Abstract
Disrupted regulation of extracellular glutamate in the central nervous system contributes to and can exacerbate the acute pathophysiology of traumatic brain injury (TBI). Previously, we reported increased extracellular glutamate in the striatum of anesthetized rats 2 days after diffuse brain injury. To determine the mechanism(s) responsible for increased extracellular glutamate, we used enzyme-based microelectrode arrays (MEAs) coupled with specific pharmacological agents targeted at in vivo neuronal and glial regulation of extracellular glutamate. After TBI, extracellular glutamate was significantly increased in the striatum by (∼90%) averaging 4.1±0.6 μM compared with sham 2.2±0.4 μM. Calcium-dependent neuronal glutamate release, investigated by local application of an N-type calcium channel blocker, was no longer a significant source of extracellular glutamate after TBI, compared with sham. In brain-injured animals, inhibition of glutamate uptake with local application of an excitatory amino acid transporter inhibitor produced significantly greater increase in glutamate spillover (∼ 65%) from the synapses compared with sham. Furthermore, glutamate clearance measured by locally applying glutamate into the extracellular space revealed significant reductions in glutamate clearance parameters in brain-injured animals compared with sham. Taken together, these data indicate that disruptions in calcium-mediated glutamate release and glial regulation of extracellular glutamate contribute to increased extracellular glutamate in the striatum 2 days after diffuse brain injury. Overall, these data suggest that therapeutic strategies used to regulate glutamate release and uptake may improve excitatory circuit function and, possibly, outcomes following TBI.
Key words: LY 379268, dl-threo-β-benzyloxyaspartate, ω-conotoxin, (S)-4-carboxyphenylglycine
Introduction
The central nervous system requires strict regulation of extracellular glutamate to guard against aberrant neuronal signaling and possible excitotoxicity. Disrupted glutamate regulation plays a pivotal role in the acute pathophysiology of traumatic brain injury (TBI) through the initiation of secondary injury cascades (Bullock et al., 1998; McIntosh et al., 1996). The primary physical and mechanical forces of a TBI disrupt the blood–brain barrier and cellular membranes, leading to a release of intracellular contents into the extracellular space (Bullock et al., 1998; Schmidt and Grady, 1993). Furthermore, disruptions in ionic regulation after TBI create a massive depolarization, or wave of depolarizations, which promotes excessive neuronal glutamate release (Katayama et al., 1990). Moreover, post-traumatic reductions in the expression of excitatory amino acid transporters (EAATs) responsible for removing glutamate from the extracellular space leads to further increases in extracellular glutamate levels (Rao et al., 1998; van Landeghem et al., 2001, 2006; Yi and Hazell, 2006; Yi et al., 2005). High extracellular glutamate propagates secondary injury cascades through the activation of glutamate receptors, increasing ionic flux, disrupting ionic homeostasis, activating calcium-dependent proteases and phospholipases, uncoupling ATP synthesis, and producing reactive oxygen species (Faden et al., 1989; Hall et al., 1994; McIntosh et al., 1996; Yi and Hazell, 2006).
Glutamate is normally released into the extracellular space by both neuronal and non-neuronal sources (Jabaudon et al., 1999; Jensen et al., 2000). Once in the extracellular space, glutamate binds to both ionotropic glutamate receptors and metabotropic glutamate receptors (mGluRs) for signal propagation. For the removal of glutamate from the extracellular space, five high affinity sodium-dependent EAATs – GLAST, GLT-1, EAAC, EAAT4, and EAAT5 – are expressed throughout the central nervous system (Danbolt, 2001). Glutamate uptake is driven by electrochemical gradients across neurons and glia with the majority (∼90%) of glutamate uptake being performed by two glial transporters in the striatum, GLAST and GLT-1 (Danbolt, 2001; Rothstein et al., 1996; Tanaka et al., 1997).
Clinically, a multitude of studies have detected significant increases in extracellular glutamate levels in the days following injury, with some reports correlating increases in extracellular glutamate with unfavorable outcomes (Bullock et al., 1995, 1998; Chamoun et al., 2010; Hlatky et al., 2004; Hutchinson et al., 2002; Koura et al., 1998; Persson and Hillered 1992; Reinert et al., 2000; Timofeev et al., 2011; Vespa et al., 1998; Yamamoto et al., 1999). These studies emphasize the significance of glutamate dysregulation in the acute pathophysiology and consequences of TBI. Unlike the clinical studies, multiple animals studies report increases in extracellular glutamate at the time of the injury, which returns to baseline concentrations within a few hours (Faden et al., 1989; Globus et al., 1995; Katoh et al., 1997; Katayama et al., 1990; Matsushita et al., 2000; Nilsson et al., 1990; Palmer et al., 1993; Rose et al., 2002; Stoffel et al., 1997; 2002). Because experimental TBI has failed to model the glutamate dysregulation seen in the clinic, the role of glutamate excitotoxicity in the acute pathophysiology of TBI has been challenged and remains unclear (Carbonell and Grady, 1999; Obrenovitch 1999).
Using novel enzyme-based microelectrode arrays (MEAs) to measure extracellular glutamate, we previously reported significant increases in extracellular glutamate and evoked glutamate release in the striatum 2 days after midline fluid percussion brain injury (Hinzman et al., 2010). The purpose of this study was to examine the regulation of extracellular glutamate by both neurons and glia to identify specific mechanism(s) responsible for the increased extracellular glutamate in the rat striatum 2 days after diffuse brain injury. Using targeted pharmacological agents, we examined how both neurons and glia release and regulate extracellular glutamate. First, we examined the contribution of calcium-mediated neuronal glutamate release to extracellular glutamate by locally applying an N-type calcium channel blocker (ω-conotoxin). Second, we examined if transient activation of the group II mGluRs (mGluR2/3) would improve neuronal regulation of extracellular glutamate by locally applying an mGluR2/3 agonist (LY 379268). Third, we examined non-neuronal sources of extracellular glutamate such as the glial expressed cystine/glutamate exchanger (xc-) by locally applying an xc- blocker ([S]-4-carboxyphenylglycine [CPG]). Fourth, we examined how glia regulate extracellular glutamate by inhibiting glutamate uptake with a competitive non-transportable EAATs blocker (dl-threo-β-benzyloxyaspartate [TBOA]). Finally, we directly tested the ability of glia to clear glutamate from the extracellular space.
Methods
Animals
Male Sprague–Dawley rats (300–400g; Harlan Laboratories Inc., Indianapolis, IN) were used for all experiments (n=45) The animals were housed in a 12 h light/dark cycle with food and water available ad libitum according to the Association for Assessment and Accreditation of Laboratory Animal Care International. Animals were acclimated to their environment for at least 1 week before any experiments. After surgery, animals were evaluated daily for postoperative care by a physical examination of the rat, screening the animals' breathing, gait, coat appearance, dehydration, body appearance, locomotion, and food and water intake. Animal care was approved by the University of Kentucky Institutional Animal Care and Use Committee.
Midline fluid percussion injury (FPI)
Animals were subjected to midline FPI as previously described (Hinzman et al., 2010; Lifshitz, 2008; Thomas et al., 2011). Rats were initially anesthetized with isoflurane at 5% for 5 min and placed in a stereotaxic frame in a sterile surgery hood with continuously delivered isoflurane at 2%. The head was shaved and a 70% alcohol and betadine solution was applied to the skin. Eyes were treated with artificial tears and body temperature was maintained with a delta-phase heating pad at ∼ 37°C (Braintree Scientific, Braintree, MA). A midline incision was made and fascia removed from the skull. A 4.7 mm trephine was used for the craniotomy, centered on the sagittal suture between bregma and lambda without disrupting the underlying dura or superior sagittal sinus. Anchoring screws were secured into pilot holes remote from the craniotomy. An injury hub was constructed from a Luer-Loc hub (20 gauge needle). The outer diameter of the hub was beveled to match the craniotomy. The injury hub was fitted into the craniotomy and cyanoacrylate gel was applied to seal the skull–hub interface. To secure the injury hub to the anchoring screws, methyl-methacrylate (Hygenic Corp., Akron, OH) was applied. The hub was filled with sterile saline and the ends of the incision sutured to prevent the skin from tearing. The female Luer-Loc injury hub was connected to the male Luer-Loc hub of the injury device (Custom Design and Fabrication, Virginia Commonwealth University, Richmond, VA). Before reflexive responses returned, the pendulum hammer of the injury device was dropped onto the fluid-filled piston to induce a moderate injury. Sham-injured animals underwent the same procedure without induction of injury. The fluid pressure pulse for the brain injury was 2.0±0.01 atm. The injury hub was removed and any bleeding controlled with Gelfoam (Pharmacia, Kalamazoo, MI). The incision was closed with staples. Righting reflex recovery times were recorded for the injury group, from the time of impact to the time when animals' spontaneously righted. Brain injured animals had righting times of 437±19 sec (n=23). Sham-injured animals had righting reflex <15 sec (n=22). No animals died from complications caused by the injury.
Enzyme-based MEAs
Ceramic-based MEAs, consisting of four platinum recording sites (15×333 μm) arranged vertically in dual pairs, were prepared and selected for in vivo recordings as previously described (Burmeister et al., 2002; Day et al., 2006; Hascup et al., 2007; Hinzman et al., 2010; Stephens et al., 2011). Briefly, a glutamate-oxidase solution covered one pair of recording sites to allow for the enzymatic conversion of glutamate to α-ketoglutarate and the generation of the reporter molecule, H2O2. The small amount of H2O2 generated by glutamate-oxidase on the surface of the MEA had a minimal impact on the production of reactive oxygen species in the local area because of the small amount of glutamate-oxidase coated on the surface of the MEA, the oxidation of H2O2 by the MEA, and the limited time of the MEA in the tissue. An inactive protein matrix covered the other pair of recording sites (sentinel sites). The two different coatings allowed the use of a self-referencing technique, in which the background current of the sentinel sites could be subtracted from the current of the glutamate-oxidase sites, thereby producing a more selective glutamate measure allowing the determination of resting levels of glutamate (Burmeister and Gerhardt, 2001; Burmeister et al., 2002). Furthermore, a size exclusion layer of 1,3–phenylenediamine (mPD) was electroplated onto the platinum recording sites to restrict the passage of large molecule interferents (ascorbic acid and dopamine) from reaching the platinum recording sites to further improve the selectivity of the MEAs for glutamate detection (Hinzman et al., 2010).
MEA calibration
Constant potential amperometry was performed with the MEAs using the FAST-16 mkII system (Quanteon Inc., LLC, Nicholasville, KY). A potential of +0.7 V versus an Ag/AgCl reference was applied to oxidize the reporter molecule, H2O2. The resulting current was amplified and digitized by the recording system. Calibrations were conducted to test the capability of the MEA to selectively measure glutamate and to generate a standard curve for the conversion of current to concentration of glutamate. The parameters tested were limit of detection (three times the standard deviation of the baseline noise), selectivity (ability of the MEA to measure glutamate compared to a major interferent, ascorbic acid), and slope of the electrode (the linear increase in current caused by additions of glutamate). The average limit of detection was 0.99±0.1 μM, selectivity was 301±57 (glutamate: ascorbic acid), and slope was 8.7±0.6 pA/μM (n=113 glutamate recording sites). Calibrations were conducted before in vivo experimentation to ensure that MEAs were functional before implantation, and prior results have shown similar performance of the MEAs between pre- and post-experimental testing (Hinzman et al., 2010).
MEA/micropipette assembly
For local application of solutions into the rat brain, glass micropipettes (1 mm o.d. 0.58 mm i.d., A-M Systems, Inc., Everett, WA) were pulled (Kopf Instruments, Tujunga, CA), and the tips of the micropipettes were bumped against a piece of glass to create an opening at the tip with an i.d. of 10–15 μm. The micropipettes were placed centrally among all four platinum recording sites and mounted 50–100 μm above the MEA. Micropipettes were filled with 1) 10 μM ω-conotoxin GVIA, N-type calcium channel blocker, (Alomone Labs, Jerusalem, Israel); 2) 100 μM LY 379268, mGluR2/3 receptor agonist, (Tocris, Ellisville, MO); 3) 50 μM (S)-4-CPG, xc- blocker, (Tocris, Ellisville, MO); 4) 100 μM TBOA, EAATs competitive inhibitor, (Tocris, Ellisville, MO); 5) 500 μM glutamate (Sigma-Aldrich, St. Louis, MO); or 6) physiological saline (Day et al., 2006; Hascup et al., 2010). All drugs were dissolved to their final concentrations in physiological saline. Solutions were sterile filtered (0.20 μm) and adjusted to a pH of 7.4. The micropipette was connected to a Picospritzer III (Parker-Hannifin, Cleveland, OH) with settings adjusted to consistently deliver volumes between 12.5 and 350 nL. Pressure was applied from 2 to 30 psi for 0.3–3 sec. Volume displacement was monitored with the use of a stereomicroscope fitted with a reticule to determine and control the volume of solution that was locally applied by pressure ejection. (Friedemann and Gerhardt, 1992)
In vivo anesthetized recordings of extracellular glutamate
Two days after midline FPI, rats were anesthetized with urethane (1.25 g/kg i.p.) (Sigma-Aldrich) and prepared for in vivo electrochemical recordings as previously described (Day et al., 2006; Hascup et al., 2007; Hinzman et al., 2010; Stephens et al., 2009; Thomas et al., 2009, 2011). Briefly, animals were placed in a stereotaxic frame and body temperature was maintained at ∼37°C with a water pad connected to a recirculating water bath. A craniotomy was performed to provide access to the striatum (AP: +1.0 mm, ML: +/- 2.5 mm, DV: −3.5, −4.0, −4.5, −5.0 mm), (Paxinos and Watson, 1998). An Ag/AgCl reference wire was implanted into the lateral parietal cortex. The MEA was lowered into the brain using a microdrive (MO-10, Narishige International, East Meadow, NY). All MEA recordings were performed at a frequency of 2 Hz.
To measure resting extracellular glutamate, the background current from the sentinel site was subtracted from the current of the enzyme site. Then, the resulting current (pA) was converted to glutamate concentration by dividing the current by the slope (pA/μM) obtained during the calibration. To examine the effect of drugs on extracellular glutamate, 100 nL of physiological saline was locally applied to assess potential dilution effects. Then, the solution in the micropipette was exchanged and 100 nL of the drug of interest was locally applied into the striatum using the same application parameters. The maximum increase/decrease in extracellular glutamate and area above/below the curve (to examine the extent of changes in extracellular glutamate with respect to time) were calculated for both local application of saline and the drugs of interest. To examine glutamate clearance, varying volumes of 500 μM glutamate were applied into the extracellular space. Glutamate signals with amplitudes in the 5–50 μM range were analyzed, as this was the physiological range of KCl-evoked glutamate release seen in sham and brain-injured rats (Hinzman et al., 2010). Glutamate clearance was analyzed for the following parameters: 1) amplitude (μM); 2) Trise (sec), the time for the signal to reach the peak amplitude; 3) T80 (sec), the time for the signal to decay by 80% from the peak amplitude; and 4) k-1 (sec −1), the slope of the linear regression of the natural log transformation of the decay over time (Hinzman et al., 2010; Nickell et al., 2007; Thomas et al., 2009).
Quantitative gene expression (rtPCR)
Two days post-injury, sham and brain-injured animals were transcardially perfused with cold saline, the brains rapidly removed from the skull and dissected into 2 mm slices using a chilled rat brain matrix, and a 1 mm diameter biopsy was removed from the dorsal striatum. The mRNA content was stabilized (RNAlater, Qiagen Corp) and stored frozen. Isolated mRNA (RNeasy, Qiagen Corp) was quantified (NanoDrop ND-1000 spectrophotometer), converted to complementary DNA (cDNA; High Capacity cDNA Archive Kit, Applied Biosystems Inc.) and then used as a template for selected commercially available gene expression assays for quantitative real-time PCR (StepONE, Applied Biosystems). The Applied Biosystems TaqMan® Gene Expression Assays (GLT-1: Rn00568080_m1; GLAST: Rn00570130_m1; 18s rRNA: 4352930E) were used for EAAT gene expression. Within each animal, relative gene expression was normalized to the 18s rRNA endogenous control and the expression level in the sham/vehicle group using the 2-ΔΔCT method (Livak and Schmittgen, 2001), which relates gene expression to the PCR cycle number at which the fluorescence signals exceed a threshold above baseline. Relative gene expression was compared between sham and brain-injured animals using a two-tailed t test.
Statistical analyses
Amperometric data were analyzed using custom MATLAB® - based software. For both resting extracellular glutamate and analysis of glutamate clearance, multiple depths in the striatum were averaged per animal, as there were no subregional differences detected. All data were analyzed using a two-tailed t test. Data are presented as mean±SEM, and statistical significance was defined as p<0.05.
Results
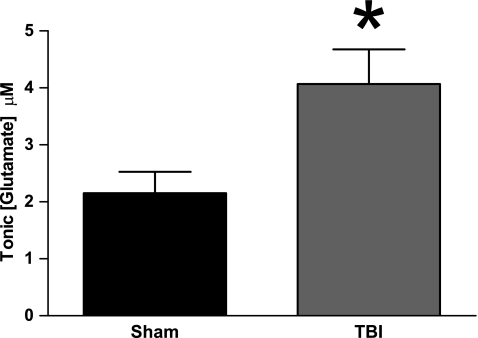
Increased extracellular glutamate in the striatum of brain-injured rats
Previously, our laboratory has reported increased extracellular glutamate in the striatum 2 days after midline fluid percussion injury (Hinzman et al., 2010). Here, we confirm the original finding by detecting significantly higher extracellular glutamate after TBI than in sham (t30=2.681, p=0.012) (Fig. 1). Extracellular glutamate averaged 2.2±0.4 μM (sham) versus 4.1±0.6 μM (TBI). As we detected significant increases in extracellular glutamate after TBI, we set out to pharmacologically manipulate the regulation of glutamate by both neurons and glia to determine the possible mechanism(s) responsible for the increased extracellular glutamate after TBI.
FIG. 1.
Increased extracellular glutamate in the striatum of brain-injured rats 2 days after midline fluid percussion brain injury, (n=16, *p<0.05).
Reduced calcium channel-dependent glutamate release in the striatum of brain-injured rats
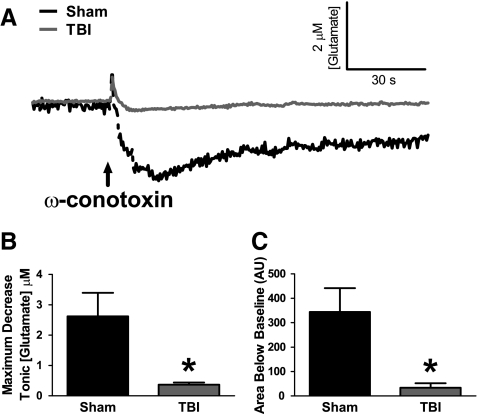
By applying an N-type calcium channel blocker (ω-conotoxin) into the extracellular space, we examined the contribution of calcium-mediated vesicular glutamate release on extracellular glutamate. In sham-injured animals, local application of ω-conotoxin produced a significant (∼80%) decrease in extracellular glutamate and reduced glutamate for a longer period of time (area under curve) compared with local application of saline (Table 1). After TBI, local application of ω-conotoxin failed to reduce the extracellular glutamate concentration compared with local application of saline (Table 1). Averaged group responses to the local application of ω-conotoxin into the striatum in both sham and TBI groups demonstrated the reduced efficacy of ω-conotoxin on extracellular glutamate after TBI (Fig. 2A). The maximum decrease in extracellular glutamate in the presence of ω-conotoxin was significantly smaller after TBI compared with sham (t6=2.901, p=0.027) (Fig. 2B). Also, the extent of reduced glutamate with respect to time (area under curve) was significantly reduced after TBI as compared with sham (t6=3.113, p=0.021) (Fig. 2C). Therefore, in sham-injured animals, calcium-mediated neuronal glutamate release was a significant source to extracellular glutamate, whereas calcium-mediated neuronal glutamate release was no longer a significant source to extracellular glutamate in brain-injured animals. Consequently, increased calcium-dependent glutamate release is unlikely to be a predominant mechanism for increased extracellular glutamate after TBI.
Table 1.
Effect of Local Application of Drugs on Extracellular Glutamate in Sham and Traumatic Brain Injured (TBI) Animals
| |
Maximum change from baseline (glutamate) μM |
Area under curve (AU) |
||
|---|---|---|---|---|
| Local application | Sham | TBI | Sham | TBI |
| Saline | −0.7±0.2 | −0.4±0.1 | 40.7±21.1 | 11.6±5.1 |
| ω-conotoxin | −2.6±0.8* | −0.4±0.1 | 343.8±97.8* | 33.7±18.6 |
| Saline | 0.4±0.4 | 1.0±0.1 | 2.4±2.1 | 2.3±2.3 |
| TBOA | 5.2±1.7* | 8.5±0.8* | 26.3±5.3* | 53.6±9.4* |
Mean±SEM; n=4.
p<0.05 (treatment vs. saline).
TBOA, dl-threo-β-benzyloxyaspartate.
FIG. 2.
Reduced calcium channel-dependent glutamate release in the striatum of brain-injured rats. (A) Baseline-matched traces of extracellular glutamate demonstrate the average effect of 100 nL local application (↑) of 10 μM ω-conotoxin, N-type calcium channel blocker, on extracellular glutamate in sham (black) and TBI animals (gray). (B) The maximum decrease in extracellular glutamate from local application of ω-conotoxin was significantly smaller after TBI than in sham-injured animals. (C) The extent of reduced extracellular glutamate with respect to time (area under curve) was significantly reduced after TBI compared with sham-injured animals (n=4, *p<0.05).
Activation of mGluR2/3 failed to reduce extracellular glutamate in the striatum of sham and brain-injured rats
Additional studies were performed to investigate other potential presynaptic changes in glutamate regulation after TBI. By applying an mGluR2/3 agonist (LY 379268) into the extracellular space, we examined if transient activation of the glutamate autoreceptors, mGluR2/3, would reduce neuronal glutamate release and thereby improve regulation of extracellular glutamate. Local application of LY 379268 failed to produce a significant decrease in extracellular glutamate compared with local application of saline in sham and after TBI (data not shown). Therefore, insufficient mGluR2/3 activation is unlikely to be responsible for increased extracellular glutamate after TBI.
Inhibition of the glial xc- failed to reduce extracellular glutamate in the striatum of sham and brain-injured rats
Another glial source of extracellular glutamate may be derived from the xc-, which is predominantly localized to glia. Considerable attention has recently been paid to the xc- and its possible role in glutamate dysregulation by glia in drug abuse (Kalivas, 2009). By applying an xc- blocker (CPG) into the extracellular space, we examined the contribution of glutamate release through the xc- on extracellular glutamate. Local application of CPG failed to produce a significant decrease in glutamate compared with local application of saline in sham and after TBI (data not shown). Therefore, glial glutamate release through the xc- is not a significant source of extracellular glutamate in sham and TBI animals, and glutamate release through the xc- is unlikely to be a mechanism for increased extracellular glutamate after TBI.
Increased glutamate spillover from the inhibition of glutamate uptake in the striatum of brain-injured rats
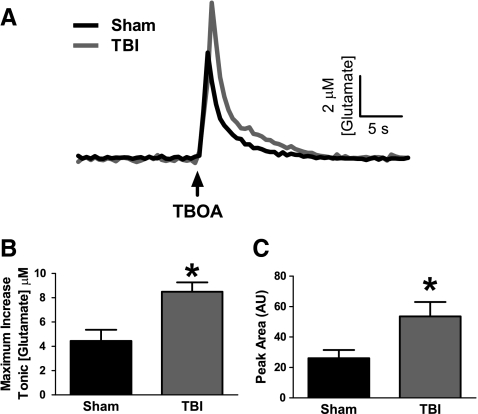
We further examined if disruptions in the regulation of extracellular glutamate by glia were responsible for the increased extracellular glutamate after TBI. Glutamate uptake through the EAATs, predominantly localized to glia, is the primary mechanism for regulating extracellular glutamate. By locally applying a competitive non-transportable EAAT blocker (TBOA) into the extracellular space, we examined how transient inhibition of glutamate uptake alters the extracellular glutamate concentration. In sham-injured animals, local application of TBOA produced a rapid and significant increase in the extracellular glutamate concentration and produced higher levels of glutamate for a longer period of time (area under curve) compared with local application of saline (Table 1). After TBI, local application of TBOA produced a rapid and significant increase in the extracellular glutamate concentration and produced higher levels of glutamate for a longer period of time (area under curve) compared with local application of saline (Table 1). Averaged group responses from the local application of TBOA within the striatum in both sham and TBI groups demonstrated increased amounts of glutamate spillover from inhibition of glutamate uptake after TBI (Fig. 3A). The maximum increase in extracellular glutamate from local application of TBOA was significantly greater after TBI than sham (t6=3.391, p=0.015) (Fig. 3B). Also, after TBI, more glutamate remained in the extracellular space for a longer period of time (area under curve) than in sham (t6=2.521, p=0.045) (Fig. 3C). Therefore, the increased glutamate spillover from the transient inhibition of glutamate uptake in brain-injured animals suggested that decreased glutamate uptake may play a role in the increase in extracellular glutamate after TBI.
FIG. 3.
Increased glutamate spillover from inhibition of glutamate uptake in the striatum of brain-injured rats. (A) Traces of extracellular glutamate showing the average response from 100 nL local application (↑) of 100 μM dl-threo-β-benzyloxyaspartate (TBOA), excitatory amino acid transporter blocker, in sham (black) and TBI (gray). (B) The maximum increase in extracellular glutamate from local application of TBOA was significantly elevated after TBI compared with sham-injured animals. (C) The extent of higher extracellular glutamate with respect to time (area under curve) was significantly increased after TBI compared with sham-injured animals (n=4, *p<0.05).
Using quantitative real-time PCR, we examined if decreased gene expression of glial EAATs (GLAST and GLT-1) was responsible for the increased sensitivity to the inhibition of the EAATs to TBOA. In the dorsal striatum, the relative expression of GLAST and GLT-1 was decreased, but not significantly after TBI compared with sham; parenthetical data shows (sham:TBI), GLAST (1.02±0.13: 0.80±0.06) (t6=1.58, p=0.164), GLT-1 (1.01±0.06: 0.84±0.16) (t6=0.95, p=0.378). Therefore, any injury-related changes in glial glutamate transporters were not evident as changes in gene expression.
Decreased glutamate clearance in the striatum of brain-injured rats
We further explored post-traumatic decreases in glutamate uptake by examining how sham and brain-injured animals were able to clear exogenous applications of glutamate within the striatum. Local application of small volumes of glutamate into the extracellular space allowed us to mimic endogenous glutamate release and examine clearance of glutamate back to baseline values. The primary mechanism for removing glutamate from the extracellular space is glutamate uptake via EAATs. Diffusion of glutamate also plays a role in lowering the extracellular glutamate concentration, but is limited by the extracellular anatomy (Danbolt, 2001). Representative recordings demonstrate slower glutamate clearance after TBI than in sham-injured animals (Fig. 4A). Because the amplitude of the glutamate signals was similar between sham (30.4±1.6 μM) and TBI (32.4±1.9 μM) (t14=0.8139, p=0.429) (Fig. 4B), we compared specific parameters of glutamate clearance (Nickell et al., 2007; Thomas et al., 2009, 2011). After local application of glutamate, glutamate levels spiked and rapidly returned to baseline concentrations as glutamate diffused from the local area, and through uptake of glutamate from the extracellular space. We calculated the Trise, the time for the signal to reach the maximum amplitude, to examine if brain injury altered diffusion of glutamate in the extracellular space. The Trise was not significantly altered after TBI (1.8±0.1 sec) compared with sham (1.9±0.1 sec) (t14=1.129, p=0.278) (Fig. 4C). As no differences in diffusion were detected after injury, any reductions in glutamate clearance likely resulted from decreases in glutamate uptake. TBI produced a significant decrease in k-1, a measure of glutamate uptake, (0.31±0.05 s−1), compared with sham (0.48±0.07 s−1) (t14=2.166, p=0.048) (Fig. 4D). Also, TBI produced a significantly longer T80, time for the signal to decay by 80% from the peak amplitude (4.5±0.6 sec), compared with sham (3.2±0.2 sec) (t14=2.184, p=0.047) (Fig. 4E). Therefore, TBI produced significant decreases in glutamate clearance without altering diffusion rates of extracellular glutamate between sham and TBI. Functional decreases in glutamate uptake are likely to be a primary mechanism responsible for increased extracellular glutamate after TBI.
FIG. 4.
Decreased glutamate clearance in the striatum of brain-injured rats. (A) Representative glutamate signals in the striatum from local application of 500 μM glutamate (↑) in sham (black) and TBI (gray) showed significant decreases in glutamate uptake parameters after TBI. (B) The amplitude of the glutamate signal was similar between sham and TBI groups. (C) Trise, an indicator of glutamate diffusion, was similar between sham and TBI groups. (D) k-1, an indicator of glutamate uptake rate, was significantly reduced after TBI compared with sham-injured animals. (E) T80, an indicator of glutamate clearance, was significantly longer after TBI compared with sham-injured animals (n=8, *p<0.05).
Discussion
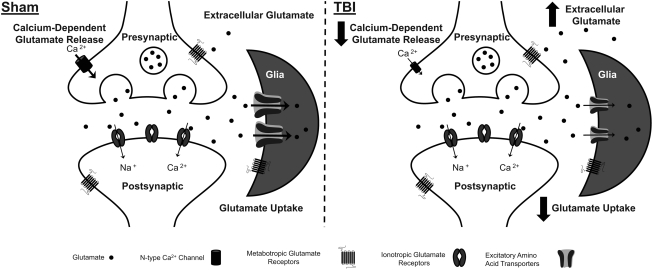
The purpose of this study was to examine the regulation of extracellular glutamate by both neurons and glia to determine the specific mechanism(s) responsible for the increase in extracellular glutamate in the rat striatum 2 days after diffuse brain injury. By combining glutamate-sensitive MEAs with targeted pharmacological agents, we were able to examine the neuronal and glial contributions to the release and regulation of extracellular glutamate. In sham-injured animals, presynaptic calcium channel-dependent neuronal glutamate release significantly contributed to extracellular glutamate. Glia were also found to regulate extracellular glutamate as inhibition of EAATs produced a significant increase in extracellular glutamate. Two days after diffuse brain injury, we detected significant post-traumatic disruptions in the regulation of extracellular glutamate by both neurons and glia. In brain-injured animals, the contribution of presynaptic calcium channel dependent neuronal glutamate release to extracellular glutamate was significantly decreased compared with sham. In addition, TBI produced increased glutamate spillover from the inhibition of glutamate uptake in the striatum of brain-injured rats. Furthermore, brain-injured animals exhibited slower clearance of extracellular glutamate, primarily due to decreases in glutamate uptake; however, not necessarily because of decreases in GLAST and GLT-1 gene expression. These results are depicted in schematic format to highlight possible sites of dysregulation in the injury-induced increase in extracellular glutamate (Fig. 5).
FIG. 5.
Schematic depicting the regulation of extracellular glutamate in the rat striatum in sham and brain-injured animals. In sham-injured animals, neurons release glutamate into the extracellular space that is dependent on calcium entry through N-type calcium channels. Once in the extracellular space, glutamate acts on both metabotropic and ionotropic glutamate receptors for signal propagation. Glutamate is removed from the extracellular space by excitatory amino acid transporters, predominantly localized to glia. Glutamate uptake is the primary mechanism for removal of extracellular glutamate and is responsible for regulating the concentration extracellular glutamate. After TBI, we detected post-traumatic disruptions in the regulation of extracellular glutamate by both neurons and glia. In brain-injured animals, calcium-dependent glutamate release via N-type calcium channels was no longer a source of extracellular glutamate. Also, the uptake of glutamate by excitatory amino acid transporters was reduced in brain-injured animals. Post-traumatic decreases in glutamate uptake was probably the primary mechanism responsible for increased extracellular glutamate after TBI.
Calcium channel dependent neuronal glutamate release contributes extracellular glutamate in sham-injured animals
In sham-injured animals, local application of ω-conotoxin produced a significant ∼80% decrease in the extracellular glutamate, suggesting that the majority of extracellular glutamate measured by MEAs is of a neuronal origin. This result is similar to another study using MEAs, where local application of ω-conotoxin (mixed channel type) produced a significant ∼50% decrease in the extracellular glutamate concentration in awake freely moving animals (Hascup et al., 2010). Collectively, these results confirm that MEAs measure extracellular glutamate derived, in part, from calcium-dependent neuronal release. In contrast to the MEA studies, extracellular glutamate sampled by microdialysis has been previously shown to be insensitive to sodium/calcium channel blockers; suggesting that the extracellular pool of glutamate sampled with microdialysis is of a non-neuronal origin (Timmerman and Westerink, 1997). The discrepancy that MEAs detect neuronally derived glutamate, whereas microdialysis samples extracellular glutamate derived from non-neuronal origins, may be because of the inherent differences in the methodologies. The extensive damage to the local brain area during implantation of the microdialysis probe and the large area of tissue sampled (1–4 mm in length), restricts microdialysis measurements of glutamate to large regions far beyond synapses (Borland et al., 2005; Hillered et al., 2005; Jaquins-Gerstl and Michael, 2009; Obrenovitch, 1999, Obrenovitch et al., 2000; Timmerman and Westerink 1997). Furthermore, the inadequate temporal resolution of microdialysis (∼1–20 min) prevents microdialysis from detecting the rapid dynamic changes in extracellular glutamate that occur in the order of milliseconds to seconds (Diamond, 2005). The MEA technologies have improved sampling capabilities for the detection of extracellular glutamate in vivo. The improved spatial resolution (333 μm in length), limited damage to the local area from implantation of the device, and fast temporal resolution (2 Hz) allow MEAs to detect the fast dynamics of glutamate closer to synapses and detect extracellular glutamate that is neuronally derived (Day et al., 2006; Hascup et al., 2007, 2009, 2010; Rutherford et al., 2007).
Reduced calcium channel dependent neuronal glutamate release in brain-injured rats
In contrast to sham-injured animals, local application of ω-conotoxin failed to significantly reduce the extracellular glutamate concentration compared with saline after TBI. This shift in the source of glutamate release from neuronal sources in sham to non-neuronal sources after TBI could be explained by two different mechanisms. First, there could be a post-traumatic decrease in neuronal glutamate release with a concurrent increase in non-neuronal sources of glutamate. Non-neuronal sources of extracellular glutamate can include: 1) micropores in cellular membranes from the primary injury forces that release glutamate into the extracellular space because of the higher concentrations of glutamate found in the cytosol or blood (Koizumi et al., 1997; Schmidt and Grady, 1993); 2) release of glutemate from activated microglia in response to inflammation after TBI (Brown and Neher, 2010); 3) release of glutamate from the xc-, which may be enhanced after injury for the production of glutathione, a major cellular antioxidant (Baker et al., 2002; Domercq et al., 2007); 4) reversal of EAATs because of ionic and metabolic disruptions after TBI transporting glutamate into the extracellular space (Gemba et al., 1994; Hamann et al., 2002; Phillis et al., 2000). Overall, the observed reduction in presynaptic calcium channel dependent vesicular glutamate release after TBI may be a compensatory mechanism for increases in non-vesicular sources of glutamate.
A second mechanism that may contribute to the decrease in presynaptic calcium channel dependent glutamate release after TBI is a post-traumatic alteration in the mechanism of vesicular glutamate release that is independent of calcium entry through the N-type calcium channel. Neurons are capable of vesicular release independent of calcium entry from the extracellular space, as internal calcium stores can mobilize from the mitochondria and endoplasmic reticulum for vesicular release (Neher and Sakaba 2008; Ryglewski et al., 2007). After midline FPI, neurons have exhibited disrupted calcium regulation with increasing intracellular calcium levels (McGinn et al., 2009; Sun et al., 2008). The disrupted internal calcium regulation and a post-traumatic shift utilizing internal calcium stores for vesicular release may explain why blocking the N-type calcium channel with ω-conotoxin failed to reduce extracellular glutamate in brain-injured animals.
Neuronal regulation of extracellular glutamate (mGluR2/3)
Transient activation of the glutamate autoreceptors (mGluR2/3) have been reported to regulate neuronal glutamate release (Bond et al., 1999), potentially restoring extracellular glutamate levels in the injured brain. The mGluR2/3's are G-protein linked receptors that negatively regulate adenylate cyclase and decrease levels of cyclic adenosine monophosphate (Danbolt, 2001). The mGluR2/3 act as negatively coupled autoreceptors providing presynaptic inhibition of glutamate release (Allen et al., 1999; Battaglia et al., 1997; Di Iorio et al., 1996; Nakanishi 1994; Schoepp 2001). In sham-injured animals, local application of LY 379268 failed to significantly reduce extracellular glutamate compared with saline, whereas a previous study in awake animals detected a significant ∼20% decrease in the extracellular glutamate from local application of LY 379268 (Hascup et al., 2010). The discrepancy between our results and the aforementioned study may be because of the confounding effects of anesthesia, as urethane has been shown to dramatically reduce extracellular glutamate by ∼ 60–80% (Rutherford et al., 2007).
We hypothesized that local transient activation of mGluR2/3 would reduce excessive synaptic glutamate release and lower extracellular glutamate, as we have previously shown increased amounts of neuronal glutamate release from local depolarization in the rat striatum 2 days after TBI (Hinzman et al., 2010). Furthermore, multiple reports have shown LY 379268 to be neuroprotective in ischemia and TBI models (Bond et al., 2000; Cai et al., 1999; Movsesyan and Faden, 2006; Stover et al., 2003). However, in brain-injured animals, local application of LY 379268 failed to reduce extracellular glutamate compared to saline. The inability of LY 379268 to reduce extracellular glutamate was also seen in a prior study, where a single i.p. injection of LY 379268 improved EEG activity and reduced contusion volume, but failed to reduce extracellular glutamate; suggesting that LY 379268 works to provide neuroprotection through mechanisms other than solely reducing extracellular glutamate (Stover et al., 2003).
Glial source of extracellular glutamate (xc-)
We also examined glial glutamate release through the xc-, a potential non-neuronal source of extracellular glutamate, by locally applying xc- blocker (CPG). The xc-, predominantly localized to glia, is an antiporter internalizing cystine with simultaneous release of glutamate; xc- plays a vital role in the prevention of oxidative damage by importing cystine for the production of glutathione, a major antioxidant in the cell (Albrecht et al., 2010). The xc- has been proposed as a major source of extracellular glutamate, with CPG producing a significant ∼ 60% decrease in extracellular glutamate sampled by microdialysis (Baker et al., 2002). In our study, local application of CPG failed to significantly reduce extracellular glutamate compared with saline in both sham-injured and TBI animals. Similar to our data, multiple reports have detected no significant change in extracellular glutamate with application of CPG in both anesthetized and awake animals (Hascup et al., 2010; Lupinsky et al., 2010; Melendez et al., 2005). In general, the data reported here and from prior reports suggest a limited role for the xc- as a source of extracellular glutamate.
Glutamate uptake through EAATs regulates extracellular glutamate
In sham-injured animals, we examined the role of EAATs in regulating extracellular glutamate by locally applying the EAAT competitive blocker, TBOA. Local application of TBOA into the extracellular space produced a robust increase in the extracellular glutamate concentration, as TBOA inhibited glutamate uptake by the EAATs, allowing more glutamate to escape from the synapses. Prior studies, using both microdialysis and MEAs, have detected similar increases in the extracellular concentration of glutamate with inhibition of EAATs, which equates to increases in extracellular glutamate ranging from ∼ 125% to 800% (Day et al., 2006; Hascup et al., 2010; Montiel et al., 2005; Tovar et al., 2009,). Collectively, our data and the previous reports support the importance of EAATs in regulating extracellular glutamate.
Increased glutamate spillover from the inhibition of glutamate uptake in brain-injured rats
Similar to sham animals, in brain-injured animals local application of TBOA into the extracellular space produced a robust increase in extracellular glutamate. In addition, TBI resulted in a significantly greater increase in the amount of glutamate spillover from the synapses of brain-injured animals compared with sham-injured animals. The increase in glutamate spillover when challenged with an uptake inhibitor suggests either a post-traumatic decrease in EAATs expression or function. Although we did not detect a significant decrease in the expression of GLAST and GLT-1 genes, multiple reports have detected post-traumatic reductions in the expression of EAATs in both the clinic and in experimental models of TBI (Rao et al., 1998; van Landeghem et al., 2001, 2006; Yi and Hazell, 2006; Yi et al., 2005).
Decreased glutamate clearance in brain-injured rats
Rapid application of glutamate into the extracellular space allowed us to mimic endogenous glutamate release events to examine the ability of EAATs to take up glutamate in vivo. Here, we report significant decreases in glutamate clearance after TBI. The post-traumatic decrease in glutamate clearance seems to be caused by decreases in glutamate uptake and not by alterations in the diffusion of glutamate within the extracellular space. If TBI drastically altered the tortuosity of the extracellular space, we would expect a post-traumatic change in the diffusion of glutamate in the extracellular space (Nicholson et al., 2000). However, we detected no significant change in the Trise as glutamate diffused from the point source (micropipette) to the MEA, suggesting that the decreased glutamate clearance is caused by decreased glutamate uptake. Post-traumatic reductions in glutamate uptake can play a pivotal role in the pathophysiology of TBI increasing the activation of metabotropic and ionotropic glutamate receptors. Pericontusional tissue exhibits decreased expression of EAATs in both the clinic and in experimental models of TBI (Rao et al., 1998; van Landeghem et al., 2001, 2006; Yi and Hazell 2006; Yi et al., 2005). Furthermore, knockdown expression of GLAST and GLT-1 produces increased neuronal damage in an experimental model of TBI (Rao et al., 2001). These studies demonstrate the importance of maintaining proper glutamate uptake to prevent propagation of secondary injury cascades and development of a contusion. Early post-traumatic disruptions in glutamate clearance may play a role in the development of late-onset gain-of- function behavioral morbidity that is correlated with changes in glutamate hypersensitivity (Thomas et al., 2011). Although we do not know if the disruptions in the regulation of extracellular glutamate will continue at later time points or indefinitely, prior work has detected persistent changes in glutamate excitability in the thalamus and somatosensory cortex (Thomas et al., 2011). Decreased glutamate uptake is likely the primary mechanism for increased extracellular glutamate after injury. With reduced glutamate uptake, more glutamate can spill out from the synapse increasing extracellular glutamate over time. Furthermore, reduced glutamate uptake is likely responsible for the increased KCl-evoked glutamate release seen in our prior study (Hinzman et al., 2010). A decrease in glutamate uptake would allow more glutamate to escape from the synapse, producing larger amplitudes during evoked release. Previously, we did not detect any post-traumatic decrease in glutamate clearance parameters from KCl-evoked glutamate release, most likely because of the disruption in the electrochemical gradient for depolarization, which is circumvented by local application of glutamate itself.
Conclusion
Using MEAs and targeted pharmacological agents, we were able to examine how neurons and glia release and regulate extracellular glutamate in vivo. Two days after diffuse brain injury, the contribution of calcium-mediated glutamate release via the N-type calcium channel was significantly reduced. Also, TBI produced a significant decrease in glutamate clearance that was primarily caused by decreases in glutamate uptake. Future work should examine if enhancing expression of the EAATs, either by viral mediated expression or by stimulating expression through the use of beta-lactam antibiotics (Harvey et al., 2011; Rothstein et al., 2005), produces an overall improvement in glutamate regulation and behavioral outcomes in experimental models of TBI. In addition, drugs that can improve the function and/or surface expression of EAATs remain possible therapeutic targets to improve outcomes following TBI.
Acknowledgments
The authors thank Francois Pomerleau and Peter Huettl for technical assistance on the glutamate recordings, and Amanda Bolton for assistance on the rtPCR. Support provided by F31NS067899, USPHS DA017186, USPHS NS3978, AG013494, NSF (ERC) EEC-0310723 DARPA N6601-09-2080, and Kentucky Spinal Cord and Head Injury Research Trust Grant 7-11.
Author Disclosure Statement
Greg A. Gerhardt is the principal owner of Quanteon LLC. Jorge E. Quintero has served as consultant to Quanteon LLC.
References
- Albrecht P. Lewerenz J. Dittmer S. Noack R. Maher P. Methner A. Mechanisms of oxidative glutamate toxicity: the glutamate/cystine antiporter system xc- as a neuroprotective drug target. CNS Neurol. Disord. Drug Targets. 2010;9:373–382. doi: 10.2174/187152710791292567. [DOI] [PubMed] [Google Scholar]
- Allen J.W. Ivanova S.A. Fan L. Espey M.G. Basile A.S. Faden A.I. Group II metabotropic glutamate receptor activation attenuates traumatic neuronal injury and improves neurological recovery after traumatic brain injury. J. Pharmacol. Exp. Ther. 1999;290:112–120. [PubMed] [Google Scholar]
- Baker D.A. Xi Z.X. Shen H. Swanson C.J. Kalivas P.W. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia G. Monn J.A. Schoepp D.D. In vivo inhibition of veratridine-evoked release of striatal excitatory amino acids by the group II metabotropic glutamate receptor agonist LY354740 in rats. Neurosci. Lett. 1997;229:161–164. doi: 10.1016/s0304-3940(97)00442-4. [DOI] [PubMed] [Google Scholar]
- Bond A. Jones N.M. Hicks C.A. Whiffin G.M. Ward M.A. O'Neill M.F. Kingston A.E. Monn J.A. Ornstein P.L. Schoepp D.D. Lodge D. O'Neill M.J. Neuroprotective effects of LY379268, a selective mGlu2/3 receptor agonist: investigations into possible mechanism of action in vivo. J. Pharmacol. Exp. Ther. 2000;294:800–809. [PubMed] [Google Scholar]
- Bond A. Ragumoorthy N. Monn J.A. Hicks C.A. Ward M.A. Lodge D. O'Neill M.J. LY379268, a potent and selective Group II metabotropic glutamate receptor agonist, is neuroprotective in gerbil global, but not focal, cerebral ischaemia. Neurosci. Lett. 1999;273:191–194. doi: 10.1016/s0304-3940(99)00663-1. [DOI] [PubMed] [Google Scholar]
- Borland L.M. Shi G. Yang H. Michael A.C. Voltammetric study of extracellular dopamine near microdialysis probes acutely implanted in the striatum of the anesthetized rat. J. Neurosci. Methods. 2005;146:149–158. doi: 10.1016/j.jneumeth.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Brown G.C. Neher J.J. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol. Neurobiol. 2010;41:242–247. doi: 10.1007/s12035-010-8105-9. [DOI] [PubMed] [Google Scholar]
- Bullock R. Zauner A. Myseros J.S. Marmarou A. Woodward J.J. Young H.F. Evidence for prolonged release of excitatory amino acids in severe human head trauma. Relationship to clinical events. Ann. N. Y. Acad. Sci. 1995;765:290–298. doi: 10.1111/j.1749-6632.1995.tb16586.x. [DOI] [PubMed] [Google Scholar]
- Bullock R. Zauner A. Woodward J.J. Myseros J. Choi S.C. Ward J.D. Marmarou A. Young H.F. Factors affecting excitatory amino acid release following severe human head injury. J. Neurosurg. 1998;89:507–518. doi: 10.3171/jns.1998.89.4.0507. [DOI] [PubMed] [Google Scholar]
- Burmeister J.J. Gerhardt G.A. Self-referencing ceramic-based multisite microelectrodes for the detection and elimination of interferences from the measurement of L-glutamate and other analytes. Anal. Chem. 2001;73:1037–1042. doi: 10.1021/ac0010429. [DOI] [PubMed] [Google Scholar]
- Burmeister J.J. Pomerleau F. Palmer M. Day B.K. Huettl P. Gerhardt G.A. Improved ceramic-based multisite microelectrode for rapid measurements of L-glutamate in the CNS. J. Neurosci.Methods. 2002;119:163–171. doi: 10.1016/s0165-0270(02)00172-3. [DOI] [PubMed] [Google Scholar]
- Cai Z. Xiao F. Fratkin J.D. Rhodes P.G. Protection of neonatal rat brain from hypoxic-ischemic injury by LY379268, a Group II metabotropic glutamate receptor agonist. Neuroreport. 1999;10:3927–3931. doi: 10.1097/00001756-199912160-00037. [DOI] [PubMed] [Google Scholar]
- Carbonell W.S. Grady M.S. Evidence disputing the importance of excitotoxicity in hippocampal neuron death after experimental traumatic brain injury. Ann. N. Y. Acad. Sci. 1999;890:287–298. doi: 10.1111/j.1749-6632.1999.tb08005.x. [DOI] [PubMed] [Google Scholar]
- Chamoun R. Suki D. Gopinath S.P. Goodman J.C. Robertson C. Role of extracellular glutamate measured by cerebral microdialysis in severe traumatic brain injury. J. Neurosurg. 2010;113:564–570. doi: 10.3171/2009.12.JNS09689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt N.C. Glutamate uptake. Prog.Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Day B.K. Pomerleau F. Burmeister J.J. Huettl P. Gerhardt G.A. Microelectrode array studies of basal and potassium-evoked release of L-glutamate in the anesthetized rat brain. J.Neurochem. 2006;96:1626–1635. doi: 10.1111/j.1471-4159.2006.03673.x. [DOI] [PubMed] [Google Scholar]
- Di Iorio P. Battaglia G. Ciccarelli R. Ballerini P. Giuliani P. Poli A. Nicoletti F. Caciagli F. Interaction between A1 adenosine and class II metabotropic glutamate receptors in the regulation of purine and glutamate release from rat hippocampal slices. J. Neurochem. 1996;67:302–309. doi: 10.1046/j.1471-4159.1996.67010302.x. [DOI] [PubMed] [Google Scholar]
- Diamond J.S. Deriving the glutamate clearance time course from transporter currents in CA1 hippocampal astrocytes: transmitter uptake gets faster during development. J. Neurosci. 2005;25:2906–2916. doi: 10.1523/JNEUROSCI.5125-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domercq M. Sanchez–Gomez M.V. Sherwin C. Etxebarria E. Fern R. Matute C. System xc- and glutamate transporter inhibition mediates microglial toxicity to oligodendrocytes. J. Immunol. 2007;178:6549–6556. doi: 10.4049/jimmunol.178.10.6549. [DOI] [PubMed] [Google Scholar]
- Faden A.I. Demediuk P. Panter S.S. Vink R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science. 1989;244:798–800. doi: 10.1126/science.2567056. [DOI] [PubMed] [Google Scholar]
- Friedemann M.N. Gerhardt G.A. Regional effects of aging on dopaminergic function in the Fischer-344 rat. Neurobiol Aging. 1992;13:325–332. doi: 10.1016/0197-4580(92)90046-z. [DOI] [PubMed] [Google Scholar]
- Gemba T. Oshima T. Ninomiya M. Glutamate efflux via the reversal of the sodium-dependent glutamate transporter caused by glycolytic inhibition in rat cultured astrocytes. Neuroscience. 1994;63:789–795. doi: 10.1016/0306-4522(94)90523-1. [DOI] [PubMed] [Google Scholar]
- Globus M.Y. Alonso O. Dietrich W.D. Busto R. Ginsberg M.D. Glutamate release and free radical production following brain injury: effects of posttraumatic hypothermia. J. Neurochem. 1995;65:1704–1711. doi: 10.1046/j.1471-4159.1995.65041704.x. [DOI] [PubMed] [Google Scholar]
- Hall E.D. Andrus P.K. Yonkers P.A. Smith S.L. Zhang J.R. Taylor B.M. Sun F.F. Generation and detection of hydroxyl radical following experimental head injury. Ann. N.Y. Acad.Sci. 1994;738:15–24. doi: 10.1111/j.1749-6632.1994.tb21785.x. [DOI] [PubMed] [Google Scholar]
- Hamann M. Rossi D.J. Marie H. Attwell D. Knocking out the glial glutamate transporter GLT-1 reduces glutamate uptake but does not affect hippocampal glutamate dynamics in early simulated ischaemia. Eur. J. Neurosci. 2002;15:308–314. doi: 10.1046/j.0953-816x.2001.01861.x. [DOI] [PubMed] [Google Scholar]
- Harvey B.K. Airavaara M. Hinzman J. Wires E.M. Chiocco M.J. Howard D.B. Shen H. Gerhardt G. Hoffer B.J. Wang Y. Targeted over-expression of glutamate transporter 1 (glt-1) reduces ischemic brain injury in a rat model of stroke. PLoS One. 6, 2011:e22135. doi: 10.1371/journal.pone.0022135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascup E.R. af Bjerken S. Hascup K.N. Pomerleau F. Huettl P. Stromberg I. Gerhardt G.A. Histological studies of the effects of chronic implantation of ceramic-based microelectrode arrays and microdialysis probes in rat prefrontal cortex. Brain Res. 2009;1291:12–20. doi: 10.1016/j.brainres.2009.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascup E.R. Hascup K.N. Stephens M. Pomerleau F. Huettl P. Gratton A. Gerhardt G.A. Rapid microelectrode measurements and the origin and regulation of extracellular glutamate in rat prefrontal cortex. J. Neurochem. 2010;115:1608–1620. doi: 10.1111/j.1471-4159.2010.07066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascup K.N. Rutherford E.C. Quintero J.E. Day B.K. Nickell J.R. Pomerleau F. Huettl P. Burmeister J.J. Gerhardt G.A. Electrochemical Methods for Neuroscience. CRC Press; Boca Raton, FL: 2007. Second-by-second measures of L-glutamate and other neurotransmitters using enzyme-based microelectrode arrays. Chapter 19. [PubMed] [Google Scholar]
- Hillered L. Vespa P.M. Hovda D.A. Translational neurochemical research in acute human brain injury: the current status and potential future for cerebral microdialysis. J. Neurotrauma. 2005;22:3–41. doi: 10.1089/neu.2005.22.3. [DOI] [PubMed] [Google Scholar]
- Hinzman J.M. Thomas T.C. Burmeister J.J. Quintero J.E. Huettl P. Pomerleau F. Gerhardt G.A. Lifshitz J. Diffuse brain injury elevates tonic glutamate levels and potassium-evoked glutamate release in discrete brain regions at two days post-injury: an enzyme-based microelectrode array study. J. Neurotrauma. 2010;27:889–899. doi: 10.1089/neu.2009.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlatky R. Valadka A.B. Goodman J.C. Contant C.F. Robertson C.S. Patterns of energy substrates during ischemia measured in the brain by microdialysis. J. Neurotrauma. 2004;21:894–906. doi: 10.1089/0897715041526195. [DOI] [PubMed] [Google Scholar]
- Hutchinson P.J. O'Connell M.T. Al-Rawi P.G. Kett–White C.R. Gupta A.K. Maskell L.B. Pickard J.D. Kirkpatrick P.J. Increases in GABA concentrations during cerebral ischaemia: a microdialysis study of extracellular amino acids. J. Neurol. Neurosurg. Psychiatry. 2002;72:99–105. doi: 10.1136/jnnp.72.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabaudon D. Shimamoto K. Yasuda–Kamatani Y. Scanziani M. Gahwiler B.H. Gerber U. Inhibition of uptake unmasks rapid extracellular turnover of glutamate of nonvesicular origin. Proc. Natl. Acad. Sci. U. S. A. 1999;96:8733–8738. doi: 10.1073/pnas.96.15.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaquins–Gerstl A. Michael A.C. Comparison of the brain penetration injury associated with microdialysis and voltammetry. J. Neurosci Methods. 2009;183:127–135. doi: 10.1016/j.jneumeth.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J.B. Pickering D.S. Schousboe A. Depolarization-induced release of [(3)H]D-aspartate from GABAergic neurons caused by reversal of glutamate transporters. Int. J. Dev. Neurosci. 2000;18:309–315. doi: 10.1016/s0736-5748(99)00099-4. [DOI] [PubMed] [Google Scholar]
- Kalivas P.W. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Katayama Y. Becker D.P. Tamura T. Hovda D.A. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J. Neurosurg. 1990;73:889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- Katoh H. Sima K. Nawashiro H. Wada K. Chigasaki H. The effect of MK-801 on extracellular neuroactive amino acids in hippocampus after closed head injury followed by hypoxia in rats. Brain Res. 1997;758:153–162. doi: 10.1016/s0006-8993(97)00213-8. [DOI] [PubMed] [Google Scholar]
- Koizumi H. Fujisawa H. Ito H. Maekawa T. Di X. Bullock R. Effects of mild hypothermia on cerebral blood flow-independent changes in cortical extracellular levels of amino acids following contusion trauma in the rat. Brain Res. 1997;747:304–312. doi: 10.1016/s0006-8993(96)01240-1. [DOI] [PubMed] [Google Scholar]
- Koura S.S. Doppenberg E.M. Marmarou A. Choi S. Young H.F. Bullock R. Relationship between excitatory amino acid release and outcome after severe human head injury. Acta Neurochir. Suppl. 1998;71:244–246. doi: 10.1007/978-3-7091-6475-4_70. [DOI] [PubMed] [Google Scholar]
- Lifshitz J. Chen Z.X. Xu X.-M. Zhang J. Animal Models of Acute Neurological Injuries. The Humana Press Inc.; Totowa, NJ: 2008. Fluid percussion injury. [Google Scholar]
- Livak K.J. Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lupinsky D. Moquin L. Gratton A. Interhemispheric regulation of the medial prefrontal cortical glutamate stress response in rats. J. Neurosci. 2010;30:7624–7633. doi: 10.1523/JNEUROSCI.1187-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita Y. Shima K. Nawashiro H. Wada K. Tsuzuki N. Miyazawa T. Real time monitoring of glutamate following fluid percussion brain injury with hypoxia in the rat. Acta Neurochir. Suppl. 2000;76:207–212. doi: 10.1007/978-3-7091-6346-7_42. [DOI] [PubMed] [Google Scholar]
- McGinn M.J. Kelley B.J. Akinyi L. Oli M.W. Liu M.C. Hayes R.L. Wang K.K. Povlishock J.T. Biochemical, structural, and biomarker evidence for calpain-mediated cytoskeletal change after diffuse brain injury uncomplicated by contusion. J. Neuropathol. Exp. Neurol. 2009;68:241–249. doi: 10.1097/NEN.0b013e3181996bfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh T.K. Smith D.H. Meaney D.F. Kotapka M.J. Gennarelli T.A. Graham D.I. Neuropathological sequelae of traumatic brain injury: relationship to neurochemical and biomechanical mechanisms. Lab. Invest. 1996;74:315–342. [PubMed] [Google Scholar]
- Melendez R.I. Vuthiganon J. Kalivas P.W. Regulation of extracellular glutamate in the prefrontal cortex: focus on the cystine glutamate exchanger and group I metabotropic glutamate receptors. J. Pharmacol. Exp. Ther. 2005;314:139–147. doi: 10.1124/jpet.104.081521. [DOI] [PubMed] [Google Scholar]
- Montiel T. Camacho A. Estrada–Sanchez A.M. Massieu L. Differential effects of the substrate inhibitor l-trans-pyrrolidine-2,4-dicarboxylate (PDC) and the non-substrate inhibitor DL-threo-beta-benzyloxyaspartate (DL-TBOA) of glutamate transporters on neuronal damage and extracellular amino acid levels in rat brain in vivo. Neuroscience. 2005;133:667–678. doi: 10.1016/j.neuroscience.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Movsesyan V. A. Faden A.I. Neuroprotective effects of selective group II mGluR activation in brain trauma and traumatic neuronal injury. J. Neurotrauma. 2006;23:117–127. doi: 10.1089/neu.2006.23.117. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Metabotropic glutamate receptors: synaptic transmission, modulation, and plasticity. Neuron. 1994;13:1031–1037. doi: 10.1016/0896-6273(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Neher E. Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59:861–872. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Nicholson C. Chen K.C. Hrabetova S. Tao L. Diffusion of molecules in brain extracellular space: theory and experiment. Prog. Brain Res. 2000;125:129–154. doi: 10.1016/S0079-6123(00)25007-3. [DOI] [PubMed] [Google Scholar]
- Nickell J. Salvatore M.F. Pomerleau F. Apparsundaram S. Gerhardt G.A. Reduced plasma membrane surface expression of GLAST mediates decreased glutamate regulation in the aged striatum. Neurobiol. Aging. 2007;28:1737–1748. doi: 10.1016/j.neurobiolaging.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Nilsson P. Hillered L. Ponten U. Ungerstedt U. Changes in cortical extracellular levels of energy-related metabolites and amino acids following concussive brain injury in rats. J. Cereb. Blood Flow Metab. 1990;10:631–637. doi: 10.1038/jcbfm.1990.115. [DOI] [PubMed] [Google Scholar]
- Obrenovitch T.P. High extracellular glutamate and neuronal death in neurological disorders. Cause, contribution or consequence? Ann. N.Y. Acad. Sci. 1999;890:273–286. doi: 10.1111/j.1749-6632.1999.tb08004.x. [DOI] [PubMed] [Google Scholar]
- Obrenovitch T.P. Urenjak J. Zilkha E. Jay T.M. Excitotoxicity in neurological disorders—the glutamate paradox. Int. J. Dev. Neurosci. 2000;18:281–287. doi: 10.1016/s0736-5748(99)00096-9. [DOI] [PubMed] [Google Scholar]
- Palmer A.M. Marion D.W. Botscheller M.L. Swedlow P.E. Styren S.D. DeKosky S.T. Traumatic brain injury-induced excitotoxicity assessed in a controlled cortical impact model. J. Neurochem. 1993;61:2015–2024. doi: 10.1111/j.1471-4159.1993.tb07437.x. [DOI] [PubMed] [Google Scholar]
- Persson L. Hillered L. Chemical monitoring of neurosurgical intensive care patients using intracerebral microdialysis. J. Neurosurg. 1992;76:72–80. doi: 10.3171/jns.1992.76.1.0072. [DOI] [PubMed] [Google Scholar]
- Phillis J.W. Ren J. O'Regan M.H. Transporter reversal as a mechanism of glutamate release from the ischemic rat cerebral cortex: studies with DL-threo-beta-benzyloxyaspartate. Brain Res. 2000;880:224. doi: 10.1016/s0006-8993(00)02755-4. [DOI] [PubMed] [Google Scholar]
- Rao V.L. Baskaya M.K. Dogan A. Rothstein J.D. Dempsey R.J. Traumatic brain injury down-regulates glial glutamate transporter (GLT-1 and GLAST) proteins in rat brain. J. Neurochem. 1998;70:2020–2027. doi: 10.1046/j.1471-4159.1998.70052020.x. [DOI] [PubMed] [Google Scholar]
- Rao V.L. Dogan A. Bowen K.K. Todd K.G. Dempsey R.J. Antisense knockdown of the glial glutamate transporter GLT-1 exacerbates hippocampal neuronal damage following traumatic injury to rat brain. Eur. J. Neurosci. 2001;13:119–128. [PubMed] [Google Scholar]
- Reinert M. Khaldi A. Zauner A. Doppenberg E. Choi S. Bullock R. High extracellular potassium and its correlates after severe head injury: relationship to high intracranial pressure. Neurosurg. Focus. 2000;8:e10. doi: 10.3171/foc.2000.8.1.2027. [DOI] [PubMed] [Google Scholar]
- Rose M.E. Huerbin M.B. Melick J. Marion D.W. Palmer A.M. Schiding J.K. Kochanek P.M. Graham S.H. Regulation of interstitial excitatory amino acid concentrations after cortical contusion injury. Brain Res. 2002;943:15–22. doi: 10.1016/s0006-8993(02)02471-x. [DOI] [PubMed] [Google Scholar]
- Rothstein J.D. Dykes–Hoberg M. Pardo C.A. Bristol L.A. Jin L. Kuncl R.W. Kanai Y. Hediger M.A. Wang Y. Schielke J.P. Welty D.F. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rothstein J.D. Patel S. Regan M.R. Haenggeli C. Huang Y.H. Bergles D.E. Jin L. Dykes Hoberg M. Vidensky S. Chung D.S. Toan S.V. Bruijn L.I. Su Z.Z. Gupta P. Fisher P.B. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Rutherford E.C. Pomerleau F. Huettl P. Stromberg I. Gerhardt G.A. Chronic second-by-second measures of L-glutamate in the central nervous system of freely moving rats. J. Neurochem. 2007;102:712–722. doi: 10.1111/j.1471-4159.2007.04596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryglewski S. Pflueger H.J. Duch C. Expanding the neuron's calcium signaling repertoire: intracellular calcium release via voltage-induced PLC and IP3R activation. PLoS Biol. 2007;5:e66. doi: 10.1371/journal.pbio.0050066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R.H. Grady M.S. Regional patterns of blood-brain barrier breakdown following central and lateral fluid percussion injury in rodents. J. Neurotrauma. 1993;10:415–430. doi: 10.1089/neu.1993.10.415. [DOI] [PubMed] [Google Scholar]
- Schoepp D.D. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J. Pharmacol. Exp. Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- Stephens M.L. Quintero J.E. Pomerleau F. Huettl P. Gerhardt G.A. Age-related changes in glutamate release in the CA3 and dentate gyrus of the rat hippocampus. Neurobiol Aging. 2011;32:811–820. doi: 10.1016/j.neurobiolaging.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel M. Eriskat J. Plesnila M. Aggarwal N. Baethmann A. The penumbra zone of a traumatic cortical lesion, a microdialysis study of excitatory amino acid release. Acta Neurochir. Suppl. 1997;70:91–93. doi: 10.1007/978-3-7091-6837-0_28. [DOI] [PubMed] [Google Scholar]
- Stoffel M. Plesnila N. Eriskat J. Furst M. Baethmann A. Release of excitatory amino acids in the penumbra of a focal cortical necrosis. J. Neurotrauma. 2002;19:467–477. doi: 10.1089/08977150252932415. [DOI] [PubMed] [Google Scholar]
- Stover J.F. Sakowitz O.W. Beyer T.F. Dohse N.K. Kroppenstedt S.N. Thomale U.W. Schaser K.D. Unterberg A.W. Effects of LY379268, a selective group II metabotropic glutamate receptor agonist on EEG activity, cortical perfusion, tissue damage, and cortical glutamate, glucose, and lactate levels in brain-injured rats. J. Neurotrauma. 2003;20:315–326. doi: 10.1089/089771503765172273. [DOI] [PubMed] [Google Scholar]
- Sun D.A. Deshpande L.S. Sombati S. Baranova A. Wilson M.S. Hamm R.J. DeLorenzo R.J. Traumatic brain injury causes a long-lasting calcium (Ca2+)-plateau of elevated intracellular Ca levels and altered Ca2+ homeostatic mechanisms in hippocampal neurons surviving brain injury. Eur. J. Neurosci. 2008;27:1659–1672. doi: 10.1111/j.1460-9568.2008.06156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K. Watase K. Manabe T. Yamada K. Watanabe M. Takahashi K. Iwama H. Nishikawa T. Ichihara N. Kikuchi T. Okuyama S. Kawashima N. Hori S. Takimoto M. Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Thomas T.C. Grandy D.K. Gerhardt G.A. Glaser P.E. Decreased dopamine D4 receptor expression increases extracellular glutamate and alters its regulation in mouse striatum. Neuropsychopharmacology. 2009;34:436–445. doi: 10.1038/npp.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T.C. Hinzman J. Gerhardt G.A. Lifshitz J. Hypersensitive glutamate signaling correlates with the development of late-onset behavioral morbidity in diffuse brain-injured circuitry. J Neurotrauma. 2012;29:187–200. doi: 10.1089/neu.2011.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman W. Westerink B.H. Brain microdialysis of GABA and glutamate: what does it signify? Synapse. 1997;27:242–261. doi: 10.1002/(SICI)1098-2396(199711)27:3<242::AID-SYN9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Timofeev I. Carpenter K.L. Nortje J. Al-Rawi P.G. O'Connell M.T. Czosnyka M. Smielewski P. Pickard J.D. Menon D.K. Kirkpatrick P.J. Gupta A.K. Hutchinson P.J. Cerebral extracellular chemistry and outcome following traumatic brain injury: a microdialysis study of 223 patients. Brain. 2011;134:484–494. doi: 10.1093/brain/awq353. [DOI] [PubMed] [Google Scholar]
- Tovar Y.R.L.B., Santa–Cruz L.D.Zepeda A.Tapia R.2009Chronic elevation of extracellular glutamate due to transport blockade is innocuous for spinal motoneurons in vivo Neurochem. Int. 54186–191. [DOI] [PubMed] [Google Scholar]
- van Landeghem F.K. Stover J.F. Bechmann I. Bruck W. Unterberg A. Buhrer C. von Deimling A. Early expression of glutamate transporter proteins in ramified microglia after controlled cortical impact injury in the rat. Glia. 2001;35:167–179. doi: 10.1002/glia.1082. [DOI] [PubMed] [Google Scholar]
- van Landeghem F.K. Weiss T. Oehmichen M. von D.A. Decreased expression of glutamate transporters in astrocytes after human traumatic brain injury. J. Neurotrauma. 2006;23:1518–1528. doi: 10.1089/neu.2006.23.1518. [DOI] [PubMed] [Google Scholar]
- Vespa P. Prins M. Ronne–Engstrom E. Caron M. Shalmon E. Hovda D.A. Martin N.A. Becker D.P. Increase in extracellular glutamate caused by reduced cerebral perfusion pressure and seizures after human traumatic brain injury: a microdialysis study. J. Neurosurg. 1998;89:971–982. doi: 10.3171/jns.1998.89.6.0971. [DOI] [PubMed] [Google Scholar]
- Yamamoto T. Rossi S. Stiefel M. Doppenberg E. Zauner A. Bullock R. Marmarou A. CSF and ECF glutamate concentrations in head injured patients. Acta Neurochir. Suppl. 1999;75:17–19. doi: 10.1007/978-3-7091-6415-0_4. [DOI] [PubMed] [Google Scholar]
- Yi J.H. Hazell A.S. Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochem. Int. 2006;48:394–403. doi: 10.1016/j.neuint.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Yi J.H. Pow D.V. Hazell A.S. Early loss of the glutamate transporter splice-variant GLT-1v in rat cerebral cortex following lateral fluid-percussion injury. Glia. 2005;49:121–133. doi: 10.1002/glia.20099. [DOI] [PubMed] [Google Scholar]