Abstract
Equisetum arvense, commonly known as the field horsetail, has potential as a new functional food ingredient. However, little information is available on its side effects, and the general toxicity of Equisetum arvense has yet to be examined in detail. In the present study, we evaluated the influence of administration in diet at doses of 0, 0.3, 1 and 3% for 13 weeks in male and female F344 rats. No toxicity was detected with reference to clinical signs, body weight, urinalysis, hematology and serum biochemistry data and organ weights. Microscopic examination revealed no histopathological lesions associated with treatment. In conclusion, the no-observed-adverse-effect level (NOAEL) for Equisetum arvense was determined to be greater than 3% in both sexes of F344 rat (males and females: >1.79 g/kg BW/day and >1.85 g/kg BW/day, respectively) under the conditions of the present study.
Keywords: Equisetum arvense, horsetail, NOAEL, toxicity, F344 rat
Introduction
Equisetum arvense, commonly known as the field horsetail, is a bushy perennial featuring rhizomatous stem formation 1 . The plant contains abundant minerals such as silicon and calcium 2 , as well as small amounts of pharmacologically active compounds, and has been used as a traditional medicine to stop bleeding, heal ulcers and wounds and treat tuberculosis and kidney diseases. It is available as a dried extract in powdered form or a liquid extract, and its biological effects include anti-oxidant 3 , 4 , anti-convulsant and sedative actions 5 , as demonstrated in recent in vitro and in vivo studies. In particular, it is expected to lower high blood pressure due to its diuretic effect, which was previously observed in rats and humans 6 . Therefore, Equisetum arvense may have potential as new functional food ingredient.
Recently, no genetic toxicity of Equisetum arvense was reported in a reverse mutation test, chromosomal aberration test or micronucleus test 7 . Additionally, the LD50 value of Equisetum arvense was found to exceed 5000 mg/kg in a single-dose toxicity study in rats 7 .
However, it was reported to cause skin dermatitis in people allergic to tobacco smoke 8 . Furthermore, little is known about its side effects when continuously administered via the oral route.
In the present study, we therefore examined the general toxicity of Equisetum arvense administered in diet for 13 weeks to male and female F344 rats.
Materials and Methods
Animals and test chemical
Five-week-old F344 rats (40 males and 40 females) were obtained from Charles River Laboratories Japan, Inc. (Kanagawa, Japan) and housed in a room maintained under a 12-h light/dark cycle at constant temperature (23 ± 1°C) and humidity (50 ± 5%). Test chemicals (Lot.183-3194G, Cross Co., Ltd., Osaka, Japan), a yellow-green to yellow fresh powder, were prepared every month from Equisetum arvense, extracted with hot water and mixed with powdered CRF-1 basal diet (Oriental Yeast, Tokyo, Japan) at each concentration. The diet of each group was changed twice a week during the experiment.
Experimental design
All procedures were approved by the Institutional Animal Care and Use Committee of Osaka City University Medical School. After acclimation for 1 week, male and female rats at six weeks of age were randomly divided into eight groups of 10 rats each. Dosage selections were based on estimated intake for humans as follows. We expected that people consumed 5 mg/kg Equisetum arvense daily as a supplement. To ensure a safety factor of 100-fold, the 1% dose was set to feed at an approximate dosage level of 500 mg/kg, and 3 and 0.3% doses were chosen as the high and low dose groups using a common ratio of about 3. The animals received diets containing Equisetum arvense at doses of 0, 0.3, 1 and 3%, respectively, for 13 weeks. They were observed daily for clinical signs and mortality. Body weight and food consumption were measured weekly. Fresh urine samples were collected from all animals at week 13. Urinalysis using N-multistix SG-L and Clinitek Status (Siemens, Tokyo, Japan) was performed for the following parameters: glucose (GLU), bilirubin (BIL), ketone (KET), specific gravity (SG), protein (PRO), urobilinogen (URO), occult blood (OB), pH and nitrite (NIT). At the end of the experiment, all animals were fasted overnight and euthanized by exsanguination under ether anesthesia. Blood was taken from the abdominal aorta for hematology and serum biochemistry analyses. Hematological examinations included the following parameters: white blood cell count (WBC), red blood cell count (RBC), hemoglobin concentration (Hb), hematocrit (Ht), platelet count (PLT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC). Serum biochemistry was performed to examine total proteins (TP), albumin (ALB), albumin/globulin ratio (A/G), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma glutamyl transpeptidase (γ-GTP), creatinine (CRN), triglycerides (TG), total cholesterol (T-Cho), total bilirubin (T-Bil), blood urea nitrogen (BUN), sodium (Na), potassium (K), chloride (Cl), calcium (Ca) and inorganic phosphorus (P). Analyses for hematology and serum biochemistry were conducted at Mitsubishi Chemical Medience Corp., (Osaka, Japan) using automatic analyzing machines, XT-2100 (Sysmex, Hyogo, Japan) and H7700 (Hitachi, Tokyo, Japan), for blood and serum analyses, respectively. At sacrifice, the heart, liver, spleen, kidneys, adrenals, testes, brain and thymus were excised and weighed. In addition to these organs, the lymph nodes (cervical and mesenteric), aorta, salivary gland, bone (sternum, femur), bone marrow, trachea, thyroid, tongue, esophagus, stomach, duodenum, small intestine, large intestine, pancreas, urinary bladder, seminal vesicles, prostate gland, epididymis, ovaries, uterus, vagina, pituitary gland, sciatic nerve, skeletal muscle, spinal cord, eyes and harderian glands were excised and fixed in 10% buffered formalin. Testes were fixed in Bouin's solution. Paraffin-embedded tissue sections of all organs and tissues were routinely prepared and stained with hematoxylin and eosin for histopathological examination. All organs and tissues in the control and high-dose groups were examined. Histopathological examination was also extended to all tissues of the low- and medium-dose groups if lesions were frequently found in the high-dose group.
Immunohistochemistry
The avidin-biotin complex (ABC) method was used to demonstrate alpha2u-globulin expression in the kidney. After deparaffinization, kidney sections were treated in a microwave oven sequentially with 3% H2O2, normal rabbit serum, goat polyclonal anti- alpha2u-globulin antibody (AF586, R&D Systems, Inc., Minneapolis, MN, USA) diluted 1:300, biotin-labeled secondary antibody and avidin-biotin complex (Vectastain ABC kit, Funakoshi, Tokyo, Japan). Tissue sections were lightly counterstained with hematoxylin. Kidney tissue sections without addition of primary antibody were used as negative controls. For positive controls, kidneys of rats administered KBrO3, which is known to increase alpha2u-globulin, were subjected to analysis.
Statistics
The Dunnett’s multiple range test was employed for comparison of body weights, urinalysis, organ weights (both absolute and relative), hematology and serum biochemistry data between the control and treated groups. For all comparisons, p-values less than 0.05 were considered to be statistically significant (Stat Light, Yukms Co., Ltd., Tokyo, Japan).
Results
Clinical observations and survival
No deaths or obvious clinical signs were noted in any of the animals throughout the experimental period. During the experiment, the body weights and cumulative body weight gains in all treatment groups were similar to those of the controls (Fig. 1). The total intake of Equisetum arvense (g/rat) for 13 weeks was 0 (0%), 3.8 (0.3%), 12.3 (1%) and 36.4 (3%) in males and 0 (0%), 2.3 (0.3%), 7.9 (1%) and 24.2 (3%) in females (Table 1). There was no difference in food consumption.
Fig. 1.
Body weight changes in F344 rats given Equisetum arvense . Symbols represent the means.
Table 1. Intake of Equisetum arvense during the Experiment.
Urinalysis
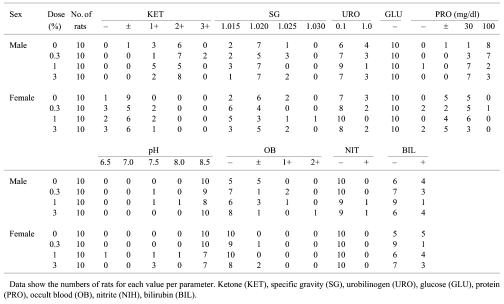
There were no significant differences in any of the parameters among the groups, but the protein levels in the 1 and 3% male groups tended to decrease (Table 2).
Table 2. Urinalysis Data for F344 Rats Given Equisetum arvense .
Hematology and serum biochemistry
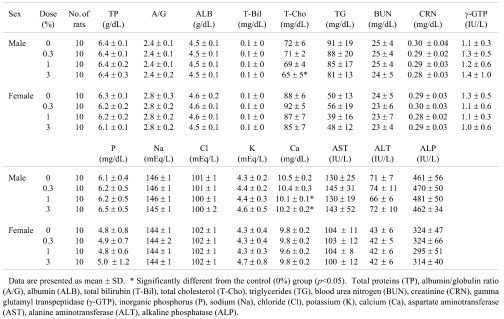
Significant alterations were observed in hematological parameters (Table 3), including increased MCH in the 0.3% males, increased PLT in the 1% females and decreased MCH in the 1% females (p<0.05), but no dose dependence was apparent. In addition, a trend towards a dose-dependent decrease in WBC was found in the females. Data for serum biochemistry are shown in Table 4. There were significant decreases in Ca in the 1 and 3% male groups (p<0.01 and p<0.05) and in T-Cho in the 3% males (p<0.05). A trend towards a dose-dependent decrease in TG was found in the males.
Table 3. Hematological Data for F344 Rats Given Equisetum arvense .
Table 4. Serum Biochemistry Results for F344 Rats given Equisetum arvense .
Organ weights
Absolute and relative organ weights are summarized in Table 5. There were no significant differences in any organ weights. A tendency towards a decrease in the absolute weights of the adrenals was observed in the males.
Table 5. Organ Weights of F344 Rats Given Equisetum arvense .
Macroscopic and microscopic findings
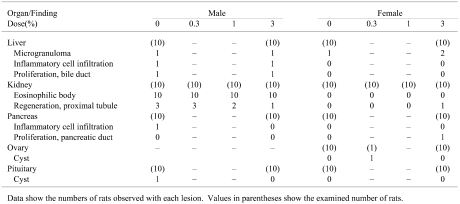
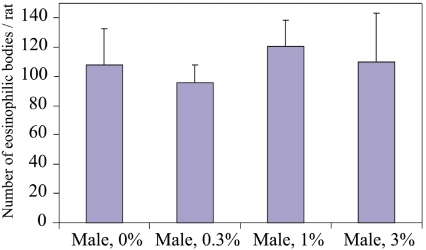
No treatment-related macroscopic changes were observed in any of the animals at sacrifice. Histopathologically, spontaneous inflammatory and proliferative lesions in the liver and pancreas, microgranulomas in the liver, atrophy in kidneys and cysts in the ovary and pituitary glands were diagnosed. All the changes were of minimal grade. Eosinophilic bodies and alpha2u-globulin expression in the proximal tubules of the kidneys were observed in all male rats, including the 0% group (Fig. 2). Extended microscopic examination of kidneys in the 0.3 and 1% male groups showed no histopathological changes compared with the 0% group (Table 6). The number of eosinophilic bodies per rat among the groups was not significantly different (Fig. 3). Moreover, no treatment-related findings were revealed in other tissues and organs of the male or female rats.
Fig. 2.
Eosinophilic body with overexpression of alpha2u-globulin in the rat kidney. Kidney sections from male control rats were used to confirm whether eosinophilic bodies (arrowheads) were also positive for alpha2u-globulin. Some epithelial cells in proximal tubules were positive. a: HE stain. b: immunohistochemistry for alpha2u-globulin. Scale bar shows 100 μm.
Table 6. Histopathological Findings for F344 Rats Given Equisetum arvense .
Fig. 3.
Number of eosinophilic bodies in male rat kidneys. No significant differences were observed among the groups. The column and whisker represent the mean and standard deviation, respectively.
Discussion
In the present study, dietary administration with Equisetum arvense for 13 weeks was not found to alter body weight in either gender of rat. The decline of the urine protein levels after administration of Equisetum arvense in the 1 and 3% male groups may be related to its diuretic effect, which was previously reported 6 . Although the WBC in females and the weights of the adrenals in males were dose-dependently decreased, no histopathological changes related to inflammation and atrophy of adrenals were observed. Furthermore, although a significant decrease in the concentration of serum Ca was noted in the 1 and 3% groups in males, all values were within the physiological range previously observed in our lab. A significant decrease in T-Cho and a tendency for decrease in TG were seen in a dose-related manner in males, but no histopathological changes pointing to altered lipid metabolism were observed in the liver.
Histopathological examination of kidneys revealed the existence of intracytoplasmic granules, diagnosed as eosinophilic bodies, seen in the proximal tubules in all male groups (Fig. 2a), but not in the female rats. These granules were demonstrated to be alpha2u-globulin by immunohistochemistry (Fig. 2b). Abnormal accumulation of alpha2u-globulin is known to be related to nephropathy and renal carcinogenesis in male rats 9 , 10 . As no difference in the number of eosinophilic bodies was seen between the control and treated groups, administration of Equisetum arvense clearly exerted no effects on the formation of alpha2u-globulin.
In an earlier toxicological report for rats 11 , dietary administration of 4% Equisetum arvense with 0.5% cholesterol and 0.15% sodium cholate for 14 days caused dermatitis of the neck, head and back skin in about 20-65% of rats, with no significant differences in the serum IgE levels. However, clinical signs, such as redness and edema, were not observed in this 13-week dietary administration study. Therefore, it is likely that Equisetum arvense alone does not induce dermatitis in rats and that the changes observed in the earlier study were probably due to coadminstration of excess amounts of cholesterol and/or sodium cholate with Equisetum arvense 11 .
In humans, it was reported that seborrhoeic dermatitis occurred in some patients, transdermal absorption of Equisetum arvense after passive inhalation of tobacco smoke 8 . Smoke components, such as nicotine and formaldehyde are considered to cause allergic contact dermatitis 12 .
However, the results of the present study showed no adverse effects of Equisetum arvense when administered alone in diet. Taking into accounts these reports, we predict that additional factors such as a potential allergen of tobacco might influence the side effects of Equisetum arvense.
In conclusion, we determined the no-observed-adverse-effect level (NOAEL) for Equisetum arvense to be more than 3% in both male and female rats (males and females: >1.79 g/kg BW/day and >1.85 g/kg BW/day, respectively) under the conditions of the present study.
References
- 1.Nijole B, Valentinas S, Steponas C. The spread of field horsetail (Equisetum arvense L.) in drained areas of Lithuania: Reasons and consequences, and possibilities for its control. Acta Agriculturae Scandinavica. 2006;56:25–30. [Google Scholar]
- 2.Hodson MJ, White PJ, Mead A, Broadley MR. Phylogenetic variation in the silicon composition of plants. Annals of Botany. 2005;96:1027–1046. doi: 10.1093/aob/mci255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Myagmar BE, Aniya Y. Free radical scavenging action of medicinal herbs from Mongolia. Phytomedicine. 2000;7:221–229. doi: 10.1016/S0944-7113(00)80007-0. [DOI] [PubMed] [Google Scholar]
- 4.Dos Santos JG, Jr, Hoffmann MM, Marcela BM, Maria NB, Damasseno Maia F, Kalyne AL. Cognitive enhancement in aged rats after chronic administration of Equisetum arvense L. with demonstrated antioxidant properties in vitro. Pharmacol Biochem Behav. 2005;81:593–600. doi: 10.1016/j.pbb.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Dos Santos JG, Jr, Blanco MM, Do Monte FH, Russi M, Lanziotti VM, Leal LK, Cunha GM. Sedative and anticonvulsant effects of hydroalcoholic extract of Equisetum arvense . Fitoterapia. 2005;76:508–513. doi: 10.1016/j.fitote.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Wright CI, Van-Buren L, Kroner CI, Koning MM. Herbal medicines as diuretics: review of the scientific evidence. J Ethnopharmcol. 2007;114:1–31. doi: 10.1016/j.jep.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 7.Miwa Y, Sakuma R. A safety toxicology study of Equisetum arvense L. Pharmacometrics. 2009;76:61–69. [Google Scholar]
- 8.Sudan BJ. Seborrhoeic dermatitis induced by nicotine of horsetails (Equisetum arvense L.) Contact Dermatitis. 1985;13:201–202. doi: 10.1111/j.1600-0536.1985.tb02548.x. [DOI] [PubMed] [Google Scholar]
- 9.Doi AM, Hill G, Seely J, Hailey JR, Kissling G, Bucher JR. Alpha 2u-globulin nephropathy and renal tumors in national toxicology program studies. Toxicol Pathol. 2007;35:533–540. doi: 10.1080/01926230701338941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao GN. Diet and kidney diseases in rats. Toxicol Pathol. 2002;30:651–656. doi: 10.1080/01926230290166733. [DOI] [PubMed] [Google Scholar]
- 11.Maeda H, Miyamoto K, Sano T. Occurrence of dermatitis in rats fed a cholesterol diet containing field horsetail (Equisetum arvense L.) J Nutr Sci Vitaminol. 1997;43:553–563. doi: 10.3177/jnsv.43.553. [DOI] [PubMed] [Google Scholar]
- 12.Glick ZR, Saedi N, Ehrlich A. Allergic contact dermatitis from cigarettes. Dermatitis. 2009;20:6–13. [PubMed] [Google Scholar]