Abstract
Type VI secretion systems (T6SSs) are involved in the pathogenicity of several Gram-negative bacteria. Based on sequence analysis, we found that a cluster of Escherichia coli virulence factors (EVF) encoding a putative T6SS exists in the genome of the meningitis-causing E. coli K1 strain RS218. The T6SS-associated deletion mutants exhibited significant defects in binding to and invasion of human brain microvascular endothelial cells (HBMEC) compared with the parent strain. Hcp family proteins (the hallmark of T6SS), including Hcp1 and Hcp2, were localized in the bacterial outer membrane, but the involvements of Hcp1 and Hcp2 have been shown to differ in E. coli-HBMEC interaction. The deletion mutant of hcp2 showed defects in the bacterial binding to and invasion of HBMEC, while Hcp1 was secreted in a T6SS-dependent manner and induced actin cytoskeleton rearrangement, apoptosis, and the release of interleukin-6 (IL-6) and IL-8 in HBMEC. These findings demonstrate that the T6SS is functional in E. coli K1, and two Hcp family proteins participate in different steps of E. coli interaction with HBMEC in a coordinate manner, e.g., binding to and invasion of HBMEC, the cytokine and chemokine release followed by cytoskeleton rearrangement, and apoptosis in HBMEC. This is the first demonstration of the role of T6SS in meningitis-causing E. coli K1, and T6SS-associated Hcp family proteins are likely to contribute to the pathogenesis of E. coli meningitis.
INTRODUCTION
Escherichia coli is the leading cause of neonatal Gram-negative bacterial meningitis, but the pathogenesis of neonatal E. coli meningitis remains incompletely understood. Previous studies showed that E. coli K1 penetration into the brain, the essential step in the development of meningitis, requires a high level of bacteremia and E. coli binding to and invasion of human brain microvascular endothelial cells (HBMEC), which constitute the blood-brain barrier (BBB) (20, 27). Several E. coli determinants have been identified to contribute to meningitis, which include the K1 capsule, Ibe proteins, OmpA, type 1 fimbriae, flagella, CNF1, and NlpI (17, 18, 19, 27, 29, 39, 40, 46), but the pathogenesis of E. coli meningitis has not been completely elucidated.
Recently, a new secretion system, named the type VI secretion system (T6SS), was identified and characterized in several Gram-negative pathogens (7, 9, 13, 26, 31, 44). Based on current information, the T6SS represents a complex secretion machinery and contributes to pathogenicity in many bacteria (24, 26, 31, 34, 37, 50). Hcp (hemolysin-coregulated protein) and VgrG (valine glycine repeat) may represent the components or effector proteins of T6SS (3, 30, 31). The ATP hydrolytic activity of ClpV forms oligomeric complexes to energize the system for Hcp1 secretion (26). IcmF, as a component of the T6SS apparatus, is required for the function of T6SS, and DotU is essential for the secretion function through stabilizing the multiprotein complex in the membrane (3, 44). The data from enteroaggregative E. coli (EAEC) showed that the T6SS in a 117-kb pathogenicity island may be an important mediator involved in aggregative adherence to host cell surfaces (7).
In silico analysis showed that more than 10 orthologs of known T6SS components are present in most genome-se-quenced pathogenic E. coli strains, including enterohemorrhagic Escherichia coli (EHEC) strains EDL933 and Sakai, enteropathogenic Escherichia coli (EPEC) strain B171, uropathogenic Escherichia coli (UPEC) strains 536, UTI89, and CFT073, avian pathogenic Escherichia coli (APEC) strain APEC01, EAEC strain 17-2, and neonatal meningitis Escherichia coli (NMEC) strains S88 and IHE3034 (GenBank accession no. NC011742 and CP001969) (1, 23, 25, 35). Our previous comparative genome hybridization (CGH) analysis revealed that T6SS-like gene clusters, including the icmF-like component, clpV, dotU, and hcp2, are present in the RS218-derived genomic island 1 (RDI-1) region of strain RS218 (47). Of interest, the T6SS clusters have two hcp-like genes located next to each other in the chromosome of strain RS218, while hcp genes are dispersed in other bacterial genomes (7, 44). Hcp protein was first identified as a major T6SS-associated protein in Vibrio cholerae (14, 31) and was involved in forming a transportation channel between the inner and outer membranes of the bacterium as a component protein in a hexameric ring-like structure (22, 28, 35). On the other hand, Hcp is considered a secreted protein with various roles in different bacteria (7, 13, 31, 32, 43, 44). Hcp was thought to be responsible for cytotoxicity in Dictyostelium amoebae and J774 murine macrophages during V. cholerae infection (31), and specific pathogenic roles of Hcp or Hcp-like proteins were demonstrated in several pathogenic bacteria. For example, Hcp may play a role in facilitating efficient tumorigenesis in Agrobacterium tumefaciens, and Hcp1 was detected in the sera of cystic fibrosis patients and produced in vivo during Burkholderia mallei infecting humans or horses (26, 34, 44). However, little is known about the function of Hcp in extraintestinal pathogenic E. coli, especially in meningitis-causing E. coli K1.
In this study, the Hcp family proteins' secretion pathway was identified in the meningitis-causing E. coli K1 strain, and their roles in E. coli K1 interaction with HBMEC were investigated. We determined (i) roles of T6SS and Hcp family proteins in bacterial binding to and invasion of HBMEC; (ii) Hcp family proteins' secretion pathway and their cellular localization; and (iii) cytotoxic effects of Hcp family proteins in HBMEC. Our results indicate that the two Hcp family proteins have different roles in meningitis-causing E. coli K1 infection and coordinately contribute to the pathogenicity of E. coli K1 interaction with HBMEC.
MATERIALS AND METHODS
Cell and bacterial culture.
HBMEC were grown in RPMI 1640 containing 10% heat-inactivated fetal bovine serum (FBS), 10% Nu-serum (Gibco), 2 mM glutamine, 1 mM pyruvate, penicillin (100 μg/ml), streptomycin (100 μg/ml), 1% essential amino acids (Gibco), and 1% vitamins (Gibco). All E. coli K1 strains were grown at 37°C in Luria-Bertani (LB) broth with appropriate antibiotics and shaking. All E. coli strains and plasmids used in this study are listed in Table 1.
Table 1.
Strains and plasmids used in the current study
| Strain or plasmid | Characteristic(s) | Source |
|---|---|---|
| Strains | ||
| RS218 | O18:K1:H7; a clinical isolate from the cerebrospinal fluid of a newborn infant with meningitis | Laboratory stock |
| ΔT6SS | RS218 mutant with T6SS core component gene clusters deletion | This study |
| ΔRDI-1 | RS218 mutant with whole RDI-1 gene clusters deletion | Laboratory stock |
| ΔevfC | RS218 mutant with evfC gene deletion | This study |
| Δhcp1 | RS218 mutant with hcp1 deletion | This study |
| Δhcp2 | RS218 mutant with hcp2 deletion | This study |
| Δhcps | RS218 mutant with hcp1 and hcp2 deletion | This study |
| pAC-evfC/ΔevfC | Complementation for ΔevfC using pACYC184 as the vector | This study |
| pAC-hcp2/Δhcp2 | Complementation for Δhcp2 using pACYC184 as the vector | This study |
| Plasmids | ||
| pACYC184 | pACYC184 containing chloramphenicol resistance gene | Laboratory stock |
| pAC-evfC | evfC coding region was cloned into the BamHI and SalI sites of pACYC184 | This study |
| pAC-hcp2 | hcp2 coding region was cloned into the ScaI and EcoRI sites of pACYC184 | This study |
| pET22b-hcp1 | E. coli overexpression vector to generate Hcp1 purified protein in BL21 | This study |
| pQE80-hcp1 | E. coli overexpression vector to generate C-terminal His-tagged protein Hcp1 in RS218 | This study |
| pQE80-hcp2 | E. coli overexpression vector to generate C-terminal His-tagged protein Hcp2 in RS218 | This study |
| pCX-hcp1 | hcp1 coding region was cloned into the EcoRI and NdeI sites of pCX340; tetracycline resistance | This study |
| pCX-hcp2 | hcp2 coding region was cloned into the EcoRI and KpnI sites of pCX340; tetracycline resistance | This study |
| pQE80L | E. coli overexpression vector | Qiagen |
Construction of isogenic mutants and plasmids.
The construction of the deletion mutants in E. coli was done as previously described (5). hcp2 and evfC genes were ligated into the pACYC184 vector, and the resultant plasmids were used for complementation assays. His-tagged Hcp1 and Hcp2 proteins were expressed from pET22b-hcp1 and pQE80-hcp2, respectively. The cytosol and outer membrane fractions markers, Crp (cyclic AMP [cAMP] receptor protein) and OmpA proteins, were prepared from pQE80-crp and pET22b-ompA, respectively. All primers used in this study are listed in Table S1 in the supplemental material.
β-Lactamase fusion construction.
pCX-hcp1 and pCX-hcp2, which were used in the β-lactamase fusion assay and protein translocation assay, were constructed by following our previous protocols (49). blaM together with its upstream multiple cloning sites was cloned from pCX340 into pRS (a derivative of pSR; the difference is that in pRS, the R6kγ replication origin is upstream of the spectinomycin resistance gene), yielding pFBI (for fuse Bla in frame) (49). The hcp1 and hcp2 genes then were amplified from RS218 genomic DNA with two pairs of primers (hcp1-EcoR and hcp1-Nde-B as well as hcp2-Kpn and hcp2-EcoR [see Table S1 in the supplemental material]) and cloned into the EcoRI and NdeI or EcoRI and KpnI sites of plasmid pFBI, respectively, which gave the Hcp1-Bla and Hcp2-Bla fusion plasmids pCX-hcp1 and pCX-hcp2, respectively.
Expression and purification of His-tagged protein and polyclonal antibody preparation.
Protein expression and purification were carried out as described previously (27). Purified protein was injected into rabbits with Freund's complete adjuvant, followed by mixture with Freund's incomplete adjuvant (Sigma) for the production of polyclonal antiserum as described previously (16).
Isolation of the secretory proteins.
Secretory proteins were prepared by a previously described method with modifications (12). E. coli strains grown overnight were diluted 1:100 into M-9 minimal medium supplemented with 44 mM NaHCO3, 8 mM MgSO4, 0.4% glucose, and 0.1% Casamino Acids. Secretory proteins were harvested by centrifugation at 8,000 × g for 30 min at 4°C and were passed through 0.45-μm filters to remove bacterial cell contamination. Later, proteins in the supernatant were concentrated using 5,000-molecular-weight-cutoff spin columns (Millipore), and the concentrations of the wild-type strain and T6SS-associated mutant supernatants were assessed using a bicinchoninic acid (BCA) protein quantification kit. Fifteen μg protein was loaded into each lane for SDS-PAGE, followed by Western blot analysis.
Western blot analysis.
Samples (proteins were prepared as described above) in SDS sample buffer were boiled for 5 min, centrifuged for 2 min at 12,000 × g, and loaded onto 12% polyacrylamide gels. Proteins were transferred to nitrocellulose membranes. Membranes were blocked using 5% nonfat milk in phosphate-buffered saline (PBS) containing 0.1% Tween (PBST) and probed with a mouse anti-β-actin antibody (1:2,000; Sigma), a mouse anti-His antibody (1:2,000; Tanon), or a rabbit polyclonal antibody of Hcp1 or Hcp2 (1:5,000; this study) overnight at 4°C. Blots were washed with PBST 4 times for 5 min each. They then were probed with horseradish peroxidase (HRP)-conjugated secondary antibody (1:10,000; Sigma) at room temperature for 1 h and washed with PBST 4 times for 5 min each. The blots were developed with Super Signal West Pico chemiluminescence substrate (Pierce) followed by O-MAT X-ray film exposure.
β-Lactamase activity assay.
Bacteria were grown in brain heart infusion broth (BD-Difco) with the appropriate antibiotic and 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 37°C overnight with agitation and then centrifuged at 12,000 rpm for 15 min. Ten μl nitrocefin stock solution was added to 100 μl supernatant from each strain, and then the mixture was incubated at room temperature for up to 15 min to allow red color to develop. Spectrophotometric assays for Bla were carried out by measuring changes in absorbance at 486 nm. Nitrocefin is a chromogenic β-lactamase substrate that undergoes distinctive color change from yellow (maximum wavelength [λmax] of 390 nm at pH 7.0) to red (λmax of 486 nm at pH 7.0) as the amide bond in the β-lactam ring is hydrolyzed by β-lactamase.
Immunofluorescence assays.
Chamber slides were seeded with 2 × 104 cells per well and incubated at 37°C in 5% CO2 overnight, and serial dilutions of a freshly thawed sample of nickel-purified His-Hcp1 were added to each well. The mixtures were incubated for an additional 3, 17, and 42 h at 37°C in 5% CO2 and later were fixed for 20 min at room temperature. Following fixation, the cells were stained by phalloidin conjugate (Shengyou Biotechnology) and incubated for 45 min in the dark at room temperature. Chamber slides were photographed on an Olympus microscope model BX60 fitted with an Olympus photomicrographic system model PM-30.
Association and invasion assays in HBMEC.
E. coli invasion assays were performed using a gentamicin protection assay as described previously (39, 48). The invasion frequency was calculated by dividing the number of invaded bacteria by the CFU of the original inoculum. Results were presented as the relative invasion frequency compared to the invasion frequency of wild-type RS218, which was arbitrarily set at 100%. Association assays were performed similarly to the invasion assay, except that the gentamicin treatment step was omitted.
Flow cytometry assessment of apoptosis in vitro.
In vitro apoptosis activity was measured in triplicate in a 6-well microplate (BD Biosciences) as follows. HBMEC (2 × 105) were incubated with Hcp1 and Hcp2 proteins for 24 h at 37°C. Five microliters of annexin V-fluorescein isothiocyanate (FITC) from the apoptosis detection kit (BD Pharmingen) was added to the cell suspension and incubated at room temperature for 10 min, followed by the addition of PI (propidium iodide) and incubation at room temperature for 10 min. PI- and annexin V-treated cells were designated early apoptotic and late apoptotic cells, respectively (33).
Analysis of caspase 8 activity.
Caspase 8 activity was measured by a caspase 8 activity assay kit according to the manufacturer's instructions (Beyotime) using Ac-IETD-pNA (N-acetyl-Ile-Glu-Thr-Asp-p-nitroanilide) as the substrate. Briefly, 80 μl lysate protein of HBMEC (1 to 3 mg/ml) was mixed with 10 μl of 2 mM Ac-IETD-pNA substrate and 10 μl reaction buffer. After 2 h of incubation at 37°C, caspase 8 activity was measured in a luminescence spectrophotometer (LS-50) (λ, 405 nm).
Cytokine assessment of HBMEC supernatants.
HBMEC (1 × 106) were grown in 6-well plates and incubated with 50 μg/ml Hcp family proteins per well for 6 h at 37°C in a 5% CO2 incubator. Supernatants were collected and centrifuged to remove cell debris. The cytokine levels in the supernatant were determined by following the eBioscience FlowCytomix multiple analyte detection kit instructions. The FlowCytomix Pro software was used for data analysis (eBioscience).
Statistics.
Statistical analyses were performed using Prism 5 (GraphPad). One-tailed, paired t tests were used to determine P values.
RESULTS
The T6SS gene cluster exists in the genome of meningitis-causing E. coli K1 strains, including strain RS218.
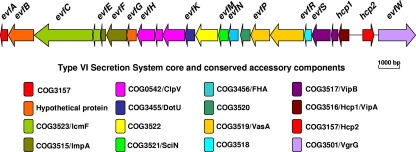
Genome sequence analysis showed that a gene cluster (GenBank accession number JN837480) encoding a putative T6SS exists in the genome of E. coli K1 strain RS218, named the evf cluster (E. coli virulence factors) (Fig. 1). This cluster contains evfC, evfH, evfK, hcp1, hcp2, and evfW, which are homologous to vasK (icmF-like), vasH (clpV homolog), vasF (dotU homolog), hcp1, hcp2, and vgrG genes of V. cholerae, respectively, and they have been shown to represent core and conserved accessory components in the T6SS. It has been shown that the open reading frame (ORF) orders of the T6SS gene clusters in different bacteria vary, although they share core ORFs (35). In this work, we found that the ORF order and component genes of the T6SS in E. coli strain RS218 are different from those of other bacteria, including Vibrio, Yersinia, and Pseudomonas. The demonstration of the two hcp-like genes located next to each other in the chromosome of E. coli K1 strain RS218 has not been found in other bacteria, whose hcp genes are dispersed in the chromosomes. In addition, the T6SS in RS218 contains some unique unknown genes, including evfB, evfG, evfL, evfO, and evfR, implying that they play some unknown roles in the function of the newly identified T6SS in E. coli K1 RS218. The T6SS in strain RS218 is located in the genomic island called RDI-1 and adjacent to the tRNA gene aspV (45), suggesting that this gene cluster is acquired by horizontal gene transfer (HGT). Our previous CGH study showed that 8 of 10 representative meningitis-causing E. coli K1 isolates harbor the whole T6SS cluster, while the remaining 2 strains contain part of the T6SS gene cluster (Table 2), suggesting that T6SS genes are prevalent in meningitis-causing E. coli K1 isolates (47).
Fig 1.
Schematic diagram of the genetic organization of RS218 T6SS gene clusters. The T6SS whole-gene cluster of ORFs with different colors and different direction arrows is shown in the diagram. RS218 evfC, evfH, evfK, hcp1, hcp2, and evfW are the homologs of known vasK, clpV, dotU, hcp1, hcp2, and vgrG genes in V. cholerae, respectively. The database of Clusters of Orthologous Groups of proteins (COGs) was obtained from the National Center of Biotechnology Information (http://www.ncbi.nlm.nih.gov/COG/new/).
Table 2.
Distribution of selected T6SS coding genes among 10 E. coli K1 strainsa
| ORF | Annotation | Presence of T6SS coding genes in strainsb |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RS218 | C5 | IHE3034 | S88 | S95 | A90 | RS167 | E334 | EC10 | RS168 | ||
| hcp2 | Homolysin-coregulated protein | + | + | + | + | + | + | + | + | − | +/− |
| evfS | T4 phage gene 25-like lysozyme | + | + | + | + | + | + | + | + | − | − |
| evfN | Signal transduction | + | + | + | + | + | + | + | + | − | − |
| evfM | Lipoprotein | + | + | + | + | + | + | + | + | − | − |
| evfK | Putative membrane protein | + | + | + | + | + | + | + | + | − | − |
| evfG | Hypothetical protein | + | + | + | + | + | + | + | + | − | − |
| evfF | Hypothetical protein | + | + | + | + | + | + | + | + | +/− | +/− |
| evfC | IcmF-like protein | + | + | + | + | + | + | + | + | +/− | +/− |
| evfB | Hypothetical protein | + | + | + | + | + | + | + | + | − | +/− |
Only microarray data not confirmed by PCR are given.
+, Present; −, absent; +/−, divergent.
T6SS and Hcp family proteins affected the bacterial binding to and invasion of HBMEC.
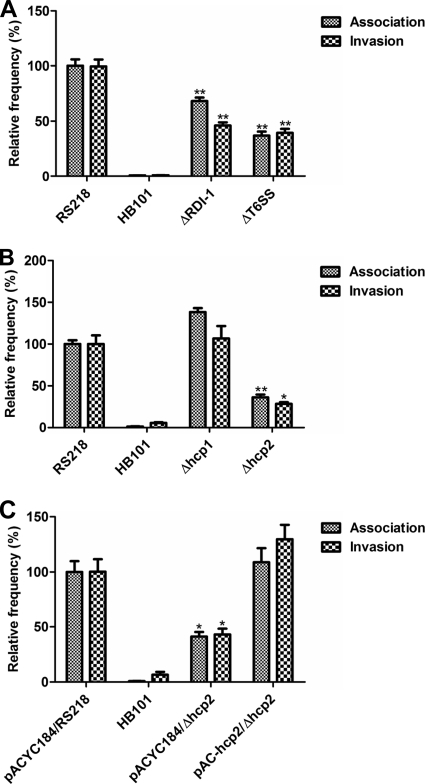
Our previous data showed that the RDI-1 deletion mutant (ΔRDI-1) of E. coli K1 strain RS218 was defective in its ability to invade HBMEC compared to that of the parental strain (45). To test whether the T6SS located in RDI-1 is responsible for this phenotype, we constructed the core component gene cluster deletion mutant (from evfB to hcp1) and examined the mutant's binding and invasion abilities in HBMEC along with those of the wild-type strain and ΔRDI-1 as positive and negative controls, respectively. The growth rate of ΔT6SS is comparable to that of the parental strain (see Fig. S1A in the supplemental material). As shown in Fig. 2A, ΔT6SS is defective in HBMEC binding and invasion, similarly to ΔRDI-1. These findings indicate that the T6SS is involved in E. coli K1 strain RS218 binding to and invasion of HBMEC.
Fig 2.
T6SS and hcp family genes of E. coli strain RS218 are involved in the bacterial binding to and invasion of HBMEC. (A) T6SS of E. coli strain RS218 is involved in the bacterial binding to and invasion of HBMEC. Both ΔRDI-1 and ΔT6SS mutants of E. coli strain RS218 exhibited significantly decreased association with and invasion of HBMEC. The ΔRDI-1 and ΔT6SS strains exhibited 68.1% ± 5.6% and 36.9% ± 6.2% (means ± standard deviations [SD]), respectively, of the association frequency of the wild-type strain, which was arbitrarily set at 100%, while their relative invasion frequencies were 46.4% ± 5.0% and 39.8% ± 6.2% (means ± SD), respectively. The error bars indicate the SD representing the means from three independent experiments. **, P < 0.01. (B) Hcp2 of E. coli strain RS218 was involved in the bacterial binding to and invasion of HBMEC. The Δhcp2 strain had relative association and invasion rates decreased to 36.2% ± 3.4% and 28.4% ± 2.0% (means ± SD) of those of the wild-type strain, respectively. The error bars indicate the SD representing the means from three independent experiments. *, P < 0.05; **, P < 0.01. (C) The complemented hcp2 strain restored the bacterial binding to and invasion of HBMEC. The hcp2 complementation strain pAC-hcp2/Δhcp2 exhibited 108.8% ± 22.1% and 129.7% ± 22.5% in HBMEC association and invasion (means ± SD) rates, respectively, compared to that of the parental strain with the vector control. The error bars indicate the SD representing the means from three independent experiments. *, P < 0.05.
Hcp is considered the hallmark of a functional T6SS and is involved in pathogenesis in different bacteria. To further examine the roles of Hcp1 and Hcp2 in strain RS218 interaction with HBMEC, we constructed the hcp1 and hcp2 deletion mutants from RS218 and performed the HBMEC association and invasion assays. We found that the growth rates of Δhcp1 and Δhcp2 are similar to those of the parental strain (see Fig. S1A in the supplemental material). Δhcp2 was defective in HBMEC binding and invasion, suggesting that Hcp2 contributes to the bacterial binding to and internalization of HBMEC, and the defect of Δhcp2 was restored by transcomplementation (Fig. 2B and C). In contrast, the Δhcp1 strain did not affect the bacterial binding to and invasion of HBMEC (Fig. 2B). These findings demonstrate that Hcp2 contributes to the interaction of E. coli K1 strain RS218 with HBMEC, supporting our hypothesis that the Hcp family protein plays a role in the bacterial interaction with the blood-brain barrier.
Hcp1 was secreted by T6SS in E. coli K1.
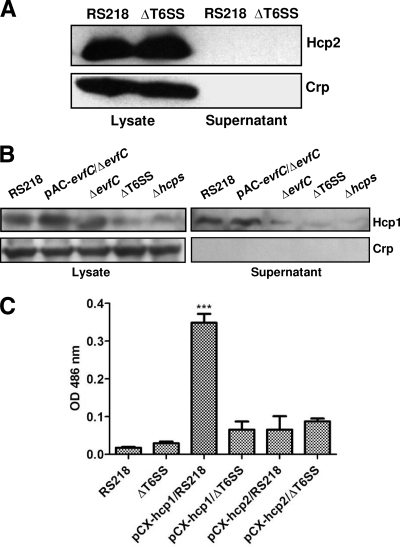
Since Hcp family genes were involved in the bacterial binding to and invasion of HBMEC, we next investigated the underlying mechanism involved in Hcp-mediated binding and invasion. Hcp family proteins can be secreted via a T6SS-dependent pathway in several bacteria and can be detected in bacterial culture supernatants (1, 2, 26, 31, 44). We therefore speculated that Hcp family proteins were secreted from E. coli K1 RS218 and affect RS218 interaction with HBMEC. We first collected the supernatants of strain RS218 and T6SS-associated mutants and then determined the Hcp family proteins by Western blotting using Hcp1 or Hcp2 polyclonal antiserum. As shown in Fig. 3A, Hcp2 was demonstrated in the lysates of RS218 but was not detected in the culture supernatant of strain RS218. In contrast, the Hcp1 protein was present in the culture supernatant of strain RS218 but was absent from all other mutants, including ΔT6SS, Δhcps (deletion of hcp1 and hcp2), and ΔevfC (a homolog of the vasK gene which has been shown to be required for the function of T6SS in V. cholerae [24]) (Fig. 3B). The lack of Hcp1 secretion was not due to defects in protein expression, as shown by the demonstration of Hcp1 in the bacterial lysates of ΔevfC. Meanwhile, the complemented strain pAC-evfC/ΔevfC restored the secretion of Hcp1, suggesting that evfC contributed to the secretion function of T6SS. The weak bands detected in the lysates of ΔT6SS and Δhcps mutants (Fig. 3B) are likely due to nonspecific reactivity with the rabbit Hcp1 polyclonal antiserum. The quality of culture supernatants was verified by the absence of the cytosolic marker cAMP receptor protein (Crp) using anti-Crp antibody (Fig. 3A and B). These findings suggest that the Hcp1 protein, not the Hcp2 protein, can be secreted through the T6SS in E. coli K1 strain RS218.
Fig 3.
Hcp1 secretion rather than Hcp2 secretion is dependent on the T6SS in E. coli RS218. (A) Hcp2 is undetected in supernatant from strain RS218. The Hcp2 secretion pathway was demonstrated by Western blotting with Hcp2 and Crp antibodies using the supernatants of E. coli strains RS218 and ΔT6SS. Crp is a cytoplasmic protein marker. (B) Hcp1 is secreted by the T6SS of RS218. Hcp1 secretion was demonstrated by Western blotting with Hcp1 and Crp antibodies using the supernatants from E. coli strains RS218, ΔT6SS, Δhcps, ΔevfC, and pAC-evfC/ΔevfC. Crp is a cytoplasmic protein marker. (C) β-Lactamase activity detection in the supernatants of various strains. pCX-hcp1/ΔT6SS, pCX-hcp2/RS218, and pCX-hcp2/ΔT6SS showed negligible β-lactamase activity, indicating no secretion, while pCX-hcp1/RS218 exhibited considerable β-lactamase activity, supporting Hcp1 secretion. The represented Bla activity data (means ± SD) represent the results from duplicate samples of two independent experiments. ***, P < 0.001. OD 486, optical density at 486 nm.
To confirm these findings, we transferred pCX-hcp1 and pCX-hcp2 (hcp1-bla and hcp2-bla fusions were made by the insertion of the bla gene after the hcp1 and hcp2 genes) into strains RS218 and ΔT6SS individually and examined the β-lactamase activity in the bacterial culture supernatants using the protocol described previously (4, 49). Under this condition, the secretion of β-lactamase is entirely dependent on the secretion of Hcp family proteins. We were only able to visualize the color change of nitrocefin in the culture supernatant of pCX-hcp1/RS218, not in that of pCX-hcp1/ΔT6SS (Fig. 3C). The culture supernatant of neither pCX-hcp2/RS218 nor pCX-hcp2/ΔT6SS induced the color change, which confirmed our earlier finding (Fig. 3A), suggesting that Hcp2 is not secreted by T6SS. Taken together, these data demonstrated that Hcp1, not Hcp2, is secreted by the T6SS in E. coli K1 strain RS218.
Hcp1 led to the rearrangement of actin cytoskeleton of HBMEC.
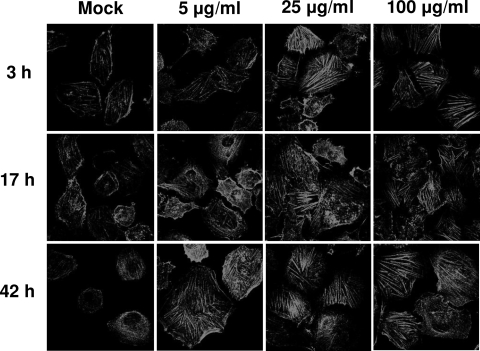
Since host cell actin cytoskeleton rearrangements are involved in the E. coli invasion of HBMEC, we examined whether secreted Hcp1 protein affected the actin cytoskeleton beneficially for the invasion function of T6SS in E. coli RS218. A progressive rearrangement of F-actin along with stress fiber formation (1,024×) was observed in response to Hcp1 in a time- and dose-dependent manner (Fig. 4). These findings suggest that the secreted Hcp1 protein can affect the actin cytoskeleton in HBMEC without affecting E. coli binding to and invasion of HBMEC.
Fig 4.
Effect of Hcp1 on F-actin distribution in HBMEC. The cells showed a time- and dose-dependent increase in stress fiber formation and cytoskeleton rearrangement in response to Hcp1 treatment compared to that of the 1× PBS (pH 7.4) negative control (Mock). Magnification, ×1,024.
Hcp1 induced apoptosis of HBMEC.
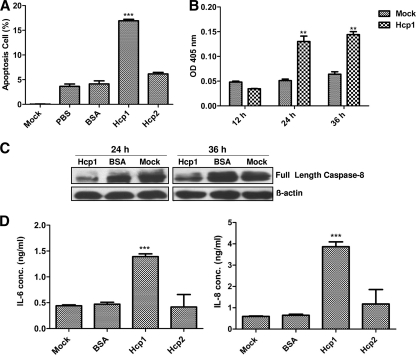
Effectors secreted by bacterial T6SS have been shown to induce host cell apoptosis (37), and apoptosis can be regarded as a strategy exploited by pathogenic bacteria to favor their own survival or spread in the host (10). We next examined whether secreted Hcp1 would induce apoptosis in HBMEC. As shown in Fig. 5A, the cells incubated with Hcp1 exhibited early apoptosis compared to that with Hcp2, bovine serum albumin (BSA), and the PBS control, which was supported by the demonstration of the fragmented DNA using agarose gel electrophoresis (data not shown).
Fig 5.
Apoptosis and cytokine release induced by Hcp1 in HBMEC. (A) The percentages of the apoptosis of cells exposed to Hcp1 and Hcp2 were determined by flow-cytometric analysis. After 24 h of incubation, the early apoptosis rate was 17% in HBMEC with Hcp1 (100 μg/ml), which was significantly higher than rates of 6% for Hcp2 (100 μg/ml), 4% for PBS, and 4% for BSA and medium negative controls (Mock). The error bars indicate the SD from three different experiments. ***, P < 0.001. (B) Colorimetric caspase 8 detection in total lysates of HBMEC. At 24 and 36 h, caspase 8 activity treated with Hcp1 (100 μg/ml) was increased compared to that of the medium treatment control (Mock). The color change was measured with a microplate reader at 405 nm. The results are represented with error bars from three experiments in triplicate. **, P < 0.01. (C) Western blot analysis revealed cleaved caspase 8 with full-length caspase 8 antibody. Comparing the bands in the negative control (Mock) and BSA control, the HBMEC treated with Hcp1 showed decreased full-length caspase 8 after 24- and 36-h treatments. (D) Cytokine release in HBMEC incubated with Hcp family proteins. Approximately 1.3 ng/ml IL-6 and 3.8 ng/ml IL-8 were released in response to Hcp1 treatment within 6 h. The results are presented as the means ± SD from duplicate samples from two individual studies. ***, P < 0.001.
Hcp1 can increase activity of caspase 8.
Caspase activation is a cellular event associated with the onset of apoptosis. Suarez et al. showed that a T6SS effector, VgrG1 of Aeromonas hydrophila, can induce the activation of caspase (36). To further explore the mechanism of apoptosis in HBMEC induced by purified Hcp1, we tested the activities of caspase 8 and caspase 3 in HBMEC treated with Hcp1 and showed that the caspase 8 activity increased significantly after 24 and 36 h of treatment compared to that of the control (Fig. 5B). In contrast, Hcp1 did not affect caspase 3 activation in HBMEC (data not shown).
In addition, Western blot analysis was applied to determine caspase 8 expression using anti-caspase 8 antibody (Santa Cruz, CA) and β-actin antibody as quality and quantity controls (Fig. 5C). Hcp1-induced apoptosis involved caspase 8 activation, leading to subunit release from the full-length caspase 8 precursor. These findings indicated that the apoptosis of HBMEC induced by Hcp1 is likely to occur via a caspase 8 pathway which has not been observed in other bacteria.
Hcp family proteins induced cytokine release in HBMEC.
Since increased levels of cytokines occurred in bacterial meningitis and Hcp family proteins were involved in strain RS218 interaction with HBMEC, we investigated the role of Hcp family proteins in cytokine and chemokine secretion in HBMEC. After 6 h of incubation, significantly higher levels of interleukin-6 (IL-6) and IL-8 were released after Hcp1 protein stimulation in HBMEC. In contrast, Hcp2 showed no significant increases in cytokine and chemokine release compared to those of the BSA control in HBMEC (Fig. 5D). This is the first report demonstrating that Hcp1, as a T6SS effector protein, induced cytokine and chemokine release in HBMEC, suggesting that the effector protein Hcp1 contributes to the inflammation associated with bacterial meningitis.
DISCUSSION
In the present study, we showed that the T6SS contributes to E. coli K1 binding to and invasion of HBMEC as well as actin cytoskeleton rearrangement, apoptosis, and the release of cytokines and chemokines in HBMEC. It has been shown that type 1 fimbriae are important determinants involved in E. coli K1 binding to and invasion of HBMEC (41), and the level of expression of type 1 fimbriae was decreased when hcp or clpV was deleted from an APEC strain (6). However, the expression of type 1 fimbriae in ΔT6SS was comparable to that of E. coli K1 RS218, as shown by a yeast aggregation assay (see Fig. S1B in the supplemental material). More importantly, ΔT6SS did not show any significant differences in the expression of the K1 capsule, NlpI, OmpA, and flagella, which were previously identified as the key bacterial determinants involved in the E. coli K1 interaction with HBMEC (see Fig. S1C to F). These findings indicate that the T6SS in E. coli K1 is directly involved in the bacterial interaction with HBMEC (Fig. 2A) instead of affecting the expression of other known bacterial determinants contributing to interaction with HBMEC.
According to previous studies, Hcp family proteins can be secreted by the T6SS and play important roles in bacterial interaction with host cells (31, 37, 43, 44). We therefore determined the secretion of the Hcp family proteins, including Hcp1 and Hcp2, in the supernatants of RS218 (wild type) and its T6SS mutants (ΔT6SS, Δhcps, and ΔevfC). Our data showed that Hcp1 was secreted via T6SS in E. coli K1 strain RS218, but there was no evidence of secretion for Hcp2 (Fig. 3A, B, and C). In contrast, Hcp2 was shown to be involved in HBMEC binding and invasion, while such HBMEC binding and invasion was not affected by Hcp1.
Since Hcp1 was secreted by T6SS but did not contribute to HBMEC binding and invasion, we hypothesized that Hcp1 is involved in eliciting inflammatory responses from HBMEC. Previous studies using HBMEC stimulated with bacterial strains, including E. coli K1 strain RS218, have shown the increased release of cytokines and chemokines (8, 11, 15, 38). Here, we showed that the purified Hcp1 protein stimulated HBMEC to release IL-6 and IL-8, which are important inflammatory mediators in eliciting inflammation and leukocyte influx in bacterial meningitis (20, 21, 42) (Fig. 5D). The released cytokines affect the permeability of the BBB, leading to an increased inflammatory response in the central nervous system (20, 42). In addition, we showed that the Hcp1 protein induced actin cytoskeleton rearrangements and apoptosis in HBMEC (Fig. 4 and 5). However, all of these cytotoxic effects of Hcp1 do not require its translocation into HBMEC (see Fig. S3 in the supplemental material). We speculate the existence of a specific receptor for Hcp1 on HBMEC surfaces, and that Hcp1 interaction with its receptor initiates inflammatory responses in HBMEC. Studies are in progress to investigate this issue.
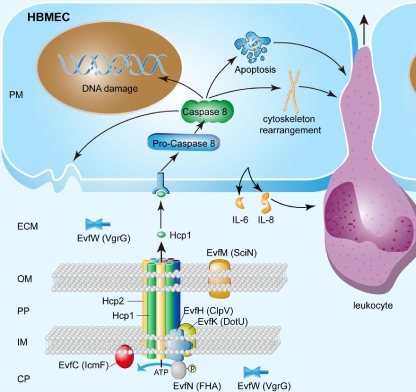
Based on our findings reported in this study, we propose a model for the T6SS and its effectors during the meningitis-causing E. coli K1 interaction with HBMEC (Fig. 6). Hcp family proteins were anchored on the bacterial membrane (see Fig. S2 in the supplemental material) and may work as a component of the T6SS apparatus on the bacterial cell membrane. The T6SS assembled on the membrane may increase the bacterial abilities of HBMEC binding and invasion although the needle structure consisted of Hcp family proteins and other proteins. Once the functional T6SS is assembled, E. coli K1 can secrete the effector proteins, such as Hcp1, and other effector proteins (demonstrated by the fact that Δhcp1 did not decrease the binding to and invasion of HBMEC [Fig. 2B]) to the outside of bacterial cells, and these effectors can induce the release of cytokines and chemokines, actin cytoskeleton changes, and host cell apoptosis in HBMEC. All of these events affect the BBB and promote the development of meningitis. In contrast, the deletion of Hcp2 decreased the E. coli K1 association with and invasion of HBMEC (Fig. 2B), and Hcp2 was localized in the outer membrane (see Fig. S2 in the supplemental material) without being secreted into HBMEC. Hcp2 may work as a bacterial component that promotes bacterial interaction with HBMEC, although the mechanism remains unknown at this time. The two Hcp family proteins, Hcp1 and Hcp2, are likely to coordinately regulate the interaction between E. coli K1 and HBMEC by playing different roles in the development of E. coli meningitis.
Fig 6.
Model for the role of T6SS in E. coli K1 interaction with HBMEC. We propose a potential model to illustrate the role of T6SS in E. coli K1 interaction with HBMEC. We propose a one-step channel through the periplasm which is mainly composed of Hcp1, Hcp2, EvfC, and other proteins. As one of the T6SS component proteins, Hcp2 is responsible for transporting cytotoxic effectors, such as the Hcp1 protein, through the channel. Once Hcp1 was secreted outside, it was recognized by the specific receptor of HBMEC and led to a series of effects, including cytoskeleton rearrangement, apoptosis, caspase 8 activation, and cytokine release, which can initiate inflammation, including the migration of leukocytes across HBMEC. CP, bacterial cytoplasm; IM, bacterial inner membrane; PP, bacterial periplasm; OM, bacterial outer membrane; ECM, extracellular milieu; and PM, host cell plasma membrane.
In summary, we reported the distribution of T6SS in meningitis-causing E. coli K1 isolates and showed for the first time the secretion pathway of an Hcp-like effector protein, Hcp1, in E. coli K1 strain RS218 that can induce cytokines release, cytoskeleton rearrangement, and apoptosis in HBMEC. Our findings suggest that the Hcp family proteins in E. coli are targets for the development of preventive or therapeutic drugs and/or vaccines against E. coli meningitis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Donna Pearce at the Johns Hopkins University School of Medicine for E. coli association and invasion assays in HBMEC. We are thankful to Eric Oswald for providing plasmid pCX340 and the RBL (Research-Based Learning) students at Shanghai Jiao Tong University School of Medicine (Xiao-Lei Shi, Hao Li, Xiao-Xi Chen, Pei Xue, and Rui Jin) for the generation of the Crp and OmpA antibodies.
This work was supported by grants from the National Natural Science Foundation of China (no. 30870100 and 81000709), the National Key Program for Infectious Diseases of China (no. 2008ZX10004-002, 2008ZX10004-009, and 2009ZX10004-712), the Program of Shanghai Subject Chief Scientist (no. 09XD1402700), and the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning.
Footnotes
Published ahead of print 19 December 2011
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1. Aschtgen MS, Gavioli M, Dessen A, Lloubes R, Cascales E. 2010. The SciZ protein anchors the enteroaggregative Escherichia coli type VI secretion system to the cell wall. Mol. Microbiol. 75:886–899 [DOI] [PubMed] [Google Scholar]
- 2. Aubert DF, Flannagan RS, Valvano MA. 2008. A novel sensor kinase-response regulator hybrid controls biofilm formation and type VI secretion system activity in Burkholderia cenocepacia. Infect. Immun. 76:1979–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cascales E. 2008. The type VI secretion toolkit. EMBO Rep. 9:735–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Charpentier X, Oswald E. 2004. Identification of the secretion and translocation domain of the enteropathogenic and enterohemorrhagic Escherichia coli effector Cif, using TEM-1 beta-lactamase as a new fluorescence-based reporter. J. Bacteriol. 186:5486–5495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Pace F, et al. 2010. The type VI secretion system plays a role in type 1 fimbria expression and pathogenesis of an avian pathogenic Escherichia coli strain. Infect. Immun. 78:4990–4998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dudley EG, Thomson NR, Parkhill J, Morin NP, Nataro JP. 2006. Proteomic and microarray characterization of the AggR regulon identifies a pheU pathogenicity island in enteroaggregative Escherichia coli. Mol. Microbiol. 61:1267–1282 [DOI] [PubMed] [Google Scholar]
- 8. Eisenhauer PB, et al. 2004. Escherichia coli Shiga toxin 1 and TNF-alpha induce cytokine release by human cerebral microvascular endothelial cells. Microb. Pathog. 36:189–196 [DOI] [PubMed] [Google Scholar]
- 9. Filloux A, Hachani A, Bleves S. 2008. The bacterial type VI secretion machine: yet another player for protein transport across membranes. Microbiology 154:1570–1583 [DOI] [PubMed] [Google Scholar]
- 10. Fiorentini C, Falzano L, Travaglione S, Fabbri A. 2003. Hijacking Rho GTPases by protein toxins and apoptosis: molecular strategies of pathogenic bacteria. Cell Death Differ. 10:147–152 [DOI] [PubMed] [Google Scholar]
- 11. Galanakis E, Di Cello F, Paul-Satyaseela M, Kim KS. 2006. Escherichia coli K1 induces IL-8 expression in human brain microvascular endothelial cells. Eur. Cytokine Netw. 17:260–265 [PubMed] [Google Scholar]
- 12. Gruenheid S, et al. 2004. Identification and characterization of NleA, a non-LEE-encoded type III translocated virulence factor of enterohaemorrhagic Escherichia coli O157:H7. Mol. Microbiol. 51:1233–1249 [DOI] [PubMed] [Google Scholar]
- 13. Hood RD, et al. 2010. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe 7:25–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ishikawa T, Rompikuntal PK, Lindmark B, Milton DL, Wai SN. 2009. Quorum sensing regulation of the two hcp alleles in Vibrio cholerae O1 strains. PLoS One 4:e6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jayasekera S, Matin A, Sissons J, Maghsood AH, Khan NA. 2005. Balamuthia mandrillaris stimulates interleukin-6 release in primary human brain microvascular endothelial cells via a phosphatidylinositol 3-kinase-dependent pathway. Microbes Infect. 7:1345–1351 [DOI] [PubMed] [Google Scholar]
- 16. Khan NA, Kim Y, Shin S, Kim KS. 2007. FimH-mediated Escherichia coli K1 invasion of human brain microvascular endothelial cells. Cell Microbiol. 9:169–178 [DOI] [PubMed] [Google Scholar]
- 17. Khan NA, et al. 2003. Outer membrane protein A and cytotoxic necrotizing factor-1 use diverse signaling mechanisms for Escherichia coli K1 invasion of human brain microvascular endothelial cells. Microb. Pathog. 35:35–42 [DOI] [PubMed] [Google Scholar]
- 18. Khan NA, et al. 2002. Cytotoxic necrotizing factor-1 contributes to Escherichia coli K1 invasion of the central nervous system. J. Biol. Chem. 277:15607–15612 [DOI] [PubMed] [Google Scholar]
- 19. Kim KJ, Elliott SJ, Di Cello F, Stins MF. 2003. The K1 capsule modulates trafficking of E. coli-containing vacuoles and enhances intracellular bacterial survival in human brain microvascular endothelial cells. Cell Microbiol. 5:245–252 [DOI] [PubMed] [Google Scholar]
- 20. Kim KS. 2003. Neurological diseases: pathogenesis of bacterial meningitis: from bacteremia to neuronal injury. Nat. Rev. Neurosci. 4:376–385 [DOI] [PubMed] [Google Scholar]
- 21. Ledesma MA, et al. 2010. The hemorrhagic coli pilus (HCP) of Escherichia coli O157:H7 is an inducer of proinflammatory cytokine secretion in intestinal epithelial cells. PLoS One 5:e12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leiman PG, et al. 2009. Type VI secretion apparatus and phage tail-associated protein complexes share a common evolutionary origin. Proc. Natl. Acad. Sci. U. S. A. 106:4154–4159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lloyd AL, Henderson TA, Vigil PD, Mobley HL. 2009. Genomic islands of uropathogenic Escherichia coli contribute to virulence. J. Bacteriol. 191:3469–3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma AT, McAuley S, Pukatzki S, Mekalanos JJ. 2009. Translocation of a Vibrio cholerae type VI secretion effector requires bacterial endocytosis by host cells. Cell Host Microbe 5:234–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moriel DG, et al. 2010. Identification of protective and broadly conserved vaccine antigens from the genome of extraintestinal pathogenic Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 107:9072–9077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mougous JD, et al. 2006. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science 312:1526–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Parthasarathy G, Yao Y, Kim KS. 2007. Flagella promote Escherichia coli K1 association with and invasion of human brain microvascular endothelial cells. Infect. Immun. 75:2937–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pell LG, Kanelis V, Donaldson LW, Howell PL, Davidson AR. 2009. The phage lambda major tail protein structure reveals a common evolution for long-tailed phages and the type VI bacterial secretion system. Proc. Natl. Acad. Sci. U. S. A. 106:4160–4165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prasadarao NV, et al. 1996. Outer membrane protein A of Escherichia coli contributes to invasion of brain microvascular endothelial cells. Infect. Immun. 64:146–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pukatzki S, Ma AT, Revel AT, Sturtevant D, Mekalanos JJ. 2007. Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proc. Natl. Acad. Sci. U. S. A. 104:15508–15513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pukatzki S, et al. 2006. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc. Natl. Acad. Sci. U. S. A. 103:1528–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rao PS, Yamada Y, Tan YP, Leung KY. 2004. Use of proteomics to identify novel virulence determinants that are required for Edwardsiella tarda pathogenesis. Mol. Microbiol. 53:573–586 [DOI] [PubMed] [Google Scholar]
- 33. Russo TA, et al. 2005. E. coli virulence factor hemolysin induces neutrophil apoptosis and necrosis/lysis in vitro and necrosis/lysis and lung injury in a rat pneumonia model. Am. J. Physiol. Lung Cell. Mol. Physiol. 289:L207–L216 [DOI] [PubMed] [Google Scholar]
- 34. Schell MA, et al. 2007. Type VI secretion is a major virulence determinant in Burkholderia mallei. Mol. Microbiol. 64:1466–1485 [DOI] [PubMed] [Google Scholar]
- 35. Shrivastava S, Mande SS. 2008. Identification and functional characterization of gene components of type VI secretion system in bacterial genomes. PLoS One 3:e2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Suarez G, et al. 2010. A type VI secretion system effector protein, VgrG1, from Aeromonas hydrophila that induces host cell toxicity by ADP ribosylation of actin. J. Bacteriol. 192:155–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Suarez G, et al. 2008. Molecular characterization of a functional type VI secretion system from a clinical isolate of Aeromonas hydrophila. Microb. Pathog. 44:344–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tenenbaum T, et al. 2007. Streptococcus agalactiae invasion of human brain microvascular endothelial cells is promoted by the laminin-binding protein Lmb. Microbes Infect. 9:714–720 [DOI] [PubMed] [Google Scholar]
- 39. Teng CH, et al. 2005. Escherichia coli K1 RS218 interacts with human brain microvascular endothelial cells via type 1 fimbria bacteria in the fimbriated state. Infect. Immun. 73:2923–2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Teng CH, et al. 2010. NlpI contributes to Escherichia coli K1 strain RS218 interaction with human brain microvascular endothelial cells. Infect. Immun. 78:3090–3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Teng CH, et al. 2006. Effects of ompA deletion on expression of type 1 fimbriae in Escherichia coli K1 strain RS218 and on the association of E. coli with human brain microvascular endothelial cells. Infect. Immun. 74:5609–5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Furth AM, Roord JJ, van Furth R. 1996. Roles of proinflammatory and anti-inflammatory cytokines in pathophysiology of bacterial meningitis and effect of adjunctive therapy. Infect. Immun. 64:4883–4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Williams SG, Varcoe LT, Attridge SR, Manning PA. 1996. Vibrio cholerae Hcp, a secreted protein coregulated with HlyA. Infect. Immun. 64:283–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu HY, Chung PC, Shih HW, Wen SR, Lai EM. 2008. Secretome analysis uncovers an Hcp-family protein secreted via a type VI secretion system in Agrobacterium tumefaciens. J. Bacteriol. 190:2841–2850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xie Y, et al. 2006. Identification and characterization of Escherichia coli RS218-derived islands in the pathogenesis of E. coli meningitis. J. Infect. Dis. 194:358–364 [DOI] [PubMed] [Google Scholar]
- 46. Xie Y, Yao Y, Kolisnychenko V, Teng CH, Kim KS. 2006. HbiF regulates type 1 fimbriation independently of FimB and FimE. Infect. Immun. 74:4039–4047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yao Y, Xie Y, Kim KS. 2006. Genomic comparison of Escherichia coli K1 strains isolated from the cerebrospinal fluid of patients with meningitis. Infect. Immun. 74:2196–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yao Y, et al. 2009. The type III secretion system is involved in the invasion and intracellular survival of Escherichia coli K1 in human brain microvascular endothelial cells. FEMS Microbiol. Lett. 300:18–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yu H, Kim KS. 2010. Ferredoxin is involved in secretion of cytotoxic necrotizing factor 1 across the cytoplasmic membrane in Escherichia coli K1. Infect. Immun. 78:838–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zheng J, Leung KY. 2007. Dissection of a type VI secretion system in Edwardsiella tarda. Mol. Microbiol. 66:1192–1206 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.