Abstract
The asymmetric unit of the title complex, [Sr5(C8H4O4)4(HCO2)2]n, contains three independent SrII ions, one of which is located on an inversion center. In the crystal, the SrII ions (coordination numbers 8, 9 and 12) are connected by two crystallographically distinct benzene-1,2-dicarboxylate ligands and one formate ligand, forming a two-dimensional polymer parallel to (001).
Related literature
For general background to metal coordination polymers, see: Kitagawa et al. (2004 ▶). For related structures, see: Stein & Ruschewitz (2005 ▶); Zhang et al. (2009 ▶); Wang et al. (2010 ▶). 
Experimental
Crystal data
[Sr5(C8H4O4)4(HCO2)2]
M r = 1184.58
Triclinic,

a = 7.0292 (3) Å
b = 10.2892 (4) Å
c = 12.5439 (5) Å
α = 91.361 (2)°
β = 90.407 (2)°
γ = 104.998 (2)°
V = 876.00 (6) Å3
Z = 1
Mo Kα radiation
μ = 7.65 mm−1
T = 295 K
0.20 × 0.18 × 0.15 mm
Data collection
Bruker APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2010 ▶) T min = 0.310, T max = 0.393
15465 measured reflections
4295 independent reflections
3585 reflections with I > 2σ(I)
R int = 0.033
Refinement
R[F 2 > 2σ(F 2)] = 0.028
wR(F 2) = 0.060
S = 1.04
4295 reflections
268 parameters
H-atom parameters constrained
Δρmax = 0.97 e Å−3
Δρmin = −0.39 e Å−3
Data collection: APEX2 (Bruker, 2010 ▶); cell refinement: SAINT (Bruker, 2010 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: DIAMOND (Brandenburg, 2010 ▶); software used to prepare material for publication: SHELXTL (Sheldrick, 2008 ▶).
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811044977/lh5360sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811044977/lh5360Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
This research was supported by National Science Council, Taiwan (NSC99–2113-M-033–005-MY2) and by the Center-of-Excellence (COE) Program on Membrane Technology of the Ministry of Education (MOE).
supplementary crystallographic information
Comment
The increasingly rapid development of metal coordination polymers over the past two decades has attracted considerable attention due to their structural diversity and important applications (Kitagawa et al., 2004). benzene-1,2-dicarboxylate acid (H2BDC) has been successively applied to construct to strontium (Stein & Ruschewitz, 2005), lead (Zhang et al., 2009), and tin complexes (Wang et al., 2010). Here we report the crystal structure of the title complex.
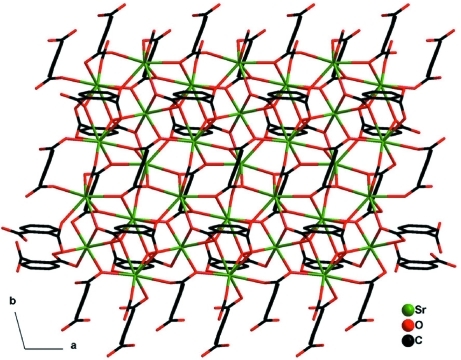
The title compound contains three crystallographically independent SrII ions, with coordination numbers 12 (Sr1, located on an inversion center), 8 (Sr2) and 9 (Sr3). The Sr—O distances range from 2.467 (2) to 2.9332 (19) Å. The coordination geometry of the Sr(II) ions is shown in Fig. 1. In the crystal, the SrII ions are connected by two crystallographically distinct benzene-1,2-dicarboxylate ligands and one formate ligand, to form a two-dimensional polymer parallel to (001) [Fig. 2].
Experimental
Solvothermal reactions were carried out at 423 K for 2 d in a Teflon-lined acid digestion bomb with an internal volume of 23 ml followed by slow cooling at 6 K/h to room temperature. A single-phase product consisting of transparent colorless crystals of was obtained from a mixture of Sr(NO3)2 (0.0847 g,0.4 mmol), H2ortho-BDC (0.0332 g, 0.2 mmol), DMF (5.0 ml) and H2O (1.0 ml).
Refinement
H atoms were placed in ideal geometries, with C—H = 0.93 Å and Uiso(H)= 1.2Ueq(C).
Figures
Fig. 1.
Part of the title structure, showing 50% probability displacement ellipsoids. [symmetry codes: (i) 1 - x, -y, -z; (ii) 1 + x, y, z; (iii) 2 - x, -y, -z; (iv) 1 - x, -1 - y, -z; (v) 2 - x, -1 - y, -z; (vi) x, 1 + y, z].
Fig. 2.
The layer structure of the title compound viewed along the c axis.
Crystal data
| [Sr5(C8H4O4)4(HCO2)2] | Z = 1 |
| Mr = 1184.58 | F(000) = 572 |
| Triclinic, P1 | Dx = 2.245 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 7.0292 (3) Å | Cell parameters from 8942 reflections |
| b = 10.2892 (4) Å | θ = 2.6–28.3° |
| c = 12.5439 (5) Å | µ = 7.65 mm−1 |
| α = 91.361 (2)° | T = 295 K |
| β = 90.407 (2)° | Lamellar, colorless |
| γ = 104.998 (2)° | 0.20 × 0.18 × 0.15 mm |
| V = 876.00 (6) Å3 |
Data collection
| Bruker APEXII CCD diffractometer | 4295 independent reflections |
| Radiation source: fine-focus sealed tube | 3585 reflections with I > 2σ(I) |
| graphite | Rint = 0.033 |
| Detector resolution: 8.3333 pixels mm-1 | θmax = 28.3°, θmin = 1.6° |
| φ and ω scans | h = −9→9 |
| Absorption correction: multi-scan (SADABS; Bruker, 2010) | k = −13→13 |
| Tmin = 0.310, Tmax = 0.393 | l = −16→16 |
| 15465 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.028 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.060 | H-atom parameters constrained |
| S = 1.04 | w = 1/[σ2(Fo2) + (0.0254P)2 + 0.4547P] where P = (Fo2 + 2Fc2)/3 |
| 4295 reflections | (Δ/σ)max = 0.001 |
| 268 parameters | Δρmax = 0.97 e Å−3 |
| 0 restraints | Δρmin = −0.39 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Sr1 | 1.0000 | 0.0000 | 0.0000 | 0.01600 (9) | |
| Sr2 | 0.86724 (3) | −0.37889 (2) | −0.11377 (2) | 0.01683 (7) | |
| Sr3 | 0.57129 (3) | 0.21010 (2) | −0.05482 (2) | 0.01681 (7) | |
| O1 | 0.6246 (3) | −0.05156 (18) | −0.08904 (15) | 0.0184 (4) | |
| O2 | 0.8372 (3) | −0.14323 (19) | −0.17205 (16) | 0.0219 (4) | |
| O3 | 0.2714 (3) | 0.03708 (19) | −0.14874 (16) | 0.0227 (4) | |
| O4 | 0.1994 (3) | −0.17952 (19) | −0.11074 (16) | 0.0209 (4) | |
| O5 | 0.7838 (3) | −0.26762 (19) | 0.05581 (15) | 0.0215 (4) | |
| O6 | 1.0850 (3) | −0.18764 (19) | 0.12185 (17) | 0.0254 (5) | |
| O7 | 0.5072 (3) | −0.61995 (19) | 0.10329 (16) | 0.0233 (4) | |
| O8 | 0.8151 (3) | −0.54513 (18) | 0.05059 (15) | 0.0190 (4) | |
| O9 | 0.6767 (3) | −0.6166 (2) | −0.19012 (17) | 0.0274 (5) | |
| O10 | 0.8059 (4) | −0.4615 (3) | −0.30783 (19) | 0.0432 (6) | |
| C1 | 0.6836 (4) | −0.1025 (3) | −0.1717 (2) | 0.0168 (6) | |
| C2 | 0.5647 (4) | −0.1157 (3) | −0.2734 (2) | 0.0178 (6) | |
| C3 | 0.6460 (4) | −0.1431 (3) | −0.3689 (2) | 0.0268 (7) | |
| H3A | 0.7758 | −0.1489 | −0.3701 | 0.032* | |
| C4 | 0.5356 (5) | −0.1618 (3) | −0.4624 (3) | 0.0316 (7) | |
| H4A | 0.5917 | −0.1788 | −0.5265 | 0.038* | |
| C5 | 0.3424 (5) | −0.1553 (3) | −0.4603 (3) | 0.0327 (8) | |
| H5A | 0.2678 | −0.1684 | −0.5231 | 0.039* | |
| C6 | 0.2587 (4) | −0.1293 (3) | −0.3652 (3) | 0.0292 (7) | |
| H6A | 0.1279 | −0.1256 | −0.3643 | 0.035* | |
| C7 | 0.3690 (4) | −0.1087 (3) | −0.2717 (2) | 0.0184 (6) | |
| C8 | 0.2747 (4) | −0.0818 (3) | −0.1693 (2) | 0.0172 (6) | |
| C9 | 0.9131 (4) | −0.2604 (3) | 0.1291 (2) | 0.0166 (6) | |
| C10 | 0.8555 (4) | −0.3394 (3) | 0.2271 (2) | 0.0183 (6) | |
| C11 | 0.9164 (4) | −0.2784 (3) | 0.3253 (3) | 0.0280 (7) | |
| H11A | 0.9963 | −0.1909 | 0.3287 | 0.034* | |
| C12 | 0.8602 (5) | −0.3458 (3) | 0.4187 (3) | 0.0336 (8) | |
| H12A | 0.9028 | −0.3042 | 0.4843 | 0.040* | |
| C13 | 0.7407 (5) | −0.4750 (3) | 0.4136 (3) | 0.0343 (8) | |
| H13A | 0.7023 | −0.5208 | 0.4760 | 0.041* | |
| C14 | 0.6777 (4) | −0.5367 (3) | 0.3161 (3) | 0.0275 (7) | |
| H14A | 0.5956 | −0.6236 | 0.3136 | 0.033* | |
| C15 | 0.7349 (4) | −0.4711 (3) | 0.2222 (2) | 0.0180 (6) | |
| C16 | 0.6787 (4) | −0.5489 (3) | 0.1181 (2) | 0.0159 (6) | |
| C17 | 0.7073 (5) | −0.5772 (4) | −0.2845 (3) | 0.0377 (8) | |
| H17A | 0.6537 | −0.6374 | −0.3400 | 0.045* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Sr1 | 0.01285 (16) | 0.01455 (18) | 0.0203 (2) | 0.00311 (13) | −0.00091 (13) | 0.00027 (15) |
| Sr2 | 0.01502 (12) | 0.01474 (13) | 0.02072 (15) | 0.00390 (10) | 0.00023 (10) | 0.00003 (10) |
| Sr3 | 0.01324 (12) | 0.01526 (13) | 0.02143 (15) | 0.00273 (9) | 0.00058 (10) | 0.00130 (10) |
| O1 | 0.0151 (9) | 0.0180 (10) | 0.0201 (11) | 0.0006 (8) | −0.0004 (8) | −0.0016 (8) |
| O2 | 0.0151 (9) | 0.0235 (11) | 0.0280 (12) | 0.0065 (8) | −0.0020 (8) | 0.0008 (9) |
| O3 | 0.0229 (10) | 0.0179 (10) | 0.0287 (12) | 0.0080 (8) | −0.0001 (9) | −0.0042 (9) |
| O4 | 0.0140 (9) | 0.0210 (10) | 0.0263 (12) | 0.0015 (8) | 0.0003 (8) | 0.0058 (9) |
| O5 | 0.0269 (10) | 0.0175 (10) | 0.0216 (11) | 0.0086 (8) | −0.0045 (8) | −0.0001 (8) |
| O6 | 0.0189 (10) | 0.0213 (11) | 0.0352 (13) | 0.0027 (8) | 0.0041 (9) | 0.0083 (9) |
| O7 | 0.0153 (9) | 0.0236 (11) | 0.0287 (12) | 0.0013 (8) | −0.0013 (8) | −0.0036 (9) |
| O8 | 0.0186 (9) | 0.0184 (10) | 0.0213 (11) | 0.0065 (8) | 0.0053 (8) | 0.0022 (8) |
| O9 | 0.0330 (12) | 0.0234 (11) | 0.0267 (13) | 0.0084 (9) | −0.0005 (9) | 0.0044 (10) |
| O10 | 0.0558 (16) | 0.0426 (15) | 0.0303 (14) | 0.0106 (13) | 0.0049 (12) | 0.0044 (12) |
| C1 | 0.0116 (12) | 0.0135 (13) | 0.0230 (16) | −0.0008 (10) | 0.0000 (11) | 0.0018 (12) |
| C2 | 0.0172 (13) | 0.0157 (13) | 0.0198 (15) | 0.0031 (11) | −0.0012 (11) | −0.0003 (11) |
| C3 | 0.0213 (14) | 0.0315 (17) | 0.0276 (18) | 0.0070 (13) | 0.0027 (13) | −0.0010 (14) |
| C4 | 0.0345 (18) | 0.041 (2) | 0.0199 (17) | 0.0101 (15) | 0.0023 (13) | −0.0011 (15) |
| C5 | 0.0356 (18) | 0.043 (2) | 0.0197 (17) | 0.0106 (15) | −0.0081 (14) | −0.0037 (15) |
| C6 | 0.0212 (14) | 0.0410 (19) | 0.0272 (18) | 0.0119 (13) | −0.0062 (12) | −0.0037 (15) |
| C7 | 0.0169 (13) | 0.0144 (13) | 0.0238 (16) | 0.0038 (11) | 0.0010 (11) | 0.0003 (12) |
| C8 | 0.0085 (11) | 0.0192 (14) | 0.0243 (16) | 0.0046 (10) | −0.0050 (10) | −0.0009 (12) |
| C9 | 0.0193 (13) | 0.0128 (13) | 0.0194 (15) | 0.0072 (11) | 0.0027 (11) | −0.0014 (11) |
| C10 | 0.0169 (13) | 0.0180 (14) | 0.0193 (15) | 0.0035 (11) | −0.0010 (11) | −0.0004 (12) |
| C11 | 0.0282 (16) | 0.0227 (16) | 0.0287 (18) | −0.0007 (13) | −0.0039 (13) | −0.0041 (14) |
| C12 | 0.0386 (19) | 0.038 (2) | 0.0212 (18) | 0.0048 (15) | −0.0021 (14) | −0.0069 (15) |
| C13 | 0.0440 (19) | 0.0368 (19) | 0.0199 (18) | 0.0057 (16) | 0.0035 (15) | 0.0068 (15) |
| C14 | 0.0295 (16) | 0.0211 (15) | 0.0293 (18) | 0.0019 (13) | 0.0020 (13) | 0.0021 (14) |
| C15 | 0.0144 (12) | 0.0196 (14) | 0.0199 (15) | 0.0043 (11) | 0.0018 (11) | −0.0003 (12) |
| C16 | 0.0172 (13) | 0.0129 (13) | 0.0194 (15) | 0.0071 (10) | −0.0019 (11) | 0.0023 (11) |
| C17 | 0.0385 (19) | 0.037 (2) | 0.039 (2) | 0.0135 (16) | 0.0002 (16) | −0.0016 (17) |
Geometric parameters (Å, °)
| Sr1—O3i | 2.641 (2) | O6—C9 | 1.251 (3) |
| Sr1—O3ii | 2.641 (2) | O6—Sr3iii | 2.6281 (19) |
| Sr1—O2iii | 2.661 (2) | O7—C16 | 1.248 (3) |
| Sr1—O2 | 2.661 (2) | O7—Sr2iv | 2.6335 (18) |
| Sr1—O6iii | 2.6729 (19) | O7—Sr3viii | 2.729 (2) |
| Sr1—O6 | 2.6729 (19) | O8—C16 | 1.277 (3) |
| Sr1—O1 | 2.7742 (17) | O8—Sr2v | 2.6710 (18) |
| Sr1—O1iii | 2.7742 (17) | O8—Sr3viii | 2.9331 (19) |
| Sr1—O5iii | 2.8848 (19) | O9—C17 | 1.262 (4) |
| Sr1—O5 | 2.8848 (19) | O9—Sr3viii | 2.467 (2) |
| Sr1—O4i | 2.9242 (19) | O10—C17 | 1.256 (4) |
| Sr1—O4ii | 2.9242 (19) | C1—C2 | 1.504 (4) |
| Sr2—O5 | 2.536 (2) | C2—C3 | 1.384 (4) |
| Sr2—O10 | 2.556 (2) | C2—C7 | 1.396 (4) |
| Sr2—O2 | 2.6087 (19) | C3—C4 | 1.384 (4) |
| Sr2—O9 | 2.620 (2) | C3—H3A | 0.9300 |
| Sr2—O7iv | 2.6335 (18) | C4—C5 | 1.378 (4) |
| Sr2—O8v | 2.6710 (18) | C4—H4A | 0.9300 |
| Sr2—O8 | 2.6766 (18) | C5—C6 | 1.384 (5) |
| Sr2—O4ii | 2.6789 (18) | C5—H5A | 0.9300 |
| Sr3—O9vi | 2.467 (2) | C6—C7 | 1.383 (4) |
| Sr3—O1i | 2.6128 (19) | C6—H6A | 0.9300 |
| Sr3—O6iii | 2.6281 (19) | C7—C8 | 1.502 (4) |
| Sr3—O3 | 2.6314 (19) | C8—Sr1vii | 3.125 (3) |
| Sr3—O4i | 2.6938 (19) | C8—Sr3i | 3.420 (3) |
| Sr3—O5i | 2.7104 (18) | C9—C10 | 1.490 (4) |
| Sr3—O7vi | 2.729 (2) | C10—C11 | 1.383 (4) |
| Sr3—O1 | 2.8361 (19) | C10—C15 | 1.400 (4) |
| Sr3—O8vi | 2.9332 (19) | C11—C12 | 1.382 (4) |
| O1—C1 | 1.271 (3) | C11—H11A | 0.9300 |
| O1—Sr3i | 2.6129 (19) | C12—C13 | 1.376 (5) |
| O2—C1 | 1.255 (3) | C12—H12A | 0.9300 |
| O3—C8 | 1.250 (3) | C13—C14 | 1.380 (4) |
| O3—Sr1vii | 2.641 (2) | C13—H13A | 0.9300 |
| O4—C8 | 1.264 (3) | C14—C15 | 1.382 (4) |
| O4—Sr2vii | 2.6789 (18) | C14—H14A | 0.9300 |
| O4—Sr3i | 2.6938 (19) | C15—C16 | 1.510 (4) |
| O4—Sr1vii | 2.9242 (19) | C16—Sr3viii | 3.190 (3) |
| O5—C9 | 1.275 (3) | C17—H17A | 0.9300 |
| O5—Sr3i | 2.7104 (18) | ||
| O3i—Sr1—O3ii | 180.00 (9) | O3—Sr3—O1 | 65.33 (5) |
| O3i—Sr1—O2iii | 72.69 (6) | O4i—Sr3—O1 | 76.04 (6) |
| O3ii—Sr1—O2iii | 107.31 (6) | O5i—Sr3—O1 | 124.22 (5) |
| O3i—Sr1—O2 | 107.31 (6) | O7vi—Sr3—O1 | 141.68 (6) |
| O3ii—Sr1—O2 | 72.69 (6) | O9vi—Sr3—O8vi | 71.45 (6) |
| O2iii—Sr1—O2 | 180.0 | O1i—Sr3—O8vi | 109.25 (5) |
| O3i—Sr1—O6iii | 103.66 (6) | O6iii—Sr3—O8vi | 82.40 (6) |
| O3ii—Sr1—O6iii | 76.34 (6) | O3—Sr3—O8vi | 161.74 (5) |
| O2iii—Sr1—O6iii | 102.22 (6) | O4i—Sr3—O8vi | 62.49 (5) |
| O2—Sr1—O6iii | 77.78 (6) | O5i—Sr3—O8vi | 100.39 (5) |
| O3i—Sr1—O6 | 76.34 (6) | O7vi—Sr3—O8vi | 45.82 (5) |
| O3ii—Sr1—O6 | 103.66 (6) | O1—Sr3—O8vi | 132.73 (5) |
| O2iii—Sr1—O6 | 77.78 (6) | O9vi—Sr3—C16vi | 86.49 (7) |
| O2—Sr1—O6 | 102.22 (6) | O1i—Sr3—C16vi | 89.85 (6) |
| O6iii—Sr1—O6 | 180.0 | O6iii—Sr3—C16vi | 104.15 (6) |
| O3i—Sr1—O1 | 68.69 (6) | O3—Sr3—C16vi | 142.52 (6) |
| O3ii—Sr1—O1 | 111.31 (6) | O4i—Sr3—C16vi | 63.56 (6) |
| O2iii—Sr1—O1 | 131.93 (5) | O5i—Sr3—C16vi | 83.02 (6) |
| O2—Sr1—O1 | 48.07 (5) | O7vi—Sr3—C16vi | 22.65 (6) |
| O6iii—Sr1—O1 | 61.89 (6) | O1—Sr3—C16vi | 139.48 (6) |
| O6—Sr1—O1 | 118.11 (6) | O8vi—Sr3—C16vi | 23.60 (6) |
| O3i—Sr1—O1iii | 111.31 (6) | O9vi—Sr3—C8i | 143.07 (7) |
| O3ii—Sr1—O1iii | 68.69 (6) | O1i—Sr3—C8i | 48.65 (6) |
| O2iii—Sr1—O1iii | 48.07 (5) | O6iii—Sr3—C8i | 81.07 (6) |
| O2—Sr1—O1iii | 131.93 (5) | O3—Sr3—C8i | 111.74 (6) |
| O6iii—Sr1—O1iii | 118.11 (6) | O4i—Sr3—C8i | 19.62 (6) |
| O6—Sr1—O1iii | 61.89 (6) | O5i—Sr3—C8i | 119.24 (6) |
| O1—Sr1—O1iii | 180.0 | O7vi—Sr3—C8i | 77.15 (6) |
| O3i—Sr1—O5iii | 120.83 (6) | O1—Sr3—C8i | 66.95 (6) |
| O3ii—Sr1—O5iii | 59.17 (6) | O8vi—Sr3—C8i | 78.75 (6) |
| O2iii—Sr1—O5iii | 68.95 (6) | C16vi—Sr3—C8i | 73.62 (6) |
| O2—Sr1—O5iii | 111.05 (6) | O9vi—Sr3—Sr1vii | 125.58 (5) |
| O6iii—Sr1—O5iii | 46.67 (5) | O1i—Sr3—Sr1vii | 41.76 (4) |
| O6—Sr1—O5iii | 133.33 (5) | O6iii—Sr3—Sr1vii | 144.06 (4) |
| O1—Sr1—O5iii | 108.47 (5) | O3—Sr3—Sr1vii | 38.86 (4) |
| O1iii—Sr1—O5iii | 71.53 (5) | O4i—Sr3—Sr1vii | 109.12 (4) |
| O3i—Sr1—O5 | 59.17 (6) | O5i—Sr3—Sr1vii | 44.41 (4) |
| O3ii—Sr1—O5 | 120.83 (6) | O7vi—Sr3—Sr1vii | 84.43 (4) |
| O2iii—Sr1—O5 | 111.05 (6) | O1—Sr3—Sr1vii | 82.91 (4) |
| O2—Sr1—O5 | 68.95 (6) | O8vi—Sr3—Sr1vii | 130.22 (4) |
| O6iii—Sr1—O5 | 133.33 (5) | C16vi—Sr3—Sr1vii | 106.72 (5) |
| O6—Sr1—O5 | 46.67 (5) | C8i—Sr3—Sr1vii | 90.35 (4) |
| O1—Sr1—O5 | 71.53 (5) | C1—O1—Sr3i | 119.45 (16) |
| O1iii—Sr1—O5 | 108.47 (5) | C1—O1—Sr1 | 89.98 (14) |
| O5iii—Sr1—O5 | 180.0 | Sr3i—O1—Sr1 | 99.39 (6) |
| O3i—Sr1—O4i | 46.64 (5) | C1—O1—Sr3 | 129.32 (16) |
| O3ii—Sr1—O4i | 133.36 (5) | Sr3i—O1—Sr3 | 108.81 (6) |
| O2iii—Sr1—O4i | 59.32 (5) | Sr1—O1—Sr3 | 96.94 (5) |
| O2—Sr1—O4i | 120.68 (5) | C1—O2—Sr2 | 126.39 (17) |
| O6iii—Sr1—O4i | 65.35 (6) | C1—O2—Sr1 | 95.62 (17) |
| O6—Sr1—O4i | 114.65 (6) | Sr2—O2—Sr1 | 98.51 (6) |
| O1—Sr1—O4i | 73.41 (5) | C8—O3—Sr3 | 121.66 (16) |
| O1iii—Sr1—O4i | 106.59 (5) | C8—O3—Sr1vii | 100.71 (17) |
| O5iii—Sr1—O4i | 75.06 (5) | Sr3—O3—Sr1vii | 102.43 (7) |
| O5—Sr1—O4i | 104.94 (5) | C8—O4—Sr2vii | 135.86 (16) |
| O3i—Sr1—O4ii | 133.36 (5) | C8—O4—Sr3i | 114.66 (15) |
| O3ii—Sr1—O4ii | 46.64 (5) | Sr2vii—O4—Sr3i | 109.39 (6) |
| O2iii—Sr1—O4ii | 120.68 (5) | C8—O4—Sr1vii | 87.01 (16) |
| O2—Sr1—O4ii | 59.32 (5) | Sr2vii—O4—Sr1vii | 90.77 (5) |
| O6iii—Sr1—O4ii | 114.65 (6) | Sr3i—O4—Sr1vii | 96.68 (6) |
| O6—Sr1—O4ii | 65.35 (6) | C9—O5—Sr2 | 112.02 (16) |
| O1—Sr1—O4ii | 106.59 (5) | C9—O5—Sr3i | 132.22 (17) |
| O1iii—Sr1—O4ii | 73.41 (5) | Sr2—O5—Sr3i | 115.44 (7) |
| O5iii—Sr1—O4ii | 104.94 (5) | C9—O5—Sr1 | 86.87 (15) |
| O5—Sr1—O4ii | 75.06 (5) | Sr2—O5—Sr1 | 94.66 (6) |
| O4i—Sr1—O4ii | 180.00 (5) | Sr3i—O5—Sr1 | 94.48 (5) |
| O5—Sr2—O10 | 153.40 (7) | C9—O6—Sr3iii | 138.19 (17) |
| O5—Sr2—O2 | 75.35 (6) | C9—O6—Sr1 | 97.19 (16) |
| O10—Sr2—O2 | 88.68 (7) | Sr3iii—O6—Sr1 | 104.80 (7) |
| O5—Sr2—O9 | 125.53 (6) | C16—O7—Sr2iv | 143.91 (17) |
| O10—Sr2—O9 | 50.80 (7) | C16—O7—Sr3viii | 99.92 (17) |
| O2—Sr2—O9 | 128.29 (6) | Sr2iv—O7—Sr3viii | 111.61 (7) |
| O5—Sr2—O7iv | 66.86 (6) | C16—O8—Sr2v | 118.00 (16) |
| O10—Sr2—O7iv | 88.28 (7) | C16—O8—Sr2 | 121.54 (15) |
| O2—Sr2—O7iv | 72.55 (6) | Sr2v—O8—Sr2 | 115.75 (6) |
| O9—Sr2—O7iv | 75.32 (6) | C16—O8—Sr3viii | 89.57 (15) |
| O5—Sr2—O8v | 101.16 (6) | Sr2v—O8—Sr3viii | 102.86 (6) |
| O10—Sr2—O8v | 105.39 (7) | Sr2—O8—Sr3viii | 99.56 (6) |
| O2—Sr2—O8v | 129.55 (6) | C17—O9—Sr3viii | 153.6 (2) |
| O9—Sr2—O8v | 95.28 (6) | C17—O9—Sr2 | 91.1 (2) |
| O7iv—Sr2—O8v | 153.14 (6) | Sr3viii—O9—Sr2 | 114.79 (8) |
| O5—Sr2—O8 | 68.06 (6) | C17—O10—Sr2 | 94.3 (2) |
| O10—Sr2—O8 | 123.11 (7) | O2—C1—O1 | 122.6 (3) |
| O2—Sr2—O8 | 143.15 (6) | O2—C1—C2 | 118.3 (3) |
| O9—Sr2—O8 | 73.66 (6) | O1—C1—C2 | 119.0 (2) |
| O7iv—Sr2—O8 | 88.90 (6) | O2—C1—Sr1 | 60.21 (14) |
| O8v—Sr2—O8 | 64.25 (6) | O1—C1—Sr1 | 65.40 (14) |
| O5—Sr2—O4ii | 85.44 (6) | C2—C1—Sr1 | 162.29 (18) |
| O10—Sr2—O4ii | 106.46 (7) | C3—C2—C7 | 119.5 (3) |
| O2—Sr2—O4ii | 63.25 (6) | C3—C2—C1 | 119.7 (2) |
| O9—Sr2—O4ii | 147.58 (6) | C7—C2—C1 | 120.6 (3) |
| O7iv—Sr2—O4ii | 132.53 (6) | C2—C3—C4 | 120.5 (3) |
| O8v—Sr2—O4ii | 66.31 (6) | C2—C3—H3A | 119.7 |
| O8—Sr2—O4ii | 116.43 (6) | C4—C3—H3A | 119.7 |
| O5—Sr2—C17 | 144.39 (8) | C5—C4—C3 | 119.8 (3) |
| O10—Sr2—C17 | 25.29 (8) | C5—C4—H4A | 120.1 |
| O2—Sr2—C17 | 108.87 (8) | C3—C4—H4A | 120.1 |
| O9—Sr2—C17 | 25.52 (8) | C4—C5—C6 | 120.3 (3) |
| O7iv—Sr2—C17 | 80.49 (8) | C4—C5—H5A | 119.9 |
| O8v—Sr2—C17 | 101.96 (8) | C6—C5—H5A | 119.9 |
| O8—Sr2—C17 | 98.66 (8) | C7—C6—C5 | 120.2 (3) |
| O4ii—Sr2—C17 | 128.81 (8) | C7—C6—H6A | 119.9 |
| O5—Sr2—C9 | 21.42 (6) | C5—C6—H6A | 119.9 |
| O10—Sr2—C9 | 174.73 (7) | C6—C7—C2 | 119.7 (3) |
| O2—Sr2—C9 | 87.44 (6) | C6—C7—C8 | 119.3 (2) |
| O9—Sr2—C9 | 130.15 (7) | C2—C7—C8 | 121.0 (3) |
| O7iv—Sr2—C9 | 87.16 (6) | O3—C8—O4 | 123.9 (3) |
| O8v—Sr2—C9 | 79.84 (6) | O3—C8—C7 | 117.3 (2) |
| O8—Sr2—C9 | 59.46 (6) | O4—C8—C7 | 118.8 (2) |
| O4ii—Sr2—C9 | 74.88 (6) | O3—C8—Sr1vii | 56.14 (14) |
| C17—Sr2—C9 | 155.22 (9) | O4—C8—Sr1vii | 69.17 (15) |
| O5—Sr2—Sr1 | 46.07 (4) | C7—C8—Sr1vii | 164.05 (18) |
| O10—Sr2—Sr1 | 127.95 (6) | O3—C8—Sr3i | 108.26 (18) |
| O2—Sr2—Sr1 | 41.23 (4) | O4—C8—Sr3i | 45.72 (12) |
| O9—Sr2—Sr1 | 163.24 (5) | C7—C8—Sr3i | 115.95 (16) |
| O7iv—Sr2—Sr1 | 88.05 (4) | Sr1vii—C8—Sr3i | 79.71 (6) |
| O8v—Sr2—Sr1 | 100.66 (4) | O6—C9—O5 | 122.1 (3) |
| O8—Sr2—Sr1 | 108.71 (4) | O6—C9—C10 | 119.3 (2) |
| O4ii—Sr2—Sr1 | 47.09 (4) | O5—C9—C10 | 118.6 (2) |
| C17—Sr2—Sr1 | 150.09 (7) | O6—C9—Sr1 | 59.12 (13) |
| C9—Sr2—Sr1 | 49.25 (5) | O5—C9—Sr1 | 68.79 (14) |
| O5—Sr2—Sr3viii | 99.78 (4) | C10—C9—Sr1 | 153.70 (18) |
| O10—Sr2—Sr3viii | 82.14 (6) | O6—C9—Sr2 | 97.14 (17) |
| O2—Sr2—Sr3viii | 147.27 (4) | O5—C9—Sr2 | 46.56 (13) |
| O9—Sr2—Sr3viii | 31.50 (5) | C10—C9—Sr2 | 126.17 (17) |
| O7iv—Sr2—Sr3viii | 75.82 (4) | Sr1—C9—Sr2 | 78.21 (6) |
| O8v—Sr2—Sr3viii | 83.16 (4) | C11—C10—C15 | 119.4 (3) |
| O8—Sr2—Sr3viii | 42.44 (4) | C11—C10—C9 | 118.8 (3) |
| O4ii—Sr2—Sr3viii | 149.44 (4) | C15—C10—C9 | 121.7 (2) |
| C17—Sr2—Sr3viii | 56.95 (7) | C12—C11—C10 | 121.0 (3) |
| C9—Sr2—Sr3viii | 99.28 (5) | C12—C11—H11A | 119.5 |
| Sr1—Sr2—Sr3viii | 145.840 (8) | C10—C11—H11A | 119.5 |
| O9vi—Sr3—O1i | 164.51 (6) | C13—C12—C11 | 119.4 (3) |
| O9vi—Sr3—O6iii | 73.93 (6) | C13—C12—H12A | 120.3 |
| O1i—Sr3—O6iii | 121.54 (6) | C11—C12—H12A | 120.3 |
| O9vi—Sr3—O3 | 103.04 (7) | C12—C13—C14 | 120.3 (3) |
| O1i—Sr3—O3 | 71.31 (6) | C12—C13—H13A | 119.9 |
| O6iii—Sr3—O3 | 113.33 (6) | C14—C13—H13A | 119.9 |
| O9vi—Sr3—O4i | 123.48 (6) | C13—C14—C15 | 120.9 (3) |
| O1i—Sr3—O4i | 67.42 (6) | C13—C14—H14A | 119.6 |
| O6iii—Sr3—O4i | 69.37 (6) | C15—C14—H14A | 119.6 |
| O3—Sr3—O4i | 130.36 (6) | C14—C15—C10 | 119.0 (3) |
| O9vi—Sr3—O5i | 87.78 (6) | C14—C15—C16 | 118.4 (2) |
| O1i—Sr3—O5i | 76.83 (6) | C10—C15—C16 | 122.4 (2) |
| O6iii—Sr3—O5i | 159.68 (6) | O7—C16—O8 | 122.4 (3) |
| O3—Sr3—O5i | 61.60 (6) | O7—C16—C15 | 120.0 (2) |
| O4i—Sr3—O5i | 129.85 (6) | O8—C16—C15 | 117.5 (2) |
| O9vi—Sr3—O7vi | 96.29 (6) | O7—C16—Sr3viii | 57.43 (14) |
| O1i—Sr3—O7vi | 75.32 (6) | O8—C16—Sr3viii | 66.83 (14) |
| O6iii—Sr3—O7vi | 126.54 (6) | C15—C16—Sr3viii | 161.68 (17) |
| O3—Sr3—O7vi | 120.04 (6) | O10—C17—O9 | 123.8 (3) |
| O4i—Sr3—O7vi | 74.30 (6) | O10—C17—Sr2 | 60.42 (19) |
| O5i—Sr3—O7vi | 63.16 (6) | O9—C17—Sr2 | 63.39 (18) |
| O9vi—Sr3—O1 | 120.34 (6) | O10—C17—H17A | 118.1 |
| O1i—Sr3—O1 | 71.19 (6) | O9—C17—H17A | 118.1 |
| O6iii—Sr3—O1 | 61.57 (5) | Sr2—C17—H17A | 177.4 |
Symmetry codes: (i) −x+1, −y, −z; (ii) x+1, y, z; (iii) −x+2, −y, −z; (iv) −x+1, −y−1, −z; (v) −x+2, −y−1, −z; (vi) x, y+1, z; (vii) x−1, y, z; (viii) x, y−1, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: LH5360).
References
- Brandenburg, K. (2010). DIAMOND Crystal Impact GbR, Bonn, Germany.
- Bruker (2010). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Kitagawa, S., Kitaura, R. & Noro, S. (2004). Angew. Chem. Int. Ed. 43, 2334–2375. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Stein, I. & Ruschewitz, U. (2005). Acta Cryst. E61, m141–m143.
- Wang, X., Liu, L., Makarenko, T. & Jacobson, A. J. (2010). Cryst. Growth Des. 10, 3752–3756.
- Zhang, L., Li, Z. J., Lin, Q. P., Qin, Y. Y., Zhang, J., Yin, P. X., Cheng, J. K. & Yao, Y. G. (2009). Inorg. Chem. 48, 6517–6525. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811044977/lh5360sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811044977/lh5360Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report