Abstract
Purpose
Self-drilling screws (SDS) and self-tapping screws (STS) allow for quicker bone insertion and are associated with increased anchorage. This is an experimental in vivo comparison of anterior cervical SDS and STS in the post-insertion acute and chronic phases.
Methods
Thirty C2–C6 vertebrae from six Santa Inês hair sheep were used. Each screw design was randomly assigned to five of each spinal level. Insertion torque was measured using a torque device. Three animals were killed in each phase. Vertebrae were randomly assigned to pullout tests and histomorphometrical bone–screw interface evaluation (percent screw–bone contact and bone density inside and outside the threaded area). Statistical significance was set at P < 0.05.
Results
SDS insertion torque was greater than STS (P = 0.0001). SDS pullout strength was significantly greater than STS in the acute and chronic phases (P = 0.0001, 0.0003, respectively). SDS percent screw–bone contact and inside area bone density were significantly greater in both phases. No outside area bone density differences were observed in either phase.
Conclusions
SDS had higher insertion torque and better anchorage than STS in both phases. SDS percent bone–screw contact and inside area bone density were higher in both phases.
Keywords: Biomechanics, Bone screws, Histology, Osseointegration, Spine
Introduction
Anterior cervical fixation using a plate and screws is a common procedure used to provide mechanical stability in the treatment of various diseases of the cervical spine including tumour, trauma, or degeneration [1, 16]. Multiple factors influence anterior cervical plating strength and stability, including bone mineral density [8, 31], bone geometry [27], plate and screw type [31], screw length [5], bone screw interface [10, 15, 30], and screw orientation [22, 28]. Early devices used non-self-tapping screws and required use of a screw that penetrated the posterior cortex of the vertebral body (bicortical purchase) [24, 27]. Non-self-tapping screws require a drilled pilot hole followed by the use of a tap to create threads in the bone before screw insertion. The bicortical purchase was a drawback that delayed acceptance of this device worldwide, particularly in the US. As a result, new-generation systems were developed using screws fixed to the implant (angle stable screws) that did not require bicortical purchase [35].
Self-tapping screws (STS) and self-drilling screws (SDS) were originally developed for maxillofacial surgery and were later adapted to the new-generation anterior cervical fixation systems. STS and SDS were developed to reduce the number of tools and surgical steps without compromising screw pullout. STS are inserted directly into a pre-drilled hole without tapping the screw thread [12, 36]. SDS allows direct insertion into the bone without the need for pre-drilling and tapping [9, 12, 34].
The goal of the present study was to experimentally compare in vivo cervical SDS and STS used in anterior cervical plates according to biomechanical and histomorphometrical parameters in the post-insertion acute and chronic phases.
Methods
All experiments were conducted in accordance with National Institutes of Health Guidelines for the Welfare of Experimental Animals, and its methodology was reviewed and approved by the local Animal Care Use Committee (protocol number 011/2005). Cervical vertebrae of the C2–C6 segments of 6 healthy male Santa Inês sheep with a mean body weight of 35.7 ± 4.8 kg were used (n = 30 vertebrae).
The mineral density of the vertebrae was assessed by dual X-ray absorptiometry (DEXA) using the QDR system (version 11-2:5; Hologic 4500W, Waltham, MA, USA). The mean (SD) bone mineral density of the specimens was 0.32 ± 0.002 g/cm3.
The screws used in the study were composed of commercially pure titanium and were of the same length (14 mm), the same external diameter (4 mm), and the same internal diameter (3.2 mm). The STS (CSLP®; Synthes, Paoli, PA, USA) had a 1.25 mm pitch and the SDS (Vectra®; Synthes) had a 1.68 mm pitch (Fig. 1).
Fig. 1.
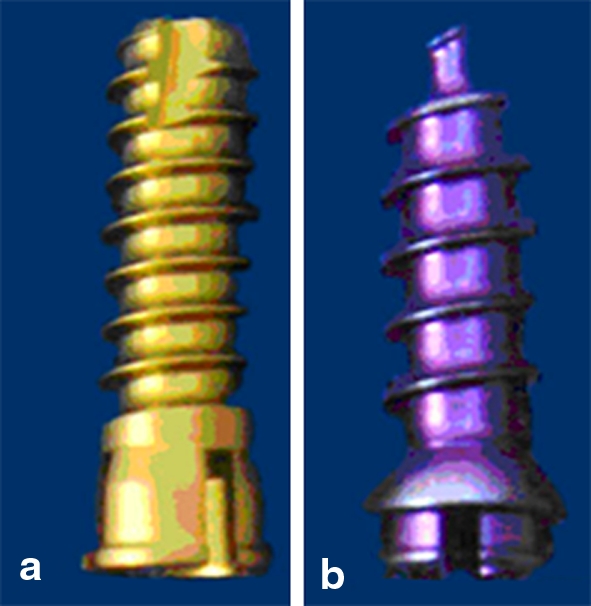
Photograph of self-tapping (a) and self-drilling (b) anterior cervical screws
Surgical protocol
In all surgical procedures, the animals were anesthetized with an intramuscular solution consisting of xylazine 0.5 mg/kg (Dopaser®; Calier, Barcelona, Catalunya, Spain) + acepromazine 0.1 mg/kg (Acepram®; Univet, São Paulo, São Paulo, Brazil) + ketamine 2 mg/kg (Ketamina®; Agener União Saúde Animal, São Paulo, São Paulo, Brazil) for induction followed by a maintenance solution of ketamine (1 g) + xylazine (100 g) + guaiacol glyceryl ether 50 g (Quimibrás, Rio de Janeiro, Rio de Janeiro, Brazil) in saline infused through a venous line (3 mL/kg/h) during the procedure. The cervical spine was exposed anteriorly followed by exposure of the anterior surface of the C2–C6 vertebrae. Screws were assigned in a pairwise fashion to each of the five spinal levels (C2–C6) such that each cervical body would contain one SDS and one STS. Prior to the STS insertion, pilot holes were placed using a 2.5 mm drill. Each SDS has a sharp threaded tip that allows for penetration of the outer cortical bone without the use of a drill, and a pilot hole was not placed for screw insertion.
All screws were inserted manually under direct visualization according to the manufacturer’s specifications. The screws were inserted perpendicular to the anterior surface of the vertebral body and 2 mm was left exposed to simulate the height of an anterior plate. Screws were inserted by hand using a custom driver with a torque calibration system. In the animals killed in the acute phase, the insertion torque was measured with a torque device (TL-500/MKMT-1; Mackena Corporation, São Paulo, SP, Brazil) that provided the maximum torque reached during screw insertion. The animals were randomly assigned to be killed immediately after screw placement (acute phase) or 8 weeks after the surgical procedure (chronic phase). In the animals of the acute group (n = 3), the cervical vertebrae (C2–C6; n = 15) were removed right after screw placement. The individual vertebral bodies were separated from one another using a scalpel and prepared for mechanical and histological study. In the animals of the chronic group (n = 3), the wounds were closed in layers using degradable atraumatic 3–0 polyglycan suture material (Vycril 2.0; Ethicon, Sommerville, NJ, USA) and the skin was sutured using Nylon 3.0 (Mononylon 3.0; Ethicon). Postoperative analgesia was obtained using tramadol hydrochloride (União Química Farmaceutica Nacional; Pouso Alegre, Minas Gerais, Brazil). The animals were killed after 8 weeks using an overdose of anesthetic drugs and the cervical vertebrae (C2–C6; n = 15) were removed. The individual vertebral bodies were separated from one another using a scalpel and prepared for mechanical and histological assessment.
Biomechanical assessment (Pullout test)
Ten vertebrae from 3 animals killed immediately after screw placement (acute phase) and 10 vertebrae from 3 animals killed after 8 weeks (chronic phase) were randomly assigned to the screw pullout test.
Mechanical pullout tests were performed at room temperature on moistened vertebrae using an Emic® universal testing machine (DL 10000; EMIC, São José dos Pinhais, PR, Brazil) working with a load cell capacity of 2,000 N. The axial traction velocity was 2 mm/min with a previous load of 50 N and a holding time of 10 s. The calf vertebrae were placed in a pullout-holding device, the system was aligned, and pullout force (F) was vertically applied to the head screw until the pullout. The strengths were obtained using Tesc 3.13 software (Fig. 2).
Fig. 2.
Diagram illustrating the experimental set-up for pullout testing. Right details of the screw–pullout device connection
Histomorphometrical analysis
Five vertebrae from the three animals killed immediately after screw placement (acute phase) and five vertebrae from the three animals killed after 8 weeks (chronic phase) were randomly assigned for the histomorphometrical analysis.
The screws together with 5 mm of surrounding bone were separated from the cervical vertebrae and fixed in 10% neutral buffered formalin. The specimens were subsequently dehydrated in an ascending series of ethyl alcohols and infiltrated with methyl methacrylate. The hardened blocks were sectioned using a microtome (Microslice 2; Ultratec, Santa Ana, CA, USA) along the long axis of each screw to obtain sections of about 70 μm, which were then stained with alizarin red and Stevenel’s blue for light microscopy analysis.
Blind quantitative histomorphometrical analysis was performed using a Leica DM LB2 light microscope using images acquired at 25× and 100× magnification and a Leica camera (Leica DC300 F; Leica Microsystems GmbH, Wetzlar, Germany) coupled with the light microscope. Histomorphometrical measurements included bone–implant contact (percentage of linear measurement along the axial wall of the sectioned implant), percentage of bone inside the screw thread in a measuring frame, and percentage of bone outside the screw thread (rectangular area adjacent to the screw thread) with a length equivalent to the number of screw threads in the measuring frame and a height equivalent to double the height of the screw thread (Fig. 3). The measurement frame was the same for both screw modalities, covering the length of three threads for SDS and four threads for STS.
Fig. 3.
Micrograph of the trabecular bone–implant interface illustrating the histomorphometrical measurement. The dotted line indicates the bone–implant contact. The smaller rectangle delimits the area selected for the measurement of bone percentage inside the screw thread. The larger rectangle delimits the cancellous bone area outside the screw thread. Alizarin red and Stevenel’s blue staining. ×25 magnification
Statistical analysis
The Mixed Effects Linear Model using SAS® 9.0 (SAS Institute Inc., Cary, NC, USA) software was used to compare the biomechanical and histomorphometrical measurements using a level of significance of P < 0.05.
Results
Insertion torque
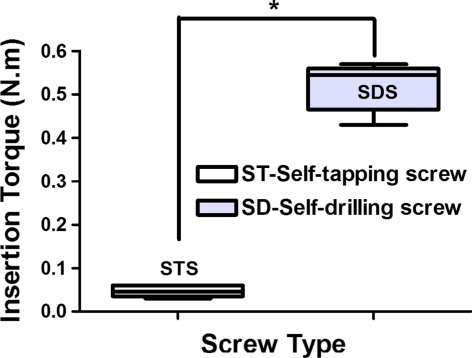
The insertion torque was measured in all screws of the acute phase group (15 SDS and 15 STS). The mean insertion torque required was 0.518 ± 0.051 N m for SDS and 0.047 ± 0.013 for STS. SDS insertion torque was significantly greater than that of STS (P < 0.0001; Fig. 4).
Fig. 4.
Graph showing the insertion torque (mean and standard deviation) of self-tapping screws (STS) and self-drilling screws (SDS) in the acute phase. The asterisk (*) indicates a statistically significant difference (P < 0.0001)
Pullout strength
A total of 10 SDS and 10 STS pullout tests were performed in each phase, i.e., acute (postoperative) and chronic phase (8 weeks) after screw insertion. The mean SDS pullout strength was significantly greater than that of STS in the acute (473.44 ± 81.47 N vs. 237.12 ± 24.97 N; P < 0.0001) and chronic phase (595.75 ± 122.43 N vs. 430.02 ± 101.47 N; P < 0.0003; Fig. 5).
Fig. 5.
Graph showing the pullout strength (mean and standard deviation) of self-tapping (STS) and self-drilling (SDS) screws after insertion (acute phase) and 8 weeks later. The asterisks indicate a statistically significant difference (*P < 0.0001 and ** P < 0.0003)
Histomorphometry
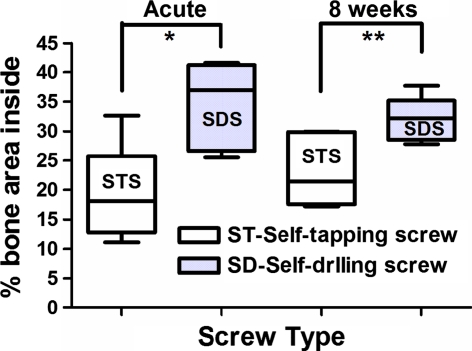
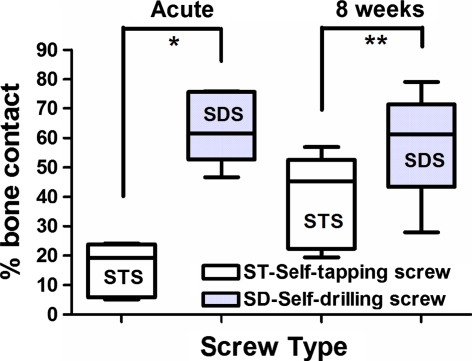
A total of five histomorphometrical analyses were performed with each screw modality in each phase (acute and chronic). SDS bone–implant contact values were significantly higher than those of STS in both the acute (63.62 ± 12.24% vs. 15.71 ± 9.22%; P < 0.0004) and chronic (58.12 ± 18.69% vs. 38.99 ± 15.92%; P < 0.04) phases. The percentage of bone inside the screw thread area was greater in the SDS compared with that of the STS in the acute (34.54 ± 7.45% vs. 19.03 ± 8.22%; P < 0.0005) and chronic (31.94 ± 3.80% vs. 23.25 ± 6.24%; P < 0.013) phases. The percentage of bone outside the screw thread area was statistically similar between the two screw modalities in both the acute (33.99 ± 11.7% vs. 25.20 ± 8.48%; P = 0.1) and chronic (32.16 ± 5.26 vs. 33.942 ± 13.28; P = 0.71) phases (Figs. 6, 7, 8, 9, 10).
Fig. 6.
Graph showing the percentage of bone inside the screw thread area (mean and standard deviation) of self-tapping screws (STS) and self-drilling screws (SDS) after insertion (acute phase) and 8 weeks later. The asterisks indicate a statistically significant difference (*P < 0.0005 and **P < 0.013)
Fig. 7.
Graph showing the percentage of bone–implant contact (mean and standard deviation) of self-tapping screws (STS) and self-drilling screws (SDS) after insertion (acute phase) and 8 weeks later. The asterisks indicate a statistically significant difference (*P < 0.004 and **P < 0.04)
Fig. 8.
Graph showing the percentage of bone outside the screw thread area (mean and standard deviation) of self-tapping screws (STS) and self-drilling screws (SDS) after insertion (acute phase) and 8 weeks later. No statistical difference was found
Fig. 9.
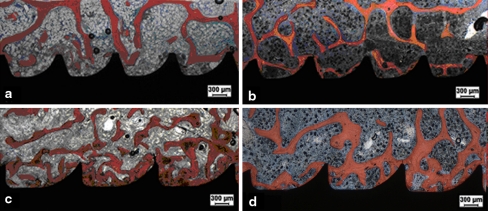
Micrographs of the trabecular bone–implant interface illustrating the histomorphometric results in the acute and chronic phases. a Self-tapping screw (STS) in the acute phase. b STS in the chronic phase (8 weeks). c Self-drilling screw (SDS) in the acute phase. d SDS in the chronic phase (8 weeks). Alizarin red and Stevenel’s blue staining. ×25 magnification
Fig. 10.
Micrographs of the trabecular bone–implant interface illustrating the histomorphometrical results in the acute and chronic phases. a Self-tapping screws (STS) in the acute phase. b STS in the chronic phase (8 weeks). c Self-drilling screws (SDS) in the acute phase. d SDS in the chronic phase (8 weeks). Alizarin red and Stevenel’s blue staining. ×25 magnification
Discussion
Anterior cervical plates have been used to provide rigid internal fixation to the cervical spinal segment, and secure screw anchorage is essential for an uneventful anterior cervical plate fixation [17]. Most of the published data concerning cervical screws have used autopsied bone [15, 16, 27, 29, 32] or synthetic materials [3, 5, 23]. Screws used for anterior plate fixation differ in material, design, dimension, and insertion technique [15]. In living bone, the holding power of a screw is inseparably a function of the weakest element in the bone–screw composite, the bone adjacent to the screw [33]. The holding power will not only be dependent on the screw design but also on the changes induced in bone by insertion trauma, the reaction of bone to the implant, and the resorption and remodelling as a result of healing [33]. The goal of our study was to explore the in vivo biomechanical properties of anterior cervical screws and correlate this with the histological changes taking place in the adjacent bone.
While animal models may closely represent the mechanical and physiological human clinical situation and allow for the evaluation of materials in loaded and unloaded situations and the study of implant bone interface [26], it should be remembered that this is only an approximation, as each animal model has unique advantages and disadvantages. Although differences in bone density exist between humans and sheep [18], sheep bone dimensions are suitable for the implantation of human implants [21]. Sheep and humans have similar patterns of bone growth, so sheep remain a valuable model for human bone turnover and remodelling activity [4, 6, 25].
SDS and STS were originally developed for maxillofacial surgery and were later adopted for spinal surgery use. The rationale for STS and SDS development was to reduce the number of tools and surgical steps without compromising screw pullout strength [12]. The avoidance of bone necrosis secondary to the large amount of heat generated during drilling of cortical bone [7, 13, 33] is also another advantage of SDS. However, this advantage may not be valid for vertebral screws, considering that their application to cancellous bone does not require powerful drilling that generates heat as is required for cortical bone. Greater injury to the bone occurs when a large amount of heat is generated during drilling. Thermal necrosis of the surrounding bone has been observed, leading to osteolysis around the screw and stability loss [11, 33].
The pullout strength in the acute and chronic phases was higher in SDS than in STS. The anchorage of the screw is directly related to the contact between the surface of the screw thread and the surrounding bone [13]. The greater percentage of screw–bone contact in the screw thread area and the higher bone density inside the thread area of SDS explain the higher pullout strength of SDS in the acute and chronic phases. Similar results were reported in biomechanical and histomorphometrical studies of SDS and STS used in maxillofacial surgery [13, 20, 38].
Claims have been made that SDS and STS can cause bone necrosis and result in fibrous tissue formation, leading to loss of screw holding power. The insertion of SDS involves a radial displacement of bone by the conical tip of the screw, which may cause damage to bone [14]. No bone necrosis or fibrous tissue formation was observed with either type of screw used in our study. It has been reported that pressure generated by the SDS core could produce atrophy or cracks in bone tissue around the screw [2, 14], but our results do not support this statement regarding vertebral cancellous bone. We observed that insertion of SDS and STS into vertebral cancellous bone did not cause harm to surrounding bone, and the percentage of screw–bone contact and bone density inside the thread area of the screw increased after 8 weeks. Heidemann et al. [14] compared the amount of bone remodelling inside the STS and SDS thread and observed that a significant amount of residual bone was observed inside the SDS thread. The results of the histomorphometrical evaluation agree with the theoretical hypothesis that drill-free screws should have a higher screw–bone contact than any other type of screws that are inserted after drilling. The insertion of SDS promotes the transport and deposition of bone debris around the screw due to the screw’s conical shaft [14].
The bone outside the screw thread area did not seem to be affected by the placement of either screw type. No difference in bone area was observed in the two screw types in the acute or chronic phases. The pressure generated by screw insertion that could produce atrophy or cracks in the surrounding bone [12] did not alter the surrounding bone outside the screw thread.
SDS and STS pullout strength were both higher 8 weeks after insertion into bone. The same increase in screw holding power was observed after screw insertion into cortical bone in an unloaded screw system similar to that in our experimental model [33].
In the interpretation and analysis of our results, it should be considered that the screws used in our study were not of the same design. Screw design and other parameters such as screw diameter, screw length, and bone mineral density ensure a secure bone–screw interface and affect screw insertion torque and pullout strength [15].
Hitchon et al. [15] observed no significant difference in pullout strength between STS and SDS with the same design at three different lengths (12, 14, and 16 mm), although the insertional torque required for SDS was greater. The highest SDS pullout strength used in our study may be related to screw design and not only the self-drilling feature. Screw design is an important factor in screw pullout strength, and the SDS screws used in our study had a longer pitch compared to STS. We also observed greater insertional torque for SDS as reported in other studies [12, 37]. The higher insertional torque for SDS is because greater force is needed to tap the SDS threads. Each SDS has a sharp threaded tip that enables penetration of the outer cortex without pre-drilling. The threads are guided along a rotation axis up to the screw head. The higher insertional torque of SDS compared to STS might present a problem in thick cortical bone (>3 mm) [7, 12, 36], but this is not a problem for their use in cervical fixation considering their insertion into cancellous bone. The SDS design influences screw pullout resistance, and conically shaped screws have shown the best results in cancellous bone [19].
The present study limitations are mainly directed to two aspects. The first is related to the lack of an associated plate to the screws that would simulate closely the situation clinically observed. The second factor to be considered is that the screws were not submitted to load, so the adjacent bone healed undisturbed. Despite those limitations, the present study is based on validated experimental models in the literature and the values obtained are comparable.
Comparison of SDS and STS pullout strength showed that SDS had higher insertional torque, higher acute and chronic pullout strength associated with greater bone–screw contact percentage inside the screw thread, and greater bone density inside the thread. However, the better biomechanical resistance and greater amount of bone inside the thread of SDS cannot be exclusively attributed to its drill-free insertion; the screw design should also be considered a feature having influence on the superiority of the SDS used in this study. Nevertheless, the self-drilling used in our study allowed for insertion into the anterior aspect of the cervical body without the need for bone drilling or tapping, and reduced operation time for screw insertion due to the easy and rapid screw insertion was accompanied by an increase in primary screw stability in the acute and chronic phases after insertion. SDS required fewer tools and surgical steps without compromising pullout strength or causing adjacent bone necrosis.
Acknowledgments
This study was sponsored by the National Council for Scientific and Technological Development (CNPq).
Conflict of interest
There are no conflicts of interest of any kind related to this study.
References
- 1.Aebi M, Zuber K, Marchesi D. Treatment of cervical spine injuries with anterior plating. Indications, techniques, and results. Spine (Phila Pa 1976) 1991;16:S38–S45. doi: 10.1097/00007632-199103001-00008. [DOI] [PubMed] [Google Scholar]
- 2.Champy M, Lodde JP, Schmitt R, et al. Mandibular osteosynthesis by miniature screwed plates via a buccal approach. J Maxillofac Surg. 1978;6:14–21. doi: 10.1016/S0301-0503(78)80062-9. [DOI] [PubMed] [Google Scholar]
- 3.Chapman JR, Harrington RM, Lee KM, et al. Factors affecting the pullout strength of cancellous bone screws. J Biomech Eng. 1996;118:391–398. doi: 10.1115/1.2796022. [DOI] [PubMed] [Google Scholar]
- 4.Chavassieux P, Pastoureau P, Boivin G, et al. Dose effects on ewe bone remodeling of short-term sodium fluoride administration—a histomorphometric and biochemical study. Bone. 1991;12:421–427. doi: 10.1016/8756-3282(91)90031-D. [DOI] [PubMed] [Google Scholar]
- 5.Conrad BP, Cordista AG, Horodyski M, et al. Biomechanical evaluation of the pullout strength of cervical screws. J Spinal Disord Tech. 2005;18:506–510. doi: 10.1097/01.bsd.0000140196.99995.65. [DOI] [PubMed] [Google Scholar]
- 6.Boer FC, Patka P, Bakker FC, et al. New segmental long bone defect model in sheep: quantitative analysis of healing with dual energy X-ray absorptiometry. J Orthop Res. 1999;17:654–660. doi: 10.1002/jor.1100170506. [DOI] [PubMed] [Google Scholar]
- 7.Ellis JA, Jr, Laskin DM. Analysis of seating and fracturing torque of bicortical screws. J Oral Maxillofac Surg. 1994;52:483–486. doi: 10.1016/0278-2391(94)90346-8. [DOI] [PubMed] [Google Scholar]
- 8.Grubb MR, Currier BL, Shih JS, et al. Biomechanical evaluation of anterior cervical spine stabilization. Spine (Phila Pa 1976) 1998;23:886–892. doi: 10.1097/00007632-199804150-00009. [DOI] [PubMed] [Google Scholar]
- 9.Guntermann J, Gellrich NC, Schramm A, et al. The Synthes 1.5 and 2.0 self-drilling screws indication in maxillofacial surgery. J Craniomaxillofac Surg. 1998;26:65. [Google Scholar]
- 10.Hadjipavlou AG, Nicodemus CL, al-Hamdan FA, et al. Correlation of bone equivalent mineral density to pull-out resistance of triangulated pedicle screw construct. J Spinal Disord. 1997;10:12–19. doi: 10.1097/00002517-199702000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Heidemann W, Gerlach KL. Clinical applications of drill free screws in maxillofacial surgery. J Craniomaxillofac Surg. 1999;27:252–255. doi: 10.1016/S1010-5182(99)80037-7. [DOI] [PubMed] [Google Scholar]
- 12.Heidemann W, Gerlach KL, Grobel KH, et al. Drill Free Screws: a new form of osteosynthesis screw. J Craniomaxillofac Surg. 1998;26:163–168. doi: 10.1016/S1010-5182(98)80007-3. [DOI] [PubMed] [Google Scholar]
- 13.Heidemann W, Terheyden H, Gerlach KL. Analysis of the osseous/metal interface of drill free screws and self-tapping screws. J Craniomaxillofac Surg. 2001;29:69–74. doi: 10.1054/jcms.2000.0179. [DOI] [PubMed] [Google Scholar]
- 14.Heidemann W, Terheyden H, Gerlach KL. In vivo studies of screw-bone contact of drill-free screws and conventional self-tapping screws. Mund Kiefer Gesichtschir. 2001;5:17–21. doi: 10.1007/PL00010793. [DOI] [PubMed] [Google Scholar]
- 15.Hitchon PW, Brenton MD, Coppes JK, et al. Factors affecting the pullout strength of self-drilling and self-tapping anterior cervical screws. Spine (Phila Pa 1976) 2003;28:9–13. doi: 10.1097/00007632-200301010-00004. [DOI] [PubMed] [Google Scholar]
- 16.Johnston TL, Karaikovic EE, Lautenschlager EP, et al. Cervical pedicle screws vs. lateral mass screws: uniplanar fatigue analysis and residual pullout strengths. Spine J. 2006;6:667–672. doi: 10.1016/j.spinee.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Kaiser MG, Haid RW, Jr, Subach BR, et al. Anterior cervical plating enhances arthrodesis after discectomy and fusion with cortical allograft. Neurosurgery. 2002;50:229–236. doi: 10.1097/00006123-200202000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Liebschner MA. Biomechanical considerations of animal models used in tissue engineering of bone. Biomaterials. 2004;25:1697–1714. doi: 10.1016/S0142-9612(03)00515-5. [DOI] [PubMed] [Google Scholar]
- 19.Lohr J, Gellrich NC, Buscher P, et al. Comparative in vitro studies of self-boring and self-tapping screws. Histomorphological and physical-technical studies of bone layers] Mund Kiefer Gesichtschir. 2000;4:159–163. doi: 10.1007/s100060050189. [DOI] [PubMed] [Google Scholar]
- 20.Mischkowski RA, Kneuertz P, Florvaag B, et al. Biomechanical comparison of four different miniscrew types for skeletal anchorage in the mandibulo-maxillary area. Int J Oral Maxillofac Surg. 2008;37:948–954. doi: 10.1016/j.ijom.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 21.Newman E, Turner AS, Wark JD. The potential of sheep for the study of osteopenia: current status and comparison with other animal models. Bone. 1995;16:277S–284S. doi: 10.1016/8756-3282(95)00026-a. [DOI] [PubMed] [Google Scholar]
- 22.Ogon M, Haid C, Krismer M, et al. Comparison between single-screw and triangulated, double-screw fixation in anterior spine surgery. A biomechanical test. Spine (Phila Pa 1976) 1996;21:2728–2734. doi: 10.1097/00007632-199612010-00005. [DOI] [PubMed] [Google Scholar]
- 23.Oktenoglu BT, Ferrara LA, Andalkar N, et al. Effects of hole preparation on screw pullout resistance and insertional torque: a biomechanical study. J Neurosurg. 2001;94:91–96. doi: 10.3171/spi.2001.94.1.0091. [DOI] [PubMed] [Google Scholar]
- 24.Omeis I, DeMattia JA, Hillard VH, et al. History of instrumentation for stabilization of the subaxial cervical spine. Neurosurg Focus. 2004;16:E10. doi: 10.3171/foc.2004.16.1.11. [DOI] [PubMed] [Google Scholar]
- 25.Pastoureau P, Arlot M, Caulin F, Barlet J, Meunier P, Delmas P (1989) Effects of oophorectomy on biochemical and histological indices of bone turnover in ewes. J Bone Miner Res 4:S237, abstract 477
- 26.Pearce AI, Richards RG, Milz S, et al. Animal models for implant biomaterial research in bone: a review. Eur Cell Mater. 2007;13:1–10. doi: 10.22203/ecm.v013a01. [DOI] [PubMed] [Google Scholar]
- 27.Pitzen T, Barbier D, Tintinger F, et al. Screw fixation to the posterior cortical shell does not influence peak torque and pullout in anterior cervical plating. Eur Spine J. 2002;11:494–499. doi: 10.1007/s00586-002-0447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez-Olaverri JC, Hasharoni A, DeWal H, et al. The effect of end screw orientation on the stability of anterior instrumentation in cyclic lateral bending. Spine J. 2005;5:554–557. doi: 10.1016/j.spinee.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 29.Ronderos JF, Jacobowitz R, Sonntag VK, et al. Comparative pull-out strength of tapped and untapped pilot holes for bicortical anterior cervical screws. Spine (Phila Pa 1976) 1997;22:167–170. doi: 10.1097/00007632-199701150-00007. [DOI] [PubMed] [Google Scholar]
- 30.Ruland CM, McAfee PC, Warden KE, et al. Triangulation of pedicular instrumentation. A biomechanical analysis. Spine (Phila Pa 1976) 1991;16:S270–S276. doi: 10.1097/00007632-199106001-00019. [DOI] [PubMed] [Google Scholar]
- 31.Ryken TC, Clausen JD, Traynelis VC, et al. Biomechanical analysis of bone mineral density, insertion technique, screw torque, and holding strength of anterior cervical plate screws. J Neurosurg. 1995;83:325–329. [PubMed] [Google Scholar]
- 32.Ryken TC, Goel VK, Clausen JD, Traynelis VC (1995) Assessment of unicortical and bicortical fixation in a quasistatic cadaveric model. Role of bone mineral density and screw torque. Spine (Phila Pa 1976) 20:1861–1867 [DOI] [PubMed]
- 33.Schatzker J, Sanderson R, Murnaghan JP (1975) The holding power of orthopedic screws in vivo. Clin Orthop Relat Res 108:115–126 [DOI] [PubMed]
- 34.Schimming R, Gutwald R, Gellrich N, et al. Selfdrilling screws: clinical experiences in cranio- and maxillofacial surgery Int J Oral Maxillofac Surg 19992826. 10.1016/S0901-5027(99)80743-710065644 [DOI] [Google Scholar]
- 35.Scholz M, Reyes PM, Schleicher P, et al. A new stand-alone cervical anterior interbody fusion device: biomechanical comparison with established anterior cervical fixation devices. Spine (Phila Pa 1976) 2009;34:156–160. doi: 10.1097/BRS.0b013e31818ff9c4. [DOI] [PubMed] [Google Scholar]
- 36.Sowden D, Schmitz JP. AO self-drilling and self-tapping screws in rat calvarial bone: an ultrastructural study of the implant interface. J Oral Maxillofac Surg. 2002;60:294–299. doi: 10.1053/joms.2002.30585. [DOI] [PubMed] [Google Scholar]
- 37.Su YY, Wilmes B, Honscheid R, Drescher D. Comparison of self-tapping and self-drilling orthodontic mini-implants: an animal study of insertion torque and displacement under lateral loading. Int J Oral Maxillofac Implants. 2009;24:404–411. [PubMed] [Google Scholar]
- 38.Wu X, Deng F, Wang Z, et al. Biomechanical and histomorphometric analyses of the osseointegration of microscrews with different surgical techniques in beagle dogs. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:644–650. doi: 10.1016/j.tripleo.2008.05.031. [DOI] [PubMed] [Google Scholar]