Introduction
Allergy to foods is an increasing problem in the American population; shellfish allergy affects approximately 2% of the adult US population and can affect up to 3.1% of adults ages 41–60[1]. Reactions include classical immediate hypersensitivity such as urticaria, swelling, difficulty breathing and in some cases anaphylaxis[1].
Tropomyosin, a 34kDa muscle protein, is a major shrimp allergen and binds IgE in at least two thirds of allergic patients[2]. However; other shrimp antigens such as shrimp tRNA, Pen m2 and sarcoplasmic calcium binding protein have also been reported [3–5]. Tropomyosin is a pan-allergen, with similar amino acid sequence and allergenic properties in shellfish such as lobster and crab, as well as other arthropods, such as cockroach and mites[6–8] Cross reactivity to arthropod tropomyosin and shellfish occurs frequently in shrimp allergic patients; tropomyosin is a major allergen from many invertebrate species[6, 9, 10].
Immunotherapy is a treatment in which the allergen is administered in increasing doses, either sublingually or subcutaneously, in an effort to tolerize T cells [11–14]. Immunotherapy has been a successful method for desensitizing patients with allergies, including sensitivities to cat, pollen, and bee venom [15–19]. Sublingual immunotherapy is generally considered safer than injections; however, in the case of severe food allergies systemic reactions may still occur. Immunotherapy with short peptides, or T cell epitopes, has also been proposed as a safer method of desensitization, as peptides are too short to crosslink IgE on mast cells and cause life threatening anaphylaxis.
T cell epitope immunotherapy has been reported to desensitize patient's allergic reaction to the bee venom phospholipase A2 protein, Cry j 1, a Japanese Cedar pollinosis antigen and Fel d 1, the protein involved in cat allergies[20–23]. The mechanism, as in traditional therapy, is thought to involve induction of IL-10 secreting Treg cells which down-regulate the activity of Th2 T lymphocytes [24–29]. In addition, IL-10 is known to provoke B cell switching to IgG4, which may act as a blocking antibody to IgE[13],[11],[17]. IL-10 can also decrease eosinophil and neutrophil recruitment.
In this study, we describe the clinical characteristics and result of skin testing and IgE responses to different shellfishand seafood antigens in a cohort of shrimp allergic patients. We also demonstrate that in vitro stimulation with native tropomyosin protein and tropomyosin-derived peptides produce dose-dependent T-cell proliferative responses in shrimp allergic patients. Our results are important for future identification of candidate T-cell epitopes that can be used for immunotherapy of patients with shrimp allergy.
Materials and Methods
Study Population
Patients and controls were recruited based on history of allergy to shrimp that included the time between exposure and reaction. Reactions included urticaria, erythema, swelling, vomiting, diarrhea, rhinitis, coughing, dypsnea, and loss of consciousness. After written consent, individuals were skin prick tested with commercially available allergen extracts of shrimp (family Penaeidae), crab (Callinectes spp), lobster (Homarus americanus), mollusks (Mercenaria spp., Ostrea/Crassostrea, Placopecten magellanicus) and fish species (Salmo salar, Salmo salar, Oncorhynchus mykiss, Thunnus sp.), as well as saline solution as a negative control and histamine as a positive control (Greer,Lenoir,NC). Allergen solutions were applied on the volar skin of the arm, and wheal and flare reactions were read after 15–20 min. Mean wheal and flare diameters were calculated from the largest and perpendicular diameters. The Institutional Review Board at the University of Utah approved this protocol.
Total and specific IgE levels
Sera obtained from patients and controls were tested for total and specific IgE levels to shellfish-derived extracts using Immunocap assays according to the manufacturer's recommendations (Phadia, Uppsala, Sweden). Tropomyosin specific immunocaps were made using purified biotinylated P. monodon tropomyosin conjugated to streptavidin immunocaps at 0.25 mg/ml according to the manufacturer's recommendations.
T-cell proliferation assays
T-cell proliferation assays were performed in peripheral blood mononuclear cells (PBMC) from patients and controls added to 96-well round-bottom plates in culture media containing different concentration of purified, native tropomyosin and tropomyosin-derived peptides as indicated. Cultures were incubated for six days and pulsed overnight with tritiated thymidine. Radioactive label incorporation during T-cell proliferation was measured by liquid scintillation spectroscopy. T-cell proliferation was also measured using flow cytometric analysis of cells stained with carboxyfluorescein succinimidyl ester (CFSE) [30]. PBMC were stained with 0.5 μM CFSE and cultured for 7 days in the presence or absence of tropomyosin (1 μg/ml). Dosage of tropomyosin was determined by previous dose response curves ranging from 0–10 μg/ml. At the end of the 7-day culture, cells were stained with fluorescent-labeled monoclonal antibodies against CD4+, CD45RA+ and CD45RO+ and cells analyzed by flow cytometry using FlowJo software (Tree Star Inc., Ashland, OR).
The distributions of proliferation results were tested by the D'Agostino & Pearson normality test. According to the results of normality test, variables were analyzed by the paired t test or Mann Whitney test. Levels of significance are reported as two- tailed p values. A p value <0.05 was considered significant. These data analyses were performed with the aid of Prims 5 software (GraphPad, La Jolla, CA).
Antigens and peptides
Tropomyosin was purified from frozen Black Tiger (Penaeus monodon) shrimp (Aquarius Fish Market, Salt Lake City, UT). Black Tiger shrimp is one of the major cultivated shrimp species worldwide. Briefly, 500 g of frozen Black Tiger shrimp (Aquarius Fish Company, Salt Lake City, UT) was blended in a food processor and extracted in 20 mM KCl, 1 mM KHCO3, 0.1mM dithiothreitol for 10 minutes, for a total of five times. Extract was discarded and the shrimp homogenate was filtered through cheese cloth and extracted three times in 1 liter of 95% ethanol for ten minutes and extracted three times in 1 liter of diethyl ether for 10 minutes. Extract was discarded and the shrimp homogenate was dried 2 hours or overnight in the hood and extracted overnight in high salt buffer. Proteins were ammonium sulfate precipitated from shrimp extract, solubilized and tropomyosin was precipitated by reducing the pH. The precipitate was solubilized and dialyzed into PBS[31]. Purity was validated by 12% Bis Tris SDS-PAGE (Figure 1) (Life Technologies, Grand Island, NY). The most abundant purified protein was identified as shrimp tropomyosin by mass spectrometry; peptides were analyzed using a nano-LC/MS/MS system comprised of a nano-LC pump (2D-ultra, Eksigent) and a LTQ-FT mass spectrometer (ThermoElectron Corporation, San Jose, CA) and a MASCOT full database search (Matrix Science, Boston, MA).
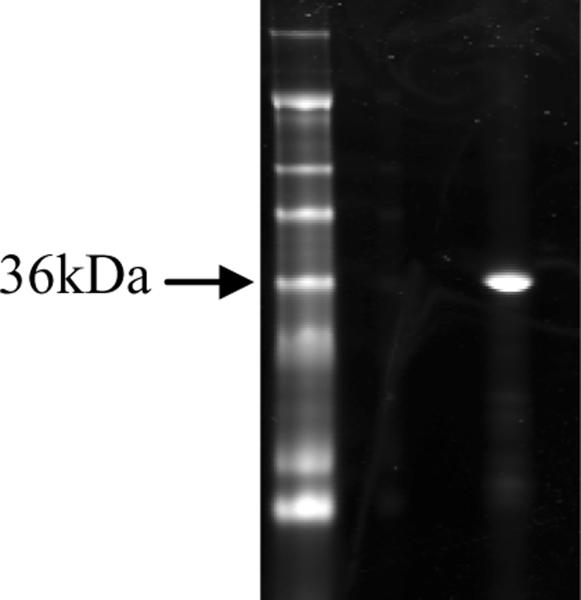
Figure 1.
SDS PAGE of purified P. monodon tropomyosin. Lane 1: Marker (SeeBlue Plus2, Life Technologies, Grand Island, NY). Lane 2: 1 μg purified protein. The arrow points to the 36kDa marker. The predicted band size of shrimp tropomyosin is 34 kDa.
Synthetic peptides were obtained from Mimotopes Ltd (Melbourne, Australia) in sets consisting of 15-mers overlapping by 12 amino acids spanning the P. monodon tropomyosin molecule. Based on the scale of synthesis (~4 mg/peptide), each peptide was solubilized in 30 μl of dimethyl sulfoxide and brought up to 10 mM in PBS.
Results
Clinical history, skin prick testing, shrimp specific IgE levels and T cell proliferation in response to shrimp tropomyosin were characterized in 58 subjects, including thirty eight shrimp allergic patients and 20 negative controls with no history of shrimp allergy. History included questions regarding immediate type I reactions such as hives, swelling, runny nose and tightness of the chest as well as abdominal symptoms such as diarrhea and vomiting. Reactivity to allergens measured by skin prick testing included shrimp, as well as other crustaceans, mollusks and fish (Table 1). Classical immediate type I allergic reactions were reported by 29 out of 38 patients (76%), whereas abdominal symptoms, including vomiting and diarrhea, were reported in nine (24%) of the allergic subjects. Only two out of twenty negative controls (10%) were positive by skin prick testing.
| Patient | Skin Prick | Total IgE | Shrimp | Tropo | Lobster | Crab | Cod | Tuna | Salmon | Mackerel | Trout | Clam | Oyster | Scallop |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | + | 944 | 8.15 | 2.69 | 7.93 | 7.12 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | 1.77 | 2.83 | 2.57 |
| 19 | + | 68.0 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | |
| 20 | + | 236 | 28.1 | 12.1 | 24.6 | 20.8 | <0.35 | 0.76 | <0.35 | <0.35 | <0.35 | 4.03 | 2.40 | 5.36 |
| 23 | + | 109 | 4.33 | 1.07 | 1.79 | 1.79 | <.35 | <0.35 | <0.35 | <0.35 | <0.35 | 0.48 | 0.45 | 0.37 |
| 24 | + | 417 | 1.94 | <0.35 | 0.48 | 0.5 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 |
| 25 | + | 40.8 | 0.84 | 0.43 | 0.98 | 0.95 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 |
| 26 | + | 86.8 | <0.35 | <0.35 | 2.55 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | |
| 27 | + | 22.3 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | |
| 29 | + | 122 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | |
| 31 | + | 278 | 40.5 | 13.5 | 26.5 | 22 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | 6.49 | 5.60 | 4.62 |
| 32 | + | 111 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | |
| 33 | + | 1074 | 30.6 | 12.0 | 24.6 | 27.0 | 1.42 | 3.57 | 2.74 | 2.21 | 0.98 | 8.02 | 2.94 | 5.71 |
| 35 | + | 40.3 | 0.47 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 |
| 37 | + | 172 | 11.4 | 0.47 | <0.35 | 2.49 | <0.35 | <0.35 | <0.35 | 1.64 | <0.35 | <0.35 | <0.35 | <0.35 |
| 39 | + | 8.16 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | |
| 40 | + | 12.8 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | |
| 42 | + | 85.2 | 1.31 | 0.40 | <0.35 | 1.06 | <.35 | 0.38 | <.35 | 0.79 | <0.35 | <0.35 | <0.35 | <0.35 |
| 43 | + | 408 | 20.3 | 0.48 | 0.41 | 0.62 | <.35 | <.35 | <.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 |
| 44 | + | 52.6 | 1.9 | 0.67 | 0.73 | <0.35 | <0.35 | 0.39 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | |
| 45 | + | 12.5 | 1.32 | 0.48 | <0.35 | 0.83 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 |
| 46 | + | 406 | 50.8 | 26.9 | 47.0 | 44.9 | <0.35 | 0.86 | 0.36 | <.35 | <.35 | 11.3 | 5.55 | 4.77 |
| 47 | + | 213 | 0.65 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 |
| 4 | − | 21.8 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | |
| 8 | − | 68.9 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | |
| 10 | − | 872 | 1.49 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 |
| 15 | − | 79.0 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | |
| 30 | − | 216 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | |
| 38 | − | 95.3 | <0.35 | <0.35 | <.35 | <.35 | <.35 | <.35 | <.35 | <.35 | <.35 | <.35 | <.35 | |
| 41 | − | 227 | 1.65 | 0.55 | 1.27 | 1.27 | <0.35 | <0.35 | 0.45 | 0.47 | 0.42 | <0.35 | <0.35 | 0.39 |
The distribution of total and specific IgE levels in the cohort of 29 patients with classical immediate type I allergic manifestations and 20 negative controls are shown in Figure 2 and Table 1. Total IgE levels were significantly increased in the 29 patients (median: 109 IU/ml, range: 8.16 – 1,074) compared to the 20 controls (median: 30.95 IU/ml, range: 3.16 – 153; p= 0.0024) (Figure 2A). Eleven out of 29 patients (38%) had total IgE levels above the normal reference range (0–180 IU/ml), whereas none of the controls had elevated total IgE levels. IgE levels to shrimp extracts were also significantly increased in the 29 patients (median: 0.84 kU/L, range: 0.35 – 50.80) compared to the 20 controls (median: 0.1 kU/L, range: 0.1 – 0.21; p< 0.0001) (Figure 2B). Seventeen out of 29 patients (59%) had shrimp-specific IgE levels above the normal reference range (>35 kU/L), whereas none of the control had elevated shrimp-specific IgE levels.
Figure 2.
Distribution of total IgE (A) and specific IgE levels to shrimp extracts (B) in the cohort of 29 patients with classical immediate type I allergic manifestations and 20 negative controls. Horizontal lines represent median values. Negative values differ slightly due to a slight change in the manufacturer's non-reactive baseline values.
Patients with increased IgE levels to shrimp extracts were tested for IgE levels to shrimp tropomyosin and extracts from other shellfish species. We observed that 12 out of 17 patients (70%) with elevated IgE levels to shrimp extracts in serum also had elevated IgE levels to shrimp tropomyosin. Elevated IgE levels in shrimp tropomyosin reactive patients were also observed to extracts from crab (88%), lobster (65%), scallop (41%), clam (35%), oyster (35%), tuna (24%), salmon (24%), trout (12%), and cod (6%) (Table 1).
T cell proliferation in response to native tropomyosin stimulation in vitro
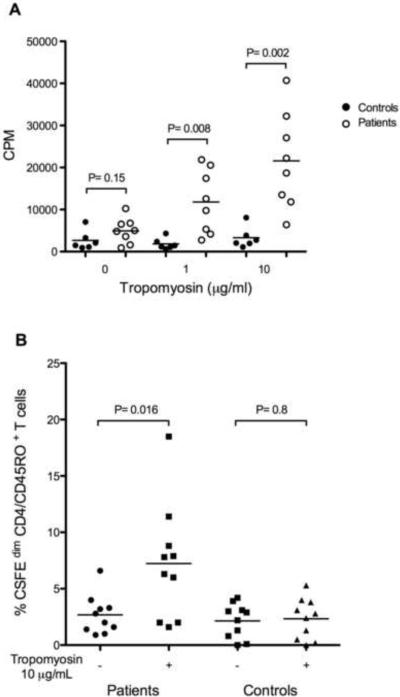
T cell proliferation in response to tropomyosin challenge was measured in PBMC from shrimp allergic patients and controls in vitro. Remarkably, the proliferative response of T cells from shrimp allergic patients to tropomyosin was significantly increased as compared with the response of T cells from controls (Figure 3A). Proliferation was also measured by flow cytometry by incorporation of CFSE, which is sequestered inside the cytoplasm of viable cells and diluted during cell proliferation. Using this method to analyze the proliferation of subsets of CD4+ T cells including naïve T cells (CD4+, CD45RA+) and memory T cells (CD4+, CD45RO+), we found a significant increase in the number of proliferating CD4+ T cells of the memory subset (CD4+, CD45RO+) after tropomyosin stimulation (Figure 3B), whereas the increase in the number of proliferating CD4+ T cells of the naïve subset (CD4+, CD45RA+) was minimal (data not shown).
Figure 3.
(A) T-cell proliferation in response to native tropomyosin stimulation in vitro. P. monodon tropomyosin was added to PBMCs from patients (N=8) and controls (N=6) at concentrations indicated on the x axis. Counts per minute (CPM) of incorporated tritiated thymidine from triplicate 6-day cultures are shown for each individual. Patients represented are: 2, 20, 31, 33, 35, 37, 41, 46, and six negative controls (5, 6, 14, 16, 18, 21). (B) Percentage of proliferating memory T cells (CFSEdim CD4/CD45RO+) in patients (N=10) and controls (N=10) in the presence or absence of 10 μg/mL of tropomyosin from P. monodon. Horizontal lines represent median values. Patients represented are: 2, 20, 27, 31, 33, 35, 37, 43, 45, 46 and negative controls 5, 8, and 16 ARUP controls*) *Patients were positive for skin prick testing and history. Controls were individuals tested negative for skin prick, and normal IgE total and IgE shrimp.
Specific T-cell responses to tropomyosin-derived peptides
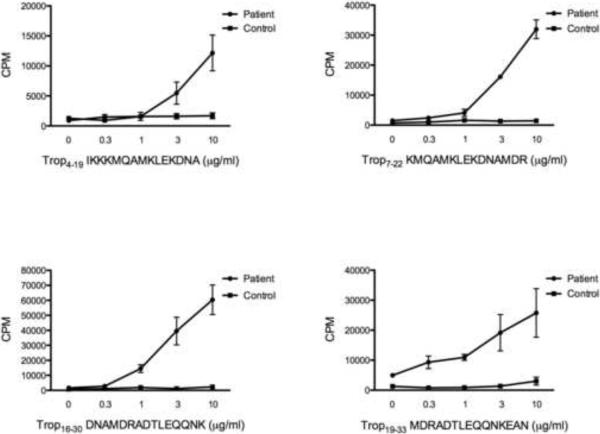
Overlapping 15-mer peptides derived from the P. monodon tropomyosin protein sequence were tested for their ability to stimulate T-cell proliferation in selected shrimp allergic patients. Several peptides from tropomyosin protein elicited strong dose dependent proliferative responses in shrimp allergic patients compared to HLA-DR matched controls, suggesting that multiple peptides from the tropomyosin protein can be recognized by T cells in a variety of HLA backgrounds (Figure 4). Moreover, a tropomyosin-specific T-cell line established from one patient by sorting and expanding proliferating T cells exhibited dose dependent proliferation in response to tropomyosin, but not to a control peptide from Hepatitis C virus (Figure 5).
Figure 4.
Examples of T-cell proliferative responses to stimulation with tropomyosin-derived peptides. P. monodon tropomyosin-derived peptides were added to PBMCs from patients and controls at concentrations indicated on the x axis. Averages and standard error of counts per minute (CPM) of incorporated tritiated thymidine from triplicate 6-day cultures are shown for each experiment.
Figure 5.
Proliferative response of a tropomyosin-specific T-cell line. P. monodon tropomyosin and Hepatitis C peptide (VIKGGRHLIFCHPKKKCD) were added to the T-cell line at concentrations indicated on the x axis. Averages and standard error of counts per minute (CPM) of incorporated tritiated thymidine from triplicate 6-day cultures are shown for each experiment.
Discussion
A cohort of shrimp allergic patients has been identified and characterized on the basis of history as well as skin prick testing, analysis of serum IgE levels, and lymphocyte proliferation in response to shrimp tropomyosin. Most shrimp allergic subjects exhibited a classical immediate type hypersensitivity characterized by positive skin prick tests, a history of swelling, urticaria and respiratory difficulties following shrimp ingestion. 76% of patients with a history of immediate shrimp sensitivity had a positive skin prick test. The existence of a group of patients with a history indicative of shrimp allergy but with negative skin tests suggests that these patients have lost sensitivity, possibly as part of the natural history of this disease. Furthermore, 59% of subjects with immediate type reactions according to history were positive for shrimp specific IgE, indicating that approximately a third of subjects have non-IgE mediated allergy or that enough time had elapsed since shrimp ingestion that IgE antibodies had disappeared from the circulation. Of shrimp IgE positive subjects, 70% were also positive for shrimp tropomyosin specific IgE, suggesting that 30% of allergic patients may be allergic to other shrimp proteins, such as tRNA, Pen m2 and sarcoplasmic calcium binding protein, similar to findings in other studies[3–5]. Allergic reactions to crustaceans and mollusks are often cross-reactive, which is explained by the highly conserved amino acid sequences of tropomyosins among these species [6–8]. Consistent with sequence conservation of tropomyosin, we observed elevated IgE levels to extracts from crab, lobster, scallop, clam, oyster, tuna, salmon, trout, and cod which may be cross reactive to tropomyosin.
Allergic responses are initially mediated through the presentation of allergens in the context of HLA class II molecules to naïve CD4 T cells that mature into CD4 memory T cells. CD4 memory T cells stimulate B cell differentiation into plasma cells that secrete allergen specific IgE antibodies. Upon the re-exposure to the allergen, memory CD4 T cells will re-stimulate specific IgE antibody production. In this study we demonstrate that patients with clinical history of shrimp allergy and increased shrimp-specific IgE levels have dose dependent proliferation of memory CD4+ T cells after in vitro stimulation with P. monodon tropomyosin. To our knowledge this is the first study showing that tropomyosin, the most important shrimp-derived allergen, elicits memory T cell proliferation in the context of HLA class II-restricted antigen presentation.
Presently, the primary therapy for shellfish allergy is the avoidance of shellfish food. While this is effective, it requires careful attention to the diet, and even then, allergenic food can be disguised in mixtures of other foods and produced severe reactions. Desensitization shots can be dangerous due to the risk of anaphylaxis. Therefore, a vaccine using short peptides is likely to be much safer and avoid the risk of anaphylaxis. Recent published reports on T-cell epitope peptide vaccines for the treatment of allergic diseases are most promising.
T cell epitope immunotherapy has been reported to desensitize patient's allergic reaction to the bee venom phospholipase A2 protein, Cry j 1, a Japanese Cedar pollinosis antigen and Fel d 1, the protein involved in cat allergies[20–23]. The mechanism, as in traditional therapy, is thought to involve induction of IL-10 secreting Treg cells which down-regulate the activity of Th2 T lymphocytes[24–29]. In addition, IL-10 is known to provoke B cell switching to IgG4, which may act as a blocking antibody to IgE[13],[11],[17]. IL-10 can also decrease eosinophil and neutrophil recruitment. In the case of cat allergy, treatment of 16 sensitive patients with T-cell peptides from the major cat allergen, Fel d 1, had profound effects; cutaneous late phase reactions, stimulated by Fel d 1, diminished strikingly, and lymphocyte proliferation and PBMC production of IL-4, Il-13 and interferon-γ, stimulated by whole cat dander, decreased significantly. In addition, IL-10 production by PBMC increased significantly and the ability of the T cell peptide treated patients to tolerate exposure to cats significantly improved[23]. In patients with honey bee venom allergy, treatment with T cell peptides from phospholipase A2 specifically reduced proliferation of PBMC to phospholipase A2 and production of TH1 and TH2 cytokines[20]. After two months of peptide immunotherapy, three of the five patients tolerated sting challenge without anaphylaxis, although two patients developed erythema and urticaria with mild angioedema. Treatment of 12 additional bee venom sensitive patients with T-cell peptides from phospholipase A2 showed reductions in cutaneous late-phase reactions, PBMC proliferation, production of IL-13 and interferon-γ, and increases in IL-10 production[32]. These results encourage belief that administration of peptides corresponding to T cell epitopes may prove effective for allergen desensitization.
In this study, overlapping 15-mer peptides from the P. monodon tropomyosin protein sequence were used to stimulate T-cell proliferation in selected shrimp allergic patients. The finding that multiple peptides from tropomyosin protein elicit strong dose dependent proliferative responses in patients compared to HLA-DR matched controls, strongly suggests that combination of peptides from tropomyosin protein can be used to modulate T-cell responses of shrimp allergic patients in a variety of HLA backgrounds. Further studies are necessary to precisely map and validate tropomyosin-derived T-cell epitopes and to determine the patterns of presentation by different HLA class II molecules.
Our results suggest an approach to exploit T-cell epitope vaccination as a desensitization therapy for allergy to shellfish. Knowledge of T-cell epitopes of shrimp tropomyosin will set the stage of future trials of T-cell epitope peptide therapy in allergy to shellfish. This is important because shrimp allergy can be disabling and potentially fatal. Severely affected individuals who might be afraid to visit a seafood restaurant because of cross contamination would at least be able to accompany their friends and perhaps partake in small amounts of shrimp. At the very least, severe consequences like anaphylactic shock could be eliminated with this approach.
Acknowledgements
This research was supported by a grant from the Food Allergy Initiative, NIH R21AI088589 and NIH 5R03AI076386-02. Mass spectrometry was supported by the University of Utah Mass Spectrometry Core Lab.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of seafood allergy in the United States determined by a random telephone survey. J Allergy Clin Immunol. 2004;114(1):159. doi: 10.1016/j.jaci.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 2.Shanti KN, Martin BM, Nagpal S, Metcalfe DD, Rao PV. Identification of tropomyosin as the major shrimp allergen and characterization of its IgE-binding epitopes. J Immunol. 1993;151(10):5354. [PubMed] [Google Scholar]
- 3.Nagpal S, Metcalfe DD, Rao PV. Identification of a shrimp-derived allergen as tRNA. J Immunol. 1987;138(12):4169. [PubMed] [Google Scholar]
- 4.Yu CJ, Lin YF, Chiang BL, Chow LP. Proteomics and immunological analysis of a novel shrimp allergen, Pen m 2. J Immunol. 2003;170(1):445. doi: 10.4049/jimmunol.170.1.445. [DOI] [PubMed] [Google Scholar]
- 5.Ayuso R, Grishina G, Ibanez MD, Blanco C, Carrillo T, Bencharitiwong R, Sanchez S, Nowak-Wegrzyn A, Sampson HA. Sarcoplasmic calcium-binding protein is an EF-hand-type protein identified as a new shrimp allergen. J Allergy Clin Immunol. 2009;124(1):114. doi: 10.1016/j.jaci.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 6.Leung PS, Chow WK, Duffey S, Kwan HS, Gershwin ME, Chu KH. IgE reactivity against a cross-reactive allergen in crustacea and mollusca: evidence for tropomyosin as the common allergen. J Allergy Clin Immunol. 1996;98(5 Pt 1):954. doi: 10.1016/s0091-6749(96)80012-1. [DOI] [PubMed] [Google Scholar]
- 7.Leung PS, Chen YC, Gershwin ME, Wong SH, Kwan HS, Chu KH. Identification and molecular characterization of Charybdis feriatus tropomyosin, the major crab allergen. J Allergy Clin Immunol. 1998;102(5):847. doi: 10.1016/s0091-6749(98)70027-2. [DOI] [PubMed] [Google Scholar]
- 8.Santos AB, Chapman MD, Aalberse RC, Vailes LD, Ferriani VP, Oliver C, Rizzo MC, Naspitz CK, Arruda LK. Cockroach allergens and asthma in Brazil: identification of tropomyosin as a major allergen with potential cross-reactivity with mite and shrimp allergens. J Allergy Clin Immunol. 1999;104(2 Pt 1):329. doi: 10.1016/s0091-6749(99)70375-1. [DOI] [PubMed] [Google Scholar]
- 9.Fernandes J, Reshef A, Patton L, Ayuso R, Reese G, Lehrer SB. Immunoglobulin E antibody reactivity to the major shrimp allergen, tropomyosin, in unexposed Orthodox Jews. Clin Exp Allergy. 2003;33(7):956. doi: 10.1046/j.1365-2222.2003.01722.x. [DOI] [PubMed] [Google Scholar]
- 10.Ayuso R, Lehrer SB, Reese G. Identification of continuous, allergenic regions of the major shrimp allergen Pen a 1 (tropomyosin) Int Arch Allergy Immunol. 2002;127(1):27. doi: 10.1159/000048166. [DOI] [PubMed] [Google Scholar]
- 11.Rolland JM, Gardner LM, O'Hehir RE. Allergen-related approaches to immunotherapy. Pharmacol Ther. 2009;121(3):273. doi: 10.1016/j.pharmthera.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Broide DH. Immunomodulation of allergic disease. Annu Rev Med. 2009;60:279. doi: 10.1146/annurev.med.60.041807.123524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gleich GJ, Zimmermann EM, Henderson LL, Yunginger JW. Effect of immunotherapy on immunoglobulin E and immunoglobulin G antibodies to ragweed antigens: a six-year prospective study. J Allergy Clin Immunol. 1982;70(4):261. doi: 10.1016/0091-6749(82)90062-8. [DOI] [PubMed] [Google Scholar]
- 14.Khinchi MS, Poulsen LK, Carat F, Andre C, Hansen AB, Malling HJ. Clinical efficacy of sublingual and subcutaneous birch pollen allergen-specific immunotherapy: a randomized, placebo-controlled, double-blind, double-dummy study. Allergy. 2004;59(1):45. doi: 10.1046/j.1398-9995.2003.00387.x. [DOI] [PubMed] [Google Scholar]
- 15.Smith H, White P, Annila I, Poole J, Andre C, Frew A. Randomized controlled trial of high-dose sublingual immunotherapy to treat seasonal allergic rhinitis. J Allergy Clin Immunol. 2004;114(4):831. doi: 10.1016/j.jaci.2004.06.058. [DOI] [PubMed] [Google Scholar]
- 16.Bilo MB, Severino M, Cilia M, Pio A, Casino G, Ferrarini E, Campodonico P, Milani M. The VISYT trial: Venom Immunotherapy Safety and Tolerability with purified vs nonpurified extracts. Ann Allergy Asthma Immunol. 2009;103(1):57. doi: 10.1016/S1081-1206(10)60144-5. [DOI] [PubMed] [Google Scholar]
- 17.Nieminen K, Valovirta E, Savolainen J. Clinical outcome and IL-17, IL-23, IL-27 and FOXP3 expression in peripheral blood mononuclear cells of pollen-allergic children during sublingual immunotherapy. Pediatr Allergy Immunol. 2009 doi: 10.1111/j.1399-3038.2009.00920.x. [DOI] [PubMed] [Google Scholar]
- 18.Gadermaier E, Flicker S, Aberer W, Egger C, Reider N, Focke M, Vrtala S, Kundi M, Valenta R. Analysis of the Antibody Responses Induced by Subcutaneous Injection Immunotherapy with Birch and Fagales Pollen Extracts Adsorbed onto Aluminum Hydroxide. Int Arch Allergy Immunol. 2009;151(1):17. doi: 10.1159/000232567. [DOI] [PubMed] [Google Scholar]
- 19.Dahl R, Kapp A, Colombo G, de Monchy JG, Rak S, Emminger W, Rivas MF, Ribel M, Durham SR. Efficacy and safety of sublingual immunotherapy with grass allergen tablets for seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;118(2):434. doi: 10.1016/j.jaci.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Muller U, Akdis CA, Fricker M, Akdis M, Blesken T, Bettens F, Blaser K. Successful immunotherapy with T-cell epitope peptides of bee venom phospholipase A2 induces specific T-cell anergy in patients allergic to bee venom. J Allergy Clin Immunol. 1998;101(6 Pt 1):747. doi: 10.1016/S0091-6749(98)70402-6. [DOI] [PubMed] [Google Scholar]
- 21.Bateman EA, Ardern-Jones MR, Ogg GS. Identification of an immunodominant region of Fel d 1 and characterization of constituent epitopes. Clin Exp Allergy. 2008;38(11):1760. doi: 10.1111/j.1365-2222.2008.03098.x. [DOI] [PubMed] [Google Scholar]
- 22.Masuyama K, Chikamatsu K, Ikagawa S, Matsuoka T, Takahashi G, Yamamoto T, Endo S. Analysis of helper T cell responses to Cry j 1-derived peptides in patients with nasal allergy: candidate for peptide-based immunotherapy of Japanese cedar pollinosis. Allergol Int. 2009;58(1):63. doi: 10.2332/allergolint.08-OA-0008. [DOI] [PubMed] [Google Scholar]
- 23.Oldfield WL, Larche M, Kay AB. Effect of T-cell peptides derived from Fel d 1 on allergic reactions and cytokine production in patients sensitive to cats: a randomised controlled trial. Lancet. 2002;360(9326):47. doi: 10.1016/s0140-6736(02)09332-7. [DOI] [PubMed] [Google Scholar]
- 24.Campbell JD, Buckland KF, McMillan SJ, Kearley J, Oldfield WL, Stern LJ, Gronlund H, van Hage M, Reynolds CJ, Boyton RJ, Cobbold SP, Kay AB, Altmann DM, Lloyd CM, Larche M. Peptide immunotherapy in allergic asthma generates IL-10-dependent immunological tolerance associated with linked epitope suppression. J Exp Med. 2009;206(7):1535. doi: 10.1084/jem.20082901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moingeon P, Batard T, Fadel R, Frati F, Sieber J, Van Overtvelt L. Immune mechanisms of allergen-specific sublingual immunotherapy. Allergy. 2006;61(2):151. doi: 10.1111/j.1398-9995.2006.01002.x. [DOI] [PubMed] [Google Scholar]
- 26.Francis JN, Till SJ, Durham SR. Induction of IL-10+CD4+CD25+ T cells by grass pollen immunotherapy. J Allergy Clin Immunol. 2003;111(6):1255. doi: 10.1067/mai.2003.1570. [DOI] [PubMed] [Google Scholar]
- 27.Bohle B, Kinaciyan T, Gerstmayr M, Radakovics A, Jahn-Schmid B, Ebner C. Sublingual immunotherapy induces IL-10-producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation. J Allergy Clin Immunol. 2007;120(3):707. doi: 10.1016/j.jaci.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 28.Bellinghausen I, Metz G, Enk AH, Christmann S, Knop J, Saloga J. Insect venom immunotherapy induces interleukin-10 production and a Th2-to-Th1 shift, and changes surface marker expression in venom-allergic subjects. Eur J Immunol. 1997;27(5):1131. doi: 10.1002/eji.1830270513. [DOI] [PubMed] [Google Scholar]
- 29.Akdis CA, Blesken T, Akdis M, Wuthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998;102(1):98. doi: 10.1172/JCI2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quah BJ, Warren HS, Parish CR. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat Protoc. 2007;2(9):2049. doi: 10.1038/nprot.2007.296. [DOI] [PubMed] [Google Scholar]
- 31.Greaser ML, Gergely J. Reconstitution of troponin activity from three protein components. J Biol Chem. 1971;246(13):4226. [PubMed] [Google Scholar]
- 32.Tarzi M, Klunker S, Texier C, Verhoef A, Stapel SO, Akdis CA, Maillere B, Kay AB, Larche M. Induction of interleukin-10 and suppressor of cytokine signalling-3 gene expression following peptide immunotherapy. Clin Exp Allergy. 2006;36(4):465. doi: 10.1111/j.1365-2222.2006.02469.x. [DOI] [PubMed] [Google Scholar]