Abstract
Background
Acute kidney injury (AKI) duration following cardiac surgery is associated with poor survival in a dose-dependent manner. However, it is not known what peri-operative risk factors contribute to prolonged AKI and delayed recovery. We sought to identify peri-operative risk factors that predict duration of AKI, a complication that effects short and long term survival.
Methods
We studied 4,987 consecutive cardiac surgery patients from 2002 through 2007. AKI was defined as a ≥0.3 (mg/dL) or ≥50% increase in SCr from baseline. Duration of AKI was defined by the number of days AKI was present. Step-wise multivariable negative binomial regression analysis was conducted using peri-operative risk factors for AKI duration. C-index was estimated by Kendall’s tau.
Results
AKI developed in 39% of patients with a median duration of AKI at 3 days and ranged from 1 to 108 days. Patients without AKI had duration of zero days. Independent predictors of AKI duration included baseline patient and disease characteristics, operative and post-operative factors. Prediction for mean duration of AKI was developed using coefficients from the regression model and externally validated the model on 1,219 cardiac surgery patients in a separate cardiac surgery cohort (TRIBE-AKI). The C-index was 0.65 (p<0.001) for the derivation cohort and 0.62 (p<0.001) for the validation cohort.
Conclusion
We identified and externally validated peri-operative predictors of AKI duration. These risk-factors will be useful to evaluate a patient’s risk for the tempo of recovery from AKI after cardiac surgery and subsequent short and long term survival. The level of awareness created by working with these risk factors have implications regarding positive changes in processes of care that have the potential to decrease the incidence and mitigate AKI.
Keywords: acute kidney injury, cardiac surgery, risk model, risk prediction, Statistics, risk analysis/modeling, Surgery, complications, Kidney, CABG, Heart Valve surgery
Introduction
Acute kidney injury (AKI) is a common complication following cardiac surgery and is strongly associated with increased morbidity, mortality and length of hospitalization.[1] Using the Acute Kidney Injury Network (AKIN) definition of AKI, the incidence of post-operative AKI in cardiac surgery is higher than previously thought and has a more profound influence on survival than is appreciated by many cardiac surgeons. Morbidity and mortality have been demonstrated to be directly proportional not only to the severity of AKI by the magnitude of the peak rise in serum creatinine[2, 3], it is also related to the duration of AKI[4, 5]. The ability to discriminate between patients that are at high risk of developing AKI during the peri-operative hospitalization of high importance, both with regard to predicting short and long term mortality and the implications for prevention and mitigation.
Several studies have investigated the predictive ability of patient and limited procedural risk factors for developing new dialysis-dependent renal failure.[6–9] These models have performed well in predicting severe AKI as defined as a 2.0 mg/dL or 2-fold increase in serum creatinine or new dialysis.[7–10] Other investigations have targeted the prediction of large immediate post-operative declines in renal function.[11–13] However, investigations have not been undertaken to investigate the predictive ability of patient and peri-operative risk factors for the duration of AKI as a marker of AKI severity.
While severe post-operative renal insufficiency is of extreme importance, the importance of milder forms of AKI have been underappreciated. The newer definitions of AKI such as AKIN and RIFLE (discussed below) have increased our awareness of this complication and by looking at the duration of AKI as well at its severity, there are clear implications for survival and opportunities for prevention and mitigation.[14–16]
Therefore, we sought to evaluate the predictive ability of patient and peri-operative risk factors for AKI and the duration of AKI among consecutive patients undergoing cardiac surgery with external validation by the Translational Research Investigating Biomarker Endpoints (TRIBE) consortium.[17, 18] We hypothesized that patient, procedural (surgical and perfusion), and immediate post-surgical processes or events could predict the development of AKI and duration of AKI thereby providing surgical teams with a risk tool to identify patients at increased risk for AKI and long durations of AKI, which are directly proportional to increased mortality.[4]
Material and Methods
Derivation Cohort
From 2002 to 2007, we prospectively enrolled 4,987 consecutive cardiac surgery patients and retrospectively analyzed the prospective cohort. Patients were excluded if they had a history of dialysis-dependent renal failure (N=70). Among the remaining patients, 86 were excluded due to invalid procedure dates that could not be reconciled and therefore the duration of AKI could not be calculated, totaling 4,831 patients.
Maine Medical Center (MMC) is one of the centers in the Northern New England Cardiovascular Disease Study Group consortium, and in this analysis, the data from this single center (MMC) was analyzed. The data was collected in a comprehensive database prospectively with the intention of analyzing it retrospectively at a future date. The information is de-identified and managed in accordance with HIPAA regulations. As the privacy and safety of each individual patient in the database was not at risk; therefore, the Maine Medical Center Institutional Review Board at has approved this study and waived the need for patient consent.
Validation Cohort
The TRIBE-AKI cohort: was used for external validation. This is a cohort of prospectively enrolled adults undergoing cardiac surgery (coronary artery bypass grafting or valve surgery) who were at high risk for AKI, at six academic medical centers in North America between July 2007 and December 2009. High risk for AKI was defined by the presence of one or more of the following: emergency surgery, preoperative serum creatinine> 2 mg/dL (> 177 μmol/L), ejection fraction <35% or grade 3 or 4 left ventricular dysfunction, age > 70 years, diabetes mellitus, concomitant coronary artery bypass grafting (CABG) and valve surgery, or repeat revascularization surgery. Patients with evidence of AKI before surgery, prior kidney transplantation, or end-stage renal disease were excluded.[17, 18]
AKI and Duration of AKI for derivation and validation cohorts
AKI was determined by measuring the percent and absolute change from the last pre-operative SCr prior to surgery to the highest post-operative SCr using the Acute Kidney Injury Network (AKIN) definition of a ≥0.3 (mg/dL) or ≥50% increase in SCr from baseline.[15]SCr was measured on a daily basis until 48 hours after surgery; this was followed by additional days of SCr measurement based on the attending provider’s discretion. All laboratory SCr measures were performed per the hospitals standing protocol. The standard of care at Maine Medical Center is to determine creatinine measurements on the first and second post operative days after cardiac surgery. Nearly all of the creatinine rises occur within this time frame. If there is no elevation in SCr in the first 48 hours post op, no further SCrs are ordered unless specifically indicated. If the SCr rises post-operative, then it is clinically indicated to follow that value, allowing us the convenience of being able to measure the duration of the rise above baseline when the retrospective analysis is done. This standard of care was useful in extracting the data from our database and the hospital laboratory records, and made it unnecessary to require more SCr determinations for research purposes. AKI duration was calculated at each post-operative SCr measurement compared to the last pre-operative SCr using the AKIN criteria.[15] Duration of AKI was then defined by the number of days AKI was present as described previously.[4]
Statistical Analysis
Baseline patient and disease characteristics were summarized by χ2 tests, students t-test or Wilcoxon Ranksum tests were appropriate. Degrees of freedom for the χ2 tests depended on the number of groups. We first conducted univariable comparisons of potential patient and procedural risk factors for any occurrence of AKI using univariable logistic regression analysis (Table 1). Baseline estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease equation (mL/min/1.73 m2).[19] The primary endpoint of this study is length of AKI duration in days, which is coded as zero for those who did not experience any AKI. Negative binomial regression was used to model the number of days of AKI in terms of baseline patient characteristics. This approach to regression delivers coefficients which when exponentiated can be interpreted as incident rate ratios for one more day of duration. A backwards stepwise approach was used to identify a final model. We used predicted values from the negative binomial regression model, which can be interpreted as the mean number of days of AKI duration. We assessed the discriminatory ability of these predicted days of AKI duration using the concordancy index (calculated as 0.5 + 0.5*tau where tau is Kendall’s tau-b measured of correlation). This was done internally, as well as externally using the TRIBE data. An on-line calculator was developed from the negative binomial regression model coefficients and can be found at: http://yale.edu/tribeaki/aki_duration_calc.html. External validation of the C-index for each category of AKI duration and the devised AKI duration scoring system were conducted by the TRIBE consortium. All analyses were conducted using Stata 9.2 (College Station, TX).
Table 1.
Univariate Associations Between Risk Factors and AKI Duration
| Variables | Patients without AKI (%) | Patients with AKI (%) | IRR | 95%CI | P-value |
|---|---|---|---|---|---|
| Patients (number) | 2932 (60.7) | 1899 (39.3) | |||
| Pre-operative | |||||
| Age 60–69 | 954 (32.5) | 531 (28.0) | 1.64 (1.38–1.95) | <0.001 | |
| Age 70–79 | 762 (26.0) | 713 (35.6) | 2.85 (2.41–3.38) | <0.001 | |
| Age 80+ | 206 (7.0) | 270 (14.2) | 3.77 (2.97–4.77) | <0.001 | |
| Male | 2069 (70.6) | 1329 (70.0) | 0.89 (0.77–1.02) | 0.101 | |
| Diabetes | 853 (29.1) | 694 (36.6) | 1.45 (1.26–1.67) | <0.001 | |
| Hypertension | 1821 (62.1) | 1371 (72.2) | 1.29 (1.12–1.49) | <0.001 | |
| Congestive heart failure | 528 (18.0) | 514 (27.1) | 2.23 (1.91–2.61) | <0.001 | |
| Vascular disease | 554 (18.9) | 508 (26.8) | 1.70 (1.46–2.00) | <0.001 | |
| White blood cell count >12,000 | 213 (7.3) | 174 (9.2) | 1.73 (1.36–2.20) | <0.001 | |
| Estimated GFR<60 (mL/min) | 469 (16.0) | 581 (30.6) | 2.39 (2.05–2.79) | <0.001 | |
| Prior CABG surgery | 185 (6.3) | 120 (6.3) | 1.58 (1.20–2.06) | 0.001 | |
| Intra-aortic balloon pump | 211 (7.2) | 148 (7.8) | 1.83 (1.43–2.35) | <0.001 | |
| Number of pRBC units, mean±SD | 0.04±0.32 | 0.09±0.50 | 1.34 (1.12–1.60) | 0.001 | |
| Volume of fluids (L), mean±SD | 1257±655 | 1277±577 | 1.00 (0.99–1.00) | 0.061 | |
| Peri-operative | |||||
| CABG | 2080 (70.9) | 1228 (64.7) | Ref | ||
| Valve | 488 (16.6) | 286 (15.1) | 1.18 (0.98–1.41) | 0.080 | |
| CABG/Valve | 364 (12.4) | 385 (20.4) | 2.31 (1.92–2.76) | <0.001 | |
| Off-pump surgery | 210 (7.2) | 76 (4.0) | 0.43 (0.32–0.58) | <0.001 | |
| Number of valves, mean±SD | 0.32±0.52 | 0.39±0.56 | 1.53 (1.36–1.73) | <0.001 | |
| Number of anastomoses, mean±SD | 2.74±1.67 | 2.86±1.66 | 1.02 (0.98–1.06) | 0.433 | |
| Pump time (min), mean±SD | 110±54 | 124±55 | 1.01 (1.01–1.01) | <0.001 | |
| Pump time >120 (min) | 1093 (37.3) | 879(46.3) | 2.07 (1.81–2.36) | <0.001 | |
| Cross-clamp time (min), mean±SD | 69.7±40.3 | 77.4±38.6 | 1.01 (1.01–1.01) | <0.001 | |
| Cardioplegia time (min), mean±SD | 20.1±7.6 | 20.7±6.6 | 1.02 (1.01–1.03) | <0.001 | |
| Blood cardioplegia | 2439 (83.2) | 1644 (86.6) | 1.22 (1.01–1.46) | 0.038 | |
| Cold cardioplegia | 1482 (50.6) | 802 (42.2) | 0.88 (0.77–1.01) | 0.068 | |
| Cardioplegia hot shot | 2558 (87.2) | 1692 (89.1) | 1.11 (0.90–1.36) | 0.333 | |
| Retrograde autologous priming (RAP) | 1722 (58.7) | 1140 (60.0) | 0.85 (0.75–0.98) | 0.023 | |
| Volume of fluids on bypass (mL), mean±SD | 1925±2151 | 2213±2494 | 1.00 (1.00–1.00) | <0.001 | |
| Prime volume (mL), mean±SD | 1150±535 | 1190±559 | 1.00 (1.00–1.00) | <0.001 | |
| Blood prime units, mean±SD | 0.09±0.46 | 0.20±0.65 | 1.56 (1.36–1.79) | <0.001 | |
| Number of pRBCs units, mean±SD | 0.51±1.24 | 0.87±1.76 | 1.33 (1.26–1.40) | <0.001 | |
| Highest blood temperature, mean±SD | 37.5±0.41 | 37.5±0.70 | 1.00 (0.92–1.09) | 0.911 | |
| Lowest venous saturation, mean±SD | 69.87±6.27 | 69.82±6.35 | 1.00 (0.99–1.01) | 0.549 | |
| Total volume of heparin>50,000 units | 1031 (35.2) | 700 (36.9) | 0.97 (0.84–1.11) | 0.619 | |
| Last potassium on bypass, mean±SD | 5.58±3.32 | 5.58±3.54 | 1.00 (0.98–1.01 | 0.795 | |
| Nadir hematocrit on bypass, mean±SD | 23.24±3.29 | 22.56±3.39 | 0.91 (0.90–.93) | <0.001 | |
| Nadir hematocrit<20 on bypass | 332 (11.3) | 320 (16.9) | 1.62 (1.34–1.97) | <0.001 | |
| Ultrafiltration (hemoconcentration on bypass) | 136 (4.6) | 139 (7.3) | 1.74 (1.31–2.30) | <0.001 | |
| Return to bypass | 219 (7.5) | 204 (10.7) | 1.61 (1.28–2.03) | <0.001 | |
| Aprotinin use | 1157 (39.5) | 936 (49.4) | 2.08 (1.82–2.37) | <0.001 | |
| Post-operative | |||||
| Number of pRBCs units, mean±SD | 0.59±1.55 | 1.44±3.04 | 1.34 (1.29–1.39) | <0.001 | |
| Inotropes ≥2 at 48hrs | 40 (1.4) | 125 (6.6) | 5.17 (3.66–7.30) | <0.001 | |
| Number of inotropes at 4 hrs, mean±SD | 0.45±0.69 | 0.75±0.88 | 2.04 (1.88–2.22) | <0.001 | |
| Number of inotropes at 48 hrs, mean±SD | 0.10±0.38 | 0.37±0.78 | 2.66 (2.37–2.98) | <0.001 | |
| Low cardiac output failure | 125 (4.3) | 233 (12.3) | 3.76 (2.96–4.78) | <0.001 | |
AKI: acute kidney injury; CABG: coronary artery bypass graft surgery; GFR: glomerular filtration rate; IRR: Incidence Rate Ratio; pRBC: packed red blood cells; SD: standard deviation.
Results
From 2002 to 2007, 39.3 percent of patients developed AKI after cardiac surgery (1,886/4,837). The average number of index admission serum creatinine measures per patient was 9.6. Median duration of AKI lasted was 3 days and ranged from 1 to 108 days. Univariate associations between patient, procedural and immediate post-operative risk factors are summarized in Table 1. The first column in Table 1 refers to the proportion of all patients that have the risk factor of interest; the second column denotes the proportion of patients with AKI that have that risk factor of interest. We identified risk factors on multivariable analysis for longer AKI duration: Pre-operative factors included age, male sex, diabetes, hypertension, vascular disease, eGFR<60 (ml/min/m2), and the number of packed red blood cells transfused prior to surgery; operative factors included off-pump surgery, pump time ≥ 120 minutes, clamptime (minutes), aprotinin, nadir hematocrit on bypass, and use of ultrafiltration; post-operative factors included number of packed red blood cells transfused after the procedure, number of inotropes at 4 and 48hrs (Table 2). Operative elements that were protective against AKI duration were prior CABG surgery, off-pump surgery, total fluids on bypass, return to bypass, and using more than 2 inotropes at 48 hours after surgery. C-index was statistically significant at 0.66 (p<0.001). The total score can be used to look up the risk of AKI duration: http://yale.edu/tribeaki/aki_duration_calc.html. To calculate the mean number of expected AKI days:
Table 2.
Multivariate Prediction of AKI Duration
| Risk Factors | IRR | 95%CI | P-Value |
|---|---|---|---|
| Pre-operative | |||
| Age 60 | 1.29 | (1.11–1.51) | 0.001 |
| Age 70 | 1.85 | (1.58–2.16) | <0.001 |
| Age 80 | 2.56 | (2.07–3.17) | <0.001 |
| Male | 1.29 | (1.12–1.48) | <0.001 |
| Diabetes | 1.28 | (1.13–1.45) | <0.001 |
| Hypertension | 1.29 | (1.14–1.46) | <0.001 |
| Vascular disease | 1.18 | (1.03–1.36) | 0.018 |
| Estimated GFR<60 | 1.80 | (1.56–2.07) | <0.001 |
| Number of pRBC units | 1.17 | (1.02–1.34) | 0.026 |
| Prior CABG surgery | 0.78 | (0.62–0.99) | 0.047 |
| Peri-operative | |||
| Off-pump surgery | 0.73 | (0.54–0.99) | 0.043 |
| Cross-clamp time (min) | 1.00 | (1.00–1.00) | 0.036 |
| Pump time >120 (min) | 1.19 | (1.03–1.39) | 0.023 |
| Volume of fluids on bypass (mL) | 0.99 | (0.99–0.99) | 0.036 |
| Cold cardioplegia | 0.84 | (0.75–0.95) | 0.005 |
| Nadir hematocrit on bypass | 0.97 | (0.95–0.99) | 0.011 |
| Nadir hematocrit<20 on bypass | 0.79 | (0.64–0.97) | 0.022 |
| Aprotinin use | 1.16 | (1.02–1.32) | 0.021 |
| Ultrafiltration used | 1.33 | (1.05–1.70) | 0.020 |
| Return to bypass | 0.77 | (0.62–0.95) | 0.015 |
| Post-operative | |||
| Number of pRBCs | 1.20 | (1.16–1.24) | <0.001 |
| Inotropes ≥2 at 48hrs | 0.68 | (0.46–0.99) | 0.049 |
| Number of inotropes at 4 hrs, mean±SD | 1.27 | (1.16–1.39) | <0.001 |
| Number of inotropes at 48 hrs | 1.76 | (1.53–2.04) | <0.001 |
| C-Index | 0.66 | <0.001 | |
CABG: coronary artery bypass graft; IRR: Incidence Rate Ratio; GFR: glomerular filtration rate; pRBC: packed red blood cells.
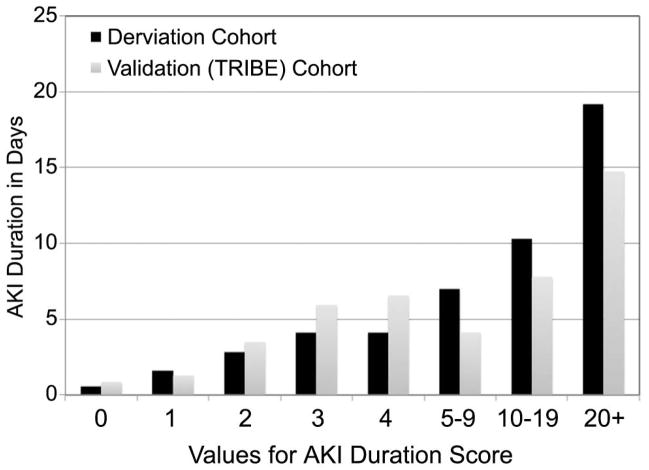
The models were externally validated using data from 1,219 cardiac surgery patients from the TRIBE cohort. TRIBE had 35 percent of patients developing AKI with similar baseline risk factors (Table 3). The TRIBE validation cohort resulted in similar prediction of AKI duration and a statistically significant C-index of 0.71 (p<0.001). Figure 1 demonstrates the predicted duration of AKI and actual duration of AKI in the derivation (NNE) and validation (TRIBE) cohorts.
Table 3.
Risk Factors of TRIBE Validation Cohort
| Variables | Patients without AKI (%) | Patients with AKI (%) |
|---|---|---|
| Patients (number) | 793 (65.0) | 426 (35.0) |
| Pre-operative | ||
| Age 60–69 | 161 (20.3) | 83 (19.5) |
| Age 70–79 | 404 (50.9) | 205 (48.1) |
| Age 80+ | 121 (15.3) | 85 (20.0) |
| Male | 526 (66.3) | 300 (70.4) |
| Diabetes | 314 (39.6) | 197 (46.2) |
| Hypertension | 608 (76.7) | 353 (82.9) |
| Congestive heart failure | 175 (22.1) | 139 (32.6) |
| Estimated GFR<60 (mL/min) | 244 (30.8) | 180 (42.3) |
| Prior CABG surgery | 99 (12.5) | 56 (13.2) |
| Peri-operative | ||
| CABG | 395 (49.8) | 190 (44.6) |
| Valve | 234 (29.5) | 120 (28.2) |
| CABG/Valve | 163 (20.5) | 116 (27.2) |
| Off-pump surgery | 73 (9.2) | 31 (7.3) |
| Number of anastomoses, mean±SD | 2.4±1.2 | 2.3±1.2 |
| Pump time (min), mean±SD | 106.3±52.7 | 128.5±69.0 |
| Pump time >120 (min) | 239 (30.1) | 208 (48.8) |
| Blood cardioplegia | 697 (87.9) | 380 (89.2) |
| Cold cardioplegia | 697 (87.9) | 380 (89.2) |
| Number of pRBCs units, mean±SD | 2.5±1.9 | 2.8±2.3 |
| Aprotinin use | 24 (3.0) | 13 (3.1) |
| Post-operative | ||
| Inotropes ≥2 at 4hrs | 31 (3.9) | 170 (39.9) |
| Inotropes ≥2 at 48hrs | 22 (2.8) | 125 (29.3) |
AKI: acute kidney injury; CABG: coronary artery bypass graft surgery; GFR: glomerular filtration rate; SD: standard deviation; pRBC: packed red blood cells.
Figure 1.
The duration of acute kidney injury (AKI) is plotted by the predicted duration of AKI in the derivation cohort (NNE, Black bars) and the validation cohort (TRIBE, Gray bars).
Comment
In our large prospective cardiac surgery cohort, we evaluated the predictive ability of baseline patient risk factors and peri-operative risk factors for the development AKI and AKI duration. After rigorous testing of these risk factors in univariate and multivariable logistic and multinomial logistic regression, we discovered thirteen patient and peri-operative risk factors predictive of AKI and the duration of AKI.
Prior Studies of AKI Prediction
Based on our previous work, AKI duration appears to be a better indicator of in-hospital outcomes (mortality, length of stay and total costs) than the outcome of AKI alone.[4] Prior studies have largely investigated clinical risk factors for new onset of dialysis-dependent renal failure occurring during the post-operative period after cardiac surgery. Clinical risk factors have included older age,[7, 13] sex,[11] race,[7] diabetes,[7–9, 13, 20, 21] peripheral vascular disease,[21, 22] baseline eGFR[9, 22] (or baseline SCr),[6–8, 13, 20, 21] poor ejection fraction,[6, 8, 9, 22] New York Heart Association class,[6–8, 13, 21, 22] congestive heart failure,[11, 20] prior acute myocardial infarction,[7, 11, 21] atrial fibrillation,[21] lung disease or chronic obstructive pulmonary disease,[6–8, 22] pre-operative intra-aortic balloon pump,[6, 8, 9, 22] emergent surgery,[8, 9] type of surgery,[6–9, 13] reoperation,[6–8, 20, 22] and low cardiac output failure or use of more than two inotropes.[11, 13] Only three studies investigated perfusion characteristics such as cardiopulmonary bypass time >120 or >180 minutes.[11, 13, 20, 21]
Other studies investigated clinical risk factors for AKI as an endpoint, a more common renal outcome among cardiac surgery patients. Three studies investigate clinical risk factors for AKI including older age,[12, 13] sex,[11, 12] baseline SCr,[13] prior myocardial infarction,[11] intra-aortic balloon pump,[11, 12] prior heart surgery,[12] New York Heart Association class,[13] congestive heart failure,[11, 12] hypertension,[12] pulse pressure,[11] diabetes or blood glucose,[12, 13] inflammation,[12] type of surgery,[13] and low cardiac output failure or use of 2 or more inotropes.[11, 13] Of these studies, Only two studies investigated perfusion characteristics including cardiopulmonary bypass time >120 minutes,[11, 13] and low central venous pressure > 14 cm H2O.[13] In our investigation, we determined the predictive utility of clinical risk factors for AKI and AKI duration. Similar to other AKI models, we found age, male sex, hypertension, diabetes, and cardiopulmonary bypass times >120 minutes to be predictive of AKI duration. Our modeling of AKI duration has added to the literature by exploring the role of operative techniques and risk factors including the predictive ability red blood cell transfusion, use of ultrafiltration (hemoconcentration on bypass)and post-operative inotropes.
Englberger validated the Cleveland Clinic,[8] Society for Thoracic Surgeons (STS),[7] and Toronto[9] models originally developed for AKI requiring dialysis as an endpoint. He demonstrated these models performed well in predicting severe AKI (2.0 mg/dL or 2-fold increase in SCr or dialysis) with the Cleveland risk score (ROC 0.77; 95%CI 0.74–0.80) and STS model (ROC 0.76; 95%CI 0.73–0.80) performing better than the Toronto model (ROC 0.71; 95%CI 0.67–0.75).[10] Our modeling focused on predicting the duration of AKI with pre- and peri-operative risk factors with an easy-to-use on-line calculator, which can be found at: http://yale.edu/tribeaki/aki_duration_calc.html. The use of the on-line calculator demonstrates the generalizeability of the prediction model with regard to its clinical usefulness. The calculator helps the practitioner see, in real time, how modifiable perioperative variables impact outcomes.
Strengths and Limitations
There are limitations to consider for this prediction modeling. First, we developed the prediction modeling based on a prospectively collected, retrospectively analyzed cohort from a single institution. Second, the C-statistic from the derivation cohort and validation cohort were low, suggesting unmeasured factors not captured by this cohort could improve the prediction of AKI duration. Three, SCr labs were drawn each day until 48 hours after surgery on all patients; after 48 hours, SCr labs were ordered on a per-provider discretion until hospital discharge and may be subject to ascertainment bias. However, our study also has notable strengths. We developed the prediction modeling for AKI duration using a modern prospective cohort of consecutive patients with a wide mix of comorbid conditions, age, gender, race, and therefore allows for adequate generalizability of our findings to other cardiac surgery centers. We externally validated this model by the TRIBE-AKI cohort included high-risk patients for developing AKI, which may have limited the precision of the external validation, however the validation cohort exceeded the C-index for the derivation cohort and had a similar AKI event rate. TRIBE-AKI also did not have patient information on any vascular disease, use of ultrafiltration (hemoconcentration on bypass), or pre- or post-operative red blood cell transfusion, which may have contributed to lower C-index. We also included post-operative risk factors for AKI duration, specifically post-operative transfusion, and indicators for low-cardiac output failure. We believe these near-post-operative events (4–48 hours after surgery) are important to include in the modeling and can be added to the model when evaluating risk. While these may be competing endpoints, they play a crucial role as indicators of anemia and hypo-perfusion. We have identified a broad range of risk factors for AKI duration surpassing previous work by evaluating detailed operative characteristics, techniques and exposures.
Conclusions
Previously, we reported on the direct proportionality of AKI duration and long-term mortality.4 The post-operative complication of AKI in cardiac surgery is currently underrated and its prevention and mitigation may have long term implications similar to such things as the use of the internal mammary artery in coronary artery bypass surgery and aspirin in myocardial infarction. In this investigation we have evaluated a wide range of patient and operative risk factors for the prediction of AKI and AKI duration. A tool for the surgeon to predict and potentially mitigate AKI has the potential to take its place in the surgeon’s armamentarium of prediction models. We identified risk factors associated with longer durations of AKI, including baseline patient and disease characteristics and peri- and post-operative factors. Some of these risk factors are modifiable and can be used to prevent AKI and/or long durations of AKI, thereby minimizing the risk of developing cardiac surgery associated AKI and its survival implications. Future clinical trials for AKI should focus on enrolling patients at higher risk of longer duration of AKI. We have provided an externally validated prediction tool for determining the risk of longer durations of AKI.
Acknowledgments
Dr. Brown is supported by grant number K01HS018443 from the Agency for Healthcare Research and Quality. Dr. Parikh is supported by grant R01HL085757 from the National Institutes of Health. Dr. Coca is funded by the career development grant K23DK08013 from the National Institutes of Health, by the Hartford Foundation Center of Excellence in Aging at Yale Subspecialty Scholar Award, and by the American Society of Nephrology-ASP Junior Development Award in Geriatric Nephrology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:844–61. doi: 10.2215/CJN.05191107. [DOI] [PubMed] [Google Scholar]
- 2.Hobson CE, Yavas S, Segal MS, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–53. doi: 10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 3.Bihorac A, Yavas S, Subbiah S, et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249:851–8. doi: 10.1097/SLA.0b013e3181a40a0b. [DOI] [PubMed] [Google Scholar]
- 4.Brown JR, Kramer RS, Coca SG, Parikh CR. Duration of acute kidney injury impacts long-term survival after cardiac surgery. Ann Thorac Surg. 2010;90:1142–8. doi: 10.1016/j.athoracsur.2010.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coca SG, King JT, Jr, Rosenthal RA, Perkal MF, Parikh CR. The duration of postoperative acute kidney injury is an additional parameter predicting long-term survival in diabetic veterans. Kidney Int. 2010;78:926–33. doi: 10.1038/ki.2010.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chertow GM, Lazarus JM, Christiansen CL, et al. Preoperative renal risk stratification. Circulation. 1997;95:878–84. doi: 10.1161/01.cir.95.4.878. [DOI] [PubMed] [Google Scholar]
- 7.Mehta RH, Grab JD, O’Brien SM, et al. Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation. 2006;114:2208–16. doi: 10.1161/CIRCULATIONAHA.106.635573. quiz 2208. [DOI] [PubMed] [Google Scholar]
- 8.Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16:162–8. doi: 10.1681/ASN.2004040331. [DOI] [PubMed] [Google Scholar]
- 9.Wijeysundera DN, Karkouti K, Dupuis JY, et al. Derivation and validation of a simplified predictive index for renal replacement therapy after cardiac surgery. Jama. 2007;297:1801–9. doi: 10.1001/jama.297.16.1801. [DOI] [PubMed] [Google Scholar]
- 10.Englberger L, Suri RM, Li Z, et al. Validation of clinical scores predicting severe acute kidney injury after cardiac surgery. Am J Kidney Dis. 2010;56:623–31. doi: 10.1053/j.ajkd.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 11.Aronson S, Fontes ML, Miao Y, Mangano DT. Risk index for perioperative renal dysfunction/failure: critical dependence on pulse pressure hypertension. Circulation. 2007;115:733–42. doi: 10.1161/CIRCULATIONAHA.106.623538. [DOI] [PubMed] [Google Scholar]
- 12.Brown JR, Cochran RP, Leavitt BJ, et al. Multivariable prediction of renal insufficiency developing after cardiac surgery. Circulation. 2007;116:I139–43. doi: 10.1161/CIRCULATIONAHA.106.677070. [DOI] [PubMed] [Google Scholar]
- 13.Palomba H, de Castro I, Neto AL, Lage S, Yu L. Acute kidney injury prediction following elective cardiac surgery: AKICS Score. Kidney Int. 2007;72:624–31. doi: 10.1038/sj.ki.5002419. [DOI] [PubMed] [Google Scholar]
- 14.Lopes JA, Fernandes P, Jorge S, et al. Acute kidney injury in intensive care unit patients: a comparison between the RIFLE and the Acute Kidney Injury Network classifications. Crit Care. 2008;12:R110. doi: 10.1186/cc6997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robert AM, Kramer RS, Dacey LJ, et al. Cardiac surgery-associated acute kidney injury: a comparison of two consensus criteria. Ann Thorac Surg. 2010;90:1939–43. doi: 10.1016/j.athoracsur.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 17.Shlipak MG, Coca SG, Wang Z, et al. Presurgical Serum Cystatin C and Risk of Acute Kidney Injury After Cardiac Surgery. Am J Kidney Dis. 2011 doi: 10.1053/j.ajkd.2011.03.015. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zappitelli M, Krawczeski CD, Devarajan P, et al. Early postoperative serum cystatin C predicts severe acute kidney injury following pediatric cardiac surgery. Kidney Int. 2011 doi: 10.1038/ki.2011.123. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 20.Rahmanian PB, Filsoufi F, Castillo JG, et al. Predicting postoperative renal failure requiring dialysis, and an analysis of long-term outcome in patients undergoing valve surgery. J Heart Valve Dis. 2008;17:657–65. [PubMed] [Google Scholar]
- 21.Rahmanian PB, Kwiecien G, Langebartels G, et al. Logistic risk model predicting postoperative renal failure requiring dialysis in cardiac surgery patients. Eur J Cardiothorac Surg. 2011 doi: 10.1016/j.ejcts.2010.12.051. In Press. [DOI] [PubMed] [Google Scholar]
- 22.Eriksen BO, Hoff KR, Solberg S. Prediction of acute renal failure after cardiac surgery: retrospective cross-validation of a clinical algorithm. Nephrol Dial Transplant. 2003;18:77–81. doi: 10.1093/ndt/18.1.77. [DOI] [PubMed] [Google Scholar]