Background: The human proton-coupled folate transporter (PCFT) may exist as homo-oligomers.
Results: PCFT monomers form oligomers, and wild-type and inactive mutant PCFT monomers show dominant-positive activity when co-expressed.
Conclusion: Wild-type PCFT may rescue the mutant phenotype via formation of hetero-oligomers.
Significance: Better understanding of PCFT oligomerization may identify therapeutic applications for hereditary folate maladsorption and delivery of PCFT-targeted chemotherapy drugs for cancer.
Keywords: Folate, Membrane Proteins, Molecular Pharmacology, Proton Pumps, Transport Drugs, Transporters, Antifolate, Oligomer, Proton-coupled Folate Transporter, Transporter
Abstract
The proton-coupled folate transporter (PCFT; SLC46A1) is a proton-folate symporter that is abundantly expressed in solid tumors and normal tissues, such as duodenum. The acidic pH optimum for PCFT is relevant to intestinal absorption of folates and could afford a means of selectively targeting tumors with novel cytotoxic antifolates. PCFT is a member of the major facilitator superfamily of transporters. Because major facilitator superfamily members exist as homo-oligomers, we tested this for PCFT because such structures could be significant to PCFT mechanism and regulation. By transiently expressing PCFT in reduced folate carrier- and PCFT-null HeLa (R1-11) cells and chemical cross-linking with 1,1-methanediyl bismethanethiosulfonate and Western blotting, PCFT species with molecular masses approximating those of the PCFT dimer and higher order oligomers were detected. Blue native polyacrylamide gel electrophoresis identified PCFT dimer, trimer, and tetramer forms. PCFT monomers with hemagglutinin and His10 epitope tags were co-expressed in R1-11 cells, solubilized, and bound to nickel affinity columns, establishing their physical associations. Co-expressing YPet and ECFP*-tagged PCFT monomers enabled transport and fluorescence resonance energy transfer in plasma membranes of R1-11 cells. Combined wild-type (WT) and inactive mutant P425R PCFTs were targeted to the cell surface by surface biotinylation/Western blots and confocal microscopy and functionally exhibited a “dominant-positive” phenotype, implying positive cooperativity between PCFT monomers and functional rescue of mutant by WT PCFT. Our results demonstrate the existence of PCFT homo-oligomers and imply their functional and regulatory impact. Better understanding of these higher order PCFT structures may lead to therapeutic applications related to folate uptake in hereditary folate malabsorption, and delivery of PCFT-targeted chemotherapy drugs for cancer.
Introduction
Folates are essential for cell growth and tissue regeneration. The biologic role of folate cofactors derives from their participation in one-carbon transfer reactions, leading to nucleotide precursors, serine, and methionine (1). Because mammalian cells cannot synthesize folates de novo, membrane transport of extracellular folates is essential. Three primary transport routes are involved in folate internalization, including the reduced folate carrier (RFC),4 the proton-coupled folate transporter (PCFT), and the folate receptors. These transporters differ in terms of mechanism, and each system typically plays a unique role in mediating folate transport across epithelia and into systemic tissues (2).
The human PCFT (hPCFT; SLC46A1) is a proton-folate symporter that functions optimally at acidic pH by coupling the downhill flow of protons to the uphill transport of folates (3–5). The role of hPCFT in intestinal folate absorption was established by demonstrating loss-of-function mutations in hPCFT in patients with the rare autosomal inherited disorder, hereditary folate malabsorption (HFM) (5). To date, 17 unique hPCFT mutations have been reported in ethnically varied kindreds (5–15). Although proton-coupled, this transporter is also functional at more physiologic pH, at which it retains appreciable affinity for pemetrexed (16), a newer antifolate currently approved for treating mesothelioma and non-squamous, non-small cell lung cancer (17–19). In addition to proximal small intestine, PCFT is expressed in other normal tissues, such as liver and kidney, which do not experience low pH conditions (2). In terms of cancer, a prominent low pH transport route was identified in 29 of 32 human solid tumor cell lines (20), and abundant hPCFT transcripts were detected by real-time PCR in a large cohort (n = 53) of human tumor sublines from an assortment of lineages (21). The interstitial pH of solid tumors is reportedly acidic (22, 23), conditions under which hPCFT would provide an important route of cytotoxic antifolate uptake if expressed at sufficient levels. Indeed, results of our recent studies imply that hPCFT could offer a unique means for selectively targeting solid tumors with cytotoxic antifolates that are not substrates for the ubiquitously expressed RFC (21, 24–26).
Reflecting its biological and therapeutic importance, a number of studies have begun to explore key structural determinants of hPCFT function (Fig. 1). There are two N-glycosylation consensus sites (Asn-58 and Asn-68) in the hPCFT loop domain between transmembrane domains (TMDs) 1 and 2. Based on results with substituted cysteine-scanning mutagenesis of a Cys-less hPCFT and accessibilities to thiol-reactive agents (27) and on studies of hemagglutinin (HA) epitope accessibilities to epitope-specific antibody (28, 29), hPCFT has 12 TMDs with N and C termini directed to the cytosol. Structurally and/or functionally important residues have been identified in hPCFT and include (with possible roles) Glu-185 (which is required for proton coupling) (30), His-247 and Ser-172 (which modulate folate and proton access to a high affinity binding site) (31), His-281 (which is important for substrate binding) (31), Arg-376 (which impacts proton and substrate binding) (12), and Asp-156 (which contributes to PCFT protein stability) (13) (Fig. 1). In addition, residues mapping to a well conserved β-turn in a region between TMDs 2 and 3 (DXXGRR; positions 109–114) were implicated as functionally important (6, 8, 13, 32, 33) (Fig. 1). From loss of transport for R113C hPCFT, a model was proposed in which TMDs 1, 3, 4, and 6 form a hydrophobic binding pocket into which Arg-113 protrudes (32). However, this has not been experimentally validated.
FIGURE 1.
hPCFT topology model. A topology model for hPCFT is shown, including 12 TMDs and internal N and C termini. The seven Cys residues are shown as black circles. Structurally and functionally important residues, as described in the “Introduction,” are labeled as black squares. The DXXGRR motif (positions 109–114) is shown as gray circles. Pro-425, for which its Arg mutant was used in current study, is shown as a black triangle. N-Glycosylation occurs at Asn-58 and Asn-68 (also shown as black circles).
Like RFC, PCFT belongs to the major facilitator superfamily of transporters. Because numerous major facilitator superfamily proteins, including LacS, AE1, GLUT1, TetA, and human RFC (hRFC), have been reported to exist as oligomers (e.g. dimers, tetramers, etc.) (34–40), we decided to explore this important question for hPCFT, given the potential mechanistic and regulatory ramifications of such structures. In this report, we use an assortment of powerful biochemical and molecular methods to assess the existence and potential functional impact of oligomeric hPCFT.
EXPERIMENTAL PROCEDURES
Reagents
[3′,5′,7-3H]Mtx (20 Ci/mmol) was purchased from Moravek Biochemicals (Brea, CA). Unlabeled Mtx was provided by the Drug Development Branch, NCI, National Institutes of Health (Bethesda, MD). Synthetic oligonucleotides were obtained from Invitrogen (Carlsbad, CA). Tissue culture reagents and supplies were purchased from assorted vendors with the exception of fetal bovine serum, which was purchased from Hyclone Technologies (Logan, UT). The cross-linking reagent 1,1-methanediyl bismethanethiosulfonate (MTS-1-MTS) was purchased from Toronto Research Chemicals (Toronto, Canada).
Generation of hPCFT and Other Plasmid Constructs
An expression construct for deglycosylated hPCFT with a Myc epitope tag (dghPCFTMyc-His6) and Gln substituted for Asn at positions 58 and 68 was generated by site-directed mutagenesis, using wild-type (WT) Myc-His6-tagged hPCFT (wthPCFTMyc-His6) in pCDNA3.1 (24) as template. To prepare WT HA-tagged hPCFT (wthPCFTHA) with an HA epitope insertion (YPYDVPDYA) at amino acid 459, preceded by a 3-amino acid (GTQ) linker, the hPCFT cDNA fragment was excised from the wthPCFTMyc-His6 construct by digestion with BamHI and KpnI and cloned upstream of the HA tag in pCDNA3 after deleting the hRFC cDNA fragment from a carboxyl-terminal HA-tagged hRFC construct (41). Carboxyl-HA-tagged human thiamine transporter 1, ThTr1HA, was generated from ThTr1 in pBlueScriptII KS(−) (42) with the same strategy as for wthPCFTHA above. WT Myc-tagged hPCFT (wthPCFTMyc) was prepared from wthPCFTHA by inserting a Myc epitope (EQKLISEEDL) in place of the HA tag, followed by a stop codon. A FLAG epitope (DYKDDDDK) was inserted at the hPCFT N terminus just after Met-1 of wthPCFTMyc to generate wtFLAGhPCFTMyc, and from this construct, wtFLAGhPCFTMyc-His10 was generated by inserting a His10 immediately following the Myc sequence. Deglycosylated hPCFT (dgFLAGhPCFTMyc-His10) was also prepared from wtFLAGhPCFTMyc-His10 by substitution of Asn-58 and Asn-68 with glutamine.
To prepare fluorescent protein-tagged hPCFTs for the fluorescence resonance energy transfer (FRET) experiments (see below), YPet-His and ECFP*-His cDNAs optimized for mammalian codon expression of yellow and enhanced cyan fluorescent proteins, respectively, were provided by Dr. Tomoo Ohashi (Duke University Medical Center) (43). To generate monomeric YPet (mYPet) and ECFP* (mECFP*), Ala-206 was substituted with lysine in both constructs, as described for other GFP variants (44). To clone mYPet in the pCDNA3 vector (mYPet/pCDNA3), BamHI and EcoRI restriction sites were introduced 5′ and 3′, respectively, of the mYPet cDNA fragment. The mYPet cDNA fragment was digested with BamHI and EcoRI and cloned in pCDNA3 using these same sites. mECFP*/pCDNA3 was prepared using the same strategy with BamHI and EcoRV. To attach mYPet to the C terminus of hPCFT (hPCFT-mYPet), the hPCFT cDNA fragment was excised from the wthPCFTMyc construct by HindIII and BamHI digestions and inserted upstream of the mYPet cDNA in the mYPet/pCDNA3 construct using these same sites. An analogous strategy was used to attach mECFP* to the C terminus of hPCFT (hPCFT-mECFP*), using the wthPCFTMyc and mECFP*/pCDNA3 constructs. Both hPCFT-mYPet and hPCFT-mECFP* constructs included a 2-amino acid (GS) insertion between the hPCFT and the fluorescent protein tag. To attach mYPet to the N terminus of hPCFT (mYPet-hPCFT), the hPCFT cDNA fragment was excised from the wthPCFTMyc/pCDNA3 construct by EcoRI and HpaI digestions and inserted downstream of the mYPet cDNA in mYPet/pCDNA3 at the EcoRI and HpaI sites. mYpet-hPCFT included a short (AEFHHTGKVDPH) linker between mYPet and hPCFT. To generate a tandem construct in which mYPet and mECFP* (mYPet-mECFP*) are covalently linked head-to-tail for use as a positive control for the FRET experiments, the mYPet cDNA fragment was cut from mYPet/pCDNA3 and inserted upstream of the mECFP* cDNA in mECFP*/pCDNA3 between the HindIII and BamHI sites. The resulting construct included a small (GGS) linker between mYPet and mECFP.
A clinically relevant hPCFT mutant, P425R (6), was introduced into wtFLAGhPCFTMyc-His10 to generate the P425R FLAGhPCFTMyc-His10 (hereafter, termed FLAGP425RMyc-His10). FLAGP425RHA-Myc-His10 was generated by inserting an HA in front of Myc in FLAGP425RMyc-His10.
All mutagenesis, including insertions of restriction sites and epitope insertions, was performed with the QuikChangeTM mutagenesis kit (Agilent Technologies Inc., Santa Clara, CA). Mutation primers were designed on the Agilent Technologies Web site. Sequences for the mutation primers are available upon request. All mutations were confirmed by automated DNA sequencing by Genewiz Corp. (South Plainfield, NJ).
Cell Culture
hRFC- and hPCFT-null HeLa cells, designated R1-11-mock (16) (hereafter, designated simply R1-11) and hRFC-null R5 HeLa cells (20) were gifts of Dr. I. David Goldman (Bronx, NY). R1-11 and R5 cells were maintained in complete RPMI 1640 medium containing 10% fetal bovine serum, 2 mm l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. WT and mutant hPCFT constructs (see above) were transiently transfected into R1-11 cells with Lipofectamine-Plus reagent (Invitrogen), as described previously for the R5 HeLa cells (45). For experiments in which results for transfections with two plasmid constructs were directly compared with results for cells transfected with a single plasmid construct, total DNA amounts were maintained constant by adding empty pCDNA3 (Invitrogen) to the single transfections. For all transfections, transfection efficiencies were monitored by luciferase assays, using co-transfections with pGL4.74[hRluc/TK] vector and a Dual-Luciferase® reporter assay system (Promega, Madison, WI) with a TD-20/20 luminometer (Turner Designs, Sunnyvale, CA). With all transfections, cells were harvested after 48 h for transport and luciferase assays and for preparing plasma membranes and Western blotting (see below).
Cross-linking Experiments
R1-11 cells were transfected with dghPCFTMyc-His6 (see above), and after 48 h, cells were washed with ice-cold Dulbecco's phosphate-buffered saline (PBS) twice and then treated with MTS-1-MTS (3 Å, flexible) at a final concentration of 0.05 mm for 30 min at 4 °C. An equivalent amount of PBS or dimethyl sulfoxide (DMSO) (vehicle used for MTS-1-MTS) was added to the transfected cells as negative control. The cross-linking reactions were terminated by the addition of 5 mm N-ethylmaleimide. After washing with ice-cold PBS (two times), treated cells were harvested and stored at −20 °C. Plasma membranes were prepared by differential centrifugation (46), samples (12 μg of protein) were treated with or without dithiothreitol (DTT) (0 °C; 0–80 mm) and analyzed by 4–20% Novex® Tris/glycine gel (Invitrogen) electrophoresis (125 V, 120 min), followed by Western blotting (see below). The blots were probed with anti-hPCFT polyclonal antibody (1:2000) raised in a rabbit against hPCFT peptide 445CKADPHLEFQQFPQSP459 (Invitrogen) and purified on an Affi-Gel 10 peptide affinity column, as described by the manufacturer (Bio-Rad, Hercules, CA).
Protein Association Study with His SpinTrapTM Chromatography
R1-11 cells were co-transfected with wtFLAGhPCFTMyc-His10 or FLAGP425RMyc-His10 with wthPCFTHA in different DNA amounts (4200, 3150, and 2100 ng/sample) as 1:1 ratios for both constructs. Transfected cells were harvested and disrupted (sonication), and membranes were solubilized by 1% n-dodecyl-β-d-maltoside for 2.5 h at 4 °C. One-tenth of the solubilized samples were spared as whole cell lysates to monitor protein expression levels by Western blots. The remainder was passed through His SpinTrapTM columns (GE Healthcare; Buckinghamshire, United Kingdom) with 20 mm sodium phosphate buffer (pH 7.4) containing 0.1% n-dodecyl-β-d-maltoside, 500 mm NaCl, EDTA-free proteinase inhibitor (Roche Applied Science, Indianapolis, IN), and 120 mm imidazole in both equilibration and washing buffers. The bound proteins were eluted with the above buffer, containing 500 mm imidazole. The whole cell lysates and the samples in the eluates were fractionated on 4–20% Tris/glycine gels (Invitrogen), followed by Western blotting.
Membrane Preparations and Western Blot Analysis of Plasma Membrane and Cell Surface hPCFT Proteins
Plasma membrane preparations, SDS-PAGE, and electrotransfer to polyvinylenedifluoride (PVDF) membranes (Pierce, Rockford, IL) were performed exactly as reported previously (45). Detection and quantitation of immunoreactive proteins used anti-FLAG (Sigma, St. Louis, MO), anti-HA (Covance, Emeryville, CA), or anti-His6 (GenScript, Piscataway, NJ) antibodies and IRDye800-conjugated secondary antibody with an Odyssey® infrared imaging system (LI-COR, Lincoln, NE). To quantitate the broadly banding hPCFT forms by densitometry, identical areas were “boxed” and corrected for background signal (i.e. an identical boxed “blank” region). Na+/K+ ATPase was used as a loading control (mouse antibody from Novus Biologicals, Littleton, CO). Densitometry used the Odyssey software (version 3.0).
For some experiments, the Cell Surface Labeling Accessory Pack (Thermo Scientific, Rockford, IL) was used to biotinylate and isolate surface membrane proteins prior to SDS-PAGE and Western analysis. Briefly, cells were incubated with 0.25 mg/ml sulfo-NHS-SS-biotin in PBS for 30 min at 4 °C and then solubilized with lysis buffer. The lysates were centrifuged to remove the insoluble fraction. The supernatants were incubated with immobilized NeutrAvidinTM gel slurry for 1 h at room temperature, after which the beads were washed five times with wash buffer containing protease inhibitors (Roche Applied Science). The proteins were eluted with 1× SDS-PAGE sample buffer (47) containing 50 mm DTT, and analyzed by SDS-PAGE/Western blotting. If isolation of biotinylated surface proteins was followed by deglycosylation, the biotinylated surface proteins were eluted with 10 mm Tris-Cl (pH 7.5), containing 0.5% SDS, 50 mm DTT, and protease inhibitor mixture. For deglycosylation, 50 mm sodium phosphate (pH 7.5), 1% Nonidet P-40, and N-glycosidase F (1000 units; New England Biolabs, Ipswich, MA) were added, and the reaction was incubated overnight at 37 °C.
Blue Native Polyacrylamide Gel Electrophoresis (BN-PAGE)
The NativePAGETM Novex® BisTris gel system (Invitrogen) was used. Briefly, dgFLAGhPCFTMyc-His10 was transfected into R1-11 cells (see above); cells were harvested after 48 h. Plasma membranes were prepared by differential centrifugation, and the final membrane particulate fractions were suspended in 1× NativePAGETM sample buffer (50 mm BisTris, 6 n HCl, 10% glycerol, 0.001% Ponceau S, pH 7.2) containing 50 mm DTT, 1% digitonin, and protease inhibitors. Samples were incubated at 4 °C for 1 h and then at 25 °C for 30 min with rocking. Following centrifugation (12,000 rpm for 10 min at 4 °C), the supernatants were transferred to fresh tubes. Each sample (15 μl) was combined with 5 μl of 1× NativePAGETM sample buffer containing 1% digitonin, followed by 1 μl of 5% NativePAGETM G-250 just prior to electrophoresis. Samples were fractionated along with NativeMarkTM unstained protein standards (Invitrogen) as described by the manufacturer using an XCell SureLockTM minicell (Invitrogen) and NativePAGETM Novex® 4–16% BisTris gels. Proteins were transferred to a PVDF membrane using the XCell IITM blot module and NuPAGE® transfer buffer. Protein standards were stained with Coomassie Blue (CB) G250, and the immunoreactive hPCFT proteins were detected with anti-FLAG antibody, as described above.
FRET
R1-11 cells (2 × 105) were seeded in 35-mm glass bottom Microwell dishes (MatTek, Ashland, MA) 24 h before transfections with Lipofectamine-Plus reagents (see above). A total of 700 ng of DNA were used per dish (350 ng for each construct; for single transfections, 350 ng of pCDNA3 were added to maintain constant DNA). After 48 h and just prior to the FRET experiments, the medium in the dish was replaced with Leibovitz's L-15 medium (Invitrogen). FRET between YPet- and ECFP*-tagged hPCFT was assessed by calculating the sensitized emission (the YPet emission upon ECFP* excitation) from confocal donor and acceptor images, acquired separately using a laser-scanning confocal fluorescence microscope. All confocal microscopy work was performed in the Microscopy, Imaging, and Cytometry Resources Core at the Wayne State University School of Medicine. The imaging equipment consisted of a Leica TCS SP5MP confocal microscope system (Leica Microsystems, Heidelberg, Germany), equipped with an argon laser (maximum luminous power at the focal plane is less than 30 milliwatts) with 458- and 514-nm lines. ECFP* fluorescence protein was excited using the optimized donor excitation with the 458-nm laser line. Spectral detection bandwidth of the Leica SP5 channels was set up to balance minimal cross-talk with optimal collection efficiency. ECFP* fluorescence protein emission was detected between 460 and 490 nm; YPet was excited with a 514-nm argon laser, and emission was detected between 528 and 603 nm. FRET between ECFP* and YPet fluorescent proteins was calculated using the FRET-sensitized emission module of the Leica confocal software (LCS 2.61.1537), applying the equation, FRET = (B − A × β − C × γ)/C (48), where A represents ECFP* emission (by ECFP* excitation), B is FRET emission (by ECFP* excitation), C is YPet emission (by YPet excitation), β is the correction factor for donor cross-talk (B/A when only ECFP* is expressed), and γ is the correction factor for acceptor cross-excitation (B/C when only YPet is expressed). Factors β and γ in the acquired images were 0.427 and 0.055, respectively. In all FRET experiments, positive and negative FRET controls were analyzed, including an ECFP*-YPet tandem construct, and co-transfected separate ECFP* and YPet constructs, respectively. FRET efficiencies were averaged from 15 separate cells for each experimental sample and control. FRET image raw data were processed using ImageJ software (W.S. Rasband, ImageJ, National Institutes of Health, Bethesda, MD). Multiple images were collected and analyzed. Representative images are shown. Statistical analysis was performed to compare results for the experimental samples with those for the negative control.
Confocal Microscopy
For confocal microscopy, R1-11 cells were plated and transfected in Lab-Tek®II chamber slides (Nalge Nunc International, Naperville, IL). Transfections with one (wtFLAGhPCFTMyc-His10/pCDNA3 vector, wthPCFTHA/pCDNA3 vector, FLAGP425RMyc-His10/pCDNA3 vector) and two (wtFLAGhPCFTMyc-His10/wthPCFTHA, wthPCFTHA/FLAGP425RMyc-His10) hPCFT constructs were performed so as to identify hPCFT monomer expression and localization. After 48 h, cells were fixed with 3.3% paraformaldehyde (in PBS), permeabilized with 0.1% Triton X-100 (in PBS), and stained with primary antibodies, followed by incubation with secondary antibodies (49). The primary antibodies used were mouse anti-FLAG (Sigma) and goat anti-HA polyclonal antibodies (Abcam, Cambridge, MA). Fluorescent secondary antibodies included Alexa Fluor® 568 donkey anti-goat IgG (H+L) and Alexa Fluor® 488 donkey anti-mouse IgG (H+L) (Invitrogen). Cross-reactivities between primary antibodies (anti-FLAG and anti-HA) and between secondary antibodies were also tested. Slides were visualized with a Zeiss LSM-510 META NLO using a 63× water immersion lens, with the same parameter setting for all samples. Confocal work was done in the Microscopy, Imaging, and Cytometry Resources Core at Wayne State University School of Medicine.
Membrane Transport Experiments and WT/Mutant Mixing Experiments
Cellular uptake of [3H]Mtx (0.5 μm) was measured over 2 min at 37 °C in 60-mm dishes in MES-buffered saline (20 mm MES, 140 mm NaCl, 5 mm KCl, 2 mm MgCl2, and 5 mm glucose) at pH 5.5, as described (24). Levels of intracellular radioactivity were expressed as pmol/mg protein, calculated from direct measurements of radioactivity and protein contents (50) of the cell homogenates.
To systematically determine the impact of co-expressing WT with inactive P425R hPCFT, R1-11 cells were transfected with plasmids encoding wtPCFTHA and FLAGP425RHA-Myc-His10 in defined ratios, including 0:1.0, 0.2:0.8, 0.3:0.7, 0.4:0.6, 0.5:0.5, 0.6:0.4, 0.7:0.3, 0.8:0.2, and 1.0:0, while maintaining constant total plasmid. After 48 h, hPCFT transport was assayed at pH 5.5 with [3H]Mtx. Another aliquot of cells was biotinylated with sulfo-NHS-SS-biotin, after which biotinylated cell surface proteins were isolated on immobilized avidin, eluted, and analyzed by SDS-PAGE and Western blots following deglycosylation with N-glycosidase F. Deglycosylated WT and P425R proteins were resolved (molecular masses differ by ∼3.2 kDa), thus permitting quantification on Western blots with anti-HA antibody. hPCFT transport activities in excess of the low residual level for P425R were plotted against the fraction of WT hPCFT to total hPCFT forms (WT plus mutant). If hPCFT monomers function independently (i.e. monomeric hPCFT is the “minimal functional unit”), transport activity should closely reflect the amounts of WT hPCFT and increase linearly with increasing ratios of WT to total surface hPCFT forms (34, 51–54). However, if there is functional interaction between hPCFT monomers, total transport activity should increase or decrease quadratically with the increasing fraction of active hPCFT. These methods are completely analogous to those previously described for numerous other oligomeric transporters, including hRFC (34, 51–54).
RESULTS
Identification of Higher Order hPCFT Structures with Bifunctional Thiol-reactive Cross-linker MTS-1-MTS
hPCFT has seven cysteines (Cys-21, Cys-66, Cys-151, Cys-229, Cys-298, Cys-328, and Cys-397), including three in the TMDs (Cys-151, Cys-229, and Cys-397), two in intracellular loop domains (Cys-21 and Cys-328), and two in extracellular loop domains (Cys-66 and Cys-298) (Fig. 1). To begin to examine the possibility of whether hPCFT exists as a higher order oligomeric complex, we used an intermediate length (3 Å) methane thiosulfonate bifunctional cross-linker, MTS-1-MTS, capable of cross-linking vicinal Cys residues. The experimental design involved transfecting hPCFT-null R1-11 cells with a functional (∼60% of WT) Myc-His6-tagged hPCFT construct (dghPCFTMyc-His6) in which the N-glycosylation consensus sites at Asn-58 and Asn-68 were mutated to Gln (to avoid complications of N-glycosylation in data analysis) and then treating the transfectants with MTS-1-MTS to cross-link vicinal thiols. Following cross-linking, plasma membranes were prepared, and membrane proteins were analyzed by SDS-PAGE and Western blotting with hPCFT-specific antibody.
Although the predicted molecular mass of hPCFT is 52.8 kDa, non-cross-linked dghPCFTMyc-His6 migrated as a ∼45-kDa species (Fig. 2, labeled a in lane 1) with detection by hPCFT-specific antibody. MTS-1-MTS treatment of dghPCFTMyc-His6-expressing R1-11 cells resulted in a unique band at ∼90 kDa (Fig. 2, labeled b in lane 3) and substantial amounts of higher mass species (>180 kDa; labeled c) clearly distinct from ∼45-kDa monomeric dghPCFTMyc-His6 and not present in control cells treated with solvent (DMSO) in lieu of cross-linker or PBS. Based on relative intensities, cross-linked species comprised a substantial amount of the immunoreactive hPCFT. Essentially identical results were obtained when dghPCFTMyc-His6 forms were detected with Myc epitope-specific antibody (not shown). When cross-linked samples were treated with DTT (beginning at 1.25 mm) prior to SDS-PAGE, bands b and c disappeared, indicating a reversal of cross-linking (Fig. 2, lanes 4–10). Although a ∼60-kDa species was detected with hPCFT antibody in samples treated with DTT, this was not seen when Myc-specific antibody was used.
FIGURE 2.
Detection of putative hPCFT oligomers with a MTS homobifunctional cross-linker. The dghPCFTMyc-His6 construct was transiently expressed in hRFC- and hPCFT-null HeLa R1-11 cells. Cells were treated with the MTS-1-MTS bifunctional cross-linker at 4 °C for 30 min. Following cross-linking, plasma membranes were prepared, and membrane proteins were analyzed by a 4–20% Novex® Tris/glycine gel under non-reducing conditions and Western blotting with hPCFT-specific polyclonal antibody. In the absence of DTT treatment, unique bands (b and c (lane 3)) were identified that were not seen in the absence of cross-linker (lanes 1 and 2). Treatment of cross-linked proteins with DTT prior to running the gradient gel reversed cross-linking for bands b and c (lanes 4–10). In the figure, a ∼60-kDa protein was detected with hPCFT-specific antibody in cross-linked samples treated with DTT. This band was also detected in non-cross-linked samples treated with reducing agents (e.g. DTT or 2-mercaptoethanol). Although species a–c were all detected when the blot shown was probed with cMyc antibody (not shown), the ∼60-kDa species was not. NS, nonspecific.
The nature of the cross-linked species b and c is not certain and may involve cross-links of dghPCFTMyc-His6 with non-hPCFT proteins. However, their molecular masses relative to that for monomeric dghPCFTMyc-His6 are entirely consistent with their identities as higher order hPCFT homo-oligomers (dimer, tetramer, etc.). Accordingly, these results demonstrate the feasibility of the existence of homo-oligomeric hPCFT.
BN-PAGE of dgFLAGhPCFTMyc-His10
BN-PAGE has been used extensively to study multiprotein complexes, including oligomeric structures of membrane proteins (55). BN-PAGE uses CB G-250, which binds nonspecifically to all proteins and is itself negatively charged. Because CB does not act as a detergent, BN-PAGE preserves oligomeric protein structures while offering a higher resolution than other separation techniques, such as gel filtration or sucrose density ultracentrifugation, that are commonly used to determine molecular sizes and the relative abundances of oligomeric proteins (56). Because the digitonin bound to proteins is replaced by the amphipathic CB dye during BN-PAGE and is increased for membrane proteins over soluble proteins, in order to accurately calculate molecular masses for membrane proteins from soluble standard proteins, the equation MBNP/1.8 = MAA was used (where MBNP represents the apparent mass directly calculated from BN-PAGE, and MAA is the actual molecular mass based on the amino acid sequence) (57).
When functional dgFLAGhPCFTMyc-His10 was analyzed by BN-PAGE with Western blots probed with anti-FLAG antibody, three major bands were observed (labeled (hPCFT)4, (hPCFT)3, and (hPCFT)2 in Fig. 3). The MBNP values, calculated from the median migrations of the major hPCFT species (indicated by the arrows) and a standard curve generated from soluble protein standards were 374, 278, and 190 kDa, respectively. Using the above conversion factor, the MAA values of these immunoreactive dgFLAGhPCFTMyc-His10 bands were calculated as 208, 154, and 105 kDa, approximating the molecular masses predicted for tetrameric, trimeric, and dimeric hPCFTs, respectively. Thus, hPCFT would seem to exist as higher order complexes when electrophoresis is performed under conditions that do not disrupt protein-protein interactions.
FIGURE 3.
Demonstration of putative hPCFT oligomers on blue native polyacrylamide gels. dgFLAGhPCFTMyc-His10 was transfected into R1-11 cells, and cells were harvested 48 h later. Plasma membranes were prepared for BN-PAGE, as described under “Experimental Procedures.” The sample and protein standards were run on a 4–16% BisTris gel and then transferred to a PVDF membrane. The protein standard lane was cut out, and the membrane was stained separately with CB G250. The rest of the blot was processed for Western analysis and developed with anti-FLAG (primary) and IRDye800-conjugated (secondary) antibodies. Immunoreactive proteins were detected on an Odyssey infrared imager. The figure shows putative tetrameric ((hPCFT)4), trimeric ((hPCFT)3), and dimeric ((hPCFT)2) species, as described under “Results.”
Association of Co-expressed HA- and FLAG/Myc-His10-tagged hPCFT Monomers
In additional experiments to confirm PCFT oligomerization, we co-expressed wthPCFTHA and wtFLAGhPCFTMyc-His10 proteins in hPCFT-null R1-11 cells. A reporter construct encoding Renilla luciferase under control of a thymidine kinase promoter (pGL4.74[hRluc/TK]) was co-transfected to control for minor (<5%) differences in transfection efficiencies. In transport assays, normalized [3H]Mtx (0.5 μm, 2 min) uptake into the dually transfected cells (relative transport = 1.58 ± 0.07 (n = 7)) approximated the sum of levels recorded for wthPCFTHA and wtFLAGhPCFTMyc-His10 individually (relative transport = 1.00 and 0.76 ± 0.06, respectively (n = 7)) (i.e. the sum of transport levels for individually transfected wthPCFTHA and wtFLAGhPCFTMyc-His10 was not statistically significantly different from that measured when both constructs were transfected together) (Fig. 4A).
FIGURE 4.
Co-transfections of WT and P425R hPCFTs. wtFLAGhPCFTMyc-His10, wthPCFTHA, and FLAGP425RMyc-His10 were transiently transfected into HeLa R1-11 cells either singly or in combination. Transfection efficiencies were monitored with pGL4.74[hRluc/TK] vector, which encodes Renilla luciferase under control of a thymidine kinase promoter, and luciferase assays. A, cells were assayed for transport at pH 5.5 with [3H]Mtx (0.5 μm) for 2 min at 37 °C. Transport results are normalized to Renilla luciferase activities; results are expressed relative to those for wthPCFTHA and are reported as mean values ± S.E. (error bars) from seven independent experiments. Compared with wthPCFTHA, the increases of relative transport of both dual transfectants (wthPCFTHA plus wtFLAGhPCFTMyc-His10 and wthPCFTHA plus FLAGP425RMyc-His10) are significantly higher (p < 0.01; paired t test). B, results are shown for surface hPCFT proteins labeled with sulfo-NHS-SS-biotin (0.25 mg/ml) and isolated on immobilized NeutrAvidinTM gel. Samples were analyzed by SDS-PAGE and Western blotting with His6- and HA-specific antibodies. Na+/K+ ATPase was used as a loading control. The molecular mass markers for SDS-PAGE are noted. NS, nonspecific.
To confirm plasma membrane targeting of the wthPCFTHA and wtFLAGhPCFTMyc-His10 proteins, transfectants were treated with sulfo-NHS-SS-biotin to biotinylate hPCFT molecules at the cell surface via exposed amines. Cells were lysed, and biotinylated proteins were bound to strepavidin-agarose (NeutrAvidinTM) and then eluted with DTT and analyzed on Western blots probed with anti-His6 and anti-HA antibodies. By this assay, both wthPCFTHA and wtFLAGhPCFTMyc-His10 were targeted to the cell surface (Fig. 4B, lanes 2–4). Due to marked differences in sensitivity of detection of low levels of surface hPCFT with anti-His6 and anti-HA antibodies, the major ∼45-kDa unglycosylated hPCFT forms were most obvious when the blot was probed with anti-His6 antibody, although low levels of higher molecular mass glycosylated forms could be discerned. Detection with the anti-FLAG antibody was even less effective for these analyses (not shown). With anti-HA antibody, both the unglycosylated and glycosylated species were detected. Surface expression of wthPCFTHA and wtFLAGhPCFTMyc-His10 in single or co-transfected R1-11 cells was further confirmed by confocal microscopy with indirect immunofluorescence staining by HA and FLAG epitope-specific primary antibodies and Alexa Fluor® 488 (FLAG) and Alexa Fluor® 568 (HA)-conjugated secondary antibodies (Fig. 5A). Again, both wtFLAGhPCFTMyc-His10 and wthPCFTHA were expressed at the cell surface either singly or in combination, consistent with our surface biotinylation results, although there was some staining of intracellular structures, as reported previously (24). There was no staining of untransfected R1-11 cells (not shown).
FIGURE 5.
Immunofluorescence staining of HA- and FLAG-tagged WT and mutant hPCFTs. A and B, results are shown for HeLa R1-11 cells transfected with wtFLAGhPCFTMyc-His10 (panels a, b, and d), wthPCFTHA (panels c, d, e, h, i, and j), and FLAGP425RMyc-His10 (panels f and g), either singly or together. Cells were fixed with 3.3% paraformaldehyde, permeabilized with 0.1% Triton X-100, and stained with Alexa Fluor® 488-conjugated (for FLAG-tagged proteins; green) or Alexa Fluor® 568-conjugated (for HA-tagged hPCFT; red) secondary antibodies. Slides were visualized with a Zeiss LSM-510 META NLO using a 63× water immersion lens. Staining is shown for the individual fluors and as a merged image.
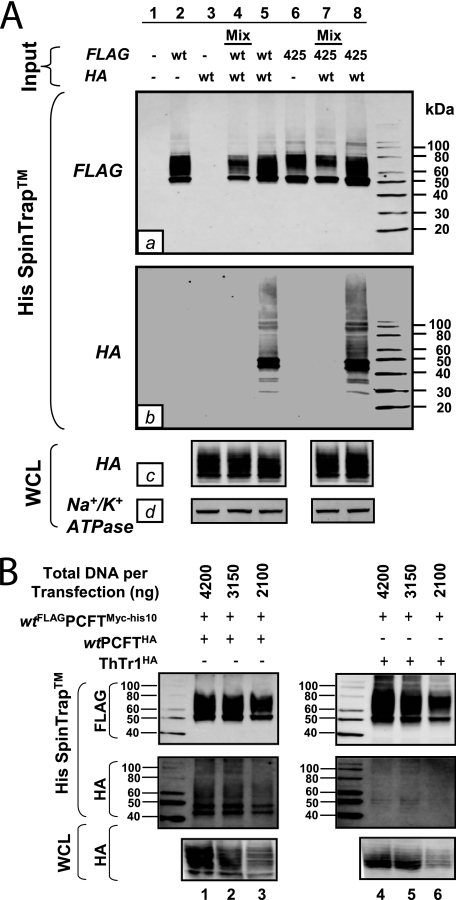
To further confirm whether there is a direct association between hPCFT monomers, singly (wthPCFTHA or wtFLAGhPCFTMyc-His10) or co-transfected (wthPCFTHA plus wtFLAGhPCFTMyc-his10) cells were harvested, and total cell proteins were solubilized by 1% n-dodecyl-β-d-maltoside, followed by purification with His SpinTrapTM affinity columns (see “Experimental Procedures”). Samples were eluted and then analyzed by 4–20% Tris/glycine gels/Western blots with epitope-specific antibodies. As expected, wtFLAGhPCFTMyc-His10 but not wthPCFTHA proteins from singly transfected samples bound to the His SpinTrapTM columns (through the His10 tag) and were detected with FLAG antibody (Fig. 6A, a, lanes 2 and 3, respectively). When wtFLAGhPCFTMyc-His10 and wthPCFTHA proteins were co-expressed in R-11 cells, both proteins were retained on the nickel columns and were detected with FLAG and HA antibodies, respectively (Fig. 6A, a and b, lane 5). However, when extracts from singly transfected samples were mixed just prior to nickel chromatography, wtFLAGhPCFTMyc-His10 but not wthPCFTHA proteins were bound to the column (Fig. 6A, a and b, lane 4). The lack of detection of wthPCFTHA protein in the “mixed” sample was not due to low level expression, because with constant loading (reflected in Na+/K+ ATPase levels in Fig. 6A, d, lanes 3–5), wthPCFTHA proteins were comparably expressed in whole cell lysates (labeled WCL) from the single and double transfected samples (Fig. 6A, c, lanes 3–5). Thus, co-expression is clearly obligatory to the association between wthPCFTHA and wtFLAGhPCFTMyc-His10 proteins.
FIGURE 6.
Co-association of hPCFT monomers. R1-11 cells were co-transfected with wtFLAGhPCFTMyc-His10 or FLAGP425RMyc-His10, together with wthPCFTHA in fixed DNA amounts (4200 ng of DNA/sample) (A) or were co-transfected with wtFLAGhPCFTMyc-His10 combined with ThTr1HA or wthPCFTHA with different DNA amounts (4200, 3150, and 2100 ng/sample) (B) as 1:1 ratios for both constructs. Transfected cells were harvested, sonicated, and solubilized (whole cell lysate (WCL)), and the homogenates were fractionated on His SpinTrapTM columns, as detailed under “Experimental Procedures.” The whole cell lysates and the nickel column eluate fractions were separated by 4–20% Tris/glycine gels, followed by Western blotting with FLAG (labeled a in panel A) or HA (labeled b and c in panel A) antibodies. Loading for whole cell lysates was normalized by probing with antibody to Na+/K+ ATPase (labeled d in panel D).
To establish the specificity of association between hPCFT monomers, we repeated this experiment over a range of plasmid amounts (2100, 3150, and 4200 ng, respectively, as 1:1 ratios) for the wtFLAGhPCFTMyc-His10 and wthPCFTHA constructs and for constructs encoding wtFLAGhPCFTMyc-His10 with a functionally non-related transporter, HA-tagged human thiamine transporter (ThTr1HA). In this experiment, wthPCFTHA efficiently bound to the His SpinTrapTM column via its association with wtFLAGhPCFTMyc-His10 and showed a distinct dose dependence (Fig. 6B, left panels, lanes 1–3). Conversely, ThTr1HA protein binding to the His SpinTrapTM column at any concentration was effectively nominal, despite high levels of ThTr1HA protein expression in whole cell lysates and efficient binding of wtFLAGhPCFTMyc-His10 protein to the nickel matrix (Fig. 6B, right panels, lanes 4–6).
Collectively, these results establish that wtFLAGhPCFTMyc-His10 and wthPCFTHA are efficiently expressed in hPCFT-null R1-11 HeLa cells, whereupon they are targeted to the plasma membrane surface to restore membrane transport activity. Our co-association experiments with co-expressed wtFLAGhPCFTMyc-His10 and wthPCFTHA proteins and His SpinTrapTM column binding strongly and directly support conclusions from our cross-linking and BN-PAGE experiments, namely that hPCFT exists as a higher order homo-oligomer composed of associating hPCFT monomers at the plasma membrane surface. Our inability to detect an analogous complex between wtFLAGhPCFTMyc-His10 and ThTr1HA proteins strongly argues that the association between hPCFT monomers is highly specific.
FRET Analysis of mECFP*- and mYPet-tagged PCFT in Situ
FRET is a non-invasive technique designed to monitor formation of multiprotein or homo-oligomeric complexes in living cells, thus complementing biochemical approaches for studying protein oligomerization. Typically, fluorescence-tagged proteins with overlapping emission and excitation spectra (e.g. CFP and YFP) are co-expressed in cells, and fluorescence is monitored at the emission wavelength for the acceptor fluorochrome (58). To adapt this approach to studies of hPCFT oligomerization, it was necessary to first generate “monomeric” YPet and ECFP* forms (see “Experimental Procedures”) to eliminate potential interference resulting from oligomerization of the fluorescence proteins (43, 44, 59). To generate monomeric forms of YPet and ECFP*, Ala-206 was replaced with lysine (44). A positive control (mYPet-mECFP*) was also prepared in which mYPet was covalently linked “head-to-tail” to mECFP* (Fig. 7A, top). To confirm their expression, the monomeric and fused YPet and ECFP* constructs were initially transfected into another HeLa subline (R5) and analyzed on Western blots with GFP antibody capable of reacting with YPet and ECFP* proteins. As shown in Fig. 7A (bottom), all fluorescent proteins expressed well, and all showed the expected molecular masses (27.4 kDa for mYPet, 27.3 kDa for mECFP*, and 55 kDa for mYPet-mECFP*) on Western blots.
FIGURE 7.
Sensitized FRET emission between hPCFT monomers fused with mYPet and mECFP*. A, schematic of the mYPet and mECFP* tandem construct and their fusion constructs with hPCFT is shown in the upper panel. In the lower panel, results for determination of expression levels of individual mYPet and mECFP* proteins and the mYPet-mECFP* fusion protein by Western blotting are shown. Total cell lysates were prepared from non-transfected R5 HeLa cells and cells transiently transfected with mYPet, mECFP*, or mYPet-mECFP* constructs. Proteins were analyzed by SDS-PAGE and Western blots with detection using the Odyssey infrared imaging system and GFP (primary, mouse polyclonal; Abcam) and IRDye800-conjugated (secondary) antibodies. B, mYPet and mECFP* hPCFT fusion proteins were characterized for expression and transport function. Plasma membranes were prepared from non- and mock-pCDNA3 R1-11 cells and R1-11 cells transiently transfected with individual mYPet-hPCFT, hPCFT-mECFP*, and hPCFT-mYPet constructs. Plasma membrane preparations were isolated and separated by SDS-PAGE, followed by Western blot analysis and detection with the Odyssey infrared imaging system, using hPCFT-specific (top) and GFP-specific (middle) antibodies and IRDye800-conjugated secondary antibody. Transfected cells were also assayed for membrane transport (bottom). [3H]Mtx (0.5 μm) uptakes were measured for 2 min at 37 °C. Representative transport results are shown. C, for FRET assays, mYPet plus mECFP* (negative control), mYPet-mECFP* tandem (positive control), mYPet-hPCFT plus hPCFT-mECFP*, and hPCFT-mYPet plus hPCFT-mECFP* constructs were transiently transfected into R1-11 cells. R1-11 cells were also singly transfected with mYPet, mECFP*, mYPet-hPCFT, hPCFT-mECFP*, and hPCFT-mYPet constructs as controls (not shown). Forty-eight hours post-transfection, mYPet and mECFP* images were acquired on a confocal microscope, and total donor (mECFP*), total acceptor (mYPet), and sensitized FRET emissions (NET FRET) were calculated, as described under “Experimental Procedures.” FRET efficiency values from 15 separate cells (for each experimental sample and controls, as well) were used for statistical analysis. FRET efficiencies for both hPCFT dual transfections (mYPet-hPCFT plus hPCFT-mECFP* and hPCFT-mYPet plus hPCFT-mECFP*) were significantly higher than that for the negative control (mYPet plus mECFP*), with p < 0.0001 (***) and p = 0.0007 (**) (paired t test), respectively. For the co-transfections, the FRET efficiency of mYPet-hPCFT plus hPCFT-mECFP* is significantly higher than that of hPCFT-mYPet plus hPCFT-mECFP* (p = 0.0003; paired t test). D, images collected in the FRET channel were calculated, as described under “Experimental Procedures.” Representative images for each sample are shown. Calculated FRET values for both test samples (mYPet-hPCFT plus hPCFT-mECFP* and hPCFT-mYPet plus hPCFT-mECFP*) and controls (mYPet plus mECFP*, mYPet-mECFP*) are represented using a pseudocolor scale. NET FRET signals for both hPCFT samples were found mainly in the cell surface membranes, whereas significant FRET was observed intracellularly for the mYPet-mECFP* positive control. Error bars, S.E.
To tag hPCFT, mYPet was fused to either the N or C terminus (termed mYPet-hPCFT and hPCFT-mYPet, respectively), and mECFP* was attached to the C terminus of hPCFT (hPCFT-mECFP*), as depicted in Fig. 7A (top). By combining the N- or C-terminal mYPet-tagged hPCFT with the hPCFT-mECFP* construct in a suitable co-expression model, it was possible to assess relative orientations of individual hPCFT monomers with respect to each other (i.e. “head-to-head” or “head-to-tail”) in putative oligomeric hPCFT complexes.
Initially, the individual hPCFT fusion constructs were transfected into R1-11 cells and then assayed for [3H]Mtx transport and protein levels on Western blots. All constructs restored high and similar levels of transport activity, well in excess of the residual low level in hPCFT-null R1-11 cells (Fig. 7B, bottom). The hPCFT proteins were detected on Western blots with hPCFT-specific (Fig. 7B, top) and GFP-specific (middle) antibodies. The C-terminal tagged constructs (both mYPet and mECFP*) showed substantially reduced signals with the hPCFT antibody, probably due to masking of the carboxyl-terminal peptide epitope resulting from its proximity to the fluorescent protein tag.
For FRET experiments, R1-11 HeLa cells were transfected with the various constructs both singly and in combination. Transfected DNAs were maintained constant with empty pCDNA3 plasmid. Transfections (including negative and positive controls) were as follows: (i) mYPet, (ii) mECFP*, (iii) mYPet plus mECFP*, (iv) mYPet-mECFP*, (v) mYPet-hPCFT, (vi) hPCFT-mYPet, (vii) hPCFT-mECFP*, (viii) mYPet-hPCFT plus hPCFT-mECFP*, and (ix) hPCFT-mYPet plus hPCFT-mECFP*. FRET was measured with a confocal microscope as an increase in acceptor fluorescence resulting from donor excitation (i.e. sensitized emission FRET). Images collected in the FRET channel were corrected for fluorescence bleed-through. Representative FRET images are shown in Fig. 7D. Calculated FRET values of both test samples and controls are represented using a pseudocolor scale. Fig. 7D clearly shows FRET for the mYPet-mECFP* positive control (b) and the mYPet-hPCFT plus hPCFT-mECFP* (c) and hPCFT-mYPet plus hPCFT-mECFP* (d) constructs, at levels far exceeding that for the mYPet plus mECFP* negative control (a). This establishes close proximity (within 100 Å) of the fluorescently tagged hPCFT monomers and of mYPet and mECFP*. For the fluorescence protein-tagged hPCFT constructs, the majority of FRET was unambiguously localized to the cell surface, although there was some intracellular FRET for a few cells, consistent with our finding of intracellular hPCFT by indirect immunofluorescence staining (Fig. 5A). To quantitate FRET for statistical analysis, we calculated mean values of the intensities from FRET images over the cell surfaces of 15 individual cells. Values were compared with the negative (mYPet plus mECFP*) and positive (mYPet-mECFP*) controls. Calculated FRET efficiencies (reported as mean values ± S.E.) of the mYPet-hPCFT plus hPCFT-mECFP* (0.21 ± 0.02) and hPCFT-mYPet plus hPCFT-mECFP* (0.13 ± 0.01) co-transfections, as well as the mYPet-mECFP* positive control (0.51 ± 0.01), were all significantly higher than that of the mYPet plus mECFP* negative control (0.08 ± 0.01), with p < 0.0001 for mYPet-mECFP* and mYPet-hPCFT plus hPCFT-mECFP* and p = 0.0007 for hPCFT-mYPet plus hPCFT-mECFP* (by paired t test; Fig. 7C).
These results strongly support the notion that hPCFT oligomers form in situ and that these protein-protein complexes localize to the plasma membrane of transfected R1-11 cells. Interestingly, the differences in FRET efficiencies for mYPet-hPCFT plus hPCFT-mECFP* (0.21 ± 0.02) and hPCFT-mYPet plus hPCFT-mECFP* (0.13 ± 0.01) were statistically significant (p = 0.0003, by paired t test), suggesting that the association between monomeric hPCFT molecules shows a preferential (head-to-tail) orientation.
Co-expression of WT and Mutant hPCFT Monomers
Co-expression of WT and inactive mutant protein monomers within the same cell is a powerful approach to study potential functionally important monomer interactions within homo-oligomeric membrane transporters (34, 40, 51, 53). As long as transfection efficiencies are carefully controlled for (e.g. by co-transfections with a reporter gene construct), detection of a functionally dominant phenotype (negative or positive dominance) can provide powerful insights into the mechanistic or regulatory significance of these higher order structures.
Among the inactive hPCFT mutants identified in patients with HFM (5–15), P425R in the loop-TMD 12 junction of the hPCFT protein (Fig. 1) resulting from a C to G transition at position 1274 of the pcft coding sequence was of particular interest. When expressed in WT HeLa cells, P425R hPCFT, unlike most hPCFT mutants associated with HFM, was reported to show nominal transport function associated with at least some level of surface expression (6). In hPCFT-null R1-11 cells, FLAG/Myc-His10-tagged P425R hPCFT showed ∼5.1% of WT transport of [3H]Mtx (Fig. 4A). The P425R hPCFT protein was significantly targeted to the membrane surface in R1-11 cells, as reflected in its biotinylation with sulfo-NHS-SS-biotin and detection by Western blotting (Fig. 4B, lane 5) and in its imunofluorescence staining and detection by confocal microscopy (Fig. 5B, f). Immunofluorescence staining (both surface and intracellular) of P425R hPCFT was somewhat greater than was observed for WT hPCFT (Fig. 5, compare B (f) with A (a)). On Western blots probed with anti-His6 antibody, with protein loading normalized to Na+/K+ ATPase, the level of surface (biotinylated) FLAGP425RMyc-His10 hPCFT exceeded that for wtFLAGhPCFTMyc-His10 by 2.48 ± 0.63-fold (S.E.) (n = 4) (Fig. 4B, lanes 5 and 2, respectively).
To establish the possible physical association between inactive P425R hPCFT and WT hPCFT in oligomers and its potential impact on transport function, we co-transfected R1-11 cells with equivalent amounts of wthPCFTHA and FLAGP425RMyc-His10 hPCFT expression constructs, analogous to the wthPCFTHA/wtFLAGhPCFTMyc-His10 co-transfections described above (see parallel results for WT hPCFT depicted in Figs. 4–6). A Renilla luciferase reporter construct (pGL4.74[hRluc/TK]) was again included in the transfections to correct for minor differences in transfection efficiencies. After 48 h, membrane transport of [3H]Mtx (0.5 μm) was assayed (along with luciferase activities) (Fig. 4A), and surface proteins were biotinylated with sulfo-NHS-SS-biotin and then isolated and analyzed on Western blots with anti-FLAG and anti-HA antibodies (Fig. 4B). FLAGP425RMyc-His10 and wthPCFTHA hPCFTs were co-localized by immunostaining with anti-FLAG and Alexa Fluor® 488-conjugated antibodies and with anti-HA and Alexa Fluor® 568-conjugated antibodies, respectively (Fig. 5B). Results for the WT/mutant dual transfections were compared with those for R1-11 cells transfected with WT/WT constructs and singly transfected with wthPCFTHA or FLAGP425RMyc-His10 hPCFT. Co-association of WT/mutant hPCFT monomers was assayed with His SpinTrapTM columns (Fig. 6A).
Analogous to the WT hPCFT monomers, wthPCFTHA and FLAGP425RMyc-His10 hPCFT co-associated on His SpinTrapTM columns when co-expressed (Fig. 6A, lanes 6–8), establishing their association in higher order mutant-WT hetero-oligomeric complexes. Interestingly, in membrane transport assays with [3H]Mtx as transport substrate, net transport for combined wthPCFTHA and FLAGP425RMyc-His10 hPCFT was significantly increased (1.51 ± 0.12-fold; n = 7; p < 0.01 by paired t test), well above the level for wthPCFTHA alone, despite the fact that only wthPCFTHA was appreciably active in the single transfections (Fig. 4A). There were no significant differences in the relative levels of wthPCFTHA protein between the single and co-transfected samples on Western blots (Fig. 4B, lanes 3 and 6). Although there was a slight reproducible increase in the level of surface FLAGP425RMyc-His10 hPCFT (1.78 ± 0.27-fold; n = 4) in the presence of wthPCFTHA (Fig. 4B, lanes 5 and 6), based on its low level transport activity, this was insufficient to account for the disproportionate increase in net transport. Immunofluorescence staining of WT and mutant hPCFTs co-localized primarily at the cell surface, although intracellular structures were also stained, as noted above (Fig. 5B, g–i). There was a slight increased staining of FLAGP425RMyc-His10 hPCFT in the presence of wthPCFTHA (Fig. 5B, g) compared with FLAGP425RMyc-His10 hPCFT alone (f). These results imply that intracellular trafficking and surface expression of the inactive P425R hPCFT mutant are increased in the presence of WT PCFT.
Importantly, the transport data imply that the activity of the P425R mutant is at least partly restored via its association with a WT hPCFT monomer. To examine this possibility systematically, we co-expressed wtPCFTHA and FLAGP425RHA-Myc-His10 PCFT in defined ratios, based on amounts of plasmid DNA used for transfections (i.e. 0:1.0, 0.2:0.8, 0.3:0.7, 0.4:0.6, 0.5:0.5, 0.6:0.4, 0.7:0.3, 0.8:0.2, and 1.0:0). Surface hPCFT proteins were labeled with sulfo-NHS-SS-biotin, isolated, and deglycosylated, so that amounts of the WT and mutant hPCFT forms could be resolved by SDS-PAGE and quantified by Western blotting and densitometry. Net transport rates of [3H]Mtx were plotted versus the fractional compositions of WT hPCFT of the total (WT plus mutant) hPCFT forms. By this analysis, a linear relationship results if the monomers function independently of their oligomeric status (34, 51–54). However, if interactions (positive or negative) occur between WT and mutant monomers, the results should conform to a distinctly non-linear (quadratic) pattern, depending on whether the effect of WT/mutant co-association is dominant-positive or -negative and on the oligomerization state (dimer, trimer, etc.) (34, 51–54). Results with mutant and WT PCFT are shown in Fig. 8 for three independent experiments (as mean values ± S.E.) and clearly establish a strong positive deviation from linearity, suggesting cooperative interactions between hPCFT monomers that result in a net positive impact on carrier function, consistent with transport results in Fig. 4A.
FIGURE 8.
Dominant-positive interactions between WT and inactive P425R hPCFT. wtPCFTHA and FLAGP425RHA-Myc-His10 constructs were transiently transfected into R1-11 cells. The cells were assayed for transport with [3H]Mtx (0.5 μm) over 2 min at pH 5.5 and at 37 °C. Surface hPCFT proteins were labeled with sulfo-NHS-SS-biotin. The biotinylated proteins were isolated, deglycosylated, and analyzed by 4–20% Tris/glycine gels and Western blotting with HA-specific antibody, as described under “Experimental Procedures.” Relative levels of WT and P425R proteins were measured by densitometry. A shows the linear (dashed) line predicted for functionally non-interacting hPCFT monomers, as described under “Results.” The data shown are for transport activities in excess of the low residual level for P425R plotted against the fraction of WT hPCFT in the WT/P425R hPCFT mixtures, as calculated from densitometry measurements from three independent experiments. Results are mean values ± S.E. (error bars). Statistics and correlation coefficients were calculated by Prism software (version 4.0). A clear non-linear dose response is observed (solid line). B shows a representative Western blot of deglycosylated wtPCFTHA and FLAGP425RHA-Myc-His10 proteins. The calculated fractions of WT hPCFT to total hPCFT proteins from each sample are noted below each lane with S.E. values from three independent experiments in parentheses. Experimental details are provided under “Experimental Procedures”.
DISCUSSION
In this report, we used an assortment of powerful biochemical and molecular approaches to demonstrate the existence of hPCFT oligomers. (i) By protein cross-linking, oligomeric hPCFT appeared to predominate over monomeric carrier. (ii) On BN-PAGE, dimeric hPCFT was the major species. (iii) HA and FLAG/His10 epitope-tagged hPCFT proteins co-localized to plasma membranes in transiently transfected R1-11 HeLa cells. (iv) By co-expression of His10-tagged and HA-tagged hPCFT proteins and nickel affinity chromatography, clear evidence of association between hPCFT monomers was obtained. Co-folding and specificity of this hPCFT monomer association was confirmed by mixing individually expressed hPCFT monomers prior to nickel chromatography and by co-expressing His10-tagged hPCFT with HA-tagged human ThTr1. (v) Further, FRET was detected at the cell surface for YPet and ECFP*-tagged hPCFTs, confirming that hPCFT monomer associations occurred in situ. Collectively, these results argue for a physical association between hPCFT monomers and the existence of hPCFT oligomers at the cell surface. Although the involvement of associations of hPCFT with additional non-hPCFT proteins is also possible, the molecular masses of the higher order hPCFT complexes by protein cross-linking or by BN-PAGE imply the existence of homo-oligomeric hPCFT.
The functional impact of these associations was strongly implied by evidence of a dominant-positive phenotype when the inactive P425R hPCFT was co-expressed with WT hPCFT monomer. This appeared to involve co-folding and increased surface trafficking of P425R hPCFT to the cell surface, as reflected in increased surface levels of mutant hPCFT protein by surface biotinylation and Western blotting, and by indirect immunofluorescence and confocal microscopy. However, the net transport activity of combined P425R plus WT hPCFTs increased disproportionately to the levels of individual mutant/WT transport activities and to expression levels of WT and mutant hPCFT proteins. This was further tested by co-expressing P425R and WT hPCFTs in defined molar ratios, with levels of surface mutant and WT proteins measured by surface biotinylation and deglycosylation so that mutant and WT forms could be simultaneously detected on Western blots. In these experiments, when net transport was plotted versus the fraction of surface WT hPCFT (34, 51–53), a distinct positive non-linearity was detected, strongly implying functional “rescue” of the mutant monomer, independent of its intracellular trafficking but rather mediated allosterically via monomer interactions across the oligomer interface(s). Thus, functional cooperation between hPCFT monomers in facilitating transport of folate substrates appears likely.
Based on the “alternate access model” for secondary transporters such as Lac Y (60), we suggest an analogous reaction scheme for hPCFT-mediated transport adapted from that of Unal et al. (30), which incorporates a functional impact of hPCFT oligomerization. The scheme shown in Fig. 9 is based on a dimeric model for hPCFT suggested by our BN-PAGE results. We assume that both hPCFT monomers in homodimeric hPCFT undergo the transport cycle concurrently via six distinct stages. The scheme starts with an outward facing unloaded hPCFT dimer composed of two WT monomers. The ordered binding of co-transported protons (step 1) and substrates (step 2) to both monomers triggers a conformational change that results in a membrane transition of hPCFT monomers from an outward to the inward facing state (step 3). This is followed by an ordered release of substrates (step 4) and protons (step 5) from both monomers into the cytoplasm and, finally, return of both unloaded monomers to their outward facing state to complete the transport cycle (step 6). Intrinsic to this model is a functional cooperativity between associated hPCFT monomers that effect substrate and proton binding and release in tandem. Based on our results, we hypothesize that the association of WT monomer with inactive P425R effects a conformational shift that activates the inactive mutant monomer, permitting loading of both substrates and protons (steps 1 and 2), coincident movement to assume an inward facing state (step 3), and substrate and proton release (steps 4 and 5). Although it is possible that other hPCFT mutants may show increased activity when oligomerized with WT PCFT, analogous to P425R, this is by no means obligatory. Indeed, based on the scheme depicted in Fig. 9, a dominant-negative phenotype can as easily be envisaged. This, along with additional studies of the structural determinants and mechanistic impact of hPCFT oligomerization, will be the topic of future reports. Interestingly, the hPCFT primary structure includes GXXXG motifs in TMD 2 (amino acids 93–97) and TMD 4 (amino acids 155–159), analogous to “dimerization motifs” implicated in the oligomerization of other amphipathic proteins (61, 62).
FIGURE 9.
Proposed reaction scheme for hPCFT-mediated cellular uptake involving cooperative interactions between hPCFT monomers. Based on the “alternate access model” for secondary transporters, such as Lac Y (60), adapted from that of Unal et al. (30) for monomeric PCFT, an analogous reaction scheme is depicted for hPCFT-mediated transport that incorporates the functional impact of hPCFT oligomerization. The model starts from the outward facing unloaded dimer, followed by the ordered binding of the co-transported protons (step 1) and (anti)folate substrates (step 2), which triggers a conformational change resulting in simultaneous transition of the two hPCFT monomers to an inward facing state (step 3). This is followed by an ordered release of substrates (step 4) and then protons (step 5) into the cytoplasm. The unloaded homo-oligomeric unit then returns to the outward facing state (step 6) to complete the transport cycle. In this model, the two hPCFT monomers are suggested to function cooperatively in facilitating substrate and proton binding, conformational changes, and substrate and proton release. For further details, see “Discussion.”
Given the critical roles of hPCFT in intestinal absorption of dietary folates and of mutant hPCFT in HFM (5–15) and in the selective delivery of cytotoxic antifolates for targeting solid tumors (21, 24–26), our findings of a functionally important oligomerization for hPCFT are particularly significant. For instance, in HFM, our findings may explain why all HFM patients thus far described have mutations in both pcft gene alleles because loss of a single pcft allele on hPCFT function would probably not be detected (5–15). Although hPCFT mutations in other diseases have not been reported, based on previous experiences with RFC and Mtx (63), for hPCFT-selective antifolates (21, 24–26), such mechanisms seem likely. Drawing from our results with P425R hPCFT, better understanding of the structural and regulatory determinants of hPCFT oligomerization may lead to novel approaches for therapeutically rescuing functionally impaired hPCFT mutants, perhaps with hPCFT peptidomimetics or small molecules. These topics are under active investigation in this laboratory and will be reported elsewhere.
Acknowledgments
We thank Dr. I. David Goldman for the generous gift of RFC- and hPCFT-null R1-11 and RFC-null R5 HeLa cells. We thank Dr. Tomoo Ohashi (Duke University Medical Center) for providing the YPet-His and ECFP*-His cDNAs for the FRET experiments. We thank Dr. Ellis Neufeld (Children's Hospital Boston) for providing the human thiamine transport construct in pBlueScriptII KS(−).
This work was supported, in whole or in part, by National Institutes of Health (NIH), NCI, Grant R01 CA53535. The Microscopy, Imaging and Cytometry Resources Core is supported, in part, by NIH Center Grant P30CA22453 (to the Barbara Ann Karmanos Cancer Institute) and the Perinatology Research Branch of NICHD, NIH, Wayne State University.
- RFC
- reduced folate carrier
- BN-PAGE
- blue native PAGE
- CB
- Coomassie Blue
- HFM
- hereditary folate malabsorption
- hPCFT
- human proton-coupled folate transporter
- hRFC
- human reduced folate carrier
- MTS-1-MTS
- 1,1-methanediyl bismethanethiosulfonate
- Mtx
- methotrexate
- PCFT
- proton-coupled folate transporter
- sulfo-NHS-SS-biotin
- sulfo-N-hydroxysuccinimide-SS-biotin
- TMD
- transmembrane domain
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- ECFP*
- enhanced cyan fluorescent protein with K26R/N164H mutation
- mECFP*
- monomeric ECFP*
- mYPet
- monomeric YPet
- FRET
- fluorescence resonance energy transfer
- PVDF
- polyvidylidene difluoride
- PAGE
- polyacrylamide gel electrophoresis
- YPet
- FRET-optimized yellow fluorescent protein.
REFERENCES
- 1. Stokstad E. L. R. (ed) (1990) Historical Perspective on Key Advances in the Biochemistry and Physiology of Folates, pp. 1–21, Wiley-Liss, New York [Google Scholar]
- 2. Zhao R., Matherly L. H., Goldman I. D. (2009) Membrane transporters and folate homeostasis. Intestinal absorption and transport into systemic compartments and tissues. Expert. Rev. Mol. Med. 11, e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhao R., Goldman I. D. (2007) The molecular identity and characterization of a proton-coupled folate transporter–PCFT. Biological ramifications and impact on the activity of pemetrexed. Cancer Metastasis Rev. 26, 129–139 [DOI] [PubMed] [Google Scholar]
- 4. Nakai Y., Inoue K., Abe N., Hatakeyama M., Ohta K. Y., Otagiri M., Hayashi Y., Yuasa H. (2007) Functional characterization of human proton-coupled folate transporter/heme carrier protein 1 heterologously expressed in mammalian cells as a folate transporter. J. Pharmacol. Exp. Ther. 322, 469–476 [DOI] [PubMed] [Google Scholar]
- 5. Qiu A., Jansen M., Sakaris A., Min S. H., Chattopadhyay S., Tsai E., Sandoval C., Zhao R., Akabas M. H., Goldman I. D. (2006) Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell 127, 917–928 [DOI] [PubMed] [Google Scholar]
- 6. Zhao R., Min S. H., Qiu A., Sakaris A., Goldberg G. L., Sandoval C., Malatack J. J., Rosenblatt D. S., Goldman I. D. (2007) The spectrum of mutations in the PCFT gene, coding for an intestinal folate transporter, that are the basis for hereditary folate malabsorption. Blood 110, 1147–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Min S. H., Oh S. Y., Karp G. I., Poncz M., Zhao R., Goldman I. D. (2008) The clinical course and genetic defect in the PCFT gene in a 27-year-old woman with hereditary folate malabsorption. J. Pediatr. 153, 435–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lasry I., Berman B., Straussberg R., Sofer Y., Bessler H., Sharkia M., Glaser F., Jansen G., Drori S., Assaraf Y. G. (2008) A novel loss-of-function mutation in the proton-coupled folate transporter from a patient with hereditary folate malabsorption reveals that Arg-113 is crucial for function. Blood 112, 2055–2061 [DOI] [PubMed] [Google Scholar]
- 9. Borzutzky A., Crompton B., Bergmann A. K., Giliani S., Baxi S., Martin M., Neufeld E. J., Notarangelo L. D. (2009) Reversible severe combined immunodeficiency phenotype secondary to a mutation of the proton-coupled folate transporter. Clin. Immunol. 133, 287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meyer E., Kurian M. A., Pasha S., Trembath R. C., Cole T., Maher E. R. (2010) A novel PCFT gene mutation (p.Cys66LeufsX99) causing hereditary folate malabsorption. Mol. Genet. Metab. 99, 325–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Atabay B., Turker M., Ozer E. A., Mahadeo K., Diop-Bove N., Goldman I. D. (2010) Mutation of the proton-coupled folate transporter gene (PCFT-SLC46A1) in Turkish siblings with hereditary folate malabsorption. Pediatr. Hematol. Oncol. 27, 614–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mahadeo K., Diop-Bove N., Shin D., Unal E. S., Teo J., Zhao R., Chang M. H., Fulterer A., Romero M. F., Goldman I. D. (2010) Properties of the Arg-376 residue of the proton-coupled folate transporter (PCFT-SLC46A1) and a glutamine mutant causing hereditary folate malabsorption. Am. J. Physiol. Cell Physiol. 299, C1153–C1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shin D. S., Min S. H., Russell L., Zhao R., Fiser A., Goldman I. D. (2010) Functional roles of aspartate residues of the proton-coupled folate transporter (PCFT-SLC46A1). A D156Y mutation causing hereditary folate malabsorption. Blood 116, 5162–5169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mahadeo K. M., Diop-Bove N., Ramirez S. I., Cadilla C. L., Rivera E., Martin M., Lerner N. B., DiAntonio L., Duva S., Santiago-Borrero P. J., Goldman I. D. (2011) Prevalence of a loss-of-function mutation in the proton-coupled folate transporter gene (PCFT-SLC46A1) causing hereditary folate malabsorption in Puerto Rico. J. Pediatr. 159, 623–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shin D. S., Mahadeo K., Min S. H., Diop-Bove N., Clayton P., Zhao R., Goldman I. D. (2011) Identification of novel mutations in the proton-coupled folate transporter (PCFT-SLC46A1) associated with hereditary folate malabsorption. Mol. Genet. Metab. 103, 33–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao R., Qiu A., Tsai E., Jansen M., Akabas M. H., Goldman I. D. (2008) The proton-coupled folate transporter. Impact on pemetrexed transport and on antifolate activities compared with the reduced folate carrier. Mol. Pharmacol. 74, 854–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vogelzang N. J., Rusthoven J. J., Symanowski J., Denham C., Kaukel E., Ruffie P., Gatzemeier U., Boyer M., Emri S., Manegold C., Niyikiza C., Paoletti P. (2003) Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J. Clin. Oncol. 21, 2636–2644 [DOI] [PubMed] [Google Scholar]
- 18. Hanna N., Shepherd F. A., Fossella F. V., Pereira J. R., De Marinis F., von Pawel J., Gatzemeier U., Tsao T. C., Pless M., Muller T., Lim H. L., Desch C., Szondy K., Gervais R., Shaharyar, Manegold C., Paul S., Paoletti P., Einhorn L., Bunn P. A., Jr. (2004) Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J. Clin. Oncol. 22, 1589–1597 [DOI] [PubMed] [Google Scholar]
- 19. Ciuleanu T., Brodowicz T., Zielinski C., Kim J. H., Krzakowski M., Laack E., Wu Y. L., Bover I., Begbie S., Tzekova V., Cucevic B., Pereira J. R., Yang S. H., Madhavan J., Sugarman K. P., Peterson P., John W. J., Krejcy K., Belani C. P. (2009) Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer. A randomized, double-blind, phase 3 study. Lancet 374, 1432–1440 [DOI] [PubMed] [Google Scholar]
- 20. Zhao R., Gao F., Hanscom M., Goldman I. D. (2004) A prominent low-pH methotrexate transport activity in human solid tumors. Contribution to the preservation of methotrexate pharmacologic activity in HeLa cells lacking the reduced folate carrier. Clin. Cancer Res. 10, 718–727 [DOI] [PubMed] [Google Scholar]
- 21. Kugel Desmoulin S., Wang L., Hales E., Polin L., White K., Kushner J., Stout M., Hou Z., Cherian C., Gangjee A., Matherly L. H. (2011) Therapeutic targeting of a novel 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl antifolate to human solid tumors based on selective uptake by the proton-coupled folate transporter. Mol. Pharmacol. 80, 1096–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Helmlinger G., Yuan F., Dellian M., Jain R. K. (1997) Interstitial pH and pO2 gradients in solid tumors in vivo. High-resolution measurements reveal a lack of correlation. Nat. Med. 3, 177–182 [DOI] [PubMed] [Google Scholar]
- 23. Raghunand N., Altbach M. I., van Sluis R., Baggett B., Taylor C. W., Bhujwalla Z. M., Gillies R. J. (1999) Plasmalemmal pH gradients in drug-sensitive and drug-resistant MCF-7 human breast carcinoma xenografts measured by 31P magnetic resonance spectroscopy. Biochem. Pharmacol. 57, 309–312 [DOI] [PubMed] [Google Scholar]
- 24. Desmoulin S. K., Wang Y., Wu J., Stout M., Hou Z., Fulterer A., Chang M. H., Romero M. F., Cherian C., Gangjee A., Matherly L. H. (2010) Targeting the proton-coupled folate transporter for selective delivery of 6-substituted pyrrolo[2,3-d]pyrimidine antifolate inhibitors of de novo purine biosynthesis in the chemotherapy of solid tumors. Mol. Pharmacol. 78, 577–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang L., Cherian C., Desmoulin S. K., Polin L., Deng Y., Wu J., Hou Z., White K., Kushner J., Matherly L. H., Gangjee A. (2010) Synthesis and antitumor activity of a novel series of 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl antifolate inhibitors of purine biosynthesis with selectivity for high affinity folate receptors and the proton-coupled folate transporter over the reduced folate carrier for cellular entry. J. Med. Chem. 53, 1306–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang L., Desmoulin S. K., Cherian C., Polin L., White K., Kushner J., Fulterer A., Chang M. H., Mitchell-Ryan S., Stout M., Romero M. F., Hou Z., Matherly L. H., Gangjee A. (2011) Synthesis, biological, and antitumor activity of a highly potent 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl antifolate inhibitor with proton-coupled folate transporter and folate receptor selectivity over the reduced folate carrier that inhibits β-glycinamide ribonucleotide formyltransferase. J. Med. Chem. 54, 7150–7164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhao R., Unal E. S., Shin D. S., Goldman I. D. (2010) Membrane topological analysis of the proton-coupled folate transporter (PCFT-SLC46A1) by the substituted cysteine accessibility method. Biochemistry 49, 2925–2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Unal E. S., Zhao R., Qiu A., Goldman I. D. (2008) N-Linked glycosylation and its impact on the electrophoretic mobility and function of the human proton-coupled folate transporter (HsPCFT). Biochim. Biophys. Acta 1778, 1407–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qiu A., Min S. H., Jansen M., Malhotra U., Tsai E., Cabelof D. C., Matherly L. H., Zhao R., Akabas M. H., Goldman I. D. (2007) Rodent intestinal folate transporters (SLC46A1). Secondary structure, functional properties, and response to dietary folate restriction. Am. J. Physiol. Cell Physiol. 293, C1669–C1678 [DOI] [PubMed] [Google Scholar]
- 30. Unal E. S., Zhao R., Goldman I. D. (2009) Role of the glutamate 185 residue in proton translocation mediated by the proton-coupled folate transporter SLC46A1. Am. J. Physiol. Cell Physiol. 297, C66–C74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Unal E. S., Zhao R., Chang M. H., Fiser A., Romero M. F., Goldman I. D. (2009) The functional roles of the His-247 and His-281 residues in folate and proton translocation mediated by the human proton-coupled folate transporter SLC46A1. J. Biol. Chem. 284, 17846–17857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lasry I., Berman B., Glaser F., Jansen G., Assaraf Y. G. (2009) Hereditary folate malabsorption. A positively charged amino acid at position 113 of the proton-coupled folate transporter (PCFT/SLC46A1) is required for folic acid binding. Biochem. Biophys. Res. Commun. 386, 426–431 [DOI] [PubMed] [Google Scholar]
- 33. Subramanian V. S., Marchant J. S., Said H. M. (2008) Apical membrane targeting and trafficking of the human proton-coupled transporter in polarized epithelia. Am. J. Physiol. Cell Physiol. 294, C233–C240 [DOI] [PubMed] [Google Scholar]
- 34. Veenhoff L. M., Heuberger E. H., Poolman B. (2001) The lactose transport protein is a cooperative dimer with two sugar translocation pathways. EMBO J. 20, 3056–3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dahl N. K., Jiang L., Chernova M. N., Stuart-Tilley A. K., Shmukler B. E., Alper S. L. (2003) Deficient HCO3− transport in an AE1 mutant with normal Cl− transport can be rescued by carbonic anhydrase II presented on an adjacent AE1 protomer. J. Biol. Chem. 278, 44949–44958 [DOI] [PubMed] [Google Scholar]
- 36. Taylor A. M., Zhu Q., Casey J. R. (2001) Cysteine-directed cross-linking localizes regions of the human erythrocyte anion-exchange protein (AE1) relative to the dimeric interface. Biochem. J. 359, 661–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zottola R. J., Cloherty E. K., Coderre P. E., Hansen A., Hebert D. N., Carruthers A. (1995) Glucose transporter function is controlled by transporter oligomeric structure. A single, intramolecular disulfide promotes GLUT1 tetramerization. Biochemistry 34, 9734–9747 [DOI] [PubMed] [Google Scholar]
- 38. Yin C. C., Aldema-Ramos M. L., Borges-Walmsley M. I., Taylor R. W., Walmsley A. R., Levy S. B., Bullough P. A. (2000) The quarternary molecular architecture of TetA, a secondary tetracycline transporter from Escherichia coli. Mol. Microbiol. 38, 482–492 [DOI] [PubMed] [Google Scholar]
- 39. Hickman R. K., Levy S. B. (1988) Evidence that TET protein functions as a multimer in the inner membrane of Escherichia coli. J. Bacteriol. 170, 1715–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hou Z., Matherly L. H. (2009) Oligomeric structure of the human reduced folate carrier. Identification of homo-oligomers and dominant-negative effects on carrier expression and function. J. Biol. Chem. 284, 3285–3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Payton S. G., Haska C. L., Flatley R. M., Ge Y., Matherly L. H. (2007) Effects of 5′-untranslated region diversity on the posttranscriptional regulation of the human reduced folate carrier. Biochim. Biophys. Acta 1769, 131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fleming J. C., Tartaglini E., Steinkamp M. P., Schorderet D. F., Cohen N., Neufeld E. J. (1999) The gene mutated in thiamine-responsive anemia with diabetes and deafness (TRMA) encodes a functional thiamine transporter. Nat. Genet. 22, 305–308 [DOI] [PubMed] [Google Scholar]
- 43. Ohashi T., Galiacy S. D., Briscoe G., Erickson H. P. (2007) An experimental study of GFP-based FRET, with application to intrinsically unstructured proteins. Protein Sci. 16, 1429–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zacharias D. A., Violin J. D., Newton A. C., Tsien R. Y. (2002) Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296, 913–916 [DOI] [PubMed] [Google Scholar]
- 45. Hou Z., Stapels S. E., Haska C. L., Matherly L. H. (2005) Localization of a substrate binding domain of the human reduced folate carrier to transmembrane domain 11 by radioaffinity labeling and cysteine-substituted accessibility methods. J. Biol. Chem. 280, 36206–36213 [DOI] [PubMed] [Google Scholar]
- 46. Matherly L. H., Czajkowski C. A., Angeles S. M. (1991) Identification of a highly glycosylated methotrexate membrane carrier in K562 human erythroleukemia cells up-regulated for tetrahydrofolate cofactor and methotrexate transport. Cancer Res. 51, 3420–3426 [PubMed] [Google Scholar]
- 47. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 48. Wouters F. S., Verveer P. J., Bastiaens P. I. (2001) Imaging biochemistry inside cells. Trends Cell Biol. 11, 203–211 [DOI] [PubMed] [Google Scholar]
- 49. Witt T. L., Stapels S. E., Matherly L. H. (2004) Restoration of transport activity by co-expression of human reduced folate carrier half-molecules in transport-impaired K562 cells. Localization of a substrate binding domain to transmembrane domains 7–12. J. Biol. Chem. 279, 46755–46763 [DOI] [PubMed] [Google Scholar]
- 50. Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 [PubMed] [Google Scholar]
- 51. Bamber L., Harding M., Monné M., Slotboom D. J., Kunji E. R. (2007) The yeast mitochondrial ADP/ATP carrier functions as a monomer in mitochondrial membranes. Proc. Natl. Acad. Sci. U.S.A. 104, 10830–10834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. MacKinnon R. (1991) Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature 350, 232–235 [DOI] [PubMed] [Google Scholar]
- 53. Yerushalmi H., Lebendiker M., Schuldiner S. (1996) Negative dominance studies demonstrate the oligomeric structure of EmrE, a multidrug antiporter from Escherichia coli. J. Biol. Chem. 271, 31044–31048 [DOI] [PubMed] [Google Scholar]
- 54. Hou Z., Cherian C., Drews J., Wu J., Matherly L. H. (2010) Identification of the minimal functional unit of the homo-oligomeric human reduced folate carrier. J. Biol. Chem. 285, 4732–4740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bisetto E., Giorgio V., Di Pancrazio F., Mavelli I., Lippe G. (2007) Characterization of oligomeric forms from mammalian F0F1-ATP synthase by BN-PAGE. The role of detergents. Ital. J. Biochem. 56, 254–258 [PubMed] [Google Scholar]
- 56. Swamy M., Siegers G. M., Minguet S., Wollscheid B., Schamel W. W. (2006) Blue native polyacrylamide gel electrophoresis (BN-PAGE) for the identification and analysis of multiprotein complexes. Sci. STKE 2006, pl4. [DOI] [PubMed] [Google Scholar]
- 57. Heuberger E. H., Veenhoff L. M., Duurkens R. H., Friesen R. H., Poolman B. (2002) Oligomeric state of membrane transport proteins analyzed with blue native electrophoresis and analytical ultracentrifugation. J. Mol. Biol. 317, 591–600 [DOI] [PubMed] [Google Scholar]
- 58. Tsien R. Y. (1998) The green fluorescent protein. Annu. Rev. Biochem. 67, 509–544 [DOI] [PubMed] [Google Scholar]
- 59. Shaner N. C., Steinbach P. A., Tsien R. Y. (2005) A guide to choosing fluorescent proteins. Nat. Methods 2, 905–909 [DOI] [PubMed] [Google Scholar]
- 60. Abramson J., Smirnova I., Kasho V., Verner G., Kaback H. R., Iwata S. (2003) Structure and mechanism of the lactose permease of Escherichia coli. Science 301, 610–615 [DOI] [PubMed] [Google Scholar]
- 61. Polgar O., Ierano C., Tamaki A., Stanley B., Ward Y., Xia D., Tarasova N., Robey R. W., Bates S. E. (2010) Mutational analysis of threonine 402 adjacent to the GXXXG dimerization motif in transmembrane segment 1 of ABCG2. Biochemistry 49, 2235–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Duan P., Wu J., You G. (2011) Mutational analysis of the role of GXXXG motif in the function of human organic anion transporter 1 (hOAT1). Int. J. Biochem. Mol. Biol. 2, 1–7 [PMC free article] [PubMed] [Google Scholar]
- 63. Matherly L. H., Hou Z., Deng Y. (2007) Human reduced folate carrier. Translation of basic biology to cancer etiology and therapy. Cancer Metastasis Rev. 26, 111–128 [DOI] [PubMed] [Google Scholar]