ZnO films towards 100 ppm of SO2 was 2.8, whereas the result from Lupan et al. [26] was less than 0.5. Likewise flame-made ZnO films showed sensitivity towards 200, 300 and 500 ppm of ethanol as 18.2, 22.4 and 27.5 respectively which were higher than the values reported by Singh et al. [28] and Hieu et al. [31].
Table 3.
Sensitivity of flame-made undoped ZnO nanoparticles towards different concentrations of NO2, C2H5OH and SO2 gases under the operating temperature of 300 °C.
| Gas concentration | Sensitivity |
|---|---|
| 1, 5, 10, 20, 30 and 50 ppm of NO2 | 2.7, 6.2, 11.8, 18.5, 26.7 and 33 |
| 100 ppm of SO2 | 2.8 |
| 200,300, and 500 ppm of ethanol | 18.2, 22.4 and 27.5 |
4. Conclusions
In summary, we have shown that FSP is a promising technique for the synthesis of high purity nano-sized materials with controlled size and crystallinity in a single step, exemplified by the fabrication of an undoped ZnO array sensor that can sense NO2, C2H5OH and SO2 gases. The undoped ZnO-based NO2 gas sensor showed the lowest detection limit of 1 ppm with short response and recovery time. Moreover, the sensors showed a high selectivity towards NO2 at 300 °C when compared with C2H5OH and SO2 gases, respectively. The sensitivity of undoped ZnO film towards 50 ppm of NO2, C2H5OH and SO2 were 33, 7 and 3 respectively.
Acknowledgments
The authors gratefully acknowledge the financial support from the Office of Higher Education Commision, Ministry of Education; the Graduate School and Department of Chemistry, Faculty of Science, Chiang Mai University, Thailand; the National Nanotechnology Center and the National Electronics and Computer Technology Center, Pathumthani, Thailand.
References
- 1.Cheng XL, Zhao H, Huo LH, Gao S, Zhao JG. ZnO nanoparticulate thin film: Preparation, characterization and gas-sensing property. Sens. Actuat. B Chem. 2004;102:248–252. [Google Scholar]
- 2.Nunes P, Fernandes B, Fortunato E, Vilarinho P, Martins R. Performances presented by zinc oxide thin films deposited by spray pyrolysis. Thin Solid Films. 1999;337:176–179. [Google Scholar]
- 3.Jiaqiang X, Yuping C, Yadong L, Jianian S. Gas sensing properties of ZnO nanorods prepared by hydrothermal method. J. Mater. Sci. 2005;40:2919–2921. [Google Scholar]
- 4.Baruwati B, Kumar DK, Manorama SV. Hydrothermal synthesis of highly crystalline ZnO nanoparticles: A competitive sensor for LPG and EtOH. Sens. Actuat. B Chem. 2006;119:676–682. [Google Scholar]
- 5.Li D, McCann JT, Xia Y. Electrospinning: A simple and versatile technique for producing ceramic nanofibers and nanotubes. J. Am. Ceram. Soc. 2006;89:1861–1869. [Google Scholar]
- 6.Gao T, Wang TH. Synthesis and properties of multipod-shaped ZnO nanorods for gas-sensor applications. Appl. Phys. A. 2005;80:1451–1454. [Google Scholar]
- 7.Umar A, Suh EK, Hahn YB. Growth and optical properties of large-quantity single-crystalline ZnO rods by thermal evaporation. J. Phys. D. 2007;40:3478–3484. [Google Scholar]
- 8.Sun ZP, Liu L, Zhang L, Jia DZ. Rapid synthesis of ZnO nano-rods by onestep room-temperature, solid-state reaction and their gas-sensing properties. Nanotechnology. 2006;17:2266–2270. [Google Scholar]
- 9.Rout CS, Krishna SH, Vivekchand SRC, Govindaraj A, Rao CNR. Hydrogen and ethanol sensors based on ZnO nanorods, nanowires and nanotubes. Chem. Phys. Lett. 2006;418:586–590. [Google Scholar]
- 10.Li C, Li L, Du Z, Yu H, Xinag Y, Li Y, Cai Y, Wang T. Rapid and ultrahigh ethanol sensing based on Au-coated ZnO nanorods. Nanotechnology. 2008;19:35501-1–35501-4. doi: 10.1088/0957-4484/19/03/035501. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Zhu CL, Xiao G. Reduced-temperature ethanol sensing characteristics of flower-like ZnO nanorods synthesized by a sonochemical method. Nanotechnology. 2006;17:4537–4541. doi: 10.1088/0957-4484/17/18/002. [DOI] [PubMed] [Google Scholar]
- 12.Guo G, Guo J, Tao D, Choy WCH, Zhao L, Qian W, Wang Z. A simple method to prepare multi-walled carbon nanotubes/ZnO nanoparticles composites. Appl. Phys. A. 2007;89:525–528. [Google Scholar]
- 13.Yu K, Zhang Y, Luo L, Wang W, Zhu Z, Wang J, Cui Y, Ma H, Lu W. Growth and optical properties of quadrangular zinc oxide nanorods on copper-filled porous silicon. Appl. Phys. A. 2004;79:443–446. [Google Scholar]
- 14.Maedler L, Kammler HK, Mueller R, Pratsinis SE. Controlled synthesis of nanostructured particles by flame spray pyrolysis. J. Aerosol. Sci. 2002;33:369–389. [Google Scholar]
- 15.Gergintschew Z, Forster H, Kositza J, Schipanski D. Two-dimensional numerical simulation of semiconductor gas sensors. Sens. Actuat. B Chem. 1995;26:170–173. [Google Scholar]
- 16.Egashira M, Shimizu Y, Takao Y, Sako S. Variations in I–V characteristics of oxide semiconductors induced by oxidizing gases. Sens Actuat B Chem. 1996;35–36:62–67. [Google Scholar]
- 17.Xu J, Pan Q, Shun Y, Tian Z. Grain size control and gas sensing properties of ZnO gas sensor. Sens. Actuat. B Chem. 2000;66:277–279. [Google Scholar]
- 18.Koshizaki N, Oyama T. Sensing characteristics of ZnO-based NO sensor. Sens. Actuat. B Chem. 2000;66:119–121. [Google Scholar]
- 19.Rao GST, Rao DT. Gas sensitivity of ZnO based thick film sensor to NH3 at room temperature. Sens. Actuat. B Chem. 1999;55:166–169. [Google Scholar]
- 20.Law M, Kind H, Kim F, Messer B, Yang P. Photochemical sensing of NO2 with SnO2 nanoribbon nanosensors at room temperature. Angew. Chem. Int. Ed. 2002;41:2405–2408. doi: 10.1002/1521-3773(20020703)41:13<2405::AID-ANIE2405>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Ghimbeu CM, Schoonman J, Lumbreras M, Siadat M. Electrostatic spradeposited zinc oxide films for gas sensor applications. Appl. Surf. Sci. 2007;253:7483–7489. [Google Scholar]
- 22.Cho PS, Kim KW, Lee JH. NO2 sensing characteristics of ZnO nanorods prepared by hydrothermal method. J. Electroceram. 2006;17:975–978. [Google Scholar]
- 23.Sadek AZ, Wlodarski W, Kalantar-zadeh K, Choopun S. ZnO Nanobelt Based Conductometric H2 and NO2 Gas Sensors. IEEE Sens. J. 2005 doi: 10.1109/ICSENS.2005.1597952. [DOI] [Google Scholar]
- 24.Sadek AZ, Choopun S, Wlodarski W, Ippolito SJ, Kalantar-zadeh K. Characterization of ZnO nanobelt-based gas sensor for H2, NO2, and hydrocarbon sensing. J. IEEE Sens. 2007;7:919–924. [Google Scholar]
- 25.Koshizaki N, Oyama T. Sensing characteristics of ZnO-based NO sensor. Sens. Actuat. B Chem. 2000;66:119–121. [Google Scholar]
- 26.Lupan O, Chow L, Chai G. A single ZnO tetrapod-based sensor. Sens. Actuat. B Chem. 2009;141:511–517. [Google Scholar]
- 27.Zhang WD, Zhang WH, Ma XY. Tunable ZnO nanostructures for ethanol sensing. J. Mater. Sci. 2009;44:4677–4682. [Google Scholar]
- 28.Singh RC, Singh O, Singh MP, Chandi PS. Synthesis of zinc oxide nanorods and nanoparticles by chemical route and their comparative study as ethanol sensors. Sens. Actuat. B Chem. 2008;135:352–357. [Google Scholar]
- 29.Jing Z, Zhan J. Fabrication and gas-sensing properties of porous ZnO nanoplates. Adv. Mater. 2008;20:4547–4551. [Google Scholar]
- 30.Xu J, Zhang Y, Chen Y, Xiang Q, Pan Q, Shi L. Uniform ZnO nanorods can be used to improve the response of ZnO gas sensor. Mater. Sci. Eng. B. 2008;150:55–60. [Google Scholar]
- 31.Hieu NV, Chien ND. Low-temperature growth and ethanol-sensing characteristics of quasi-one-dimensional ZnO nanostructures. Phys. B. 2008;403:50–56. [Google Scholar]
- 32.Oh E, Choi H-Y, Jung S-H, Cho S, Kim JC, Lee K-H, Kang S-W, Kim J, Yun J-Y. High-performance NO2 gas sensor based on ZnO nanorod grown by ultrasonic irradiation. Sens. Actuat. B Chem. 2009;141:239–243. [Google Scholar]
- 33.Wan Q, Li QH, Chen YJ, He XL, Li JP, Lin CL, Wang TH. Fabrication and ethanol sensing characteristics of ZnO nanowire gas sensors. Appl. Phys. Lett. 2004;84:3654–3656. [Google Scholar]
- 34.Shinde VR, Gujar TP, Lokhande CD. Enhanced response of porous ZnO nanobeads towards LPG:Effect of Pd sensitization. Sens. Actuat. B Chem. 2007;123:701–706. [Google Scholar]
- 35.Gonga H, Hua JQ, Wang JH, Onga CH, Zhub FR. Nano-crystalline Cu-doped ZnO thin film gas sensor for CO. Sens. Actuat. B Chem. 2006;115:247–251. [Google Scholar]