Abstract
d-Amino acid oxidase (DAO) is a hydrogen peroxide-generating enzyme that uses a d-amino acid as a substrate. We hypothesized that DAO may protect against bacterial infection, because hydrogen peroxide is one of the most important molecules in the antibacterial defense systems in mammals. We show here that DAO suppressed the growth of Staphylococcus aureus in a manner that depended on the concentration of DAO and d-amino acid in vitro. Addition of catalase abolished the bacteriostatic activity of DAO. Although DAO plus d-Ala showed less bactericidal activity, addition of myeloperoxidase (MPO) greatly enhanced the bactericidal activity of DAO. Furthermore, DAO was able to utilize bacterial lysate, which contains d-Ala derived from peptidoglycan; this could produce hydrogen peroxide with, in the presence of myeloperoxidase, formation of hypochlorous acid. This concerted reaction of DAO and MPO led to the bactericidal action. In vivo experiments showed that DAO−/− (mutant) mice were more susceptible to S. aureus infection than were DAO+/+ (wild-type) mice. These results suggest that DAO, together with myeloperoxidase, may play an important role in antibacterial systems in mammals.
INTRODUCTION
Hydrogen peroxide (H2O2), a major reactive oxygen species (ROS), demonstrates bactericidal activity against various microorganisms (14). In mammals, immune cells (macrophages and neutrophils) utilize ROS, such as H2O2 and superoxide anion radical, to eliminate infecting bacteria. Furthermore, in the presence of H2O2, myeloperoxidase (MPO) oxidizes halide ions (Cl− and I−) to produce hypohalous acid (hypochlorous acid [HOCl] and hypoiodous acid). Hypohalous acid is one of the most active ROS and is toxic to various microbes. Neutrophils are major immune cells that utilize HOCl to eliminate infecting bacteria. For neutrophils, a primary source of the H2O2 used to generate HOCl is NADPH oxidase (24). H2O2 may also have an additional significant role in chronic granulomatous disease, which is a hereditary disease caused by abnormal H2O2 production in neutrophils that is primarily due to defective NADPH oxidase (7, 9).
d-Amino acid oxidase (DAO) is another H2O2-generating enzyme, which uses flavin adenine dinucleotide (FAD) as a coenzyme. Biochemically, DAO catalyzes oxidative deamination of d-amino acids to yield corresponding α-keto acids, with molecular oxygen (O2) used as an electron acceptor and, thus, formation of H2O2. DAO exists in many organisms, from yeasts to mammals, but not in bacteria. In mammals, DAO is abundant in the kidney, liver, and brain. However, the biological roles of DAO in mammals remain unclear. In the brain, for example, DAO is associated with neurotransmission via modulation of d-serine levels. The enzymatic activity of DAO has been correlated with the incidence of schizophrenia (8).
Moreover, several researchers found DAO in human and porcine neutrophils, which suggests an important antibacterial role for DAO in neutrophils. Robinson et al. reported that DAO is located on the cell surface and DAO is internalized with foreign objects, such as bacteria (26). Reports that purified porcine DAO has antibacterial activity against Escherichia coli in vitro and that its activity was enhanced by addition of d-amino acids, as well as by binding of DAO to bacterial cell walls, have appeared in the literature (10, 29).
d-Amino acids (e.g., d-Ala and d-Glu) are integral components of the peptidoglycan of bacterial cell walls and are missing in mammals. We thus hypothesized that DAO may utilize d-Ala generated by target bacteria and that peptidoglycan synthesis may be involved in defense against microbial infections, because d-Ala is ubiquitous in bacteria. DAO activity is also reportedly absent in the kidney in most germ-free mice and is partially restored by injection of d-Ala (21), which suggests that DAO may be induced by cell wall components and thus may protect hosts against bacterial infection. However, some researchers reported no difference in DAO activity in germ-free mice and conventional mice (23).
In this study, we investigated the potential role of DAO as an antibacterial defense enzyme by using a porcine recombinant DAO, as well as in vitro and in vivo experiments with DAO-deficient mice and Staphylococcus aureus. The combined effect of DAO and MPO was also investigated.
MATERIALS AND METHODS
Materials.
The S. aureus strain ATCC 25923 was used in this study. ddY mice were purchased from Japan SLC, Inc., Shizuoka, Japan. Soybean casein digest (SCD) broth was purchased from Nissui Co., Tokyo, Japan. Leupeptin, phenylmethylsulfonyl fluoride (PMSF), and FAD were purchased from Sigma-Aldrich Chemical Co., St. Louis, MO. 3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) and SBT [N,N′-bis (2,4-disulfobenzyl) toluidine] was purchased from Dojindo Chemical Laboratories, Kumamoto, Japan. TaKaRa Ex Taq was purchased from Takara Bio Inc., Shiga, Japan. PflMI restriction enzyme was purchased from New England BioLabs (Ipswich, MA). SCD agar, isopropyl-β-d-thiogalactopyranoside, carbenicillin, polyoxyethylene sorbitan monolaurate (Tween 20), ammonium sulfate, Tris, EDTA, sodium dodecyl sulfate (SDS), proteinase K, casein sodium, and others were purchased from Wako Pure Chemical Industries, Ltd., Osaka, Japan.
Production and purification of recombinant DAO.
Recombinant porcine DAO was prepared as described previously (12). Briefly, the E. coli BL21(DE3) strain harboring a pET3c plasmid encoding porcine DAO was cultured in LB medium containing 50 μg/ml carbenicillin as usual, and 0.01 mM isopropyl-β-d-thiogalactopyranoside was added to allow expression of DAO in E. coli. After culture for 20 h at 37°C, the bacterial pellets were sonicated (150 W; 30 min) in 20 mM pyrophosphate buffer (pH 8.2) at 4°C. DAO was obtained by means of ammonium sulfate precipitation after heat denaturation, followed by DEAE cellulose column chromatography. The purity of the DAO was checked by means of SDS-polyacrylamide gel electrophoresis (PAGE) with Coomassie brilliant blue staining (purity > 90%).
Assay for bacteriostatic and bactericidal activities. (i) Bacteriostatic activity.
S. aureus was cultured until the mid-log phase of growth in SCD broth with reciprocal shaking at 1.5 Hz and 37°C. Then, the bacteria were washed twice with physiological saline (0.85%), and 1 × 106 CFU/ml of S. aureus was incubated with DAO (2 to 40 μg/ml), d-amino acids (d-Ala [1 to 10 mM], d-Pro [0.3 to 10 mM], d-Glu [10 mM], or d-Asp [10 mM]), and catalase (1 mg/ml) in SCD broth at 37°C for 5 h. The optical density at 570 nm was used to compare relative bacterial cell numbers for the different conditions tested.
(ii) Bactericidal activity.
S. aureus (1 × 106 CFU/ml), which was prepared as described above, was incubated with DAO (10 to 200 μg/ml), d-amino acids in the same concentration range mentioned above, and MPO (3.2 U/ml) in 50 mM phosphate-citrate buffer (pH 5.5), or the bacteria were incubated with DAO (10 to 50 μg/ml) and the above-described d-amino acids in 10 mM phosphate-buffered saline (PBS) (pH 7.4) at 37°C in a shaking (1.5 Hz) water bath for 30 min. Duplicate 100-μl aliquots of each dilution were added to 15 ml of SCD agar and mixed well in petri dishes for colony formation. The numbers of colonies formed were determined after overnight incubation at 37°C.
Measurement of hydrogen peroxide production.
Hydrogen peroxide was measured by colorimetric assay using peroxidase-coupled oxidation of o-dianisidine. DAO (5 μg/ml), bacterial lysate (0.5 to 1.0 mg/ml), and horseradish peroxidase (6.6 μg/ml) were incubated in 50 mM phosphate buffer (pH 7.4) containing o-dianisidine (75 μg/ml) at 25°C, and then absorbance at 460 nm was measured. Bacterial lysate was prepared by acid hydrolysis of S. aureus, hydrolysis in 6 M HCl at 110°C for 24 h, and was then kept in a desiccator containing pellets of NaOH under vacuum to remove the HCl and dried to obtain a powder.
Measurement of hypohalous acid generation.
The amount of hypohalous acid (HOCl) was measured by a colorimetric method based on SBT. Briefly, an H2O2-generating system (200 μg/ml DAO and 10 mM d-Ala), 0.8 or 1.6 U/ml MPO, 200 mM NaCl, and 5 mg/ml potassium iodide were incubated with 0.1 mg/ml SBT in 50 mM phosphate citrate buffer (pH 5.5), after which the absorbance at 675 nm was measured (27). In another experiment, a lysate of S. aureus was used instead of d-Ala.
Genotyping.
Genomic DNA was extracted from the auricles of ddY mice after incubation of auricular tissue in lysis buffer (10 mM Tris-HCl, 10 mM EDTA, 0.5% SDS, 0.5 mg/ml proteinase K) at 55°C for 6 h, after which the genomic DNA was purified by phenol-chloroform extraction and ethanol precipitation. The mouse Dao gene was amplified by means of PCR using 5′-mDAO and 3′-mDAO primers. The PCR products were also subjected to phenol-chloroform extraction and ethanol precipitation, followed by digestion with the restriction enzyme PflMI. The digested DNA fragments were then separated using 1% agarose gel electrophoresis. The primer sequences used for mouse Dao genotyping were as follows: 5′-mDAO, ATGTACGAAGCTGGAGGACAGAGGGG, and 3′-mDAO, CAAGCAGACAGGGCAAGCTCTTCATGG.
Quantification of bactericidal activity in kidneys.
Six-week-old female ddY mice were injected with 1 × 107 CFU of S. aureus via the tail vein. Three days after bacterial challenge, the mice were sacrificed and the kidneys were excised. The kidneys were homogenized in 1% CHAPS in RPMI medium, and the homogenates were serially diluted with physiological saline. Duplicate 100-μl aliquots of each dilution were added to 15 ml of SCD agar at about 45°C and mixed vigorously in petri dishes. The numbers of viable bacteria were assessed by counting the colonies formed on SCD agar plates after overnight incubation at 37°C.
Measurement of DAO enzymatic activity.
Kidneys or peritoneal neutrophils were homogenized using a Polytron homogenizer (Kinematica AG, Lucerne, Switzerland) or by ultrasonication (type Dr. Hielscher, UP50H homogenizer, tip drip type) in ice-cold suspension buffer (100 mM Tris-HCl, pH 8.0) containing a mixture of protease inhibitors (1 mM PMSF, 10 μg/ml leupeptin, and 2.5 mM EDTA). The tissue lysates obtained were then centrifuged (12,000 × g; 15 min at 4°C), and each supernatant specimen was used for measurement of DAO enzymatic activity. The DAO activity in the tissue lysates was determined on the basis of formation of α-keto acid (pyruvic acid) during the reaction between d-Ala and DAO, as described previously (11).
Preparation of mouse peritoneal neutrophils.
Mouse peritoneal neutrophils were elicited by using a peritoneal injection of 3 ml of 6% casein sodium salt dissolved in physiological saline and harvested via peritoneal lavage 6 h later with 5 ml of PBS. Contaminating erythrocytes were removed by causing them to burst in hypotonic saline solution (0.2% NaCl); isotonicity was then restored with a rebalancing solution (1.9% NaCl), followed by centrifugation. Approximately 1 × 107 neutrophils were obtained from 10-week-old female ddY mice. The purity of the neutrophils was evaluated by means of Giemsa staining under a microscope.
Bactericidal activity of mouse neutrophils with wild-type (DAO+/+) and DAO-deficient (DAO−/−) mice.
S. aureus bacteria at the mid-log phase in SCD broth as described above were washed twice with physiological saline and suspended in PBS containing 1 mM MgCl2 and 1.5 mM CaCl2, and the bacteria were treated with 10% pooled mouse serum at 37°C for 15 min for endocytosis after opsonization of S. aureus with mouse serum. Then, neutrophils were added to the bacteria at a 1:1 ratio of bacteria to neutrophils (4 × 106 cells/ml) in PBS supplemented with 1 mM glucose and 10% mouse serum for opsonization and incubated at 37°C. After 90 min of incubation, each sample was diluted in 0.2% Tween 20 and incubated for 5 min at room temperature to lyse the neutrophils and release phagocytosed bacteria, after which samples were vortexed vigorously and duplicate 100-μl aliquots were plated on 15-ml agar gel plates of SCD medium, followed by overnight culture at 37°C. The viable bacteria were then counted.
RESULTS
In vitro bacteriostatic activity of DAO.
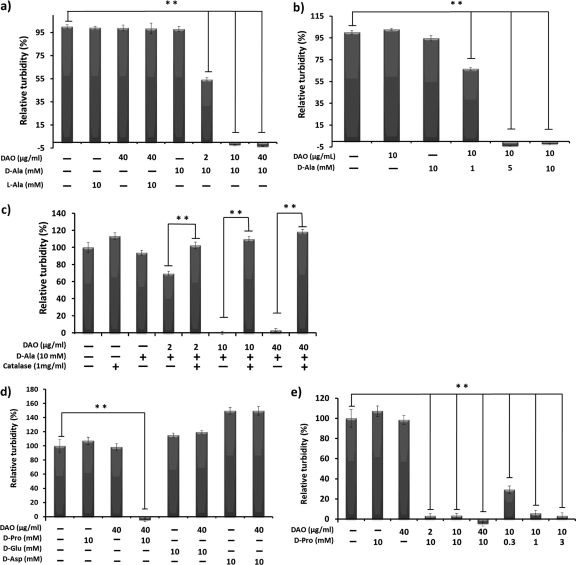
We first examined the bacteriostatic activity of DAO against S. aureus. S. aureus bacteria were incubated with porcine DAO at various concentrations in the presence of 10 mM d-Ala in SCD medium. A concentration of 2 μg/ml DAO showed 50% bacterial growth suppression, and complete growth suppression was observed with DAO at more than 10 μg/ml (Fig. 1a). Similar concentration-dependent bacteriostatic activity was seen with d-Ala in the presence of 10 μg/ml of DAO (Fig. 1b). This bacteriostatic activity of DAO was not observed with the addition of l-Ala (Fig. 1a). Further, addition of 1 mg/ml catalase abolished the bacteriostatic effect of DAO completely (Fig. 1c), which suggests that H2O2 is the major effector of DAO-induced bacteriostatic effects. Though addition of d-Glu and d-Asp could not suppress S. aureus bacterial growth, d-Pro suppressed the growth of S. aureus more extensively than d-Ala in a dose-dependent manner (Fig. 1d and e).
Fig 1.
Bacteriostatic activity of DAO against S. aureus. (a and b) S. aureus (1 × 106 CFU/ml), which was grown until the mid-log phase in SCD broth, was incubated with increasing concentrations of DAO in the presence of 10 mM d-Ala (a) or increasing concentrations of d-Ala in the presence of 10 μg/ml DAO (b) in SCD broth at 37°C for 5 h, and then the optical density at 570 nm was measured. (c) S. aureus (1 × 106 CFU/ml) was incubated with DAO and d-Ala, with or without 1 mg/ml (5,000 to 10,000 U/ml) of bovine catalase, and then the optical density at 570 nm was measured. (d and e) S. aureus (1 × 106 CFU/ml) was incubated with DAO in the presence of d-amino acids (d-Pro [0.3 to 10 mM], d-Glu [10 mM], or d-Asp [10 mM]) in SCD broth at 37°C for 5 h, and then the optical density at 570 nm was measured. The values are means and standard deviations (SD). **, statistically significant differences (P < 0.01) by Student's t test.
Enhancement of the bactericidal effect of DAO with MPO in vitro.
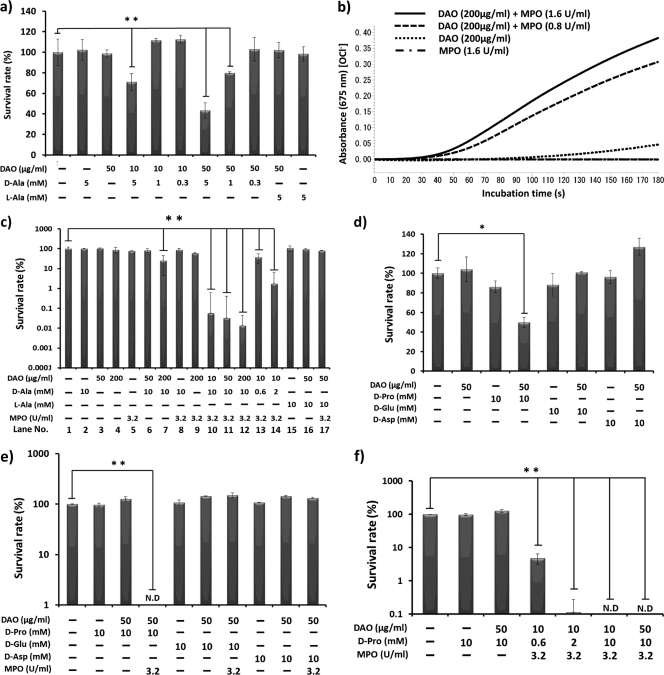
We also examined the bactericidal activity of DAO against S. aureus. DAO showed only weak bactericidal activity, even in the presence of 5 mM d-Ala; about 40% decreases in viable bacteria were observed (Fig. 2a). Next, we examined the bactericidal activity of DAO in relation to that of MPO. Generation of hypohalous acid was detected by means of the SBT-based colorimetric method as described in Materials and Methods (27). Hypohalous acid was generated when the H2O2-generating system (DAO and d-Ala) was mixed with MPO and chloride ion (Fig. 2b). These results indicated that DAO serves as an H2O2 donor for MPO to generate HOCl.
Fig 2.
Generation of HOCl and bacterial killing by the DAO–MPO–d-amino acid system. (a and d) S. aureus (1 × 106 CFU/ml) was incubated with DAO (10 to 50 μg/ml) and d-amino acid (d-Ala [0.3 to 5 mM], d-Pro [10 mM], d-Glu [10 mM], or d-Asp [10 mM]) in 10 mM phosphate-buffered saline (pH 7.4) at 37°C for 30 min, and then each reaction mixture was diluted in physiological saline, mixed with warm SCD agar, and plated on petri dishes. The bacterial colonies were counted after overnight culture at 37°C. The values are expressed relative to the control. (b) DAO (200 μg/ml) was incubated with or without MPO in 50 mM phosphate citrate buffer (pH 5.5) for the indicated time in the system containing 10 mM d-Ala and 0.1 mg/ml SBT, an indicator of HOCl generation, and absorbance at 675 nm was then measured. (c, e, and f) S. aureus (1 × 106 CFU/ml) was incubated with a combination of DAO (10 to 200 μg/ml), d-amino acid (d-Ala [0.6 to 10 mM], d-Pro [0.6 to 10 mM], d-Glu [10 mM], or d-Asp [10 mM]), and MPO (3.2 U/ml) for 30 min in 50 mM phosphate citrate buffer (pH 5.5). The viable bacteria were counted by colony-forming assay as described above. The values are means and SD. N.D., not detected. ** and *, statistically significant differences (P < 0.01 and P < 0.05, respectively) by Student's t test.
We next examined the bactericidal activity of the DAO-MPO system. DAO alone showed no bactericidal activity at pH 5.5, but coincubation of DAO and d-Ala showed significant bactericidal activity; about 60% bacterial killing was achieved compared with the control under our experimental conditions (Fig. 2c, lane 7). When three components, DAO, d-Ala, and MPO, were incubated with bacteria, the highest bactericidal activity (more than 99% killing) among the conditions tested was obtained (Fig. 2c, lanes 10 to 14). Similar results were obtained using d-Pro; coincubation of d-Pro, DAO, and MPO showed the highest bactericidal activity against S. aureus (Fig. 2e and f); however, this was not the case with d-Glu and d-Asp (Fig. 2d and e).
Use of bacterial d-amino acid by DAO and generation of HOCl in the presence of MPO.
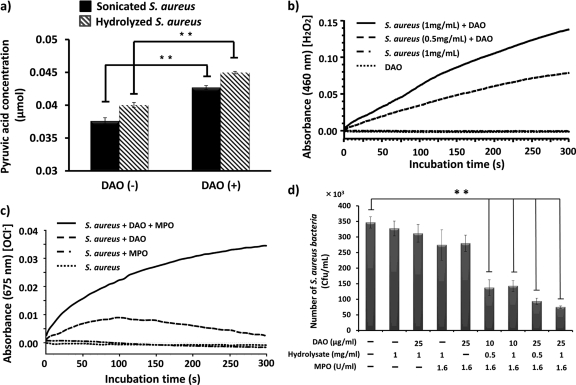
Essentially all bacteria produce d-amino acids for the biosynthesis of peptidoglycan, the crucial component of the bacterial cell wall. We investigated whether DAO can use the bacterial lysate, which contains d-amino acids. Use of a d-amino acid, such as d-Ala, for DAO was examined by measuring the production of pyruvic acid, as well as generation of H2O2. Acid hydrolysate of S. aureus was used as the source of the bacterial d-amino acid. Incubation of the lysate with DAO led to significantly increased pyruvic acid production (Fig. 3a). Furthermore, generation of H2O2 was measured by incubation of the lysate of S. aureus and DAO (Fig. 3b), and HOCl was also generated in the presence of MPO (Fig. 3c). Indeed, coincubation with bacterial lysate, DAO, and MPO produced significant bactericidal activity (Fig. 3d). These results indicate that DAO can utilize bacterial lysates as a source of d-amino acid to generate H2O2, which is converted HOCl by MPO.
Fig 3.
Utilization of bacterial cell wall-derived d-amino acid by DAO and generation of the bactericidal molecule HOCl. (a) Lysate of S. aureus (1 mg/ml) was incubated with 0.2 mg/ml DAO for 30 min at 37°C, and then pyruvic acid production was quantified as described in Materials and Methods. (b) Lysate of S. aureus (0.5 to 1 mg/ml) was incubated with DAO (5 μg/ml) in 50 mM phosphate buffer at 25°C for the indicated time to generate H2O2. H2O2 was measured by use of peroxidase-coupled oxidation of o-dianisidine (460 nm) as described in Materials and Methods. (c) Lysate of S. aureus (1 mg/ml) was incubated with DAO (200 μg/ml) plus MPO (1.6 U/ml) in the presence of SBT in acetate buffer (pH 5.5), and then absorbance at 675 nm was measured for HOCl generation. (d) S. aureus (1 × 106 CFU/ml) was incubated with the lysate of S. aureus (0.5 to 1.0 mg/ml), DAO (10 to 25 μg/ml), and MPO (1.6 U/ml) in 50 mM phosphate citrate buffer (pH 5.5) at 37°C for 30 min. Each reaction mixture was diluted with physiological saline, mixed with warm SCD agar, and plated on petri dishes. The bacterial colonies were counted after overnight culture at 37°C. The values are means and SD. **, statistically significant differences (P < 0.01) by Student's t test.
Difference in DAO activitin in DAO+/+ and DAO−/− mice and their susceptibilities to bacterial infection.
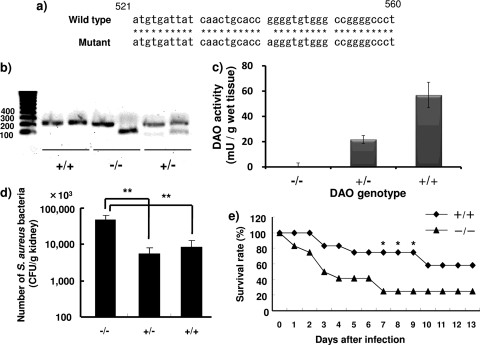
Certain ddY mice reportedly have a single-nucleotide substitution (541 G→A) in the Dao gene (Fig. 4a) (19). This nucleotide polymorphism results in the glycine (GGG)-to-arginine (AGG) amino acid substitution, which inactivates the enzyme activity of mouse DAO (19). We therefore sorted ddY mice into wild-type (DAO+/+) and DAO-deficient (DAO−/−) mice by using a restriction fragment length polymorphism (RFLP) method (Fig. 4b). Among 46 female ddY mice from Japan SLC, Inc., that were tested, 6 were wild-type mice (+/+), 16 were homozygous mutant mice (−/−), and 24 were heterozygous mice (+/−).
Fig 4.
The Dao variant gene in ddY mice and its role in bacterial infection. (a) DNA sequences of the Dao genes in wild-type (DAO+/+) and mutant (DAO−/−) mice. Asterisks indicate identical nucleotide sequences. (b) The mouse Dao gene was amplified via a pair of 5′-mDAO and 3′-mDAO primers, followed by digestion with PflMI restriction enzyme. These digested fragments were applied to gel electrophoresis in a 1% agarose gel. (c) DAO activity in kidney homogenates was measured as described in Materials and Methods. (d) Fate of S. aureus after injection of 1 × 107 CFU/mouse. Three days after i.v. bacterial infection, viable bacteria in kidney homogenates were counted as described in Materials and Methods (n = 7 to 8). The values are means and SD. **, statistically significant differences (P < 0.01) by Student's t test. (e) Female ddY mice, 6 weeks old, were injected with S. aureus, 1 × 108 CFU/mouse, via the tail vein. The survival rates of the DAO+/+ and DAO−/− groups were checked daily (n = 9). *, statistically significant differences (P < 0.05) by the chi-square test.
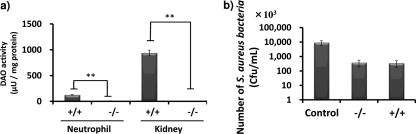
DAO activity in kidneys of ddY mice with different DAO expression levels was measured as described in Materials and Methods. Consistent with the results of Dao genotyping, DAO enzyme activity in the kidneys of DAO+/+ mice was highest, and the lowest DAO activity occurred in kidneys of DAO−/− mice (56.9 mU/g wet tissue versus 0.04 mU/g wet tissue). DAO+/− mice showed about half the wild-type DAO activity (21.7 mU/g wet tissue) (Fig. 4c). To investigate the susceptibilities of mice with different DAO expression levels, ddY mice were injected intravenously (i.v.) with S. aureus via the tail vein as a renal bacterial infection model, and the number of viable bacteria in the kidneys was determined. The numbers of viable bacteria in the kidneys of the DAO+/+ and DAO+/− mice were significantly reduced (to about 10%) compared with the numbers in the kidneys of DAO−/− mice (Fig. 4d). No significant difference was seen between DAO+/+ and DAO+/− mice. We also examined the survival rate of ddY mice infected with S. aureus. After 2 weeks, 2 of 9 DAO−/− mice survived, whereas 5 of 9 DAO+/+ mice survived (Fig. 4e).
DAO activity and bactericidal activity of neutrophils derived from DAO variant mice.
We also examined the DAO activity of peritoneal neutrophils derived from DAO+/+ and DAO−/− mice. The DAO activity of neutrophils derived from DAO−/− mice was 9.4 μU/mg protein, whereas that of neutrophils derived from DAO+/+ mice was 125 μU/mg protein (Fig. 5a). Incubation with mouse peritoneal neutrophils dramatically reduced the viability of S. aureus, although no significant difference was seen between DAO+/+ and DAO−/− mice (Fig. 5b).
Fig 5.
DAO activity of mouse peritoneal neutrophils and antibacterial activity of DAO−/− and DAO+/+ mouse peritoneal neutrophils. (a) Peritoneal neutrophils were elicited using 6% casein sodium salt. The DAO activities of mouse neutrophils and kidney were measured, as evaluated by pyruvic acid formation. (b) S. aureus (4 × 106 CFU/ml) was incubated with or without equal numbers of mouse peritoneal neutrophils (1:1) at 37°C for 30 min. Serial dilutions were mixed with SCD agar on petri dishes, and the bacterial colonies were counted after overnight culture at 37°C. The values are means and SD. **, statistically significant differences (P < 0.01) by Student's t test.
DISCUSSION
In our previous studies, we reported that DAO showed selective cytotoxicity against various cancer cells in vitro and in vivo (12, 13). DAO-mediated cytotoxicity was nullified by the addition of catalase, which suggested that DAO exerted cytotoxic effects via production of H2O2. In this study, we examined the potential bacteriostatic and bactericidal activities of DAO in vitro and in vivo, as well as the role of DAO in MPO-dependent killing. For this purpose, we prepared recombinant DAO obtained from different sources (i.e., pig, mouse, and human). We found that porcine DAO yielded both more DAO and higher enzyme activity than did the other DAOs (data not shown). We therefore utilized recombinant porcine DAO for our in vitro study. The recombinant DAO showed a single band, as judged from an SDS-PAGE gel stained with Coomassie brilliant blue. The relative activity of recombinant DAO was 5.3 U/mg protein, which is equivalent to that of purified DAO from porcine kidney.
In the present study, we observed that in the presence of 10 mM d-Ala, DAO showed concentration-dependent bacteriostatic activity against S. aureus, with the 50% inhibitory concentration (IC50) of DAO almost 2 μg/ml (Fig. 1a). In the presence of 2 μg/ml of DAO, d-Ala showed a concentration-dependent bacteriostatic effect, and incubation of S. aureus with more than 5 mM d-Ala completely suppressed bacterial growth (Fig. 1b). Such DAO-mediated bacteriostatic activity was abolished by addition of catalase (Fig. 1c). This result clearly indicates the important role of H2O2 in the bacteriostatic activity of DAO. In this setting, l-Ala, d-Glu, and d-Asp did not show any bacteriostatic activity toward S. aureus even in the presence of DAO (Fig. 1a and d). This is because l-Ala, d-Glu, and d-Asp are not preferred substrates for DAO, and thus, there was no production of H2O2. d-Pro, which is a preferable substrate for DAO, showed more potent bacteriostatic activity against S. aureus (Fig. 1d and e). These results indicate that DAO needs available d-amino acids, such as d-Ala or d-Pro, to exhibit bacteriostatic activity.
Compared to the bacteriostatic activity of DAO, the bactericidal activity of d-Ala plus DAO was very weak (Fig. 2a and d);10 μg/ml DAO plus 5 mM d-Ala decreased the viable bacteria only to about 35%, at which point growth of S. aureus was completely suppressed (Fig. 1a and b). We further examined the bactericidal activity of the combination of DAO and MPO. MPO is an abundant protein in neutrophils (∼5% of cellular protein) and is rich in granules (4). MPO is also found in the extracellular compartment after degranulation of granules (15). The biochemical function of MPO is oxidation of the Cl− using H2O2 under acidic pH to produce HOCl. The resultant HOCl shows highly potent bactericidal activity (1).
In the present study, we showed that incubation of DAO and d-Ala with MPO in the presence of chloride ion at pH 5.5 generated HOCl (Fig. 2a). In this assay system, H2O2 was supplied via the reaction of DAO with d-Ala, after which MPO oxidized the chloride ion to generate HOCl. We then showed the bactericidal activity of DAO in combination with MPO. It was realized that MPO significantly enhanced the bactericidal activity of DAO when d-Ala or d-Pro was present (Fig. 2). Maximum bactericidal activity was observed when S. aureus was incubated with DAO, d-pro followed by d-Ala, and MPO; more than 99% of S. aureus bacteria were killed (Fig. 2c). Concerted bactericidal activity of DAO and MPO was not observed with the addition of l-Ala, d-Glu, or d-Asp (Fig. 2c and e) because these three amino acids are not preferable substrates for DAO reaction.
Peptidoglycan of S. aureus consists of alternating units of N-acetylglucosamine and N-acetylmuramic acid and a pentapeptide containing l-Ala, d-Ala, and d-Glu for structural integrity (28). d-Ala and d-Glu are generated by amino acid racemase and also by d-amino acid transaminase in bacteria (25). We next examined the availability of d-amino acids derived from bacteria for the substrate of DAO. By the incubation of bacterial lysate with DAO, the levels of pyruvic acid, a reaction product of DAO plus d-Ala, and H2O2 were elevated (Fig. 3a and b). Generation of H2O2 was observed by incubation of DAO and bacterial lysate, although the level of H2O2 was not sufficient to suppress the growth of S. aureus in SCD broth (data not shown). However, in the presence of MPO, concerted bactericidal activity against S. aureus was observed with DAO plus MPO and bacterial lysate. Namely, mixing of DAO (25 μg/ml), MPO (1.6 U/ml), and bacterial lysate (1 mg/ml) showed 80% killing of viable bacteria, whereas without MPO no bactericidal activity was observed (Fig. 3d). Thus, this bactericidal activity is thought to be mediated by the generation of HOCl (Fig. 3c). In fact, according to the literature, the culture medium of S. aureus contains d-amino acids at the mM level. The availability of d-Ala for DAO to utilize for the generation of H2O2 is considered to come from de novo-synthesized (by racemase) d-Ala. Importantly, DAO and MPO reportedly bind to bacterial cell walls and exhibit bacteriostatic and bactericidal activities (2, 29). This cell wall-binding property is advantageous for more localized and concentrated generation of antibacterial molecules, such as H2O2 and HOCl (2, 29). These results thus indicate that DAO and MPO can act synergistically in an antibacterial system.
Konno and Yasumura reported genetic polymorphism in the Dao gene in ddY mice (19). A fraction of ddY mice possess a single-nucleotide polymorphism in the Dao gene: a nucleotide change from GGG to AGG, which causes a glycine-to-arginine amino acid substitution. This amino acid substitution disrupted DAO enzyme activity without affecting the DAO expression level (19). We thus grouped ddY mice on the basis of genetic polymorphism by using the RFLP method, as described previously. Of 46 female ddY mice, 16 were double mutants (DAO−/−), 24 were heterozygous (DAO+/−), and 6 were wild type (DAO+/+). In agreement with genotyping of the Dao gene, DAO−/− mice showed the lowest DAO activity (0.04 mU/g wet tissue) and DAO+/+ mice had the highest DAO activity (56.9 mU/g wet tissue). DAO+/− mice had almost half the DAO activity (21.7 mU/g wet tissue) of DAO+/+ mice.
We also examined the antibacterial role of DAO in an in vivo setting using DAO+/+ and DAO−/− mice. Intravenous injection of S. aureus resulted in severe renal infection. Compared with DAO−/− mice, DAO+/+ mice had significantly reduced (to less than 10%) numbers of viable bacteria in the kidney (Fig. 4d). Consistent with this result, DAO+/+ mice demonstrated greater resistance to S. aureus infection (i.e., had a higher survival rate) than DAO−/− mice (Fig. 4e).
The neutrophil is one of the most important defenses against microbial infection, and its dysfunction causes severe chronic infection (3, 17). Therefore, we examined whether neutrophils derived from DAO−/− mice had lower bactericidal activity than neutrophils derived from DAO+/+ mice. In fact, peritoneal neutrophils derived from DAO+/+ mice showed higher DAO activity than did those derived from DAO−/− mice (Fig. 5a). However, contrary to our expectation, no difference in bactericidal activity was observed for neutrophils derived from DAO+/+ and those derived from DAO−/− mice (Fig. 5b). This result may be due to the relatively low H2O2 generation potential of DAO compared with NADPH oxidase, which is the major source of H2O2 in neutrophils. DAO may not affect the net amount of H2O2 in neutrophils, and thus, it may not be the primary antibacterial component in neutrophils. However, it may be one component operating in antimicrobial defense, which may become important in specific cases, for example, NADPH oxidase deficiency. As mentioned above, DAO activity was higher in the kidneys of DAO+/+ and DAO+/− mice, about 56.9 mU/g wet tissue and 21.7 mU/g wet tissue, respectively, than in DAO−/− mice. This activity is thought to be enough to reduce bacterial growth in the kidney, because a DAO concentration of 10 μg/ml suppressed bacterial growth, as shown in Fig. 1a and b. However, we could not show direct evidence that kidney homogenates exhibit bactericidal activity toward S. aureus (data not shown). This may be because, in this experimental workup, cellular integrity was completely destroyed, and formation of a phagosome and accumulation of antibacterial protein and supply of d-Ala at a localized site were not complete enough to show bactericidal activity, although more studies are needed to confirm this possibility. In this regard, Zhang et al. reported the potential binding of DAO to bacterial cell walls, and DAO showed higher antibacterial activity than a theoretical amount of H2O2, as calculated from the unit activity of DAO (30). Such H2O2 generation in the vicinity of bacteria may be advantageous for avoiding H2O2 degradation by kidney catalase before the H2O2 can act against the bacteria. Furthermore, neutrophils are recruited to the infected site, and abundant MPO may be available at sites of bacterial infection, which can exert the synergistic effect of DAO with MPO. In fact, in this study, we found that DAO+/+ mice had less infection in the kidney and a higher survival rate after intravenous infection with S. aureus than DAO−/− mice.
S. aureus, used in the present study, is a catalase-producing bacterium and so is able to degrade H2O2 to water and oxygen. Production of catalase is well correlated with susceptibility to hydrogen peroxide treatment and with virulence and survival in the polymorph nuclear phagocyte (16, 22), though catalase-negative S. aureus may be more susceptible to treatment with DAO, d-Ala, and MPO, which remains to be elucidated. We also noticed that catalase-negative bacteria, such as Streptococcus spp., were more resistant to hydrogen peroxide treatment and DAO treatment (data not shown). This may be due to other anti-ROS enzymes, such as peroxidases, filling the role of catalase (6).
Based on the results shown in Fig. 1 and 2, DAO requires free d-amino acids, such as d-Ala or d-Pro, in order to exert bacteriostatic and bactericidal activities. Two d-amino acid sources of supply may be available in the in vivo setting: the infecting and growing bacteria and the enterobacteria. It has been reported that S. aureus possesses DAO-reactive materials, free d-amino acid, in the cell pool at 3.6 μmol/100 mg (dry weight) of cells (5). Also, approximately 1 mM d-amino acids are secreted from bacteria in the bacterial culture medium (20). Konno et al. observed that the d-amino acids that accumulated in urine and tissues derived from enterobacteria in DAO−/− mice, but not in normal mice, which indicates that DAO continuously catabolizes d-amino acids and thus generates H2O2 by means of endogenously derived d-amino acids in the kidney or other tissues (18). These possibilities remain to be elucidated.
These observations suggest that DAO exhibits bacteriostatic activity and bactericidal activity in the presence of MPO in vitro. The substrate of DAO, d-amino acids, may be supplied from infecting bacteria or endogenous bacteria in vivo to some extent. Thus, DAO, in combination with MPO, may be an antibacterial system in mammals, especially in the kidney but perhaps also in the lung and liver, which utilizes d-amino acids derived from endogenous bacteria in infections.
ACKNOWLEDGMENT
This work was supported by Grant-in-Aid for Scientific Research 21791016 from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Footnotes
Published ahead of print 25 January 2012
REFERENCES
- 1. Albrich JM, McCarthy CA, Hurst JK. 1981. Biological reactivity of hypochlorous acid: implications for microbicidal mechanisms of leukocyte myeloperoxidase. Proc. Natl. Acad. Sci. U. S. A. 78:210–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allen RC, Stephens JT., Jr 2011. Myeloperoxidase selectively binds and selectively kills microbes. Infect. Immun. 79:474–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baehner RL, Nathan DG, Karnovsky ML. 1969. The metabolic and bactericidal defect in chronic granulomatous disease. Vox Sang. 17:35–36 [PubMed] [Google Scholar]
- 4. Bainton DF, Ullyot JL, Farquhar MG. 1971. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J. Exp. Med. 134:907–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhattacharyya SK, Banerjee AB. 1974. d-Amino acids in the cell pool of bacteria. Folia Microbiol. 19:43–50 [DOI] [PubMed] [Google Scholar]
- 6. Brenot A, King KY, Janowiak B, Griffith O, Caparon MG. 2004. Contribution of glutathione peroxidase to the virulence of Streptococcus pyogenes. Infect. Immun. 72:408–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Briggs RT, Karnovsky ML, Karnovsky MJ. 1977. Hydrogen peroxide production in chronic granulomatous disease. A cytochemical study of reduced pyridine nucleotide oxidases. J. Clin. Invest. 59:1088–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chumakov I, et al. 2002. Genetic and physiological data implicating the new human gene G72 and the gene for D-amino acid oxidase in schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 99:13675–13680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clifford DP, Repine JE. 1982. Hydrogen peroxide mediated killing of bacteria. Mol. Cell Biochem. 49:143–149 [DOI] [PubMed] [Google Scholar]
- 10. Cline MJ, Lehrer RI. 1969. d-Amino acid oxidase in leukocytes: a possible d-amino-acid-linked antimicrobial system. Proc. Natl. Acad. Sci. U. S. A. 62:756–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. D'Aniello A, et al. 1993. Biological role of D-amino acid oxidase and D-aspartate oxidase. Effects of D-amino acids. J. Biol. Chem. 268:26941–26949 [PubMed] [Google Scholar]
- 12. Fang J, et al. 2008. Oxystress inducing antitumor therapeutics via tumor-targeted delivery of PEG-conjugated D-amino acid oxidase. Int. J. Cancer 122:1135–1144 [DOI] [PubMed] [Google Scholar]
- 13. Fang J, Sawa T, Akaike T, Maeda H. 2002. Tumor-targeted delivery of polyethylene glycol-conjugated D-amino acid oxidase for antitumor therapy via enzymatic generation of hydrogen peroxide. Cancer Res. 62:3138–3143 [PubMed] [Google Scholar]
- 14. Haase LW. 1950. Bactericidal action of hydrogen peroxide, peroxides, and oxidizing compounds. Pharmazie 5:436–437 [PubMed] [Google Scholar]
- 15. Jaovisidha P, Peeples ME, Brees AA, Carpenter LR, Moy JN. 1999. Respiratory syncytial virus stimulates neutrophil degranulation and chemokine release. J. Immunol. 163:2816–2820 [PubMed] [Google Scholar]
- 16. Kanafani H, Martin SE. 1985. Catalase and superoxide dismutase activities in virulent and nonvirulent Staphylococcus aureus isolates. J. Clin. Microbiol. 21:607–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klebanoff SJ, White LR. 1969. Iodination defect in the leukocytes of a patient with chronic granulomatous disease of childhood. N. Engl. J. Med. 280:460–466 [DOI] [PubMed] [Google Scholar]
- 18. Konno R, Niwa A, Yasumura Y. 1990. Intestinal bacterial origin of D-alanine in urine of mutant mice lacking D-amino-acid oxidase. Biochem. J. 268:263–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Konno R, Yasumura Y. 1983. Mouse mutant deficient in D-amino acid oxidase activity. Genetics 103:277–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lam H, et al. 2009. d-Amino acids govern stationary phase cell wall remodeling in bacteria. Science 325:1552–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lyle LR, Jutila JW. 1968. d-Amino acid oxidase induction in the kidneys of germ-free mice. J. Bacteriol. 96:606–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mandell GL. 1975. Catalase, superoxide dismutase, and virulence of Staphylococcus aureus. In vitro and in vivo studies with emphasis on staphylococcal-leukocyte interaction. J. Clin. Invest. 55:561–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nagata Y, Yamada R, Nagasaki H, Konno R, Yasumura Y. 1991. Administration of D-alanine did not cause increase of D-amino acid oxidase activity in mice. Experientia 47:835–838 [DOI] [PubMed] [Google Scholar]
- 24. Paul BB, Strauss RR, Jacobs AA, Sbarra AJ. 1970. Function of H(2)O(2), myeloperoxidase, and hexose monophosphate shunt enzymes in phagocytizing cells from different species. Infect. Immun. 1:338–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pucci MJ, Thanassi JA, Ho HT, Falk PJ, Dougherty TJ. 1995. Staphylococcus haemolyticus contains two D-glutamic acid biosynthetic activities, a glutamate racemase and a D-amino acid transaminase. J. Bacteriol. 177:336–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Robinson JM, Briggs RT, Karnovsky MJ. 1978. Localization of D-amino acid oxidase on the cell surface of human polymorphonuclear leukocytes. J. Cell Biol. 77:59–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sakamoto R, et al. 2003. A new water-soluble chromogenic indicator: an application to the determination of chlorine in aqueous solutions. Anal. Sci. 19:1445–1447 [DOI] [PubMed] [Google Scholar]
- 28. Schleifer KH. 1975. Chemical structure of the peptidoglycan, its modifiability and relation to the biological activity. Z. Immunitatsforsch. Exp. Klin. Immunol. 149:104–117 [PubMed] [Google Scholar]
- 29. Zhang H, Yang Q, Sun M, Teng M, Niu L. 2004. Hydrogen peroxide produced by two amino acid oxidases mediates antibacterial actions. J. Microbiol. 42:336–339 [PubMed] [Google Scholar]
- 30. Zhang M, et al. 2011. Behavioral characterization of a mutant mouse strain lacking D-amino acid oxidase activity. Behav. Brain Res. 217:81–87 [DOI] [PubMed] [Google Scholar]