Abstract
The purpose of this study was to develop and investigate a new noninvasive approach to quantify left ventricular (LV) pressures using subharmonic emissions from microbubbles. A Sonix RP ultrasound scanner with PA4-2 phased array transducer was used in pulse inversion grayscale mode. Unprocessed radiofrequency data were obtained for 5 seconds (n=3) with pulsed wave Doppler from the aorta and/or LV of 4 canines during Sonazoid infusion. Simultaneous pressure measurements were obtained using Millar manometer. Subharmonic data (in dB) were extracted and processed. The resulting calibration factor (mmHg/dB), from the aorta, was used to estimate LV pressures. Errors ranged from 0.19 to 2.50 mmHg when estimating these pressures using the aortic calibration factor from the respective canines; but were considerably higher (0.64-8.98 mmHg) when a mean aortic calibration factor was used. In conclusion, subharmonic emissions from ultrasound contrast agents have the potential to noninvasively monitor LV pressures.
Keywords: contrast echocardiography, subharmonic microbubble signals, noninvasive pressure estimation
Knowledge of left ventricular (LV) pressures is important for diagnosis, treatment and management of patients with several cardiac abnormalities (1, 2). Invasive hemodynamic monitoring with right heart catheterization has remained the clinical standard of care for these patients (1). However, the potential complications associated with this invasive technique (1) underscore the need for reliable noninvasive methods to measure intra-cardiac pressures (2). Microbubble based ultrasound contrast agents (UCA) are approved in the United States for LV opacification studies, but have the potential to be applied much more broadly. A promising approach to monitor pressure changes with UCA relies on the fact that these gas bubbles exposed to pressure changes exhibit volume pulsations. Furthermore, the difference in compressibility between the shell encapsulated microbubbles and the surrounding medium (i.e., blood) enhances the backscattered/reflected signals from these microbubbles. Thus, when microbubbles are imaged by conventional ultrasound machines, they reflect soundwaves over a range of frequencies with the greatest amplitude at the insonation frequency. Additional peaks of reflectance occur at multiples (harmonics) of that frequency, as well as at intermediate harmonics, the largest of which is the subharmonic frequency at half of the insonation frequency. We have found that the amplitude of this subharmonic signal is sensitive to changes in pressure and have proposed a technique that utilizes subharmonic signals for pressure estimation called subharmonic aided pressure estimation (SHAPE) (3). This technique is based on the principle that the subharmonic amplitude in the received signal spectrum is linearly (r2=0.96) and inversely related to increase in ambient pressures (3). Previously, the use of SHAPE to monitor pressure changes in the aorta of open-chest canines using single element transducers was demonstrated (4). However, the use of single element transducers (without imaging capability) and open-chest measurements preclude clinical investigations. Therefore, the goal of this pilot study was to assess the potential of SHAPE to determine LV pressures noninvasively, with a commercially available ultrasound scanner.
Animal Preparation
This research study was approved by the IACUC of Thomas Jefferson University and conducted in accordance with NIH guidelines. Four mongrel dogs weighing 22.5±1.00 kg were studied. Intravenous injection of Propofol (Abbott Laboratories, Chicago, IL; dose: 7 ml/kg) was used as initial anesthetic, while a facemask with Isoflurane 2-4% (Iso-thesia; Abbott Laboratories, Chicago, IL) maintained sedation. The canines were placed on a warming blanket to maintain normal body temperature. Based on prior studies, Sonazoid microbubbles (GE Healthcare, Oslo, Norway) were selected for this study(5). An 18-gauge catheter was placed in a forelimb vein for Sonazoid infusion (0.015 μl/kg/min). A 5F solid state catheter tip manometer (SPR-350, Millar Instruments, Inc., Houston, TX) was used as the reference standard and was introduced at the site for pressure measurements under ultrasound guidance. Post-study, the canines were sacrificed by an intravenous injection of Beuthanasia (0.25 mg/kg).
Data Acquisition
A commercial Sonix RP ultrasound scanner with PA4-2 phased array probe (2.5 MHz center frequency; Ultrasonix, Richmond, BC, Canada) was operated with pulse inversion grayscale imaging to scan the canines (closed-chest). The accumulated signal from two ultrasound pulses (with a phase difference of 180°) was acquired from the pulsed wave (PW) Doppler gate placed at the region of interest (i.e., in LV or aorta; Figure 1). A synchronization signal from the ultrasound scanner triggered an oscilloscope (9350 AM, LeCroy, Chestnut Ridge, NY) for simultaneous acquisition of the pressure catheter data on a computer through Labview (Version 8.0, National Instruments Corp., Austin, TX). The subharmonic response from UCA and its ability to track ambient pressures depend on insonation frequency and incident acoustic pressure (5). Thus, transmit frequency was maintained at 2.5 MHz (5), while incident acoustic output from the scanner was varied from −8 to 0 dB. Ultrasound radiofrequency (RF) data was acquired for 5 seconds (n=3) simultaneously with the pressure catheter data from the aorta of 2 canines and the LV of 4 canines cumulating to 90 acquisitions.
Figure 1.


Ultrasound Data Acquisition
Grayscale ultrasound image of the left ventricle without (A) and with (B) UCA. Solid arrow in panel A indicates the pressure catheter; dotted arrow in both panels indicates the pulsed wave Doppler gate used to acquire the ultrasound data.
Data Processing and Statistical Analysis
Data processing was performed offline using Matlab (Version 7.8.0, The Mathworks, Inc., Natick, MA). Unprocessed RF data for each accumulated pulse (Figure 2-A) was transformed to the Fourier domain and the subharmonic signal amplitude (in dB; Figure 2-B) was extracted as the average signal in a 40% bandwidth around the subharmonic frequency (i.e., 1.25 MHz). This data was processed using a median filter to eliminate noise spikes. The range of the subharmonic signal (i.e., maximum minus minimum subharmonic amplitude) was compared from each pulse contour (after eliminating noisy pulses) for each incident acoustic pressure. The incident acoustic pressure with maximum stable subharmonic range was selected for LV pressure tracking as shown in Figure 2-C (to avoid loss of microbubbles’ pressure sensitivity due to bubble collapse at high incident acoustic pressures or lack of subharmonic signals at low acoustic pressures). The calibration factor in mmHg/dB was calculated using least square regression analyses from the aortic data (utilizing both subharmonic data and pressure values). This calibration factor was applied to the subharmonic data from the LV of the individual canine to determine the mean LV diastolic pressure (M-LVDP), minimum LV diastolic pressure (LVDmin), LV end diastolic pressure (LVEDP) and LV peak systolic pressure (LVPSP). For two other canines, LV pressure estimates were obtained with the mean calibration factor from the first two canines. The subharmonic estimates were compared to the manometer pressures. Two tailed paired t-tests were conducted for each LV pressure parameter obtained from SHAPE and the reference standard (p-values<0.05 were considered significant).
Figure 2.



Ultrasound Data Processing
Steps for extracting and processing the subharmonic signal. Panels A and B illustrate a typical signal from a pulsed wave Doppler gate and its frequency domain representation. Fundamental and subharmonic signals within the bandwidth of the transducer are labeled (Panel B). Panel C illustrates the processed subharmonic signal (blue) and the pressure catheter data (red) - note the inverse relationship which is in agreement with documented literature (3–5)).
Analysis of the Calibration Factor (mmHg/dB) from Aorta (Table 1)
Table 1.
Subharmonic-pressure calibration factor from the aorta
| Calibration factor in the aorta (mmHg/dB) | Standard error of the estimates (mmHg) | Mean aortic pressures (mmHg)
|
||
|---|---|---|---|---|
| SHAPE* | Catheter | |||
| Canine 1 | −4.929 | 4.32 | 67.98 | 68.29 |
| Canine 2 | −4.920 | 7.13 | 59.61 | 62.10 |
subharmonic aided pressure estimation
Catheter Calibration factor calculated from the aorta of two canines shown with the standard error of the estimates from the linear regression analysis. Mean aortic pressures obtained using SHAPE and the manometer are compared.
The calibration factors obtained from the aorta of two canines verify that the subharmonic signal varied inversely with the underlying pressure waveform. The maximum error between the mean aortic pressures recorded by the SHAPE approach and the reference standard was 2.5 mmHg.
SHAPE in LV using Calibration Factor from Aorta (Table 2)
Table 2.
Left ventricular (LV) pressure measurements with aortic calibration factors
| Canine 1
|
Canine 2
|
|||||||
|---|---|---|---|---|---|---|---|---|
| SHAPE║ | Catheter | Error (mmHg) | Percent Error | SHAPE║ | Catheter | Error (mmHg) | Percent Error | |
| M-LVDP* | 20.11 | 17.61 | 2.50 | 14 | 14.21 | 13.42 | 0.79 | 6 |
| LVDmin† | 15.88 | 15.69 | 0.19 | 1 | 7.53 | 8.89 | −1.36 | −15 |
| LVEDP‡ | 22.07 | 19.74 | 2.33 | 12 | 19.11 | 16.89 | 2.22 | 13 |
| LVPSP§ | 70.23 | 68.81 | 1.42 | 2 | 83.84 | 82.11 | 1.73 | 2 |
| Heart Rate (per min.) |
109.77 | 109.86 | N/A | N/A | 105.47 | 109.86 | N/A | N/A |
mean LV diastolic pressure,
minimum LV diastolic pressure,
LV end diastolic pressure,
LV peak systolic pressure,
subharmonic aided pressure estimation
LV pressures from two canines (where data was also acquired from the aorta) using SHAPE compared to the reference standard (manometer catheter). Errors are indicated as reference standard subtracted from the SHAPE data. Percent errors are calculated using the reference standard.
For two canines where the calibration factor was obtained from the aorta, the LV pressure estimates were calculated using the known peak systolic LV pressures. Figure 3 shows a trace of LV pressure obtained using SHAPE and the manometer pressures for canine 1. Overall, errors between the SHAPE approach and the pressure catheter ranged from 0.19 to 2.5 mmHg.
Figure 3.
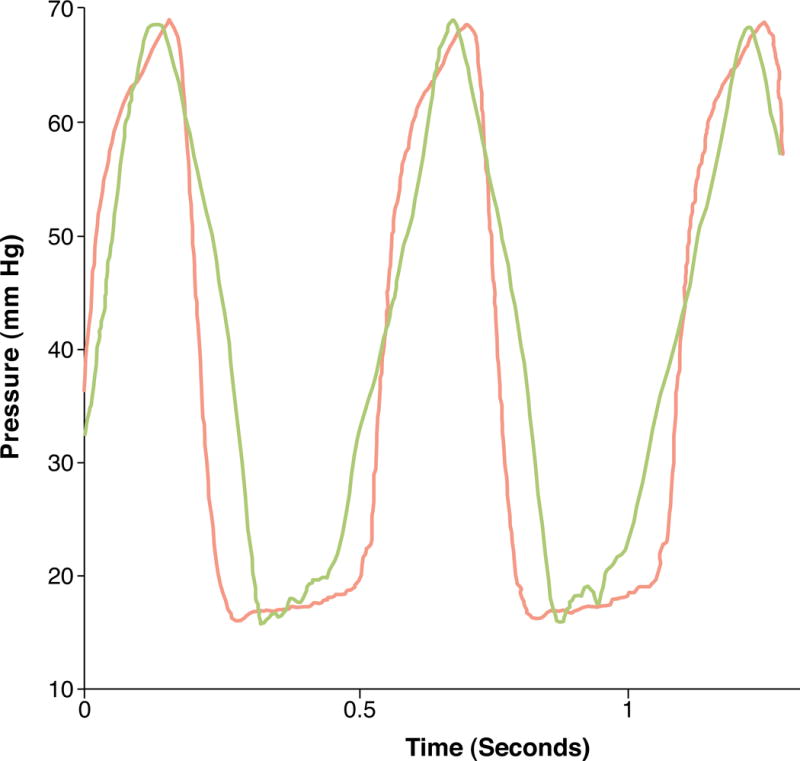
LV Pressure Tracking
LV pressure waveform obtained using the manometer (red) and the corresponding subharmonic aided pressure estimation results (blue).
SHAPE in LV using Mean Calibration Factor from Aorta of Other Canines (Table 3)
Table 3.
Left ventricular (LV) pressure measurements without aortic calibration factors
| Canine 3
|
Canine 4
|
|||||||
|---|---|---|---|---|---|---|---|---|
| SHAPE║ | Catheter | Error (mmHg) | Percent Error | SHAPE║ | Catheter | Error (mmHg) | Percent Error | |
| M-LVDP* | 13.61 | 14.25 | −0.64 | −4 | 15.43 | 13.02 | 2.41 | 19 |
| LVDmin† | 5.82 | 7.23 | −1.41 | −20 | 12.04 | 9.59 | 2.45 | 26 |
| LVEDP‡ | 12.06 | 18.13 | −6.07 | −33 | 21.99 | 13.01 | 8.98 | 69 |
| LVPSP§ | 82.66 | 79.57 | 3.09 | 4 | 83.84 | 84.59 | −0.75 | 1 |
| Heart Rate (per min.) |
99.98 | 100.71 | N/A | N/A | 94.79 | 91.55 | N/A | N/A |
mean LV diastolic pressure,
minimum LV diastolic pressure,
LV end diastolic pressure,
LV peak systolic pressure,
subharmonic aided pressure estimation
LV pressures from two canines (where data was acquired from the LV only) using SHAPE compared to the reference standard (manometer catheter). The errors are indicated as in Table 2.
For the two other canines, the mean calibration factor from the first two canines (i.e., −4.9245 mmHg/dB obtained from Table 1) was used to estimate the LV pressures. For these canines, relatively higher errors between the SHAPE approach and the pressure catheter were reported ranging from 0.64 to 8.98 mmHg.
Comparison of SHAPE LV pressures with manometer pressures (Table 4)
Table 4.
Paired Comparisons of subharmonic aided pressure estimation (SHAPE) in left ventricle (LV) and the manometer pressures for all canines.
| Difference (mmHg) (SHAPE║ – manometer pressure
|
95% Confidence Interval of the Difference (mmHg)
|
Significance (2-tailed) | ||||
|---|---|---|---|---|---|---|
| Mean | Standard deviation | Standard error of mean | Lower | Upper | ||
| M-LVDP* | 1.26 | 1.49 | 0.75 | −1.11 | 3.64 | 0.18 |
| LVDmin† | −0.03 | 1.81 | 0.91 | −2.92 | 2.85 | 0.97 |
| LVEDP‡ | 1.86 | 6.16 | 3.08 | −7.94 | 11.67 | 0.59 |
| LVPSP§ | 1.37 | 1.59 | 0.79 | −1.16 | 3.90 | 0.18 |
mean LV diastolic pressure,
minimum LV diastolic pressure,
LV end diastolic pressure,
LV peak systolic pressure
None of the differences between SHAPE and the reference standard for all the estimates of LV pressures were significant (p>0.17). The mean absolute errors for M-LVDP, LVDmin, LVEDP and LVPSP were 1.58±1.01 mmHg, 1.35±0.92 mmHg, 4.90±3.26 mmHg and 1.75±0.98 mmHg, respectively. Heart rate obtained from the frequency domain representation of SHAPE data and pressure catheter data was also in good agreement with maximum absolute error of 4.39 beats per minute (Tables 2 and 3). These results, based on estimates from all four canines, indicate that SHAPE is able to noninvasively track in vivo pressures in the LV, if the pulse pressures in the aorta are known.
Discussion
The use of microbubbles to quantify ambient pressures based on shift in resonance frequency, detection of single bubble echoes, excitation using dual frequencies, monitoring cavitation onset or dissolution time of microbubbles have been tested in vitro with errors ranging from 10 to 15 mmHg under ideal conditions. None of these techniques have documented in vivo results. Conversely, Shi et al verified empirically that subharmonic signal amplitude from UCA show ambient pressure sensitivity (3). This technique was used in vivo to investigate its efficacy (4), though invasively and finally studies were performed to identify optimum parameters for noninvasive application of SHAPE (5), which were used in this study (i.e., Sonazoid microbubbles and 2.5 MHz insonation frequency). The incident acoustic pressure required to elicit a subharmonic response sensitive to in vivo pressures will vary on a case-by-case basis based on attenuation of the incident beam and the scanning depth. Therefore acoustic output power on the scanner was varied and the level showing maximum stable subharmonic range was selected for pressure monitoring.
These results are the first in vivo study demonstrating the application of subharmonic emissions from microbubbles for noninvasive quantification of LV pressures. First, it was hypothesized that the subharmonic emissions from microbubbles can be utilized to quantify in vivo LV pressures noninvasively. The resultant pressure errors between SHAPE and the pressure catheter did not exceed 2.50 mmHg when the calibration factor from the aorta of the individual canine was used in the pressure derivation stage (cf., Table 2). Additionally, there were no statistically significant differences between pressures recorded by SHAPE and the pressure catheter (p>0.17; cf., Table 4). Second, it was hypothesized that a mean calibration factor may be used across all subjects. The errors increased up to 8.98 mmHg under this hypothesis (cf., Table 3). This suggests that the addition of aortic measurements to obtain a calibration factor from each individual subject allows LV pressure estimation with less error. The calibration factor (mmHg/dB) relates the change in subharmonic signal amplitude to change in pressure value; this relationship extends across the full range of clinical pressures (5). In cases presented with disease states like aortic stenosis, where LVPSP may differ from the peak cuff-based pressure estimate, Doppler based LV-aortic pressure gradient may be additionally incorporated for LVPSP determination. Thus, we have shown that SHAPE is in good agreement with the reference standard (maximum mean absolute error: 2.40±2.22 mmHg) based on data from four canines across all pressure estimates.
Study Limitations
A new version of the subharmonic extraction and processing algorithm will permit real-time pressure estimation in future studies, unlike the off-line data processing of this study.
The SHAPE technique is incident acoustic pressures dependent. In this study, discrete increments (of 2 dB i.e., 25% relative increase) for the incident acoustic pressures (fixed by the ultrasound scanner) were used. Thus, if the most “sensitive” incident acoustic pressure falls between the range of 25% then the SHAPE pressure sensing will not be “optimum”, thereby contributing to the errors attained in the LV pressure estimates.
The small sample size (four canines) may obscure any statistically significant difference between SHAPE estimates and the manometer pressures.
For potential clinical use, the accuracy of this technique with cuff based pressure measurements remains to be investigated.
Lastly, the hemodynamics in the canines were not altered. However, as evident in Figure 3, relative changes occur in the subharmonic amplitude of the signal in response to changes in the ambient pressures, and thus real-time pressure tracking is feasible (to monitor instantaneous hemodynamic changes, as well).
Clinical Implications
A single underlying technique to quantify LV pressures noninvasively may aid patient management in addition to the existing indices, which are mostly applicable in specific subsets of cardiac pathologies. The absolute LV pressures obtained using the SHAPE approach may be applicable to all clinical populations without the added risks and costs involved in right heart catheterization. As UCA are already approved in the United States for LV opacification, the supplementary pressure values obtained using SHAPE may provide a quantitative parameter for the diagnosis of a myriad of cardiac pathologies. Moreover, these pressures can be obtained with the same contrast administration dosage as the subharmonic signal amplitude reduction was negligible even when the concentration of the bubbles was varied by a factor of 3 (3). Therefore, we conclude that the ability of subharmonic emissions from UCA to noninvasively monitor LV pressures should now be investigated in clinical populations to verify ultimate applicability.
Acknowledgments
Funding sources: AHA grant 0655441U: Dallas, Texas; NIH R21 HL081892 and RC1 DK087365 (JRE): Bethesda, Maryland and U.S. Army Medical Research & Material Command grant W81XWH-08-1-0503 (VGH): Fort Detrick, Maryland
ABBREVIATIONS AND ACRONYMS
- LV
leftventricle/ventricular
- LVDmin
minimum LV diastolic pressure
- LVEDP
LV end diastolic pressure
- LVPSP
LV peak systolic pressure
- M-LVDP
mean LV diastolic pressure
- PW
pulsed wave
- RF
radiofrequency
- SHAPE
subharmonic aided pressure estimation
- UCA
ultrasound contrast agents
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: KD and CL: employees of Ultrasonix Medical Corporation.
References
- 1.Chatterjee K. The Swan-Ganz catheters: past, present and future: a viewpoint. Circulation. 2009;119:147–52. doi: 10.1161/CIRCULATIONAHA.108.811141. [DOI] [PubMed] [Google Scholar]
- 2.Solomon S, Stevenson L. Recalibrating the barometer: is it time to take a critical look at noninvasive approaches to measuring filling pressures? Circulation. 2009;119:13–5. doi: 10.1161/CIRCULATIONAHA.108.823591. [DOI] [PubMed] [Google Scholar]
- 3.Shi W, Forsberg F, Raichlen J, Needleman L, Goldberg B. Pressure dependence of subharmonic signals from contrast microbubbles. Ultrasound Med Biol. 1999;25:275–83. doi: 10.1016/s0301-5629(98)00163-x. [DOI] [PubMed] [Google Scholar]
- 4.Forsberg F, Liu J, Shi W, Furuse J, Shimizu M, Goldberg B. In vivo pressure estimation using subharmonic contrast microbubble signals: proof of concept. IEEE Trans Ultrason, Ferroelec Freq Contr. 2005;52:581–3. doi: 10.1109/tuffc.2005.1428040. [DOI] [PubMed] [Google Scholar]
- 5.Halldorsdottir VG, Dave JK, Leodore LM, Eisenbrey JR, Park S, Hall AL, Thomenius K, Forsberg F. Subharmonic contrast microbubble signals for noninvasive pressure estimation under static and dynamic flow conditions. Ultrason Imaging. 2011;33:153–64. doi: 10.1177/016173461103300301. [DOI] [PMC free article] [PubMed] [Google Scholar]


