Abstract
Objectives
Although healthy individuals have less force production capacity during bilateral muscle contractions compared to unilateral efforts, emerging evidence suggests that certain aspects of paretic upper limb task performance after stroke may be enhanced by moving bilaterally instead of unilaterally. We investigated whether the bilateral movement condition affects grip force differently on the paretic side of people with post-stroke hemiparesis, compared to their non-paretic side and both sides of healthy young adults.
Methods
Within a single session, we compared: 1) maximal grip force during unilateral vs. bilateral contractions on each side, and 2) force contributed by each side during a 30% submaximal bilateral contraction.
Results
Healthy controls produced less grip force in the bilateral condition, regardless of side (- 2.4% difference), and similar findings were observed on the non-paretic side of people with hemiparesis (- 4.5% difference). On the paretic side, however, maximal grip force was increased by the bilateral condition in most participants (+11.3% difference, on average). During submaximal bilateral contractions in each group, the two sides each contributed the same percentage of unilateral maximal force.
Conclusions
The bilateral condition facilitates paretic limb grip force at maximal, but not submaximal levels.
Significance
In some people with post-stroke hemiparesis, the paretic limb may benefit from bilateral training with high force requirements.
Keywords: stroke, upper extremity, interhemispheric inhibition, bilateral deficit, strength, human
INTRODUCTION
In healthy adults, maximal voluntary force production is diminished, generally 3 – 25%, when homologous muscles on the left and right sides contract simultaneously rather than individually. This phenomenon, termed the ‘bilateral deficit’, has been demonstrated for flexor and extensor muscle groups of the upper and lower extremities, and affects force production during all types (e.g. isometric, concentric, eccentric, isokinetic) of maximal contractions (Vandervoort et al., 1984, Howard and Enoka, 1991, Oda and Moritani, 1994, 1995, Weir et al., 1995, Herbert and Gandevia, 1996, Taniguchi, 1998, Li et al., 2001, Latash et al., 2002, Van Dieen et al., 2003, Kuruganti et al., 2005, Hay et al., 2006, Kuruganti and Seaman, 2006, Magnus and Farthing, 2008, Yamauchi et al., 2009). The bilateral deficit is present in both young and older adults (Owings and Grabiner, 1998, Li et al., 2003, Kuruganti and Seaman, 2006, Yamauchi et al., 2009), and is a flexible, use-dependent phenomenon that increases with unilateral training and decreases with bilateral training (Howard and Enoka, 1991, Weir et al., 1995, Taniguchi, 1997, 1998, Jakobi and Chilibeck, 2001, Kuruganti et al., 2005).
Evidence supports interhemispheric inhibition as the predominant mechanism underlying the bilateral deficit phenomenon (Archontides and Fazey, 1993). During unilateral activity, a high level of cortical activation in one hemisphere has an inhibitory influence on the homologous cortical area in the opposite hemisphere (Asanuma and Okuda, 1962, Duque et al., 2005b). This effect, which suppresses mirror movements and supports motor control of intentionally unilateral actions, occurs via transcallosal connections between homologous cortical areas. Existence of the bilateral deficit phenomenon suggests that during maximal bilateral symmetrical contractions, both sides mutually inhibit each other, limiting the intensity of cortical activation and thereby limiting maximal muscle force production.
Insufficient force production due to limited neural drive is a primary impairment post- stroke (Rosenfalck and Andreassen, 1980, Young and Mayer, 1982, Jakobsson et al., 1992, Gemperline et al., 1995, Frontera et al., 1997). Since training under conditions that optimize motor performance may lead to greater recovery (Dobkin, 2004), it is important to understand how the bilateral condition affects various aspects of movement after stroke. Prior studies show that paretic limb reaching velocity is greater during bilateral versus unilateral efforts (Harris-Love et al., 2005, Rose and Winstein, 2005, McCombe Waller et al., 2006), and that training bilaterally may improve certain aspects of paretic limb function more than unilateral training does (Lin et al., 2009, Stoykov et al., 2009). Effects of the bilateral condition on paretic limb force production, however, remain unclear.
Only two published reports, with apparently conflicting results, have begun to address this issue (Li et al., 2003, McQuade et al., 2008). In one study of ten people with chronic hemiparesis, the bilateral condition had no effect on the paretic limb's maximal elbow flexor force, although the non-paretic side produced, on average, 15% less force in the bilateral condition than it did in a unilateral effort (McQuade et al., 2008). These findings, combined with the bilateral deficit phenomenon in healthy individuals, suggest that cortical activation is not limited by the bilateral condition in the lesioned hemisphere, as it is on the non-lesioned side and in the intact nervous system. Another study, however, reported bilateral deficits of approximately 23% on both sides in seven people with stroke who performed unilateral vs. bilateral multi-finger flexion contractions (Li et al., 2003).
In this study, we investigated whether the bilateral condition affects force production differently on the paretic side after stroke, as compared to the non-paretic side and to healthy individuals. We compared maximal grip force across unilateral versus bilateral conditions within a single testing session, and included a group of healthy young adults to confirm that our protocol produced the expected bilateral deficit phenomenon in neurologically-intact individuals.
As the null hypothesis, we considered that people post-stroke might produce less maximal paretic-limb force during bilateral efforts compared to unilateral efforts, similar to the bilateral deficit normally observed. This possibility was supported by ample evidence of intact interhemispheric inhibition after stroke (Murase et al., 2004, Duque et al., 2005a, Butefisch et al., 2008, Perez and Cohen, 2009), and by the findings of Li et al. (2003) discussed above. As an alternative hypothesis, we proposed that the paretic side would benefit from the bilateral condition, and therefore would show no reduction in maximal grip force during bilateral versus unilateral efforts. This possibility was supported by the findings of McQuade et al. (2008) discussed above, reports of improved paretic limb reaching in the bilateral condition (Harris-Love et al., 2005, Rose and Winstein, 2005, McCombe Waller et al., 2006), and reports of improved outcomes after bilateral versus unilateral training (Lin et al., 2009, Stoykov et al., 2009).
Since most skilled movements performed in daily activities and post-stroke rehabilitation require only a small percentage of maximal force generating capacity (Wagner et al., 2007), influence of the bilateral condition on submaximal force production is highly relevant from a clinical perspective. We therefore included a submaximal task that required participants to maintain a pre-determined force level, using both hands to simultaneously grip separate dynamometers. As the null hypothesis, we considered that the bilateral condition might affect the paretic and non-paretic limbs similarly, and the two sides therefore would contribute equal percentages of their respective unilateral maximal force values in order to achieve the specified submaximal force level. As the alternative hypothesis, we proposed that the paretic limb would benefit from the bilateral condition at submaximal force levels, and that it therefore would contribute a higher percentage of its maximal unilateral grip force, while the non-paretic side would contribute a lower percentage of its maximal unilateral grip force.
METHODS
Participants
Sixteen people with hemiparesis due to stroke were recruited from the St. Louis metropolitan area via the Brain Recovery Core Database and the Cognitive Rehabilitation Research Group Stroke Registry at Washington University, and from local support groups for people with stroke. Potential participants were included if they had been diagnosed with stroke and had persistent upper extremity paresis, demonstrated by Medical Research Council muscle test scores that were at least one grade lower on the paretic side compared to the non-paretic side. Potential participants were excluded if they: 1) had severe aphasia as indicated by a score of 2 or 3 on the Best Language item of the National Institutes of Health Stroke Scale (NIHSS), 2) had severe hemispatial neglect, as indicated by a score of 2 on the Extinction and Inattention item on the NIHSS, 3) were unable to follow 2-step commands, 4) had musculoskeletal or other medical conditions besides stroke that limited either upper extremity, or 5) were unable to hold a grip dynamometer once it was placed in their hand. Since the bilateral deficit has been shown to be influenced by weight training and other similar athletic activities (Howard and Enoka, 1991, Taniguchi, 1997, 1998, Kuruganti et al., 2005), we also excluded people who participated in weight training, rock climbing, racquet sports, rowing, or any similar activities involving the upper extremities, at any time within the past year.
Fifteen healthy young people also participated, in order to provide reference values and to verify the presence of a bilateral deficit affecting grip force using the instrumentation and protocol employed in this study. Since the bilateral deficit has been shown to be similar across younger vs. older age groups, age-matching to the participants with hemiparesis was not considered necessary (Kuruganti et al., 2005, Yamauchi et al., 2009). Volunteers were included if they: 1) were between 20 and 35 years old, 2) had no history of any neurological disorder or musculoskeletal condition affecting their upper extremity on either side, and 3) had not participated in weight training or other similar activities within the past year. This study was approved by the Washington University Human Research Protection Office, and all participants provided informed consent prior to enrollment.
Clinical Measurements
Clinical scales were used to describe the sample of people with post-stroke hemiparesis. We assessed upper extremity function using the Action Research Arm Test (Lyle, 1981, Lang et al., 2006, Yozbatiran et al., 2008) and the Activities of Daily Living and Hand Function domains of the Stroke Impact Scale, version 3.0 (Duncan et al., 1999, Lai et al., 2002). Sensation on the palmar surface of the distal index finger was evaluated on the paretic side using Semmes-Weinstein monofilaments (Bell-Krotoski, 1991). Spasticity of the wrist flexors was assessed on the paretic side using the Modified Ashworth Scale (Bohannon and Smith, 1987). When possible, medical records were reviewed to identify lesion locations.
Maximum grip strength was measured in all participants using a Jamar grip dynamometer in its second position (Schmidt and Toews, 1970, Fess, 1992). For each side, we recorded the average score achieved in three unilateral attempts, each separated by 15 seconds of rest. This common clinical method of assessing grip strength was used in addition to the experimental protocol described below, since the grip strength values from the Jamar method can be directly compared to published normative data (Bohannon et al., 2006) and to other studies of people with post-stroke hemiparesis.
Instrumentation
For the experimental protocol, grip force was measured using two Biopac strain gauge hand dynamometers (Model SS25, Biopac Systems, Inc., Goleta, CA). The same two dynamometers were used throughout the study. Each dynamometer was paired with a custom-designed differential amplifier that applied a low pass filter at 10 Hz and a gain of 2000. Signals were sampled at 20 Hz with an A/D converter (Model USB-1208LS, Measurement Computing Corp., Norton, MA). Prior to the study, linear response characteristics were verified and scale factors were determined for each dynamometer/amplifier pair using calibration weights ranging from 0 to 218 N and procedures recommended by the manufacturer. Zero offsets were determined immediately prior to each data collection session, by collecting five seconds of data with no load applied to the dynamometers. Because of differences in design and response characteristics, the Biopac dynamometers yielded different grip force values compared to the Jamar method, although recordings from the two methods were highly correlated (r = 0.91). The Biopac design employs a strain gauge at one end of the dynamometer, resulting in attenuation of grip forces that occur at a distance from the strain gauge. In contrast, the Jamar dynamometer, which is commonly used in clinical settings, consists of a hydraulic dual-post design capable of measuring all grip force exerted along the length of its handle.
Data was acquired using custom software created with LabVIEW 8.2 (National Instruments Corp., Austin, TX). The LabVIEW program randomly assigned which dynamometer would be used in each hand, randomly selected the order of conditions presented to the participant (left unilateral, right unilateral, or bilateral), and controlled the duration of each grip force trial and rest break. The program also applied the previously determined calibration parameters and stored data to a file.
Positioning
All data for each participant were collected within a single session. The participant was seated in a chair with a table in front and a pillow behind their back. Table height was adjusted to allow for symmetrical positioning of both upper extremities with approximately 30 degrees of flexion, abduction, and internal rotation at the shoulders, 60 degrees of flexion at the elbows, and neutral forearm supination / pronation. Versa-Form vacuum-molded pillows (Patterson Medical Products, Inc., Bolingbrook, IL) were used to provide firm support and to maintain this position throughout the data collection process. This standardized positioning minimized postural adjustments and compensatory movements that might otherwise affect grip force production, and ensured constant positioning across all trials. A laptop computer on a cart was placed in front of the table, for viewing by the participant.
Maximal Grip Force Measurements
Maximal grip force was assessed first. A computer-generated random sequence specified the order of three conditions (left unilateral, right unilateral, and bilateral). The sequence was repeated three times, yielding nine trials. Each effort was separated by two minutes of rest, to minimize fatigue. Before each trial, the examiner placed a dynamometer in the hand(s) corresponding to the condition being tested and carefully positioned the upper edge of the dynamometer in the web space between the thumb and index finger. For unilateral trials, only the hand participating in the trial held a dynamometer, and the participant was asked to relax the other hand.
The participant was then instructed to watch the computer screen, wait for a green light to appear, and then squeeze as hard as possible with the appropriate hand(s). Visual feedback was provided on the screen in real time for motivation, using meters that displayed the force generated by each hand on a scale from 0 to 25 kg. The examiner also verbally encouraged maximum effort by yelling ‘Go, go, go’ loudly for the 5-second duration of each trial. After all maximal trials were completed, a 4-minute rest period was provided.
Submaximal Grip Force Measurements
Because upper extremity task performance generally utilizes only a small percentage of maximal force production capacity (Wagner et al., 2007), we examined whether potential effects of the bilateral condition on maximal force would also be observed in a submaximal task. In preparation for submaximal trials, the LabVIEW program determined peak grip force for each maximal trial, then averaged within trial types to obtain average peak grip force on each side for the unilateral and bilateral conditions. A target force level for the submaximal trials was calculated according to the following equation:
Data collection resumed with submaximal testing, which was performed only in the bilateral condition. On-screen visual feedback for the submaximal trials consisted of a single meter in the center of the screen, which displayed the combined force produced by the two hands together. The meter was scaled so that the participant's 30% target force level corresponded with the vertical position of the meter's needle, and the meter's range spanned from 26% to 34% of maximal force. Given this feedback, the participant was unaware of how much force was contributed by each hand, but could see whether they were achieving the target force level with the two hands combined.
Prior to each trial, the examiner placed a dynamometer in each hand and instructed the participant to watch the computer screen, wait for a green light to appear, then squeeze with both hands, hard enough to make the needle point straight up. Participants were consistently encouraged to use both hands. No instructions were provided regarding distribution of effort or symmetry of force production, however, because we sought to capture the “natural” sharing of forces across the two hands. Three submaximal trials were completed, each separated by one minute of rest. Each trial ended once the participant maintained total grip force within ± 2% of their 30% submaximal target force level for three seconds.
Analysis
To examine maximal force production, peak force was determined for each maximal grip force trial, then averaged across the three repetitions of each condition to yield single values for each side in each condition, for use in the statistical analyses. For submaximal trials, analysis was restricted to the 3-second interval during which the combined force of the two sides remained within the target range (30% ± 2% of the participant's maximal unilateral grip force on the two sides combined). For each side, the average amount of force recorded during that interval was determined for each trial, averaged across the three submaximal trials, then expressed as a percentage of the average peak grip force measured during maximal unilateral trials on that side.
All statistical analyses were performed using Statistica software (Version 6.1, StatSoft Inc., Tulsa, OK). Prior to running the analyses, normal distributions of each variable were verified using the Kolmogorov-Smirnov test. The criterion for statistical significance was set at p < 0.05.
To examine the effects of the bilateral condition on maximal force production, average peak grip force served as the dependent variable. In the hemiparetic group, we examined the effects of condition (unilateral vs. bilateral) and side (paretic vs. non-paretic) using 2 × 2 repeated measures analysis of variance (ANOVA), followed by Fisher LSD post-hoc tests where indicated. Fisher LSD tests were chosen to maximize the statistical power to detect differences between groups because of the relatively small sample size (Field, 2009). Both factors were within-subjects and were considered fixed effects.
For each side, we calculated the percent difference in maximal force across the unilateral vs. bilateral conditions using the following equation:
Negative % difference values indicated less force production in the bilateral condition compared to unilateral efforts (i.e. a bilateral deficit), while positive values indicated greater forces during bilateral efforts (i.e. bilateral facilitation). Spearman rho correlation coefficients were used to examine relationships between the percent difference values and participant characteristics, including time since stroke, paretic limb grip strength impairment, and paretic limb somatosensation.
To verify the existence of a grip force bilateral deficit in healthy individuals using our instrumentation and protocol, we examined effects of condition (unilateral vs. bilateral) and side (dominant vs. non-dominant) in the control group, using 2 × 2 repeated measures ANOVA. Because the side factor compared paretic vs. non-paretic in people with hemiparesis and dominant vs. non-dominant in controls, analyses were performed separately for each group, rather than together with group included as a third factor.
To examine effects of the bilateral condition on submaximal force production across the two sides, the percentage of unilateral maximal force that was utilized during submaximal trials served as the dependent variable. For each group, a paired t-test tested for differences between the two sides (paretic vs. non-paretic for participants with hemiparesis, non-dominant vs. dominant for controls).
RESULTS
Sixteen people with hemiparesis participated in this study. Demographics and results of clinical tests are provided in Table 1. Time since stroke varied from 6 weeks to 9.6 years, and exceeded 6 months in all except 3 participants. The group comprised a wide range of sensorimotor impairment and functional limitation, ranging from mild to severe. Lesion locations also varied, including five cortical, five subcortical, two that were both cortical and subcortical, and four unknown. As measured with a Jamar grip dynamometer, grip force on the paretic side ranged from 8 to 68% of the non-paretic side.
Table 1.
Characteristics of participants with hemiparesis
| Age (years) | 62 ± 13 (47 – 91) |
|---|---|
| Gender | 10 male 6 female |
| Type of stroke | 13 ischemic 3 hemorrhagic |
| Months since stroke | 34 ± 34 (1.5 – 115) |
| Paretic side | 6 right / 10 left 8 dominant / 8 non-dominant |
| Dominant side | 12 right / 4 left |
| Jamar grip strength on paretic side (kg)a | 14.7 ± 7.6 (2.0 – 27.0) |
| Jamar grip strength on non-paretic side (kg)a | 34.3 ± 11.7 (19.0 – 60.3) |
| Jamar grip strength on paretic side (as % of grip strength on non-paretic side) | 42.2 ± 17.9 (8.0 – 68.2) |
| Sensation (size of smallest Semmes Weinstein monofilament sensed in 3 of 5 trials) | 2.83 n = 8 3.61 n = 4 4.31 n = 1 4.56 n = 2 6.65 n = 1 |
| Spasticityb | 0 n = 8 1 n = 2 2 n = 4 3 n = 2 |
| Action Research Arm Testc | 34.4 ± 16.2 (8 – 56) |
| Stroke Impact Scale Activities in a Typical Day Subscaled | 66.3 ± 15.2 (35 – 98) |
| Stroke Impact Scale Hand Function Subscaled | 48.1 ± 25.0 (5 – 85) |
Values are mean ± 1 standard deviation (range), unless otherwise noted
Maximal isometric grip strength assessed during unilateral contractions with a Jamar grip dynamometer in its second position.
Modified Ashworth Scale score of the wrist flexors on paretic side. 0-4 point scale where 0 = normal resistance to passive movement
Paretic side, 0-57 point scale where 57 = normal upper extremity function
0-100 point scale where 100 = normal activity or hand function
Fifteen controls also participated, including 8 females and 7 males between 22 and 30 years of age (mean 24.4 ± 1.8 years). All were right-handed, by self report. Jamar grip strength on the dominant (right) side was 40.6 ± 12.5 kg. On the non-dominant (left) side, Jamar grip strength was 38.0 ± 13.1 kg, and was 93.3 ± 8.8 % of the dominant side.
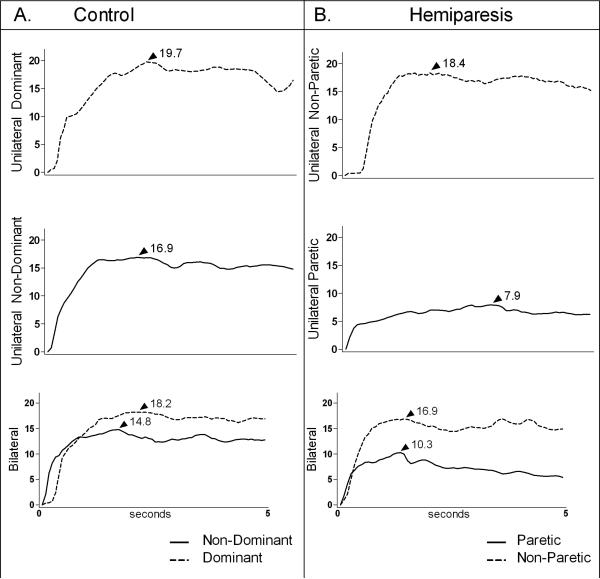
Representative data showing maximal unilateral and bilateral grip force from one control and one participant with hemiparesis are shown in Figure 1. Each side of the control participant (1A) produced less peak grip force when contracting bilaterally than it did during unilateral contractions. The same pattern was seen on the non-paretic side of the participant with hemiparesis (1B). On the paretic side, however, peak force was greater during the bilateral effort.
Figure 1.
Maximal grip force (kg) in the unilateral and bilateral conditions in one control participant (A) and one person with hemiparesis (B). Each graph represents a 5-second trial. Arrows and numerical values indicate peak force (kg). On the non-paretic side of the person with hemiparesis, and on each side of the healthy young adult, grip force was diminished in the bilateral condition compared to the unilateral condition. The paretic side, however, produced greater peak grip force in the bilateral condition than it did in the unilateral condition.
Average findings in each group are shown in Figure 2. In the control group, peak grip force averaged 2.4% less during bilateral trials than during unilateral trials (main effect of condition, p = 0.04), thus confirming the presence of a bilateral deficit affecting grip force. The effect of the bilateral vs. unilateral condition did not vary across the two sides (no condition x side interaction, p = 0.60, comparison not shown, pooled across sides in Figure 2). As expected in the control group, grip force was greater on the dominant side compared to the non-dominant side (main effect of side, p = 0.05).
Figure 2.
Maximal grip force (kg) in unilateral and bilateral conditions (mean ± 1 SE, data was normally distributed as shown by Kolmogorov-Smirnov tests, p > 0.20). In the control group (●; data pooled for dominant and non-dominant hands), peak grip force was 2.4% less in the bilateral condition than it was in the unilateral condition. For the group of participants with hemiparesis, the effect of condition differed between the two sides. The non-paretic side (■) showed 4.5% less peak grip force in the bilateral condition compared to the unilateral condition. On the paretic side (□), grip force was 11.3% greater in the bilateral condition.
In the hemiparetic group, the effect of condition differed across the two sides (condition x side interaction, p = 0.01). On the non-paretic side, peak grip force averaged 4.5% less during bilateral trials than during unilateral trials (post-hoc p = 0.04). On the paretic side, peak grip force averaged 11.3% greater during bilateral trials than during unilateral trials (post-hoc p = 0.04). As shown in individual data in Figure 3, bilateral facilitation was observed on the paretic side in 9 of the 16 participants with hemiparesis. In contrast, the non-paretic side consistently showed either a bilateral deficit or a minimal difference in force across conditions. Correlation analysis did not identify any significant relationships between the percent difference across unilateral vs. bilateral conditions, and participant characteristics including time since stroke, paretic limb grip strength impairment, and paretic limb somatosensation (Spearman rho < 0.50, p values > 0.05).
Figure 3.

Differences between unilateral and bilateral maximal force on the non-paretic (■) and paretic (□) sides in participants with hemiparesis. Negative values indicate a bilateral deficit (less force produced in the bilateral condition vs. unilateral), while positive values indicate bilateral facilitation (greater force produced in the bilateral condition vs. unilateral). Nine of the 16 participants with hemiparesis showed bilateral facilitation on the paretic side. The percent difference in force across conditions was not related to the severity of paretic limb grip force impairment. For each side, Spearman rho < 0.50 and p > 0.05.
Results of submaximal grip force trials are shown in Figure 4. Representative data from one control (4A) and one participant with hemiparesis (4B) show the time series of force generation on each side, including the last three seconds of each trial, during which the combined force of the two sides was maintained within the target range. The control participant achieved the target force level utilizing roughly 30% of each side's maximum unilateral force, and produced roughly the same amount of force on each side. The participant with hemiparesis also utilized roughly 30% of each side's maximum unilateral force, but produced less force on the paretic side than on the non-paretic side, reflecting asymmetrical strength. Figure 4C shows averaged data for each side in each group. Grip force contributed by each side is expressed as a percentage of that side's maximal unilateral force. In each group, the percentage of unilateral maximal force utilized during submaximal trials did not differ across the two sides (control group p = 0.36, hemiparetic group p = 0.25), showing that submaximal grip force was neither diminished nor facilitated by the bilateral condition. The paretic side did not use a higher percentage of maximal unilateral force than the non-paretic side did (paretic mean = 24.2%, nonparetic mean = 31.5%).
Figure 4.
Submaximal grip force in the bilateral condition in one control (A) and one person with hemiparesis (B). The last 3 seconds of data, marked with a gray block, represent the interval during which grip force of the two hands combined was maintained within the target range. Numbers indicate the amount of force each side contributed during that interval, expressed as a percentage of that side's maximal unilateral grip force. Group data (C), show no significant difference between sides in either group (p > 0.05). The lack of a significant difference between sides in the participants with hemiparesis suggests that the bilateral condition did not enhance submaximal grip force production on the paretic side.
DISCUSSION
Maximal grip force testing confirmed the presence of the bilateral deficit phenomenon affecting finger flexors on both sides of healthy controls and on the non-paretic side of participants with hemiparesis. On the paretic side, however, 9 of the 16 participants showed no bilateral deficit, and instead showed greater maximal force production during bilateral efforts compared to unilateral efforts. In the submaximal task, however, the bilateral condition did not facilitate the paretic limb's contribution. Instead, each side contributed approximately the same percentage of its respective maximal unilateral force.
An important finding of this study is that the bilateral condition enhanced maximal force production on the paretic side in some of the people with post-stroke hemiparesis. This finding extends results of a similar investigation, in which no bilateral deficit was found during maximal elbow flexor force production in ten people with chronic mild/moderate hemiparesis (McQuade et al., 2008). In addition to showing similar results in a more distal muscle group, our findings further suggest that the effect may be facilitation, rather than disinhibition, since more than half of our participants exceeded their unilateral paretic-limb performance during bilateral contractions. Together, these studies support the idea that, in some people post-stroke, the bilateral condition does not have the same negative effect on maximal descending neural drive in the lesioned hemisphere that it does in the non-lesioned hemisphere and in healthy controls.
Our results are contrary to those reported by Li et al. (2003), in which seven people with mild hemiparesis showed bilateral deficits on both sides. Since both studies assessed finger flexors, and given the lack of correlation that we showed between impairment severity and the bilateral deficit, the conflicting findings are difficult to explain based on muscle group or severity. Rather, methodological differences may explain the conflicting results. As described by Li and colleagues, they developed an alternative definition for the bilateral deficit, to allow for assessment of various single- and multi-finger combinations. Bilateral deficit was defined as the unilateral vs. bilateral difference in the force deficit, where force deficit is the difference in force produced by an individual finger contracting alone vs. force produced by the same finger while contracting in combination with other fingers on the same hand. Although results using the conventional definition were not reported (unilateral vs. bilateral difference in maximal force produced by a muscle or muscle group), the authors clarified that the two indices showed similar patterns and statistically equivalent findings in healthy individuals (Li et al., 2000). Given our results and those of McQuade et al. (2008), comparison of the two indices in people with post-stroke hemiparesis appears warranted.
In contrast to our results in the maximal force production task, there was no evidence of facilitation of the paretic limb during the submaximal task. Instead, each side contributed approximately the same percentage of its maximal unilateral force. This finding is important, since upper extremity actions performed in daily life and rehabilitation typically require only a small percentage of maximal force generating capacity (Wagner et al., 2007). If the bilateral condition facilitates paretic limb performance only at high force levels, as our results suggest, perhaps the effects of bilateral training post-stroke could be enhanced by choosing tasks with high force requirements. Because bilateral facilitation of maximal force was seen in some, but not all of our participants, there may be a subset of people post-stroke, as yet undefined, that could benefit from bilateral, high force interventions. Conversely, the bilateral condition may have little impact on training effects achieved through repetitive practice of tasks with low force requirements. Further study in this area is needed.
It is not clear from the current literature whether the bilateral deficit phenomenon occurs during submaximal force production in healthy individuals. Its presence at submaximal levels has been inferred based on high-speed isokinetic testing, which limits force levels by exploiting the force/velocity relationship of muscle contractions (Vandervoort et al., 1984, Yamauchi et al., 2009). Arguably, however, such protocols do represent maximal capacity for neural activation at the given speed, despite the limited forces achieved. Studies using transcranial magnetic stimulation (TMS) have shown that interactions between hemispheres (Svoboda et al., 2002, Perez and Cohen, 2008) and the balance of excitability across hemispheres (Perez and Cohen, 2009) can differ across different levels of muscle activation. Additional studies are needed to further understand how neural activity changes within and across hemispheres as force increases from submaximal to maximal levels, and how the bilateral condition modulates those changes.
Although measurement of grip force behavior was sufficient to achieve the purposes of this study, the lack of neurophysiological methods and functional neuroimaging can be considered a limitation. Force measurements confirmed the bilateral deficit phenomenon in healthy controls and revealed its absence on the paretic side in some of the people with hemiparesis. Numerous previous studies in healthy controls have shown that bilateral vs. unilateral differences in force production are consistently accompanied by corresponding differences in electromyographical recordings and measures of cortical activation in the primary motor area (Howard and Enoka, 1991, Oda and Moritani, 1995, Taniguchi et al., 2001, Van Dieen et al., 2003, Post et al., 2007). Those measures, therefore, likely would have added little information. Use of functional magnetic resonance imaging (fMRI) or cortical motor conduction studies, however, may have helped to expose neural mechanisms. For example, recent fMRI studies suggest that alternative networks and pathways (e.g. secondary cortical motor areas, uncrossed corticospinal projections, cerebellar circuits) may be recruited to a greater extent when moving bilaterally vs. unilaterally (Luft et al., 2004, Wu et al., 2010, Whitall et al., 2011). Future studies using fMRI may reveal whether neural pathways involving brainstem nuclei or specific secondary cortical areas (e.g. the supplementary motor area) are activated to a greater extent during bilateral force production. Future studies using TMS could further examine abnormalities in central motor conduction and changes in cortical excitability across the time course of force generation.
Limitations of this study also include the heterogeneity of participants in terms of stroke severity, time since stroke, and lesion location. Correlation analyses, however, suggested that these factors may have little influence on the effect of the bilateral condition. Limited information was available regarding lesion size, location, and pathways affected. Although the sample size was small, statistical power was adequate for testing of our hypotheses.
In summary, the bilateral condition resulted in increased maximal grip force production on the paretic side in more than half of the people with post-stroke hemiparesis, but decreased maximal grip force on the non-paretic side and on both sides of healthy controls. In a submaximal bilateral grip task, as might be expected during daily activities, each side contributed an equal percentage of its respective maximal unilateral force, thus showing no enhancement of paretic-limb performance in the submaximal bilateral task. Further investigations are needed to examine neural mechanisms and to test efficacy of bilateral training at high force levels after stroke.
HIGHLIGHTS.
Although healthy individuals produce less force during bilateral muscle contractions compared to unilateral contractions, emerging evidence suggests that the paretic limb post-stroke may benefit from bilateral movement.
The bilateral condition increased maximal grip force on the paretic side in the majority of people with post-stroke hemiparesis, but decreased maximal grip forces on the nonparetic side and on both sides of healthy controls.
In a submaximal bilateral grip task, as might be expected during daily activities, there was no enhancement of paretic-limb performance.
Acknowledgements
This study was supported in part by NIH R01HD055964 (CEL), NIH T32HD007434 (SLD), and scholarships from the Foundation for Physical Therapy, Inc (SLD). Neither author has any conflict of interest related to this manuscript. The authors acknowledge Arnold Heidbreder for engineering support, Michael Strube, PhD for statistical advice, and Sydney Schaefer, PhD for assistance with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Archontides C, Fazey JA. Inter-limb interactions and constraints in the expression of maximum force: a review, some implications and suggested underlying mechanisms. J Sports Sci. 1993;11:145–158. doi: 10.1080/02640419308729978. [DOI] [PubMed] [Google Scholar]
- Asanuma H, Okuda O. Effects of transcallosal volleys on pyramidal tract cell activity of cat. J Neurophysiol. 1962;25:198–208. doi: 10.1152/jn.1962.25.2.198. [DOI] [PubMed] [Google Scholar]
- Bell-Krotoski J. Advances in sensibility evaluation. Hand Clin. 1991;7:527–546. [PubMed] [Google Scholar]
- Bohannon RW, Peolsson A, Massy-Westropp N, Desrosiers J, Bear-Lehman J. Reference values for adult grip strength measured with a Jamar dynamometer: a descriptive meta-analysis. Physiotherapy. 2006;92:11–15. [Google Scholar]
- Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Wessling M, Netz J, Seitz RJ, Homberg V. Relationship between interhemispheric inhibition and motor cortex excitability in subacute stroke patients. Neurorehabil Neural Repair. 2008;22:4–21. doi: 10.1177/1545968307301769. [DOI] [PubMed] [Google Scholar]
- Dobkin BH. Strategies for stroke rehabilitation. Lancet Neurol. 2004;3:528–536. doi: 10.1016/S1474-4422(04)00851-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The stroke impact scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30:2131–2140. doi: 10.1161/01.str.30.10.2131. [DOI] [PubMed] [Google Scholar]
- Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG. Transcallosal inhibition in chronic subcortical stroke. Neuroimage. 2005a;28:940–946. doi: 10.1016/j.neuroimage.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Duque J, Mazzocchio R, Dambrosia J, Murase N, Olivier E, Cohen LG. Kinematically specific interhemispheric inhibition operating in the process of generation of a voluntary movement. Cereb Cortex. 2005b;15:588–593. doi: 10.1093/cercor/bhh160. [DOI] [PubMed] [Google Scholar]
- Fess EE. Grip strength. In: Casanova JS, editor. Clinical assessment recommendations. 2nd ed. American Society of Hand Therapists; Chicago: 1992. pp. 41–45. [Google Scholar]
- Field A. Discovering Statistics Using SPSS. 3rd ed. Sage Publishing Inc.; Thousand Oaks, California: 2009. [Google Scholar]
- Frontera WR, Grimby L, Larsson L. Firing rate of the lower motoneuron and contractile properties of its muscle fibers after upper motoneuron lesion in man. Muscle Nerve. 1997;20:938–947. doi: 10.1002/(sici)1097-4598(199708)20:8<938::aid-mus2>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Gemperline JJ, Allen S, Walk D, Rymer WZ. Characteristics of motor unit discharge in subjects with hemiparesis. Muscle Nerve. 1995;18:1101–1114. doi: 10.1002/mus.880181006. [DOI] [PubMed] [Google Scholar]
- Harris-Love ML, McCombe Waller S, Whitall J. Exploiting interlimb coupling to improve paretic arm reaching performance in people with chronic stroke. Arch Phys Med Rehabil. 2005;86:2131–2137. doi: 10.1016/j.apmr.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Hay D, de Souza VA, Fukashiro S. Human bilateral deficit during a dynamic multi-joint leg press movement. Hum Mov Sci. 2006;25:181–191. doi: 10.1016/j.humov.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Herbert RD, Gandevia SC. Muscle activation in unilateral and bilateral efforts assessed by motor nerve and cortical stimulation. J Appl Physiol. 1996;80:1351–1356. doi: 10.1152/jappl.1996.80.4.1351. [DOI] [PubMed] [Google Scholar]
- Howard JD, Enoka RM. Maximum bilateral contractions are modified by neurally mediated interlimb effects. J Appl Physiol. 1991;70:306–316. doi: 10.1152/jappl.1991.70.1.306. [DOI] [PubMed] [Google Scholar]
- Jakobi JM, Chilibeck PD. Bilateral and unilateral contractions: possible differences in maximal voluntary force. Can J Appl Physiol. 2001;26:12–33. doi: 10.1139/h01-002. [DOI] [PubMed] [Google Scholar]
- Jakobsson F, Grimby L, Edstrom L. Motoneuron activity and muscle fibre type composition in hemiparesis. Scand J Rehabil Med. 1992;24:115–119. [PubMed] [Google Scholar]
- Kuruganti U, Parker P, Rickards J, Tingley M, Sexsmith J. Bilateral isokinetic training reduces the bilateral leg strength deficit for both old and young adults. Eur J Appl Physiol. 2005;94:175–179. doi: 10.1007/s00421-004-1313-0. [DOI] [PubMed] [Google Scholar]
- Kuruganti U, Seaman K. The bilateral leg strength deficit is present in old, young and adolescent females during isokinetic knee extension and flexion. Eur J Appl Physiol. 2006;97:322–326. doi: 10.1007/s00421-006-0188-7. [DOI] [PubMed] [Google Scholar]
- Lai SM, Studenski S, Duncan PW, Perera S. Persisting consequences of stroke measured by the Stroke Impact Scale. Stroke. 2002;33:1840–1844. doi: 10.1161/01.str.0000019289.15440.f2. [DOI] [PubMed] [Google Scholar]
- Lang CE, Wagner JM, Dromerick AW, Edwards DF. Measurement of upper-extremity function early after stroke: properties of the action research arm test. Arch Phys Med Rehabil. 2006;87:1605–1610. doi: 10.1016/j.apmr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Latash ML, Li S, Danion F, Zatsiorsky VM. Central mechanisms of finger interaction during one- and two-hand force production at distal and proximal phalanges. Brain Res. 2002;924:198–208. doi: 10.1016/s0006-8993(01)03234-6. [DOI] [PubMed] [Google Scholar]
- Li S, Danion F, Latash ML, Li ZM, Zatsiorsky VM. Characteristics of finger force production during one and two-hand tasks. Hum Mov Sci. 2000;19:897–924. [Google Scholar]
- Li S, Danion F, Latash ML, Li ZM, Zatsiorsky VM. Bilateral deficit and symmetry in finger force production during two-hand multifinger tasks. Exp Brain Res. 2001;141:530–540. doi: 10.1007/s002210100893. [DOI] [PubMed] [Google Scholar]
- Li S, Latash ML, Yue GH, Siemionow V, Sahgal V. The effects of stroke and age on finger interaction in multi-finger force production tasks. Clin Neurophysiol. 2003;114:1646–1655. doi: 10.1016/s1388-2457(03)00164-0. [DOI] [PubMed] [Google Scholar]
- Lin KC, Chang YF, Wu CY, Chen YA. Effects of constraint-induced therapy versus bilateral arm training on motor performance, daily functions, and quality of life in stroke survivors. Neurorehabil Neural Repair. 2009;23:441–448. doi: 10.1177/1545968308328719. [DOI] [PubMed] [Google Scholar]
- Luft AR, McCombe-Waller S, Whitall J, Forrester LW, Macko R, Sorkin JD, et al. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. JAMA. 2004;292:1853–1861. doi: 10.1001/jama.292.15.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res. 1981;4:483–492. doi: 10.1097/00004356-198112000-00001. [DOI] [PubMed] [Google Scholar]
- Magnus CR, Farthing JP. Greater bilateral deficit in leg press than in handgrip exercise might be linked to differences in postural stability requirements. Appl Physiol Nutr Metab. 2008;33:1132–1139. doi: 10.1139/H08-101. [DOI] [PubMed] [Google Scholar]
- McCombe Waller S, Harris-Love M, Liu W, Whitall J. Temporal coordination of the arms during bilateral simultaneous and sequential movements in patients with chronic hemiparesis. Exp Brain Res. 2006;168:450–454. doi: 10.1007/s00221-005-0235-3. [DOI] [PubMed] [Google Scholar]
- McQuade K, Harris-Love ML, Whitall J. Maximal voluntary isometric elbow flexion force during unilateral versus bilateral contractions in individuals with chronic stroke. J Appl Biomech. 2008;24:69–74. doi: 10.1123/jab.24.1.69. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Oda S, Moritani T. Maximal isometric force and neural activity during bilateral and unilateral elbow flexion in humans. Eur J Appl Physiol Occup Physiol. 1994;69:240–243. doi: 10.1007/BF01094795. [DOI] [PubMed] [Google Scholar]
- Oda S, Moritani T. Movement-related cortical potentials during handgrip contractions with special reference to force and electromyogram bilateral deficit. Eur J Appl Physiol Occup Physiol. 1995;72:1–5. doi: 10.1007/BF00964106. [DOI] [PubMed] [Google Scholar]
- Owings TM, Grabiner MD. Normally aging older adults demonstrate the bilateral deficit during ramp and hold contractions. J Gerontol A Biol Sci Med Sci. 1998;53:B425–429. doi: 10.1093/gerona/53a.6.b425. [DOI] [PubMed] [Google Scholar]
- Perez MA, Cohen LG. Mechanisms underlying functional changes in the primary motor cortex ipsilateral to an active hand. J Neurosci. 2008;28:5631–5640. doi: 10.1523/JNEUROSCI.0093-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA, Cohen LG. Scaling of motor cortical excitability during unimanual force generation. Cortex. 2009;45:1065–1071. doi: 10.1016/j.cortex.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Post M, van Duinen H, Steens A, Renken R, Kuipers B, Maurits N, et al. Reduced cortical activity during maximal bilateral contractions of the index finger. Neuroimage. 2007;35:16–27. doi: 10.1016/j.neuroimage.2006.11.050. [DOI] [PubMed] [Google Scholar]
- Rose DK, Winstein CJ. The co-ordination of bimanual rapid aiming movements following stroke. Clin Rehabil. 2005;19:452–462. doi: 10.1191/0269215505cr806oa. [DOI] [PubMed] [Google Scholar]
- Rosenfalck A, Andreassen S. Impaired regulation of force and firing pattern of single motor units in patients with spasticity. J Neurol Neurosurg Psychiatry. 1980;43:907–916. doi: 10.1136/jnnp.43.10.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RT, Toews JV. Grip strength as measured by the Jamar dynamometer. Arch Phys Med Rehabil. 1970;51:321–327. [PubMed] [Google Scholar]
- Stoykov ME, Lewis GN, Corcos DM. Comparison of Bilateral and Unilateral Training for Upper Extremity Hemiparesis in Stroke. Neurorehabil Neural Repair. 2009 doi: 10.1177/1545968309338190. [DOI] [PubMed] [Google Scholar]
- Svoboda J, Sovka P, Stancak A. Intra- and inter-hemispheric coupling of electroencephalographic 8-13 Hz rhythm in humans and force of static finger extension. Neurosci Lett. 2002;334:191–195. doi: 10.1016/s0304-3940(02)01070-4. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y. Lateral specificity in resistance training: the effect of bilateral and unilateral training. Eur J Appl Physiol Occup Physiol. 1997;75:144–150. doi: 10.1007/s004210050139. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y. Relationship between the modifications of bilateral deficit in upper and lower limbs by resistance training in humans. Eur J Appl Physiol Occup Physiol. 1998;78:226–230. doi: 10.1007/s004210050411. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Burle B, Vidal F, Bonnet M. Deficit in motor cortical activity for simultaneous bimanual responses. Exp Brain Res. 2001;137:259–268. doi: 10.1007/s002210000661. [DOI] [PubMed] [Google Scholar]
- Van Dieen JH, Ogita F, De Haan A. Reduced neural drive in bilateral exertions: a performance-limiting factor? Med Sci Sports Exerc. 2003;35:111–118. doi: 10.1097/00005768-200301000-00018. [DOI] [PubMed] [Google Scholar]
- Vandervoort AA, Sale DG, Moroz J. Comparison of motor unit activation during unilateral and bilateral leg extension. J Appl Physiol. 1984;56:46–51. doi: 10.1152/jappl.1984.56.1.46. [DOI] [PubMed] [Google Scholar]
- Wagner JM, Dromerick AW, Sahrmann SA, Lang CE. Upper extremity muscle activation during recovery of reaching in subjects with post-stroke hemiparesis. Clin Neurophysiol. 2007;118:164–176. doi: 10.1016/j.clinph.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir JP, Housh DJ, Housh TJ, Weir LL. The effect of unilateral eccentric weight training and detraining on joint angle specificity, cross-training, and the bilateral deficit. J Orthop Sports Phys Ther. 1995;22:207–215. doi: 10.2519/jospt.1995.22.5.207. [DOI] [PubMed] [Google Scholar]
- Whitall J, Waller SM, Sorkin JD, Forrester LW, Macko RF, Hanley DF, et al. Bilateral and unilateral arm training improve motor function through differing neuroplastic mechanisms: a single-blinded randomized controlled trial. Neurorehabil Neural Repair. 2011;25:118–129. doi: 10.1177/1545968310380685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CY, Hsieh YW, Lin KC, Chuang LL, Chang YF, Liu HL, et al. Brain reorganization after bilateral arm training and distributed constraint-induced therapy in stroke patients: a preliminary functional magnetic resonance imaging study. Chang Gung Med J. 2010;33:628–638. [PubMed] [Google Scholar]
- Yamauchi J, Mishima C, Nakayama S, Ishii N. Force-velocity, force-power relationships of bilateral and unilateral leg multi-joint movements in young and elderly women. J Biomech. 2009;42:2151–2157. doi: 10.1016/j.jbiomech.2009.05.032. [DOI] [PubMed] [Google Scholar]
- Young JL, Mayer RF. Physiological alterations of motor units in hemiplegia. J Neurol Sci. 1982;54:401–412. doi: 10.1016/0022-510x(82)90203-9. [DOI] [PubMed] [Google Scholar]
- Yozbatiran N, Der-Yeghiaian L, Cramer SC. A standardized approach to performing the action research arm test. Neurorehabil Neural Repair. 2008;22:78–90. doi: 10.1177/1545968307305353. [DOI] [PubMed] [Google Scholar]