Abstract
Patients with metastatic renal cell carcinoma (RCC) undergoing cytokine or targeted therapies may show a remarkable decline in quality of life (QoL). We wanted to evaluate QoL in patients with metastatic RCC undergoing therapeutic vaccination with dendritic cells (DCs). In a cross-sectional analysis, QoL was therefore assessed in RCC patients participating in three consecutive clinical trials of DC vaccination. Before the first and after the third vaccination with DCs, patients completed a QoL questionnaire (EORTC QLQ-C30, version 3). Data were transformed into scale scores and analysed using SPSS 12.0 software. Mean values of the resulting scores obtained before and after DC vaccination were compared using students t test and Wilcoxon rank-sum test. P < 0.05 was considered statistically significant. The questionnaire was completed by 55 of 71 patients (compliance rate, 77.5%) who had a median age of 58.7 years (from 30 to 75 years). No significant reductions in functioning scales including physical, emotional and social criteria as well as symptom scores, which assess typical symptoms of tumour therapies, were observed indicating that QoL remained high during DC vaccination. Significant correlations were found between overall survival and functional as well as symptom scores. Our data indicate that DC vaccination, which is a personalised treatment modality, maintains QoL and thus represents an attractive nontoxic treatment option for patients with metastatic RCC. It will be important to identify the most effective conditions of DC vaccination including combinations with other therapeutics to maximise clinical efficacy while still preserving QoL.
Keywords: Active immunotherapy, Dendritic cells, Cancer vaccine, Renal cell carcinoma, Quality of life
Introduction
Recently, targeted therapies that interfere with angiogenesis were introduced for the treatment of metastatic renal cell carcinoma (RCC) [1]. However, treatment with these inhibitors is continuous, and side effects are common and may even be severe [2, 3]. Therefore, effective treatments, which preserve patients’ quality of life (QoL), need to be developed. Because RCC is considered to be an immune-responsive tumour, therapeutic antitumour vaccination, although experimental, represents a promising treatment strategy [4, 5].
Dendritic cells (DCs) are the most potent antigen-presenting cells that initiate a broad repertoire of immune responses [6]. DCs can also be harnessed to generate tumour immunity [6, 7]. An increasing number of phase I trials have already used ex vivo-generated DCs for antitumour vaccination [8], and several trials have been performed in metastatic RCC (reviewed by Berntsen et al. [9]). In our own trials, evidence has been obtained that administration of DCs can induce antigen-specific immunity and that it occasionally results in tumour regressions and more frequently in disease stabilisations [10–13]. Overall, evidence for clinical efficacy of DC-based immunotherapy is still relatively scarce. Draube et al. [14] recently reported results of a meta-analysis and demonstrated a statistically significant influence of DC-based immunotherapy on clinical benefit rate (CBR). The combined percentages of objective responses and stable diseases (SD) amounted to a CBR of 48% in RCC. As a proof of principle, a statistically significant effect of DC-mediated cellular immune response and of DC dose on CBR could be demonstrated [14].
In addition, the first DC-based vaccine has recently been approved by the US Food and Drug Administration (FDA), after multicentre phase III trials had demonstrated an improvement in overall survival of prostate cancer patients [15]. An observation shared by many clinical investigators using DCs for cancer treatment was the absence of severe adverse events resulting in the view that DCs are well tolerated.
To translate this general view into measureable results, we assessed QoL during DC vaccination against metastatic RCC using the core questionnaire QLQ-C30 of the EORTC.
Patients and methods
Patient selection
All patients had histologically confirmed RCC with clear cell histology and bidimensionally measurable metastatic lesions. Patients underwent tumour nephrectomy and provided informed consent before treatment start. Exclusion criteria were other immunotherapies or chemotherapy within 4 weeks before the first DC vaccination, pregnancy, lactation, other malignancies within the last 5 years, use of immunosuppressive treatments such as prednisone, azathioprine, or cyclosporine within 4 weeks before vaccination, history of autoimmune disease, presence of acute or chronic infections, HIV or viral hepatitis, or a Karnofsky Index < 60.
DC generation
DCs were generated from monocytes as described previously [10–13]. Purified monocytes were differentiated into DCs during a 5-day culture with GM-CSF and IL-4 followed by the addition of tumour antigens and control antigen (keyhole limpet haemocyanin, KLH) as well as a cocktail consisting of TNF-α, IL-1β, IL-6 and PGE2 to induce DC maturation (>85% CD83+) [16]. DC generation was successful in all patients. No contamination occurred (100% sterility).
Patient treatment
Patients were treated according to study protocols [10–13] that had previously been approved by the institutional review board of the medical faculty and of the University of Innsbruck as well as by the pharmaceutical committee of the Austrian Ministry of Health. Some patients were treated as part of a compassionate use protocol. Treatment was initiated approximately 5 weeks after nephrectomy. Patients received 3 vaccinations in monthly intervals (one treatment cycle). Of the 15 patients, who received allogeneic DCs, 7 patients received low dose of cyclophosphamide prior to each DC vaccination. The remaining 40 patients received autologous DCs and no cyclophosphamide. Aside from cyclophosphamide, which was part of the clinical protocol using allogeneic DCs [13], patients received no additional treatment during DC vaccination. Four weeks after one treatment cycle, tumour sites were examined by computerised tomography. Clinical response evaluation was based on the RECIST guidelines. Time measurements (time to progression, follow-up) started from the first vaccination (i.e. day 0). Immunotherapy was free of any charge.
QLQ-C30 questionnaire
After obtaining approval from the institutional review board (study number UN4285; SN298/4.15), we retrospectively evaluated charts of all consecutive patients treated with DC vaccination between January 2000 and March 2007. A well-accepted instrument for the evaluation of patients’ QoL outcome in cancer therapy trials is the standardised and validated QoL questionnaire (QLQ-C30, version 3) developed by the European Organisation for Research and Treatment of Cancer (EORTC) [17]. Before the first and 1 month after the third vaccination, patients independently responded to a total of 30 questions. While 28 items were scored from 1 to 4 with a lower score representing a better QoL, two items (global quality of health) were scored 1–7 points with a higher score representing a better QoL. The questionnaire permits the grouping of individual items into five functional scales (physical, role, emotional, cognitive and social), a global quality of health scale, three symptom scales (fatigue, nausea/vomiting and pain) and a number of single items assessing physical symptoms common among cancer patients (dyspnoea, sleep disturbance, appetite loss, constipation, diarrhoea), as well as the financial impact of the disease and treatment. Data were transformed into scale scores according to the EORTC QLQ-C30 scoring manual and analysed using SPSS 12.0 software. Mean values of the resulting scores obtained before and after treatment were compared using students t test and Wilcoxon rank-sum test. P < 0.05 was considered statistically significant. An overall survival variable was generated as a result of transforming overall survival time by weighting with the event variable (alive or deceased). Subsequently, a series of spearman’s rank correlations with overall survival and QoL variables were calculated.
Results
Patients’ characteristics and clinical courses
The characteristics of the study population (n = 55) are summarised in Table 1. Median overall survival of the patients who completed the questionnaire (n = 55) was 31.4 months (29 months median follow-up) and 9.9 months for the patients who did not return the questionnaire (n = 16). Median overall survival of all patients (n = 71) was 20.3 months. At the time of data analysis, 45.5% of the patients were alive. Vaccine-induced immune responses could consistently be detected in peripheral blood [10–13, 18, 19]. Two patients had a complete response with DC vaccination. One patient had lung metastases, while the other patient had suspicious lymph nodes. Both are still alive and still in complete remission, now 53 and 41 months after the first DC application. Vaccination was well tolerated. In all 55 patients, there were no grade 3 or grade 4 adverse events. Moderate, transient fever as a systemic side effect occurred in only 6 patients (11%) several hours after vaccination. Mild erythema and induration as a local side effect were observed in two patients lasting for 2 days. According to current terminology of the EORTC, fever is considered a grade 1 (<38°C) or grade 2 (>38°C) adverse event. Erythema and induration at injection sites are considered grade 1 adverse events. No relationship between vaccination details and these grade 1 or grade 2 adverse events was observed.
Table 1.
Patients’ characteristics
| Characteristics | Evaluated patients (n = 55) |
|---|---|
| Age (years) | |
| Median | 58.7 (30–75) |
| Sex | |
| Male | 37 |
| Female | 18 |
| Prior therapies | |
| Tumour nephrectomy | 55 |
| Radiotherapy | 2 |
| Immunotherapy | 5 |
| Metastatic sites | |
| Lung | 42 |
| Lymph nodes | 19 |
| Liver | 7 |
| Adrenal gland | 4 |
| Bones | 3 |
| Local recurrence | 2 |
| Treatment response | |
| CR | 2 (3.6%) |
| PR | 10 (18.2%) |
| SD | 17 (30.9%) |
| PD | 26 (47.3%) |
CR complete response, PR partial response, SD stable disease, PD progressive disease
Quality-of-life assessment
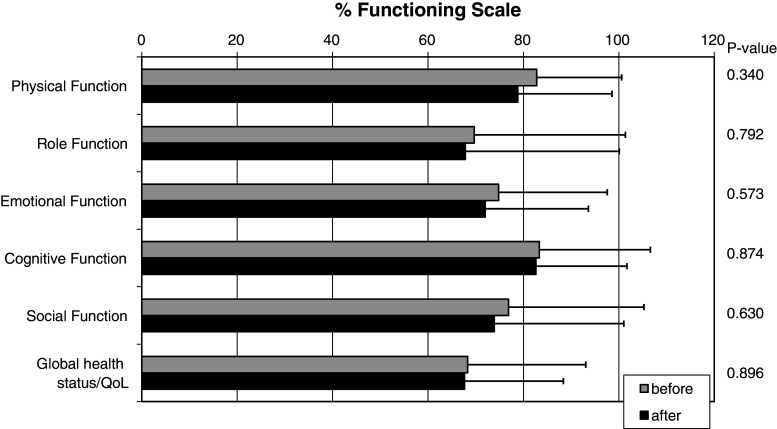
In this cross-sectional study, we used a standardised and validated QoL questionnaire (QLQ-C30 version 3) to evaluate potential changes in QoL during DC-based immunotherapy of patients with metastatic RCC. No differences in QoL were observed between study protocols using either autologous or allogeneic DCs [12, 13]. A total of 55 of 71 patients including 18 women and 37 men completed the questionnaire before the first and 1 month after the third vaccination. The compliance rate was thus 77.5%. Figure 1 illustrates patients’ functional scales (physical, role, emotional, cognitive, social) as well as global health before and after therapy. Before therapy, mean patient score was 76.0 (mean scale scores from 68.3 to 83.3). For comparison, a randomly selected sample of the Norwegian adult population (n = 1.965; mean age, 47.4), which was also analysed with the QLQ-C30 version 3, revealed a mean functional scale score of 86.0 (mean scale scores from 73.7 to 92.8) [20]. After therapy, all functional scale scores as well as global health scores remained high and mean patient score was 73.8 (mean scale scores from 67.6 to 82.6). The most notable change was a reduction in physical function, which, however, was not significant (P = 0.34) and did not exceed 4%. Global health status remained almost constant with a slight reduction of <1%.
Fig. 1.
QLQ-C30 functional scales before and after therapy
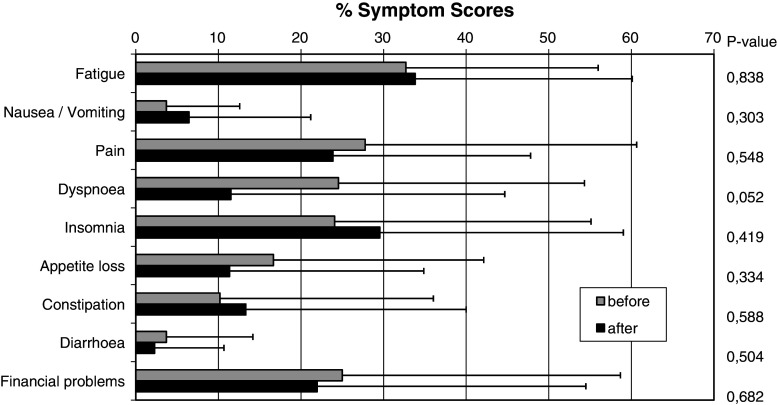
Symptom scores before and after therapy are depicted in Fig. 2. The most notable change was an improvement of dyspnoea (from 24.5 to 11.5) (P = 0.052). Other symptoms including pain, appetite loss and diarrhoea were also modestly improved, but these changes were not significant. In contrast, insomnia, fatigue, nausea/vomiting and constipation scores were slightly increased, but these changes were also not significant.
Fig. 2.
QLQ-C30 symptom scores before and after therapy
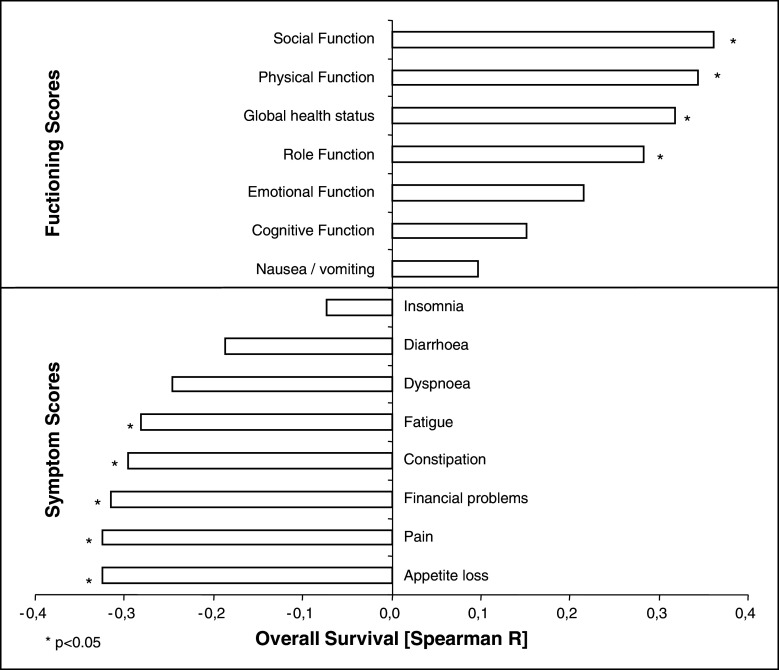
Figure 3 demonstrates the correlation between overall survival and individual functioning and symptom scores, respectively. Social function, physical function, global health status and role function showed a positive correlation with overall survival (Spearman rho from 0.283 to 0.362). Conversely, appetite loss, pain, financial problems, constipation and fatigue negatively correlated with overall survival (Spearman rho from −0.325 to −0.281). Other items like dyspnoea did not correlate with overall survival.
Fig. 3.
Correlation of overall survival with functioning and symptom scores
Discussion
In the present study, we used a standardised and validated QoL questionnaire (QLQ-C30, version 3) to evaluate possible changes in QoL during DC vaccination of patients with metastatic RCC. In 55 patients, who completed the questionnaire before the first and 1 month after the third vaccination, QoL remained high confirming the general observation by many investigators during the last decade that DC-based immunotherapy is well tolerated [8, 9]. Our findings are in agreement with two other studies that reported maintenance of QoL during DC-based immunotherapy of colorectal cancer [21] and of glioblastoma multiforme [22], respectively, however using different questionnaires.
For patients with incurable metastatic RCC, the increase in life expectancy and, simultaneously, the maintenance of physical function are primary objectives of medical intervention. For many years, IL-2 and IFN-α either alone or in combination have been used as first-line therapies of metastatic RCC [23]. Higher doses of IL-2 and IFN-α can be associated with WHO grade III and IV toxicity and may even require intensive care [24]. In one of the few studies, which assessed QoL, a significant and relatively rapid reduction in all functioning and symptom scores except in cognitive function, dyspnoea and constipation was revealed using the QLQ-C30 questionnaire [25]. The study revealed that patients’ global quality of health status decreased from 64 to 41. Changes in QoL were mainly caused by a decrease in physical functioning (from 82 to 65), psychological distress (from 77 to 61), an impairment of social activities (from 78 to 55) and a limitation in working capacity (role functioning, from 82 to 58), respectively.
Another study investigating QoL during high-dose inhalation or intravenous IL-2 treatment also using the QLQ-C30 likewise revealed a significant reduction in QoL [26]. For 15 patients treated with inhalational IL-2, the mean QoL score deteriorated significantly 1 month after treatment initiation (15.1%, P = 0.01) followed by a gradual decline after 3, 6 and 9 months and a further deterioration after 12 months of treatment. Seven patients, who received intravenous IL-2, showed a more marked, almost statistically significant deterioration in the mean QoL score during treatment (27%, P = 0.06).
Today, tyrosine kinase inhibitors (TKIs) such as sunitinib and sorafenib as well as bevacizumab, a recombinant, humanised monoclonal antibody to VEGF, or mTOR inhibitors like temsirolimus and everolimus have replaced immunotherapy with cytokines in many instances [1]. An important point in evaluating these trials is that targeted therapies cause clinically significant toxic effects that require monitoring and management [2, 3]. Grade 3/4 adverse events occurred in up to 67% of the patients [2, 3].
Little QoL data are available for the new targeted therapies. Some authors used the Functional Assessment of Cancer Therapy-Kidney Symptom Index (FKSI) to define health-related QoL. Cella et al. analysed health-related QoL in patients treated with sunitinib or IFN-α [27, 28]. In both study groups, several patients had a dose interruption because of adverse events (38% in the sunitinib group vs. 32% in the IFN-α group). One-third of the sunitinib group had a dose reduction, compared with 21% patients in the IFN-α group. In view of the numerous adverse events of both sunitinib and IFN-α, sunitinib was favoured over IFN-α regarding QoL-related symptoms. Two studies have described the effects of sorafenib on symptoms and QoL, however, using a different questionnaire [29, 30] making a side-by-side comparison impossible. However, today it is undisputable that QoL deteriorates during the first 4 weeks of treatment with all targeted therapies [31].
Absence of substantial toxicity during DC-based immunotherapy eliminates the need for intensive patient monitoring and management, which was necessary in clinical trials using TKIs. Another advantage of DC vaccination relates to the fact that DCs are given as a single administration in monthly intervals, whereas treatment with targeted therapies is continuous over a prolonged period of time and thus represents a permanent burden. No patient receiving DC vaccination required additional treatment or prolonged hospitalisation.
In the present QoL study, particular functioning and symptom scores correlated with overall survival using Spearmans’ rank correlation (Fig. 3). Functioning scores like social and physical function, global health status and a good role function that includes the working capacity positively correlated with overall survival. The preservation of physical function and physical activity has a strong impact on QoL according to Trinh et al. [32]. On the other hand, particular symptom scores negatively correlated with overall survival. Above all, appetite loss and pain seemed to have a negative influence on survival. Furthermore, constipation and fatigue negatively correlated with survival. Interestingly, financial problems showed a significant negative correlation, too, though DC vaccination therapy was free of any charge for all patients. This may reflect existential fears and general fears that are associated with this life-threatening disease and can obviously aggravate the disease course.
Our QoL study confirms by specific measurements the general observation made by many investigators during the last decade that DC vaccination is well tolerated. In multiple studies worldwide, treatment with DCs induced very little autoimmunity. This may in part be due to the limited efficacy of the DC vaccines, for which the most effective conditions are still not completely identified. However, increased clinical efficacy, for instance, in the presence of TKIs [33] or CTLA-4 blockade (ipilimumab) [15], might induce clinically relevant autoimmunity, which may then also impact on QoL. This will be an important subject of investigation in future clinical trials. New well-designed clinical trials of DC vaccination should be developed to improve clinical efficacy while preserving the QoL of patients with metastatic RCC. In this context, intermittent sunitinib treatment, which—in contrast to sorafenib—does not impair immune system functions [34] alternating with DC vaccinations might be an attractive treatment concept for RCC.
Acknowledgments
This work was supported by the COMET Center ONCOTYROL, which is funded by the Austrian Federal Ministries BMVIT/BMWFJ (via FFG) and the Tiroler Zukunftsstiftung/Standortagentur Tirol (SAT). We further appreciate the participation of the TILAK hospital holding company, who serves as a partner in the Oncotyrol research programme. Oncotyrol approved the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Ravaud A, Wallerand H, Culine S, Bernhard JC, Fergelot P, Bensalah K, Patard JJ. Update on the medical treatment of metastatic renal cell carcinoma. Eur Urol. 2008;54:315–325. doi: 10.1016/j.eururo.2008.04.056. [DOI] [PubMed] [Google Scholar]
- 2.Di Lorenzo G, Porta C, Bellmunt J, Sternberg C, Kirkali Z, Staehler M, Joniau S, Montorsi F, Buonerba C. Toxicities of targeted therapy and their management in kidney cancer. Eur Urol. 2011;59:526–540. doi: 10.1016/j.eururo.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Hutson TE, Figlin RA, Kuhn JG, Motzer RJ. Targeted therapies for metastatic renal cell carcinoma: an overview of toxicity and dosing strategies. Oncologist. 2008;13:1084–1096. doi: 10.1634/theoncologist.2008-0120. [DOI] [PubMed] [Google Scholar]
- 4.Asemissen AM, Brossart P. Vaccination strategies in patients with renal cell carcinoma. Cancer Immunol Immunother. 2009;58:1169–1174. doi: 10.1007/s00262-009-0706-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Poppel H, Joniau S, Van Gool SW. Vaccine therapy in patients with renal cell carcinoma. Eur Urol. 2009;55:1333–1342. doi: 10.1016/j.eururo.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 6.Steinman RM. Lasker basic medical research award. Dendritic cells: versatile controllers of the immune system. Nat Med. 2007;13:1155–1159. doi: 10.1038/nm1643. [DOI] [PubMed] [Google Scholar]
- 7.Tacken PJ, de Vries IJ, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7:790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 8.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–147. doi: 10.1016/S0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 9.Berntsen A, Geertsen PF, Svane IM. Therapeutic dendritic cell vaccination of patients with renal cell carcinoma. Eur Urol. 2006;50:34–43. doi: 10.1016/j.eururo.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 10.Holtl L, Rieser C, Papesh C, Ramoner R, Bartsch G, Thurnher M. CD83+ blood dendritic cells as a vaccine for immunotherapy of metastatic renal-cell cancer. Lancet. 1998;352:1358. doi: 10.1016/S0140-6736(05)60748-9. [DOI] [PubMed] [Google Scholar]
- 11.Holtl L, Rieser C, Papesh C, Ramoner R, Herold M, Klocker H, Radmayr C, Stenzl A, Bartsch G, Thurnher M. Cellular and humoral immune responses in patients with metastatic renal cell carcinoma after vaccination with antigen pulsed dendritic cells. J Urol. 1999;161:777–782. doi: 10.1016/S0022-5347(01)61767-1. [DOI] [PubMed] [Google Scholar]
- 12.Holtl L, Zelle-Rieser C, Gander H, Papesh C, Ramoner R, Bartsch G, Rogatsch H, Barsoum AL, Coggin JH, Jr, Thurnher M. Immunotherapy of metastatic renal cell carcinoma with tumor lysate-pulsed autologous dendritic cells. Clin Cancer Res. 2002;8:3369–3376. [PubMed] [Google Scholar]
- 13.Holtl L, Ramoner R, Zelle-Rieser C, Gander H, Putz T, Papesh C, Nussbaumer W, Falkensammer C, Bartsch G, Thurnher M. Allogeneic dendritic cell vaccination against metastatic renal cell carcinoma with or without cyclophosphamide. Cancer Immunol Immunother. 2005;54:663–670. doi: 10.1007/s00262-004-0629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Draube A, Klein-Gonzalez N, Mattheus S, Brillant C, Hellmich M, Engert A, von Bergwelt-Baildon M. Dendritic cell based tumor vaccination in prostate and renal cell cancer: a systematic review and meta-analysis. PLoS One. 2011;6:e18801. doi: 10.1371/journal.pone.0018801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cha E, Fong L. Immunotherapy for prostate cancer: biology and therapeutic approaches. J Clin Oncol. 2011;29:3677–3685. doi: 10.1200/JCO.2010.34.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rieser C, Bock G, Klocker H, Bartsch G, Thurnher M. Prostaglandin E2 and tumor necrosis factor alpha cooperate to activate human dendritic cells: synergistic activation of interleukin 12 production. J Exp Med. 1997;186:1603–1608. doi: 10.1084/jem.186.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 18.Rieser C, Ramoner R, Holtl L, Rogatsch H, Papesh C, Stenzl A, Bartsch G, Thurnher M. Mature dendritic cells induce T-helper type-1-dominant immune responses in patients with metastatic renal cell carcinoma. Urol Int. 1999;63:151–159. doi: 10.1159/000030438. [DOI] [PubMed] [Google Scholar]
- 19.Leonhartsberger N, Ramoner R, Putz T, Gander H, Rahm A, Falkensammer C, Bartsch G, Thurnher M. Antigen-independent immune responses after dendritic cell vaccination. Cancer Immunol Immunother. 2007;56:897–903. doi: 10.1007/s00262-006-0245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hjermstad MJ, Fayers PM, Bjordal K, Kaasa S. Health-related quality of life in the general Norwegian population assessed by the European Organization for Research and Treatment of Cancer Core Quality-of-Life Questionnaire: the QLQ = C30 (+3) J Clin Oncol. 1998;16:1188–1196. doi: 10.1200/JCO.1998.16.3.1188. [DOI] [PubMed] [Google Scholar]
- 21.Burgdorf SK, Fischer A, Myschetzky PS, Munksgaard SB, Zocca MB, Claesson MH, Rosenberg J. Clinical responses in patients with advanced colorectal cancer to a dendritic cell based vaccine. Oncol Rep. 2008;20:1305–1311. [PubMed] [Google Scholar]
- 22.Ardon H, Van Gool S, Lopes IS, Maes W, Sciot R, Wilms G, Demaerel P, Bijttebier P, Claes L, Goffin J, Van Calenbergh F, De Vleeschouwer S. Integration of autologous dendritic cell-based immunotherapy in the primary treatment for patients with newly diagnosed glioblastoma multiforme: a pilot study. J Neurooncol. 2010;99:261–272. doi: 10.1007/s11060-010-0131-y. [DOI] [PubMed] [Google Scholar]
- 23.Negrier S, Escudier B, Lasset C, Douillard JY, Savary J, Chevreau C, Ravaud A, Mercatello A, Peny J, Mousseau M, Philip T, Tursz T. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. Groupe Francais d’Immunotherapie. N Engl J Med. 1998;338:1272–1278. doi: 10.1056/NEJM199804303381805. [DOI] [PubMed] [Google Scholar]
- 24.Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13:688–696. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 25.Atzpodien J, Kuchler T, Wandert T, Reitz M. Rapid deterioration in quality of life during interleukin-2- and alpha-interferon-based home therapy of renal cell carcinoma is associated with a good outcome. Br J Cancer. 2003;89:50–54. doi: 10.1038/sj.bjc.6600996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinzer H, Mir TS, Huland E, Huland H. Subjective and objective prospective, long-term analysis of quality of life during inhaled interleukin-2 immunotherapy. J Clin Oncol. 1999;17:3612–3620. doi: 10.1200/JCO.1999.17.11.3612. [DOI] [PubMed] [Google Scholar]
- 27.Cella D. Quality of life in patients with metastatic renal cell carcinoma: the importance of patient-reported outcomes. Cancer Treat Rev. 2009;35:733–737. doi: 10.1016/j.ctrv.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Cella D, Pickard AS, Duh MS, Guerin A, Mishagina N, Antras L, Neary MP, McCann L, Hodge R, Sternberg CN (2011) Health-related quality of life in patients with advanced renal cell carcinoma receiving pazopanib or placebo in a randomised phase III trial. Eur J Cancer [Epub ahead of print] [DOI] [PubMed]
- 29.Bukowski R, Cella D, Gondek K, Escudier B. Effects of sorafenib on symptoms and quality of life: results from a large randomized placebo-controlled study in renal cancer. Am J Clin Oncol. 2007;30:220–227. doi: 10.1097/01.coc.0000258732.80710.05. [DOI] [PubMed] [Google Scholar]
- 30.Miyake H, Kurahashi T, Yamanaka K, Kondo Y, Takenaka A, Inoue TA, Fujisawa M. Impact of sorafenib on health-related quality of life in Japanese patients with metastatic renal cell carcinoma: a prospective evaluation. BJU Int. 2010;106:1643–1647. doi: 10.1111/j.1464-410X.2010.09437.x. [DOI] [PubMed] [Google Scholar]
- 31.Cella D. Beyond traditional outcomes: improving quality of life in patients with renal cell carcinoma. Oncologist. 2011;16(Suppl 2):23–31. doi: 10.1634/theoncologist.2011-S2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trinh L, Plotnikoff RC, Rhodes RE, North S, Courneya KS. Associations between physical activity and quality of life in a population-based sample of kidney cancer survivors. Cancer Epidemiol Biomarkers Prev. 2011;20:859–868. doi: 10.1158/1055-9965.EPI-10-1319. [DOI] [PubMed] [Google Scholar]
- 33.Oosterwijk E, Rathmell WK, Junker K, Brannon AR, Pouliot F, Finley DS, Mulders PF, Kirkali Z, Uemura H, Belldegrun A. Basic research in kidney cancer. Eur Urol. 2011;60:622–633. doi: 10.1016/j.eururo.2011.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hipp MM, Hilf N, Walter S, Werth D, Brauer KM, Radsak MP, Weinschenk T, Singh-Jasuja H, Brossart P. Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood. 2008;111:5610–5620. doi: 10.1182/blood-2007-02-075945. [DOI] [PubMed] [Google Scholar]