Abstract
During a population decline or disease outbreak, the true risk of specific diseases to a wild population is often difficult to determine because of a lack of baseline disease information. To better understand the risk of disease in an endangered and scientifically important population of chimpanzees (Pan trogylodytes schweinfurthii), a health monitoring program was initiated in Gombe National Park, Tanzania. As part of this health monitoring program, comprehensive necropsies with histopathology were conducted on chimpanzees (n =11; 5 male, 6 female), ranging in age from fetal to 44 yr, that were found dead between August 2004 and January 2010. In contrast to previous reports, respiratory disease was not noted as a cause of morbidity or mortality. Trauma was the most common cause of death in these 11 chimpanzees. All of the chimpanzees greater than 1 yr of age had intestinal and mesenteric parasitic granulomas associated with true strongyles consistent with Oesophagostomum spp. The relative numbers of granulomas increased with age and, in some cases, may have been a cause of weight loss and diarrhea. Simian immunodeficiency virus (SIV)cpz infection was documented in four deceased apes, all of whom exhibited varying amounts of lymphoid depletion including two females with marked CD4+ T cell loss consistent with end-stage SIVmac or human immunodeficiency virus infections. Myocardial megalokaryosis was common in chimpanzees greater than 1 mo of age; yet myocardial interstitial fibrosis, a common lesion in captive chimpanzees, was uncommon and only noted in two aged chimpanzees. These findings provide important information on causes of morbidity and mortality in wild chimpanzees, information that can be used to interpret findings during population declines and lead to better management of this population in the context of disease risk.
Keywords: Chimpanzee, pathology, Oesophagostomum spp, simian immunodeficiency virus, Gombe National Park
INTRODUCTION
Ape populations worldwide are threatened, and infectious disease is considered to be a significant risk to populations in the wild.24,29 Anthropozoo-noses are of particular concern in certain ape populations because of increased proximity to humans via overlapping human habitation and tourism.49,60 Gombe National Park in Tanzania hosts the longest continuous study of a great ape population through the Jane Goodall Institute’s research on wild chimpanzees (Pan trogylodytes schweinfurthii).44 Thus, this population is important not only because of the chimpanzee’s endangered status but also because of these ongoing, long-term behavioral studies. The park is surrounded by areas of human habitation, the chimpanzees are known to roam outside of the park, and tourists visit the park on a routine basis. All of these factors increase the risk of disease transmission between humans and apes.
Studies at Gombe span close to 50 yr and extensive life history records are available for many adult chimpanzees. Since the 1960s, the total population of chimpanzees at Gombe has declined from about 115–150 to around 100 by the end of 2008.45,48 Life history records include behavioral notes, with information about individual and group health, as well as standardized health check-sheets that were periodically filled out by field personnel.33 Analysis of these observational records collected over 47 yr identified infectious disease as the most common cause of death followed by intraspecific aggression. Most deaths due to disease occurred during epidemics, with respiratory disease predominating.61 Several of the previous major epidemics include suspected polio in 1966,11,12 respiratory disease in 1968, 1987, 1996, 2000, and 2002,12,36,41,61 and sarcoptic mange in 1997.45 However, in many of these cases, detailed postmortem evaluation, including histopathology, were not conducted and causes were inferred based on observed clinical signs. Causes of death have been inferred at other sites where chimpanzees are studied as well,40 with only rare reports of outbreaks that include pathologic or microbiologic evaluation of tissues.17,28,63 Even less is known about causes of death outside of epidemics because baseline information is largely unavailable.
In 2004, a comprehensive health monitoring program was established in Gombe that includes standardized observational health monitoring, noninvasive sample collections for virology and parasitology, as well as complete postmortem evaluations on all retrieved carcasses of not only chimpanzees but also other sympatric primate species.33,59 This program complemented an existing molecular virology survey of the chimpanzees that began in 2000. The goals of this monitoring program were to more thoroughly assess health in this valuable population, establish a database upon which further research can be based, and gain a greater understanding of baseline health issues so that true disease risks to population viability can be assessed and managed. Toward this goal, extensive effort has been made to train on-site personnel in necropsy techniques so that carcasses can be thoroughly evaluated in a safe and expeditious manner. Presented herein are the findings from the first 6 yr of necropsies conducted on chimpanzees as part of the Gombe health monitoring program.
MATERIALS AND METHODS
Gombe National Park is a 35-km2 park located at the western border of Tanzania along Lake Tanganika. This park is home to many different species of primates including three communities (Kasekela, Mitumba, and Kalande) of chimpanzees, olive baboons (Papio anubus), and red colobus monkeys (Procolobus badius). The Kasekela community is the largest (approximately 57 chimpanzees) and has been studied by Jane Goodall and colleagues since the 1960s. The other two communities, Mitumba and Kalande, are smaller (approximately 25 and 14–18 chimpanzees, respectively) and have been studied less extensively.45,48 In contrast to chimpanzees within the Kasekela community, chimpanzees of the Mitumba and Kalande communities sometimes range outside the park.45 Chimpanzees in the Kasekela and Mitumba communities are observed on a weekly basis. Chimpanzees from Kalande are not currently human habituated. From August 2004–August 2010, carcasses suitable for necropsy were available from 11 chimpanzees (4 males, 6 females, one fetus of undetermined gender). During this same time period, the total number of chimpanzees that died or disappeared from the Kasekela and Mitumba communities was 24; therefore, these 11 chimpanzees represent 46% of the total number of animals that are presumed to have died during the study period. Carcasses were recovered between 6–48 hr after death and necropsied immediately or frozen (−20°C) until trained personnel were available. Bodies were never found for 8 chimpanzees (33%) and, thus, could not be necropsied. Remaining carcasses (5, 21%) were unsuitable for necropsy.
Complete gross necropsies were performed on all retrieved carcasses by teams of field biologists, clinical veterinarians, and veterinary pathologists. All prosectors were trained in appropriate bio-safety precautions and primate necropsy techniques. Sections of brain, pituitary gland, adrenal gland, thyroid gland, eye, skin, skeletal muscle, peripheral nerve, tongue, salivary gland, trachea, esophagus, lung, heart, diaphragm, stomach, pancreas, small intestine, cecum, colon, kidney, urinary bladder, testis, uterus, ovary, mammary gland, liver, spleen, bone, bone marrow, and lymph nodes (peripancreatic, tracheobronchial, peripheral, and mesenteric) were collected, as available, and fixed in 10% neutral buffered formalin and RNAlater® (Applied Biosystems/Ambion, Austin, Texas 78744, USA). Tissues were routinely processed for histopathology, embedded in paraffin, sections cut at 3–5 μm, and stained with hematoxylin-eosin. All tissues were reviewed histologically by two board-certified veterinary pathologists (KAT, MJK). Lymphoid subsets were evaluated qualitatively and quantitatively by immunohistochemistry using previously described methods.18 Animal identification was based on Keele et al.18 and, in questionable cases (Ch-099 and Ch-036), individual identification was confirmed by microsatellite analyses of necropsy tissue samples. Pathologists were blinded to simian immunodeficiency virus (SIVcpz) status of all chimpanzees during tissue evaluation, with the exception of Ch-045.
Fecal and urine samples from chimpanzees were tested for SIVcpz-specific antibodies by chemiluminescence Western blot, and SIVcpz infection was confirmed by amplifying viral sequences from fecal RNA using previously published protocols.19,50 Additionally, presence of SIVcpz within sections of spleen was confirmed by in situ hybridization using previously described methods.7,18 Available fecal samples were also screened for chimpanzee strains of simian foamy virus (SFVcpz) and hepatitis B virus (ChHBV). Amplification of SFVcpz sequences has previously been described.32 Amplification of ChHBV sequences was performed by nested PCR of fecal DNA essentially as described,15 except for modifications in outer (forward 5′-AATCCA-CACTCCAAAAGACACCAA-3′ and reverse 5′-TTGCCGDGCAACGGGGTAAAGG-3′) and inner (forward 5′-CCGCGTCGCAGAAGATCT-CAA-3′ and reverse 5′-GAAGGCCTTRTAAG-TTGGCGAG-3′) primer sequences. These primers amplified a 1,008 base pair ChHBV-S fragment from positive human and chimpanzee control specimens.
Parasites removed from the large intestine were preserved in 70% ethanol. For identification, the parasites were placed in alcohol-glycerin solution for several weeks to clear the nematode cuticle and reveal the structures of the worm. Worms were gradually transferred to 100% glycerin and mounted on 3 × 5-in slides with cover slip pressure.6,43 Parasites were examined microscopically, measured, and identified using key characteristics outlined in standard keys and reference articles.10,30,47,53,54
After postmortem examination, carcasses were buried and soft tissue allowed to decompose for at least 1 yr. Chimpanzee carcasses were buried in flour sacks to ensure full recovery of all skeletal elements. Upon exhumation, skeletons were washed in water and allowed to thoroughly dry away from sunlight to prevent damage from UV radiation.16,64 As an additional precaution, bones from SIVcpz-positive chimpanzees were rinsed in a 10% bleach solution and then washed thoroughly in water. Skeletons from nine chimpanzees in this study (all except BB-015a and Ch-021) were inventoried and then examined for gross trauma and pathologic lesions. Lesions were characterized,4 significant findings were photographed (CAK), and photographs reviewed in the context of information from the necropsy (KAT, EVL, CAK).
RESULTS
Chimpanzee age, sex, home community, clinical history, SIVcpz, SFVcpz, and ChHBV infection status, relative quantity of parasitic granulomas, and cause of death are listed in Table 1. Trauma was the most common cause of death during this time period and affected 4/11 (36%) chimpanzees, and 4/9 (44%) adults. Four animals (Ch-024, BB-042a, Ch-045, Ch-069) presented with traumatic wounds typical of conspecific aggression including punctures from bites, open wounds, and lacerations with associated hemorrhage. One chimpanzee (Ch-045) had acute fractures of the ilium and scapula and a puncture wound to the left maxilla, while another chimpanzee (Ch-024) had a puncture wound to the calcaneous. Both Ch-045 and Ch-069 also had evidence of previous fractures (Fig. 1), including bony calluses and bony remodeling in multiple sites, although lesions in Ch-045 were more extensive, likely due to a previous fall from a tree (Wilson, unpubl. data). Ch-099 had evidence of previous trauma and was found with hind-limb paralysis of unknown cause. At necropsy, a nonunion fracture of T5 was noted with regional myelomalacia (Fig. 2). Additionally, this chimpanzee had multiple abscesses within the skeletal muscle of the inguinal region as well as evidence of bony resorption along the adjacent pubis, likely due to osteomyelitis. Mild to moderate degenerative joint disease characterized by bony remodeling on prepared skeletons was noted in all chimpanzees over 20 yr of age with severity increasing with age.23 The femoral joint (femoral head and acetabulum) was most commonly affected followed by the elbow, carpal, stifle, and tarsal joints.
Table 1.
Demographic data, history, and significant findings for individual deceased chimpanzees.
| IDa | Sex | Ageb | Community | History | SIVc | SFVc | ChHBVc | Cause of death | Parasitic granulomas |
|---|---|---|---|---|---|---|---|---|---|
| BB-015d | M | fetus | Kasekela | Possible premature birth | ND | ND | ND | Stillbirth | None |
| BB-015a | M | 6 d | Kasekela | Taken from mother | ND | ND | ND | Loss of maternal care | None |
| Ch-028 | F | 16 yr | Kasekela | Recumbent, unresponsive | − | − | − | Unknown | Moderate |
| Ch-021 | F | 20 yr | Kasekela | Emaciated | + | ND | − | AIDS-like disease | Moderate |
| Ch-036 | F | 24 yr | Kasekela | Emaciated | + | + | − | AIDS-like disease | Numerous |
| Ch-099 | F | 29 yr | Kasekela | Hind-limb paralysis | + | + | − | Trauma | Moderate |
| Ch-016 | M | 39 yr | Kasekela | Thin body condition, weak | − | + | − | Oesophagostomiasis | Numerous |
| Ch-024 | F | 44 yr | Kasekela | Multiple wounds | − | + | − | Conspecific trauma | Numerous |
| BB-042a | F | 9 mo | Mitumba | Multiple wounds | ND | ND | ND | Conspecific trauma | None |
| Ch-069 | M | 8 yr | Mitumba | Multiple puncture wounds | − | + | − | Conspecific trauma | Rare |
| Ch-045 | M | 28 yr | Mitumba | Multiple wounds | + | + | − | Conspecific trauma | Numerous |
Ch numbers represent individual chimpanzees, as reported by Keele et al., 2009.18 BB-015a and BB-015d represent offspring of female Ch-015 and BB-042a represents offspring of female Ch-042. Because fecal samples were not collected from these infants, they were not assigned Ch numbers (Keele et al., 200918).
Age, where known, represented in days (d), months (mo), or years (yr).
Simian immunodeficiency virus (SIVcpz), Simian foamy virus (SFVcpz), and chimpanzee hepatitis B virus (ChHBV) infection status negative (−), positive (+), or ND (not determined).
Figure 1.
Prepared skeletal remains of a chronic nonunion fracture of the ischium (chimpanzee Ch-045).
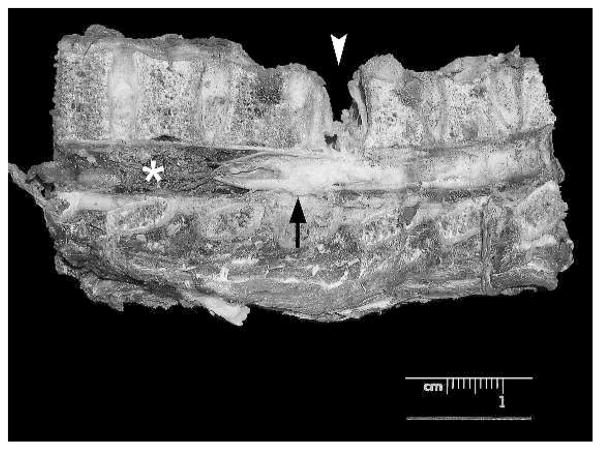
Figure 2.
Gross photograph of a formalin-fixed longitudinal section of vertebra and spinal cord with a nonunion fracture of T5 (arrowhead), regional swelling of the spinal cord (arrow), and distal myelomalacia (asterisk) (chimpanzee Ch-099).
An important lesion noted in seven chimpanzees was the presence of granulomas within the mesentery, mural granulomas within the small intestine and colon and, in one case (Ch-045), inflammation within a mesenteric artery, all of which were associated with metazoan parasites. Granulomas were characterized by abundant, variably mineralized necrotic debris surrounded by a thick, fibrous capsule containing small to moderate numbers of neutrophils, macrophages, lymphocytes, and plasma cells. Parasites were approximately 300 μm in diameter with a thick cuticle, platymyarian musculature, low lateral chords, pseudocoelom, triradiate esophagus, large intestine lined by a few multinucleated cells with a prominent brush border, and a reproductive tract (Fig. 3). A total of 15 ethanol-fixed worms (10 females and 5 males) were examined by light microscopy. Females ranged in length from 2.36–3.36 cm (mean 2.95 cm); males ranged from 2.08–2.44 cm (mean 2.27 cm). Worms were identified as Oesophagostomum (Nematoda, Strongylida, Strongyloidea, Chabertiidae, Oesophagostominae) based on the presence of a small, anteriorly-directed buccal capsule with internal and external corona radiata, copulatory bursa in the males, strongyle-type eggs in the uterus, and ventral groove and cephalic inflation posterior to the buccal cavity. The worms were identified as Oesophagostomum, subgenus Conoweberia, probably Oesophagostomum stephanostomum, because the buccal cavity was wider anteriorly than posteriorly with 6 denticles projecting into the lumen of the esophagel funnel. In general, parasitic granulomas were more numerous in older chimpanzees, and all but one individual with numerous parasitic granulomas (Ch-045) was in thin body condition.
Figure 3.
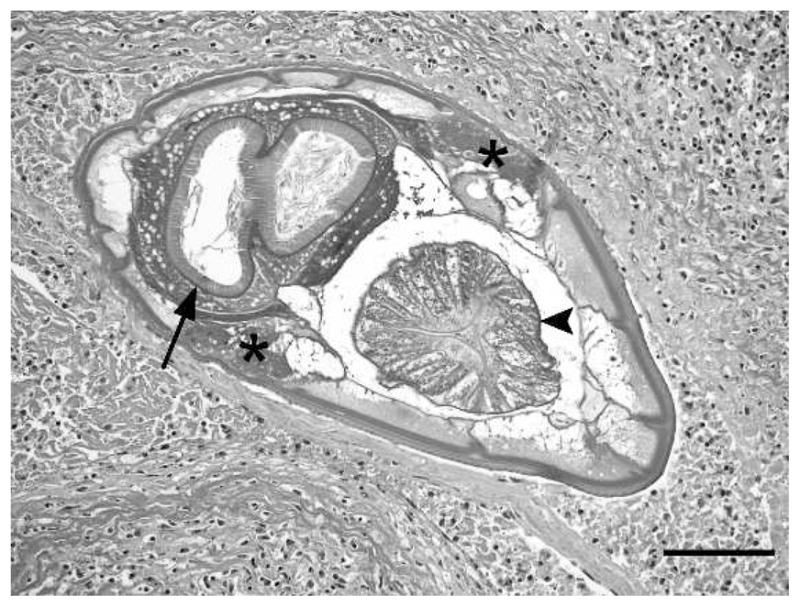
Cross-section of a true strongyle (Oesophagostomum sp.) within a mesenteric granuloma from a chimpanzee (Ch-016). Characteristic features evident in this section include low lateral chords (asterisks), triradiate esophagus (arrowhead), and large intestine with prominent brush border (arrow). Bar = 100 μm.
Three chimpanzees (Ch-021, Ch-024, and Ch-045) had intrabronchial metazoan parasites with jointed appendages and striated muscle (presumed mites). In these cases, associated inflammation was minimal. Afferent lymphatics adjacent to the mesenteric and tracheobronchial lymph nodes of one chimpanzee (Ch-021) contained 140-μm diameter round metazoan parasites (presumed filaroids) with an eosinophilic cuticle, coelomyarian musculature, pseudocoelom, and paired uteri containing numerous larva (microfilaria). Similar nematodes were present within lymphatics adjacent to a section of pancreas. No inflammation was associated with these parasites.
Four of the deceased chimpanzees were infected with SIVcpz (Ch-021, Ch-036, Ch-045, and Ch-099). At necropsy, both Ch-021 and Ch-036 had marked depletion of cortical and paracortical T-and B-lymphocytes within lymphoid tissue assessed by immunohistochemistry. Both of these chimpanzees also had evidence of emaciation including atrophy of adipose, hepatocytes, and skeletal myofibers. The two other SIVcpz-positive chimpanzees died of trauma-related causes but had depletion of CD4+ T cells, follicular hyperplasia with prominent germinal centers, and central hyalinization.18 Follicular hyperplasia with prominent germinal centers was also noted in one adult SIVcpz-negative chimpanzee from Mitumba (Ch-069). No malignancies were noted in the SIVcpz-positive chimpanzees. All SIVcpz-infected chimpanzees were infected with moderate to numerous Oesophagostomum spp. In addition to Oesophagostomum infection, Ch-021 also had filariid infection of the lymphatics. Autolytic changes in the intestinal mucosa may have obscured histologic detail, thus hindering interpretation of enteric protozoal infections. Six of the deceased apes were infected with SFVcpz (Ch-016, Ch-024, Ch-036, Ch-045, Ch-069, and Ch-099) but none was positive for ChHBV DNA. No specific lesions could be attributed to SFVcpz infection.
Marked variation in cardiac myofiber size as well as megalo- and dyskaryosis were noted in all of the subadult and adult chimpanzees (Fig. 4). The heart could not be evaluated in one newborn chimpanzee (BB-015a) due to tissue autolysis, but no lesions were noted in the stillborn fetus (BB-015d). Mild to moderate myocardial fibrosis was noted in two chimpanzees (Ch-024, Ch-045). In these cases, fibrous connective tissue extended from around small intramyocardial arterioles into the adjacent parenchyma, entrapping myocytes, some of which were atrophied. There was no evidence of medial arterial hypertrophy, arteriosclerosis, or atherosclerosis within the intramyocardial coronary arteries of these two chimpanzees.
Figure 4.
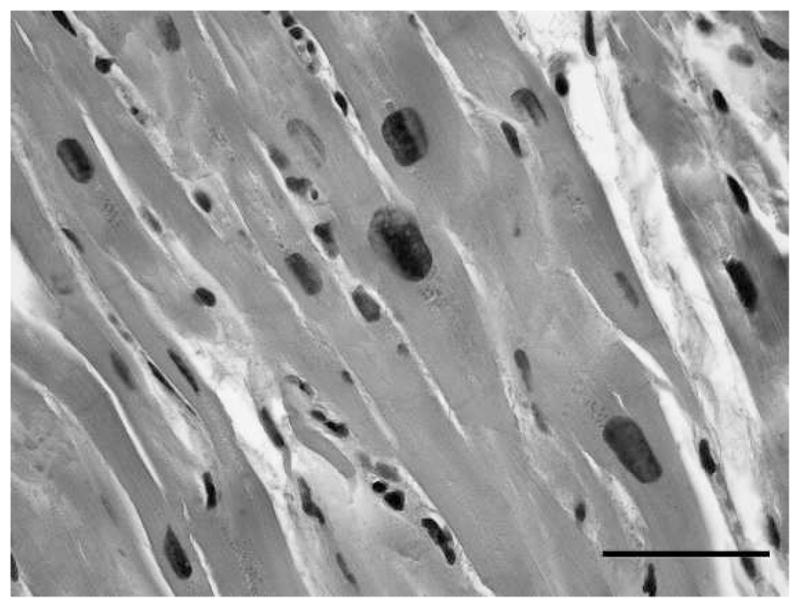
Megalokaryosis in cardiac myocytes (chimpanzee Ch-099). Note the variation in myocyte nuclear size. Bar = 50 μm.
The cause of death could not be determined for one individual (Ch-028). At necropsy, tissues were severely autolyzed due to a prolonged postmortem interval (48 hr) that hindered interpretation. This chimpanzee had secondary lesions suggestive of an infection, including lymphadenitis and periosteal new bone formation along the surface of some ribs, but no primary site of infection or inflammation was noted.
DISCUSSION
In contrast to previous reports and demographic surveys of chimpanzees from Gombe and other locations,17,28,29,40,61 disease was an uncommon cause of death in this survey and no morbidity or mortality could be attributed to an anthropozoonotic disease in these chimpanzees. Specifically, there were no cases of respiratory disease during this time period, the most common cause of death previously reported in this population.61 Additionally, lesions consistent with previous pulmonary injury (e.g., fibrosis) were not noted in individuals known to be alive during previous outbreaks. Respiratory disease has occurred in distinct epidemics in this and other chimpanzee populations;12,14,17,36,41,61 therefore, the lack of disease noted in this report could be due to several factors. First, health records prior to 2004 are sporadic; therefore, it is not known with certainty which individual chimpanzees, alive during the previous outbreaks, were symptomatic. Secondly, the nasal cavities and sinuses were not evaluated as part of the necropsy protocol; therefore, lesions associated with upper respiratory pathogens could have been missed. Thirdly, improvements in hygiene and disease-prevention protocols instituted in 2000 for researchers and tourists at Gombe may have contributed to decreased incidence of anthropozoonotic disease transmission.5 Lastly, the lack of fatalities due to respiratory disease may simply be a function of timing. During the time frame of this study, in addition to a lack of lesions within pulmonary tissues, no epidemics of fatal respiratory disease were observed in this population by behavioral researchers. However, the purpose of this study was to initiate long-term disease monitoring. Other diseases noted in this study contributed to overall morbidity and mortality, thereby providing critical baseline disease information that will give context to any future epidemics.
Granulomas with intralesional Oesophagostomum spp. within the mesentery and intestinal walls were noted in all chimpanzees older than 1 yr of age. Although quantitative evaluation of granuloma numbers has only recently been added to the necropsy protocol, relative numbers of these parasitic granulomas appear to increase with age. Previous studies have identified Oesophagostomum spp. ova in the feces of Gombe chimpanzees and baboons2,9,35,38 and similar parasite granulomas have been noted in sympatric baboons (Terio, unpubl. data). Similar parasitic granulomas have recently been reported in chimpanzees living in Taï National Park, Côted’Ivoire, Kibale National Park, Uganda, from apes in sanctuaries,26 and in both wild and captive nonhuman primates.55 Whole parasites obtained from two chimpanzees in this study were identified as Oesophagostomum subgenus Conoweberia, most likely O. stephanostomum. Several DNA sequencing studies indicate a close relationship of the nonhuman primate Oesophagostomum species and support their division into separate species in a common subgenus (Conoweberia).8 However, identification to species of the Oesophagostominae subfamily that infect nonhuman primates is difficult, and there is disagreement on the validity of some species and subgenera.10
In humans, Oesophagostomum spp. infection can result in uninodular or multinodular disease. Uninodular disease presents as a single, painful, large abdominal mass whereas, in multinodular disease, there are numerous granulomas within the colonic wall and mesentery that are associated with weight loss, diarrhea, and abdominal pain.56 Lesions in the chimpanzees described in this study, and those described in a previous study of chimpanzees from other sites,26 are consistent with the multinodular form of the disease in humans. In the previous study,26 clinical signs were only noted in apes living in sanctuaries, and authors hypothesized that self medication in wild chimpanzees was responsible for the lack of clinical signs and tolerance of the parasites. Medicinal plant use has been proposed in chimpanzees from Mahale Mountains National Park in Tanzania as a response to Oesophagostomum infections.13 In contrast to previous reports,26 several of the wild chimpanzees in this study, those that had numerous parasitic granulomas at necropsy, were also in thin condition although concurrent disease, such as the clinical SIVcpz infection (Ch-021 and Ch-036), may have contributed to weight loss in some cases. One SIVcpz-negative chimpanzee (Ch-016) with oesophagostomiasis had clinical signs of weight loss as well as diarrhea prior to death, without other predisposing factors. For several years, a wasting condition has been noted in behavioral records of chimpanzees in Gombe.61 Some of these chimpanzees have had concurrent diarrhea and clinical signs attributed to abdominal cramps that may have been due to oesophagostomiasis. In the chimpanzees living at Mahale, an “AIDS-like” wasting disease has also been reported with some individuals dying of the disease while others recovered.40 Unfortunately, no necropsies were performed and SIVcpz status was not reported, so whether this illness was due to SIVcpz, oesophagostomiasis, or both is not known at this time. Further evaluation of the parasites through quantitative analysis of ova within fecals, standardized evaluation of granuloma numbers at necropsy, and molecular characterization of the parasite in chimpanzees and other primates is necessary to understand the pathogenesis of this lesion and to determine if there are any correlates with the clinical signs.
In addition to infection with Oesophagostomum parasites, infection with lung mites was noted in three chimpanzees and one chimpanzee had lymphatic filariids. Mites are morphologically consistent with Pneumonyssus spp., which have been reported in wild as well as in captive nonhuman primates.1,21,25 Similar mites have not, to the authors knowledge, been reported in wild chimpanzee populations. Further study will be required for a more definitive identification of the lung mites infecting these chimpanzees. Lymphatic filaroid nematodes were noted in one chimpanzee in this study (Ch-021). Mansonella spp. have been previously reported within the mesentery and lymphatics of chimpanzees.58 In this case, no lesions were associated with the infection. In humans, Mansonella-associated disease is dependent on the host immune response;3 it is therefore possible that lymphoid depletion due to this chimpanzee’s advanced SIVcpz infection limited filaroid-associated disease.
Four chimpanzees within this study were chronically infected with SIVcpz. Results from three of these apes (Ch-036, Ch-045, Ch-099) were previously reported.18 The fourth chimpanzee (Ch-021) died after publication of the previous cases but was subjected to the same analysis. SIVcpz infection in all of these apes was associated with depletion of CD4+ T cells and other lymphoid lesions consistent with pathogenic infection. In two chimpanzees (Ch-021 and Ch-036), generalized cachexia in combination with marked lymphoid depletion was consistent with lesions in end-stage human immunodeficiency virus-1 human infection42 and with those noted in SIVmac-infected rhesus macaques.22 No other lesion or underlying disease was identified in these chimpanzees to explain the lymphoid depletion. There were no direct links between these four animals either through sexual partners or genetically. Two females (Ch-021 and Ch-036) were living in the same community and both became positive in 2006. While Ch-036 was likely infected by sexual transmission, Ch-021 was either pregnant or lactating during the window of infection and, therefore, was more likely infected by aggression.18 These findings challenge the dogma that SIV infections are nonpathogenic in their natural hosts.
Follicular hyperplasia is noted in the early disease stage of SIVmac-infected rhesus macaques22 and was noted in the two SIVcpz-positive chimpanzees that died of trauma-related injuries. This lesion was also present in one adult SIVcpz-negative male, suggesting that the finding of follicular hyperplasia alone is nonspecific in wild apes exposed to a multitude of immune stimulants. Quantitative evaluation of T cell subsets, however, revealed that these two SIVcpz-positive individuals had significantly fewer CD4+ T cells than did SIV-negative chimpanzees.18 Continued quantitative evaluation of T cell subsets in all retrieved carcasses will allow further assessment of lymphoid morphology in wild chimpanzees and the progression of naturally acquired SIVcpz-related disease for improved understanding of SIVcpz pathogenesis.
SFVcpz infection was noted in six chimpanzees; however, there were no observable lesions that correlated with this infection. SFVcpz infections have been previously described in this and other wild chimpanzee populations.32,37 Consistent with our findings, to date these infections have not been linked with any disease in their natural hosts31,39 nor is there evidence that SFV is pathogenic when transmitted to people.57
Cardiac myofiber megalokaryosis was noted in chimpanzees as young as 9 mo of age. Myofiber karyomegaly is reported as a common finding in many species of nonhuman primates.34 In this study, karyomegaly was not evident in the one stillborn ape from which well-preserved tissues were available. The cause of this lesion is not known, but these findings lend further credence to the presumption that this is a common and possibly incidental lesion in this species. Another common cardiac lesion in captive chimpanzees, as well as in gorillas (Gorilla gorilla gorilla) and other primates, is myocardial interstitial fibrosis, typically centered around intramyocardial coronary arteries.34 In captive chimpanzees, interstitial fibrosis is a significant cause of cardiac disease and sudden death.27,52 The pathogenesis of this lesion has been speculated to involve vasoconstriction secondary to catecholamines, as the lesion is similar to that in catecholamine toxicity and experimentally induced catecholamine cardiomyopathy.20,34,46 Myocardial fibrosis was only noted in two older chimpanzees and was generally mild, similar to that described in free-ranging gorillas.34 The affected adult male was a former alpha male of the Mitumba community and had physical evidence of acute and chronic trauma as well as behavioral notes consistent with previous fights. Catecholamine levels can be influenced by a variety of factors, including social stressors. Specifically, the impact on catecholamine levels of rank within a social hierarchy depends on many factors, including the stability of that hierarchy.51 Further research involving correlation with stress hormone levels will be necessary to better understand this lesion in both captive and wild chimpanzees.
Both the stillborn fetus (BB-015d) and the 6-day-old neonate (BB-015a) were from the same mother and represented sequential pregnancies. The neonate was one of two twins, both of which died, although the carcass from the other twin could not be recovered. This female also had a live birth previous to these two, but that infant was taken and reared by another female chimpanzee until it died at 4 mo.62 The carcass from this 4-mo-old chimpanzee was not recovered. Reduced fertility and neonatal survival has been associated with SIVcpz infection;18 however, this female has consistently tested negative for SIVcpz. The cause of the stillbirth was not identified.
While trauma, primarily conspecific, was the most significant cause of death during the evaluated study period, important information about underlying causes of morbidity, including cardiac lesions, effect of SIVcpz infection, and effects of pulmonary and mesenteric parasitism were identified from examination of these carcasses—underscoring the importance of performing a complete necropsy on all available bodies. As such, the addition of standardized necropsies to the health monitoring program has provided important information on the baseline health of chimpanzees in the Gombe ecosystem. Infrastructure is now in place so that any future disease outbreaks can be fully investigated and interpreted in the context of this background information. Because of the long-term behavioral studies of this population, results can also be interpreted in light of some clinical history, a unique component to this study absent in most field pathology surveys. Due to the importance of the ongoing behavioral studies at this site and to the close daily monitoring that researchers have been able to conduct, there has been considerable concern and hesitation toward any antemortem diagnostics or evaluation of chimpanzees that would require anesthesia. Only under exceptional circumstances (e.g., snare removal, palliative care, or testing for disease that could threaten the entire population) has immobilization of chimpanzees been approved, and immobilizations have occurred only four times in the 50 yr of the study (Lonsdorf, unpubl. data). Therefore, the information provided by this ongoing pathology survey is critical to understanding disease threats to this endangered and valuable population of chimpanzees and for understanding the importance of disease in the conservation of apes across Africa.
Acknowledgments
The authors wish to thank Dr. Anna Mosser, Dr. Ephata Kaaya, Emily Wroblewski, Jared Bakuza, Kate Detwiler, Jimmy Gray, and the field staff at Gombe Stream Research Center for assistance with research and necropsies; the histopathology laboratory at the University of Illinois Veterinary Diagnostic Laboratory for excellent technical assistance; and Dr. Chris Gardiner for consultation on mesenteric parasites. The authors also thank Tanzania National Parks, Tanzania Wildlife Research Institute, and the Tanzania Commission for Science and Technology for permission to conduct this research. Fixed tissues were imported in accordance with the Convention on International Trade in Endangered Species of Wild Fauna and Flora and on U.S. Fish and Wildlife Service regulations. This work was funded in part by the U.S. Fish and Wildlife Service Great Ape Conservation Fund, the Arcus Foundation, the Leo S. Guthman Foundation, the Davee Foundation, Jane Goodall Institute, University of Minnesota, grants from the National Science Foundation (BSC-0648481 to MW) and the National Institutes of Allergy and Infectious Diseases (R01A150529 and R01A158715 to BHH), and federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E (JDE).
Footnotes
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
LITERATURE CITED
- 1.Abbot DP, Majeed SK. A survey of parasitic lesions in wild-caught, laboratory maintained primates: (rhesus, cynomolgus, baboon) Vet Pathol. 1984;21:198–207. doi: 10.1177/030098588402100212. [DOI] [PubMed] [Google Scholar]
- 2.Bakuza JS, Nkwengulilia G. Variation over time in parasite prevalence among free-ranging chimpanzees at Gombe National Park, Tanzania. Int J Primatol. 2009;30:43–53. [Google Scholar]
- 3.Bregani ER, Tantardini F, Rovellini A. Mansonella perstans filariasis. Parassitologia. 2007;49:23–26. [PubMed] [Google Scholar]
- 4.Carter M, Pontzer H, Wrangham RW, Peterhans K. Skeletal pathology in Pan troglodytes schweinfurthii in Kibale National Park, Uganda. Am J Phys Anthropol. 2008;135:389–403. doi: 10.1002/ajpa.20758. [DOI] [PubMed] [Google Scholar]
- 5.Collins A. Health guidelines for visiting researchers in Gombe National Park to minimize risk of disease transmission among primates. Pan Africa News. 2003;10:1. [Google Scholar]
- 6.Dailey MD. Preparation of parasites for identification and cataloging. J Zoo Anim Med. 1978;9:13–15. [Google Scholar]
- 7.Estes JD, Gordon SN, Zeng M, Chahroudi AM, Dunham RM, Staprans SI, Reilly CS, Silvestri G, Haase AT. Early resolution of acute immune activation and induction of PD-1 in SIV-infected sooty mangabeys distinguishes nonpathogenic from pathogenic infection in rhesus macaques. J Immunol. 2008;180:6798–6807. doi: 10.4049/jimmunol.180.10.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gasser RB, Woods WG, Huffman MA, Blotkamp J, Polderman AM. Molecular separation of Oesophagostomum stephanostomum and Oesophagostomum bifurcum (Nematoda: Strongyloidea) from non-human primates. Int J Parasitol. 1999;29:1087–1091. doi: 10.1016/s0020-7519(99)00037-5. [DOI] [PubMed] [Google Scholar]
- 9.Gillespie TR, Lonsdorf EV, Canfield EP, Meyer DJ, Nadler Y, Raphael J, Pusey AE, Pond J, Pauley J, Mlengeya T, Travis DA. Demographic and ecological effects on patterns of parasitism in eastern chimpanzees (Pan trogylodytes schweinfurthii) in Gombe National Park, Tanzania. Am J Phys Anthropol. 2010;143:534–544. doi: 10.1002/ajpa.21348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glen DR, Brooks DR. Phylogenetic relationships of some strongylate nematodes of primates. Proc Helm Soc Wash. 1985;52:227–236. [Google Scholar]
- 11.Goodall J. Population dynamics during a 15 yr period in one community of free-living chimpanzees in the Gombe National Park, Tanzania. Z Tierpsychol. 1983;61:1–60. [Google Scholar]
- 12.Goodall J. The Chimpanzees of Gombe: Patterns of Behavior. Harvard University Press; Cambridge, Massachusetts: 1986. [Google Scholar]
- 13.Hoffman MA, Gotoh S, Turner LA, Hamai M, Yoshida K. Seasonal trends in intestinal nematode infection and medicinal plant use among chimpanzees in the Mahale Mountains, Tanzania. Primates. 1997;38:111–125. [Google Scholar]
- 14.Hosaka K. A single flu epidemic killed at least 11 chimps. Pan Africa News. 1995;2:3–4. [Google Scholar]
- 15.Hu X, Margolis HS, Purcell RH, Ebert J, Robertson BH. Identification of hepatitis B virus indigenous to chimpanzees. Proc Natl Acad Sci. 2000;97:1661–1664. doi: 10.1073/pnas.97.4.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jurmain R. Trauma, degenerative disease, and other pathologies among the Gombe chimpanzees. Am J Phys Anthropol. 1989;80:229–237. doi: 10.1002/ajpa.1330800211. [DOI] [PubMed] [Google Scholar]
- 17.Kaur T, Singh J, Tong S, Humphrey C, Clevenger D, Tan W, Szekely B, Wang Y, Li Y, Muse EA, Kiyono M, Hanamura S, Inoue E, Nakamura M, Huffman MA, Jiang B, Nishida T. Descriptive epidemiology of fatal respiratory outbreaks and detection of a human-related metapneumo-virus in wild chimpanzees (Pan troglodytes) at Mahale Mountains National Park, Western Tanzania. Am J Primatol. 2008;70:755–765. doi: 10.1002/ajp.20565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keele BF, Jones JH, Terio KA, Estes JD, Rudicell RS, Wilson ML, Li Y, Learn GH, Beasley TM, Schumacher-Stankey J, Wroblewski E, Mosser A, Raphael J, Kamenya S, Lonsdorf EV, Travis DA, Mlengeya T, Kinsel MJ, Else JG, Silvestri G, Goodall J, Sharp PM, Shaw GM, Pusey AE, Hahn BH. Increased mortality and AIDS-like immunopathology in wild chimpanzees infected with SIVcpz. Nature. 2009;460:515–519. doi: 10.1038/nature08200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keele BF, Van Heuverswyn F, Li Y, Bailes E, Takehisa J, Santiago ML, Bibollet-Ruche F, Chen Y, Wain LV, Liegeois F, Loul S, Mpoudi Ngole E, Bienvenue Y, Delaporte E, Brookfield JFY, Sharp PM, Shaw GM, Peeters M, Hahn BH. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science. 2006;313:523–526. doi: 10.1126/science.1126531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khullar M, Datta BN, Wahi PL, Chakravarti RN. Catecholamine-induced experimental cardiomyopathy—a histopathological, histochemical, and ultrastructural study. Indian Heart J. 1989;41:307–313. [PubMed] [Google Scholar]
- 21.Kim JC, Kalter SS. Pathology of pulmonary acariasis in baboons (Papio sp.) J Med Primatol. 1975;4:70–82. doi: 10.1159/000459836. [DOI] [PubMed] [Google Scholar]
- 22.King NW., Jr . Simian immunodeficiency virus infections. In: Jones TC, Mohr U, Hunt RD, editors. Nonhuman Primates I. ILSI Monographs on Pathology of Laboratory Animals. Springer-Verlag; Berlin, Heidleberg, New York: 1993. pp. 5–20. [Google Scholar]
- 23.Kirchhoff CA. PhD Dissertation. University of Minnesota; St. Paul, Minnesota: 2010. From Birth to Bones: Skeletal Evidence for Health, Disease, and Injury in the Gombe Chimpanzees. [Google Scholar]
- 24.Köndgen S, Kuhl H, Goran P, Walsh P, Schenk S, Ernst N, Blek R, Fomenty P, Mätz-Rensing K, Scwieger B, Junglen S, Ellerbrok H, Nitsche A, Briese T, Lipkin W, Pauli G, Boesch C, Leendertz F. Pandemic human viruses cause decline in endangered apes. Curr Biol. 2008;18:1–5. doi: 10.1016/j.cub.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Kooriyama T, Inaba A, Nishida T, Iwaki T. Case report of helminthes and lung mite infection in the red-tailed monkey, Cercopithecus ascanius schmidti, in Mahale Mountains National Park, Tanzania. Primates. 2010;51:183–188. doi: 10.1007/s10329-009-0185-7. [DOI] [PubMed] [Google Scholar]
- 26.Krief S, Jamart A, Mahé S, Leendertz FH, Mätz-Rensing K, Crespeau F, Bain O, Guillot J. Clinical and pathologic manifestation of oesophagostomosis in African great apes: does self-medication in wild apes influence disease progression? J Med Primatol. 2008;37:188–195. doi: 10.1111/j.1600-0684.2008.00285.x. [DOI] [PubMed] [Google Scholar]
- 27.Lammey ML, Baskin GB, Gigliotti AP, Lee DR, Ely JJ, Sleeper MM. Interstitial myocardial fibrosis in a captive chimpanzee (Pan troglodytes) population. Comp Med. 2008;58:389–394. [PMC free article] [PubMed] [Google Scholar]
- 28.Leenderz FH, Ellerbrok H, Boesch C, Couacy-Hymann E, Mätz-Rensing K, Hakenbeck R, Bergmann C, Abaza P, Junglen S, Moebius Y, Vigilant L, Formenty P, Pauli G. Anthrax kills wild chimpanzees in a tropical rainforest. Nature. 2004;430:451–452. doi: 10.1038/nature02722. [DOI] [PubMed] [Google Scholar]
- 29.Leenderz F, Pauli G, Mätz-Rensing K, Boardman W, Nunn C, Ellerbrok H, Jensen SA, Junglen S, Boesch C. Pathogens as drivers of population decline: the importance of systematic monitoring in great apes and other threatened mammals. Biol Conserv. 2006;131:325–337. [Google Scholar]
- 30.Lichtenfels JR. Keys to Genera of the Superfamily Strongyloidea. In: Anderson RC, Chabaud AG, Willmott S, editors. CIH Keys to the Nematode Parasites of Vertebrates. 7. Commonwealth Agricultural Bureau; Farnham Royal, England: 1980. [Google Scholar]
- 31.Linial M. Why aren’t foamy viruses pathogenic? Trends Microbiol. 2000;8:284–289. doi: 10.1016/s0966-842x(00)01763-7. [DOI] [PubMed] [Google Scholar]
- 32.Liu W, Worobey M, Li Y, Keele BF, Bibollet-Ruche F, Guo Y, Goepfert PA, Santiago ML, Ndjango JB, Neel C, Clifford SL, Sanz C, Kamenya S, Wilson ML, Pusey AE, Gross-Camp N, Boesch C, Smith V, Zamma K, Huffman MA, Mitani JC, Watts DP, Peeters M, Shaw GM, Switzer WM, Sharp PM, Hahn BH. Molecular ecology and natural history of simian foamy virus infection in wild-living chimpanzees. PLoS Pathogens. 2008;4:e1000097. doi: 10.1371/journal.ppat.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lonsdorf EV, Travis D, Pusey AE, Goodall J. Using retrospective health data from the Gombe chimpanzee study to inform future monitoring efforts. Am J Primatol. 2006;68:897–908. doi: 10.1002/ajp.20296. [DOI] [PubMed] [Google Scholar]
- 34.Lowenstine LJ. A primer of primate pathology: lesions and nonlesions. Tox Pathol. 2003;31:92–102. doi: 10.1080/01926230390177668. [DOI] [PubMed] [Google Scholar]
- 35.McGrew MC, Tutin CEG, Collins DA, File SK. Intestinal parasites of sympatric Pan troglodytes and Papio spp. at two sites: Gombe (Tanzania) and Mt. Assirik (Senegal) Am J Primatol. 1989;17:147–155. doi: 10.1002/ajp.1350170204. [DOI] [PubMed] [Google Scholar]
- 36.Mlengeya T. TANAPA Veterinary Department Annual Report 2000/2001. 2000. Respiratory Disease Outbreak in the Chimpanzee Population of Gombe National Park. [Google Scholar]
- 37.Morozov VA, Leendertz FH, Junglen S, Boesch C, Pauli G, Ellerbrok H. Frequent foamy virus infection in free-living chimpanzees of the Taï National Park (Côte d’Ivoire) J Gen Virol. 2009;90(pt 2):500–606. doi: 10.1099/vir.0.003939-0. [DOI] [PubMed] [Google Scholar]
- 38.Murray S, Stem C, Boudreau B, Goodall J. Intestinal parasites of baboons (Papio cynocephalus anubis) and chimpanzees (Pan trogylodytes) in Gombe National Park. J Zoo Wildl Med. 2000;31:176–178. doi: 10.1638/1042-7260(2000)031[0176:IPOBPC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 39.Murray SM, Linial ML. Foamy virus infection in primates. J Med Primatol. 2006;35:225–235. doi: 10.1111/j.1600-0684.2006.00171.x. [DOI] [PubMed] [Google Scholar]
- 40.Nishida T, Corp N, Himai M, Hasegawa T, Hiraiwa-Hasegawa M, Hosaka K, Hunt KD, Itoh N, Kawanaka K, Matsuoto-Oda A, Mitani JC, Nakamura M, Norikoshi K, Sakamaki T, Turner L, Uehara S, Zamma K. Demography, female life history, and reproductive profiles among the chimpanzees of Mahale. Am J Primatol. 2003;59:99–121. doi: 10.1002/ajp.10068. [DOI] [PubMed] [Google Scholar]
- 41.Nutter FB. Respiratory disease claims the lives of at least seven Gombe chimps. Pan African News. 1996;31:3. [Google Scholar]
- 42.Pantaleo G, Graziosi C, Demarest JF, Butini L, Montroni M, Fox CH, Orenstein JM, Kotler DP, Fauci AS. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 43.Pritchard MH, Kruse GOW. The Collection and Preservation of Animal Parasites. University of Nebraska Press; Lincoln, Nebraska and London, England: 1982. [Google Scholar]
- 44.Pusey AE, Pintea L, Wilson ML, Kamenya S, Goodall J. The contribution of long-term research at Gombe National Park to chimpanzee conservation. Conserv Biol. 2007;21:623–634. doi: 10.1111/j.1523-1739.2007.00704.x. [DOI] [PubMed] [Google Scholar]
- 45.Pusey AE, Wilson ML, Collins DA. Human impacts, disease risk and population dynamics in the chimpanzees of Gombe National Park, Tanzania. Am J Primatol. 2008;70:738–744. doi: 10.1002/ajp.20567. [DOI] [PubMed] [Google Scholar]
- 46.Rona G. Catecholamine cardiotoxicity. J Mol Cell Cardiol. 1985;17:291–306. doi: 10.1016/s0022-2828(85)80130-9. [DOI] [PubMed] [Google Scholar]
- 47.Rothamn JM, Bowman DD, Kalema-Zikusoka G, Nkurunun JB. The parasites of the gorillas in Bwindi Impenetrable National Park, Uganda. In: Newton-Fisher NE, Notman H, Paterson JD, Reynolds V, editors. Primates of Western Uganda. Springer; New York, New York: 2006. pp. 171–192. [Google Scholar]
- 48.Rudicell RS, Jones JH, Wroblewski EE, Learn GH, Li Y, Robertson JD, Greengrass E, Grossmann F, Kamenya S, Pintea L, Mjungu DC, Lonsdorf EV, Mosser A, Lehman C, Collins DA, Keele BF, Goodall J, Hahn BH, Pusey AE, Wilson ML. Impact of simian immunodeficiency virus infection on chimpanzee population dynamics. PLoS Pathogens. 2010;6(9):e1001116. doi: 10.1371/journalppat.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rwego IB, Isabirye-Basuta G, Gillespie TR, Goldberg TL. Gastrointestinal bacterial transmission among humans, mountain gorillas, and livestock in Bwindi Impenetrable National Park, Uganda. Conserv Biol. 2008;22:1600–1607. doi: 10.1111/j.1523-1739.2008.01018.x. [DOI] [PubMed] [Google Scholar]
- 50.Santiago ML, Lukasik M, Kamenya S, Li Y, Bibollet-Ruche F, Bailes E, Muller MN, Emery M, Goldenberg DA, Lwanga JS, Ayouba A, Nerrienet E, McClure HM, Heeney JL, Watts DP, Pusey AE, Collins DA, Wrangham RW, Goodall J, Brookfield JFY, Sharp PM, Shaw GM, Hahn BH. Foci of endemic simian immunodeficiency virus infection in wild-living eastern chimpanzees (Pan trogylodytes schwinfurthii) J Virol. 2003;77:7545–7562. doi: 10.1128/JVI.77.13.7545-7562.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- 52.Seiler BM, Dick EJ, Jr, Guardado-Mendoza R, Vandeberg JL, Williams JT, Mubiru JN, Hubbard GB. Spontaneous heart disease in the adult chimpanzee (Pan troglodytes) J Med Primatol. 2009;38:51–58. doi: 10.1111/j.1600-0684.2008.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skrjabin KI, Shikhobalova NP, Schultz RS, Popova TI, Boev SN, Delyamure SL. Strongylata. In: Skrjabin KI, editor. Keys to Parasitic Nematodes. Vol. 3. E. J. Brill; New York: 1952. (English Translation; Israel Program for Scientific Translation, 1961, Jerusalem) [Google Scholar]
- 54.Sleeman JM, Meader LL, Mudakikwa AB, Foster JW, Patton S. Gastrointestinal parasites of mountain gorillas (Gorilla gorilla beringei) in the Parc National des Volcans, Rwanda. J Zoo Wildl Med. 2000;31:322–328. doi: 10.1638/1042-7260(2000)031[0322:GPOMGG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 55.Stewart TB, Gasbarre LC. The veterinary importance of nodular worms (Oesophagostomum spp.) Parasitol Today. 1989;5:209–213. doi: 10.1016/0169-4758(89)90269-x. [DOI] [PubMed] [Google Scholar]
- 56.Storey PA, Faile G, Hewit E, Yelifari L, Polderman AM, Magnussen P. Clinical epidemiology and classification of human oesophagostomiasis. Trans Royal Soc Trop Med Hyg. 2000;94:177–182. doi: 10.1016/s0035-9203(00)90267-0. [DOI] [PubMed] [Google Scholar]
- 57.Switzer WM, Bhullar V, Shanmugam V, Cong ME, Parekh B, Lerche NW, Yee JL, Ely JJ, Boneva R, Chapman LE, Folks TM, Heneine W. Frequent simian foamy virus infection in persons occupationally exposed to nonhuman primates. J Virol. 2004;78:2780–2789. doi: 10.1128/JVI.78.6.2780-2789.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Toft JD, Eberhard ML. Parasitic diseases. In: Bennett BT, Abee CR, Henrickson R, editors. Nonhuman Primates in Biomedical Research: Diseases. Academic Press; San Diego, California: 1998. [Google Scholar]
- 59.Travis DA, Lonsdorf EV, Mlengeya T, Raphael J. A science-based approach to managing disease risks for ape conservation. Am J Primatol. 2008;70:745–750. doi: 10.1002/ajp.20566. [DOI] [PubMed] [Google Scholar]
- 60.Wallis J, Lee DR. Primate conservation: the prevention of disease transmission. Int J Primatol. 1999;20:803–826. [Google Scholar]
- 61.Williams JM, Lonsdorf EV, Wilson ML, Schumaker-Stankey J, Goodall J, Pusey AE. Causes of death in the Kasekela chimpanzees of Gombe National Park, Tanzania. Am J Primatol. 2008;70:766–777. doi: 10.1002/ajp.20573. [DOI] [PubMed] [Google Scholar]
- 62.Wroblewski EE. An unusual incident of adoption in a wild chimpanzee (Pan troglodytes) population at Gombe National Park. Am J Primatol. 2008;70:995–998. doi: 10.1002/ajp.20582. [DOI] [PubMed] [Google Scholar]
- 63.Wyers M, Formenty P, Cherel Y, Guigand L, Fernandez B, Boesch C, Le Guenno B. Histopathological and immunohistochemical studies of lesions associated with Ebola virus in a naturally infected chimpanzee. J Infect Dis. 1999;179:S54–59. doi: 10.1086/514300. [DOI] [PubMed] [Google Scholar]
- 64.Zihlman AL, Morbeck ME, Goodall J. Skeletal biology and individual life history of Gombe chimpanzees. J Zoolog Soc London. 1990;221:37–61. [Google Scholar]