Abstract
Objective
Growing evidence suggests that a phenotypic switch converting pancreatic acinar cells to duct-like cells can lead to pancreatic intraepithelial neoplasia (PanIN) and eventually to invasive pancreatic ductal adenocarcinoma. Histologically, the onset of this switch is characterised by the co-expression of acinar and ductal markers in acini, a lesion called acinar-to-ductal metaplasia (ADM). Transcriptional regulators required to initiate ADM still remain unknown, yet need to be identified to characterise the regulatory networks that drive ADM. Here we investigate the role of the ductal transcription factors Hepatocyte Nuclear Factor 6 (HNF6, also known as Onecut1)and SRY-related HMG box factor 9 (Sox9) in ADM.
Design
Expression of HNF6 and Sox9 is measured by immunostaining in normal and diseased human pancreas. The function of the factors is tested in cultured cells and in mouse models of ADM by a combination of gain- and loss-of-function experiments.
Results
Expression of HNF6 and Sox9 is ectopically induced in acinar cells in human ADM, as well as in mouse models of ADM. We show that these factors are required for repression of acinar genes, for modulation of ADM-associated changes in cell polarity, and for activation of ductal genes in metaplastic acinar cells.
Conclusions
HNF6 and Sox9 are new biomarkers of ADM and constitute candidate targets for preventive therapy in cases when ADM may lead to cancer. Our work also highlights that ectopic activation of transcription factors may underlie metaplastic processes occurring in other organs.
Keywords: Mice, transcription factors, pancreas, metaplasia
INTRODUCTION
Pancreatic exocrine functions are exerted by the acinar cells which produce and secrete digestive enzymes, and ductal cells which line ducts that drain the secretions to the duodenum A phenotypic switch affecting the acinar cells may cause pancreatic adenocarcinoma (PA), one of the most aggressive cancers.[1] Phenotypic analysis of PA cells reveals that they express markers of pancreatic ductal cells. However, there is evidence that PA may be initiated in acinar cells. In pancreata of humans with PA, ductal markers are expressed in acinar cells located in the vicinity of neoplastic regions.[2] Such ectopic expression of ductal markers, defined as acinar-to-ductal metaplasia (ADM), is also observed in various animal models, like pancreatic duct ligation (PDL), treatment with carcinogens or cerulein, and forced expression of transforming growth factor (TGF)α or of mutated TGFβ receptor type II (TGFβRII) in acinar cells.[3, 4, 5, 6, 7] In these models, acinar cells undergo ADM and form tubular complexes showing an intermediate phenotype between acini and ducts. Moreover, in pancreata overexpressing TGFα, ductal neoplasia develops, most likely originating from tubular complexes.[4] More recently, several studies have shown that PA can arise from acinar cells: PA develops in mice which express mutated K-ras in acinar cells.[8, 9, 10, 11, 12, 13]
PA is associated with the formation of noninvasive ductal lesions known as pancreatic intraepithelial neoplasia (PanIN)[14] which can express acinar markers.[15] Lineage tracing experiments show that tubular complexes and PanIN can originate from acinar cells.[11, 12, 13, 16] Constitutively active mutant forms of the proto-oncogene K-ras are found in a large proportion of PanINs, the overall rate for K-ras mutations increasing from 40% in PanIN1A up to 100% in PanIN3 [17]. Consequently mutated K-ras is considered as the major driver of progression to PA.[18] Mutations in other genes, such as p21, p53, p16INK4 or SMAD4, cooperate with mutated K-ras to promote this progression.[1, 19] Together, these findings suggest a model in which metaplastic acinar cells that acquire a K-ras mutation give rise to PanIN which will evolve to cancer provided that other genes are also mutated.
Clinical data show that pancreatitis, an inflammatory condition of the pancreas in which ADM is detected, may also contribute to development of PA.[20] Results obtained in mice support this model: pancreatitis accelerates PanIN formation and progression to PA when mutated K-ras is expressed in the acinar cells.[15] These data suggest that activation of a ductal gene program combined with repression of acinar gene expression in metaplastic acinar cells can lead to PA, yet how a switch from acinar to duct-like cells is determined remains unknown.
To investigate this switch, we used several models of ADM. We hypothesised that ADM is associated with inappropriate expression of ductal transcription factors in the metaplastic acinar cells. This was tested by studying the expression and function of the transcription factors HNF6, Sox9 and HNF1β in metaplastic lesions. Indeed, in mouse embryos, these factors control duct development, as well as pancreas morphogenesis and endocrine differentiation.[21, 22, 23, 24, 25] After birth, their expression persists only in duct cells, while being absent from acinar and endocrine cells. Here we show that HNF6 and Sox9 are expressed in human cells that undergo ADM. We also show that HNF6 can induce ADM and that both HNF6 and Sox9 are required in ADM for repression of acinar genes, for ADM-associated changes in cell polarity, and for activation of ductal genes in acinar cells.
MATERIALS AND METHODS
Mice
Mice received humane care according to the criteria listed by the National Academy of Sciences. Hnf6−/−, Sox9f/f, and Ela-CreERT2 mice have been described.[23, 26, 27]
Tamoxifen treatment
Six week-old ElaC-Sox9f/f mice were treated with tamoxifen (Sigma) dissolved in corn oil (Sigma) at a concentration of 30 mg/ml. Treatment consisted of an intraperitoneal injection of 100 μl combined with the gavage of another 100 μl on day 1, 3 and 5. The duct ligations and the adenovirus injections (see below) were performed 12 days after the last day of treatment.
Construction of the HNF6-expressing adenovirus
HNF6 cDNA was subcloned into the pAdTrack-CMV shuttle vector (Addgene). The resulting plasmid was linearised with PmeI and cotransformed into Escherichia coli strain BJ5183-Ad1 which contains the supercoiled adenoviral vector pAd-Easy1 (Addgene). Adenoviral recombinants were selected by kanamycin resistance and screened by restriction endonuclease digestion. The recombinant adenoviral construct was cleaved with PacI and transfected into HEK293-FT cells. Adenoviruses were propagated in this cell line, purified by a double centrifugation on CsCl gradient, and stored at −80°C. Ad-HNF6 coded for both HNF6 and GFP, their expression being under the control of independent CMV promoters.
Cell culture and adenovirus infection
266-6 cells, an acinar cell line derived from a mouse pancreatic tumor induced with an Elastase I/SV40 T antigen fusion gene, were grown in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal calf serum, L-Glutamine, and antibiotics. Ten thousand cells were seeded on 24-well plates 18h prior to adenoviral infection. Cells were infected with adenovirus expressing either HNF6 (Ad-HNF6), Sox9 (Ad-Sox9),[28] or GFP (Ad-GFP) (Vectorbiolabs) at a multiplicity of infection of 100 in 200 μl of DMEM. After one hour, 200 μl of two-fold concentrated DMEM with supplements were added to the wells. Cells were grown for 48h, then washed prior to RNA extraction or immunocytofluorescence analysis.
Western blotting
Proteins were extracted from infected 266-6 cells with RIPA buffer (150 mM NaCl, 1 mM PMSF, 1 mM EDTA, 1% Triton X100, 0.1% SDS) complemented with protease inhibitors (Sigma). For Western blot analysis, protein extracts were subjected to electrophoresis on 10% polyacrylamide gels (SDS-PAGE) and transferred onto a nitrocellulose Hybond-C membrane (Amersham). The membrane was incubated overnight at 4°C with goat anti-Amylase 1/1000 (Santa Cruz), goat anti-HNF6 1/5000 (described below) or goat anti-β-actin 1/1000 (Santa Cruz) antibodies diluted in TBS, 0.1% Tween, and 5% BSA. The membrane was then incubated with respective HRP antibodies 1/10000 (Sigma) for 2 h at room temperature. Signals were visualized by chemiluminescence.
Adenovirus injection
Adenovirus injection was performed as previously described.[29] Briefly, adult mice were anaesthetised by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (1 mg/kg). Under aseptic surgical conditions, a midline laparotomy was performed. Viscera were gently mobilised to reach the splenic lobe of the pancreas. One hundred μl of purified virus (1×1010 viruses/ml) was injected directly into 2–3 foci of the dorsal splenic lobe of the pancreas with a 3/10cc insulin syringe (Becton Dickinson). Then the viscera were placed in anatomical position and the abdominal wall and skin were sutured in separate layers. The mice received subcutaneously 1 ml of sterile phosphate-buffered saline (PBS) and food and water were given ad libitum. Mice were collected 2 or 3 days after injection of adenoviruses.
PDL and 9,10-dimethyl-1,2-benzanthracene (DMBA) treatment
Mice were anaesthetised and the pancreas was surgically exposed as mentioned above. The duct was ligated using a Surgipro II 7-0 nonabsorbable, monofilament polypropylene suture. Mice were sacrified 5 days after ligature and the pancreas was collected for immunolabeling.
For DMBA treatment, adult control and hnf6−/− mice were anaesthetised and the pancreas was surgically exposed as mentioned above. One polytetrafluoroethylene pledget soaked in 8 μl of DMBA 10 % in benzene was placed on the splenic lobe of the pancreas. Then the pancreatic parenchyma was wrapped around the pledget, resulting in a pouch that was closed using a purse-string suture. Mice were sacrificed 20 days after surgery and the pancreas was collected for immunolabeling.
Generation of a guinea pig anti-HNF6 antibody
Two peptides spanning amino acids 11-53 (GELHGVSHEPVPAPADLLGGSPHARSSVGHRGSHLPPAHPRSMc) and 61-81 (GGSGGSDYHHHHRAPEHSLAGc) of mouse HNF6 were synthesised (Schafer-N) with a C-terminal cysteine (c) added for coupling to mcKLH. Peptides were conjugated to mcKLH using Imject Maleimide Activated mcKLH Kit (Pierce) according to manufacturer’s instruction. The conjugates were purified by dialysis in PBS overnight. The two coupled KLH protein complexes were mix 1:1 and injected in four guinea pigs. They were immunised subcutaneously biweekly with 20 μg KLH-HNF6 aa11-53:KLH-HNF6 aa61-81, the first immunisation with Freund’s complete adjuvant and the next three with Freund’s incomplete adjuvant, followed by monthly immunisations with 4 μg with Freund’s incomplete adjuvant. Animals were bled 10 days after each immunisation.
Immunolabelling
266-6 cells were fixed 20 min at −20°C in methanol, washed three times in PBS, and permeabilised in PBS, 0.3% Triton X-100 for 10 min and incubated in blocking buffer (PBS, 10% BSA, 3% milk powder, 0.3% Triton X-100) for 1 h. Mouse tissue samples were fixed overnight in 4% paraformaldehyde in PBS at 4° C, and embedded in paraffin. Archival human formalin-fixed pancreatic samples from the Cliniques Universitaires Saint-Luc Pathology Bank were used to perform immunolabelling. Serial paraffin sections, 9 μm thick, were mounted on glass slides, then deparaffinised and re-hydrated. Antigen retrieval was performed by boiling the slides for 10 min in 10 mM citrate buffer, pH 6, in a microwave oven.
The primary antibodies were: guinea pig anti-HNF6 1/5000, rabbit anti-HNF6 (Santa Cruz) 1/200, rat anti-CK19 (Developmental Studies Hybridoma Bank) 1/50, CK7 (Biogenex) 1/50, rabbit anti-Cleaved Caspase3 (Cell Signaling) 1/200, rabbit anti-GFP (Abcam) 1/200, goat anti-Amylase (Santa Cruz) 1/500, rabbit anti-Sox9 (Chemicon) 1/500, Armenian hamster anti-Mucin1A (Neomarkers) 1/500, mouse anti-Ezrin (Neomarkers) 1/50, rabbit anti-HNF1β (Santa Cruz) 1/100. They were diluted in blocking buffer and incubated with the samples overnight at 4° C. Secondary antibodies and streptavidin-POD conjugate (1/100) were diluted in PBS, 10% BSA, 0.3% Triton X-100 buffer and incubated at 37° C for one hour. The immunolabelings were carried out as for immunocytofluorescence. Pictures were taken using a Cell Observer Spinning Disk confocal microscope (Zeiss) for the fluorophore labeling, or with a Mirax imaging system (Zeiss) for histological staining.
Reverse transcription-PCR (RT-PCR) and real-time quantitative PCR (Q-RT-PCR)
Total RNA was extracted from pancreata or 266-6 cells with TriPure reagent (Roche). RT-PCR and Q-RT-PCR were performed as described.[25] For quantification, absolute copy number for each mRNA was normalised to absolute β-actin mRNA copy number, using standard calibration curves. For comparison of hnf6+/+ and hnf6−/− animals, E-cadherin normalisation was also performed to take into account that the mesenchyme/epithelium ratio is increased in hnf6−/− pancreas. Sequences of the primers used for quantification can be obtained on request.
Quantification of metaplasia and apoptosis marker expression in overexpression and PDL models
For quantification, pictures from 10 randomly-chosen sections were taken per mouse (n=3 for each model). Counting and area quantification were performed with Adobe Photoshop CS2 and ImageJ software (<http://rsbweb.nih.gov/ij/>), respectively. The percentage of Sox9 deletion and of CK19- and Cleaved Caspase3-positive cells, were calculated by dividing the number of acinar cells positive for one of these markers by the total number of acinar cells. The number of acinar cells was calculated by counting acinar nuclei, using DAPI staining. Amylase, CK-19, Ezrin and Mucin1a positive pixel numbers were calculated and normalized to acinar tissue area. The positive area/acinar area ratio was arbitrarily set to 100 in wild-type mice. Bars represent means ± S.E.M. (***, P<0.001).
RESULTS
Expression of HNF6 and Sox9 in human acinar-to-ductal metaplasia
We first tested if ADM is associated with ectopic expression of ductal transcription factors in human acinar cells. In healthy pancreas, HNF6, Sox9 (figure 1A,C) and HNF1β (supplementary figure 1A) were found only in ductal cells, including the centroacinar cells. In metaplastic lesions located in the vicinity of PA regions, the expression of HNF6 and Sox9, but not that of HNF1β, extended to metaplastic acinar cells (figure 1B,D, and supplementary figure 1B). In metaplastic acinar cells, HNF6 was coexpressed with Amylase, but not with the ductal marker cytokeratin (CK) 7 (figure 1E,F). In contrast, Sox9 was not coexpressed with Amylase (figure 1E), with the exception of rare acini, in which Amylase and Sox9, were coexpressed with CK7 (arrowhead) or not (arrow) (figure 1G). Moreover, metaplastic structures with acinar-like morphology were HNF6-positive and Sox9-negative (figure 1E), whereas metaplastic structures with duct-like morphology were HNF6-negative and Sox9-positive (figure 1H). However, coexpression of both factors was observed in metaplastic cells showing intermediate morphology (data not shown). Finally, PanIN and PA cells lacked HNF6 but expressed Sox9: HNF6 was absent in PanIN1A whereas Sox9 was found in PanIN1A/B, PanIN2, and PanIN3, and cancer cells (supplementary figure 2 and data not shown). These observations indicate that HNF6 and Sox9 are ectopically expressed in metaplastic cells during ADM.
Figure 1.

HNF6 and Sox9 are expressed in human acinar-to-ductal metaplasia. (A–D) Expression of HNF6 and Sox9 in normal (A,C) and metaplastic (B,D) human pancreas. HNF6 and Sox9 are detected in duct cells, but not in acinar cells. Metaplastic areas associated with pancreatic ductal adenocarcinoma show wide expression of HNF6 and Sox9. (E–H) Immunofluorescent labelling of Amylase, HNF6, Sox9 and CK7 in metaplastic areas. HNF6 and Sox9 are coexpressed in ductal cells (cyan), and HNF6 is detected in a large proportion of metaplastic acinar cells (E). Cells positive for both Amylase and HNF6 are in most cases negative for the ductal marker CK7 (F). Sox9 is coexpressed with Amylase in a subset of metaplastic acinar cells (arrow in G) and several of these Sox9+/Amy+ cells coexpress CK7 (arrowhead). Duct-like structures positive for Sox9 and negative for HNF6 are also found (H). Dotted lines delineate a duct lumen (H).
Overexpression of HNF6 triggers ADM
To investigate if HNF6 and Sox9 can induce ADM, we overexpressed these factors in cultured cells and in pancreas. The lack of HNF1β in human ADM precluded the need to further investigate its role. Overexpression was performed by adenoviral transduction of HNF6 (Ad-HNF6) or Sox9 (Ad-Sox9), with GFP-expressing adenovirus (Ad-GFP) as a control. In the acinar 266-6 cell line, expression of HNF6, but not of GFP or Sox9, induced the ductal markers Sox9, CK19, osteopontin (OPN) [30], and repressed the acinar markers Mist1, Ptf1a, Amylase, CPA and Elastase (figure 2A). HNF1β was weakly induced by HNF6 (figure 2A). Immunostaining and western blot experiments confirmed that Sox9 was induced and Amylase repressed in cells overexpressing HNF6 (figure 2B,C and data not shown). Together, these in vitro experiments suggest that HNF6, but not Sox9, can repress acinar markers while inducing ductal markers, and that Sox9 is downstream of HNF6.
Figure 2.
Ectopic expression of HNF6 in acinar cells is associated with induction of ductal genes and repression of acinar genes in vitro. (A) Relative expression of ductal (left) and acinar (right) genes in cultured 266-6 cells. Cells were transduced with an adenovirus expressing GFP (Ad-GFP), HNF6 (Ad-HNF6), or Sox9 (Ad-Sox9) and gene expression was analyzed by Q-RT-PCR (data are means +/− SEM; n= 4; *, p<0.05; **, p<0.01). (B, C) Immunostaining for GFP, HNF6 and Amylase and Western blot for HNF6 and Amylase in cultured 266-6 cells transduced with Ad-GFP or Ad-HNF6 shows that HNF6 represses Amylase expression. Arrows, HNF6+/Amylase- cells; Arrowhead, HNF6-/Amylase+ cells. Multiplicity of infection (MOI) was 100 for Ad-GFP, 30 and 100 for Ad-HNF6. Amy, Amylase; Ela, Elastase.
We then injected pancreata in vivo with Ad-GFP, Ad-HNF6 or Ad-Sox9. After 3 days of infection, the acinar cells transduced with Ad-GFP did neither express HNF6 nor Sox9 (figure 3A). Sox9 was unable to induce HNF6 (figure 3B). In contrast, several acinar cells infected by Ad-HNF6 expressed Sox9. Surprisingly, part of the Sox9-expressing cells did not coexpress HNF6, suggesting that Sox9 expression was more stable than that of HNF6 (figure 3C). To quantify this, we looked at the temporal expression pattern of HNF6. We took advantage of the fact that Ad-HNF6 expresses both HNF6 and GFP, and compared GFP/HNF6 and GFP/Sox9 colabelling after 2 or 3 days of infection by Ad-HNF6. Almost 100% of the GFP-expressing cells were colabelled by Sox9 at the two time points, indicating that Sox9 expression was as stable as GFP in that time frame (figure 3E). In contrast, 65% of cells expressing GFP were positive for HNF6 2 days after infection, while only 35% of GFP-positive cells were HNF6-positive after 3 days (figure 3E,F). Also, the proportion of Sox9-positive cells co-expressing HNF6 diminished with time (figure 3E). All together, these results indicate that ectopic expression of HNF6 in acinar cells induces Sox9 and that Sox9 is more stable than HNF6.
Figure 3.
Ectopic expression of HNF6 in acinar cells is associated with induction of ductal genes and repression of acinar genes in vivo. (A–D) Immunolabeling and immunohistochemical staining of sections of wild-type (A–C) and ElaC-Sox9f/f (D) pancreata 3 days after infection by Ad-GFP (A), Ad-Sox9 (B), or Ad-HNF6 (C,D). Metaplasia is detected in wild-type pancreas infected with Ad-HNF6 (C). Ad-HNF6 induces Sox9, and induces the ductal marker CK19 in the cytoplasm of acinar cells, while repressing Amylase. In acinar cells, Ad-HNF6 relocates Ezrin from the apical pole to the cytoplasm. After Ad-Sox9 infection, HNF6, Amylase and Ezrin are unaffected, but marginal induction of CK19 is detectable in acinar cells (B). No induction of Sox9 is observed in ElaC-Sox9f/f after infection with Ad-HNF6 (D). Ad-HNF6 repressed Amylase in ElaC-Sox9f/f pancreata, and relocated Ezrin and CK19. Mislocalisation of Ezrin and CK19 was less severe than in the presence of Sox9 (see suppl. figure 3). (E) Percentage of GFP-positive cells that coexpressed HNF6 or Sox9, two (green) or three (yellow) days after Ad-HNF6 infection (left panel). Quantification of the percentage of Sox9-positive cells that coexpressed HNF6 at the same time points. These quantifications show that HNF6 expression is less stable than GFP and Sox9 (right panel). (F) GFP/HNF6 immunolabeling of pancreas two or three days after transduction with Ad-HNF6, which codes for both HNF6 and GFP. HNF6 is less stably expressed than GFP. (G) Cleaved Caspase3/GFP and Cleaved Caspase3/HNF6 immunolabelling of pancreas infected by Ad-GFP and Ad-HNF6, respectively. Many apoptotic cells are detected in pancreas infected with Ad-HNF6. Cl. Caspase3, Cleaved Caspase3.
Histochemical staining indicated that pancreata infected by Ad-HNF6 show metaplasia (figure 3C). This is not observed after infection with Ad-GFP (figure 3A). Ectopic expression of HNF6 in acini induced diffuse expression of CK19 and decreased that of Amylase (figure 3C), thereby recapitulating in vivo the findings in 266-cells. The number of cells with reduced amylase expression exceeded the number of cells positive for HNF6. This most likely resulted from the low stability of HNF6: when HNF6 is no longer detected, amylase expression is still reduced. Since ADM is associated with loss of acinar cell polarity,[31] we also studied the location of Ezrin and Mucin1a that are normally present at the apical pole of acinar cells (see figure 3A and figure 5A,F). After infection with Ad-HNF6, Mucin1a expression remained apical (data not shown), but Ezrin was relocated to the cytoplasm (figure 3C). After infection with Ad-Sox9, Amylase and Ezrin expression was not modified, and less than 2% of acinar cells started to express CK19 (figure 3B). Quantification of these results (supplementary figure 3) confirmed the interpretation of the immunostainings. We concluded that HNF6 can induce metaplasia by repressing acinar genes while inducing duct genes, including Sox9. Sox9 overexpression has marginal effects on CK19.
Figure 5.
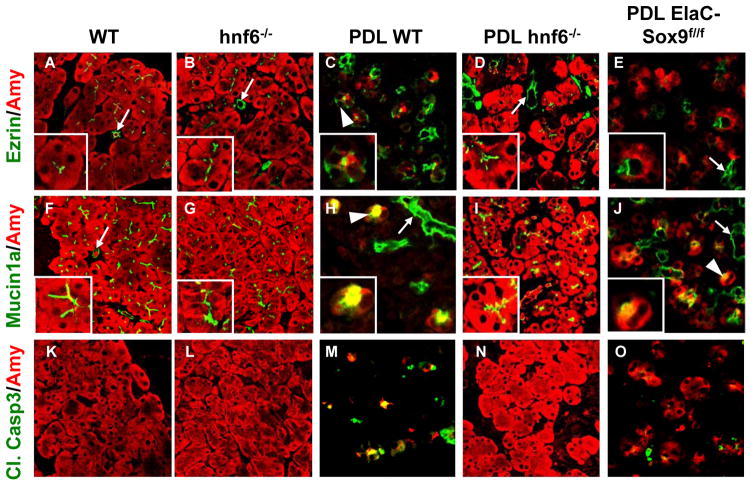
In the absence of HNF6 or Sox9 acinar cells are resistant to pancreatic duct ligation-induced loss of polarity and apoptosis. (A–E) Expression of Ezrin is located at the apical pole of acinar cells in untreated wild-type pancreas (A), but extended into the cytoplasm of metaplastic cells after PDL (C). In hnf6−/− pancreas (B), as well as in duct-ligated hnf6−/− (D) and ElaC-Sox9f/f pancreas (E), Ezrin location remains apical. Insets show magnified views of acini. (F–J) Mucin1a expression is observed at the apical pole of acinar cells in wild-type and hnf6−/− pancreas (F, G), but extended into the cytoplasm of metaplastic cells after PDL in wild-type (H) or ElaC-Sox9f/f (J) pancreas. In duct-ligated hnf6−/− pancreas (I), Mucin1a location remains apical. (K–O) Cleaved Caspase3 was induced 5 days after PDL in several acinar cells in wild-type pancreas (M), but not in hnf6−/− pancreas (N). In ElaC-Sox9f/f pancreas, the number of Cleaved Caspase3-positive cells was lower than in wild-type pancreas after PDL (O). Arrowheads point to metaplastic acini, arrows to ducts.
HNF6 and Sox9 are required for pancreatic duct ligation-induced acinar-to-ductal metaplasia
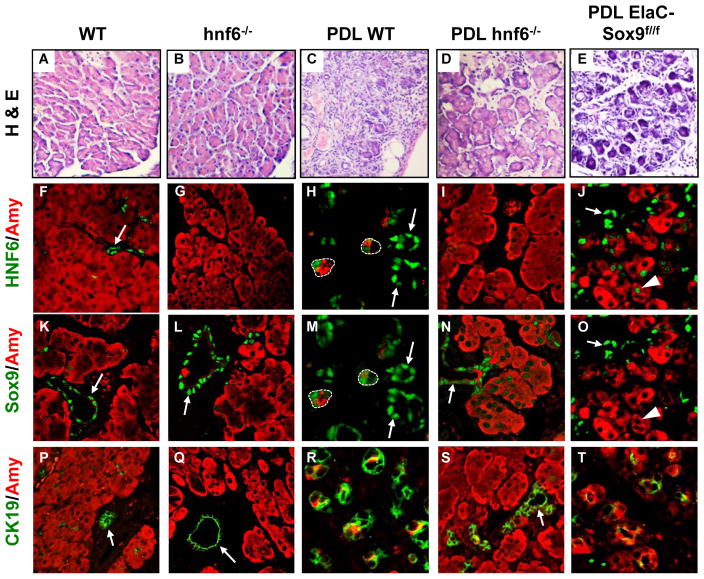
To investigate if HNF6 and Sox9 are required for ADM, we turned to mouse models of ADM. Pancreatic duct ligation (PDL) is known to induce pancreatitis with ADM and subsequent loss of acinar cells[32] (figure 4A,C). In wild-type pancreas, HNF6 was found only in ductal cells, with no expression in acinar cells (figure 4F). Sox9 was highly expressed in ductal cells whereas a few acinar cells showed low levels of Sox9 (figure 4K). After PDL, high expression of both factors was seen in metaplastic acinar cells (dotted lines in figure 4H,M). To test if HNF6 and Sox9 are required for PDL-induced ADM, we used mice knockout for these factors. We first performed PDL in hnf6−/− mice.[23] Most hnf6−/− mice die within one or two weeks after birth. They have an endocrine defect [23] and show pancreatic hypoplasia and ductal cysts.[22, 25] However, a fraction of knockout mice survive. The latter do not display acinar anomalies (figure 4A,B) and show normal acinar gene expression (unpublished). In duct-ligated hnf6−/− mice, oedema and inflammatory infiltrates were observed but, compared to wild-type duct-ligated pancreas, the integrity of the acinar tissue was less affected (figure 4B,D). As expected, in wild-type mice after PDL, CK19 and Sox9 were induced in acinar cells (figure 4K,M,P,R). In contrast, in duct-ligated hnf6−/− pancreas, expression of CK19 remained ductal (figure 4Q,S), and acinar expression of Sox9 was weak or undetectable. When detectable, this expression remained close to background (figure 4K,L,M,N). Ligated wild-type pancreas showed a number of metaplastic acini with reduced expression of Amylase (figure 4K,M,P,R) whereas Amylase expression was maintained in ligated hnf6−/− pancreas (figure 4L,N,Q,S). Quantification of the data supported our interpretation (supplementary figure 4). Histological stainings of sections corresponding to the panels shown in figure 4 are also provided in supplementary figure 5. In ligated wild-type pancreas, Ezrin and Mucin1a were no longer restricted to the apical pole but spread out in the cytoplasm (figure 5A,C,F,H), whereas they remained closely associated with the apical pole in ligated hnf6−/− pancreas (figure 5B,D,G,I). In the latter, no apoptotic cells were found (figure 5L,N), whereas apoptosis was abundant in ligated wild-type pancreas (figure 5K,M). Conversely, apoptosis was observed in pancreas infected with Ad-HNF6 while little or no apoptosis was found in pancreas infected with Ad-GFP or Ad-Sox9 (figure 3G, and data not shown). We concluded that HNF6 is required for PDL-induced repression of acinar markers and perturbed polarity.
Figure 4.
In the absence of HNF6 or Sox9, acinar cells are resistant to metaplasia induced by pancreatic duct ligation. (A–E) Haematoxylin-eosin-stained sections of pancreata, in normal condition or five days after PDL, show that acini are replaced by tubular complexes in wild-type pancreas (C), whereas they are better preserved in the absence of HNF6 (D) or less affected in the absence of Sox9 (E). (F–J) In wild-type pancreas, expression of HNF6 is only found in ducts (F), but strongly induced in metaplastic acini after PDL (H). The lack of Sox9 prevents induction of HNF6 after PDL (J), except for a few cells (arrowheads in J and O). (K–O) Sox9 expression is strongly induced in wild-type metaplastic acinar cells after PDL (M), whereas only weak induction of Sox9 is detected in the absence of HNF6 (N). Sox9 is efficiently deleted in a subset of ElaC-Sox9f/f acinar cells, and this is associated with partial resistance to acinar destruction (O). (P–T) Expression of CK19 is induced in metaplastic cells five days after PDL in wild-type (R) but not in hnf6− − (S) and to a lesser extent in ElaC-Sox9f/f (T) pancreas. Panels H and M and panels J and O show the same sections, with Sox9 labelling reset from blue to green. In panels H and M, metaplastic acini are surrounded by dotted lines. Arrows point to ducts.
We next investigated the role of Sox9 in ADM by performing PDL in mice with acinar-specific inactivation of Sox9 (Elastase CreERT2/Sox9f/f, here abbreviated ElaC-Sox9f/f).[26, 27] After tamoxifen induction, Elastase CreERT2-mediated deletion of the floxed Sox9 allele leads to a decrease in the number of Sox9-expressing acinar cells after PDL (supplementary figure 4A). Before PDL, no metaplastic lesion was observed in ElaC-Sox9f/f mice (data not shown). PDL induced pancreatitis in the mutant mice (figure 4A,E). However, in Sox9-depleted areas, more acinar cells escaped destruction, CK19 was less induced and there was less apoptosis than in duct-ligated wild-type mice (figure 4C,E,R,T; figure 5M,O and supplementary figure 4D,G). Amylase expression was maintained in the acini (figure 4J,O,T and supplementary figure 4B) and mislocalization of Ezrin was prevented (figure 5C,E and supplementary figure 4E). A limited number of Sox9-depleted cells showed HNF6 induction after PDL (arrowhead in figure 4J). However, the lack of Sox9 did not prevent PDL-induced mislocalisation of Mucin1a (figure 5H,J). We concluded that Sox9 is required for PDL-induced metaplasia but that its absence is less protective against metaplasia than the absence of HNF6.
To verify the importance of Sox9 for HNF6-induced metaplasia, Ad-HNF6 was transduced in ElaC-Sox9f/f mice (figure 3D and supplementary figure 3). In the absence of Sox9, HNF6 was less efficient in mislocating Ezrin and CK19, indicating that Sox9 mediates at least part of the metaplasia-inducing effects of HNF6. However, in the absence of Sox9, HNF6 repressed Amylase and induces apoptosis as efficiently as in the presence of Sox9, indicating that not all the effects of HNF6 are Sox9-dependent (figure 3D and supplementary figure 3A,D).
Taken together, our results show that both HNF6 and Sox9 are required for metaplasia, and that part of the effects of HNF6 depends on Sox9.
HNF6 contributes to carcinogen-induced acinar-to-ductal metaplasia
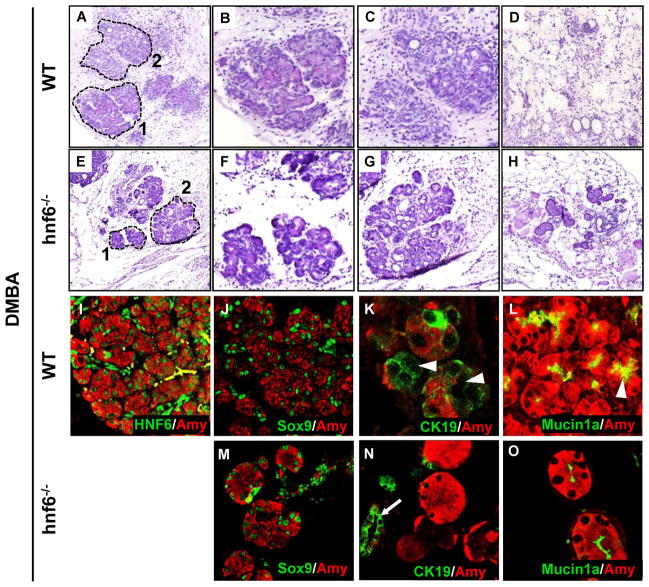
The role of HNF6 was also investigated in metaplasia induced by DMBA, a well-known carcinogen that intercalates between the DNA bases and produces random mutations.[33] In wild-type mice (figure 6A-D), DMBA perturbed acinar morphology (area 1 delineated by a dotted line in panel A, magnified in panel B) and induced formation of tubular complexes (area 2 in panel A, magnified in panel C). Total acinar cell destruction was also observed in large inflammatory regions (figure 6D). In large inflammatory regions, no persistent acinar cell was observed (n=3). In lobules adjacent to the inflammatory regions (area 1 in figure 6A, magnified in B), HNF6 and Sox9, but not HNF1β, were detected in ADM (figure 6I,J, and supplementary figure 1E). DMBA-induced ADM also showed induction of CK19 and mislocalisation of Mucin1a (figure 6K,L).
Figure 6.
HNF6 modulates acinar-to-ductal metaplasia induced by DMBA. (A–H) Hematoxilin- and eosin-stained sections of control (A–D) and hnf6−/− (E–H) pancreas treated during 20 days with DMBA. (I–L) HNF6 expression was stimulated in ducts and induced in metaplastic acinar cells after 20 days of DMBA treatment (I). This was associated with induction of Sox9 (J) and CK19 (K) in metaplastic cells (arrowheads) and with dispersal of Mucin1a (arrowheads) at a distance from the apical pole of cells (L). In I, cells in yellow are non-specifically labelled blood cells. (M–O) A number of acinar cells persisted in inflammatory regions of DMBA-treated hnf6−/− mice. The absence of HNF6 did not influence the induction of Sox9 (M) but prevented the induction of CK19 (N; arrow points to duct)) and the mislocalisation of Mucin1a (O). In wild-type sections, lobules adjacent to the inflammatory regions (area 1 in A, magnified in B) were immunostained, whereas in hnf6−/− sections, acini surrounded by a large inflammatory region were immunostained (H).
Since depletion of Sox9 in ElaC-Sox9f/f mice did not affect all acinar cells, the role of Sox9 in DMBA-induced ADM was not investigated. In contrast, we investigated the role of HNF6, by implanting DMBA in hnf6−/− pancreas. The absence of HNF6 did not prevent development of ADM (figure 6E,F) and formation of tubular complexes (figure 6G), but in contrast to wild-type pancreas, a number of acinar cells persisted in the large inflammatory regions (n=3) (figure 6H). These acinar cells ectopically expressed Sox9 (figure 6M) but showed less repression of Amylase (figure 6N,O). Moreover, in the absence of HNF6, CK19 was not induced (figure 6N) and Mucin1a location remained normal (figure 6O). We concluded that HNF6 contributes to repression of acinar markers, induction of ductal markers and perturbed polarity in DMBA-induced ADM.
DISCUSSION
In this work we hypothesised that ductal transcription factors were ectopically expressed in pancreatic acinar cells during ADM and so contribute to metaplasia. Our results show that this is the case for the transcription factors HNF6 and Sox9.[25] When ectopically expressed in acinar cells, HNF6 induces Sox9, indicating that Sox9 is downstream of HNF6. In human ADM, HNF6 is expressed predominantly in metaplastic cells which maintain overall acinar morphology and acinar marker expression, whereas Sox9 is predominantly found in metaplastic cells that display duct-like characteristics. Sox9 is detectable in PanIN, but not HNF6. These observations suggest that HNF6 expression precedes that of Sox9 in human ADM, and are in line with the finding in mice and cultured cells that HNF6 can induce Sox9. Our data are compatible with a model in which PA originates from ADM that evolves through ductal complexes and PanINs.
Ectopic expression of HNF6 is sufficient and necessary to repress acinar markers and to induce ductal markers, both in vitro and in vivo. However, these properties are not shared with Sox9. Indeed, overexpression of Sox9 cannot induce metaplasia, except for marginal induction of CK19; PDL-induced metaplasia still occurs after PDL in the absence of Sox9 but is significantly less severe than in its presence. HNF6 and Sox9 belong to the same gene cascade, HNF6 being necessary and sufficient to induce Sox9. However, the effects of HNF6 are not all mediated by Sox9, suggesting that Sox9 is not the sole effector of HNF6 in ADM. How HNF6 and Sox9 repress an acinar program while inducing a ductal program remains unknown.
Each model of ADM has its own specificity. The absence of HNF6 is less protective against ADM and acinar cell destruction in pancreas treated with DMBA, than in duct-ligated pancreas. This most probably results from the random mutagenenesis induced by DMBA, which is likely to overcome the protective effect of the absence of HNF6 in most acinar cells. Finally, in duct ligated hnf6−/− pancreas, a few cells displaying ADM were found, despite the absence of HNF6. Such cells were detected near the ligation point (data not shown), a situation reminiscent to that found near the duct ligation in MMP7−/− mice which are otherwise resistant to pancreatitis. [34]
In an earlier study the lack of HNF6 correlated with ADM and pancreatitis.[35] This was detected in mice with Pdx1-Cre-mediated inactivation of floxed hnf6 alleles. In Pdx1-Cre/hnf6loxP/loxP mice inactivation was partial, and the lack of HNF6 in ductal cells is likely to induce pancreatitis resulting from defective ductal cell function, as seen in Jagged1-deficient mice.[36] Pancreatitis would then locally promote metaplasia of acinar cells in which HNF6 has not been eliminated by the Cre recombinase. This is in contrast with our hnf6−/− mice in which all acinar cells are depleted in HNF6 and therefore resistant to metaplasia.
A 3-fold higher number of PanIN lesions was found in hnf6−/− DMBA-treated pancreas than in DMBA-treated control pancreas (data not shown). This was unexpected given that progression of ADM after DMBA treatment is slowed down in the absence of HNF6. In DMBA-treated pancreas, it has been suggested that PA arises from ductal cells.[37] Since hnf6−/− pancreata have ductal cysts lined by a multilayered epithelium[25] and harbour squamous cell metaplasia,[35] we suggest that such cysts and metaplastic lesions may sensitise the cells to DMBA, leading to increased formation of PanINs.
ADM was observed when HNF6 was ectopically expressed in several cells within pancreatic lobules. In contrast, when isolated acinar cells ectopically expressed HNF6, these cells did not undergo ADM (data not shown). Wide but not focal expression of HNF6 was associated with inflammation, suggesting that ectopic expression of HNF6 and inflammation cooperate to induce ADM. This is independent of adenoviral infection, since Ad-GFP induced inflammation but no ADM. In this respect, the effect of HNF6 is reminiscent to that of oncogenic Kras, since it is known that formation of ADM and PanIN is promoted when oncogenic Kras and inflammation are associated.[15]
Our study shows that acinar expression of HNF6 and Sox9 is characteristic of ADM in humans. Therefore, these two factors constitute new biomarkers of ADM. Since ADM is characterised by a switch from acinar to ductal phenotype, and since such a phenotypic switch can possibly lead to PA, HNF6 and Sox9 may be regulators of progression to PA. Eventually, the two factors may become targets for developing drugs that inhibit progression of preneoplastic stages to PA in cases when ADM leads to cancer, such as in families with genetic predisposition to pancreatitis or PA.
Finally, the mechanism described here, namely that ectopic expression of transcription factors of a cell type contributes to converting another cell type to the transcription factors’ cell type, may constitute a paradigm for metaplasia. For instance, chronic inflammation of the oesophagus leads to intestinal metaplasia (Barrett’s oesophagus) associated with expression of the intestinal transcription factor Cdx2, suggesting that Cdx2 may drive metaplasia.[38] The cell type origin of carcinoma does often not correlate with its histological characteristics. For instance, skin basal cell carcinoma does not originate from bulge stem cells but from resident progenitor cells of the interfollicular epidermis and the upper infundibulum, and prostate cancer derives from basal cells and not from luminal cells,[39, 40] suggesting that transcription factors from resident progenitor cells and from basal cells, respectively, may contribute to the phenotypic switch ultimately leading to carcinoma. Therefore, our findings open new venues for understanding phenotypic switches potentially leading to cancer.
Supplementary Material
SIGNIFICANCE OF THIS STUDY.
What is already known about this subject?
Acinar-to-ductal metaplasia (ADM) is found in pancreatitis both in humans and in different animal models.
Pancreatitis is a risk factor for pancreatic adenocarcinoma (PA).
Mouse models suggest that acinar cells are the cell type at the origin of PA and consequently, that ADM is a precursor lesion of PA. Transcriptional regulators controlling this phenotypic switch have not yet been identified.
What are the new findings?
HNF6 and Sox9, two transcription factors normally expressed in ductal cells, are detected in human metaplastic acinar cells associated with pancreatitis and PA. Sox9 is also expressed in pancreatic intraepithelial neoplasia and in invasive PA cells.
Ectopic expression of HNF6 in acinar cells induces metaplasia, and deletion of HNF6 and Sox9 inhibits development of ADM.
HNF6 and Sox9 activate ductal gene expression and repress acinar gene expression in metaplastic acinar cells.
How might it impact on clinical practice in the foreseeable future?
HNF6 and Sox9 are functional biomarkers of ADM.
The two factors may become targets for developing drugs that inhibit progression of preneoplastic stages of PA in families with genetic predisposition to pancreatitis or PA.
This work also suggests that metaplastic processes found in other organs are controlled by ectopic expression of transcription factors.
Acknowledgments
We thank Doris Stoffers for mice, Rolf Kemler and the Developmental Studies Hybridoma Bank (University of Iowa) for antibodies, Tong Chuan He for Sox9 adenovirus, Fabienne Foufelle for pAdTrack-CMV vector and advice, Christophe Pierreux and members of the Lemaigre laboratory for discussions, and Areej Al-Khateeb for PanIN counting.
Funding This work was supported by grants from the Fondation contre le Cancer and from the EU FP7 (Marie Curie Initial Training Network BOLD) to F.P.L. and A.G., from the Juvenile Diabetes Research Foundation to X.X., from an EFSD/MSD award and from the EU FP6 to H.H, from the NIH (CA124586) to S.F.K, and from the Université catholique de Louvain and the Centre du Cancer to P.J. Generation of the anti-HNF6 antibody was supported by BCBC grant U01DK072473 to Ole D. Madsen (as PI of Antibody Core Facility). Claude Rescan and Nicolaj Rasmussen were acknowledged for their contribution on peptide synthesis and in immunisation program, and Anette Bjerregaard and Anja Parbst Høst, for technical help. V.R. is supported by a FRSM grant of the FRS-FNRS. P.-P.P. is a Postdoctoral Researcher of the FRS-FNRS (Belgium). L.B. and P.J. are Research Associates of the FRS-FNRS.
Footnotes
Competing interest The authors declare no competing interest.
Ethics approval All animal experiments were performed with approval of the institution’s welfare committee.
Contributors PPP, AS, AG, study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; MC, acquisition of data; analysis and interpretation of data; AK, acquisition of data; analysis and interpretation of data; GV, technical support; CS, supervision of work; revision of manuscript; study concept and design; XX, acquisition of data; analysis and interpretation of data; VR, LB, adenovirus advice; JH, antibody generation; LB, HH, SFK, YD, supervision of work; revision of manuscript; study concept and design; FL, PJ, study supervision; study concept and design; analysis and interpretation of data; drafting of the manuscript.
References
- 1.Bardeesy N, Sharpless NE, DePinho RA, et al. The genetics of pancreatic adenocarcinoma: a roadmap for a mouse model. Semin Cancer Biol. 2001;11:201–18. doi: 10.1006/scbi.2000.0371. [DOI] [PubMed] [Google Scholar]
- 2.Parsa I, Longnecker DS, Scarpelli DG, et al. Ductal metaplasia of human exocrine pancreas and its association with carcinoma. Cancer Res. 1985;45:1285–90. [PubMed] [Google Scholar]
- 3.Githens S. The pancreatic duct cell: proliferative capabilities, specific characteristics, metaplasia, isolation, and culture. J Pediatr Gastroenterol Nutr. 1988;7:486–506. [PubMed] [Google Scholar]
- 4.Wagner M, Luhrs H, Kloppel G, et al. Malignant transformation of duct-like cells originating from acini in transforming growth factor transgenic mice. Gastroenterology. 1998;115:1254–62. doi: 10.1016/s0016-5085(98)70098-8. [DOI] [PubMed] [Google Scholar]
- 5.Sandgren EP, Luetteke NC, Palmiter RD, et al. Overexpression of TGF alpha in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell. 1990;61:1121–35. doi: 10.1016/0092-8674(90)90075-p. [DOI] [PubMed] [Google Scholar]
- 6.Bottinger EP, Jakubczak JL, Roberts IS, et al. Expression of a dominant-negative mutant TGF-beta type II receptor in transgenic mice reveals essential roles for TGF-beta in regulation of growth and differentiation in the exocrine pancreas. EMBO J. 1997;16:2621–33. doi: 10.1093/emboj/16.10.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jhappan C, Stahle C, Harkins RN, et al. TGF alpha overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell. 1990;61:1137–46. doi: 10.1016/0092-8674(90)90076-q. [DOI] [PubMed] [Google Scholar]
- 8.Tuveson DA, Zhu L, Gopinathan A, et al. Mist1-KrasG12D knock-in mice develop mixed differentiation metastatic exocrine pancreatic carcinoma and hepatocellular carcinoma. Cancer Res. 2006;66:242–7. doi: 10.1158/0008-5472.CAN-05-2305. [DOI] [PubMed] [Google Scholar]
- 9.De La OJ, Emerson LL, Goodman JL, et al. Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc Natl Acad Sci U S A. 2008;105:18907–12. doi: 10.1073/pnas.0810111105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Habbe N, Shi G, Meguid RA, et al. Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc Natl Acad Sci U S A. 2008;105:18913–8. doi: 10.1073/pnas.0810097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi G, Zhu L, Sun Y, et al. Loss of the acinar-restricted transcription factor Mist1 accelerates Kras-induced pancreatic intraepithelial neoplasia. Gastroenterology. 2009;136:1368–78. doi: 10.1053/j.gastro.2008.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji B, Tsou L, Wang H, et al. Ras activity levels control the development of pancreatic diseases. Gastroenterology. 2009;137:1072–82. 82 e1–6. doi: 10.1053/j.gastro.2009.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris JPt, Cano DA, Sekine S, et al. Beta-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J Clin Invest. 2010;120:508–20. doi: 10.1172/JCI40045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hruban RH, Adsay NV, Albores-Saavedra J, et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579–86. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Guerra C, Schuhmacher AJ, Canamero M, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Strobel O, Dor Y, Alsina J, et al. In vivo lineage tracing defines the role of acinar-to-ductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology. 2007;133:1999–2009. doi: 10.1053/j.gastro.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuveson DA, Hingorani SR. Ductal pancreatic cancer in humans and mice. Cold Spring Harb Symp Quant Biol. 2005;70:65–72. doi: 10.1101/sqb.2005.70.040. [DOI] [PubMed] [Google Scholar]
- 18.Almoguera C, Shibata D, Forrester K, et al. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549–54. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- 19.Maitra A, Kern SE, Hruban RH. Molecular pathogenesis of pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:211–26. doi: 10.1016/j.bpg.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Malka D, Hammel P, Maire F, et al. Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut. 2002;51:849–52. doi: 10.1136/gut.51.6.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haumaitre C, Barbacci E, Jenny M, et al. Lack of TCF2/vHNF1 in mice leads to pancreas agenesis. Proc Natl Acad Sci U S A. 2005;102:1490–5. doi: 10.1073/pnas.0405776102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacquemin P, Lemaigre FP, Rousseau GG. The Onecut transcription factor HNF-6 (OC-1) is required for timely specification of the pancreas and acts upstream of Pdx-1 in the specification cascade. Dev Biol. 2003;258:105–16. doi: 10.1016/s0012-1606(03)00115-5. [DOI] [PubMed] [Google Scholar]
- 23.Jacquemin P, Durviaux SM, Jensen J, et al. Transcription factor hepatocyte nuclear factor 6 regulates pancreatic endocrine cell differentiation and controls expression of the proendocrine gene ngn3. Mol Cell Biol. 2000;20:4445–54. doi: 10.1128/mcb.20.12.4445-4454.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seymour PA, Freude KK, Tran MN, et al. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci U S A. 2007;104:1865–70. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pierreux CE, Poll AV, Kemp CR, et al. The transcription factor hepatocyte nuclear factor-6 controls the development of pancreatic ducts in the mouse. Gastroenterology. 2006;130:532–41. doi: 10.1053/j.gastro.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Kist R, Schrewe H, Balling R, et al. Conditional inactivation of Sox9: a mouse model for campomelic dysplasia. Genesis. 2002;32:121–3. doi: 10.1002/gene.10050. [DOI] [PubMed] [Google Scholar]
- 27.Desai BM, Oliver-Krasinski J, De Leon DD, et al. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J Clin Invest. 2007;117:971–7. doi: 10.1172/JCI29988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paul R, Haydon RC, Cheng H, et al. Potential use of Sox9 gene therapy for intervertebral degenerative disc disease. Spine (Phila Pa 1976) 2003;28:755–63. [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Q, Brown J, Kanarek A, et al. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–32. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilic G, Wang J, Sosa-Pineda B. Osteopontin is a novel marker of pancreatic ductal tissues and of undifferentiated pancreatic precursors in mice. Dev Dyn. 2006;235:1659–67. doi: 10.1002/dvdy.20729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hezel AF, Gurumurthy S, Granot Z, et al. Pancreatic LKB1 deletion leads to acinar polarity defects and cystic neoplasms. Mol Cell Biol. 2008;28:2414–25. doi: 10.1128/MCB.01621-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satake K, Hiura A. A new model for pancreatitis. Pancreas. 1998;16:284–8. doi: 10.1097/00006676-199804000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Dissin J, Mills LR, Mains DL, et al. Experimental induction of pancreatic adenocarcinoma in rats. J Natl Cancer Inst. 1975;55:857–64. doi: 10.1093/jnci/55.4.857. [DOI] [PubMed] [Google Scholar]
- 34.Crawford HC, Scoggins CR, Washington MK, et al. Matrix metalloproteinase-7 is expressed by pancreatic cancer precursors and regulates acinar-to-ductal metaplasia in exocrine pancreas. J Clin Invest. 2002;109:1437–44. doi: 10.1172/JCI15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H, Ables ET, Pope CF, et al. Multiple, temporal-specific roles for HNF6 in pancreatic endocrine and ductal differentiation. Mech Dev. 2009;126:958–73. doi: 10.1016/j.mod.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Golson ML, Loomes KM, Oakey R, et al. Ductal malformation and pancreatitis in mice caused by conditional Jag1 deletion. Gastroenterology. 2009;136:1761–71. e1. doi: 10.1053/j.gastro.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 37.Bockman DE, Guo J, Buchler P, et al. Origin and development of the precursor lesions in experimental pancreatic cancer in rats. Lab Invest. 2003;83:853–9. doi: 10.1097/01.lab.0000074918.31303.5a. [DOI] [PubMed] [Google Scholar]
- 38.Hayes S, Ahmed S, Clark P. Immunohistochemical assessment for Cdx2 expression in the Barrett metaplasia-dysplasia-adenocarcinoma sequence. J Clin Pathol. 2010;64(2):110–3. doi: 10.1136/jcp.2010.075945. [DOI] [PubMed] [Google Scholar]
- 39.Youssef KK, Van Keymeulen A, Lapouge G, et al. Identification of the cell lineage at the origin of basal cell carcinoma. Nat Cell Biol. 2010;12:299–305. doi: 10.1038/ncb2031. [DOI] [PubMed] [Google Scholar]
- 40.Goldstein AS, Huang J, Guo C, et al. Identification of a cell of origin for human prostate cancer. Science. 2010;329:568–71. doi: 10.1126/science.1189992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.