Abstract
We have previously reported the design and expression of chimeric recombinant proteins as an effective platform to deliver malaria vaccines. The erythrocytic and exoerythrocytic protein chimeras described included autologous T helper epitopes genetically linked to defined B cell epitopes. Proof-of-principle studies using vaccine constructs based on the Plasmodium yoelii circumsporozoite protein (CSP) and P. yoelii merozoite surface protein-1 (MSP-1) showed encouraging results when tested individually in this mouse malaria model. To evaluate the potential synergistic or additive effect of combining these chimeric antigens, we constructed a synthetic gene encoding a hybrid protein that combined both polypeptides in a single immunogen. The multistage vaccine was expressed in soluble form in Escherichia coli at high yield. Here we report that the multistage protein induced robust immune responses to individual components, with no evidence of vaccine interference. Passive immunization using purified IgG from rabbits immunized with the hybrid protein conferred more robust protection against the experimental challenge with P. yoelii sporozoites than passive immunization with purified IgG from rabbits immunized with the individual proteins. High antibody titers and high frequencies of CD4+- and CD8+-specific cytokine-secreting T cells were elicited by vaccination. T cells were multifunctional and able to simultaneously produce interleukin-2 (IL-2), gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α). The mechanism of vaccine-induced protection involved neutralizing antibodies and effector CD4+ T cells and resulted in the control of hyperparasitemia and protection against malarial anemia. These data support our strategy of using an array of autologous T helper epitopes to maximize the response to multistage malaria vaccines.
INTRODUCTION
Malaria remains a major public health problem, even though the implementation of control measures has significantly reduced the overall transmission in the past few years (32). Parasites of the genus Plasmodium are responsible for an estimated 216 million clinical cases and over a half million deaths annually worldwide (32). The spread of multidrug-resistant strains of parasites has emphasized the need for developing novel intervention measures. Several vaccine candidates mainly focused on Plasmodium falciparum are in different phases of clinical development. Among them, RTS,S/AS02, an adjuvanted fusion protein based on the circumsporozoite protein, has reached phase 3 clinical trials (4). However, the prospect of developing a highly effective multistage vaccine that includes more than a single antigen has not been pursued vigorously.
The multistage life cycle of Plasmodium and the intricate host-parasite interactions during the course of malaria infection support the idea of targeting several antigens simultaneously for vaccine development. We have developed several chimeric recombinant proteins for proof-of-principle studies to test the feasibility of developing effective multistage subunit vaccines. Among them, two have been extensively characterized: a preerythrocytic multimeric polypeptide that incorporates linear epitopes from the Plasmodium yoelii circumsporozoite protein (CSP) and an erythrocytic chimeric protein that contains two distinct modules derived from the P. yoelii merozoite surface protein 1 (MSP-1). To design the preerythrocytic vaccine construct, a 41-mer synthetic peptide with the topology cys-T-B-CTL-cys (T represents a promiscuous CD4+ T cell epitope, B, a linear B cell epitope, and CTL, a cytotoxic CD8+-restricted T cell epitope) formulated in Montanide ISA 51 was initially tested (2). The amino- and carboxyl-terminal cysteine residues formed intermolecular disulfide bridges by spontaneous polymerization (2, 3). Both the inclusion of a promiscuous T cell epitope and the complexity of polymeric peptide species were essential for protective efficacy. To avoid the random process of polymerization of this synthetic peptide, we designed and expressed a synthetic gene engineered to contain four 41-mer sequences organized in tandem that we have named P. yoelii linear peptide chimera (PyLPC). We reported that the multimeric PyLPC formulated in the same adjuvant induced antibody and cellular immune responses comparable to those of the single 41-mer synthetic peptide (2, 26). Moreover, the chimeric recombinant protein reproduced the protective effect induced by immunization with the synthetic peptide.
PyLPC was designed to incorporate linear sequences, yet structural analyses of several erythrocytic-stage vaccine candidates have revealed that protective antibodies predominantly recognize functional domains that exhibit a complex tertiary structure (1). To test whether the strategy of using an autologous promiscuous T cell epitope to enhance the immunogenicity of linear epitopes can also be applied for nonlinear structured domains, we subsequently designed a synthetic gene encoding a chimeric recombinant protein comprising four autologous promiscuous T cell epitopes assembled in tandem and fused to the carboxy-terminal domain of the PyMSP-1 (PyMSP-119) (27). The synthetic gene was codon optimized for expression in Escherichia coli, and the chimeric protein, which we have called P. yoelii recombinant modular chimera (PyRMC), was used for comparative experiments along with a recombinant protein that only expressed the native PyMSP-119. After experimental challenge, PyRMC induced protection against both hyperparasitemia and severe anemia that was robust in comparison to the protective efficacy induced by the native PyMSP-119. Most importantly, PyRMC induced functional antibodies with the ability to protect against heterologous challenge (27).
In this study, we have assessed the potential synergistic effect of using an immunization regimen that combines PyLPC and PyRMC (PyLPC/RMC) as a multistage vaccine. We have shown that immunization with a hybrid protein composed of the N-terminal PyLPC fused to the C-terminal PyRMC resulted in the induction of strong humoral and cellular preerythrocytic and erythrocytic immunity with no evidence of interference for antigen-specific antibodies or CD4+ or CD8+ T cells. Moreover, passive immunization with purified polyclonal IgG derived from rabbits immunized with the hybrid PyLPC/RMC protein conferred more robust protection against experimental challenge with P. yoelii sporozoites than passive immunization with purified polyclonal IgG derived from rabbits immunized with the individual proteins or the mixture of antibodies. In addition to the role of neutralizing antibodies, our data also confirm the requirement of effector CD4+ T cells for protection induced by PyLPC/RMC.
MATERIALS AND METHODS
Design and biochemical characterization of the P. yoelii hybrid protein.
Synthetic genes encoding the P. yoelii preerythrocytic and the P. yoelii erythrocytic-stage antigens have been previously described (26, 27). A 624-bp synthetic Py-lpc gene, encoding a chimeric antigen based on the P. yoelii circumsporozoite protein, consists of four modules of the sequence KQISSQLTEEWS(QGPGAP)3SYVPSAEQI interspaced with KAAGPGPG spacers and an (NANP)3 amino-terminal tag sequence derived from P. falciparum circumsporozoite protein (26). The synthetic Py-rmc chimeric gene consists of four putative promiscuous T cell epitopes (PyT8, PyT53, I1620-S1631, and I1642-L1655 [27]) followed by the P. yoelii MSP-119 protein fragment containing a Plasmodium berghei CSP repeat tag sequence (PPPPNPND)2. Py-rmc was amplified using the forward primer containing a SalI restriction site (5′-ATCGTCGACGCGACCGAAATGCTGAAA-3′) and the reverse primer containing an XhoI restriction site (5′-ATCCTCGAGATCGTTCGGGTTCGGCGG-3′) (restriction sites are underlined). The product was digested with SalI and XhoI and cloned into the XhoI site of the pET24d-Py-lpc construct to create the pET24d-Py-lpc/rmc expression vector. The integrity of the vector was confirmed by restriction enzyme digestion and sequencing. The cloning strategy introduced four additional amino acids upstream (HHLD) and two additional amino acids downstream (LE) from the synthetic gene Py-rmc (Fig. 1A). The chimeric construct was transformed into E. coli BL21(DE3) cells (Novagen, Madison, WI), and protein expression induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 3 h. The 414-amino-acid hybrid protein was purified with a Ni-nitrilotriacetic acid (NTA) affinity column according to the manufacturer's instructions (Qiagen, Valencia, CA).
Fig 1.
Expression and antigenic characterization of the P. yoelii LPC/RMC recombinant hybrid protein. (A) Sequence of the PyLPC/RMC protein. The amino acid sequence is shown in single-letter code. The broken underlined sequence shows the preerythrocytic PyLPC. The underlined sequence indicates the erythrocytic PyRMC. Sequences enclosed in open boxes are four putative promiscuous CD4+ T cell epitopes included in the original PyRMC (PyT8, PyT53, I1620-S1631, and I1642-L1655) (27). Sequences enclosed in dotted boxes are the P. falciparum and P. berghei tag sequences originally included at the C-terminal end of individual proteins as an alternative for protein purification. A carboxyl-terminal 6×His tag was included for protein purification. (B) SDS-PAGE (left) and Western blot (right) analysis of the purified PyLPC/RMC protein expressed in E. coli BL21(DE3). Left, Coomassie stain after SDS-PAGE separation of the purified PyLPC/RMC (lane 1), PyLPC (lane 2), and PyRMC (lane 3) proteins. Molecular weight markers (Bio-Rad) are indicated to the left. Right, Western blot analysis of the purified PyLPC/RMC protein incubated with serum samples from mice immunized with PyLPC (lane 4), monoclonal antibody 2A10 that recognizes the P. falciparum tag sequence (16) (lane 5), serum samples from mice immunized with a synthetic peptide representing the promiscuous T cell epitope PyT8 (lane 6), serum samples from mice immunized with PyT53 (lane 7), serum samples from mice immunized with PyMSP-119 (27) (lane 8), monoclonal antibody 3D11 that recognizes the P. berghei tag sequence (33) (lane 9), or an anti-6×His tag monoclonal antibody (lane 10). (C) Antigenicity of the P. yoelii chimeric protein determined by ELISA. Recombinant chimeras were tested using anti-PyLPC/RMC (▲), anti-PyLPC (□), anti-PyRMC (♢), 2A10 ( ), 3D11 (▽), or anti-6×His tag (●). Data are presented as the geometric mean OD obtained at different concentrations of the corresponding monoclonal antibodies (10 μg/ml to 0.00047 ng/ml) or the reciprocal of the serum dilution obtained from mice immunized with P. yoelii PyLPC/RMC, PyLPC, or PyRMC (dilutions 1:1,000 to 1:209,715,200). Numbers on the x axis indicate the dilutions tested (dilution number 1 was 10 μg/ml for monoclonal antibodies or 1:1,000 for polyclonal antibodies). Antigen specificity was confirmed using preimmune serum samples as a control (data not shown). (D) Immunofluorescence images of P. yoelii parasites (sporozoites and schizonts) obtained by incubation with anti-PyLPC/RMC antibodies showing the characteristic staining patterns of CSP and MSP-1.
), 3D11 (▽), or anti-6×His tag (●). Data are presented as the geometric mean OD obtained at different concentrations of the corresponding monoclonal antibodies (10 μg/ml to 0.00047 ng/ml) or the reciprocal of the serum dilution obtained from mice immunized with P. yoelii PyLPC/RMC, PyLPC, or PyRMC (dilutions 1:1,000 to 1:209,715,200). Numbers on the x axis indicate the dilutions tested (dilution number 1 was 10 μg/ml for monoclonal antibodies or 1:1,000 for polyclonal antibodies). Antigen specificity was confirmed using preimmune serum samples as a control (data not shown). (D) Immunofluorescence images of P. yoelii parasites (sporozoites and schizonts) obtained by incubation with anti-PyLPC/RMC antibodies showing the characteristic staining patterns of CSP and MSP-1.
Western blot analysis.
Proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Electrophoresis was performed under reducing conditions on 15% polyacrylamide gels (Bio-Rad Laboratories, Hercules, CA). For Western blots, following electrophoresis, the proteins were blotted onto nitrocellulose membranes following standard procedures. The membranes were then incubated with anti-CSP species-specific monoclonal antibodies (2A10 [P. falciparum], and 3D11 [P. berghei]) or anti-His antibody at 0.2 μg/ml final concentration or antipeptide or antiprotein polyclonal antibodies that recognize individual components of the hybrid protein. The polyclonal antibodies used were elicited in mice and used at 1:5,000 serum dilution. Preimmune serum samples were also used to confirm antigen specificity.
Parasites and animals.
Female CAF1/J (H-2d/a) mice, 6 to 8 weeks of age, were purchased from the Jackson Laboratory (Bar Harbor, ME). The hybrid mice were selected based on our published data concerning the response of syngeneic mice to chimeric antigens (2) and to characterize SYVPSAEQI-specific CD8+ T cells (H-2Kd restricted). The animals were subcutaneously immunized on days 0, 20, and 40, in the base of the tail and in the interscapular area, using 10 μg of the individual proteins or 20 μg of the hybrid protein emulsified in Montanide ISA51 (Seppic, Fairfield, NJ). For dose-response experiments, protein concentrations ranging between 0.2 and 20 μg were tested. Control groups of mice received phosphate-buffered saline (PBS) alone emulsified in the same adjuvant. Twenty days after the last immunization, the mice were challenged with 100 salivary gland sporozoites per mouse. For passive transfer experiments, the mice were challenged by intravenous inoculation of 500 sporozoites per mouse. Anopheles stephensi P. yoelii 17XNL-infected mosquitoes were obtained from the Malaria Research and Reference Reagent Resource Center (MR4) (MR4 number MRA-886; ATCC, Manassas, VA). Giemsa-stained thin smears were made daily and used to quantify parasitemia by counting the percentage of infected erythrocytes from at least 1,000 counted cells per mouse using light microscopy. Additional blood samples obtained from tail vein puncture with a lancet were used to determine hemoglobin concentration using a portable hemoglobinometer (HemoCue, Inc., Lake Forest, CA). Animals were classified as severely anemic when hemoglobin levels reached 5 g/dl. Mice with a hemoglobin concentration lower than this cutoff value were euthanized.
ELISAs.
The fine specificity of the antibodies elicited by immunization with the hybrid protein was determined by enzyme-linked immunosorbent assay (ELISA) using Immulon 4HB plates (Thermo Lab Systems, Franklin, MA) coated with 1 μg/ml of the hybrid protein or individual chimeric recombinant proteins (PyLPC or PyRMC) diluted in carbonate buffer as described previously (27). Optical densities (ODs) were determined using a VERSAmax ELISA reader (Molecular Device Corporation, Sunnyvale, CA) with a 405-nm filter. The endpoint was measured as the highest dilution of serum having a change in optical density greater than the mean plus 2 standard deviations obtained using preimmune sera. The results are presented as the reciprocal of the endpoint dilution. IgG isotype profiles were also determined by ELISA as described previously (27). The affinity of antibodies was assessed by a thiocyanate elution-based ELISA as described previously (3). The procedure was similar to that described above for the standard ELISAs, with the inclusion of an extra step of adding ammonium thiocyanate.
Passive transfer experiments and T cell depletion.
Total IgGs were purified from sera obtained from rabbits immunized with either PyLPC, PyRMC, or the hybrid chimeric protein PyLPC/RMC (Covance, Denver, PA) by protein A affinity chromatography using the Affi-Gel protein A MAP II kit (Bio-Rad, Richmond, CA) and following the manufacturer's instructions. The immunoglobulin G preparations were dialyzed overnight against PBS at 4°C and sterilized by gamma irradiation. Passive transfer studies were conducted with groups of five or six female CAF1/J mice, 6 to 8 weeks of age, by intravenous inoculation of purified IgG on days −1, 0, +1, +3, and +5 relative to the day of challenge. The animals were randomized in four groups that received 500 μg/dose of purified IgGs from PyLPC-, PyRMC-, or PyLPC/RMC-immunized rabbits or IgG derived from naïve rabbits as a control. An additional group of mice received 500 μg/dose of purified IgG from PyLPC-immunized rabbits and 500 μg/dose of purified IgG from PyRMC-immunized rabbits. Rat anti-CD4 (clone GK1.5) and anti-CD8 (clone YTS169.4) monoclonal antibodies were used for in vivo depletion of lymphocyte subsets. Mice were injected intraperitoneally at 500 μg/mouse given on days −1 and +2 relative to the day of challenge. The efficiency of T cell depletion was assessed by flow cytometry analysis of splenocytes on day 5 using naïve mice.
Indirect immunofluorescence assays.
Sera obtained from mice after the third immunization with 20 μg of the hybrid protein were pooled, and the antibody reactivity against native protein was evaluated by indirect immunofluorescence. Twelve-well slides (ICN Biomedicals, Inc., Aurora, OH) were coated with P. yoelii 17XNL salivary gland sporozoites, air dried, and fixed with cold acetone. For P. yoelii-infected erythrocyte preparations, blood was drawn from P. yoelii 17XNL-infected mice into acid-citrate-dextrose (ACD) tubes. The blood samples were washed twice, using RPMI 1640 medium, and the cells adjusted to 1% hematocrit. Ten microliters of the cell suspension was added to the multiwell slides, air dried, and fixed with acetone-methanol (9:1). Several dilutions were tested, and the reactivity was evaluated using goat anti-mouse IgG Alexa Fluor 488-conjugated antibodies (Invitrogen Corporation, Carlsbad, CA). Parasite nuclei were visualized using 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) included in the anti-fade mounting medium Prolong (Invitrogen Corporation).
ELISPOT assays.
The frequency of peptide-specific T cells was determined by gamma interferon (IFN-γ)-specific enzyme-linked immunospot (ELISPOT) assays conducted ex vivo using a pool of spleen cells from four mice obtained 20 days after priming. Assays were performed in nitrocellulose microplates (Millipore, Bedford, MA) coated with rat anti-mouse IFN-γ capture antibody (BD Biosciences Pharmingen, San Diego, CA) as described previously (27). Freshly isolated spleen cells from four mice were pooled, and duplicate aliquots of different concentrations (1 × 106 and 5 × 105) were plated. T cells were activated by the addition of 10 μg/ml of PyLPC, PyRMC, or PyLPC/RMC recombinant proteins or pools of synthetic peptides representing the complete PyLPC/RMC amino acid sequence (PyLPC pool 14 peptides; PyRMC pool 1 represents the sequence upstream from PyMSP-119, 24 peptides, and PyRMC pool 2 represents the PyMSP-119 sequence, 20 peptides) and incubated for 24 h.
Flow cytometry assays.
Flow cytometry multiparametric analysis of hybrid-protein-specific T cells was done using a seven-color flow cytometry panel. The panel was used to simultaneously analyze interleukin-2 (IL-2), IFN-γ, and tumor necrosis factor alpha (TNF-α) at the single-cell level in T cells derived from splenocytes obtained 5, 11, or 19 days after a boosting immunization with the hybrid protein. A library of 58 synthetic peptides representing the complete amino acid sequence of the hybrid proteins was used for ex vivo stimulation (see above). The 15-mer synthetic peptides were overlapped by 10 amino acids. Cells were stimulated for 5 h with peptide pools or for 3 h with PyLPC/RMC at 2 μg/ml and 37°C in the presence of GolgiPlug (BD Biosciences, San Jose, CA). Cells were then incubated with ViViD dye followed by surface staining with anti-CD3 (peridinin chlorophyll protein [PerCP] Cy5.5), anti-CD4 (Alexa Fluor 700), or anti-CD8α (allophycocyanin [APC]-Cy7) for 30 min. The cells were then fixed, permeabilized, and stained with antibodies against IFN-γ (APC), TNF-α (phycoerythrin [PE]), and IL-2 (fluorescein isothiocyanate [FITC]). All the monoclonal antibodies were obtained from BioLegend (San Diego, CA). Flow cytometry analyses were performed using an LSRII (BD Biosciences, San Jose, CA), and data were analyzed using FlowJo (Tree Star, Ashland, OR). Analyses of multifunctional T cell responses were done using a Boolean analysis in FlowJo and SPICE 5.2 software (20). The lymphocytes were initially gated on ViViD− (live cells), and then CD3+ CD4+ and CD3+ CD8+ antigen-specific cytokine-secreting T cells identified.
Statistics.
Statistical analysis and graphs were done using GraphPad Prism 5.0 software (GraphPad Software, Inc., San Diego, CA). For analysis of the antibody responses, data were log-transformed to conform with the normality and variance requirements of parametric testing and compared using Student's t test. The Wilcoxon matched-pairs signed rank test was used for comparison of antibody avidity assays. For experimental challenges, differences between groups were evaluated by comparing mean area under the curve (AUC) values using one-way between-group ANOVA with post hoc Bonferroni's multiple comparison posttest. To evaluate the correlation between antibody titers and protective efficacy, the Spearman's rank correlation coefficient was calculated.
RESULTS
PyLPC/RMC chimeric hybrid protein design and biochemical characterization.
The preerythrocytic-stage recombinant chimera (PyLPC) and the erythrocytic-stage recombinant chimera (PyRMC) were designed to include P. yoelii promiscuous T cell epitopes derived from the homologous proteins. We have reported that such chimeric proteins formulated in Montanide 51 and tested individually induced robust immune responses that were protective against experimental challenge with P. yoelii sporozoites or infected erythrocytes, respectively (26, 27). However, active immunization with PyLPC or PyRMC was not able to confer sterile immunity. We therefore decided to evaluate the synergistic or additive effect of combining PyLPC and PyRMC in a single immunization regimen. A chimeric gene created by fusion of the codon-optimized Py-lpr and Py-rmc synthetic genes contained two sets of additional codons introduced during the cloning process. The first set, at the junction point where the starting methionine codon for Py-rmc was deleted, encoded the sequence HHLD. The second set of additional codons, inserted immediately preceding the hexahistidine tag, encoded the amino acids LE (Fig. 1A). The proper configuration of the cloned hybrid gene was verified by enzyme restriction analysis, and the sequence confirmed using an automatic sequencer.
The 414-amino-acid PyLPC/RMC hybrid was expressed as soluble protein in E. coli and then purified by metal chelate chromatography. Analyses by SDS-PAGE showed a single band of an apparent mobility of 52 kDa, whereas single individual parental proteins had an apparent mobility of 25 kDa (Fig. 1B). The biochemical identity of the recombinant chimeric proteins was established using ELISA and Western blot analysis and a panel of antibodies recognizing various portions of the hybrid protein. Analysis of the PyLPC/RMC showed a single major band at 52 kDa when tested with monoclonal antibodies against tag sequences (2A10, 3D11, and 6×His) or polyclonal antibodies elicited against PyLPC, PyMSP-119, or T cell epitopes expressed in PyRMC (PyT8 and PyT53) (Fig. 1B). Similarly, PyLPC/RMC was recognized in a dose-dependent manner in ELISA when serial dilutions of polyclonal and monoclonal antibodies that recognize different segments of the hybrid protein were tested (Fig. 1C). Immunofluorescence assays were also performed to determine if antibodies elicited by immunization with the hybrid chimeric protein were able to bind native P. yoelii CSP and MSP-1 (Fig. 1D). Sera from CAF1/J mice immunized with PyLPC/RMC bound P. yoelii sporozoites and merozoites at a dilution factor of 1:5,120, in contrast with the negative immunofluorescence reaction obtained with preimmune serum samples (data not shown).
Characterization of the immune responses following vaccination with the chimeric hybrid protein or the mixture of PyLPC and PyRMC proteins.
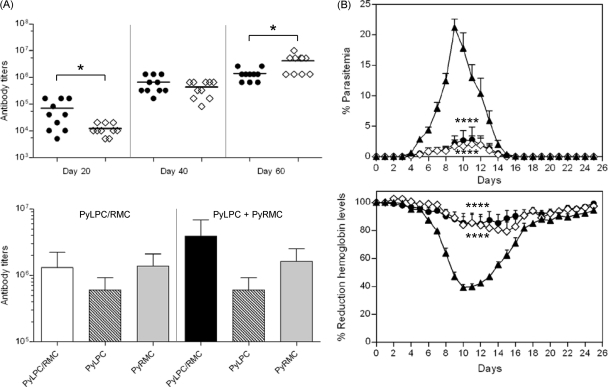
In an attempt to enhance the previously described immunogenicity and protective efficacy of PyLPC and PyRMC, we assessed in comparative experiments the response elicited by the immunization with the hybrid protein or the protein mixture using CAF1/J mice. A group of 30 mice were randomized into three groups of 10 animals per group and immunized subcutaneously three times, 20 days apart. The first group received 20 μg/dose of PyLPC/RMC formulated in Montanide ISA 51. The second group received a combination of PyLPC and PyRMC chimeric recombinant proteins at 10 μg/dose each, formulated with the same adjuvant. The control group received Montanide ISA 51 alone. After a single immunization, the total IgG antibody titers in mice immunized with the hybrid protein ranged between 1:5,120 and 1:163,840 (Fig. 2A, top). This is in contrast with the response obtained after a single immunization with the protein mixture, which induced antibody titers ranging between 1:5,120 and 1:20,480 (P = 0.029). Higher antibody titers were also obtained in the group of mice immunized with the hybrid protein after the second immunization; nevertheless, the differences were not statistically significant. The trend changed after the third immunization, with antibody titers ranging between 1:655,360 and 1:2,621,440 in mice immunized with the hybrid protein and between 1:1,310,720 and 1:10,485,760 in mice immunized with the mixture of proteins (P = 0.022) (Fig. 2A, top). However, the antibody reactivities against PyLPC or PyRMC in sera from mice immunized with the mixture of proteins were comparable to the antibody response obtained after immunization with the hybrid protein (antibody titers against PyLPC ranged between 1:327,680 and 1:1,310,720, and antibody titers for PyRMC ranged between 1:655,360 and 1:2,621,440) (Fig. 2A, bottom).
Fig 2.
(A) Top, kinetics of the antibody response to PyLPC/RMC in CAF1/J mice immunized with the hybrid chimeric protein (●) or the mixture of PyLPC and PyRMC (♢), determined by ELISA. Antibody titers were measured 20 days after each immunization. Horizontal bars represent arithmetic mean values for each group. Bottom, comparative antibody titers to individual chimeric proteins measured 20 days after the third immunization. Reciprocal endpoint ELISA titers were determined using the specified recombinant proteins and serum samples from mice immunized with PyLPC/RMC or the mixture of PyLPC and PyRMC and expressed as arithmetic mean values ± standard deviations (SD). (B) Effects of immunization with the hybrid PyLPC/RMC protein (●), the combination of PyLPC and PyRMC (♢), or placebo (▲) on the course of infection after experimental challenge with P. yoelii sporozoites (n = 10 mice). Top, summary of the course of parasitemias expressed as percentage of parasitemia (average ± SD). Bottom, impact of immunization on the hemoglobin concentration after challenge, expressed as percentage of reduction in hemoglobin concentration relative to baseline levels (average ± SD). *, P = 0.02 by t test; ****, P < 0.0001 by one-way ANOVA of AUC analysis with Bonferroni's multiple comparison posttest.
To assess the protective effect induced by immunization with PyLPC/RMC or the mixture of PyLPC and PyRMC proteins, mice were experimentally challenged with P. yoelii 17XNL sporozoites. Immunized mice reached a maximum peak of parasitemia on day 11 after experimental challenge, with mean parasitemias of 2.78% for mice that received PyLPC/RMC and 2.04% for mice that received the protein mixture (Fig. 2B, top). The maximum peak of parasitemia on day 9 was 19.87% in the adjuvant-only group. As measured by differences in the area under the curve (AUC) analysis of parasitemia versus time, there was a significant reduction in parasitemia in both the LPC/RMC and protein mixture group compared with the parasitemia in the adjuvant-immunized group (P < 0.0001). Furthermore, in comparison to baseline levels, the experimental infection caused a drop in hemoglobin levels lower than 50% in control mice immunized with adjuvant alone, resulting in anemia that did not reach the cutoff value of 5 g/dl (Fig. 2B, bottom). This is in contrast with the protective effect against anemia observed in mice immunized with the recombinant chimeric proteins. Mice immunized with these vaccine regimens had moderate decreases in hemoglobin concentration (15 to 25% from baseline levels) compared to those recorded for mice immunized with the hybrid protein or the combination of individual recombinant proteins. There was a significantly smaller hemoglobin reduction, as measured by differences in AUC analysis of hemoglobin reduction versus time, in both the PyLPC/RMC and protein mixture group compared with that in the placebo-immunized group (P < 0.0001). No differences in response were observed when the two groups immunized with the hybrid protein or the mixture of proteins were compared. There were no correlations between parasite loads and antibody titers determined after the third immunization (data not shown), suggesting a possible role of cellular immune response in protective immunity.
Potency of PyLPC/RMC in mice.
Based on the evidence that the chimeric hybrid protein induced protective efficacy similar to that induced by the mixture of PyLPC and PyRMC, we decided to evaluate only PyLPC/RMC in future studies. To determine the potency of the chimeric hybrid protein, groups of 10 CAF1/J mice were immunized three times with 0.2 μg, 1 μg, 10 μg, or 20 μg per dose. PyLPC/RMC elicited a robust antibody response at all doses tested. Following immunization with PyLPC/RMC, the antibody responses reached a plateau after the second immunization (day 40), with the exception of the animals that received 0.2 μg (Fig. 3A). After a single immunization, the antibody titers induced in mice immunized with 0.2 μg were significantly lower than the antibody titers elicited by immunization with 1 μg, 10 μg, or 20 μg (P < 0.0001). However, the differences were not significant after the second boosting (Fig. 3A). Similar trends were observed when serum samples were tested with individual components of the hybrid protein (data not shown). Nevertheless, the final antibody titers determined after the third immunization were comparable between groups and ranged between 1:655,360 and 1:5,242,880 (data not shown). No antibody responses were detected in control mice immunized with adjuvant alone (data not shown). The avidities of antibodies elicited by immunization with PyLPC/RMC were determined using a thiocyanate elution-based ELISA as described previously (3). After three immunizations, anti-PyLPC/RMC antibodies exhibited high avidity indexes (0.2 μg, 4.3 ± 1.9 M [mean ± standard deviation]; 1 μg, 4.79 ± 1.5 M; 10 μg, 6.13 ± 1.2 M; and 20 μg, 7.08 ± 0.69 M). The differences between the groups that received 20 μg and 0.2 μg or 1 μg were statistically significant (P < 0.01, Wilcoxon matched-pairs signed rank test) (Fig. 3B). Sera from mice immunized with PyLPC/RMC contained predominantly IgG1 and IgG2a (IgG1 to IgG2a ratio ranging between 4 and 8), indicating a mixed Th1/Th2-type immune response (data not shown).
Fig 3.
(A) Kinetics of the antibody response to PyLPC/RMC in CAF1/J mice immunized with different doses of the hybrid chimeric protein, determined by ELISA. Groups of 10 mice were immunized three times with 0.2 μg to 20 μg of PyLPC/RMC emulsified in Montanide ISA 51. Antibody titers were measured 20 days after each immunization and expressed as reciprocal geometric mean antibody titers ± standard deviations for mice immunized with 0.2 μg (●), 1 μg (♢), 10 μg (▲), or 20 μg (○). (B) Antibody avidity is expressed as avidity index measured as the concentration of ammonium thiocyanate required to dissociate 50% of bound antiprotein antibodies. Boxes of box-and-whisker plots summarize the medians and 25th and 75th percentiles, and whiskers indicate the upper and lower adjacent values from 10 mice. (C and D) Effects of immunization with different doses of PyLPC/RMC on the course of infection after experimental challenge with P. yoelii sporozoites. Summary of kinetics of parasitemia (C) and reduction in hemoglobin levels (D) in mice immunized with 0.2 μg (●), 1 μg (♢), 10 μg (▲), 20 μg (○), or placebo (■). Data are presented as the mean values expressed as percentage of parasitemia or the mean change in hemoglobin concentration per day determined by comparison with baseline concentrations and expressed as percentage of reduction in hemoglobin levels (n = 6). ***, P < 0.0001 by t test in comparison with results for 1, 10, or 20 μg/dose; *, P < 0.01 by Wilcoxon matched-pairs signed rank test. For panels C and D, the P values were calculated by one-way ANOVA of AUC analysis with Bonferroni's multiple comparison posttest: **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
To assess protective effect, PyLPC/RMC-immunized mice were experimentally challenged with P. yoelii 17XNL sporozoites 20 days after the last immunization. Protective immunity was induced in a dose-dependent manner. All the experimental groups immunized with 0.2, 1, 10, or 20 μg of PyLPC/RMC were protected and exhibited longer prepatent periods and delay in peak parasitemia than placebo-immunized mice, with mean peak parasitemias of 8.4% for mice immunized with 0.2 μg, 6.2% for mice immunized with 1 μg, 2.8% for mice immunized with 10 μg, and 1.2% for mice immunized with 20 μg, whereas the mean peak parasitemia for placebo-immunized mice was recorded as 23.6% on day 9. Significant differences in parasitemia levels were observed between LPC/RMC-immunized and placebo-immunized mice, as measured by differences in the AUC analysis of parasitemia versus time (Fig. 3C). Consistent with the control of parasite load, mice that received the hybrid protein significantly controlled the drop in hemoglobin concentration; the maximum decline in mean hemoglobin levels reached 34.2% from baseline levels in mice immunized with 1 μg, 37.5% in mice immunized with 10 μg, and 25.6% in mice immunized with 20 μg. These levels are in contrast with the declines in hemoglobin concentration to 60.2% from baseline level in placebo-immunized mice and 59.2% for mice immunized with 0.1 μg. Differences in the reduction of hemoglobin levels were statistically significant between groups that received 1, 10, or 20 μg of LPC/RMC and the adjuvant-immunized mice, as measured by differences in AUC analysis of hemoglobin reduction versus time (Fig. 3D).
Passive immunization experiments.
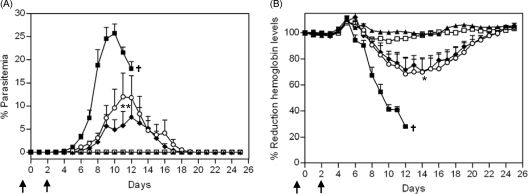
To assess the functional effect of antibodies elicited by immunization with the chimeric hybrid protein, we compared the effect of passively transferred purified IgG from rabbits immunized with PyLPC/RMC on the course of infection in CAF1/J naïve mice with the effect of the passively transferred purified IgG from rabbits immunized with PyLPC or PyRMC. An additional group of mice received a mixture of purified IgG from PyLPC- and PyRMC-immunized rabbits. The control group received purified IgG derived from naïve rabbits. To increase the stringency of the assay, we decided to use 500 sporozoites, an inoculum that represents 10 times the minimal dose required to infect 100% of normal mice.
Groups of five or six mice were passively immunized by intravenous inoculation of 500 μg of purified IgG per dose on days −1, 0, +1, +3 and +5 relative to the day of challenge. Figure 4 summarizes the results of two independent experiments. Following experimental challenge, 8 of 11 mice in the group that received purified IgG from PyLPC/RMC-immunized rabbits were protected, 6 of them with sterilizing immunity (Fig. 4A). The group of mice passively immunized with anti-PyRMC IgG showed broad individual variability and reached a mean peak parasitemia of 17.9% on day 9. Five of 11 mice developed parasitemias ranging between 15.6% and 30.1% by day 9, with hemoglobin concentrations lower than 5 g/dl, and were euthanized. Although individual mice varied widely in the time when they reached maximum parasitemia, five out of six mice that received anti-PyRMC IgG cleared the parasite infections by day 17. Nine of 11 mice that received purified IgG from PyLPC-immunized rabbits developed parasitemias ranging between 15.7% and 38.2% and were euthanized. Two mice in this experimental group controlled their parasitemia, but their hemoglobin concentrations reached baseline levels only at the end of the follow-up period (Fig. 4B). These results were in sharp contrast to the results obtained with mice that received a mixture of purified IgG from PyLPC- and PyRMC-immunized rabbits or from naïve animals. Mice with these passive transfer schemes developed high parasitemias and severe anemia and were euthanized on day 9. Consistent with complete inhibition of parasite growth, small changes in hemoglobin levels were detectable in mice that received purified IgG from PyLPC/RMC-immunized rabbits (Fig. 4B). In contrast, an accelerated reduction in hemoglobin levels was recorded in mice that received a mixture of purified IgG from PyLPC-immunized rabbits and purified IgG from PyRMC-immunized rabbits or normal IgG. All mice in these groups had hemoglobin levels lower than 5 g/dl on day 9 after challenge and were euthanized (Fig. 4B).
Fig 4.
Effects of passive immunization on the course of parasitemia (A) or hemoglobin levels (B) after experimental challenge with P. yoelii sporozoites. Results are shown for groups of mice immunized as follows: purified IgG derived from rabbits immunized with PyLPC (♦); purified IgG derived from rabbits immunized with PyRMC (▽); purified IgG derived from rabbits immunized with PyLPC/RMC (●); mixture of IgG antibodies derived from PyLPC- or PyRMC-immunized rabbits (○); and purified IgG derived from normal rabbits (△). Data are the summary of the results of two independent experiments (n = 11 mice). Error bars show standard deviations. The arrows indicate days of passive immunization with purified rabbit antibodies. †, animals that were euthanized due to severe anemia; *, P < 0.05, **, P < 0.01, and ***, P < 0.001 by one-way ANOVA of AUC analysis with Bonferroni's multiple comparison posttest in comparison to mice that received purified IgG from rabbits immunized with PyLPC/RMC.
Cellular immune responses in mice immunized with PyLPC/RMC.
The kinetics of the antibody responses using different doses of the PyLPC/RMC hybrid protein, the dose-dependent protective response to challenge with P. yoelii sporozoites, and the protective efficacy observed with passive immunization suggest a major role of antibodies in protection. However, the absence of correlation between antibody titers following active immunization and the parasite load after experimental challenge and the failure to induce a robust protection with anti-PyLPC antibodies in passive transfer experiments suggest that cellular immune responses are also involved in protection. Furthermore, the induction of high-avidity antibodies and the evidence of IgG class switching are also in support of the critical role of T helper cells in PyLPC/RMC-induced immunity.
Cellular reactivity was initially tested using ex vivo IFN-γ ELISPOT for groups of CAF1/J mice 2 weeks after a single immunization with the chimeric hybrid protein. The response to PyLPC/RMC protein ranged between 128 and 564 spot-forming cells (SFC)/106 splenocytes, compared with the baseline IFN-γ ELISPOT response in cells obtained from placebo-immunized mice that ranged between 16 and 64 (P = 0.009, data not shown). Differences were also observed after ex vivo stimulation with peptide pools (SFC ranged between 25 and 119 for the hybrid-protein-immunized mice, compared with SFC that ranged between 0 and 4 for placebo-immunized mice, P = 0.03) (data not shown).
To determine the frequency and functionality of PyLPC/RMC-specific CD4+ or CD8+ T cells, groups of CAF1/J mice were immunized twice 20 days apart, and intracellular levels of IL-2, IFN-γ, and TNF-α were determined 5, 9, and 11 days after boosting. Cytokine production by CD4+ and CD8+ T cells was assessed following ex vivo stimulation with pools of overlapping synthetic peptides covering the complete amino acid sequence of PyLPC/RMC or the recombinant protein used for immunization. A summary of the frequencies of single-cytokine-producing T cells obtained after stimulation with peptide pools is presented in Fig. 5A. Mice immunized with PyLPC/RMC had high frequencies of both CD4+ and CD8+ peptide-specific T cells. The absolute number of cytokine-producing cells increased with time, but differences in the frequency of T cell subsets were not observed. Although the three peptide pools stimulated T cells to secrete IL-2, IFN-γ, and TNF-α, LPC-specific T cells were mainly responsible for the expansion of CD8+ T cells (Fig. 5A). The PyLPC peptide pool contains the H-2Kd-restricted CTL epitope SYVPSAEQI reported in P. yoelii CSP (31). To evaluate the functionality of T cells induced by immunization with PyLPC/RMC, we analyzed the kinetics and frequency of multifunctional T cells coproducing IL-2, IFN-γ, and TNF-α. As shown in Fig. 5B, with the exception of the response of CD8+ T cells to the PyLPC peptide pool, higher proportions of triple-positive or double-positive CD4+ T cells that expressed IFN-γ (IFN-γ+ TNF-α+ or IL-2+ IFN-γ+) than of triple-positive CD8+ T cells were observed. The high proportions of double or triple positive CD4+ T cells were detected whether the cells were stimulated with the recombinant protein or pools of synthetic peptides.
Fig 5.
T cell responses following immunization with the hybrid PyLPC/RMC protein. Antigen-specific cytokine production was assessed at different time points after the final immunization. Groups of nine CAF1/J mice were immunized twice with 20 μg of PyLPC/RMC emulsified in Montanide ISA 51. The magnitudes and quality of PyLPC/RMC-specific CD4+ or CD8+ T cell responses were determined using flow cytometry multiparametric analysis. (A) Kinetics of the frequencies of CD4+ (left) or CD8+ (right) T cells producing IL-2, IFN-γ, or TNF-α upon stimulation with pools of synthetic peptides: PyLPC (closed bars), PyRMC pool 1 (hatched bars), and PyRMC pool 2 (open bars). Bars represent mean responses ± standard deviations for three mice per group. (B) To determine the proportions of multifunctional CD4+ or CD8+ T cells, Boolean gate analysis was used to identify and quantify the fraction of the total response of cells that produced three, two, or one cytokine in response to the corresponding antigen. The pie charts summarize the fractions of single, double, or triple producers for indicated groups in the experiment described for panel A.
Effect of CD4+ or CD8+ T cell depletion on protection.
In vivo depletion of CD4+ or CD8+ T cells was used to determine the role of effector T cells in protection induced by immunization with PyLPC/RMC. CAF1/J mice were immunized with the hybrid protein using the described regimen, and 4 weeks after the third immunization, T cells were depleted with anti-CD4+ (clone GK1.5) or anti-CD8+ (clone YTS169.4) monoclonal antibody or a combination of both monoclonal antibodies. Antibodies were used at 500 μg/dose intraperitoneally, given on days −1 and +2 relative to the day of challenge. Control mice immunized with PyLPC/RMC received purified normal rat IgG. The efficiency of cell depletion was monitored by flow cytometry analysis (data not shown).
After experimental challenge, all the mice immunized with placebo developed high parasitemias of over 20% between day 10 and 12 after challenge, with hemoglobin levels lower than 5 g/dl, and were euthanized (Fig. 6). Four out of six mice depleted of CD4+ T cells had peaks of parasitemia at different time points that ranged from 9 to 25.4%. Similarly, in the CD4+/CD8+ T cell-depleted group, five out of six mice had peaks of parasitemia ranging between 1.1% and 25.9% (P < 0.01 by one-way ANOVA of AUC analysis of parasitemia versus time with Bonferroni's multiple comparison posttest compared with PyLPC/RMC-immunized mice that received normal IgG). Mice that had the highest levels of parasitemia in the groups depleted of CD4+ or CD4+ CD8+ T cells also had reductions in hemoglobin levels lower than 50% in comparison to baseline levels (P < 0.05 by one-way ANOVA of AUC analysis of hemoglobin reduction versus time with Bonferroni's multiple comparison posttest compared with PyLPC/RMC-immunized mice that received normal IgG).
Fig 6.
Effects of CD4+ or CD8+ T cell depletion in CAF1/J mice immunized with PyLPC/RMC formulated in Montanide ISA 51. Mice were treated the day before challenge and again on day 2 using 500 μg of GK1.5 anti-CD4+ monoclonal antibody (♦), YTS169.4 anti-CD8+ monoclonal antibody (□), or a combination of both (○). Control mice were treated with normal rat IgG (▲). As a control for infection, placebo-immunized mice were also included (■). (A) Summary of the course of parasitemias expressed as percentage of parasitemia (average ± standard deviation [SD]). (B) Impact of immunization on the hemoglobin concentration after challenge, expressed as percentage of reduction in hemoglobin concentration relative to baseline levels (average ± SD). Data are representative of two independent experiments (n = 6 mice). The arrows indicate days of antibody treatment with specified monoclonal antibodies. †, animals that were euthanized due to severe anemia; **, P < 0.01, and *, P < 0.05 by one-way ANOVA of AUC analysis with Bonferroni's multiple comparison posttest in comparison to mice that received normal rat IgG.
PyLPC/RMC-immunized mice depleted of CD8+ T cells or immunized mice treated with normal rat IgG were protected with sterilizing immunity; only one mouse from the group that received rat IgG had two consecutive days of detectable parasitemia, with a maximum peak of 0.5% on day 11. No significant changes were observed in hemoglobin levels in mice depleted of CD8+ T cells or mice treated with normal IgG (Fig. 6).
DISCUSSION
The development of malaria vaccines has been mostly focused on single antigens. Given the complexity of the parasite life cycle and the mechanisms used by the parasite to evade the immune response, the ideal malaria vaccine should target several antigens expressed in different stages of the parasite's development. We have previously reported that immunization with chimeric recombinant proteins based on CSP or MSP-1 when tested individually induced protective immunity against malaria using a stringent challenge model (26, 27). We hypothesized that the combination of these chimeric proteins in a single immunogen would broaden the immune response by including preerythrocytic and erythrocytic vaccine components. This study demonstrates that the combination of these chimeric antigens in a vaccination regimen significantly improved the immune response, leading to a robust protective efficacy. The combination of two chimeric proteins in a single hybrid protein by genetic linkage of synthetic genes encoding individual proteins simplifies the vaccine delivery and opens the possibility of creating a multistage subunit vaccine.
We have reported that the use of autologous promiscuous T helper epitopes tailored for individual antigens is critical to enhance the magnitude and functionality of the antibody response to recombinant proteins. Also crucial for the immunogenicity of such protein immunogens was the use of a high density of T cell epitopes. This was attained by producing multimeric domains or by the inclusion of several epitopes arrayed in tandem (26, 27). Chimeric proteins containing these features are more efficient immunogens and induce more effective neutralizing antibodies than controls (26, 27). We tested the synergistic effect of PyLPC combined with PyRMC, two chimeric recombinant proteins designed to target different stages of the parasite life cycle. Here, we demonstrate that in vivo, after passive immunization, neutralization with purified IgG from PyLPC/RMC-immunized rabbits is more efficient than the neutralization effect of purified IgG from rabbits immunized with PyLPC or PyRMC (Fig. 4). In contrast, the passive transfer of a mixture of IgG antibodies elicited by immunization with PyLPC or PyRMC did not modify the kinetics of the infection in vivo. The reason for this effect is unknown at present. It is possible that passive immunization with a mixture of polyclonal antibodies interfered with parasite clearance. Alternatively, the total amount of IgG transferred (1 mg/dose instead of 500 μg/dose) could have induced a hypersensitivity reaction that led to severe malaria. Taken together, these results appear to indicate the capacity to induce more functional antibodies against sporozoites and merozoites when these antigens are delivered as components of a single chimeric protein.
Studies using the P. yoelii MSP-1 model have determined the critical role of T cells in protective immunity (12, 27). CD4+ T helper cells are essential for the induction of MSP-119 antibodies (27). In addition, as MSP-1 is also expressed in the liver stage (23), MSP-1-specific CD8+ T cells have an effect on exoerythrocytic-stage development (12). Here, we showed that immunization with a hybrid PyLPC/RMC protein induced a high frequency of CD4+ cytokine-secreting cells that recognized different epitopes of the chimeric protein. Most relevantly, CD4+ T cells were multifunctional and simultaneously produced IL-2, IFN-γ, and TNF-α. We also found that CD4+ T cell depletion but not CD8+ T cell depletion abrogated the protective effect of vaccination with the hybrid protein. CD4+ T cells with this phenotypic profile have been associated with protection against leishmaniasis and P. falciparum malaria (8, 21, 22). Multifunctional CD8+ T cell responses were also induced to the epitope expressed in the PyLPC chimeric protein. This is remarkable given that the chimeric antigen was delivered using a recombinant protein. It remains to be determined whether the induction of stronger CD8+ T cell responses by using recombinant viral vectors would improve the efficacy of the hybrid chimeric antigen described here. Interestingly, the robust responses obtained with prime-boosting immunization strategies that include recombinant viral vectors in models using MSP-1 (9, 11, 14) support the use of this approach to deliver PyLPC/RMC.
Depletion of CD4+ T cells prior to the experimental challenge abolished the protection induced by immunization with the PyLPC/RMC hybrid protein. It has been reported that effector CD4+ T cells with sporozoite specificity can target liver-stage parasites by a direct effect or through the production of IFN-γ (5, 10, 30). MSP-1-specific CD4+ T cells significantly reduce parasitemias, an effect that is also mediated by IFN-γ production (28). More recently, it has been shown that the production of IFN-γ by T cells is more robust in cells with the capacity for simultaneous production of IL-2, IFN-γ, and TNF-α (24). The relevance of multifunctional CD4+ T cells in our model is consistent with recent reports showing that multifunctional CD4+ T cells are essential for protection against sporozoite challenge in humans immunized with sporozoites under chloroquine cover (21). In this model, the exposure to preerythrocytic- and erythrocytic-stage antigens is also needed for protection. Interestingly, the protection against the experimental challenge using this vaccination approach is long lasting, persisting for at least 28 months after immunization (22). It is therefore tempting to speculate that sustained protection in malaria requires the induction of multistage and multifunctional CD4+ T cells.
A major concern for the development of multivalent vaccines is the potential for vaccine interference that would be associated with poor immune response (29). This effect has been reported using combinations of recombinant proteins (PyMSP-142 and PyMSP-8 [25] or PfMSP-142 and PfAMA-1 [17]) or using viral vectors combining PyCSP and PyMSP-142 (13). In these models, reduced antibody or CD8+ T cell responses suggest a potential adverse interaction that would preclude the use of MSP-1 as a component of a multiantigen vaccine (25). Interestingly, in the combination of PyMSP-142 and PyMSP-8, the use of a PyMSP-119--PyMSP-8 fusion protein overcame the interference in the production of MSP-119 antibodies due to the presence of CD4+ T cell epitopes in PyMSP-8.
Our results clearly show that the delivery of PyLPC and PyRMC as a hybrid protein induces a robust antibody response against individual components with no evidence of immune interference. The strength and breadth of the immune response induced by immunization with PyLPC/RMC, as indicated by the high frequencies of peptide-specific CD4+ and CD8+ cytokine-secreting cells, were considerably greater than those of the responses reported for individual proteins (26, 27). Furthermore, antibodies elicited by immunization with PyLPC/RMC were able to recognize native proteins on sporozoites and merozoites, suggesting that the structure of relevant epitopes was not modified in the expression of the chimeric protein. It seems likely that the topology of the hybrid protein, with several autologous promiscuous T cell epitopes, is responsible for the induction of balanced humoral and cellular protective immune responses.
The efficient induction of peptide-specific CD4+ and CD8+ T cell responses induced by the hybrid protein containing MSP-119 contrasts with the undetectable levels of CD4+ T cells reported for protein-based (15) or virus-vectored vaccines (11). The poor immunogenicity of MSP-119 is attributed to the complex tertiary structure of the native protein (6, 18) that interferes with antigen processing (15). We overcame this feature by expressing MSP-119 genetically linked to promiscuous T cell epitopes derived from other regions of the same native protein (27). Interestingly, a similar strategy has been used to improve the immunogenicity of the P. falciparum MSP-119 protein (19). Genetic linkage to exogenous T cell epitopes has also been reported for Plasmodium vivax MSP-119 (7). The subsequent linkage of the chimeric MSP-119 to a chimeric antigen derived from CSP enhanced the overall immune response. It is therefore feasible to design an effective malaria vaccine by selecting an array of antigens derived from proteins expressed in different stages and engineering the vaccine to contain these features.
In summary, we describe here the design and expression of a hybrid recombinant protein that includes chimeric subunit antigens derived from P. yoelii CSP and MSP-1. The hybrid PyLPC/RMC recombinant protein exhibited antigenic profiles similar to those of the original parental proteins, since the protein could be recognized by monoclonal and polyclonal antibodies specific for different segments of the chimeric protein. The hybrid protein showed a synergistic effect on protection against the challenge with sporozoites when compared to the protection conferred by individual components of the protein. Although high-affinity antibodies and multifunctional CD4+ and CD8+ T cells are elicited by immunization, only antibodies and CD4+ T cells mediate the protective effect. Thus, a desirable malaria vaccination strategy should include multistage antigens that incorporate several genetically linked autologous T cell epitopes.
ACKNOWLEDGMENTS
This research was supported by the U.S. National Institutes of Health, NIAID grant no. R01-AI064766, and the Yerkes National Primate Research Center, base grant no. RR00165, awarded by the National Center for Research Resources of the National Institutes of Health.
We thank Tracey Lamb for critical reading of the manuscript, Robert Mittler for providing the rat hybridomas for in vivo depletion, and the Malaria Research and Reference Reagent Resource Center (MR4) for providing us with P. yoelii-infected mosquitoes contributed by the New York University School of Medicine. We also thank the anonymous reviewers for their helpful comments and suggestions.
Footnotes
Published ahead of print 17 January 2012
REFERENCES
- 1. Bentley GA. 2006. Functional and immunological insights from the three-dimensional structures of Plasmodium surface proteins. Curr. Opin. Microbiol. 9:395–400 [DOI] [PubMed] [Google Scholar]
- 2. Caro-Aguilar I, Lapp S, Pohl J, Galinski MR, Moreno A. 2005. Chimeric epitopes delivered by polymeric synthetic linear peptides induce protective immunity to malaria. Microbes Infect. 7:1324–1337 [DOI] [PubMed] [Google Scholar]
- 3. Caro-Aguilar I, et al. 2002. Plasmodium vivax promiscuous T-helper epitopes defined and evaluated as linear peptide chimera immunogens. Infect. Immun. 70:3479–3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Casares S, Brumeanu T-D, Richie TL. 2010. The RTS,S malaria vaccine. Vaccine 28:4880–4894 [DOI] [PubMed] [Google Scholar]
- 5. Charoenvit Y, et al. 1999. CD4+ T-cell- and gamma interferon-dependent protection against murine malaria by immunization with linear synthetic peptides from a Plasmodium yoelii 17-kilodalton hepatocyte erythrocyte protein. Infect. Immun. 67:5604–5614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chitarra V, Holm I, Bentley GA, Petres S, Longacre S. 1999. The crystal structure of C-terminal merozoite surface protein 1 at 1.8 A resolution, a highly protective malaria vaccine candidate. Mol. Cell 3:457–464 [DOI] [PubMed] [Google Scholar]
- 7. Cunha MG, Rodrigues MM, Soares IS. 2001. Comparison of the immunogenic properties of recombinant proteins representing the Plasmodium vivax vaccine candidate MSP119 expressed in distinct bacterial vectors. Vaccine 20:385–396 [DOI] [PubMed] [Google Scholar]
- 8. Darrah PA, et al. 2007. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 13:843–850 [DOI] [PubMed] [Google Scholar]
- 9. de Cassan SC, et al. 2011. The requirement for potent adjuvants to enhance the immunogenicity and protective efficacy of protein vaccines can be overcome by prior immunization with a recombinant adenovirus. J. Immunol. 187:2602–2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Del Giudice G, et al. 1990. Peptide-primed CD4+ cells and malaria sporozoites. Immunol. Lett. 25:59–63 [DOI] [PubMed] [Google Scholar]
- 11. Douglas AD, et al. 2010. Tailoring subunit vaccine immunogenicity: maximizing antibody and T cell responses by using combinations of adenovirus, poxvirus and protein-adjuvant vaccines against Plasmodium falciparum MSP1. Vaccine 28:7167–7178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Draper SJ, et al. 2009. Recombinant viral vaccines expressing merozoite surface protein-1 induce antibody- and T cell-mediated multistage protection against malaria. Cell Host Microbe 5:95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Forbes EK, et al. 2011. Combining liver- and blood-stage malaria viral-vectored vaccines: investigating mechanisms of CD8+ T cell interference. J. Immunol. 187:3738–3750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goodman AL, et al. 2010. New candidate vaccines against blood-stage Plasmodium falciparum malaria: prime-boost immunization regimens incorporating human and simian adenoviral vectors and poxviral vectors expressing an optimized antigen based on merozoite surface protein 1. Infect. Immun. 78:4601–4612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hensmann M, et al. 2004. Disulfide bonds in merozoite surface protein 1 of the malaria parasite impede efficient antigen processing and affect the in vivo antibody response. Eur. J. Immunol. 34:639–648 [DOI] [PubMed] [Google Scholar]
- 16. Nardin EH, et al. 1982. Circumsporozoite proteins of human malaria parasites Plasmodium falciparum and Plasmodium vivax. J. Exp. Med. 156:20–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pichyangkul S, et al. 2009. Evaluation of the safety and immunogenicity of Plasmodium falciparum apical membrane antigen 1, merozoite surface protein 1 or RTS,S vaccines with adjuvant system AS02A administered alone or concurrently in rhesus monkeys. Vaccine 28:452–462 [DOI] [PubMed] [Google Scholar]
- 18. Pizarro JC, et al. 2003. Crystal structure of a Fab complex formed with PfMSP1-19, the C-terminal fragment of merozoite surface protein 1 from Plasmodium falciparum: a malaria vaccine candidate. J. Mol. Biol. 328:1091–1103 [DOI] [PubMed] [Google Scholar]
- 19. Pusic KM, et al. 2011. T cell epitope regions of the P. falciparum MSP1-33 critically influence immune responses and in vitro efficacy of MSP1-42 vaccines. PLoS One 6:e24782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roederer M, Nozzi JL, Nason MC. 2011. SPICE: exploration and analysis of postcytometric complex multivariate datasets. Cytometry A 79:167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roestenberg M, et al. 2009. Protection against a malaria challenge by sporozoite inoculation. N. Engl. J. Med. 361:468–477 [DOI] [PubMed] [Google Scholar]
- 22. Roestenberg M, et al. 2011. Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet 377:1770–1776 [DOI] [PubMed] [Google Scholar]
- 23. Sacci JB, Jr, et al. 2005. Transcriptional analysis of in vivo Plasmodium yoelii liver stage gene expression. Mol. Biochem. Parasitol. 142:177–183 [DOI] [PubMed] [Google Scholar]
- 24. Seder RA, Darrah PA, Roederer M. 2008. T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 8:247–258 [DOI] [PubMed] [Google Scholar]
- 25. Shi Q, Lynch MM, Romero M, Burns JM., Jr 2007. Enhanced protection against malaria by a chimeric merozoite surface protein vaccine. Infect. Immun. 75:1349–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Silva-Flannery LM, Cabrera-Mora M, Jiang J, Moreno A. 2009. Recombinant peptide replicates immunogenicity of synthetic linear peptide chimera for use as pre-erythrocytic stage malaria vaccine. Microbes Infect. 11:83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Singh B, Cabrera-Mora M, Jiang J, Galinski M, Moreno A. 2010. Genetic linkage of autologous T cell epitopes in a chimeric recombinant construct improves anti-parasite and anti-disease protective effect of a malaria vaccine candidate. Vaccine 28:2580–2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stephens R, et al. 2005. Malaria-specific transgenic CD4(+) T cells protect immunodeficient mice from lethal infection and demonstrate requirement for a protective threshold of antibody production for parasite clearance. Blood 106:1676–1684 [DOI] [PubMed] [Google Scholar]
- 29. Vidor E. 2007. The nature and consequences of intra- and inter-vaccine interference. J. Comp. Pathol. 137:S62–S66 [DOI] [PubMed] [Google Scholar]
- 30. Wang R, et al. 1996. Protection against malaria by Plasmodium yoelii sporozoite surface protein 2 linear peptide induction of CD4+ T cell- and IFN-gamma-dependent elimination of infected hepatocytes. J. Immunol. 157:4061–4067 [PubMed] [Google Scholar]
- 31. Weiss W, et al. 1990. Cytotoxic T cells recognize a peptide from the circumsporozoite protein on malaria-infected hepatocytes. J. Exp. Med. 171:763–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. WHO 2011. World malaria report 2011. World Health Organization, Geneva, Switzerland [Google Scholar]
- 33. Yoshida N, Nussenzweig RS, Potocnjak P, Nussenzweig V, Aikawa M. 1980. Hybridoma produces protective antibodies directed against the sporozoite stage of malaria parasite. Science 207:71–73 [DOI] [PubMed] [Google Scholar]