Abstract
Background
We aimed to characterize normal limits and determine the diagnostic accuracy for an automated quantification of 3D 82-Rubidium (Rb-82) PET/CT myocardial perfusion imaging (MPI).
Methods
We studied 125 consecutive patients undergoing Rb-82 PET/CT MPI, including patients with suspected coronary artery disease (CAD) and invasive angiography, and 42 patients with a low likelihood of CAD (LLk). Normal limits for perfusion and function were derived from LLk patients. QPET software was used to quantify perfusion abnormality at rest and stress expressed as total perfusion deficit (TPD).
Results
Relative perfusion databases did not differ in any of the 17 segments between males and females. The areas under the receiver operating characteristic curve for detection of CAD were 0.86 for identification of ≥50% and ≥70% stenosis. The sensitivity/specificity was 86%/86% for detecting ≥50% stenosis, and 93%/77% for ≥70% stenosis, respectively. Mean rest and stress left ventricular ejection fraction (LVEF) were 67±10% and 75±9%. Mean transient ischemic dilation ratio was 1.06±0.14 and mean increase in LVEF with stress was 7.4±6.1% (95th percentile of 0%).
Conclusion
Normal limits have been established for 3D Rb-82 PET/CT analysis with QPET software. Fully automated quantification of myocardial perfusion PET data shows high diagnostic accuracy for detecting obstructive CAD.
Keywords: PET/CT, myocardial perfusion imaging, quantification, 3D, coronary artery disease
Myocardial perfusion imaging (MPI) with dedicated positron emission tomography (PET) and integrated PET/computed tomography (CT) provides a highly accurate evaluation for detection of obstructive coronary artery disease (CAD).(1–3) MPI with dedicated PET and PET/CT may be more accurate than single-photon emission tomography (SPECT) in the diagnosis of obstructive CAD, especially among those undergoing pharmacologic stress.(4–6) Increased availability of PET/CT scanners, as well as improved access to the generator-produced radiotracer 82-Rubidium (Rb-82) are among factors that have lead to the increasing use of PET.
Quantitative MPI from SPECT has been shown to be accurate.(7) In addition, it is more reproducible than visual image interpretation.(8), (9) To date, however, the quantitative assessment of MPI with integrated PET/CT has been described in only in one report, which included 53 patients with correlating invasive coronary angiography (ICA).(10) Furthermore, the prior study considered data acquired on a 2D PET/CT scanner, whereas most current systems can be operated in 3D mode only, with considerably different performance parameters.(11) Our group has recently developed an improved software (QPET) for left ventricular (LV) contour detection for PET MPI, which has been applied to gated 2D PET/CT data for analysis of myocardial perfusion and left ventricular ejection fraction (LVEF) in a preliminary study.(12)
In this work, we incorporate this new dedicated PET segmentation algorithm with our method for analysis of normal limits for myocardial perfusion SPECT(13) and apply it to 3D Rb-82 PET MPI data. We develop the normal limits for the rest/adenosine stress myocardial perfusion and function, and validate quantitative myocardial perfusion analysis in comparison to ICA. This is the first report of QPET analysis of MPI data.
MATERIALS AND METHODS
Total Study Population
The total study population consisted of 125 patients who underwent rest/stress Rb-82 PET/CT MPI at our institution. All patients provided written informed consent for the use of their clinical and imaging data for research purposes.
Angiographic validation
For comparison of Rb-82 PET/CT vs. ICA, we included 83 consecutive patients (53 males) who had ICA within 6 months of rest/stress Rb-82 PET/CT MPI and did not have any of the following: (a) prior myocardial infarction or coronary revascularization; (b) non-ischemic cardiomyopathy or valvular heart disease; and (c) change in symptoms between MPI and ICA. The mean age was 70 ± 12 years and mean body mass index was 28 ± 6 kg/m2 (range 15–44). Other clinical characteristics are shown in Table 1.
Table 1.
Characteristics of patients with ICA
| Age | 70 ± 12 |
| Men | 53 (64%) |
| Body mass index (kg/m2) | 28 ± 6 |
| Hypertension | 68 (82%) |
| Diabetes | 31 (37%) |
| Hypercholesterolemia | 52 (63%) |
| Smoking | 13 (16%) |
| Typical angina | 5 (6%) |
| Atypical angina | 31 (37%) |
| Shortness of breath | 21 (25%) |
| Asymptomatic | 19 (23%) |
| Time between PET/CT MPI and ICA (days) | 15 ± 28 |
ICA invasive coronary angiography, MPI myocardial perfusion imaging
Low likelihood population
For the generation of normal limits of perfusion and function from Rb-82 PET/CT, we included an additional group of 42 patients who had a low likelihood (LLk) of CAD (<10%) based on age, sex, symptoms and coronary risk factors.(14) These patients did not have angina, shortness of breath, abnormal resting ECG, or abnormal stress ECG response (Table 2). Furthermore, to be included in the normal limits group, these patients had normal rest and stress PET perfusion findings on visual assessment (visual summed score <3), as per previous practice in generating reference limits with datasets obtained from patients with LLk of disease.(7, 13, 15)
Table 2.
Characteristics of patients with LLk of CAD
| Age | 61 ± 14 |
| Men | 21 (50%) |
| Body mass index (kg/m2) | 28 ± 8 |
| Hypertension | 29 (69%) |
| Diabetes | 11 (26%) |
| Hypercholesterolemia | 23 (55%) |
| Smoking | 2 (5%) |
LLk low likelihood, CAD coronary artery disease
PET/CT Scan Acquisition and Reconstruction
All image data were acquired in list mode on a Siemens Biograph-64 TruePoint PET/CT with the TrueV option. This 3D system consists of a 64-slice CT and a PET scanner with 4 rings of Lutetium Oxyorthosilicate (LSO) detectors with element dimensions of 4 × 4 × 20 mm3.(16) The image plane spacing is 2 mm. The dimensions for the PET axial and transaxial FOV are 216 and 605 mm respectively. The coincidence time window and the energy window are respectively 4.5 ns and 425–650 keV. The data were acquired in list mode format with CT based attenuation correction. In this study, CT scan was used only for attenuation correction. A full description of the system performance can be found in a previous report.(17) Patients were instructed to abstain from consuming any products containing caffeine for 24 h before the test. Beta-blockers and calcium-channel antagonists were discontinued for 48 h, and nitrates were withheld for at least 6 h before testing. After a 2.8 sec topogram acquisition (120 kVp, 35 mA) used for patient positioning, a CT transmission scan (120 kVp, pitch 1.5) was acquired (Figure 1). Subsequently, patients were injected with 925 to 1850 MBq (25 to 50 mCi) of Rb-82 at rest. A 6-minute rest imaging acquisition was started simultaneously with the start of the rest Rb-82 infusion. Immediately after completion of rest imaging, a second stress CT transmission scan was performed (120 kVp, pitch 1.5). Pharmacologic stress was performed by adenosine infusion (0.14 mg/kg/min for 7 min). Two minutes after the adenosine infusion, 925 to 1850 MBq (25 to 50 mCi) of Rb-82 was administered using a separate intravenous line in the other arm to prevent blousing the adenosine(18) (infusion rate 35 – 50 ml/min). Rest and stress CT transmission scans were acquired at end-expiration breath-hold, and patients were instructed to breathe normally during the PET acquisition. Patients’ emission data were reconstructed with 2D Attenuation Weighted Ordered Subsets Expectation Maximization (2 iterations and 21 subsets). CT-based attenuation, scatter, including prompt gamma,(19) decay, and random corrections were applied to the reconstructed images.
Figure 1.
Rest/stress Rb-82 PET/CT protocol.
* Adenosine; 140 µg/kg/min for 7 minutes
** CT transmission scan end-tidal expiration
Static and 8-bin ECG-gated images were generated from the list mode data. Gaussian filters with a full width at half maximum (FWHM) of 8mm for rest and stress images were used. The first 2 to 3 min of the list file was skipped to allow for blood pool clearance of Rb-82. In this study, dynamic image analysis was not considered. The next 3 min of acquisition data were used to generate sinograms for static and gated images. The quality of the registration between PET and CT was first estimated by experienced nuclear cardiology technologists using fused images in the Siemens 3D-software on a Leonardo workstation (Syngo 6.0, Siemens Medical Solution, Hoffman Estates, IL). When misalignment was identified, a manual registration matrix with 3D-translations was generated by the technologists and applied before the final reconstruction process. Both the original CT-PET alignment and the alignment after manual registration were re-checked using QPET software by an experienced imaging cardiologist as previously described.(20) Visual quality control identified patient motion in 31 cases out of 83 cases, and manual motion-correction was applied in these cases.
QPET Analysis
Segmentation of left ventricle
Transaxial PET perfusion images were automatically reoriented into short-axis and vertical and horizontal long-axis slices using software (QPET, Cedars-Sinai Medical Center, Los Angeles, California).(12) The QPET algorithm is based on the original QGS contour detection principles.(21) Briefly, an ellipsoidal model and contours are used to extract polar map samples as it was with SPECT, but with twice myocardial sampling due to the higher PET resolution.(21) For PET MPI, the algorithm was also modified to track the valve plane frame-to-frame as opposed to constraining the position of the valve plane as used for SPECT MPI calculations. As with SPECT, the mass conservation principle was applied in order to constrain the total volume of the myocardium frame-to-frame. For regional analysis, the myocardial wall segmentation was done with the 17-segment American Heart Association (AHA) model.(22)
Functional parameters
Functional assessments included left ventricular volumes, LVEF, regional wall motion and thickening, and transient ischemic dilation (TID). TID of the LV was defined as the ratio of LV volume at stress and rest.(23) All functional parameters were derived from the segmented 8-bin PET/CT data. LVEF was obtained as previously described.(24) Increase in LVEF with stress was calculated as stress LVEF − rest LVEF, and reported as the absolute difference in LVEF percentage. Regional wall motion was computed from the obtained contours by comparing the end-diastolic and end-systolic positions of the endocardial contour points for each polar map sample position. Similarly, regional wall thickening was computed by comparing the end-diastolic and end-systolic endocardial-to-epicardial distances for each polar map point.
Myocardial perfusion quantification
For the purposes of automated myocardial perfusion quantification, a study-to-study optimal count normalization factor was established by an iterative search for the minimal absolute count difference between the counts in the normal part of the myocardium, and the corresponding normal counts in the normal database. This scheme avoided normalization based on an arbitrary selection of pixels (maximum or percentile maximum).(25) Subsequently, an abnormality threshold of 3.0 average (mean absolute) deviations, which is equivalent approximately to 2.5 standard deviation (SD), was applied to estimate the extent of hypoperfusion as in myocardial perfusion SPECT quantification. The perfusion defect extent was calculated as the percentage of the total surface area of the left ventricle for which test data were below the abnormality threshold. The quantitative perfusion variable employed was total perfusion deficit (TPD), which reflects a combination of both defect severity and the extent of the defect in one parameter, as previously described.(13) Stress and ischemic (difference between stress and rest) myocardial perfusion defects on PET/CT were assessed by quantification of the TPD.(26, 27)
Normal limits for perfusion and function
Normal limits for perfusion and function were derived from a group of 42 patients. The cutoff values for abnormal TID and increase in LVEF with stress were defined as 95th percentile, rather than based on SD values due to asymmetric non-Gaussian character of the distribution.
Generation of thresholds for abnormal TPD
In determining the optimal cut-off for global and regional (per-vessel) TPD, we have considered it to be above the 95th percentile for TPD values in the LLk patients, rounded to integer values as returned by the software, similar to our analysis for SPECT MPI.(15) The resultant threshold of global TPD ≥5% is in agreement with the previously established thresholds for SPECT.(7, 15) Similarly, for per-vessel analysis, the resultant threshold of regional TPD ≥2% is agreement for quantitative measurement in each coronary artery territory. Abnormalities in the anterior and septal segments were allocated to the left anterior descending coronary artery (LAD), in the lateral segments to the left circumflex coronary artery (LCX), and in the inferior segments to the right coronary artery (RCA). For the identification of 3 vessel disease (3VD), we considered cases with ≥2% stress TPD in all LAD, LCX and RCA territories.
ICA Analysis
All ICA were interpreted visually by experienced cardiologists, who were unaware of PET/CT MPI results. Patients did not have myocardial infarction or revascularization in the interval between PET/CT MPI and ICA. A ≥50% or ≥70% luminal diameter stenosis by visual assessment was considered hemodynamically significant. Patients with left main CAD were considered to have stenosis in both the LAD and LCX vessels. Diagonal lesions were considered as LAD and obtuse marginal arteries were considered as LCX.
Statistical Analysis
Statistical analyses were performed with STATA software (version 10, StataCorp LP). All continuous variables were described as mean ± SD. For all data sets, the Shapiro-Wilk test was used for normality. Datasets did not require any adjustment to convert them to a normal distribution. Unpaired t-tests were used to compare differences in unpaired continuous data for normal database variation using p <0.01 as a cutoff. The receiver-operating-characteristic (ROC) curve analysis was performed to evaluate the ability of the quantification to predict ≥50% or ≥70% diameter stenosis of coronary arteries. ROC curves were created using a step of 0.1%, for the TPD values, and the differences between ROC curve areas (area ± SE) were compared. Differences in estimated sensitivity and specificity were compared using the McNemar’s test. In terms of the individual coronary territories, sensitivity and specificity were calculated at a cutoff point disease severity of ≥50% or ≥70% luminal diameter narrowing on ICA.
RESULTS
Automatically generated myocardial contours were evaluated by an experienced imaging cardiologist without knowledge of any clinical data, and when necessary, contours were adjusted to correspond to the myocardium (8% of cases). The software required manual adjustment of the initial LV mask of the stress contours in 6 static (7%) and 8 gated cases (10%), rest contours in 8 static (10%) and 10 gated cases (12%). All other processing was automatic.
Characterization of Normal Subjects
Normal limits for myocardial perfusion
Previously, the gender-specific databases showed differences for SPECT due to the lack of attenuation correction and differences in photon attenuation caused by variable breast geometry in females. PET scans are acquired with attenuation correction and there should not be any gender differences due to photon attenuation. Nevertheless, to determine whether there were some residual gender differences, the normal distributions of male (n = 21) and female (n = 21) studies were initially generated separately. The normal distributions did not show significant differences between genders as compared in 17-segments by t-test. Therefore, the distributions from males and females were then combined and averaged to produce a single file of mean normal-count regional distribution. This method was previously described combining the males and females databases by other groups.(10, 28) Furthermore, we estimated the power to detect a 5% difference between males and females databases to be 87%. Since the maximum difference in the segmental mean values were 2% (stress) and 3% (rest), both smaller differences than clinically meaningful, we assumed that males and females databases can be combined the results. Figure 2 shows final sex-independent mean and SD myocardial perfusion segmental count distributions at rest and stress derived from the patients with LLk of CAD. Uniform count distributions are noted throughout the myocardium with the exception of the base and apex of the heart, which is a demonstrated reduced mean count.
Figure 2.
Sex-independent polar map for rest and stress Rb-82 PET/CT mean normal myocardium distribution and SD in LLk of CAD patients (n = 42).
Normal limits for functional parameters
Normal functional parameters were also derived from each dataset with male and female (Table 3). Unpaired t-tests were used to compare the gender differences in these values and found the significant difference only in stress EDV and stress ESV. This finding that females have significantly smaller EDV and ESV is consistent with our previous report.(29) Since there were no significant differences, we combined the gender for the rest of parameters. Table 3 shows gender combined functional parameters as well as male and female, separately obtained in the LLk population. Figure 3 shows the gender combined mean normal distribution of wall motion (mm) and wall thickening (%) at rest and stress in the LLk population. Figure 3 shows the gender combined mean normal distribution of wall motion (mm) and wall thickening (%) at rest and stress in the LLk population. The cutoff value for abnormality (95th percentile) was determined to be 1.28 for TID ratio, and 0.0% for increase in LVEF with stress. In our case, the median increase in LVEF with stress was 6.1% and the range was −3.7% to 28.1%. There were 40 studies (95%) with increase in LVEF with stress greater than 0.0% and 2 studies (5%) with increase in LVEF with stress below 0%. If mean +2SD were used, the threshold would have resulted in a normal limit, which would be higher. Due to the asymmetry in the positive and negative SD values, the 95% percentile is a more robust approach for defining the normal limits.
Table 3.
Non-perfusion global normal values obtained in LLk population
| Values | Mean ± SD (total) | Mean ± SD (Male) | Mean ± SD (Female) |
|---|---|---|---|
| Rest EDV (ml) | 64 ± 18 | 69 ± 18 | 59 ± 18 |
| Rest ESV (ml) | 22 ± 11 | 25 ± 10 | 19 ± 11 |
| Stress EDV (ml) | 75 ± 21 | 83 ± 20 | 67 ± 20 |
| Stress ESV (ml) | 20 ± 11 | 24 ± 11 | 16 ± 10 |
| Rest LVEF (%) | 67 ± 10 | 64 ± 8 | 70 ± 11 |
| Stress LVEF (%) | 75 ± 9 | 72 ± 8 | 77 ± 9 |
| Increase in LVEF with stress (%) | 7.4 ± 6.1 | 7.9 ± 6.5 | 7.0 ± 5.9 |
| TID | 1.06 ± 0.14 | 1.10 ± 0.13 | 1.02 ± 0.14 |
| Rest WM (mm) | 7.0 ± 1.1 | 6.7 ± 1.0 | 7.3 ± 1.2 |
| Rest WT (%) | 44 ± 14 | 41 ± 13 | 46 ± 15 |
| Stress WM (mm) | 8.6 ± 1.3 | 8.3 ± 1.1 | 8.9 ± 1.5 |
| Stress WT (%) | 58 ± 19 | 54 ± 17 | 61 ± 21 |
LLk Low likelihood, EDV end-diastolic volume, ESV end-systolic volume, LVEF left ventricular ejection fraction, TID transient ischemic dilation, WM wall motion, WT wall thickening
Figure 3.
Mean normal distribution of wall motion (WM, mm) and wall thickening (WT, %) in LLk of CAD patients (n = 42).
ICA Findings
There were 62 patients with and 21 patients without ≥50% diameter stenosis. There were 53 patients with and 30 patients without ≥70% diameter stenosis (Table 4). Eighteen patients had 3VD with ≥50% diameter stenosis.
Table 4.
ICA findings with the number of patients in each category
| Stenosis | ||
|---|---|---|
| ≥ 50% | ≥ 70% | |
| Per Patient | 62 | 53 |
| 1VD | 24 | 26 |
| 2VD | 20 | 18 |
| 3VD | 18 | 9 |
| Per Vessel LAD | 52 | 43 |
| Per Vessel LCX | 36 | 25 |
| Per Vessel RCA | 31 | 21 |
ICA invasive coronary angiography, VD vessel disease, LAD left anterior descending artery, LCX left circumflex artery, RCA right coronary artery
Performance of Myocardial Perfusion Quantitative Analysis
Of the 83 patients who underwent PET/CT and ICA, 56 patients had perfusion defects with stress TPD ≥5%, and 51 patients had perfusion defects with ischemic-TPD ≥5%. Examples of normal perfusion and abnormal perfusion quantified with QPET are shown in Figure 4.
Figure 4.
PET images and quantitative polar maps from a patient with normal limits database (A). Uniform tracer distribution is noted with no quantitative abnormality.
Abnormal PET images in patient with a 90% proximal LAD stenosis (B). A reversible perfusion defect is seen in the anterior, septal and apical walls both on the images and the polar maps. Stress and ischemic TPD were both 15%.
Figure 5A shows ROC curves for the detection of ≥50% diameter stenosis by stress and ischemic (stress − rest) TPD. The areas under the ROC curves for stress TPD and ischemic TPD were both 0.86. Figure 5B shows ROC curves for the detection of ≥70% diameter stenosis by stress and ischemic TPD. The areas under the ROC curves for stress-TPD and ischemic-TPD were 0.86 and 0.88, respectively.
Figure 5.
ROC curves for detection of CAD [≥50% diameter stenosis (A), ≥70% diameter stenosis (B)] by measurements of stress TPD and ischemic TPD in angiographic population (n = 83). Percentage TPD was compared with presence or absence of hemodynamically significant CAD as observed by ICA. Stress TPD ≥5% and ischemia TPD≥ 5% were used as cutoff points.
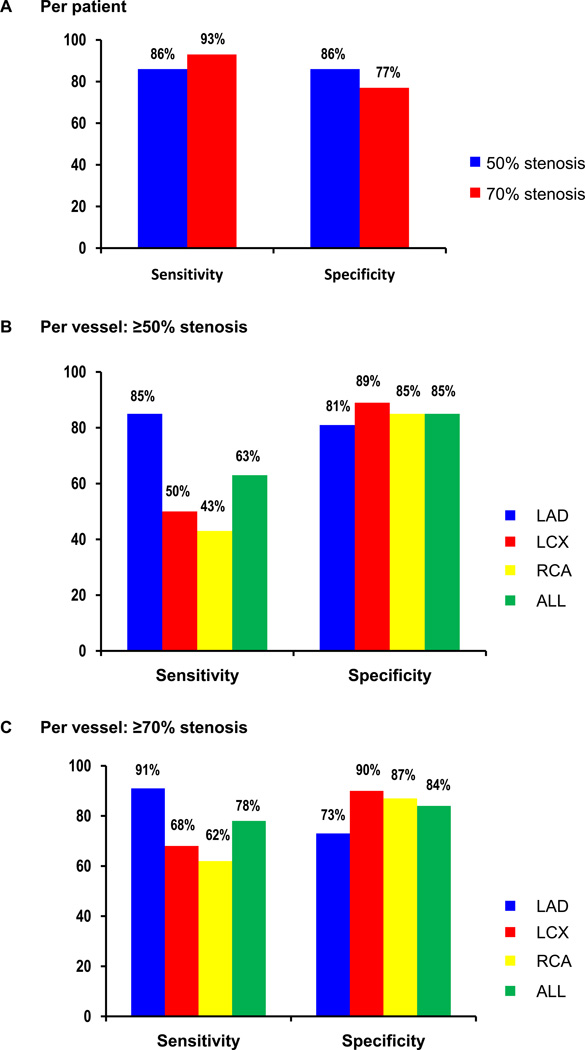
On a per patient basis, using a stress TPD ≥5%, the sensitivity (53/62) and specificity (18/21) were both 86% for the detection of ≥50% stenosis, 93% (49/53), and 77% (23/30) for the detection of ≥70% stenosis (Table 5, Figure 6A). On a per vessel basis, sensitivity was significantly higher for detection of LAD stenosis than for other vessels (p < 0.01 for all) for detecting ≥50% stenosis. When all vessels were combined, the per-vessel sensitivity and specificity were 63% and 85% for the detection of ≥50% diameter stenosis (Figure 6B). The overall vessel sensitivity was higher for ≥70% stenosis than for ≥50% stenosis (p < 0.01, Figure 6C). Similarly, for the detection of ≥70% diameter stenosis, when vessels were combined, the sensitivity and specificity were 78% and 84%. The sensitivity for detection of LAD stenosis was significantly higher than for the other vessels (p <0.01 for all). Eight out of 18 patients (44%) who had 3VD with ≥50% diameter stenosis showed TPD above the regional threshold in all respective vessels. Of 65 patients without 3VD, 62 patients (95% specificity) had normal TPD (<2%) in all vessels.
Table 5.
Results of PET and ICA findings
| ≥50% stenosis | < 50% stenosis | |
| stress TPD ≥5% | 53 | 3 |
| stress TPD <5% | 9 | 18 |
| ≥70% stenosis | < 70% stenosis | |
| stress TPD ≥5% | 49 | 7 |
| stress TPD <5% | 4 | 23 |
TPD total perfusion deficit
Figure 6.
Sensitivity and specificity per patient (A) and per vessel [B (≥50% diameter stenosis), C (≥70% diameter stenosis)] for detection of CAD by stress TPD in angiographic population.
DISCUSSION
We have comprehensively characterized normal limits and validated the diagnostic accuracy for the automated quantification (QPET) of 3D Rb-82 PET/CT MPI in the detection of obstructive CAD in comparison to ICA. The method uses modified segmentation of the LV adapted from myocardial perfusion SPECT algorithm (21) taking into account PET image characteristics.
Overall, in the previous reports of PET MPI diagnostic performance, the average sensitivity for detecting at least one coronary artery with ≥50% stenosis was 89% (range, 83%–100%), whereas the average specificity was 89% (range, 73%–100%) in 14 studies.(30) Among the 14 studies, 6 studies evaluated the diagnostic accuracy of obstructive CAD by Rb-82, and those results are comparable to our results (93% sensitivity, 77% specificity). Most of the previous results were obtained with standalone PET scanners rather than with integrated PET/CT systems. Limited data exist regarding the diagnostic accuracy of PET/CT MPI,(30) and it is known that CT attenuation correction presents difficulties due to potential image misregistration between PET and CT.(31) In addition, previous results were obtained by visual analysis in all except one report.(10) To date, two studies analyze PET/CT diagnostic performance, Sampson et al reported a sensitivity of 93% and a specificity of 83% by visual MPI assessment for detecting ≥70% stenosis in patients without known CAD by visual analysis for PET/CT operating in 2D mode.(2) In their study, the calculated specificity included the LLk patients and those with angiographically normal coronary arteries because of the small number of patients (n = 20) with angiographically normal coronary arteries.(2) In the only quantitative perfusion PET/CT analysis study to date, Santana et al reported the sensitivity and specificity as 93% and 75% for ≥50% stenosis threshold, 95% and 54% for ≥70% stenosis for 2D PET acquisition.(10) Their diagnostic results are comparable to the results reported in our study. However, ranges (SD) of normal limits for myocardial perfusion for both rest and stress Rb-82 were smaller in our study especially in the basal slice. In their reports, average SD in the basal slice (6 segments) was 17.3% for rest and 17.8% for stress whereas in our reports, the average SD was 5.1% for rest and 4.6% for stress. This may be due to the use of different software and a different normalization algorithm for calculation of normal limits. However, our average values for the normal are similar to the values reported by Santana et al except in the basal region (Figure 2).
This study validates the relative perfusion analysis for static PET MPI analysis. PET MPI also allows analysis of myocardial blood flow with Rb-82 PET, and this has been shown to be useful in identifying 3VD.(32) Although mean flow rate measurements may be obtainable from PET-based perfusion imaging, potential sources of errors in measurement, including the effect of high driving pressure and high resting flow rates, are still being characterized. Still unexplored is how mean flow rate may differentiate between patients who have epicardial stenoses and patients who have abnormalities with subendocardial microvascular perfusion. In addition, there are several technical issues in obtaining quality flow data with Rb-82, especially on the 3D systems such as count rate.(33) Thus, the analysis with relative perfusion from the static perfusion data and utilization of normal databases is still the primary diagnostic method. Quantitative rather than visual analysis of this data ensures much higher reproducibility.(8, 9) Thus, the validation of quantitative static perfusion analysis presented in this study is of high clinical relevance.
Regarding normal functional parameters, Bravo et al reported the reference range of LVEF from gated Rb-82 PET/CT 2D acquisition using 4 different commercial software packages.(34) In their report, standard SPECT software packages were used for the analysis, and mean LVEF ranged from 51% to 67% for rest and 59% to 72% for stress. The current study demonstrates higher values compared to their report, which are most likely due to the optimization of the algorithm for PET. In our preliminary work,(35) we compared SPECT and PET implementations, and this resulted in higher values that were more concordant with CT angiography. Cullom et al reported the mean TID value for normal with rest/dipyridamole stress Rb-82 PET as 0.95 in a preliminary study using a SPECT software package.(36) Our study, which used PET dedicated software showed a slightly higher value of 1.06. To our knowledge, quantitative wall motion and thickening was not previously reported for Rb-82 PET analysis and in particular not for the 3D Rb-82 PET. We have demonstrated the regional normal limits for wall motion and thickening for the QPET analysis. We have shown the increase of wall motion (average 1.6mm) and thickening (average 14%) at stress compared to rest. We also noted decreasing thickening towards the base (Figure 3) similar in the normal subjects to that reported for SPECT MPI (37) despite the fact that attenuation correction was performed on gated data unlike in myocardial perfusion SPECT.
The overall sensitivity for 3VD detection was low (44%). Such low sensitivity was the result of the criterion that TPD be positive in all vascular territories, which are not precisely determined in MPI. However, 17 out of 18 patients (94%) with 3VD showed TPD above the global threshold (TPD ≥5%) on a per patient basis (irrespective of the defect location). Adding routinely available functional information during stress may contribute to identify patients with extensive CAD.(3) Dorbala et al reported the sensitivity/specificity as 94%/55% with an increase in LVEF with stress below +5% threshold, and 59%/94% with an increase in LVEF with stress less than −5% threshold for the detection of LM/3VD.(3) In our study, we did not evaluate the incremental value of the function parameter in patients with 3VD since our population had a limited number of cases with 3VD.
Although, the limitation in sensitivity of PET for detecting 3VD (defined as detection of perfusion defects in each of 3 territories) is not resolved using relative perfusion analysis, this work focused on the analysis of static relative myocardial perfusion and did not consider the myocardial blood flow with Rb-82 PET analysis, which has been shown to be useful in identifying 3VD.(32) Mean flow rate measurements may be obtainable from PET-based perfusion imaging; however, potential sources of errors in measurement, including the effect of high driving pressure and high resting flow rates, are still being characterized. Furthermore, it is still unexplored how mean flow rate may differentiate between patients who have epicardial stenoses and patients who have abnormalities with subendocardial microvascular perfusion. Nevertheless, there are still several technical issues in obtaining quality flow data with Rb-82 such as count rate, especially for the 3D PET/CT.(33) These studies are not routinely performed by PET centers since time requirements for reconstruction time from list mode are significant and specialized software is required for their analysis. Therefore the analysis with relative perfusion from the static perfusion data and utilization of normal databases is still the primary diagnostic method. Even though perfusion data can be assessed visually, quantitative analysis ensures much higher reproducibility.(8, 9) Therefore, the analysis presented in this study is of high clinical relevance.
Although 3D Rb-82 acquisitions were previously compared to the 2D Rb-82 acquisitions for the standalone scanners,(38) the image statistics and image reconstructions applied are significantly different,(39) and this is the first study assessing diagnostic performance of the 3D PET/CT scanners for MPI. The principal advantage of 3D mode is significantly higher photon sensitivity (4–6 times),(11, 40) which can significantly reduce the tracer activity needed or shorten the acquisition time. An increase in sensitivity can improve gated imaging in particular, because in 2D mode, the image quality is severely limited by the number of counts. However, compared with 2D mode, 3D imaging still has some disadvantages due to increase scatter. The quality of 3D cardiac images was shown to be lower than(39) or, at best, comparable with that of 2D PET images.(38) Therefore, we believe it is important to provide this validation since most current scanners operate in 3D mode. In this work, we demonstrate that in general, the quantitative diagnostic performance and functional parameters are comparable to the study describing the 2D PET/CT data.
Study Limitations
The study was performed at a single center, and further studies may need to be performed to verify if the findings are applicable to other centers as well as other types of scanners. While it is expected that the same algorithms should provide the same results regardless of manufacturer, the current study analyzed data acquired only on a single specific PET/CT device. Although 3D PET scanners exhibit some similar characteristics in terms of physical performance, currently, there is little evidence in the literature to support the cross compatibility of cardiac PET systems. Thus, these results may need to be validated for other types of scanners. Normal functional parameters need to be validated in further larger study. However, the use of fully automated quantitative analysis increases the likelihood that the results of our study could be replicated elsewhere. Our study was a retrospective analysis of patients who had undergone integrated PET/CT and ICA for up to 6 months. However, the mean duration between studies was only 15 days and 88% of patients underwent both studies within 4 weeks. Moreover, patients considered for the analysis did not have a change in clinical status between the studies. The ICA was performed for clinical purposes. This practice results in a selection bias since patients with abnormal PET/CT are more likely to be referred for ICA. Specific cutoff thresholds for a given protocol may need to be confirmed in larger studies. We cannot evaluate the incremental value of the functional parameter for detecting 3VD due to a small population. We did not consider dynamic PET/CT perfusion studies in this analysis, which could further aid in detection of 3VD. However, we have considered most parameters obtained by standard PET/CT acquisition. Further studies may need to be performed to verify if the findings are applicable to other centers as well as other types of scanners. However, it should be noted that the count rate performance of this scanner is likely to be less important for the static perfusion images analyzed here than it would be for the dynamic images.
CONCLUSION
We have performed a comprehensive evaluation of automated analysis for 3D Rb-82 PET/CT scans by QPET software dedicated for PET MPI analysis. Normal limits for perfusion and function have been established. Automated quantification of 3D Rb-82 PET/CT shows high diagnostic accuracy for detecting obstructive CAD, with findings comparable to previous reports with this equipment using expert visual analysis.
Acknowledgements
The authors thank Brandi N. Huber from Cedars-Sinai Medical Center for their help with the PET acquisition. We would like to thank Arpine Oganyan for editing and proof-reading the text. Drs. Piotr Slomka, Guido Germano, and Daniel Berman receive royalties from the software employed in the study. Dr. Ludovic Le Meunier is an employee of Siemens Medical Systems, PET Division.
Footnotes
Conflict of interest statement
Some authors (DB, GG, PJS), receive royalties from the software employed in the study. LM is an employee of Siemens Medical Systems, PET Division. All others disclose no current conflict of interest.
References
- 1.Di Carli MF, Hachamovitch R. New technology for noninvasive evaluation of coronary artery disease. Circulation. 2007;115:1464–1480. doi: 10.1161/CIRCULATIONAHA.106.629808. [DOI] [PubMed] [Google Scholar]
- 2.Sampson UK, Dorbala S, Limaye A, Kwong R, Di Carli MF. Diagnostic accuracy of rubidium-82 myocardial perfusion imaging with hybrid positron emission tomography/computed tomography in the detection of coronary artery disease. J Am Coll Cardiol. 2007;49:1052–1058. doi: 10.1016/j.jacc.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Dorbala S, Vangala D, Sampson U, Limaye A, Kwong R, Di Carli MF. Value of vasodilator left ventricular ejection fraction reserve in evaluating the magnitude of myocardium at risk and the extent of angiographic coronary artery disease: a 82Rb PET/CT study. J Nucl Med. 2007;48:349–358. [PubMed] [Google Scholar]
- 4.Stewart RE, Schwaiger M, Molina E, Popma J, Gacioch GM, Kalus M, et al. Comparison of rubidium-82 positron emission tomography and thallium-201 SPECT imaging for detection of coronary artery disease. Am J Cardiol. 1991;67:1303–1310. doi: 10.1016/0002-9149(91)90456-u. [DOI] [PubMed] [Google Scholar]
- 5.Go RT, Marwick TH, MacIntyre WJ, Saha GB, Neumann DR, Underwood DA, et al. A prospective comparison of rubidium-82 PET and thallium-201 SPECT myocardial perfusion imaging utilizing a single dipyridamole stress in the diagnosis of coronary artery disease. J Nucl Med. 1990;31:1899–1905. [PubMed] [Google Scholar]
- 6.Bateman TM, Heller GV, McGhie AI, Friedman JD, Case JA, Bryngelson JR, et al. Diagnostic accuracy of rest/stress ECG-gated Rb-82 myocardial perfusion PET: comparison with ECG-gated Tc-99m sestamibi SPECT. J Nucl Cardiol. 2006;13:24–33. doi: 10.1016/j.nuclcard.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Nishina H, Slomka P, Abidov A, Yoda S, Akincioglu C, Kang X, et al. Combined supine and prone quantitative myocardial perfusion SPECT: method development and clinical validation in patients with no known coronary artery disease. J Nucl Med. 2006;47:51–58. [PubMed] [Google Scholar]
- 8.Berman D, Kang X, Gransar H, Gerlach J, Friedman J, Hayes S, et al. Quantitative assessment of myocardial perfusion abnormality on SPECT myocardial perfusion imaging is more reproducible than expert visual analysis. J Nucl Cardiol. 2009;16:45–53. doi: 10.1007/s12350-008-9018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Y, Hayes S, Ali I, Ruddy TD, Wells RG, Berman DS, et al. Automatic and visual reproducibility of perfusion and function measures for myocardial perfusion SPECT. J Nucl Cardiol. 2010;17:1050–1057. doi: 10.1007/s12350-010-9297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santana CA, Folks RD, Garcia EV, Verdes L, Sanyal R, Hainer J, et al. Quantitative (82)Rb PET/CT: development and validation of myocardial perfusion database. J Nucl Med. 2007;48:1122–1128. doi: 10.2967/jnumed.107.039750. [DOI] [PubMed] [Google Scholar]
- 11.Mawlawi O, Podoloff DA, Kohlmyer S, Williams JJ, Stearns CW, Culp RF, et al. Performance characteristics of a newly developed PET/CT scanner using NEMA standards in 2D and 3D modes. J Nucl Med. 2004;45:1734–1742. [PubMed] [Google Scholar]
- 12.Slomka P, Dorbala S, Berman D, Gerlacj J, Germano G, Di Carli M. Automated quantification and normal limits for myocardial perfusion stress/rest Rb-82 PET/CT. J Nucl Med. 2009;50(Suppl 2):1164. [Google Scholar]
- 13.Slomka P, Nishina H, Berman D, Akincioglu C, Abidov A, Friedman J, et al. Automated quantification of myocardial perfusion SPECT using simplified normal limits. J Nucl Cardiol. 2005;12:66–77. doi: 10.1016/j.nuclcard.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Diamond G, Forrester J. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300:1350–1358. doi: 10.1056/NEJM197906143002402. [DOI] [PubMed] [Google Scholar]
- 15.Nakazato R, Tamarappoo BK, Kang X, Wolak A, Kite F, Hayes SW, et al. Quantitative upright-supine high-speed SPECT myocardial perfusion imaging for detection of coronary artery disease: correlation with invasive coronary angiography. J Nucl Med. 2010;51:1724–1731. doi: 10.2967/jnumed.110.078782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonsson C, Odh R, Schnell P, Larsson S. A comparison of the imaging properties of a 3-and 4-ring biograph PET scanner using a novel extended NEMA phantom. IEEE Nucl Sci Symp Conf Rec. 2007;4:2865–2867. [Google Scholar]
- 17.Townsend D, Jakoby B, Long M, Carr C, Hubner K, Guglielmo C, et al. Performance and clinical workflow of a new combined PET/CT scanner. J Nucl Med. 2007;48(supp 2):437P. [Google Scholar]
- 18.Cerqueira MD, Verani MS, Schwaiger M, Heo J, Iskandrian AS. Safety profile of adenosine stress perfusion imaging: results from the Adenoscan Multicenter Trial Registry. J Am Coll Cardiol. 1994;23:384–389. doi: 10.1016/0735-1097(94)90424-3. [DOI] [PubMed] [Google Scholar]
- 19.Watson C, Hayden C, Casey M, Hamill J, Bendriem B. Prompt gamma correction for improved quantification in 82Rb PET. J Nucl Med. 2008;49(supp 1):64P. [Google Scholar]
- 20.Slomka P, Le Meunier L, Hayes S, Acampa W, Oba M, Haemer G, et al. Comparison of myocardial perfusion 82Rb PET performed with CT- and transmission CT-based attenuation correction. J Nucl Med. 2008;49:1992–1998. doi: 10.2967/jnumed.108.056580. [DOI] [PubMed] [Google Scholar]
- 21.Germano G, Kiat H, Kavanagh PB, Moriel M, Mazzanti M, Su HT, et al. Automatic quantification of ejection fraction from gated myocardial perfusion SPECT. J Nucl Med. 1995;36:2138–2147. [PubMed] [Google Scholar]
- 22.Cerqueira M, Weissman N, Dilsizian V, Jacobs A, Kaul S, Laskey W, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 23.Mazzanti M, Germano G, Kiat H, Kavanagh PB, Alexanderson E, Friedman JD, et al. Identification of severe and extensive coronary artery disease by automatic measurement of transient ischemic dilation of the left ventricle in dual-isotope myocardial perfusion SPECT. J Am Coll Cardiol. 1996;27:1612–1620. doi: 10.1016/0735-1097(96)00052-6. [DOI] [PubMed] [Google Scholar]
- 24.Germano G, Kiat H, Kavanagh P, Moriel M, Mazzanti M, Su H, et al. Automatic quantification of ejection fraction from gated myocardial perfusion SPECT. J Nucl Med. 1995;36:2138–2147. [PubMed] [Google Scholar]
- 25.Slomka P, Nishina H, Berman D, Kang X, Friedman J, Hayes S, et al. Automatic quantification of myocardial perfusion stress-rest change: a new measure of ischemia. J Nucl Med. 2004;45:183–191. [PubMed] [Google Scholar]
- 26.Berman D, Kang X, Hayes S, Friedman J, Cohen I, Abidov A, et al. Adenosine myocardial perfusion single-photon emission computed tomography in women compared with men. Impact of diabetes mellitus on incremental prognostic value and effect on patient management. J Am Coll Cardiol. 2003;41:1125–1133. doi: 10.1016/s0735-1097(03)00085-8. [DOI] [PubMed] [Google Scholar]
- 27.Shaw L, Berman D, Maron D, Mancini G, Hayes S, Hartigan P, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117:1283–1291. doi: 10.1161/CIRCULATIONAHA.107.743963. [DOI] [PubMed] [Google Scholar]
- 28.Grossman GB, Garcia EV, Bateman TM, Heller GV, Johnson LL, Folks RD, et al. Quantitative Tc-99m sestamibi attenuation-corrected SPECT: development and multicenter trial validation of myocardial perfusion stress gender-independent normal database in an obese population. J Nucl Cardiol. 2004;11:263–272. doi: 10.1016/j.nuclcard.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Sharir T, Kang X, Germano G, Bax J, Shaw L, Gransar H, et al. Prognostic value of poststress left ventricular volume and ejection fraction by gated myocardial perfusion SPECT in women and men: gender-related differences in normal limits and outcomes. J Nucl Cardiol. 2006;13:495–506. doi: 10.1016/j.nuclcard.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 30.Beanlands RS, Chow BJ, Dick A, Friedrich MG, Gulenchyn KY, Kiess M, et al. CCS/CAR/CANM/CNCS/CanSCMR joint position statement on advanced noninvasive cardiac imaging using positron emission tomography, magnetic resonance imaging and multidetector computed tomographic angiography in the diagnosis and evaluation of ischemic heart disease--executive summary. Can J Cardiol. 2007;23:107–119. doi: 10.1016/s0828-282x(07)70730-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slomka PJ, Le Meunier L, Hayes SW, Acampa W, Oba M, Haemer GG, et al. Comparison of myocardial perfusion 82Rb PET performed with CT- and transmission CT-based attenuation correction. J Nucl Med. 2008;49:1992–1998. doi: 10.2967/jnumed.108.056580. [DOI] [PubMed] [Google Scholar]
- 32.Parkash R, deKemp RA, Ruddy TD, Kitsikis A, Hart R, Beauchesne L, et al. Potential utility of rubidium 82 PET quantification in patients with 3-vessel coronary artery disease. J Nucl Cardiol. 2004;11:440–449. doi: 10.1016/j.nuclcard.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Klein R, Beanlands RS, deKemp RA. Quantification of myocardial blood flow and flow reserve: Technical aspects. J Nucl Cardiol. 2010;17:555–570. doi: 10.1007/s12350-010-9256-9. [DOI] [PubMed] [Google Scholar]
- 34.Bravo PE, Chien D, Javadi M, Merrill J, Bengel FM. Reference ranges for LVEF and LV volumes from electrocardiographically gated 82Rb cardiac PET/CT using commercially available software. J Nucl Med. 2010;51:898–905. doi: 10.2967/jnumed.109.073858. [DOI] [PubMed] [Google Scholar]
- 35.Slomka P, Germano G, Kavanagh P, Javadi M, Berman D, Bengel F. Evaluation of a new automatic algorithm for quantification of ECG-gated 82Rb cardiac PET. J Nucl Med. 2009;50(Suppl 2):1167. [Google Scholar]
- 36.Cullom S, Bateman T, Volker L, Hsu B, McGhie I, Case J. Transient ischemic dilation in response to dipyridamole stress with Rubidium-82 myocardial perfusion PET: Quantitative comparison of low-likelihood and CAD populations. J Nucl Med. 2007;48(Suppl2):106P. [Google Scholar]
- 37.Sharir T, Berman DS, Waechter PB, Areeda J, Kavanagh PB, Gerlach J, et al. Quantitative analysis of regional motion and thickening by gated myocardial perfusion SPECT: normal heterogeneity and criteria for abnormality. J Nucl Med. 2001;42:1630–1638. [PubMed] [Google Scholar]
- 38.Knesaurek K, Machac J, Krynyckyi BR, Almeida OD. Comparison of 2-dimensional and 3-dimensional 82Rb myocardial perfusion PET imaging. J Nucl Med. 2003;44:1350–1356. [PubMed] [Google Scholar]
- 39.Votaw JR, White M. Comparison of 2-dimensional and 3-dimensional cardiac 82Rb PET studies. J Nucl Med. 2001;42:701–706. [PubMed] [Google Scholar]
- 40.Tarantola G, Zito F, Gerundini P. PET instrumentation and reconstruction algorithms in whole-body applications. J Nucl Med. 2003;44:756–769. [PubMed] [Google Scholar]