Background: Complement C3 is expressed and secreted by human macrophages.
Results: Modified low density lipoprotein (mLDL) up-regulates C3 expression and secretion, whereas C3a stimulates mLDL uptake by macrophages.
Conclusion: Interplays between mLDL and C3 may be involved in atherosclerotic lesion formation.
Significance: Novel cross-talk has been shown between mLDL, complement component C3, and Toll-like receptor 4 in human macrophages.
Keywords: Complement, Low Density Lipoprotein (LDL), Macrophages, MAP Kinases (MAPKs), NF-κB, Nuclear Receptors, Complement C3, Liver X Receptor (LXR), Toll-like Receptor 4 (TLR4), Anaphylatoxin C3a, Modified Low Density Lipoprotein, Oxidized Low Density Lipoprotein
Abstract
Complement C3 is a pivotal component of three cascades of complement activation. C3 is expressed in human atherosclerotic lesions and is involved in atherogenesis. However, the mechanism of C3 accumulation in atherosclerotic lesions is not well elucidated. We show that acetylated low density lipoprotein and oxidized low density lipoprotein (oxLDL) increase C3 gene expression and protein secretion by human macrophages. Modified LDL (mLDL)-mediated activation of C3 expression mainly depends on liver X receptor (LXR) and partly on Toll-like receptor 4 (TLR4), whereas C3 secretion is increased due to TLR4 activation by mLDL. LXR agonist TO901317 stimulates C3 gene expression in human monocyte-macrophage cells but not in human hepatoma (HepG2) cells. We find LXR-responsive element inside of the promoter region of the human C3 gene, which binds to LXRβ in macrophages but not in HepG2 cells. We show that C3 expression and secretion is decreased in IL-4-treated (M2) and increased in IFNγ/LPS-stimulated (M1) human macrophages as compared with resting macrophages. LXR agonist TO901317 potentiates LPS-induced C3 gene expression and protein secretion in macrophages, whereas oxLDL differently modulates LPS-mediated regulation of C3 in M1 or M2 macrophages. Treatment of human macrophages with anaphylatoxin C3a results in stimulation of C3 transcription and secretion as well as increased oxLDL accumulation and augmented oxLDL-mediated up-regulation of the C3 gene. These data provide a novel mechanism of C3 gene regulation in macrophages and suggest new aspects of cross-talk between mLDL, C3, C3a, and TLR4 during development of atherosclerotic lesions.
Introduction
The complement system includes ∼30 circulating and cell membrane-bound proteins interacting with one another during complement activation. Complement activation is realized through the intermediary of three activation cascades: the classic, the alternative, and the lectin pathways. As a result of complement activation, the so-called “membrane attack complex” C5b-9 is polymerized at the terminal step of all complement activation cascades and inserted into the plasma membrane of the target pathogen cell (1). Besides the membrane attack complex, a number of intermediate molecules are generated during complement activation, such as anaphylatoxins C3a and C5a, which are small signaling molecules, involved in different processes of immune cell activation, migration, and differentiation (2), and inactivated C3b, which stimulates phagocytosis of apoptotic cells (3). Complement protein C3 is a central molecule that is indispensable for all complement activation cascades. The main source of C3 in humans is the liver (hepatocytes) (4), but C3 is also expressed at extrahepatic sites: in mononuclear phagocytes (5), fibroblasts (6), vascular endothelium (7), astroglia (8), and adipocytes (9). C3 and its degradation products are able to promote phagocytosis, activate inflammatory responses against pathogens, and regulate acquired immune system, but uncontrolled activation of C3 may result in host cell damage (10).
Atherosclerosis, a chronic inflammatory disease of blood vessels, is characterized by macrophage and modified low density lipoprotein (mLDL)3 accumulation in the artery wall (11). The implication of both innate and acquired immune systems in the process of atherogenesis is corroborated (12). There are data indicating that complement activation is also involved in atherogenesis. Components of the terminal complement pathway (13) as well as C3 and C4 (14, 15) are found in human atherosclerotic lesions. However, the roles of discrete complement components in pathogenesis of atherosclerosis are controversial. C6 deficiency has a protective effect on diet-induced atherosclerosis in rabbits (16), whereas C5 deficiency does not reduce the development of atherosclerotic lesions in apoe−/− mice (17). C3-deficient ldlr−/− mice develop more appreciable atherosclerotic lesions with increased macrophage content (18), and lack of C3, but not factor B, increases hyperlipidemia and atherosclerosis in apoe−/−ldlr−/− mice (19). Complement C3 and its cleavage products are suggested to be risk factors in the development of cardiovascular diseases (20). Serum C3 levels were shown to be associated with atherosclerosis development in humans (21, 22). Complement activation appears to exacerbate atherosclerosis through the proinflammatory effects of C3a, C5a, and C5b-9, whereas the classical pathway and inactivated C3b may have atheroprotective effects due to activating of non-inflammatory clearance of apoptotic cells by macrophages (23).
Uptake of mLDL by macrophages in the artery wall is mediated by scavenger receptors and results in formation of foam cells. The level of mLDL uptake is regulated by a variety of cytokines and signaling molecules; at the same time, macrophage transformation into foam cells leads to alteration of the pro- and anti-inflammatory cytokine expression profile of these cells (24). Despite the important role of C3 in atherogenic processes, the regulation of C3 gene expression in macrophages in atherosclerotic lesions, especially by mLDL uptake, has not been elucidated.
In the current study, we show that acetylated or oxidized LDL activate C3 gene expression and C3 protein secretion by human macrophages. The mLDL-mediated stimulation of C3 gene expression is controlled by liver X receptors (LXRs), in a ligand-dependent manner. We also show that mLDL stimulates C3 secretion via activation of Toll-like receptor 4 (TLR4), whereas C3a, a product of C3 cleavage during complement activation, increases oxidized LDL uptake and C3 expression and secretion by human macrophages. These data provide a novel mechanism of regulation of C3 expression and secretion by human macrophages and suggest novel aspects of cross-talk between the complement system, TLR4 signaling, and lipid metabolism during development of atherosclerotic lesions.
EXPERIMENTAL PROCEDURES
Chemical Inhibitors, Synthetic Ligands, and Recombinant and Purified Proteins
MAPK inhibitors and NF-κB inhibitor were purchased from Biomol: SB203580 (p38 inhibitor) (catalog no. EI-286), SP600125 (JNK1/2/3 inhibitor) (catalog no. EI-305), U0126 (MEK1/2 inhibitor) (catalog no. EI-282), and QNZ (NF-κB inhibitor) (catalog no. EI-352). LXR ligand was purchased from Biomol: TO901317 (catalog no. GR-232). Phorbol 12-myristate 13-acetate (catalog no. P8139) was purchased from Biomol. Human C3a was generated by activation of complement using zymosan A and purified as described (25). Human recombinant IL-4 (catalog no. PHC0044) was purchased from Invitrogen. Human recombinant TNFα (catalog no. T0157) and IFNγ (catalog no. I3265) were purchased from Sigma.
Antibodies
Mouse monoclonal antibodies against human β-actin (catalog no. ab3280) and against human TLR4 (catalog no. ab30667), rabbit polyclonal antibody against human LXRβ (catalog no. ab56237), and goat FITC-labeled secondary antibodies against mouse IgG (catalog no. ab6669-1) were purchased from Abcam. Mouse polyclonal antibodies against human C3 were purchased from Cytokine Ltd. Fluorochrome-labeled antibodies against human CD14 (PC5-labeled, IM0643) and CD45 (phycoerythrin-labeled, IM1833) were purchased from Beckman Coulter.
Cell Cultures and Human Peripheral Blood Monocyte-derived Macrophages
Human acute monocytic leukemia cell line THP-1 and human hepatoma cell line HepG2 were obtained from the Cell Culture Bank of the Institute of Cytology, Russian Academy of Sciences (St. Petersburg, Russia). HepG2 cells were cultivated in DMEM containing 10% fetal calf serum (FCS) (HyClone), 5% CO2 at 37 °C. THP-1 cells were cultivated in RPMI containing 10% FCS, 5% CO2 at 37 °C. The differentiation of THP-1 cells into macrophages was performed by treatment of the cells with phorbol 12-myristate 13-acetate (50 ng/ml). THP-1 cells were washed 24 h after phorbol 12-myristate 13-acetate treatment and incubated for 5 days in RPMI containing 10% FCS with culture medium replacement every 2 days. After that, THP-1 cells were incubated in RPMI containing 10% lipoprotein-deficient FCS (HyClone) for 2 days. Human peripheral blood monocytes (PBM) were isolated by Percoll-Hypaque gradient centrifugation from healthy donor blood as described (26). Mature PBM-derived macrophages were obtained after 5 days of cultivation in RPMI containing 10% FCS and incubation in RPMI containing 10% lipoprotein-deficient FCS (HyClone) for 2 days. For M1 or M2 differentiation of PBM-derived macrophages, cells were treated with IFNγ (20 ng/ml) and bacterial lipopolysaccharide (LPS) (100 ng/ml) (M1) or IL-4 (20 ng/ml) (M2) for 6 days with the culture medium and cytokine replacement every 2 days.
Plasmid Construction and Electroporation of THP-1 Cells
Primers 5′-ctcgagTCATTCCCAACCTGCTAACC-3′ and 5′-ggtaccTCCCTCCCCCATTCTACTTC-3′ (restriction sites for XhoI and KpnI, respectively, are underlined) were used to amplify the −463 to +268 region of the human C3 promoter. A truncated variant of the human C3 promoter, lacking the DR4 element, was created by amplifying the −310 to +268 region using the following primers: 5′-ctcgagTCATTCCCAACCTGCTAACC-3′ and 5′-ggtaccGGGGGTTCTCCAGACCTTAG-3′. Amplified fragments were ligated into pAT-TA vector (Eurogen) and cloned into pGL3Basic luciferase-containing reporter plasmid (Promega), digested by XhoI and KpnI restrictases. Constructed plasmids pC3(−463/+268)-Luc and pC3(−310/+268)-Luc, containing the luciferase gene under control of the −463 to +268 or −310 to +268 regions of the human C3 promoter, were verified by sequencing.
Electroporation of THP-1 cells and THP-1-derived macrophages by plasmids was performed using a Bio-Rad Gene Pulser xcell (250 V, 800 microfarads, 5 million THP-1 cells, and 2.5 μg of plasmids in 400 μl of ice-cooled PBS) as described (27). Plasmid pCMVLacZ, containing the bacterial LacZ gene under control of the late cytomegalovirus promoter, was co-transfected for normalization of luciferase activity by a β-galactosidase test. The β-galactosidase assay was performed following the standard protocols, using o-nitrophenyl-β-d-galactopyranoside as a substrate. Relative β-galactosidase activity was calculated as the A420/mg of total protein of cell lysates/h. The activity of luciferase was measured on a 20/20n luminometer (Turner BioSystems) by using the Luciferase Assay System (Promega, catalog no. E4030) in accordance with the manufacturer's guidelines. The luciferase activity is shown as relative light activity, which corresponds to the percentage of the light counts/min/mg of total protein of cell lysates relative to the control cells (relative light activity = 1 in control cells).
Animals
Male C57B/6 mice were purchased from “Rappolovo,” the Russian Medicine Academy nursery (St. Petersburg). Mice used in experiments reported here were maintained and handled in accordance with institutional ethics committee guidelines, and these experiments were performed in concordance with Ref. 62. Mice were sacrificed by cervical dislocation, and resident peritoneal macrophages were harvested from male mice (2–3 months old weighing 20–24 g) without thioglycolate stimulation. The cells harvested by peritoneal lavage were plated in 24-well plates at a cell density of 1 × 106 cells/well and incubated for 3 h in RPMI medium supplemented with 10% FCS at 37 °C, 5% CO2. After that, cells were washed three times with PBS and incubated in RPMI with 10% lipoprotein-deficient FCS (HyClone) with or without TO901317 or oxLDL for 48 h.
Reverse Transcription
Total cellular RNA was isolated by RNA STAT-60 reagent (Tel-Test) in accordance with the manufacturer's guidelines. After digestion with RNase-free DNase I (Roche Applied Science), the concentration of total RNA and RNA purity were determined using an Avaspec-2048 spectrophotometer (Avantes, The Netherlands). The ratio of optical densities at 260 and 280 nm was greater than 2.0, whereas the ratio of optical densities at 260 and 230 nm was greater than 1.7. Ribosomal RNA band integrity was confirmed by electrophoresis in 1% agarose gel. RNA (2 μg) was subjected to reverse transcription, using a dT-16 primer (Invitrogen) and Moloney murine leukemia virus reverse transcriptase (Promega) to generate first-strand cDNA.
Real-time PCR
Primers and probes for human GAPDH were described (28). Primers and probes for ABCA1 were described (29). The following sets of primers and probes were designed using Primer3 software: human β-actin (5-hACB, AGCCTTCCTTCCTGGGC; 3-hACB, CGGATGTCCACGTCACACT; h-hACB, Cy5-TGGAGTCCTGTGGCATCCACGA-RTQ2), human complement C3 (5-hC3, GAGCCAGGAGTGGACTATGTGTA; 3-hC3, CAATGGCCATGATGTACTCG; hC3-h, R6G-ACCCGACTGGTCAAGGTTCAGCTGT-BHQ1), mouse β-actin (5-m-β-actin, CTGGCACCACACCTTCTACA; 3-m-β-actin, CTTTTCACGGTTGGCCTTAG; h-m-β-actin, ROX-GCACCCTGTGCTGCTCACCG-BHQ2), mouse ABCA1 (mABCA1–5, GAGATCAAGGAACTGACCAAGATC; mABCA1–3, CCAGGAGTCCAAAGCACTCT; mABCA1-h, R6G-TCTGCATCGGCATCCCTCCC-RTQ1). The following sets of primers were used for SYBR Green real-time PCR: human TNFα (5-TNFα, ATGAGCACTGAAAGCATGATCC; 3-TNFα, GAGGGCTGATTAGAGAGAGGTC), human IL-10 (5-IL10, ACTTTAAGGGTTACCTGGGTTGC; 3-IL10, TCACATGCGCCTTGATGTCTG), human TLR4 (5-TLR4, ACTTTAAGGGTTACCTGGGTTGC; 3-TLR4, TCACATGCGCCTTGATGTCTG), human MRC1L1 (CD206) (MRC1L1-5, CGAGGAAGAGGTTCGGTTCACC; MRC1L1-3, GCAATCCCGGTTCTCATGGC), human MCP-1 (MCP1-5, CCCCAGTCACCTGCTGTTAT; MCP1-3, AGATCTCCTTGGCCACAATG), human CD14 (CD14-5, GGAAGACTTATCGACCATGGA; CD14-3, GCTGAGGTTCGGAGAAGTTG), human IL-12B (IL12B-5, GGAGTACCCTGACACCTGGA; IL12B-3, ACGCTAATGCTGGCATTTTT), human IL-15 (IL15-5, GCCCAGGGAAATCAAAAGAT; IL15–3, TGGCTCCAACAAATCAACAG); mouse complement C3 (5-mC3, GCAGGTCATCAAGTCAGGCT; 3-mC, TAGCTGGTGTTGGGCTTTTC).
The negative (no reverse transcriptase) as well as the no template control reactions were carried out to verify the absence of DNA template contamination and probe hybridization with genomic DNA for each real-time PCR. In order to optimize multiplex real-time PCRs, the conditions that provide the fastest Ct values were selected for each primer/probe set separately and in combination in the case of multiplexing. It was also ascertained during multiplex optimizing that use of all primer/probe sets in the multiplexing approach does not influence the efficiency of PCR and the Ct value in comparison with using each primer/probe set alone. The following conditions for real-time PCR were used: 95 °C for 300 s followed by 40 cycles of 95 °C for 25 s and 60 °C for 45 s for TaqMan and 5 °C for 300 s followed by 40 cycles of 95 °C for 25 s, 60 °C for 25 s, and 72 °C for 25 s for SYBR Green. The relative abundances of mRNAs of tested genes were assessed by gapdh and β-actin detection in the same reaction. The levels of mRNA of genes are presented as the results of gapdh and β-actin normalization as described (30). The number of cycles (Ct value) required to reach a threshold level of fluorescence that is ∼10 S.D. values (of fluctuations in background fluorescence) above the mean background fluorescence was determined for each PCR and primer set by use of the CFX96 real-time PCR system and automated software (Bio-Rad). The relative amount of mRNA (as a percentage of the control sample) was calculated by the relation, 2Ct(control) − Ct(sample) × 100.
ChIP Assay
Chromatin immunoprecipitation (ChIP) was performed as described previously (28). The following sets of primers were used: for ABCA1 LXR-responsive element (LXRE), 5′-CTCACTCTCGCTCGCAATTA-3′ and 5′-GAACGTCGCCCGTTTAAG-3′; for C3 LXRE, 5′-CATCCCCAGTTCACTTTTGG-3′ and 5′-CATGGAAGCCTCTGGAAGTG-3′; for the NOD1 gene, 5′-GGGCACACCTGTTTTCCAG-3′ and 5′-AAGTGATGCAGGACGAAGGAG-3′.
Immunostaining and FACS
PBM-derived macrophages were fixed with 4% formalin for 10 min at 22 °C, washed three times with PBS containing 0.1 m glycine, and incubated for 40 min at 22 °C with blocking buffer (PBS containing 1% BSA, 3% FCS, nonspecific goat IgG (1 μg/ml), and 0.02% Tween 20). For cell membrane permeabilization, cells were incubated with the addition of 0.1% Triton X-100. PBM-derived macrophages were treated with mouse monoclonal antibodies against human TLR4 (1:300 dilution) in PBS containing 1% BSA and 0.02% Tween 20 for 2 h at 22 °C, washed three times with PBS, and incubated with secondary FITC-labeled goat polyclonal antibodies against mouse IgG (1:500 dilution) in PBS containing 1% BSA and 0.02% Tween 20 for 1 h at 22 °C. After that, cells were washed three times and incubated with fluorochrome-labeled antibodies against CD14 and CD45 in PBS containing 1% BSA and 0.02% Tween 20 for 2 h at 22 °C, washed three times, and fixed in PBS containing 1% formalin for FACS. PBM-derived macrophages treated with secondary antibodies and appropriate isotype control fluorochrome-labeled antibodies but not incubated with anti-TLR4, anti-CD14, and anti-CD45 antibodies were used as a control of immunostaining specificity. FACS analysis and cell sorting were carried out using an Epics Altra flow cytometer (Beckman Coulter) and Expo32 software (Beckman Coulter).
LDL Preparation, Modification, and Labeling
Human LDL was isolated from EDTA-treated plasma by density gradient centrifugation as described (31). Low density lipoprotein was labeled with FITC as described (32). Oxidation of LDL was performed as described (31). LDL acetylation was performed as described (33).
mLDL Uptake
THP-1 macrophages or PBM-derived macrophages were incubated with FITC-labeled acLDL or oxLDL, fixed with 4% formalin for 10 min at 22 °C, and washed three times with PBS containing 0.1 m glycine. Confocal laser scanning microscopy analysis was performed using a Carl Zeiss LSM710 microscope. Lipid droplet accumulation in macrophages was estimated by cell staining with Oil Red O and hematoxylin.
Nuclear Extract Preparation and EMSA
Nuclear extracts were prepared from THP-1 cells or PBM-derived macrophages as described previously (34) with slight modifications. The following 5′-end-labeled or unlabeled with biotin synthetic oligonucleotides were purchased from Syntol (sequences of upper strands only are shown): hC3-LXRE-wt, TGGACTCATCCCCAGTTCACTTTTGGCCATGCGTTTATCAG (corresponding to the fragment of the promoter region of the human C3 gene), or hC3-LXRE-mut, TGGACTCATCCCCTTTTCACTTTTTTCCATGCGTTTATCAG (containing a mutation in the LXRE site; altered nucleotides are underlined). EMSA was performed as described (35). All EMSAs were performed in 15 μl of total reaction volume containing 50 ng of poly(dI-dC) (Roche Applied Science), 50 μg of nuclear extracts, and 50 ng of annealed biotin-labeled probes. Binding reaction was performed for 30 min at room temperature. The binding reaction mixture was separated in a 4% nondenaturing PAGE and was transferred onto a nylon (plus) membrane. Protein-bound and free probes were detected by using the streptavidin-horseradish peroxidase conjugate (Pierce) and ECL. In the competition experiments, unlabeled competitors were added to reaction mixture together with labeled probe as indicated in the figure legends.
DNA Affinity Precipitation
DNA affinity precipitation of nuclear proteins from THP-1 cells or PBM-derived macrophages was performed by using biotin end-labeled oligonucleotide as described (36). For the DNA affinity precipitation assay, biotin-labeled probes were incubated with nuclear extracts as described above and precipitated with streptavidin magnetic particles (Roche Applied Science). Precipitated proteins were analyzed by Western blotting with antibodies against LXRβ.
Enzyme-linked Immunosorbent Assay (ELISA)
Human complement C3, anaphylatoxin C3a, and TNFα in culture medium and cell lysates were detected by the sandwich ELISA with test systems purchased from Cytokine Ltd., in accordance with the manufacturer's instructions.
Statistical Analysis
Results are presented as means ± S.E. The statistical analyses of differences between compared groups were performed using an unpaired t test or Dunnett's criterion for multiple comparisons. Differences were considered statistically significant at the p < 0.05 level. All statistical analyses were performed using the program Statistica 5.0 (StatSoft).
RESULTS
mLDL and LXR Agonist TO191317 Activate C3 Gene Expression and Protein Synthesis in Human PBM- and THP-1-derived Macrophages
To test the effect of mLDL loading on the expression of the C3 gene in macrophages, we treated human PBM-derived macrophages with native or modified LDL. The level of C3 mRNA was increased in macrophages treated with acLDL or oxLDL in comparison with control cells (treated with BSA or acetylated BSA) (Fig. 1A). At the same time, native LDL did not change the level of C3 mRNA in human macrophages (Fig. 1A). Uptake of mLDL via scavenger receptors on macrophages results in formation of several oxysterols, acting as ligands for LXRs, which activate target genes in the response to oxidized cholesterol accumulation by macrophages (37). Fig. 1A shows that treatment of human PBM-derived macrophages with LXR agonist TO191317 stimulates C3 gene expression. We measured the level of ABCA1 mRNA as a control (supplemental Fig. 1A) because the ABCA1 gene is known to be strongly stimulated by mLDL uptake and LXR activation in macrophages (38). To ascertain that mLDL-mediated up-regulation of C3 gene was not a result of bacterial LPS contamination of LDL, macrophages were treated with polymyxin B. Treatment of macrophages with polymyxin B did not attenuate the effects of acLDL or oxLDL toward C3 gene activation (supplemental Fig. 1B). acLDL, oxLDL, and the LXR agonist TO191317 increased C3 gene expression in THP-1-derived macrophages (Fig. 1B). Treatment of THP-1 monocytes with TO191317 also resulted in an increase of C3 gene expression (supplemental Fig. 1C).
FIGURE 1.
oxLDL, acLDL, and LXR agonist TO191317 stimulate C3 gene and protein expression in human PBM- and THP-1-derived macrophages. A, the level of C3 mRNA in human PBM-derived macrophages (7th day of differentiation); real-time RT-PCR; 100% is the level in the macrophages treated with BSA (control cells). B, the level of C3 mRNA in human THP-1-derived macrophages 24 or 48 h after the addition of BSA, native or modified LDL, or LXR agonist TO191317; real-time RT-PCR; 100% is the level in the THP-1-derived macrophages treated with BSA. C, Western blot analysis of intracellular C3 in PBM-derived macrophages treated with BSA, native and modified LDL, or LXR agonist TO191317 48 h. D, ELISA of C3 secretion into the culture medium by PBM-derived macrophages 48 h after treatment with BSA, native and modified LDL, or LXR agonist TO191317. E, the level of C3 mRNA in human THP-1-derived macrophages. Control, THP-1 cells incubated with BSA; acLDL− and acLDL+ cells, acLDL-negative and positive THP-1-derived macrophages after cell sorting by acLDL content; real-time RT-PCR; 100% is the level in the control THP-1 macrophages. F, the level of C3 mRNA in human THP-1-derived macrophages. Control, THP-1 cells incubated with BSA (100%); AB&TLR4, TLR4-blocking antibodies; LPS, bacterial lipopolysaccharide, 100 ng/ml; real-time RT-PCR. Cells were treated with or without BSA (50 μg/ml), acetylated BSA (50 μg/ml), native LDL (50 μg/ml), acLDL (50 μg/ml), oxLDL (50 μg/ml), LPS (100 ng/ml), or TO191317 (TO; 2.5 μm) for 48 h. Values are presented as means ± S.E. (error bars) of six independent experiments. The statistical analyses of differences between compared groups were performed using an unpaired Student's t test (*, p < 0.05; **, p < 0.01) or Dunnett's criterion (#, p < 0.05). n.s., not significant.
A Western blot assay with antibodies against human C3 shows that acLDL, oxLDL, and TO191317 increased intracellular content of C3 protein in PBM-derived macrophages, whereas native LDL did not (Fig. 1C). acLDL and oxLDL also led to stimulation of C3 protein secretion by PBM-derived macrophages into the culture medium (Fig. 1D).
mLDL Uptake by THP-1-derived Macrophages Is Important for Stimulation of C3 Gene Expression
FITC-labeled acLDL was used to measure the level of acLDL uptake by THP-1-derived macrophages by using laser scanning confocal microscopy (supplemental Fig. 1D) and flow cytometry (supplemental Fig. 2). The level of acLDL accumulation inside macrophages determined by fluorescent microscopy strongly correlates with that measured by FACS (supplemental Fig. 2, A–C). Approximately 40% of cells contained FITC-labeled acLDL inside the cytoplasm 48 h after the start of incubation, whereas 60% of cells did not take up acLDL (supplemental Fig. 2C). We performed sorting of those THP-1 macrophages according to content of acLDL in the cell (acLDL-positive or -negative cells) using FACS (supplemental Fig. 2E) followed by RNA isolation from sorted acLDL− and acLDL+ cells and measurement of C3 mRNA content by using real-time RT-PCR. C3 gene expression was increased by ∼30% in acLDL-negative THP-1 macrophages in comparison with cells that were incubated in the absence of acLDL (Fig. 1E). In contrast, the level of C3 mRNA was elevated by more than 2-fold in acLDL-positive THP-1 macrophages compared with control cells (Fig. 1E). ABCA1 mRNA level was determined in the same cells to control the acLDL content-based sorting quality, and it was shown that ABCA1 gene transcription was increased only in acLDL-positive and not in acLDL-negative THP-1-derived macrophages (supplemental Fig. 1E). Similar results were obtained using FITC-conjugated oxLDL (data not shown). These data suggest the dual mode of mLDL-mediated C3 gene activation; about one-third of the effect depends on LDL signaling outside of the cell, whereas two-thirds depends on mLDL uptake.
mLDL Activates C3 Gene Expression via LXR and TLR4 Pathways in THP-1-derived Macrophages
Some components of mLDL, known as minimally oxidized or minimally modified LDL, may activate the CD14-TLR4 signaling axis in macrophages, leading to the triggering of downstream signaling pathways and thereby increasing cytokine and chemokine expression (39). It is known that another TLR4-activating agent, bacterial LPS, stimulates C3 expression in human monocyte-macrophage cells (40, 41). To determine whether mLDL activates C3 expression in a TLR4-dependent manner, TLR4-specific blocking antibodies were used. Treatment of THP-1 macrophages with blocking antibodies against TLR4 abolished LPS-induced activation of the C3 gene, whereas acLDL-mediated stimulation of C3 gene expression was inhibited only by ∼20% under the same conditions (Fig. 1F). At the same time, incubation of macrophages with acLDL did not lead to a subsequent increment of C3 mRNA level in TO191317-pretreated cells (Fig. 1F). Similar results were obtained using oxLDL (data not shown). These data suggest that moderate activation of C3 gene transcription by mLDL is mediated by TLR4, whereas the major activation effect of mLDL on the C3 gene is mediated by accumulation of LXR ligands in the cell as a result of mLDL uptake.
LXRβ Binds to Human C3 Promoter in Vitro and in Vivo
To ascertain whether the C3 gene is a direct target of LXRs, the human C3 promoter sequence was scanned for the presence of potential LXRE (DR4) sites by using nuclear hormone receptor-scan software (42). A potential LXRE was found between nucleotides −336 and −351 relative to the C3 gene transcription initiation site (Fig. 2A). EMSA was performed to test the ability of human macrophage nuclear proteins to bind with the putative C3(−336/−351)-LXRE (Fig. 2B). The binding of nuclear proteins to the probe was specific because the complex was depleted by an excess of the unlabeled C3-LXRE oligonucleotide but not by an excess of the unlabeled mutated C3-LXRE oligonucleotide (Fig. 2B). To prove LXRβ interaction with C3(−336/−351)-LXRE, we used a DNA affinity precipitation assay using THP-1-derived macrophage nuclear proteins and a biotin-labeled probe containing C3(−336/−351)-LXRE. Western blot analysis of precipitated proteins shows that LXRβ interacts with C3(−336/−351)-LXRE, and an excess of the unlabeled C3-LXRE oligonucleotide but not unlabeled mutated C3-LXRE oligonucleotide decreased the level of LXRβ in the precipitate (Fig. 2C). LXRβ from PBM-derived macrophage nuclear extracts was also bound with C3(−336/−351)-LXRE (Fig. 2D). These data show that the human C3 promoter contains a functional LXRE, which is able to bind LXRβ. Interestingly, alignment of sequences of the human and mouse 5′-region of the C3 gene indicates that the LXRE site is not conserved between human and mouse C3 promoters (the mouse C3 promoter contains deletions and nucleotide substitutions in the region corresponding to the human LXRE) (Fig. 2A). Treatment of mouse peritoneal macrophages with TO191317 or oxLDL did not up-regulate the mouse C3 gene (Fig. 2D) but stimulated ABCA1 gene expression (positive control) (supplemental Fig. 1F). These results show species-specific regulation of the C3 gene by LXR agonist. TO191317 did not lead to the stimulation of C3 gene expression in HepG2 cells (Fig. 2F), but under the same conditions, the level of ABCA1 gene expression was increased in TO191317-treated HepG2 cells (supplemental Fig. 1G).
FIGURE 2.
LXRβ binds LXRE within the human C3 promoter in human macrophages. A, the sequence of LXRE in the human C3 promoter (wild type (hC3-LXRE wt) and mutated (hC3-LXRE mut) LXRE in the human C3 promoter). Sequences of the region of the mouse and the human C3 promoter corresponding to LXRE in the human C3 promoter are shown. B, electrophoretic mobility shift assay of the human macrophage nuclear extract proteins with hC3-LXRE. Shown are a biotin-labeled (Biot.) and biotin-unlabeled (No Biot.) probe containing hC3-LXRE and flanking regions and a DNA-protein complex (C). C and D, DNA affinity precipitation assay using THP-1-derived (C) or PBM-derived (D) macrophage nuclear extract proteins and biotin-labeled probe containing hC3-LXRE followed by Western blot analysis of precipitated proteins using antibodies against LXRβ. E, the level of C3 mRNA in mouse peritoneal macrophages (PM); real-time RT-PCR; 100% is the level in the control peritoneal macrophages. Cells were treated with or without TO191317 (TO; 2.5 μm) or oxLDL (50 μg/ml) for 48 h. F, level of C3 mRNA in human HepG2 cells; real-time RT-PCR; 100% is the level in the control HepG2 cells. Cells were treated with or without TO191317 (2.5 μm) for 48 h. Values are presented as means ± S.E. of six independent experiments. G, ChIP assay with PBM- and THP-1-derived macrophages and HepG2 cells. C3 LXRE, LXRE in the human C3 promoter; ABCA1 LXRE, LXRE in the human ABCA1 promoter; NOD1, the sequence in the human NOD1 gene (specificity control). M, molecular weight marker; AB&LXRβ, antibodies against LXRβ; AB&Actin, antibodies against β-actin. H, luciferase assay of plasmids containing a fragment of the human C3 promoter with (pC3(−463/+268)-Luc) or without (pC3(−310/+268)-Luc) LXRE transfected into THP-1 monocytes and THP-1-derived macrophages treated with or without TO191317 (2.5 μm) for 24 h. Values are presented as means ± S.E. (error bars) of four independent experiments. The statistical analyses of differences between compared groups were performed using an unpaired Student's t test (*, p < 0.05) or Dunnett's criterion (#, p < 0.05). n.s., not significant.
To validate the binding of LXRβ to the human C3 promoter in vivo, a ChIP assay with anti-LXRβ antibodies was performed with PBM- and THP-1-derived macrophages. As a negative control, the anti-β-actin antibodies were used. The essential enrichment of the specific PCR signal was identified with primers specific for the human C3 promoter and ABCA1 promoter (positive control) in the precipitate with anti-LXRβ antibodies versus anti-β-actin ones in both PBM- and THP-1-derived macrophages (Fig. 2G). In the same experiment, there was not enrichment of PCR signal with primers specific for the human NOD1 gene (negative control). Next, a ChIP assay was performed to compare the levels of LXRβ binding with the human C3 promoter in macrophages and human hepatoma HepG2 cells. Fig. 2G shows that in contrast to macrophages, there was no enrichment of the C3-specific PCR band in ChIP with anti-LXRβ antibodies in HepG2 cells, whereas LXRE of the ABCA1 promoter was immunoprecipitated in the same experiment. These results provide evidence that LXRβ binds to the LXRE sequence of the C3 promoter in human macrophages but not HepG2 cells. Moreover, our results indicate that the LXRE of the human C3 promoter is occupied by HNF4α, a nuclear receptor highly expressed in hepatocytes, in HepG2 cells (data not shown). We suggest that HNF4α displaces LXRs from the LXRE of the C3 promoter in HepG2 cells. These data indicate that LXR activation regulates C3 gene expression in a cell type-specific manner.
Deletion of LXRE of C3 Promoter Abrogates Induction of C3 Transcription by LXR Agonist TO191317
Transfection of plasmids containing deletion variants of the human C3 promoter linked with the luciferase gene shows that deletion of C3(−336/−351)-LXRE significantly decreased activity of the human C3 promoter in both THP-1 monocytes and THP-1-derived macrophages (Fig. 2H). TO191317 stimulated activity of the human C3 promoter containing C3(−336/−351)-LXRE, whereas a truncated variant of the human C3 promoter, lacking the LXRE, did not respond to the treatment of THP-1 monocytes or THP-1-derived macrophages with TO191317 (Fig. 2H).
LXR Agonist TO191317 Increases C3 Protein Synthesis but Not Secretion by Human PBM-derived Macrophages
Because LXR activation increases C3 gene transcription in human macrophages, ELISA was used to determine C3 protein content in the cell lysates and the culture medium of PBM-derived macrophages incubated with TO191317 at different time points. Treatment of macrophages with TO191317 led to an increase of C3 protein content in the cell after 48 h of incubation (Fig. 3A). Despite elevation of the intracellular C3 protein level, LXR activation did not increase C3 concentration in the culture medium of macrophages (Fig. 3B). Treatment of THP-1 macrophages with TO191317 also resulted in elevation of the level of intracellular but not secreted C3 protein (data not shown).
FIGURE 3.
C3 transcription and secretion under the impact of mLDL and NF-κB or MAPK inhibitors in human macrophages. A and B, ELISA of C3 protein content in PBM-derived macrophage lysates (A) and in the culture medium (B). Cells were incubated with or without LXR agonist TO191317 (TO; 2.5 μm) for 24 or 48 h. C, ELISA of C3 protein content in the culture medium of PBM-derived macrophages; cells were treated with or without oxLDL (50 μg/ml, 48 h) and TLR4-specific blocking antibodies (AB&TLR4; 1 μg/ml). D, ELISA of C3 protein content in the culture medium of THP-1-derived macrophages; cells were treated with or without oxLDL or acLDL (50 μg/ml, 48 h) and TLR4-specific blocking antibodies (1 μg/ml). E, the level of C3 mRNA in human THP-1-derived macrophages; 100% is the level in the control THP-1 cells (untreated with inhibitors). F and G, ELISA of C3 protein content in the culture medium (F) and cell lysates (G) of THP-1-derived macrophage. Control, THP-1 cells untreated with inhibitors. Cells were treated with SB203580 (p38 inhibitor) (25 μm) (p38 inh), SP600125 (JNK1/2/3 inhibitor) (10 μm) (JNK inh), U0126 (MEK1/2 inhibitor) (10 μm) (MEK inh), QNZ (NF-κB inhibitor) (10 nm) (NF-κB inh), and BSA (50 μg/ml) or oxLDL (50 μg/ml) for 48 h. Values are presented as means ± S.E. (error bars) of six independent experiments. The statistical analyses of differences between compared groups were performed using an unpaired Student's t test (*, p < 0.05; **, p < 0.01) or Dunnett's criterion (#, p < 0.05).
mLDL Stimulates C3 Protein Secretion via TLR4 Activation in PBM- and THP-1-derived Macrophages
To determine whether mLDL stimulates C3 protein secretion by human macrophages, we performed ELISA to measure C3 protein concentration in the culture medium. Treatment of PBM-derived macrophages with oxLDL significantly increased C3 protein secretion after 48 h of incubation (Fig. 3C). Interestingly, inhibition of TLR4 by using TLR4-specific blocking antibodies abolished the oxLDL-mediated effect on C3 secretion (Fig. 3C). As has been noted above, inactivation of TLR4 leads to only a 20% decrease in oxLDL-mediated stimulation of C3 gene transcription, so this down-regulation of the C3 gene seems not to be responsible for blocking of oxLDL-induced C3 secretion. To exclude the probable effect of contamination of oxLDL with LPS, we show that treatment of THP-1 macrophages with polymyxin B did not inhibit the effects of mLDL toward C3 secretion (data not shown). Simultaneously, blocking of TLR4 resulted in an abrogation of oxLDL- and acLDL-induced stimulation of C3 secretion by THP-1-derived macrophages (Fig. 3D). The level of acLDL or oxLDL uptake by macrophages is not reduced significantly after 48 h of incubation in experiments with TLR4-specific blocking antibodies, as has been shown by measuring of accumulation of FITC-labeled acLDL and oxLDL or lipid droplets in the THP-1 macrophages (supplemental Fig. 3, A and B). Thus, the observed effects of inhibition of TLR4 on mLDL-stimulated secretion of C3 protein do not depend on an alteration in mLDL uptake efficiency in THP-1 macrophages. Taken together, these data demonstrate that mLDL activates C3 protein secretion by human macrophages via a TLR4-dependent pathway.
Role of NF-κB and MAPK Pathways in TLR4-dependent Activation of C3 Expression, Synthesis, and Secretion by THP-1-derived Macrophages Treated with oxLDL
Activation of TLR4 by exogenous or endogenous ligands up-regulates a number of immune genes by utilizing NF-κB and MAPK (ERK1/2, JNK, and p38) signaling pathways (43). To estimate signaling pathways that are involved in the TLR4-dependent regulation of C3 gene expression and protein secretion by oxLDL, we used NF-κB and MAPK inhibitors. Treatment of THP-1 macrophages with MEK1/2-ERK1/2 inhibitor appreciably reduced the level the of oxLDL-mediated increase of C3 gene expression, whereas inhibition of the NF-κB, JNK, or p38 pathway did not result in any significant abolishment of oxLDL-mediated up-regulation of the C3 gene (Fig. 3E). Inactivation of JNK, p38, or MEK1/2 abolished the oxLDL-mediated increase of intracellular C3 protein content in THP-1 macrophages (Fig. 3F). Inhibition of NF-κB but not p38, JNK, or MEK1/2 abated the level of C3 protein secretion induced by oxLDL in THP-1 macrophages (Fig. 3G). In addition, analysis of oxLDL uptake by THP-1 macrophages indicates that inhibition of JNK but not the NF-κB, MEK1/2, or p38 pathway results in suppression of oxLDL accumulation in THP-1 cells (supplemental Fig. 3C). Taken together, these data suggest that oxLDL up-regulates C3 gene expression via the TLR4-MEK1/2-ERK1/2 pathway, whereas oxLDL-dependent stimulation of C3 protein secretion seems to be mediated by NF-κB activation in human macrophages.
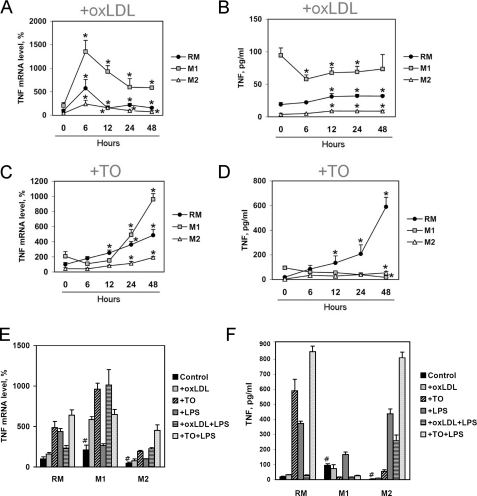
C3 Gene Expression and Protein Secretion Is Increased in IFNγ/LPS-treated Classically Activated (M1) and Decreased in IL-4-treated Alternatively Activated (M2) Human Macrophages
The microbial and cytokine environment drives macrophages to express specialized and polarized functional properties (44). The proinflammatory activities of classically activated (M1) macrophages are enhanced in the presence of LPS and/or Th1 cytokines, such as IFNγ or IL-12. Stimulation of macrophages with Th2 cytokines IL-4 or IL-13 results in an alternative macrophage activation (M2) (45). To study the impact of oxLDL and LXR agonist TO191317 on C3 expression and secretion in several functional macrophage stages, resting or classically or alternatively activated macrophages were treated with those compounds for different times (Fig. 4, A–C). Treatment of human PBM-derived macrophages with IFNγ/LPS during differentiation up-regulated C3 gene expression by 2.5-fold and C3 protein secretion by 1.8-fold in comparison with resting macrophages (RM) (Fig. 4, A–E). Differentiation of PBM-derived macrophages with IL-4 in the culture medium resulted in a decrease of the level of C3 mRNA by 5.6-fold and of C3 protein content in the culture medium by 5.2-fold (Fig. 4, A–E). As a control, the levels of TNFα expression and secretion, as well as IL-12B, IL-15, MCP-1, MRC1L1 (CD206), and IL-10 mRNA levels were measured in RM, M1, and M2 PBM-derived macrophages (supplemental Fig. 4), and obtained results are in accordance with described characteristics of classically and alternatively activated macrophages (45). C3 expression and secretion are also up-regulated in M1 and down-regulated in M2 as compared with resting THP-1-derived macrophages (supplemental Fig. 5).
FIGURE 4.
C3 transcription and secretion and surface expression of CD14 and TLR4 are differently regulated by oxLDL and LXR agonist TO191317 in human resting and classically (M1) and alternatively (M2) activated PBM-derived macrophages. A and B, the level of C3 mRNA (A) determined by real-time RT-PCR and C3 secretion (B) determined by ELISA in resting or classically or alternatively activated PBM-derived macrophages 0–48 h after the addition of oxLDL (50 μg/ml). C, level of C3 mRNA determined by real-time RT-PCR in resting or classically or alternatively activated PBM-derived macrophages 0–48 h after the addition of LXR agonist TO191317 (TO; 2.5 μm). D, the level of C3 mRNA in human PBM-derived macrophages; real-time RT-PCR; 100% is the level in the resting macrophages treated with BSA. E, ELISA of C3 protein content in the culture medium of PBM-derived macrophages. Values are presented as means ± S.E. (error bars) of six independent experiments. The statistical analyses of differences were performed separately for values in RM, M1, or M2 groups using an unpaired Student's t test (*, p < 0.05) or Dunnett's criterion (for comparison of RM, M1, and M2) (#, p < 0.05). F, level of CD14 mRNA in human PBM-derived macrophages; real-time RT-PCR; 100% is the level in the resting macrophages treated with BSA. G, FACS analysis of surface CD14 expression in human PBM-derived macrophages; the percentage of CD14+ cells is indicated. H, level of TLR4 mRNA in human PBM-derived macrophages; real-time RT-PCR; 100% is the level in the resting macrophages. I, FACS analysis of surface TLR4 expression in human PBM-derived macrophages. Medians of surface TLR4 expression are indicated. Values are presented as means ± S.E. of six independent experiments. The statistical analyses of differences between compared groups were performed using Dunnett's criterion (#, p < 0.05). PBM-derived macrophages were treated with BSA (20 ng/ml) (RM), with IFNγ (20 ng/ml) and bacterial LPS (100 ng/ml) (M1) or IL-4 (20 ng/ml) (M2) for 6 days. Cells were treated with oxLDL (50 μg/ml) and/or LXR agonist TO191317 (2.5 μm) and/or LPS (100 ng/ml) for 0–48 h.
oxLDL and LXR Agonist TO191317 Differently Activate C3 Gene Expression and Protein Secretion by Resting, M1, and M2 PBM-derived Macrophages
oxLDL stimulated C3 gene expression in resting, M1, and M2 macrophages in a time-dependent manner (Fig. 4A) and activated C3 secretion 24–48 h after its addition in resting and M2, but not M1, macrophages (Fig. 4B). oxLDL-mediated stimulation of C3 expression and secretion by macrophages was dose-dependent (supplemental Fig. 6, A and B). TO191317 increased C3 gene expression in all lines of macrophage polarization in a time-dependent (Fig. 4C) and dose-dependent (supplemental Fig. 6C) manner but, as was stated above, did not stimulate C3 secretion by macrophages (Figs. 1D and 3B). Treatment of resting human PBM-derived macrophages with oxLDL resulted in an increase of C3 mRNA level by 2.3-fold 48 h after its addition (Fig. 4D). The levels of oxLDL-induced stimulation of the C3 gene were 1.8-fold in M1 and 2.5-fold in M2 macrophages at that time point (Fig. 4D). LXR activation with TO191317 resulted in a stimulation of C3 gene transcription by 3.6-, 2.3-, and 5.1-fold in resting, M1, and M2 macrophages 48 h after addition, respectively (Fig. 4D). Interestingly, M2 polarizations of macrophages diminished oxLDL uptake (supplemental Fig. 7), which is in accordance with published data (46), but it did not repress the level of oxLDL- or TO191317-mediated up-regulation of the C3 gene (Fig. 4D). Indeed, the level of TO191317-induced activation of C3 gene expression was increased in M2 versus resting macrophages (Fig. 4D).
Treatment of resting, M1, or M2 PBM-derived macrophages with oxLDL stimulated both secretion and intracellular accumulation of C3 protein, whereas TO191317 led to an increase of intracellular C3 protein content but not secreted C3 in those cells 48 h after addition (Fig. 4E and supplemental Fig. 8). Interestingly, the oxLDL-mediated increase of intracellular C3 protein content was similar in resting, M1, and M2 macrophages, but the levels of oxLDL-induced secretion of C3 protein were decreased in M1 and M2 macrophages in comparison with resting macrophages (Fig. 4E).
The ChIP assay shows that the level of LXRβ binding with the human C3 promoter was increased in M2 macrophages as compared with resting macrophages (supplemental Fig. 9). These results appear to provide a partial explanation of the increased response of the C3 gene to LXR agonist TO191317 in M2 macrophages.
It is well studied that mLDL binds to CD14 and activates TLR4 on macrophages (47). Because mLDL seems to activate C3 protein secretion via the CD14-TLR4 pathway, we determined CD14 mRNA level and protein content of the membrane form of CD14 in human PBM-derived macrophages. CD14 mRNA level was increased in M1 and decreased in M2 in comparison with resting macrophages (Fig. 4F). Similarly, surface CD14 expression was increased in M1 and decreased in M2 as compared with resting macrophages in terms of CD14-positive cells (Fig. 4G). Interestingly, incubation of macrophages with oxLDL resulted in an increase of CD14-positive cells in the cases of resting and M2 macrophages but not M1 macrophages (Fig. 4G). Moreover, average surface CD14 expression was significantly reduced in oxLDL-treated versus oxLDL-untreated M1 macrophages (Fig. 4G). Next, we measured the levels of TLR4 mRNA as well as surface TLR4 expression in PBM-derived macrophages. The level of TLR4 mRNA was decreased in M2 but not in M1 as compared with resting macrophages (Fig. 4H). However, FACS analysis shows that the membrane expression of TLR4 was significantly lower in both M1 and M2 macrophages than in resting macrophages (Fig. 4I). Although the decreased level of membrane form of TLR4 in M2 macrophages may be explained by down-regulation of the TLR4 gene by IL-4 in those cells, reduction of surface TLR4 in M1 macrophages appears to be a result of specific down-regulation of membrane TLR4 at the post-translational level by LPS, and this observation is in accordance with published data (48). TLR4 was described as a target for positive regulation by LXR ligands and oxLDL in human macrophages (40). We show that treatment of human macrophages with oxLDL or TO191317 resulted in a stronger stimulation of membrane TLR4 expression in the resting macrophages as compared with M1 or M2 macrophages (supplemental Fig. 10). Thus, these results suggest that decreased levels of oxLDL-induced C3 protein secretion in M1 and M2 macrophages may be, at least partly, explained by down-regulation of surface CD14 and TLR4 expression in those cells.
oxLDL and LXR Agonist TO191317 Potentiate LPS-induced C3 Gene Expression and Protein Secretion in Human Macrophages
Long term LXR activation enhances the response of macrophages to LPS in terms of TNFα and MCP-1 secretion due to induction of TLR4 expression (49). On the other hand, oxLDL inhibits LPS-induced TNFα gene expression and protein synthesis in human macrophages via reduction of LPS-induced binding of NF-κB to DNA (50). We show that treatment of PBM-derived macrophages with 100 ng/ml LPS for 48 h increased C3 gene expression in resting, M1, and M2 macrophages but did not result in a stimulation of C3 secretion by these cells (Fig. 4, D and E). oxLDL potentiates LPS-induced stimulation of C3 gene expression in resting and M2 but not in M1 macrophages (Fig. 4D). Interestingly, simultaneous action of oxLDL and LPS resulted in increased C3 protein secretion as compared with oxLDL alone only in M2 macrophages, whereas in oxLDL-treated resting or M1 macrophages, the addition of LPS did not lead to a further increase of C3 secretion (Fig. 4E). Treatment of human macrophages with TO191317 for 48 h enhanced response to LPS in terms of C3 gene expression and protein secretion in resting, M2, and, to a lesser extent, in M1 macrophages (Fig. 4, D and E). To establish the specificity of oxLDL- and TO191317-mediated potentiation of LPS-induced secretion of C3 protein, we measured TNFα gene expression and TNFα protein secretion by macrophages treated with several combinations of oxLDL, TO191317, and LPS (Fig. 5, A–F). TNFα is known as a direct target for positive regulation by LXR ligands due to LXRE in its promoter region (51). oxLDL up-regulated the TNFα gene in resting, M1, and M2 macrophages with maximum activation of transcription at 6 h after the addition (Fig. 5A) and stimulated TNFα secretion in a time- and dose-dependent manner in resting and M2, but not M1, macrophages (Fig. 5B and supplemental Fig. 11, A and B). TO191317 stimulated TNFα gene expression and protein secretion in a time- and dose-dependent manner in resting and M2 macrophages, whereas this LXR agonist led to an increase of TNFα transcription but not secretion by M1 macrophages (Fig. 5, C and D, and supplemental Fig. 11, C and D). Treatment of macrophages with LPS for 48 h resulted in an increase of TNFα mRNA level in resting and M2, but not in M1, macrophages (Fig. 5E). oxLDL diminished the level of LPS-induced increase of TNFα mRNA transcription in resting macrophages but potentiated LPS response in M1 and M2 macrophages (Fig. 5E). LXR agonist TO191317 led to stronger induction of the TNFα gene in response to LPS in resting and M2 macrophages but did not affect LPS-mediated up-regulation of the TNFα gene in M1 macrophages (Fig. 5E). oxLDL and LXR agonist TO191317 stimulated TNFα secretion by resting human macrophages (Fig. 5F). Interestingly, TO191317 also stimulated TNFα secretion by M2 macrophages but led to a decrease of TNFα in the culture medium of M1 macrophages (Fig. 5F). oxLDL suppressed LPS-induced activation of TNFα secretion by resting, M1, and M2 macrophages (Fig. 5F). At the same time, TO191317 stimulated LPS-induced activation of TNFα secretion by resting and M2 macrophages, whereas treatment of M1 macrophages with TO191317 resulted in abolishment of LPS-mediated activation of TNFα secretion (Fig. 5F). Taken together, these results suggest that, despite similar effects of oxLDL and LXR agonist TO191317 directed to up-regulation of C3 and TNFα transcription in human macrophages, those compounds differently regulate C3 and TNFα secretion and potentiate LPS response in a gene- and macrophage polarization-specific manner (supplemental Fig. 12).
FIGURE 5.
TNFα expression and secretion by human resting and classically (M1) and alternatively (M2) activated PBM-derived macrophages. A and B, level of TNFα mRNA (A) determined by real-time RT-PCR and TNFα secretion (B) determined by ELISA in resting and classically or alternatively activated PBM-derived macrophages 0–48 h after the addition of oxLDL (50 μg/ml). C and D, the level of TNFα mRNA (C) determined by real-time RT-PCR and TNFα secretion (D) determined by ELISA in resting and classically or alternatively activated PBM-derived macrophages 0–48 h after the addition of LXR agonist TO191317 (TO; 2.5 μm). E, level of TNFα mRNA in human PBM-derived macrophages; real-time RT-PCR; 100% is the level in the macrophages treated with BSA. F, ELISA of TNFα protein content in the culture medium of PBM-derived macrophages. PBM-derived macrophages were treated with BSA (20 ng/ml) (RM), with IFNγ (20 ng/ml) and bacterial LPS (100 ng/ml) (M1) or IL-4 (20 ng/ml) (M2) for 6 days. Cells were treated with oxLDL (50 μg/ml) and/or LXR agonist TO191317 (2.5 μm) and/or LPS (100 ng/ml) for 48 h. Values are presented as means ± S.E. (error bars) of six independent experiments. The statistical analyses of differences were performed separately for values in the RM, M1, or M2 group using an unpaired Student's t test (*, p < 0.05) or Dunnett's criterion (for comparison of RM, M1, and M2) (#, p < 0.05).
C3a Stimulates oxLDL Uptake by Human Macrophages in Vitro
C3a was shown to influence immune properties of dendritic cells (52, 53) and monocyte-macrophage cells (54), and a 5–10 nm concentration of C3a was enough to realize its signaling functions toward those cells. Treatment of human PBM-derived macrophages with oxLDL led to an increase of the level of C3a in the culture medium (supplemental Fig. 13). Because C3a was found in human atherosclerotic lesions, and expression of C3a receptor has been identified in human atherosclerotic lesions and in macrophages (2, 55, 56), we studied whether C3a influences oxLDL uptake in human PBM-derived macrophages. Treatment of PBM-derived macrophages with C3a (C3a was added to macrophages at the 4th or at the 2nd and 4th days of differentiation, and cells were differentiated until the 6th day; oxLDL was added to macrophages at the 4th day of differentiation) resulted in an increase of oxLDL uptake and lipid droplet accumulation in comparison with C3a-untreated cells (Fig. 6A and supplemental Fig. 14). Macrophages incubated with C3a for 4 days showed more enhanced oxLDL uptake than cells treated with C3a for 2 days (Fig. 6A and supplemental Fig. 14).
FIGURE 6.
C3a stimulates oxLDL uptake and C3 expression and secretion by human PBM-derived macrophages in vitro. A, FACS analysis of FITC-labeled oxLDL accumulation (50 μg/ml; 48 h) in PBM-derived macrophages treated with or without C3a (10 nm). C3a (10 nm) was added to the cells (at the 4th day of differentiation) at the same time as oxLDL (shown as C3a 2 days) or 2 days before and with oxLDL (at the 2nd and 4th days of differentiation) (shown as C3a 4 days). Medians of fluorescence are indicated. B–D, the level of C3 mRNA (B) determined by real-time RT-PCR and secreted (C) and intracellular (D) C3 protein determined by ELISA in human PBM-derived macrophages treated with C3a (10 nm) for 0–72 h. E and F, level of C3 mRNA (E) determined by real-time RT-PCR and secreted C3 (F) determined by ELISA in human PBM-derived macrophages treated with or without C3a (10 nm), oxLDL (50 μg/ml), or bacterial LPS (100 ng/ml) for 48 h. Values are presented as means ± S.E. (error bars) of five independent experiments. The statistical analyses of differences between compared groups were performed using an unpaired Student's t test (*, p < 0.05) or Dunnett's criterion (#, p < 0.05).
C3a Activates C3 Gene Expression and Protein Secretion by Human Macrophages
Treatment of PBM-derived human macrophages with C3a resulted in fast elevation of the level of C3 mRNA with a maximum at 24 h, whereas the effect of C3a on C3 gene expression was undetectable at the mRNA level 72 h after treatment (Fig. 6B). Secreted and intracellular C3 were also increased 12–24 h after treatment of macrophages with C3a (Fig. 6, C and D). C3a also increased C3 gene expression in oxLDL-treated macrophages, resulting in further C3 gene up-regulation 48 h after treatment (Fig. 6E). C3a stimulated C3 secretion in oxLDL-treated cells, which led to a cumulative increase of C3 in the culture medium (Fig. 6F). By contrast with oxLDL, stimulation of C3 gene expression by LPS was not increased by C3a, and C3a did not stimulate C3 protein secretion in LPS-treated macrophages (Fig. 6, E and F). Thus, C3a-dependent effects on C3 expression and secretion appear to be inhibited by LPS but enhanced by oxLDL in human macrophages.
DISCUSSION
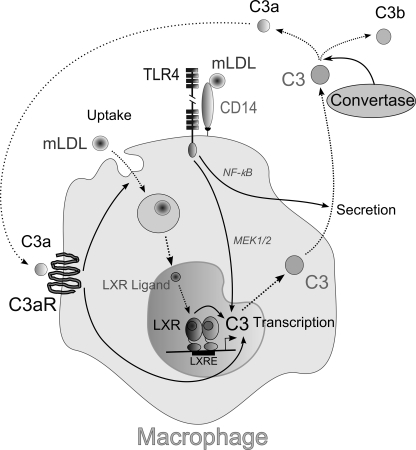
A principal finding of the present study is that mLDL positively regulates C3 gene expression in human but not in mouse macrophages. Both C3 mRNA and protein were found in human atherosclerotic lesions (14, 15). On the other hand, atherosclerotic lesions contain oxidatively modified LDL, which has a wide variety of immunological properties associated with atherosclerosis (57). Being taken up by macrophages, mLDL leads to formation of oxysterols, which are ligands for LXRs (37). In addition, mLDL has been found to bind to CD14 and activate Toll-like receptors on macrophages (47). We show that mLDL activates C3 expression after uptake by macrophages, and mLDL-mediated up-regulation of the C3 gene appears to be mediated by LXRs, whereas activation of the TLR4-MEK-ERK pathway by mLDL has a minor effect on stimulation of C3 gene expression (Fig. 7). In contrast to C3 gene transcription, C3 protein secretion is stimulated by mLDL via TLR4-dependent activation of the NF-κB pathway (Fig. 7). We found the LXRE site in the human C3 promoter and showed that LXRβ is bound with this regulatory site in human macrophages in vivo and in vitro. The LXRE site is not conserved between humans and mice, which seems to explain the species-specific regulation of C3 expression by LXRs. Interestingly, the TLR4 gene was found to be a target for regulation by LXRs in human but not in mouse macrophages due to the LXRE site in the human TLR4 promoter, which is not conserved in mice (49). We also show that the C3 gene is regulated by LXRs in a cell type-specific manner because LXR agonist TO191317 does not stimulate C3 gene transcription in human hepatoma cell line HepG2, and LXRE in the C3 promoter is not occupied by LXRβ in HepG2 cells.
FIGURE 7.
Hypothetical scheme illustrating a possible mechanism of mLDL-mediated regulation of C3 expression and secretion by human macrophages. mLDL is taken up by macrophage, which results in intracellular oxidized cholesterol accumulation. Oxidized cholesterol (LXR ligand) activates C3 gene transcription via LXR interacting with LXRE inside the human C3 gene promoter. Interaction of mLDL with CD14 and TLR4 stimulates both C3 gene transcription (via the MEK1/2-ERK1/2 signaling pathway) and C3 protein secretion (via NF-κB activation) by macrophage. Produced as a result of complement activation, anaphylatoxin C3a interacts with C3a receptor (C3aR) and promotes C3 transcription and mLDL uptake by macrophage. The arrows show activation, and the dotted line arrows show transport of C3 and C3 cleavage products inside or outside of the cell. For a further explanation, see “Results” and “Discussion.”
Treatment of macrophages with various cytokines and/or bacterial components leads to classical (M1) or alternative (M2) activation of macrophages (44, 45). We show that IFNγ/LPS-treated macrophages (M1) have an augmented level of C3 transcription and secretion, whereas C3 is down-regulated in IL-4-treated macrophages (M2). oxLDL and LXR agonist TO191317 stimulate C3 expression in resting, M1, and M2 macrophages, and the amplitude of TO191317-mediated activation of the C3 gene is increased in M2 as compared with resting macrophages, probably due to increased LXRβ binding with LXRE in the C3 promoter in M2 macrophages. The TNFα gene is known to be positively regulated by an LXR agonist (51), and, in contrast to the C3 gene, TNFα activation by TO191317 is comparable in resting, M1, and M2 macrophages. On the other hand, the levels of oxLDL-induced secretion of C3 are down-regulated in M1 and M2 macrophages, probably due to diminished surface TLR4 expression in oxLDL-treated and untreated M1 and M2 as compared with resting macrophages. In support of this suggestion, we have found that LPS-induced activation of the TNFα gene is also abolished in M1 and M2 in comparison with resting macrophages. oxLDL-mediated decrease of surface CD14 in M1 macrophages also may explain reduced response of these cells in terms of oxLDL-induced C3 secretion.
Pretreatment of mouse macrophages with oxLDL or minimally modified LDL enhances LPS-induced expression and secretion of different proinflammatory cytokines and chemokines (58, 59), and this process appears to be partly mediated by activation of TLR4 signaling by mLDL in mouse macrophages (39). On the contrary, oxLDL inhibits LPS-induced TNFα gene expression and protein synthesis in human macrophages (50). Our data show that oxLDL potentiates LPS-induced up-regulation of the C3 gene in resting and M2 macrophages and overstimulates LPS-induced C3 protein secretion in M2 macrophages. Interestingly, oxLDL and LXR agonist TO191317 differently influence LPS-induced TNFα gene expression and protein secretion, depending on the functional stage of human macrophages. For instance, TO191317 potentiates LPS-stimulated secretion of TNFα by resting and M2 macrophages but abrogates an increase of both spontaneous and LPS-activated TNFα secretion in M1 macrophages. It was shown that ligand-activated LXR decreases the induction of several inflammatory genes, such as iNOS, COX-2, MMP-9, and various chemokines, in response to LPS, TNFα, and IL-1β stimuli (60). The observed differences between oxLDL- or LXR agonist-dependent regulation of LPS-induced activation of C3 and TNFα genes in resting, M1, and M2 macrophages might be explained by different implications of anti-inflammatory activities of LXRs in dependence on macrophage functional stage.
We show that C3a up-regulates oxLDL uptake by human macrophages. In addition, C3a activates C3 gene expression and secretion in both oxLDL-treated and untreated macrophages. The C3 gene was shown to be up-regulated by C3a in human keratinocytes (61), but the mechanism of this activation still has not been elucidated. Thus, C3a may be involved in positive feedback regulation of both oxLDL-dependent C3 secretion and C3a generation (Fig. 7).
Taken together, our results show that LXR ligands, which are generated in mLDL-laden macrophage, activate the C3 gene via LXR binding with LXRE inside the human C3 gene promoter. In addition, mLDL activates the CD14-TLR4 signaling axis, stimulating thereby both C3 gene transcription and C3 protein secretion by macrophage (Fig. 7). Moreover, anaphylatoxin C3a, which may be produced as a result of C3 cleavage by convertases, promotes C3 expression and secretion and mLDL uptake by macrophage. Certainly, these data are based on an in vitro study, so the conclusion that all of the described mechanisms are realized in in vivo macrophages should be assessed with caution and is a subject of further investigation. Nevertheless, our results suggest a novel role of oxLDL and LXRs in regulation of C3 expression and secretion by human macrophages and show interplays between the complement system, TLR4, and mLDL metabolism, which may occur during development of atherosclerotic lesions.
Supplementary Material
Acknowledgments
We thank Dr. A. D. Denisenko, Dr. V. N. Kokriakov, and Dr. E. P. Kisseleva (Institute of Experimental Medicine Russian Academy of Medical Sciences, St. Petersburg, Russia) for helpful critical discussion and Dr. S. A. Podzorova (Pavlov Institute of Physiology of the Russian Academy of Sciences, St. Petersburg, Russia) for methodological assistance with the laser scanning confocal microscope. We thank Ekaterina Grinevich and Alexander Efremov for assistance with human and mouse macrophage preparation experiments.
This work was supported by Russian Fund of Basic Research Grant 11-04-02012.

This article contains supplemental Figs. 1–14.
- mLDL
- modified low density lipoprotein
- oxLDL
- oxidized low density lipoprotein
- acLDL
- acetylated low density lipoprotein
- LXR
- liver X receptor
- LXRE
- LXR-responsive element
- PBM
- peripheral blood monocyte(s)
- RM
- resting macrophage(s).
REFERENCES
- 1. Walport M. J. (2001) Complement. First of two parts. N. Engl. J. Med. 344, 1058–1066 [DOI] [PubMed] [Google Scholar]
- 2. Klos A., Tenner A. J., Johswich K. O., Ager R. R., Reis E. S., Köhl J. (2009) The role of the anaphylatoxins in health and disease. Mol. Immunol. 46, 2753–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ricklin D., Hajishengallis G., Yang K., Lambris J. D. (2010) Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 11, 785–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alper C. A., Johnson A. M., Birtch A. G., Moore F. D. (1969) Human C3. Evidence for the liver as the primary site of synthesis. Science 163, 286–288 [DOI] [PubMed] [Google Scholar]
- 5. Einstein L. P., Hansen P. J., Ballow M., Davis A. E., 3rd, Davis J. S., 4th, Alper C. A., Rosen F. S., Colten H. R. (1977) Biosynthesis of the third component of complement (C3) in vitro by monocytes from both normal and homozygous C3-deficient humans. J. Clin. Invest. 60, 963–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Katz Y., Strunk R. C. (1988) Synovial fibroblast-like cells synthesize seven proteins of the complement system. Arthritis Rheumat. 31, 1365–1370 [DOI] [PubMed] [Google Scholar]
- 7. Warren H. B., Pantazis P., Davies P. F. (1987) The third component of complement is transcribed and secreted by cultured human endothelial cells. Am. J. Pathol. 129, 9–13 [PMC free article] [PubMed] [Google Scholar]
- 8. Lévi-Strauss M., Mallat M. (1987) Primary cultures of murine astrocytes produce C3 and factor B, two components of the alternative pathway of complement activation. J. Immunol. 139, 2361–2366 [PubMed] [Google Scholar]
- 9. Choy L. N., Rosen B. S., Spiegelman B. M. (1992) Adipsin and an endogenous pathway of complement from adipose cells. J. Biol. Chem. 267, 12736–12741 [PubMed] [Google Scholar]
- 10. Sahu A., Lambris J. D. (2001) Structure and biology of complement protein C3, a connecting link between innate and acquired immunity. Immunol. Rev. 180, 35–48 [DOI] [PubMed] [Google Scholar]
- 11. Ross R. (1999) Atherosclerosis. An inflammatory disease. N. Engl. J. Med. 340, 115–126 [DOI] [PubMed] [Google Scholar]
- 12. Getz G. S. (2005) Thematic review series. The immune system and atherogenesis. Immune function in atherogenesis. J. Lipid Res. 46, 1–10 [DOI] [PubMed] [Google Scholar]
- 13. Niculescu F., Rus H. (1999) Complement activation and atherosclerosis. Mol. Immunol. 36, 949–955 [DOI] [PubMed] [Google Scholar]
- 14. Yasojima K., Schwab C., McGeer E. G., McGeer P. L. (2001) Complement components, but not complement inhibitors, are up-regulated in atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 21, 1214–1219 [DOI] [PubMed] [Google Scholar]
- 15. Hansson G. K., Holm J., Kral J. G. (1984) Accumulation of IgG and complement factor C3 in human arterial endothelium and atherosclerotic lesions. Acta Pathol. Microbiol. Immunol. Scand. A 92, 429–435 [DOI] [PubMed] [Google Scholar]
- 16. Schmiedt W., Kinscherf R., Deigner H. P., Kamencic H., Nauen O., Kilo J., Oelert H., Metz J., Bhakdi S. (1998) Complement C6 deficiency protects against diet-induced atherosclerosis in rabbits. Arterioscler. Thromb. Vasc. Biol. 18, 1790–1795 [DOI] [PubMed] [Google Scholar]
- 17. Patel S., Thelander E. M., Hernandez M., Montenegro J., Hassing H., Burton C., Mundt S., Hermanowski-Vosatka A., Wright S. D., Chao Y. S., Detmers P. A. (2001) ApoE(-/-) mice develop atherosclerosis in the absence of complement component C5. Biochem. Biophys. Res. Commun. 286, 164–170 [DOI] [PubMed] [Google Scholar]
- 18. Buono C., Come C. E., Witztum J. L., Maguire G. F., Connelly P. W., Carroll M., Lichtman A. H. (2002) Influence of C3 deficiency on atherosclerosis. Circulation 105, 3025–3031 [DOI] [PubMed] [Google Scholar]
- 19. Persson L., Borén J., Robertson A. K., Wallenius V., Hansson G. K., Pekna M. (2004) Lack of complement factor C3, but not factor B, increases hyperlipidemia and atherosclerosis in apolipoprotein E−/− low density lipoprotein receptor−/− mice. Arterioscler. Thromb. Vasc. Biol. 24, 1062–1067 [DOI] [PubMed] [Google Scholar]
- 20. Onat A., Can G., Rezvani R., Cianflone K. (2011) Complement C3 and cleavage products in cardiometabolic risk. Clin. Chim. Acta 412, 1171–1179 [DOI] [PubMed] [Google Scholar]
- 21. Muscari A., Massarelli G., Bastagli L., Poggiopollini G., Tomassetti V., Drago G., Martignani C., Pacilli P., Boni P., Puddu P. (2000) Relationship of serum C3 to fasting insulin, risk factors, and previous ischaemic events in middle-aged men. Eur. Heart J. 21, 1081–1090 [DOI] [PubMed] [Google Scholar]
- 22. Ajjan R., Grant P. J., Futers T. S., Brown J. M., Cymbalista C. M., Boothby M., Carter A. M. (2005) Complement C3 and C-reactive protein levels in patients with stable coronary artery disease. Thromb. Haemost. 94, 1048–1053 [DOI] [PubMed] [Google Scholar]
- 23. Mevorach D., Mascarenhas J. O., Gershov D., Elkon K. B. (1998) Complement-dependent clearance of apoptotic cells by human macrophages. J. Exp. Med. 188, 2313–2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Galkina E., Ley K. (2009) Immune and inflammatory mechanisms of atherosclerosis. Annu. Rev. Immunol. 27, 165–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ischenko A., Sayah S., Patte C., Andreev S., Gasque P., Schouft M. T., Vaudry H., Fontaine M. (1998) Expression of a functional anaphylatoxin C3a receptor by astrocytes. J. Neurochem. 71, 2487–2496 [DOI] [PubMed] [Google Scholar]
- 26. Bennett S., Breit S. N. (1994) Variables in the isolation and culture of human monocytes that are of particular relevance to studies of HIV. J. Leukoc. Biol. 56, 236–240 [DOI] [PubMed] [Google Scholar]
- 27. Maess M. B., Buers I., Robenek H., Lorkowski S. (2011) Improved protocol for efficient nonviral transfection of premature THP-1 macrophages. Cold Spring Harb. Protoc. 2011, pdb.prot5612 [DOI] [PubMed] [Google Scholar]
- 28. Mogilenko D. A., Dizhe E. B., Shavva V. S., Lapikov I. A., Orlov S. V., Perevozchikov A. P. (2009) Role of the nuclear receptors HNF4 α, PPAR α, and LXRs in the TNF α-mediated inhibition of human apolipoprotein A-I gene expression in HepG2 cells. Biochemistry 48, 11950–11960 [DOI] [PubMed] [Google Scholar]
- 29. Mogilenko D. A., Shavva V. S., Dizhe E. B., Orlov S. V., Perevozchikov A. P. (2010) PPARγ activates ABCA1 gene transcription but reduces the level of ABCA1 protein in HepG2 cells. Biochem. Biophys. Res. Commun. 402, 477–482 [DOI] [PubMed] [Google Scholar]
- 30. Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, RESEARCH0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Khan B. V., Parthasarathy S. S., Alexander R. W., Medford R. M. (1995) Modified low density lipoprotein and its constituents augment cytokine-activated vascular cell adhesion molecule-1 gene expression in human vascular endothelial cells. J. Clin. Invest. 95, 1262–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dardik B. N., Schwartzkopf C. D., Stevens D. E., Chatelain R. E. (2000) A quantitative assay for the non-covalent association between apolipoprotein[a] and apolipoprotein B. An alternative measure of Lp[a] assembly. J. Lipid Res. 41, 1013–1019 [PubMed] [Google Scholar]
- 33. Jialal I., Chait A. (1989) Differences in the metabolism of oxidatively modified low density lipoprotein and acetylated low density lipoprotein by human endothelial cells: inhibition of cholesterol esterification by oxidatively modified low density lipoprotein. J. Lipid Res. 30, 1561–1568 [PubMed] [Google Scholar]
- 34. Andrews N. C., Faller D. V. (1991) A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 19, 2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rodgers J. T., Patel P., Hennes J. L., Bolognia S. L., Mascotti D. P. (2000) Use of biotin-labeled nucleic acids for protein purification and agarose-based chemiluminescent electromobility shift assays. Anal. Biochem. 277, 254–259 [DOI] [PubMed] [Google Scholar]
- 36. Orlov S. V., Kuteykin-Teplyakov K. B., Ignatovich I. A., Dizhe E. B., Mirgorodskaya O. A., Grishin A. V., Guzhova O. B., Prokhortchouk E. B., Guliy P. V., Perevozchikov A. P. (2007) Novel repressor of the human FMR1 gene. Identification of p56 human (GCC)n-binding protein as a Krüppel-like transcription factor ZF5. FEBS J. 274, 4848–4862 [DOI] [PubMed] [Google Scholar]
- 37. Calkin A. C., Tontonoz P. (2010) Liver X receptor signaling pathways and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 30, 1513–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schmitz G., Langmann T. (2005) Transcriptional regulatory networks in lipid metabolism control ABCA1 expression. Biochim. Biophys. Acta 1735, 1–19 [DOI] [PubMed] [Google Scholar]
- 39. Miller Y. I., Viriyakosol S., Worrall D. S., Boullier A., Butler S., Witztum J. L. (2005) Toll-like receptor 4-dependent and -independent cytokine secretion induced by minimally oxidized low density lipoprotein in macrophages. Arterioscler. Thromb. Vasc. Biol. 25, 1213–1219 [DOI] [PubMed] [Google Scholar]
- 40. Nichols W. K. (1984) LPS stimulation of complement (C3) synthesis by a human monocyte cell line. Complement 1, 108–115 [DOI] [PubMed] [Google Scholar]
- 41. Sutton M. B., Strunk R. C., Cole F. S. (1986) Regulation of the synthesis of the third component of complement and factor B in cord blood monocytes by lipopolysaccharide. J. Immunol. 136, 1366–1372 [PubMed] [Google Scholar]
- 42. Sandelin A., Wasserman W. W. (2005) Prediction of nuclear hormone receptor response elements. Mol. Endocrinol. 19, 595–606 [DOI] [PubMed] [Google Scholar]
- 43. Barton G. M., Medzhitov R. (2003) Toll-like receptor signaling pathways. Science 300, 1524–1525 [DOI] [PubMed] [Google Scholar]
- 44. Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686 [DOI] [PubMed] [Google Scholar]
- 45. Martinez F. O., Sica A., Mantovani A., Locati M. (2008) Macrophage activation and polarization. Front. Biosci. 13, 453–461 [DOI] [PubMed] [Google Scholar]
- 46. Chinetti-Gbaguidi G., Baron M., Bouhlel M. A., Vanhoutte J., Copin C., Sebti Y., Derudas B., Mayi T., Bories G., Tailleux A., Haulon S., Zawadzki C., Jude B., Staels B. (2011) Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARγ and LXRα pathways. Circ. Res. 108, 985–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Miller Y. I., Chang M. K., Binder C. J., Shaw P. X., Witztum J. L. (2003) Oxidized low density lipoprotein and innate immune receptors. Curr. Opin. Lipidol. 14, 437–445 [DOI] [PubMed] [Google Scholar]
- 48. Bosisio D., Polentarutti N., Sironi M., Bernasconi S., Miyake K., Webb G. R., Martin M. U., Mantovani A., Muzio M. (2002) Stimulation of Toll-like receptor 4 expression in human mononuclear phagocytes by interferon-γ. A molecular basis for priming and synergism with bacterial lipopolysaccharide. Blood 99, 3427–3431 [DOI] [PubMed] [Google Scholar]
- 49. Fontaine C., Rigamonti E., Nohara A., Gervois P., Teissier E., Fruchart J. C., Staels B., Chinetti-Gbaguidi G. (2007) Liver X receptor activation potentiates the lipopolysaccharide response in human macrophages. Circ. Res. 101, 40–49 [DOI] [PubMed] [Google Scholar]
- 50. Ohlsson B. G., Englund M. C., Karlsson A. L., Knutsen E., Erixon C., Skribeck H., Liu Y., Bondjers G., Wiklund O. (1996) Oxidized low density lipoprotein inhibits lipopolysaccharide-induced binding of nuclear factor-κB to DNA and the subsequent expression of tumor necrosis factor-α and interleukin-1β in macrophages. J. Clin. Invest. 98, 78–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Landis M. S., Patel H. V., Capone J. P. (2002) Oxysterol activators of liver X receptor and 9-cis-retinoic acid promote sequential steps in the synthesis and secretion of tumor necrosis factor-α from human monocytes. J. Biol. Chem. 277, 4713–4721 [DOI] [PubMed] [Google Scholar]
- 52. Li K., Anderson K. J., Peng Q., Noble A., Lu B., Kelly A. P., Wang N., Sacks S. H., Zhou W. (2008) Cyclic AMP plays a critical role in C3a-receptor-mediated regulation of dendritic cells in antigen uptake and T-cell stimulation. Blood 112, 5084–5094 [DOI] [PubMed] [Google Scholar]
- 53. Peng Q., Li K., Anderson K., Farrar C. A., Lu B., Smith R. A., Sacks S. H., Zhou W. (2008) Local production and activation of complement up-regulates the allostimulatory function of dendritic cells through C3a-C3aR interaction. Blood 111, 2452–2461 [DOI] [PubMed] [Google Scholar]
- 54. Fischer W. H., Jagels M. A., Hugli T. E. (1999) Regulation of IL-6 synthesis in human peripheral blood mononuclear cells by C3a and C3a(desArg). J. Immunol. 162, 453–459 [PubMed] [Google Scholar]
- 55. Oksjoki R., Laine P., Helske S., Vehmaan-Kreula P., Mäyränpää M. I., Gasque P., Kovanen P. T., Pentikäinen M. O. (2007) Receptors for the anaphylatoxins C3a and C5a are expressed in human atherosclerotic coronary plaques. Atherosclerosis 195, 90–99 [DOI] [PubMed] [Google Scholar]
- 56. Oksjoki R., Kovanen P. T., Pentikäinen M. O. (2003) Role of complement activation in atherosclerosis. Curr. Opin. Lipidol. 14, 477–482 [DOI] [PubMed] [Google Scholar]
- 57. Hansson G. K., Libby P., Schönbeck U., Yan Z. Q. (2002) Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ. Res. 91, 281–291 [DOI] [PubMed] [Google Scholar]
- 58. Groeneweg M., Kanters E., Vergouwe M. N., Duerink H., Kraal G., Hofker M. H., de Winther M. P. (2006) Lipopolysaccharide-induced gene expression in murine macrophages is enhanced by prior exposure to oxLDL. J. Lipid Res. 47, 2259–2267 [DOI] [PubMed] [Google Scholar]
- 59. Wiesner P., Choi S. H., Almazan F., Benner C., Huang W., Diehl C. J., Gonen A., Butler S., Witztum J. L., Glass C. K., Miller Y. I. (2010) Low doses of lipopolysaccharide and minimally oxidized low density lipoprotein cooperatively activate macrophages via nuclear factor κB and activator protein-1. Possible mechanism for acceleration of atherosclerosis by subclinical endotoxemia. Circ. Res. 107, 56–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hong C., Tontonoz P. (2008) Coordination of inflammation and metabolism by PPAR and LXR nuclear receptors. Curr. Opin. Genet. Dev. 18, 461–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Purwar R., Wittmann M., Zwirner J., Oppermann M., Kracht M., Dittrich-Breiholz O., Gutzmer R., Werfel T. (2006) Induction of C3 and CCL2 by C3a in keratinocytes. A novel autocrine amplification loop of inflammatory skin reactions. J. Immunol. 177, 4444–4450 [DOI] [PubMed] [Google Scholar]
- 62. Institute of Laboratory Animal Research, Commission on Life Sciences, National Research Council (1996) Guide for the Care and Use of Laboratory Animals, National Academy Press; Washington, D. C. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.