Abstract
In Parkinson’s disease, subthalamic nucleus (STN) neurons burst fire with increased periodicity and synchrony. This may entail abnormal release of glutamate, the major source of which in STN is cortical afferents. Indeed, the cortico-subthalamic pathway is implicated in the emergence of excessive oscillations, which are reduced, as are symptoms, by dopamine-replacement therapy or deep brain stimulation (DBS) targeted to STN. Here we hypothesize that glutamatergic synapses in the STN may be differentially modulated by low-frequency stimulation (LFS) and high-frequency stimulation (HFS), the latter mimicking deep brain stimulation. Recordings of evoked and spontaneous excitatory post synaptic currents (EPSCs) were made from STN neurons in brain slices obtained from dopamine-intact and chronically dopamine-depleted adult rats. HFS had no significant effect on evoked (e) EPSC amplitude in dopamine-intact slices (104.4±8.0%) but depressed eEPSCs in dopamine-depleted slices (67.8±6.2%). Conversely, LFS potentiated eEPSCs in dopamine-intact slices (126.4±8.1%) but not in dopamine-depleted slices (106.7±10.0%). Analyses of paired-pulse ratio, coefficient of variation, and spontaneous EPSCs suggest that the depression and potentiation have a presynaptic locus of expression. These results indicate that the synaptic efficacy in dopamine-intact tissue is enhanced by LFS. Furthermore, the synaptic efficacy in dopamine-depleted tissue is depressed by HFS. Therefore the therapeutic effects of DBS in Parkinson’s disease appear mediated, in part, by glutamatergic cortico-subthalamic synaptic depression and implicate dopamine-dependent increases in the weight of glutamate synapses, which would facilitate the transfer of pathological oscillations from the cortex.
Keywords: subthalamic nucleus, primary motor cortex, 6-hyroxydopamine, deep brain stimulation
The cardinal motor symptoms of Parkinson’s disease (PD) are coincident with the loss of independent neuronal activity in the cortex and nuclei of the basal ganglia (BG). Thus, in the presence of normal dopaminergic “drive,” the activity of BG neurons is largely desynchronized. However, in idiopathic PD and its experimental models, neurons of the external globus pallidus (GP) and subthalamic nucleus (STN), amongst others in the BG, loose their independence and show increases in burst firing and synchronization (Filion and Tremblay, 1991; Bergman et al., 1994; Nini et al., 1995). During the slow-wave activity prominent in sleep and general anesthesia, this pathological activity favors low-frequency oscillations (<2 Hz) that are synchronized to ongoing slow cortical rhythms (Magill et al., 2001; Mallet et al., 2008a). During wakefulness or analogous brain states, this pathological activity favors oscillations at beta (15–30 Hz) frequencies (Brown et al., 2001; Sharott et al., 2005; Kühn et al., 2006; Mallet et al., 2008a,b), which may underlie rigidity and bradykinesia (Levy et al., 2000; Eusebio et al., 2011). Pathological beta oscillations and motor symptoms may be reversed by dopamine replacement therapy (Brown et al., 2001; Kühn et al., 2006), or by lesions or high-frequency (>100 Hz) stimulation (“deep brain stimulation” or DBS) directed to STN (Bergman et al., 1994; Eusebio et al., 2011).
Using an organotypic culture preparation, Plenz and Kitai (1999) proposed that the GP and STN constitute a central pacemaker responsible for oscillatory activity, at least at low frequencies (<3 Hz). However, this proposal has not been supported in vitro (Loucif et al., 2005) or in vivo (Magill et al., 2000, 2001). Instead, these studies point to a critical role of the cortex and STN in sculpting BG neuronal activity through the glutamatergic “hyperdirect” cortico-subthalamic pathway (Fujimoto and Kita, 1993; Maurice et al., 1998; Nambu et al., 2000; Magill et al., 2001, 2004; Sharott et al., 2005; Mallet et al., 2008a,b; Litvak et al., 2011). Moreover, several reports indicate that, although the STN is the target of choice for surgical intervention therapies in PD, stimulation of the motor cortex also provides relief from symptoms (Pagni et al., 2003; Drouot et al., 2004; Lefaucheur et al., 2004).
As stimulation parameters for DBS are similar to the “tetanizing” protocols used for the study of synaptic plasticity in many brain areas, we hypothesized that the beneficial effects of DBS arise, at least in part, as a consequence of the modulation of the efficacy of cortico-subthalamic synapses and a reversal of the pathological effects of dopamine depletion. Indeed, dopamine depletion leads to the generation of large compound excitatory postsynaptic currents (EPSCs) in the STN (Ammari et al., 2011), presumably as a consequence of a loss of inhibitory control of excitatory inputs by dopamine acting at presynaptic dopamine D2 receptors (Shen and Johnson, 2000).
In this study, we have used high-frequency stimulation (HFS) of glutamatergic STN afferents to mimic DBS, and low-frequency stimulation (LFS) to mimic pathological bursting activity, in both dopamine-intact and chronically dopamine-depleted brain slices. We show that LFS increases the weight of glutamatergic synaptic inputs in dopamine intact tissue, whereas HFS elicits synaptic depression in dopamine-depleted tissue.
EXPERIMENTAL PROCEDURES
All the animals in this study were used in accordance with the Animals (Scientific Procedures) Act, 1986 (UK) and the European Communities Council Directive (80/609/EEC).
Brain slice preparation
Parasagittal brain slices (300 μm thick) were prepared from young adult (45–52 days of age, 180–220 g) male Wistar rats. These animals were inbred from a colony originally obtained from Charles River (Kent, UK). Animals were first anaesthetized using isoflurane (2% in O2) and decapitated. Slices were cut on a DTK-1000 Microslicer (Dosaka, Japan) in ice-cold sucrose-based solution containing (in mM) sucrose, 206; KCl, 2; MgSO4, 1.6; NaHCO3, 25.9; NaH2PO4, 1.25; glucose, 10; and CaCl2, 2.16. Slices were then transferred and incubated at room temperature for 1 h in artificial cerebrospinal fluid (aCSF) containing (in mM) NaCl, 126; KCl, 2.95; MgSO4, 1.6; NaHCO3, 25.9; NaH2PO4, 1.25; glucose, 10; CaCl2, 2; and ascorbic acid, 0.2. Both solutions were continuously aerated with 95% O2 and 5%CO2, maintaining pH at 7.3 and 310 mOsm. Slices were then transferred to a recording chamber and perfused with aCSF at 34 °C at a rate of 2 ml min−1. The whole STN and individual STN neurons were visualized by differential interference contrast (DIC) infrared microscopy using an Olympus BX51W1 microscope with a CCD camera (KP-M1; Hitachi, Tokyo, Japan).
Electrophysiology
Whole-cell voltage-clamp recordings were made using borosilicate glass pipettes (3–4 MÙ resistance). Electrodes were filled with an internal solution containing (in mM) HEPES, 40; QX-314, 5; EGTA, 0.6; NaCl, 4; d-gluconate, 100; TEA-Cl, 5; ATP-Na, 4; and GTP-Na, 0.3 (titrated with CsOH to pH 7.25) at 295 mOsm. The concentration of EGTA was set at 0.6 mM so as not to compromise potential plasticity involving calcium-dependent postsynaptic mechanisms. Synaptic currents were recorded at a holding potential of −60 mV using an Axopatch 200B amplifier (Molecular Devices Corp., Sunnyvale, CA, USA). Series resistance (range: 3–20 MÙ) was monitored throughout each experiment. Results from cells where series resistance changed over 20% or was >20 MÙ were discarded. The GABAA receptor channel blocker picrotoxin (50 μM) was present in the perfusate throughout all recordings. In experiments from dopamine-intact controls, the nonspecific dopamine receptor agonist apomorphine was bath applied at a concentration of 20 μM.
Stimulation protocols
Focal electrical stimulation was carried out using glass electrodes filled with normal aCSF. To evoke excitatory postsynaptic currents (eEPSCs) mediated by the cortico-subthalamic pathway, each stimulating electrode was placed in the rostral internal capsule, at a distance of at least 200 μm from the recorded STN cell. To assess the lasting effects of LFS and HFS, pairs of stimuli were delivered every 10 s, with an interstimulus interval of 50 ms. Stimulation was carried out via a DS-3 constant-current isolated stimulator (Digitimer Ltd., Welwyn Garden City, UK), and its parameters (amplitude and width) were adjusted on a cell-by-cell basis to produce 75% of maximum eEPSC amplitude. Stable baseline recordings were made for at least 10 min before HFS or LFS.
HFS consisting of 100 pulses at 100 Hz was chosen to mimic DBS. LFS was generated by delivering 40 Hz stimulation (10 pulses) every 1 s for 5 min. This latter paradigm mimics the pathological activity of STN neurons that emerges during the slow-wave activity brain state in anaesthetized 6-hydroxydopamine-lesioned rats (Magill et al., 2001). HFS and LFS paradigms were programed in Clampex 10.2.
Data acquisition and analysis
Data were low-pass filtered at 2 kHz by an 8-pole Bessel filter (Axopatch 200B amplifier), and digitized at 10 kHz using a Digidata 1440A. Analysis was performed off-line using Clampfit 10.2 (Molecular Devices, Sunnyvale, CA, USA). Paired EPSCs (P1 and P2) were evoked by single-shock electrical stimulation of equal strength and duration, at an interstimulus interval of 50 ms and any changes in the paired pulse ratio (PPR) taken as indication of presynaptic locus of expression (Thomson, 2000). PPRs were calculated by dividing the mean amplitude of second response (P2) by the mean amplitude of first response (P1), thus avoiding facilitatory bias (Kim and Alger, 2001). Ten minutes of baseline (pre-LFS or HFS) data were compared with 10 min epochs of data throughout the duration of recording after LFS or HFS. Only data showing stable baseline amplitudes, defined as those having a coefficient of variation <0.15, were analyzed using one-way ANOVAs.
The interevent interval (IEI) of spontaneous EPSCs (sEPSCs) was analyzed off-line using Mini-Analysis Programe (Synaptosoft, USA). The significance of shifts in cumulative probability distributions was assessed using the Kolmogorov–Smirnov test (KS test). Ten minutes of data were compared with the number of events always being >100. To compare pooled data before and after stimulation, we determined median IEI values for each cell and calculated the mean of these median values for each cell population. All data are expressed as mean±SEM and significance for all statistical tests was set at P<0.05.
To confirm the locus of plasticity, coefficient of variation (CV) analysis was also undertaken (Bekkers and Stevens, 1990). This is a form of nonstationary fluctuation analysis that allows synaptic characteristics to be determined when it is impractical to create conditions for altered release probability. Quantal theory describes changes in release probability (P) and number of release sites (N) at a presynaptic locus, whereas changes in response amplitude (Q) are postsynaptic. The mean amplitude of an EPSC represents the product of N, P, and Q, and the coefficient of variation (CV−2) is proportional to NP/1–P. Therefore, any presynaptic changes will affect CV−2 and be independent of Q. Thus, using a plot of mean amplitude (Q) against CV−2, a response that moves along the x-axis indicates a postsynaptic locus of expression, whereas a response that moves along the diagonal indicates a presynaptic locus of expression.
6-hydroxydopamine lesions and behavioral testing
Unilateral 6-hydroxydopamine (6-OHDA) lesions were carried out on 150–180 g rats, as described previously (Magill et al., 2001). Briefly, anesthesia was induced and maintained with isoflurane (1.5–2.5% in O2). Twenty five minutes before the injection of 6-OHDA, all animals received a bolus of desipramine (25 mg kg−1 i.p.; Sigma) to minimize the uptake of 6-OHDA by noradrenergic neurons (Schwarting and Huston, 1997). Immediately before use, the neurotoxin 6-OHDA (hydrochloride salt; Sigma) was dissolved in ice-cold 0.9% w/v NaCl solution containing 0.02% w/v ascorbate to a final concentration of 4 mg/ml. Three micro liters of 6-OHDA solution was then injected at a rate of 0.6 μl/min into the region adjacent to the medial substantia nigra (4.5 mm posterior of bregma, 1.2 mm lateral to the midline, and 7.9 mm ventral to the dura).
The extent of the dopamine cell lesion was assessed by the forelimb stepping test (Olsson et al., 1995). Behavioral tests were performed 12 or 15 days after the injection of toxin. Each animal was tested twice per day for three consecutive days. Each of the animals used for electrophysiology showed significant deficits in forelimb stepping on the side contralateral to the lesion (ipsilateral 13.5±1.6 steps vs. contralateral 0.47±0.13 steps, n=10, P<0.0001). These observed behavioral changes indicate loss of the majority of dopamine neurons. Indeed, behavioral deficits of this magnitude not only correlate with significant reductions in tyrosine hydroxylase-expressing (dopaminergic) neurons but also with increased oscillatory activity in the BG (Tseng et al., 2005). All electrophysiological recordings were performed 2–5 weeks after injection of toxin, which corresponds to a time when the pathophysiological state has stabilized at a maximum level (Pan and Walters, 1988; Vila et al., 2000).
RESULTS
In the presence of picrotoxin (50 μM), stimulation within the internal capsule elicited a short-latency (monosynaptic) inward current that reversed around 0 mV and was blocked by glutamate receptor antagonists CNQX (10 μM) and d-AP5 (50 μM). At a stimulation frequency of 0.1 Hz, no potentiation or rundown of these synaptic responses and no changes in intrinsic properties such as resting membrane potential (monitored by changes in holding current) or input resistance were observed.
A previous study (Shen et al., 2003) indicated that the response of STN neurons to HFS was highly variable, such that potentiation, depression, or no changes were observed. We hypothesized that this could be owing to differing concentrations of dopamine release in STN within each brain slice (Cragg et al., 2004), leading, in turn, to an inconsistent level of dopamine receptor activation. Therefore, in control experiments (slices from dopamine-intact rats), we added the nonspecific dopamine receptor agonist apomorphine (20 μM) to the perfusate to mimic the “tonic” dopamine receptor activation that likely occurs locally in the STN in the intact brain (see Cragg et al., 2004) and provide maximum contrast between control slices and those slices obtained from 6-OHDA-lesioned animals. Addition of apomorphine (20 μM) elicited an inward current indicative of membrane depolarization, and an increase in input conductance, as previously observed after application of both receptor agonists and exogenous dopamine (Cragg et al., 2004; Loucif et al., 2008). These postsynaptic effects were accompanied by a decrease in eEPSC amplitude consistent with previous observations. For each stimulation protocol, we assessed evoked (e)EPSC (P1) amplitudes and paired pulse ratios (P2/P1), and we performed a CV analysis of spontaneous (s)EPSCs.
In the first series of experiments, we examined the effect of HFS, consisting of 100 pulses at 100 Hz used to mimic DBS, on glutamatergic synaptic responses of STN neurons in slices from control (dopamine-intact) animals. No significant changes in P1 EPSP amplitude (104.4±8.0%, n=8 neurons, P>0.05, Fig. 1A, B) or PPR (P2/P1, 0.87±0.09 and 0.87±0.07 at baseline and post-HFS, respectively, n=8, P>0.05, Fig. 1B, C) were observed after HFS. We next addressed the synaptic variability using CV analysis, which again yielded no evidence for changes in Q, N, or P (Fig. 1D). Furthermore, no change in the IEI of sEPSCs was observed 20 min following HFS (P=0.8, KS test, Fig. 1E, F). Therefore, in dopamine-intact slices and in the presence of dopamine receptor activation, HFS had no effect on the efficacy of the glutamatergic synapses.
Fig. 1. No effect of high-frequency stimulation on glutamatergic EPSCs in STN neurons in dopamine-intact slices.
(A) Normalized evoked EPSC amplitude (P1) 10 min before and 30 min after application of HFS (n=8 neurons). Each data point represents the mean (± SEM) of six evoked responses per neuron that were averaged across all neurons. Initiation of HFS (100 pulses at 100 Hz) is at time zero. (B) Paired evoked EPSCs (P1 and P2, average of 10 consecutive traces) separated by an interstimulus interval of 50 ms before (black) and 20 min after (red) HFS. (C) Paired pulse ratio (P2/P1) 10 min before and 30 min after application of HFS (n=8). (D) Shows the relative changes in normalized P1 EPSC amplitude plotted against the relative changes in CV-2 induced by HFS for each neuron recorded (white circles) and their average (black). (E) Raw traces showing spontaneous EPSCs before (black) and 20 min after (red) HFS (five consecutive sweeps). (F) Cumulative probability plots of interevent interval (IEI) before (black) and after (red) HFS (P>0.05).
We next examined the effect of HFS on glutamatergic synaptic responses of STN neurons in slices obtained from dopamine-depleted animals. In contrast to the scenario in slices from dopamine-intact animals, HFS of the cortico-subthalamic pathway in dopamine-depleted animals induced a short-term depression of P1 amplitude, lasting for approximately 2 min, followed by a longer lasting depression (67.8±6.2%, n=6, P<0.01 Fig. 2A, B), which was recorded for at least 30 min. The depression of P1 following HFS was accompanied by a significant change in PPR (0.89±0.07 and 0.97±0.03 at baseline and post-HFS, respectively, n=6, P<0.05, Fig. 2B, C). A significant presynaptic component was also evident in the CV analysis, which showed a pronounced movement along the diagonal toward origin that is indicative of a reduction in release probability and/or number of release sites (Fig. 2D). These data correlated with the effect of HFS on sEPSCs because the IEI was significantly increased 20 min after stimulation, as compared with baseline (P<0.005, KS test, Fig. 2E, F). Thus HFS, which had no effect on excitatory inputs to STN neurons in dopamine-intact slices, produces a marked synaptic depression of glutamate responses after chronic dopamine depletion. This plasticity showed a clear presynaptic component in its locus of expression.
Fig. 2. High-frequency stimulation depresses glutamatergic EPSCs in STN neurons in dopamine-depleted slices.
(A) Normalized eEPSC (P1) amplitude 10 min before and 30 min after application of HFS (n=6 neurons). (B) Paired evoked EPSCs (P1 and P2) before (black) and 20 min after (red) HFS. (C) Paired pulse ratio 10 min before and 30 min after application of HFS (n=6). (D) Shows the relative changes in normalized P1 EPSC amplitude plotted against the relative changes in CV−2 induced by HFS for each recording (white circles) and their average (black). (E) Raw traces showing sEPSCs before (black) and 20 min after (red) HFS. (F) Cumulative probability plots of IEIs before (black) and after (red) HFS (P<0.005).
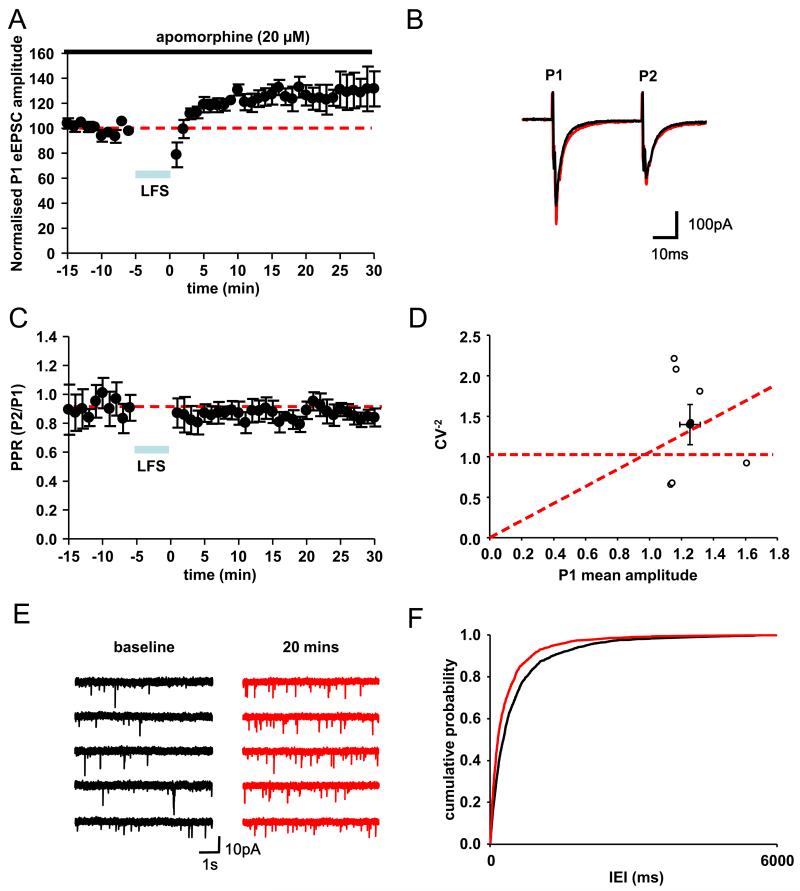
In a third series of experiments, we examined the effect of LFS (40 Hz stimulation, 10 pulses every 1 s for 5 min) to mimic the cortically driven bursting activity seen in the STN in vivo (Magill et al., 2001). In dopamine-intact slices (with 20 μM apomorphine), LFS promoted a potentiation of P1 amplitude for the following 30 min (126.4±8.1%, n=7, P<0.01, Fig. 3A, B). Although no significant changes in PPR were observed (0.79±0.1 and 0.75±0.06 at baseline and post-LFS, respectively, n=7, P>0.05, Fig. 3B, C), analysis of CV showed movement along the diagonal away from the origin indicative of increases in either probability of release and/or number of release sites (Fig. 3D), that is, in the opposite direction from changes observed after HFS in dopamine-depleted slices. These data correlated with analysis of sEPSC and a decrease in IEI (increase in frequency) 20 min after LFS (P<0.05, KS test, Fig. 3E, F). Therefore, in contrast to HFS, LFS in dopamine-intact slices produces a marked potentiation in synaptic weight of glutamatergic synapses, which also showed a presynaptic locus of expression.
Fig. 3. Low-frequency stimulation potentiates glutamatergic EPSCs in STN neurons in dopamine-intact slices.
(A) Normalized evoked EPSC (P1) amplitude 10 min before and 30 min after application of LFS (n=7 neurons). Duration of LFS (10 pulses at 40 Hz delivered every second for 5 min) is indicated by blue bar. Each data point represents the mean (± SEM) of six evoked responses per neuron that were averaged across all neurons. (B) Typical paired EPSCs (P1 and P2, average of 10 consecutive traces in one neuron) evoked before (black) and 20 min after (red) LFS. (C) Paired pulse ratio (P2/P1) 10 min before and 30 min after application of LFS (n=7). (D) Shows the relative changes in normalized P1 EPSC amplitude plotted against the relative changes in CV−2 induced by LFS for each neuron recorded (white) and their average (black). (E) Raw traces showing sEPSCs before (black) and 20 min after (red) LFS (five consecutive sweeps) (F) Cumulative probability plots of interevent interval (IEI) before (black) and after (red) LFS (P<0.001).
Finally, we examined the effect of LFS on glutamatergic synaptic responses of STN neurons in slices obtained from dopamine-depleted animals. In this case, LFS induced a short-lived depression that immediately followed the stimulation protocol, but no significant longer-lasting changes in P1 amplitude were observed (106.7±10.0%, n=6, P>0.05, Fig. 4A, B). There was also no significant change in PPR (0.97±0.09 and 1.01±0.08 at baseline and post-LFS, respectively, P>0.05 Fig. 4B, C). There was a small change in CV along the diagonal (Fig. 4D), but this was not associated with amplitude changes in P1, PPR, and IEI of sEPSCs (P>0.05, KS test, Fig. 4E, F). Therefore, the significant potentiation of glutamatergic EPSCs induced by LFS in dopamine-intact slices is not replicated in slices obtained from dopamine-depleted animals.
Fig. 4. No effect of low-frequency stimulation on glutamatergic EPSCs in STN neurons in dopamine-depleted slices.
(A) Normalized eEPSC (P1) amplitude 10 min before and 30 min after application of HFS (n=6). (B) Paired evoked EPSCs (P1 and P2) before (black) and 30 min after (red) LFS. (C) Paired pulse ratio (P2/P1) 10 min before and 30 min after application of LFS (n=6). (D) Shows the relative changes in normalized P1 EPSC amplitude plotted against the relative changes in CV−2 induced by LFS. (E) Raw traces showing sEPSCs before (black) and 20 min after (red) HFS. (F) Cumulative probability plots of interevent interval (IEI) before (black) and after (red) LFS (P>0.05).
DISCUSSION
We have shown that LFS, which mimics pathological cortically driven bursting activity in STN, promoted potentiation of glutamatergic (cortico-subthalamic) synaptic inputs but only in dopamine-intact tissue. Similarly, HFS promotes synaptic depression but only in dopamine-depleted tissue, indicating that the beneficial effects of DBS may be mediated, in part, by depression of cortico-subthalamic synapses (Fig. 5). Taken together, these results suggest that the synaptic weight of the cortico-subthalamic pathway is dynamically modulated by ambient concentrations of dopamine, such that it is reduced in dopamine-intact animals and increased in the dopamine-depleted Parkinsonian state. We propose that the increase in the relative weight of cortico-subthalamic synapses in the Parkinsonian animal is one way by which the STN becomes sensitized to oscillatory cortical inputs, resulting in increased pathological bursting activity in STN that is coherent with cortical activity (Magill et al., 2001; Sharott et al., 2005; Mallet et al., 2008a,b).
Fig. 5. Summary scheme of frequency selectivity and dopamine dependence of plasticity at cortico-subthalamic synapses.

This figure illustrates the potential increase in synaptic weight of cortico-subthalamic input promoted by the depletion of dopamine, the definitive pathology in Parkinson’s disease. High-frequency stimulation (HFS) of glutamatergic inputs to STN, which mimics deep brain stimulation (DBS), has no effect in dopamine-intact slices but elicits synaptic depression in dopamine-depleted slices. In contrast, low-frequency stimulation (LFS), which mimics pathological bursting, has no effect in dopamine-depleted but elicits synaptic potentiation in the dopamine-intact slices.
Experimental considerations
The STN contains a relatively homogenous population of glutamatergic projection neurons (Nakanishi et al., 1987; Beurrier et al., 1999; Bevan et al., 2002; Loucif et al., 2008). It receives extensive, topographically organized inputs from ipsilateral frontal neocortex, including motor and premotor areas (Smith et al., 1998; Nambu et al., 2000). Afferent inputs arising in these cortical areas have a powerful synchronizing effect on ensembles of STN neurons (Magill et al., 2004), and provide a good substrate for the entrainment of STN firing to ongoing cortical oscillations (Magill et al., 2000, 2001; Mallet et al., 2008a,b).
Unfortunately, the synaptic weight of cortico-subthalamic inputs cannot be directly tested by simply measuring basal synaptic transmission in control and dopamine-depleted slices. This would require that the cortex is the only source of glutamatergic input to STN. This is not the case as glutamatergic neurons of the intralaminar thalamus and mesopontine tegmentum also innervate the STN (Bevan et al., 1995; Bevan and Bolam, 1995). One would also have to assume that these inputs are unaffected by dopamine-depletion. However, given that the cortex provides the majority of the glutamatergic inputs to STN that course through the internal capsule (Kitai and Deniau, 1981; Nambu et al., 2000), we assume that our electrical stimuli evoked glutamate release from cortically derived axon terminals. We have only assessed spontaneous EPSCs because assessment of miniature EPSCs in the presence of tetrodotoxin, the usual strategy for determination of presynaptic versus postsynaptic effects, is not compatible with HFS and LFS. We acknowledge that data relating to changes in sEPSC frequency alone cannot be used to definitely determine locus of action. However, changes in sEPSC frequency, without changes in holding potential or input resistance, combined with paired pulse and CV data provides ample evidence for a presynaptic component of plasticity.
STN neurons recorded in vivo from 6-OHDA-lesioned animals display a 2–3-fold increase in the magnitude of their bursting activity in time with the cortex as compared with STN neurons in dopamine-intact controls (Magill et al., 2001). This abnormal bursting during slow-wave sleep may also lead to the reinforcement of synaptic weights, particularly along the cortico-subthalamic pathway. The LFS protocol utilized in this study mimics this pathological, slow busting activity, whereas HFS at 100 Hz mimics DBS. We acknowledge that longer duration HFS may produce more robust plastic changes, and that this may be viewed more analogous to DBS. However, as short duration stimulations were sufficient to induce plasticity, and longer duration stimulations compromise cell viability, the stimulation protocols employed here allow extended good-quality recordings and tests of plasticity, while relating to the protocols used clinically. Indeed, both LFS and HFS employed here are similar to those used in many other synaptic plasticity studies both in vitro and in vivo.
Mechanism of DBS
As the beneficial effects of DBS targeted to the STN were similar to STN lesions, the mechanism originally proposed for DBS efficacy involved functional inhibition of the STN, including through depolarization block and/or functional deafferentation (Benazzouz et al., 1993; Benazzouz and Hallett, 2000). More recently, multiple effects of DBS have been described including local changes in neuronal excitability, enhanced GABA and glutamate release, axonal activation and axonal block (see Deniau et al., 2010 for review) as well as stimulus induced modulation of pathological activity (Hammond et al., 2008).
The fact that lesions of the STN have beneficial effects in animal models of PD, and that the behavioral effects of HFS were similar to those of lesions, led to the STN being the preferred site of stimulation (Benazzouz et al., 1993; Krack et al., 2003). However, this does not necessarily establish a leading role for the STN in generating all pathological activity in Parkinsonism. Indeed there is an argument that the STN is an efficacious site of stimulation because of its small volume and nodal point within BG circuitry. Recent evidence has implicated the cortex not only in the generation of pathological activity but also as a potential target both for pharmacological and surgical interventions in modulating such activity. Thus, HFS of cortex appears to normalize firing rates in the Parkinsonian STN and reduce akinesia and bradykinesia (Drouot et al., 2004; Pagni et al., 2005). Furthermore, repetitive transcranial magnetic stimulation improves motor performance in PD (Siebner et al., 2000; Khedr et al., 2003; Lefaucheur et al., 2004; Fregni et al., 2006). These findings are supplemented by reports that DBS of the STN promotes antidromic stimulation of cortical networks implicated in the generation of pathological oscillations in PD (Li et al., 2007; Lehmkuhle et al., 2009), with the magnitude of antidromic activation correlating with the suppression of pathological beta activity. In addition, any antidromic (and thence, orthodromic) activation of neuronal networks within the cortex would directly modulate and perhaps negate the oscillatory activity being transferred from cortex to STN. However, one of the most compelling pieces of evidence implicating the cortico-subthalamic pathway in the beneficial effects of HFS is a recent optogenetic study that allowed the precise role of different nuclei and axonal projections to be determined in behaving Parkinsonian rodents. Thus, the therapeutic effects of DBS were only accounted for by direct stimulation of afferents projecting to the STN, and specifically, those arising from layer V cortical neurons, again implicating the cortico-subthalamic pathway (Gradinaru et al., 2009).
The frequency- and dopamine-dependency of changes in the plasticity profile of the cortico-subthalamic pathway presented here add further to the weight of evidence that this pathway plays a central role in PD pathology. Low-frequency stimulation potentiated glutamatergic (cortico-subthalamic) synaptic inputs in dopamine-intact tissue only, and HFS promoted synaptic depression in dopamine-depleted tissue only, suggesting that the synaptic weight of the cortico-subthalamic pathway is dynamically modulated by dopamine. Furthermore, these results further promote the view that the beneficial effects of DBS are at least in part owing to stimulation of the cortico-subthalamic pathway.
Synaptic plasticity
Although motor symptoms of PD reappear within seconds/minutes after DBS ceases (Temeperli et al., 2003), several pieces of evidence indicate a mechanistic role for synaptic plasticity in the therapeutic effects of DBS. The maximum benefits on tremor and rigidity are reached with a delay of minutes after the switching on of DBS, whereas the delay in reaching maximum benefit for akinesia may be minutes to hours (Krack et al., 2002). Furthermore, it is often necessary to adjust and refine stimulus parameters over the lifetime of DBS therapy (Krack et al., 2002), indicating some slowly evolving synaptic or other change associated with disease progression. Moreover, it has long been recognized that the stimulation frequency is the key to determining clinical efficacy in Parkinsonism. When DBS is directed to the STN, clinical benefits become evident at stimulation frequencies above 50 Hz, whereas stimulation at lower frequencies (5–20 Hz) exacerbates the symptoms of rigidity and bradykinesia (Moro et al., 2002; Fogelson et al., 2005; Eusebio et al., 2008).
With regard to movement disorders, plasticity in the cortico-striatal pathway has been studied extensively (Calabresi et al., 1992, 1997, 2000; see Kreitzer and Malenka, 2008 for review). In response to high-frequency stimulation (HFS—trains of pulses at 100 Hz), the glutamatergic cortico-striatal synapses undergo either long-term potentiation (LTP) or long-term depression (LTD), with dopaminergic signaling necessary for the induction of such plasticity (Calabresi et al., 1997; Kreitzer and Malenka, 2005). However, Shen et al. (2008) reported that dopamine is not necessarily required for the induction of synaptic plasticity, but it is critical for the bidirectional, spike timing-dependent plasticity. Dopamine, acting in concert with other neurotransmitters and neuromodulators, such as glutamate, adenosine, and cannabinoids, displays concentration-dependent actions at D1 and D2 receptors that determine the direction of plasticity. Our data show that this is also the case with the cortico-subthalamic pathway, where chronic depletion of dopamine does not eliminate plasticity, but rather, determines the plasticity profile in response to different stimulation protocols. As acute administration of dopamine antagonists does not replicate the chronic changes in oscillatory dynamics associated with dopamine deletion, (Mallet et al., 2008b) and the presence (or absence) of dopamine may only play a role in the induction of plasticity, we have not attempted to reverse the synaptic depression associated with HFS by acute administration of the dopamine agonist apomorphine.
With the exception of one published report (Shen et al., 2003), stimulus-induced long-term adaptations in the synaptic weight of glutamatergic inputs to the STN have not been studied. However, Shen et al. (2003) showed that HFS (100 Hz for 1 min) of inputs to STN induces three forms of synaptic plasticity at glutamatergic synapses, including short-term potentiation (STP), LTP, and LTD. The STP and the LTD have paired-pulse characteristics that are consistent with changes in presynaptic action, whereas the characteristics of the LTP are more consistent with a postsynaptic action. Although we do not rule out a postsynaptic locus for some forms of synaptic plasticity in STN, the potentiation and depression of the cortico-subthalamic pathway induced by LFS and HFS, respectively, that we define here have presynaptic components that relate to changes in the probability of release or the number of release sites. Furthermore, synaptic depression induced by HFS only occurs in the dopamine-depleted state. Consistent with these findings, EPSCs in STN in dopamine-depleted animals are amplified and followed by bursts of polysynaptic EPSCs, both of which are attenuated by HFS (Ammari et al., 2011).
Effect on pathological oscillations
Synchronized beta-frequency oscillations in the cortex and BG structures (including STN) are considered pathological because not only do they correlate with rigidity and brady-kinesia but also because both beta activity and symptoms are simultaneously suppressed by dopamine replacement (Priori et al., 2004; Kühn et al., 2006, 2009) or DBS (Gaynor et al., 2008; Eusebio et al., 2011). This excessive synchronization of activity is accompanied by increased coherence across cortico-basal ganglia networks and is thought to limit computational capacity through reduced information coding capacity (see Hammond et al., 2007).
In this study, we show that, in dopamine-intact tissue, LFS increases the weight of glutamatergic synaptic inputs and that, in the dopamine-depleted state, the beneficial effects of HFS are consistent with synaptic depression. As HFS reduces glutamate input and DBS reduces beta oscillations and motor symptoms, then beta activity and other pathological changes might be supported by cortico-subthalamic input that is abnormally strengthened after dopamine loss. Further support for this idea can be found in a recent computational modeling study (Moran et al., 2011). The increase in the relative weight of cortico-subthalamic synapses is therefore one way by which the STN becomes sensitized to oscillatory cortical inputs, resulting in increased pathological bursting activity that is coherent with cortical activity. As the cortex appears to play a critical role in manifestation of pathological oscillations, it provides an alternative, less invasive site of stimulation, which still requires optimum stimulation paradigms to be determined for best symptomatic relief.
Acknowledgments
This work is supported by Parkinson’s UK grant G-071 and The Medical Research Council, UK.
Abbreviations
- BG
basal ganglia
- DBS
deep brain stimulation
- eEPSC
evoked excitatory postsynaptic current
- EPSC
excitatory post synaptic current
- GP
globus pallidus
- HFS
high-frequency stimulation
- IEI
inter-event interval
- LFS
low-frequency stimulation
- PD
Parkinson’s disease
- PPR
paired pulse ratio
- STN
subthalamic nucleus
- STP
short-term potentiation
- 6-OHDA
6-hydroxydopamine
REFERENCES
- Ammari R, Bioulac B, Garcia L, Hammond C. The subthalamic nucleus becomes a generator of bursts in the dopamine-depleted state. Its high frequency stimulation dramatically weakens transmission to the globus pallidus. Front Syst Neurosci. 2011;5:43. doi: 10.3389/fnsys.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers JM, Stevens CF. Presynaptic mechanism for long-term potentiation in the hippocampus. Nature. 1990;346:724–729. doi: 10.1038/346724a0. [DOI] [PubMed] [Google Scholar]
- Benazzouz A, Gross C, Féger J, Boraud T, Bioulac B. Reversal of rigidity and improvement in motor performance by subthalamic high-frequency stimulation in MPTP-treated monkeys. Eur J Neurosci. 1993;5(4):382–389. doi: 10.1111/j.1460-9568.1993.tb00505.x. [DOI] [PubMed] [Google Scholar]
- Benazzouz A, Hallett M. Mechanism of action of deep brain stimulation. Neurology. 2000;55(12 Suppl 6):S13–S16. [PubMed] [Google Scholar]
- Bergman H, Wichmann T, Karmon B, Delong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Francis CM, Bolam JP. The glutamate-enriched cortical and thalamic input to neurons in the subthalamic nucleus of the rat: convergence with GABA-positive terminals. J Comp Neurol. 1995;361(3):491–511. doi: 10.1002/cne.903610312. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Bolam JP. Cholinergic, GABAergic, and glutamate-enriched inputs from the mesopontine tegmentum to the subthalamic nucleus in the rat. J Neurosci. 1995;15(11):7105–7120. doi: 10.1523/JNEUROSCI.15-11-07105.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MD, Magill PJ, Hallworth NE, Bolam JP, Wilson CJ. Regulation of the timing and pattern of action potential generation in rat subthalamic neurones in vitro by GABA-A IPSPs. J Neurophysiol. 2002;87:1348–1362. doi: 10.1152/jn.00582.2001. [DOI] [PubMed] [Google Scholar]
- Beurrier C, Congar P, Bioulac B, Hammond C. Subthalamic nucleus neurones switch from single-spike activity to burst-firing mode. J Neurosci. 1999;19(2):599–609. doi: 10.1523/JNEUROSCI.19-02-00599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Di Lazzaro V. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson’s disease. J Neurosci. 2001;21:1033–1038. doi: 10.1523/JNEUROSCI.21-03-01033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Maj R, Pisani A, Mercuri NB, Bernardi G. Long-term synaptic depression in the striatum: physiological and pharmacological characterization. J Neurosci. 1992;12(11):4224–4233. doi: 10.1523/JNEUROSCI.12-11-04224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Saiardi A, Pisani A, Baik JH, Centonze D, Mercuri NB, Bernardi G, Borrelli E. Abnormal synaptic plasticity in the striatum of mice lacking dopamine D2 receptors. J Neurosci. 1997;17(12):4536–4544. doi: 10.1523/JNEUROSCI.17-12-04536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Marfia GA, Pisani A, Sancesario G, Bernardi G. Synaptic transmission in the striatum: from plasticity to neurodegeneration. Prog Neurobiol. 2000;61(3):231–265. doi: 10.1016/s0301-0082(99)00030-1. [DOI] [PubMed] [Google Scholar]
- Cragg SJ, Baufreton J, Xue Y, Bolam JP, Bevan MD. Synaptic release of dopamine in the subthalamic nucleus. Eur J Neurosci. 2004;20(7):1788–1802. doi: 10.1111/j.1460-9568.2004.03629.x. [DOI] [PubMed] [Google Scholar]
- Deniau JM, Degos B, Bosch C, Maurice N. Deep brain stimulation mechanisms: beyond the concept of local functional inhibition. Eur J Neurosci. 2010;32(7):1080–1091. doi: 10.1111/j.1460-9568.2010.07413.x. [DOI] [PubMed] [Google Scholar]
- Drouot X, Oshino S, Jarraya B, Besret L, Kishima H, Remy P, Dauguet J, Lefaucheur JP, Dolle F, Conde F, Bottlaender M, Peschanski M, Keravel Y, Hantraye P, Palfi S. Functional recovery in a primate model of Parkinson’s disease following motor cortex stimulation. Neuron. 2004;5:769–778. doi: 10.1016/j.neuron.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Eusebio A, Chen CC, Lu CS, Lee ST, Tsai CH, Limousin P, Hariz M, Brown P. Effects of low-frequency stimulation of the subthalamic nucleus on movement in Parkinson’s disease. Exp Neurol. 2008;209(1):125–130. doi: 10.1016/j.expneurol.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusebio A, Thevathasan W, Doyle Gaynor L, Pogosyan A, Bye E, Foltynie T, Zrinzo L, Ashkan K, Aziz T, Brown P. Deep brain stimulation can suppress pathological synchronisation in parkinsonian patients. J Neurol Neurosurg Psychiatry. 2011;82(5):569–573. doi: 10.1136/jnnp.2010.217489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filion M, Tremblay L. Abnormal spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res. 1991;547:142–151. [PubMed] [Google Scholar]
- Fogelson N, Pogosyan A, Kühn AA, Kupsch A, van Bruggen G, Speelman H, Tijssen M, Quartarone A, Insola A, Mazzone P, Di Lazzaro V, Limousin P, Brown P. Reciprocal interactions between oscillatory activities of different frequencies in the subthalamic region of patients with Parkinson’s disease. Eur J Neurosci. 2005;22(1):257–266. doi: 10.1111/j.1460-9568.2005.04179.x. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Santos MC, Lima M, Vieira AL, Rigonatti SP, Silva MT, Barbosa ER, Nitsche MA, Pascual-Leone A. Non-invasive cortical stimulation with transcranial direct current stimulation in Parkinson’s disease. Mov Disord. 2006;21(10):1693–1702. doi: 10.1002/mds.21012. [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Kita H. Response characteristics of subthalamic neurons to the stimulation of the sensorimotor cortex in the rat. Brain Res. 1993;609(1-2):185–192. doi: 10.1016/0006-8993(93)90872-k. [DOI] [PubMed] [Google Scholar]
- Gaynor LM, Kühn AA, Dileone M, Litvak V, Eusebio A, Pogosyan A, Androulidakis AG, Tisch S, Limousin P, Insola A, Mazzone P, Di Lazzaro V, Brown P. Suppression of beta oscillations in the subthalamic nucleus following cortical stimulation in humans. Eur J Neurosci. 2008;28(8):1686–1695. doi: 10.1111/j.1460-9568.2008.06363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324(5925):354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trends Neurosci. 2007;30:357–364. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Hammond C, Ammari R, Bioulac B, Garcia L. Latest view on the mechanism of action of deep brain stimulation. Mov Disord. 2008;23(15):2111–2121. doi: 10.1002/mds.22120. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Farweez HM, Islam H. Therapeutic effect of repetitive transcranial magnetic stimulation on motor function in Parkinson’s disease patients. Eur J Neurol. 2003;10(5):567–572. doi: 10.1046/j.1468-1331.2003.00649.x. [DOI] [PubMed] [Google Scholar]
- Kim J, Alger BE. Random response fluctuations lead to spurious paired-pulse facilitation. J Neurosci. 2001;21:9608–9619. doi: 10.1523/JNEUROSCI.21-24-09608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitai ST, Deniau JM. Cortical inputs to the subthalamus: intracellular analysis. Brain Res. 1981;214(2):411–415. doi: 10.1016/0006-8993(81)91204-x. [DOI] [PubMed] [Google Scholar]
- Krack P, Fraix V, Mendes A, Benabid AL, Pollak P. Post-operative management of subthalamic nucleus stimulation for Parkinson’s disease. Mov Disord. 2002;17(Suppl 3):S188–S197. doi: 10.1002/mds.10163. [DOI] [PubMed] [Google Scholar]
- Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, Koudsie A, Limousin PD, Benazzouz A, LeBas JF, Benabid AL, Pollak P. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 2003;349(20):1925–1934. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J Neurosci. 2005;25(45):10537–10545. doi: 10.1523/JNEUROSCI.2959-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60(4):543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn AA, Kupsch A, Schneider GH, Brown P. Reduction in subthalamic 8–35 Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease. Eur J Neurosci. 2006;23(7):1956–1960. doi: 10.1111/j.1460-9568.2006.04717.x. [DOI] [PubMed] [Google Scholar]
- Kühn AA, Tsui A, Aziz T, Ray N, Brücke C, Kupsch A, Schneider GH, Brown P. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson’s disease relates to both bradykinesia and rigidity. Exp Neurol. 2009;215(2):380–387. doi: 10.1016/j.expneurol.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Drouot X, Von Raison F, Ménard-Lefaucheur I, Cesaro P, Nguyen JP. Improvement of motor performance and modulation of cortical excitability by repetitive transcranial magnetic stimulation of the motor cortex in Parkinson’s disease. Clin Neurophysiol. 2004;115(11):2530–2541. doi: 10.1016/j.clinph.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Lehmkuhle MJ, Bhangoo SS, Kipke DR. The electrocorticogram signal can be modulated with deep brain stimulation of the subthalamic nucleus in the hemiparkinsonian rat. J Neurophysiol. 2009;102(3):1811–1820. doi: 10.1152/jn.90844.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Hutchison WD, Lozano AM, Dostrovsky JO. High-frequency synchronization of neuronal activity in the subthalamic nucleus of parkinsonian patients with limb tremor. J Neurosci. 2000;20:7766–7775. doi: 10.1523/JNEUROSCI.20-20-07766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Arbuthnott GW, Jutras MJ, Goldberg JA, Jaeger DJ. Resonant antidromic cortical circuit activation as a consequence of high-frequency subthalamic deep-brain stimulation. J Neurophysiol. 2007;98(6):3525–3537. doi: 10.1152/jn.00808.2007. [DOI] [PubMed] [Google Scholar]
- Litvak V, Jha A, Eusebio A, Oostenveld R, Foltynie T, Limousin P, Zrinzo L, Hariz MI, Friston K, Brown P. Resting oscillatory cortico-subthalamic connectivity in patients with Parkinson’s disease. Brain. 2011;134:359–374. doi: 10.1093/brain/awq332. [DOI] [PubMed] [Google Scholar]
- Loucif KC, Wilson CL, Baig R, Lacey MG, Stanford IM. Functional interconnectivity between the globus pallidus and the subthalamic nucleus in the mouse brain slice. J Physiol. 2005;567:977–987. doi: 10.1113/jphysiol.2005.093807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loucif AJ, Woodhall GL, Sehirli US, Stanford IM. Depolarisation and suppression of burst firing in the mouse subthalamic nucleus by dopamine D1/D5 receptor activation of a cyclic nucleotide gated non-specific cation conductance. Neuropharmacology. 2008;55:94–105. doi: 10.1016/j.neuropharm.2008.04.025. [DOI] [PubMed] [Google Scholar]
- Magill PJ, Bolam JP, Bevan MD. Relationship of activity in the subthalamic nucleus-globus pallidus network to cortical electroencephalogram. J Neurosci. 2000;20:820–833. doi: 10.1523/JNEUROSCI.20-02-00820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magill PJ, Bolam JP, Bevan MD. Dopamine regulates the impact of the cerebral cortex on the subthalamic nucleus-globus pallidus network. Neuroscience. 2001;106:313–330. doi: 10.1016/s0306-4522(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Magill PJ, Sharott A, Bevan MD, Brown P, Bolam JP. Synchronous unit activity and local field potentials evoked in the subthalamic nucleus by cortical stimulation. J Neurophysiol. 2004;92(2):700–714. doi: 10.1152/jn.00134.2004. [DOI] [PubMed] [Google Scholar]
- Mallet N, Pogosyan A, Márton LF, Bolam JP, Brown P, Magill PJ. Parkinsonian beta oscillations in the external globus pallidus and their relationship with subthalamic nucleus activity. J Neurosci. 2008a;28:14245–14258. doi: 10.1523/JNEUROSCI.4199-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Pogosyan A, Sharott A, Csicsvari J, Bolam JP, Brown P, Magill PJ. Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J Neurosci. 2008b;28:4795–4806. doi: 10.1523/JNEUROSCI.0123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice N, Deniau JM, Glowinski J, Thierry AM. Relationships between the prefrontal cortex and the basal ganglia in the rat: physiology of the corticosubthalamic circuits. J Neurosci. 1998;18:9539–9546. doi: 10.1523/JNEUROSCI.18-22-09539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran RJ, Mallet N, Litvak V, Dolan RJ, Magill PJ, Friston KJ, Brown P. Alterations in brain connectivity underlying beta oscillations in Parkinsonism. PLoS Comput Biol. 2011;7:e1002124. doi: 10.1371/journal.pcbi.1002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro E, Esselink RJ, Xie J, Hommel M, Benabid AL, Pollak P. The impact on Parkinson’s disease of electrical parameter settings in STN stimulation. Neurology. 2002;59(5):706–713. doi: 10.1212/wnl.59.5.706. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Kita H, Kitai ST. Electrical membrane properties of rat subthalamic neurones in an in vitro slice preparation. Brain Res. 1987;437:35–44. doi: 10.1016/0006-8993(87)91524-1. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Hamada I, Kita H, Imanishi M, Akazawa T, Ikeuchi Y, Hasegawa N. Excitatory cortical inputs to pallidal neurons via the subthalamic nucleus in the monkey. J Neurophysiol. 2000;84(1):289–300. doi: 10.1152/jn.2000.84.1.289. [DOI] [PubMed] [Google Scholar]
- Nini A, Feingold A, Slovin H, Bergman H. Neurons in the globus pallidus do not show correlated activity in the normal monkey, but phase-locked oscillations appear in the MPTP model of parkinsonism. J Neurophysiol. 1995;74(4):1800–1805. doi: 10.1152/jn.1995.74.4.1800. [DOI] [PubMed] [Google Scholar]
- Olsson M, Nikkhah G, Bentlage C, Björklund A. Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci. 1995;15(5):3863–3875. doi: 10.1523/JNEUROSCI.15-05-03863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagni CA, Zeme S, Zenga F. Further experience with extradural motor cortex stimulation for the treatment of advanced Parkinson’s disease: report of 3 new cases. J Neurosurg Sci. 2003;47:189–193. [PubMed] [Google Scholar]
- Pagni CA, Zeme S, Zenga F, Maina R. Extradural motor cortex stimulation in advanced Parkinson’s disease: the Turin experience: technical case report. Neurosurgery. 2005;57(4):E402. doi: 10.1227/01.neu.0000176857.66627.01. [DOI] [PubMed] [Google Scholar]
- Pan HS, Walters JR. Unilateral lesion of the nigrostriatal pathway decreases the firing rate and alters the firing pattern of globus pallidus neurons in the rat. Synapse. 1988;2(6):650–656. doi: 10.1002/syn.890020612. [DOI] [PubMed] [Google Scholar]
- Plenz D, Kital ST. A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus. Nature. 1999;400:677–682. doi: 10.1038/23281. [DOI] [PubMed] [Google Scholar]
- Priori A, Foffani G, Pesenti A, Tamma F, Bianchi AM, Pellegrini M, Locatelli M, Moxon KA, Villani RM. Rhythm-specific pharmacological modulation of subthalamic activity in Parkinson’s disease. Exp Neurol. 2004;189(2):369–379. doi: 10.1016/j.expneurol.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Schwarting RK, Huston JP. Behavioral and neurochemical dynamics of neurotoxic meso-striatal dopamine lesions. Neurotoxicology. 1997;18(3):689–708. [PubMed] [Google Scholar]
- Sharott A, Magill PJ, Harnack D, Kupsch A, Meissner W, Brown P. Dopamine depletion increases the power and coherence of beta-oscillations in the cerebral cortex and subthalamic nucleus of the awake rat. Eur J Neurosci. 2005;21(5):413–422. doi: 10.1111/j.1460-9568.2005.03973.x. [DOI] [PubMed] [Google Scholar]
- Shen KZ, Johnson SW. Presynaptic dopamine D2 and muscarine M3 receptors inhibit excitatory and inhibitory transmission to rat subthalamic neurones in vitro. J Physiol. 2000;525(2):331–341. doi: 10.1111/j.1469-7793.2000.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen KZ, Zhu ZT, Munhall A, Johnson SW. Synaptic plasticity in rat subthalamic nucleus induced by high-frequency stimulation. Synapse. 2003;50(4):314–319. doi: 10.1002/syn.10274. [DOI] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321(5890):848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner HR, Rossmeier C, Mentschel C, Peinemann A, Conrad B. Short-term motor improvement after sub-threshold 5-Hz repetitive transcranial magnetic stimulation of the primary motor hand area in Parkinson’s disease. J Neurol Sci. 2000;178(2):91–94. doi: 10.1016/s0022-510x(00)00370-1. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86(2):353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Temeperli P, Ghika J, Villemure JG, Burkhard PR, Bogousslavsky J, Vingerhoets FJ. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology. 2003;60:78–81. doi: 10.1212/wnl.60.1.78. [DOI] [PubMed] [Google Scholar]
- Thomson AM. Molecular frequency filters at central synapses. Prog Neurobiol. 2000;62:159–196. doi: 10.1016/s0301-0082(00)00008-3. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Kargieman L, Gacio S, Riquelme LA, Murer MG. Consequences of partial and severe dopaminergic lesion on basal ganglia oscillatory activity and akinesia. Eur J Neurosci. 2005;22:2579–2586. doi: 10.1111/j.1460-9568.2005.04456.x. [DOI] [PubMed] [Google Scholar]
- Vila M, Périer C, Féger J, Yelnik J, Faucheux B, Ruberg M, Raisman-Vozari R, Agid Y, Hirsch EC. Evolution of changes in neuronal activity in the subthalamic nucleus of rats with unilateral lesion of the substantia nigra assessed by metabolic and electrophysiological measurements. Eur J Neurosci. 2000;12:337–344. doi: 10.1046/j.1460-9568.2000.00901.x. [DOI] [PubMed] [Google Scholar]