Abstract
We report here the interesting case of a 5-year-old male cynomolgus monkey with goblet cell hyperplasia and thickening of the muscular layer throughout the small intestine without exhibiting any clinical symptoms. Necropsy examination showed diffuse thickening of the intestinal wall from the jejunum to the ileum, with an appearance likened to a rubber tube. Histopathologically, marked thickening was observed in both the mucosal and muscular layers in the jejunum and ileum, and slight thickening was observed in the duodenum. Goblet cell hyperplasia with extension of the circular folds and villi was prominently observed. The mucosal surface was covered with a thick mucus layer containing desquamated mucosal epithelial cells, and both the inner and outer muscular layers were markedly thickened due to smooth muscle hypertrophy. Neither macroscopic nor histopathological examination identified any causative factors, such as infection, enteritis and intestinal stenosis, or obstruction that may have caused development of this lesion. Given these observations, this case may simply be considered of spontaneous goblet cell hyperplasia and muscular layer thickening in the small intestine of a cynomolgus monkey.
Keywords: goblet cell hyperplasia, muscular layer thickening, intestinal smooth muscle, cynomolgus monkey
Among the mucosal epithelial cells in the intestine, intestinal goblet cells are well-known to fulfill a protective function through synthesis and secretion of mucins in response to a number of stimuli, including cholinergic nerves or agents, hormonal influence, inflammatory factors, lectins and bacterial toxins, as well as other irritants and chemical factors. 1 In certain animal species, these stimuli may induce goblet cell hyperplasia. 2 – 8 Smooth muscle cells, which surround the gastrointestinal tract, are vital to maintaining proper gastrointestinal function. These cells occasionally exhibit cellular adaptations such as hypertrophy or hyperplasia in response to muscular cell injury and hormonal or mechanical stimulation. 9 Spontaneously occurring hypertrophy or hyperplasia of smooth muscle has been reported in pigs, horses, cats and humans. 9 – 11 However, although data regarding spontaneous lesions in cynomolgus monkeys (Macaca fascicularis) would be helpful in assessing compound toxicity in nonclinical toxicity studies, reports of spontaneous lesions in the alimentary tracts of these monkeys are limited except for those related to parasites. Here, we report a case of non-symptomatic goblet cell hyperplasia and muscular layer thickening throughout the small intestine in a cynomolgus monkey.
A male cynomolgus monkey born and raised in a primate colony in China was purchased from Guangxi Grandforest Scientific Primate Company, Ltd. (Guangxi, PR China) at four years of age. The animal was housed by itself in a stainless-steel cage (600 mm W × 700 mm D × 1200 mm H) with an artificial lighting cycle of 12 hours (7:00 to 19:00), an average temperature of 25°C (range: 18–28°C), an average relative humidity of 60% (range: 40–70%) and 17.3 ventilation changes/hour on average (range: 13.8–20.8 changes/hour). The monkey was fed a commercial primate diet (PS; Oriental Yeast Co., Ltd., Tokyo, Japan) and allowed ad libitum access to water. The treatment and handling of the animal were approved based on the Guidelines for Animal Experimentation issued by Astellas Pharma Inc., which in turn were based on the International Guiding Principles for Biomedical Research Involving Animals published by the Council for International Organizations of Medical Sciences (CIOMS). 12 Serological examination for viruses (B-virus; simian immunodeficiency virus; simian T-cell leukemia virus-I; simian retrovirus I, II and V; ebola; and hepatitis A and B), bacterial examination using medium culture (Mycobacterium tuberculosis, Shigella spp., Salmonella spp.) and microscopic or fecal smear examination for parasites (lice, fleas, ticks, Giardia, Strongyloides, Entamoeba) were conducted, with results confirming no infection with any of the above agents. At 5 years of age, the monkey was allocated to the control group of a 1-week repeated oral dose toxicity study. There were no noteworthy findings in clinical observation, body weight, food consumption, hematology and blood chemistry throughout the quarantine, acclimation and toxicity study periods. The monkey was euthanized under ketamine anesthesia at the end of the study period, and a full internal and external macroscopic examination was performed. Another monkey that had shown no abnormalities and had been purchased at the same time from the same breeder as the animal in question was used as a reference control for gross and histological pathology (Fig. 1B and Figs. 2B, D, F, H).
Fig. 1.
Gross appearance of the mucosal surface of the ileum from the present case (A) and a normal monkey (B). A: Thickening of the mucosal folds and conglomerated mucus (arrows) on the mucosal surface. B) Normal appearance.
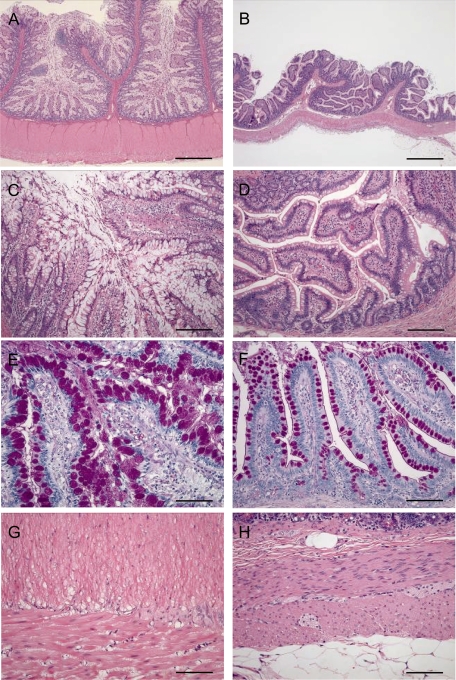
Fig. 2.
Photomicrographs of the ileum from the present case (A, C, E, G) and a normal monkey (B, D, F, H). A: Marked thickening of both the mucosal and muscular layers. C: Hyperplasia of the goblet cells with extension of the villi. E: PAS-positive reaction in the mucus of hyperplastic goblet cells. G: Marked thickening of both the inner and outer muscular layers due to smooth muscle hypertrophy. B, D, F, H: Normal appearance. A, B: H&E, bar=1000 μm. C, D: Higher magnification of A and B, H&E, bar=200 μm. E, F: PAS, bar=100 μm. G, H: H&E, bar=100 μm.
Necropsy examination showed diffuse thickening of the intestinal wall (approximately 5 mm), including the mucosal and muscular layers, from the jejunum to the ileum, and the intestine had the general appearance of a rubber tube. Mucosal folds were more elongated in the examined animal than in the control. Whitish, conglomerated mucus masses (3–7 mm in diameter) were observed on the mucosal surface (Fig. 1A). In contrast, no abnormalities were noted on macroscopic examination of the duodenum and large intestine. In addition, the thymus was atrophied, and the mesenteric lymph node was enlarged.
For routine histopathological examination, most systemic organs and tissues were fixed in 10% neutral buffered formalin, and paraffin-embedded sections were prepared and stained with hematoxylin and eosin (H&E). For the specimens of small intestine, staining with periodic acid Schiff (PAS) and alcian blue (pH 1.0 and 2.5) for polysaccharides, phosphotungstic acid hematoxylin for muscle fibers, Masson’s trichrome for collagen fibers and Gram, Giemsa and Wartin-Starry stains for bacteria was additionally performed. To assess the proliferating activity of mucosal epithelia and intestinal smooth muscle, immunohistochemical staining was also performed using proliferating cell nuclear antigen antibody (PCNA; Novocastra, Newcastle upon Tyne, UK) and the labeled streptavidin-biotin method (LSAB®2 System-HRP; DAKO A/S, Glostrup, Denmark).
Histologically, marked thickening was observed in both the mucosal and muscular layers from the jejunum to the ileum (Fig. 2A), and slight thickening was observed in the duodenum. In the mucosa, goblet cell hyperplasia with extension of the circular folds and villi was prominently observed (Figs. 2A, C). The mucosal surface throughout the small intestine was covered with a thick mucus layer containing desquamated mucosal epithelial cells (Fig. 2C). The goblet cells were markedly swollen due to increased intracellular mucus. On staining, the mucus showed a positive reaction for PAS (Fig. 2E) and was stained blue in alcian blue staining (pH 2.5); the results were identical to those obtained on examination of the mucus in samples of normal intestine (Fig. 2F). No cellular or structural atypia was observed in the epithelial cells. In addition, the reactivity of PCNA immunostaining in the intestinal mucosa appeared markedly similar to observations of reactivity made on normal intestinal tissue samples.
Minimal to slight inflammatory cell infiltration consisting primarily of lymphocytes and plasma cells was observed in the lamina propria of the duodenum and jejunum, but not to an obvious degree in the ileum. No organisms such as protozoa or parasites were found in the intestine, and no pathogenic bacteria were identified by Gram, Giemsa or Wartin-Starry stains. Smooth muscle fibers were hypertrophied, and both the inner and outer muscular layers were markedly thickened (Fig. 2G). Nuclei stained positive with PCNA were scattered in the hypertrophic smooth muscle but were not detected in the intestinal muscular layer samples obtained from the control animal. No histopathologic abnormalities were observed in the nerve plexuses. In the gastric mucosa, the amount of intracellular mucus of the superficial mucous cells was increased, and the gastric pit was slightly extended. In addition to these changes, thymic involution and lymphoid follicular hyperplasia in the mesenteric lymph node were observed. No abnormal findings were observed in other organs or tissues.
The animal in the present case clearly showed goblet cell hyperplasia and smooth muscular hypertrophy from the jejunum to the ileum, including marked thickening of the mucosal and muscular layers, without exhibiting any clinical symptoms. Given that this phenomenon was observed only in a control group monkey and not in any animals treated with the test article, we consider this a spontaneous occurrence.
The intestinal epithelium is covered by a protective mucus gel composed predominantly of mucin glycoproteins that are synthesized and secreted by goblet cells. 1 Goblet cell hyperplasia has been reported in relation to infection of nematodes, including Nippostrongylus brasiliensis, Strongyloides ratti, Trichuris muris and Trichinella spiralis. 2 In a study involving experimental infection of baboons with Schistosoma mansoni, stimulation of the intestinal mucosa was found to induce goblet cell hyperplasia and villus atrophy. 3 Mucins from goblet cells play an important role in trapping and removing intestinal nematodes from the gut. Several studies have demonstrated that intestinal microbes may induce goblet cell hyperplasia in bacterial enteritis associated with Yersinia enterocolitica, Escherichia coli, and Campylobacter-like organisms. 4 , 5 However, in the present case, no findings related to nematode infection or bacterial enteritis were observed in macroscopic or histopathological examination.
Mucoid enteropathy, 6 which is characterized by dehydration, diarrhea and normal or subnormal temperature, is a serious digestive disease afflicting young rabbits and involves hyperplasia of goblet cells without inflammatory changes in the duodenum, jejunum or ileum. Mucoid enteropathy may be related to gut dysfunction or may be due to direct pathogenic agents. 7 One study conducted on a macaque with protein-losing enteropathy showing chronic diarrhea and progressive weight loss found striking proliferation of goblet cells with excess mucin production. 8 However, as clinical abnormalities such as diarrhea and weight loss were not noted as general signs in the present case, we believe that our findings are not comparable to mucoid enteropathy in rabbits or protein-losing enteropathy in macaques.
Another remarkable characteristic of this case is the observed thickening of the intestinal muscular layer resulting from the smooth muscle hypertrophy and hyperplasia, which were also evident based on PCNA immunostaining. Muscular layer thickening due to intestinal smooth muscle hypertrophy or hyperplasia has been previously described to occur spontaneously in horses with intestinal diverticulum, pigs infected with Lawsonia intracellularis, cats with gastrointestinal parasitism or secondary to neoplastic intestinal obstruction and humans. 9 , 10 Furthermore, several experiments in laboratory animals have reproduced compensatory muscular hypertrophy via surgically-induced stenosis or partial resection. 9 However, the animal in the present case had no clinical history of intestinal stenosis or obstruction and showed no gross findings indicative of alimentary passage failure. One study in rats reported that inflammation in the intestinal tract may induce hypertrophy of intestinal smooth muscle. 11 In the present case as well, we cannot completely deny involvement of inflammation in the muscular layer, because minimal to slight inflammatory cell infiltration was observed in the small intestine and lymphoid follicular hyperplasia in the mesenteric lymph node. However, the smooth muscle change was not considered to be induced by inflammation because this lesion was most prominent in the ileum and was without any accompanying inflammation.
To our knowledge, no case similar to the present case has been reported previously in any other species, and thus, the pathogenesis of our findings and the relationship between them remains unclear. Therefore, this case may simply be a rare occurrence resulting from unknown pathogenesis in a cynomolgus monkey.
Acknowledgments
We thank Ms. M Murata and Ms. K Okazaki of Astellas Research Technologies Co., Ltd., for their skillful technical assistance.
References
- 1.Specian RD, Oliver MG. Functional biology of intestinal goblet cells. Am J Physiol. 1991;260:C183–C193. doi: 10.1152/ajpcell.1991.260.2.C183. [DOI] [PubMed] [Google Scholar]
- 2.Khan WI, Collins SM. Immune-mediated alteration in gut physiology and its role in host defence in nematode infection. Parasite Immunol. 2004;26:319–326. doi: 10.1111/j.0141-9838.2004.00715.x. [DOI] [PubMed] [Google Scholar]
- 3.Farah IO, Nyindo M. Acute schistosomiasis mansoni in the baboon Papio anubis gives rise to goblet-cell hyperplasia and villus atrophy that are modulated by an irradiated cercarial vaccine. Parasitol Res. 1997;83:281–284. doi: 10.1007/s004360050247. [DOI] [PubMed] [Google Scholar]
- 4.Dillehay DL, Paul KS, Boosinger TR, Fox JG. Enterocecocolitis associated with Escherichia coli and Campylobacter-like organisms in a hamster (Mesocricetus auratus) colony. Lab Anim Sci. 1994;44:12–16. [PubMed] [Google Scholar]
- 5.Lal M, Kaur H, Gupta LK, Sood NK. Histopathological and ultrastructual alterations of induced Yersinia enterocolitica infection in mice. Indian J Pathol Microbiol. 2004;47:559–564. [PubMed] [Google Scholar]
- 6. Flatt RE, Weisbroth SH, Kraus AL.Metabolic, traumatic, mycotic, and miscellaneous deseases of rabbits. In: The biology of the laboratory rabbit. Weisbroth SH, Flatt RE and Kraus AL (eds). Academic Press, New York. 435–451.1974.
- 7.Rodriguez-De Lara R, Cedillo-Pelaez C, Constantino-Casas F, Fallas-Lopez M, Cobos-Peralta MA, Gutierrez-Olvera C, Juarez-Acevedo M, Miranda-Romero LA. Studies on the evolution, pathology, and immunity of commercial fattening rabbits affected with epizootic outbreaks of diarrhoeas in Mexico: a case report. Res Vet Sci. 2008;84:257–268. doi: 10.1016/j.rvsc.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodger RF, Bronson RT, McIntyre KW, Nicolosi RJ. Protein-losing enteropathy in six macaques. J Am Vet Med Assoc. 1980;177:863–866. [PubMed] [Google Scholar]
- 9.Bettini G, Muracchini M, Della Salda L, Preziosi R, Morini M, Guglielmini C, Sanguinetti V, Marcato PS. Hypertrophy of intestinal smooth muscle in cats. Res Vet Sci. 2003;75:43–53. doi: 10.1016/s0034-5288(03)00041-9. [DOI] [PubMed] [Google Scholar]
- 10.Lawson GH, Gebhart CJ. Proliferative enteropathy. J Comp Pathol. 2000;122:77–100. doi: 10.1053/jcpa.1999.0347. [DOI] [PubMed] [Google Scholar]
- 11.Blennerhassett MG, Bovell FM, Lourenssen S, McHugh KM. Characteristics of inflammation-induced hypertrophy of rat intestinal smooth muscle cell. Dig Dis Sci. 1999;44:1265–1272. doi: 10.1023/a:1026669409229. [DOI] [PubMed] [Google Scholar]
- 12. The Council for International Organizations of Medical Sciences (CIOMS). International guiding principles for biomedical research involving animals. Altern Lab Anim. 12: ii–. 1985. [PubMed]