Abstract
Monoamine transporters terminate synaptic neurotransmission and are molecular targets for antidepressants and psychostimulants. Fluorescent reporters can monitor real-time transport and are amenable for high-throughput screening. However, until now, their use has mostly been successful to study the catecholamine transporters but not the serotonin (5HT) transporter. Here, we use fluorescence microscopy, electrophysiology, pharmacology, and molecular modeling to compare fluorescent analogs of 1-methyl-4-phenylpyridinium (MPP+) as reporters for the human serotonin transporter (hSERT) in single cells. The fluorescent substrate 4-(4-(dimethylamino)phenyl)-1-methylpyridinium (APP+) exhibits superior fluorescence uptake in hSERT-expressing HEK293 cells than other MPP+ analogs tested. APP+ uptake is Na+- and Cl−-dependent, displaced by 5HT, and inhibited by fluoxetine, suggesting APP+ specifically monitors hSERT activity. ASP+, which was previously used to study catecholamine transporters, is 10 times less potent than APP+ at inhibiting 5HT uptake and has minimal hSERT-mediated uptake. Furthermore, in hSERT-expressing oocytes voltage-clamped to −60 mV, APP+ induced fluoxetine-sensitive hSERT-mediated inward currents, indicating APP+ is a substrate, whereas ASP+ induced hSERT-mediated outward currents and counteracted 5HT-induced hSERT currents, indicating ASP+ possesses activity as an inhibitor. Extra-precise ligand receptor docking of APP+ and ASP+ in an hSERT homology model showed both ASP+ and APP+ docked favorably within the active region; accordingly, comparable concentrations are required to elicit their opposite electrophysiological responses. We conclude APP+ is better suited than ASP+ to study hSERT transport fluorometrically.
Keywords: Fluorescence, In Vivo Imaging, Neurotransmitter Transport, Neurotransmitters, Transport, APP+, ASP+, SLCA4, Fluorescent Reporter, Transporter Uptake
Introduction
Serotonin (5HT)3 plays a role in the regulation of many behaviors (1, 2), and disturbances in the serotonergic system are implicated in a myriad of diseases (3–7). Following neurotransmission the serotonin transporter (SERT) clears 5HT from the synaptic cleft (8–10). Numerous antidepressants such as fluoxetine or citalopram inhibit re-uptake of 5HT by SERT (11), and drugs of abuse, such as 3,4-methylenedioxymethamphetamine (MDMA) induce reverse 5HT transport through the transporter (12). SERT is a member of the solute carrier 6 gene family consisting of numerous ion-coupled co-transporters, such as the norepinephrine and dopamine transporters (13–15). These transporters use the Na+ ionic electrochemical potential to concentrate substrates against their concentration gradient (15–18). Traditionally, co-transport is described by the alternating access model where ions and substrate are transported with a fixed stoichiometry (19, 20); however, a competing model describes a channel within the transporter capable of conducing currents mediated by substrate and ions. These substrate-induced uncoupled currents are found in many neurotransmitter transporters (21–25). Additionally, in the absence of substrate, a constitutive leak current exists, which, for SERT, can be uncovered with inhibitors such as fluoxetine (25).
Traditionally, monoamine transporter activity has been assessed by radiolabeled uptake assays; however, these biochemical assays have poor temporal resolution and do not yield spatial information of the transport process. Conversely, fluorescent substrates of monoamine transporters are advantageous tools to study mechanistic properties of SERT because they provide a continuous signal that can be measured with single live-cell imaging. In addition to the temporal and spatial advantages of fluorescent substrates, their use is amenable for high-throughput screening (27, 28).
Previously, we characterized 4-(4-(dimethylamino)styryl)-N-methylpyridinium (ASP+) as a fluorescent reporter for uptake activity for both the human norepinephrine transporter (hNET) and the human dopamine transporter (hDAT) and utilized it to study biophysical properties of hNET such as substrate-protein stoichiometry and substrate dwell time (29, 30). Although ASP+ was transported well by both hNET and hDAT, it was transported poorly by the human serotonin transporter (hSERT) (29–31). Therefore, we designed alternative fluorescent substrates of hSERT based on the structure of 1-methyl-4-phenylpyridinium (MPP+), a known monoamine transporter substrate that has high-affinity for transport by the catecholamine transporters (32–34). Although a fluorescent analog of MPP+ has been marketed, it was only tested as a reagent for high-throughput screening (28, 35). Moreover, many properties for this class of fluorescent MPP+ analogs are unknown, such as their electrophysiological profile, their interaction within the transporter, their subcellular localization, and their fluorescent characterization in single live-cell imaging. In this study, we tested several fluorescent MPP+ analogs, selected the most efficient fluorescent substrate for hSERT, 4-(4-(dimethylamino)phenyl)-1-methylpyridinium (APP+), and thoroughly characterized its fluorescent activity profile for hSERT in single cells.
Additionally, we compared the efficiency of APP+ to target hSERT against the fluorescent compound ASP+ that has previously been used to study hDAT and hNET (27, 29, 30, 36–38). We discovered several differences between the two fluorescent compounds. In particular, ASP+ exhibits negligible uptake through hSERT, and unlike APP+, ASP+ exhibits binding-associated fluorescence on the plasma membrane; however, this ASP+ signal in hSERT-expressing cells is indistinguishable from signal in parental cells. Furthermore, APP+ is much stronger at inhibiting uptake of radiolabeled 5HT as compared with ASP+. More strikingly, electrophysiological data indicate that ASP+ exhibits inhibitor-like behavior, whereas APP+ behaves as an hSERT substrate. Lastly, ligand receptor docking of the compounds in a homology model based on the crystallized bacterial leucine transporter (39) shows that both APP+ and ASP+ dock favorably within the active region of hSERT, which could explain their functional efficacy to induce their opposing electrophysiological effects on hSERT. These findings indicate APP+ is a better fluorescent substrate than ASP+ to study hSERT.
EXPERIMENTAL PROCEDURES
Maintenance of Parental and HEK293 Cells Stably Expressing hSERT (hSERT-HEK Cells)
Cells were prepared in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2 mm l-glutamine, penicillin (100 units/ml), streptomycin (100 μg/ml), and G418. Trypsin-released cells were plated in MatTek glass-bottomed dishes pretreated with poly-l-lysine (MatTek, Ashland, MA) at 100,000–150,000 cells per dish and allowed to grow for 24–48 h before measurements. The medium for parental HEK293 cells lacks G418.
Solutions for All Experiments Using hSERT-HEK Cells
Krebs-Ringer Hepes (KRH) buffer consists of the following: 120 mm NaCl, 1.3 mm KCl, 2.2 mm CaCl2, 1.2 mm MgSO4, 10 mm HEPES, and 1 g/liter glucose, pH 7.4. For the ionic dependence experiments, Na+ was replaced with equimolar choline, and Cl− was replaced with equimolar gluconate.
Fluorescence Image Acquisition
Experiments were performed at room temperature (23–25 °C) unless noted otherwise. hSERT-HEK or HEK293 cells were mounted on a Zeiss 510 confocal microscope (Zeiss) and focused with differential interference contrast. The culture medium was discarded, and the cells were mounted immediately on the microscope and refocused. As soon as image acquisition began, the desired treatment was applied. An argon laser tuned to 488 nm was used to excite APP+, its analogs, and ASP+. Emission filters used were 505–550 nm for APP+ and its analogs and 585–615 nm for ASP+. Gain (contrast) and offset (brightness) of the photomultiplier tube was set to avoid saturation at the highest fluorophore concentration. Microscopy was performed with a 10 × 0.8 numerical aperture water objective, a 40 × 1.3 numerical aperture water objective, or a 40 × 1.4 numerical aperture oil objective.
Total Fluorescence Intensity
The cellular spectra were acquired under “lambda” mode after exposure to each synthesized fluorescent compound for 5 min, in which emitted fluorescence was acquired at 10-nm intervals. To determine total fluorescence, the spectra curves are integrated.
Time-lapse Acquisition and Analysis
Time-lapses consisted of a series of images taken over a defined period of time (from 30 s to 10 min as indicated) with acquisition rates ranging from one image every second to one image every 5 s. Fluorescent image analysis was performed using LSM software, AIM (Zeiss). Fluorescence accumulation was measured as average pixel intensity within specified regions of interest identified in the differential interference contrast channel images, which represent individual cells. Fluorescence accumulation was averaged for at least 30 cells per time-lapse. The fluorescence intensity was plotted in arbitrary fluorescent units (AFU) over time. To determine Vmax, Vmin, km, and the Hill coefficient n, values were fitted to the Hill equation, y = Vmax + (Vmin − Vmax) × xn/(kn + xn) using Origin 8 (OriginLab Corp., Northampton, MA).
Plasma Membrane Co-localization and Line Scans
Co-localization studies were performed by incubating cells at 37 °C for 15 min with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI, Invitrogen) and co-applying 10 μm APP+ for 5 min. Then images were merged for the APP+ channel and DiI channel. DiI was excited with an argon laser tuned to 549 nm, and the emission filter used was 560–615 nm. Line scans were performed by selecting regions from the outer edges of cells as determined by DiI labeling of the plasma membrane.
Statistics
S.E. was calculated for fluorescence accumulation in all cells at each time point in a time-lapse, and S.E. for each plot are shown as merged Y-error bars.
Expression of hSERT in Xenopus Oocytes
Oocytes were harvested and prepared from adult Xenopus laevis females following standard procedures (40, 41). We selected stage V–VI oocytes for cRNA injection within 24 h of isolation. cRNA was transcribed from the pOTV vector using mMessage Machine T7 kit (Ambion Inc., Austin, TX). Each oocyte was injected with 30 ng of cRNA using a Nanoject AutoOocyteInjector (Drummond Scientific Co., Broomall, PA) and incubated at 18 °C for 5–10 days in Ringer's solution supplemented with sodium pyruvate (550 μg/ml), streptomycin (100 μg/ml), tetracycline (50 μg/ml), and 5% dialyzed horse serum.
Electrophysiology
We performed two-electrode voltage-clamp (TEVC) experiments as described previously (42). Recordings were done at room temperature (23–25 °C). Electrodes having resistances from 1–5 megohms are filled with 3 m KCl. X. laevis oocytes are voltage-clamped to −60 mV (unless otherwise noted) with a GeneClamp 500 (Axon Instruments), and the holding current was recorded using Clampex 10 (Axon Instruments). Standard extracellular buffer was perfused until stable baseline currents were obtained, followed by experimental drugs. The extracellular buffer consisted of the following: 120 mm NaCl, 7.5 mm HEPES, 5.4 mm potassium gluconate, 1.2 mm calcium gluconate, pH 7.4.
Competition Assay
Radiolabeled assay protocols were performed as described previously (43, 44). First, hSERT-HEK cells were plated on poly-l-lysine-coated, 24-well tissue culture plates at 10,000 cells per well for 2–3 days to obtain ∼90% cell confluence. Then, medium was removed by aspiration, and the hSERT-HEK cells were washed with room temperature KRH buffer. Cells were incubated for 10 min at 37 °C with mixtures of radiolabeled substrate, and the compounds were tested at a broad range of concentrations. The assay mixture was aspirated, and cells were washed three times with ice-cold (4 °C) KRH buffer. Lastly, cells were solubilized with ice-cold Ecoscint H (National Diagnostics), and the remaining [3H]5HT was measured using a scintillation counter.
Competition Assay Analysis and Cheng-Prusoff Correction
Concentration-response curves for APP+ and ASP+ inhibition of [3H]5HT uptake into hSERT-HEK cells were used to obtain the IC50. Briefly, [3H]5HT accumulation was measured in hSERT-HEK cells in the presence of increasing APP+ and ASP+ concentrations, and these values were normalized to the highest [3H]5HT reading, which is the value in the absence of competing compound. The data were fitted to the Hill equation, y = Vmax + (Vmin − Vmax) × xn/(kn + xn) (Origin 8), and to correct for substrate concentration, the Cheng-Prusoff equation was employed: ki = IC50/(1 + [S]/km), where ki is the binding affinity of the inhibitor, IC50 is the functional strength of the inhibitor, [S] is the substrate concentration, and km is the affinity of the substrate for the enzyme.
RESULTS
Fluorescent MPP+ Analogs
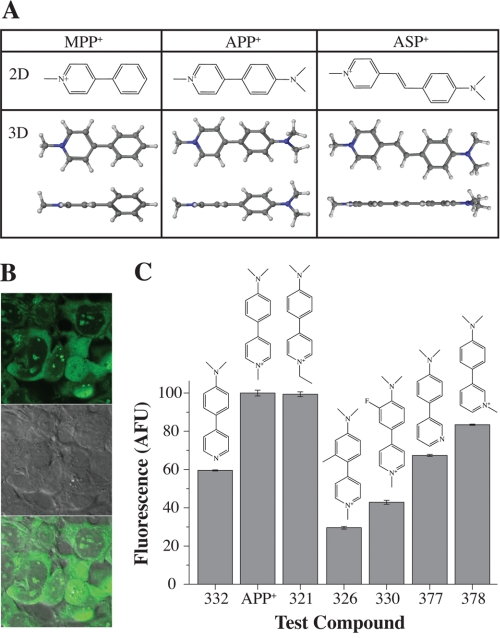
Addition of an electron donating dimethyl amino group to the phenyl ring of MPP+ resulted in a fluorescent compound called 4-(4-(dimethylamino)phenyl)-1-methylpyridinium (APP+) (Fig. 1A, top row), which was transported by hSERT-expressing HEK293 (hSERT-HEK) cells (Fig. 1B). APP+ co-localizes with MitoTracker and SYTO-17, indicating APP+ stains mitochondria and nucleoli, respectively (supplemental Fig. 1). The fluorescence intensity for APP+ and a series of APP+ analogs was measured in hSERT-HEK cells. APP+ and compound 321 emitted the most fluorescence, whereas compounds 326, 330, and 375 emitted the weakest fluorescence (Fig. 1C). All tested APP+ analogs displayed similar fluorescence localization inside hSERT-HEK cells, and peak emission was measured at 520–525 nm, with the highest emission peak seen with APP+ (supplemental Fig. 2). We pursued characterization of APP+ to assess its utility to study hSERT activity in single cells.
FIGURE 1.
Structures of MPP+ and its fluorescent analogs APP+ and ASP+ and screening for a fluorescent substrate of hSERT. A, the addition of an electron donating dimethyl amine group to the phenyl ring of MPP+ results in the fluorescent compound APP+, which can be compared with ASP+, the previously characterized fluorescent substrate for hNET (chemical structures shown in two-dimensional (2D) views, top row). Two three-dimensional (3D) views showing energy minimized MPP+, APP+, and ASP+ (bottom row). Although the aromatic rings in APP+ do not attain a co-planar conformation and favor twisted dihedral angles, ASP+ adopts a co-planar conformation. B, images of hSERT-HEK cells exposed to fluorescent compounds (from top to bottom: APP+ only, differential interference contrast, and APP+ and differential interference contrast merged). C, fluorescence intensity was calculated by integrating emission spectra curves for each compound. Fluorescence was normalized to the brightest compound, APP+. Structure nomenclature: 332, N,N-dimethyl-4-(pyridin-4-yl)aniline; APP+, 4-(4-(dimethylamino)styryl)-N-methylpyridinium; 321, 4-(4-(dimethylamino)phenyl)-1-ethylpyridinium; 326, 4-(4-(dimethylamino)-2-methylphenyl)-1-methylpyridinium; 330, 4-(4-(dimethylamino)-3-fluorophenyl)-1-methylpyridinium; 377, N,N-dimethyl-4-(pyridin-3-yl)aniline; 378, 3-(4-(dimethylamino)phenyl)-1-methylpyridinium.
APP+ Displays Two Distinct Fluorescence Accumulation Rates
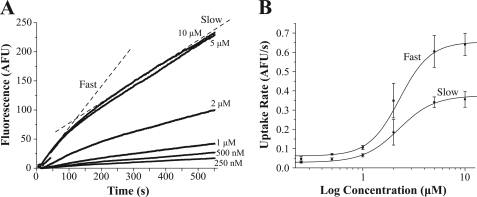
We first established a concentration-response curve of APP+ by performing time-lapses of hSERT-HEK cells exposed to APP+ at concentrations ranging from 250 nm to 10 μm (Fig. 2A). APP+ fluorescence accumulation yielded a biphasic plot that corresponds with two distinct rates of fluorescence accumulation, an initial “fast” phase and a secondary “slow” phase, indicated by dotted lines over the 10 μm APP+ accumulation curve (Fig. 2A). To compare the rates of APP+ fluorescence accumulation, we fitted straight lines to the slow and fast components for each concentration, plotted these slope values against concentration, and then fitted to the Hill equation. The Vmax and km for the fast phase were 0.65 ± 0.07 AFU/s and 2.29 ± 0.65 μm, respectively, and for the slow phase the Vmax and km were 0.38 ± 0.04 AFU/s and 2.36 ± 0.55 μm, respectively (Fig. 2B). The Hill coefficient was 2.91 ± 1.09 for the fast component and 2.53 ± 0.68 for the slow component.
FIGURE 2.
APP+ displays two rates of uptake. A, APP+ accumulation depends on concentration. Time-lapses of hSERT-HEK cells exposed to different concentrations of APP+ (from 250 nm to 10 μm) were acquired at a rate of one image per second for 10 min. Dotted lines represent the two rates observed, the initial (slow) and subsequent (fast) components. B, to determine the Vmax and km, straight lines were fitted, and slopes were obtained for both the slow and fast phases acquired at each concentration. For the fast phase, measurements from 20–100 s were used, and for the slow phase, the measurements from 200 s to the end of the acquisition were used. Slopes were plotted against concentration and these values were fit to the Hill equation, y = Vmin + (Vmax − Vmin) × xn/(kn + xn). The Vmax, Vmin, and km for the fast phase were 0.65 ± 0.07 AFU/s, 0.062 ± 0.008 AFU/s, and 2.29 ± 0.65 μm, respectively, and for the slow phase, the Vmax,Vmin, and km were 0.38 ± 0.04 AFU/s, 0.029 ± 0.003 AFU/s, and 2.36 ± 0.55 μm, respectively. The Hill coefficient was 2.91 ± 1.09 for the fast component and 2.53 ± 0.68 for the slow component.
APP+ Is a Substrate for hSERT
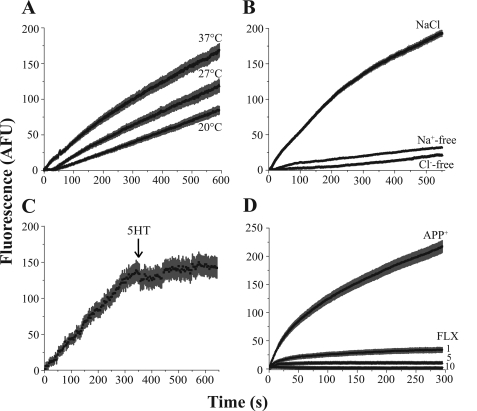
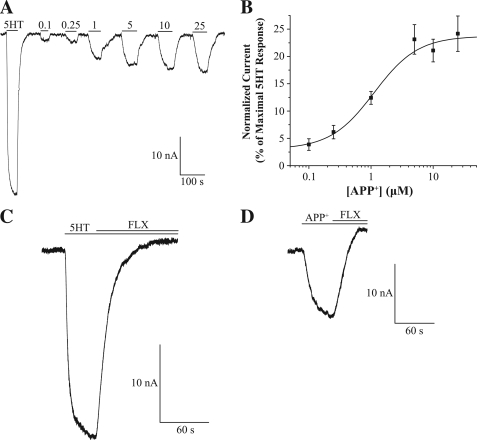
To characterize APP+ as a suitable substrate to study hSERT activity in single cells, we performed a series of time-lapse experiments measuring 2 μm APP+ accumulation. Because monoamine transporter uptake is temperature-sensitive (45), we measured APP+ accumulation at 20, 27, and 37 °C. As expected, temperature induced altered APP+ accumulation by hSERT, which was more noticeable for the initial fast uptake rate (Fig. 3A). Removal of Na+ or Cl− eliminated APP+ accumulation in hSERT-expressing cells (Fig. 3B), which is in agreement with the ionic dependence for monoamine transporter uptake (8, 46). Furthermore, application of the endogenous hSERT substrate 5HT (10 μm) while measuring APP+ accumulation induced an immediate decrease in APP+ accumulation, which suggests APP+ uptake is mediated by hSERT (Fig. 3C). Lastly, to verify that APP+ is specifically transported through hSERT, we measured APP+ accumulation in the presence of the specific hSERT inhibitor fluoxetine. Although the low fluoxetine concentration (1 μm) only partially diminished the APP+ signal, the higher fluoxetine concentrations (5–10 μm) completely abolished APP+ uptake (Fig. 3D).
FIGURE 3.
APP+ is a fluorescent substrate of hSERT. Time-lapses of hSERT-HEK cells exposed to 2 μm APP+. A, temperature regulates APP+ accumulation. APP+ fluorescence accumulation is greater when measured under physiological (37 °C) temperature, and much lower at 20 °C as compared with the moderate (27 °C) temperature. B, removal of both Na+ and Cl− diminished APP+ accumulation. C, an immediate decrease in APP+ accumulation occurs when 5HT (10 μm) was added 6 min into the time-lapse (indicated by arrow). D, co-treatment with fluoxetine (FLX; from 1 to 10 μm) abolishes APP+ fluorescence accumulation.
ASP+ Is Less Efficient Than APP+ at Targeting hSERT
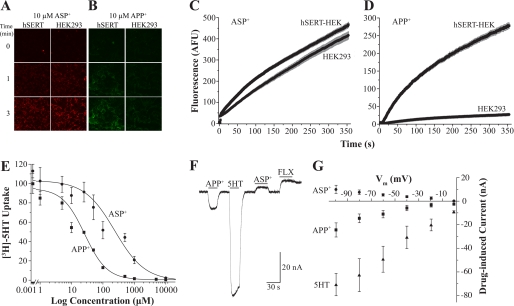
We have demonstrated that APP+ is an adequate fluorescent substrate for hSERT in single cells. However, because ASP+ has not been thoroughly studied as an hSERT fluorescent substrate, we sought to test APP+ against ASP+ under similar conditions. ASP+ did not elicit visible levels of fluorescence in hSERT-HEK cells until 10 μm or higher concentrations were employed; however, the signal elicited at these ASP+ concentrations is similarly bright in both hSERT-HEK and parental cells and falls within the noise for all time points measured (Fig. 4A, [ASP+] = 10 μm). In addition, ASP+ fluorescence accumulation rates were parallel between hSERT-HEK and parental cells for all concentrations tested (1–30 μm), indicating nonspecific ASP+ uptake (Fig. 4C, [ASP+] = 10 μm). Conversely, the APP+ (10 μm) signal appears much brighter in hSERT-HEK cells than in parental cells (Fig. 4B), and APP+ (2 μm) fluorescence uptake is greater in hSERT-HEK cells as compared with parental cells (Fig. 4D). These discernable differences are clearly observed at APP+ concentrations from 1–10 μm (data not shown). To assess the affinity of APP+ and ASP+ at hSERT, we produced concentration-response curves for inhibition of [3H]5HT uptake in hSERT-HEK cells with increasing APP+ or ASP+ concentrations (Fig. 4E). Fitting to the Hill equation and subsequent correction for substrate concentration with the Cheng-Prusoff equation yielded ki of 19.7 ± 2.23 μm for APP+ and a weaker ki of 180.1 ± 20.3 μm for ASP+. For comparison, the ki for 5HT is reported as 1.7 ± 1 μm (47). The Hill coefficients for inhibition of [3H]5HT uptake were 1.23 ± 0.08 for APP+ and 0.91 ± 0.15 for ASP+.
FIGURE 4.
Comparing the effects of ASP+ and APP+ on hSERT. A and B, images of hSERT-HEK or parental HEK293 cells before (row labeled with 0) and after exposure to 10 μm ASP+ (A) or APP+ (B) for 1 or 3 min. ASP+ fluoresces red and labels the exterior membrane of cells in both hSERT-HEK and parental cells, whereas APP+, which fluoresces green, seems to accumulate only inside of hSERT-HEK cells. C and D, time-lapses comparing fluorescence accumulation rates between hSERT-HEK and HEK293 cells for ASP+ (30 μm; C) and APP+ (5 μm;D). E, concentration-response curve for APP+ and ASP+ inhibition of [3H]5HT uptake into hSERT-HEK cells. [3H]5HT accumulation was measured in hSERT-HEK cells in the presence of increasing APP+ and ASP+ concentrations and normalized to data in the absence of the competing substrate. The data were fit to the Hill equation, y = Vmax + (V0 − Vmax) × xn/(kn + xn), and ki values were determined using the Cheng-Prusoff equation to correct for substrate concentration. The fits yield ki (APP+) = 19.7 ± 2.23 μm and ki (ASP+) = 180.1 ± 20.3 μm. Values are represented as means ± S.E. (n = 3). The Hill coefficients for ASP+ and APP+ are 0.91 ± 0.15 and 1.23 ± 0.08, respectively. F, electrophysiological effect of substrates on hSERT. Currents in an hSERT-expressing X. laevis oocyte clamped to −60 mV are measured in response to 10 μm 5HT, APP+, ASP+, and 1 μm fluoxetine (FLX). Bars display perfusion duration of each compound. G, the effect of voltage (from 0 to −100 mV) on hSERT-induced currents. Currents induced by 5HT, APP+, ASP+ are plotted relative to the baseline set as 0 at each potential (n = 4). Control (uninjected) oocytes show no response to 5HT, ASP+, or APP+ (not shown).
APP+ Acts as an hSERT Substrate, whereas ASP+ Behaves Like an hSERT Inhibitor
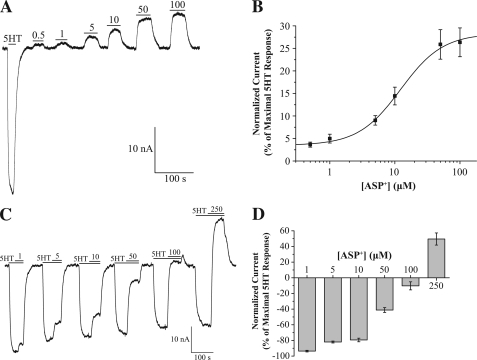
Because transporter currents reflect the effect of substrates and inhibitors, we employed TEVC and measured hSERT currents in Xenopus oocytes in response to 10 μm 5HT, APP+, ASP+, and 1 μm fluoxetine (holding potential = −60 mV). Although APP+ induced an hSERT-mediated inward current, similar in effect to the endogenous substrate of hSERT, 5HT, ASP+ induced an hSERT-mediated outward current, which is similar to the effect the hSERT inhibitor fluoxetine exerts on hSERT (Fig. 4F). Control oocytes (not expressing hSERT) do not induce currents when exposed to 5HT, ASP+, APP+, or fluoxetine (data not shown). Voltage dependence was assessed by stepping the holding potential in oocytes (from −100 to 0 mV), and measured APP+- and ASP+- induced inward and outward hSERT-mediated currents, respectively, which are plotted as drug-induced currents relative to baseline set to 0 (Fig. 4G). A concentration-response curve of hSERT currents in response to APP+ was produced by applying from 0.1 to 25 μm APP+ to hSERT-expressing oocytes (Fig. 5A), and average data from several recordings were fit to the Hill equation, which yielded km = 1.13 ± 0.28 μm and n = 1.23 ± 0.60 (Fig. 5B). The hSERT inhibitor fluoxetine (1 μm) blocked inward currents produced in response to 2 μm 5HT and 10 μm APP+ (compare Fig. 5, C and D). A concentration-response curve for ASP+ was determined using concentrations from 0.5 to 100 μm (Fig. 6A), and fitting average data from several recordings yielded km = 12.25 ± 2.71 μm and n = 1.34 ± 0.31 (Fig. 6B). Because the hSERT-mediated outward current induced by ASP+ resembles the action of transporter inhibitors, we tested its utility as an inhibitor. Application of ASP+ (1–250 μm) inhibited 5HT-induced hSERT-mediated currents in a dose-dependent manner, and notably, ASP+ imposed an outward current at the highest ASP+ concentration tested (Fig. 6, C and D).
FIGURE 5.
APP+ exhibits substrate-like activity at hSERT. A, APP+ concentration-response curve. Representative trace of hSERT currents are measured in response to APP+ (0.1–25 μm) applied to hSERT-expressing X. laevis oocyte clamped to −60 mV. B, summary data were normalized to 5 μm 5HT-induced currents and fit to the Hill equation, y = Imax + (Imin − Imax) × xn/(kn + xn) (n = 8). The Imax, Imin, km, and Hill coefficient were 23.75 ± 2.51, 3.02 ± 2.08, 1.13 ± 0.28 μm, and 1.23 ± 0.60, respectively. C, application of fluoxetine (FLX; 1 μm) blocks the 5HT-induced hSERT current ([5HT] = 2 μm). D, application of fluoxetine (1 μm) blocks the APP+-induced hSERT current ([APP+] = 10 μm).
FIGURE 6.
ASP+ exhibits inhibitor-like activity at hSERT. A, ASP+ concentration-response curve. Representative trace of hSERT currents are measured in response to APP+ (0.5–100 μm) applied to hSERT-expressing X. laevis oocyte clamped to −60 mV. B, summary data were normalized to 2 μm 5HT-induced currents and fit to the Hill equation, y = Imax + (Imin − Imax) × xn/(kn + xn) (n = 8). The Imax, Imin, km, and Hill coefficient were 28.32 ± 2.17, 3.48 ± 0.44, 12.25 ± 2.71 μm, and 1.34 ± 0.31, respectively. C, ASP+ inhibits 5HT-induced hSERT currents. During currents induced by 5HT (2 μm), ASP+ is co-applied at indicated concentrations (from 1 to 250 μm, as indicated above upper bars in traces) (n = 8). D, summary data of ASP+ inhibition of 5HT-induced hSERT currents (from currents in C).
APP+ and ASP+ Do Not Display Measureable Fluorescence on hSERT
Due to the inherent ability ASP+ possesses to fluoresce while interacting at hNET, we previously were able to take advantage of ASP+ to study ASP+-hNET stoichiometry and measure the residence time of ASP+ on hNET before being transported (30). These studies were successful because the ASP+ signal displayed on the plasma membrane of hNET-expressing cells was sensitive and specific over parental cells (29, 30). Although we were able to mimic the initial rapid ASP+ binding phase (previously observed in hNET-expressing cells) in hSERT-HEK cells (supplemental Video 1, labeled with an arrow in supplemental Fig. 3A, and clearly seen 10 s after ASP+ application, supplemental Fig. 3A, inset), these ASP+ concentrations that elicited signal on the plasma membrane (10 μm and higher) displayed comparable fluorescence binding in parental cells (Fig. 4, A and C). Furthermore, pretreatment of hSERT-HEK cells with the hSERT inhibitor paroxetine did not quell ASP+ plasma membrane fluorescence (supplemental Fig. 4). These data strongly imply that ASP+ is not suitable to discern plasma membrane labeling in hSERT-HEK cells solely attributed to specific ASP+ interaction on hSERT.
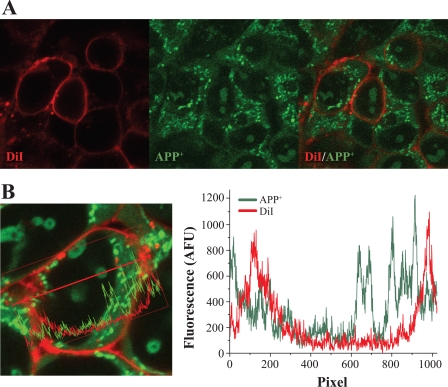
We next studied whether APP+ would elicit specific signal at the plasma membrane of hSERT-HEK cells. Because we had noticed that APP+ time-lapses (at a rate of 1 Hz) displayed no rapid fluorescent signal associated with membrane binding (Figs. 2A, 3, and 4D), we tried to resolve a potential initial binding phase on the plasma membrane that may not be discernable at slow image acquisition rates; however, even increasing the image acquisition rate to 5 Hz failed to yield a plasma membrane binding phase (arrow in supplemental Fig. 3B). Interestingly, APP+ fluorescence increased diffusely inside the cells without any visible APP+ fluorescence while being transported by hSERT (supplemental Video 2, and supplemental Fig. 3B, inset, after 10 s of APP+ exposure). Another attempt to determine whether APP+ yields any visible fluorescence at the plasma membrane was to establish APP+ co-localization with the lipophilic plasma membrane marker DiI in hSERT-HEK cells; however, no overlap between the two signals was detected (Fig. 7A). To look for APP+/DiI co-localization in more detail, dual-channel line scans through individual cells were performed, but no discernable co-localization was obtained, as indicated by asynchronous peaks (Fig. 7B, plot) corresponding to APP+ and DiI fluorescence intensity along a line scan through an individual hSERT-HEK cell (Fig. 7B, red arrow). Subsequently, we sought co-localization of DiI to additional APP+ analogs that had been shown to induce hSERT-mediated outward currents in TEVC recordings (data not shown), which suggests they bind to hSERT in a manner similar to inhibitors. However, neither compound produced signal at the plasma membrane as shown by their lack of co-localization with DiI (supplemental Fig. 5). In another effort to detect APP+ binding at hSERT, we employed the hSERT mutant C109A/G338C that has been shown to lack 5HT transport while maintaining similar substrate binding affinity (48). Because the strong intracellular fluorescence due to APP+ transport would be absent, any fluorescent signal measured would be attributed to APP+-mutant hSERT interaction. Following exposure to 3 μm APP+ for 5 min, cells transiently transfected with wild-type hSERT cells exhibited bright fluorescence signal; conversely, hardly any APP+ signal was observed in cells transiently transfected with C109A/G338C hSERT or in parental cells (supplemental Fig. 6, top row). Lastly, increasing APP+ concentration to 50 μm did not elicit a fluorescent signal on the plasma membrane in either parental cells or cells transiently transfected with wild-type or C109A/G338C hSERT (supplemental Fig. 6, bottom row). As expected, 50 μm APP+ entry was comparable in all conditions tested, which is explained by nonspecific diffusion or by the presence of endogenous organic cation transporters, which transport substrates, such as MPP+ and ASP+, with low affinity and high capacity (49–53).
FIGURE 7.
APP+ is not fluorescent at the plasma membrane. A, plasma membrane of hSERT-HEK cells is labeled with DiI (red, left) and APP+ (green, middle) is added to cells to determine co-localization (DiI and APP+ merged, right). B, line scan (red arrow) through an hSERT-HEK cell with APP+/DiI (left) and plotted fluorescence intensity of line scan through cell (right).
Docking of APP+ and ASP+ to hSERT
We sought to examine how the structural difference between APP+ and ASP+ affects their interaction with hSERT by utilizing a homology model of hSERT based on the crystal structure of LeuTAa (39), and employing extra-precise ligand-receptor docking, which yields information about how tightly compounds dock within the active region of hSERT. Docking was performed with APP+ and ASP+ at their low energy state (supplemental Fig. 7). Although 5HT, APP+, and ASP+ all docked favorably within the established active region of hSERT (supplemental Fig. 8, A–C) with respective G-scores of −10.95, −7.61, and −8.90 kcal/mol (values for energies measured are shown in supplemental Table 1), only 5HT displays significantly improved docking over both APP+ and ASP+, whereas the difference in binding scores between APP+ and ASP+ is within uncertainty of the method. Zoomed images display interactions of docked 5HT, APP+, and ASP+ at their most energetically favorable positions to residues within the active region of hSERT (supplemental Fig. 8, D–F). The hSERT amino acids whose side chains are within 3 Å or less of docked compounds (listed in supplemental Table 2) are mostly from transmembrane helices 1, 3, 6, and 8, which have been shown to form the active region where substrates bind (39).
DISCUSSION
Fluorescent monoamine transporter substrates provide a significant technical improvement to study substrate transport and binding over traditional radiolabeled substrate uptake assays. Fluorescent substrates in conjunction with confocal microscopy enable the measurement of real-time activity in individual cells. When we first established the fluorescent compound ASP+ as a substrate for monoamine transporters, we determined the substrate-to-transporter stoichiometry and the residence time of ASP+ on hNET before being transported (29, 30, 37). Subsequently, ASP+ was successfully utilized to study the regulation of hDAT activity by several dopamine receptors (36, 38). In addition, further studies validated the use of ASP+ as a useful fluorescent substrate amenable for high-throughput methods for which ASP+ uptake by hNET was effective with low μm affinity (27, 31). However, ASP+ had marginal effectiveness as an hSERT substrate; in fact, to see substantial hSERT-mediated uptake incubation for long time periods was required. In one instance, measurements taken 10–60 min after hSERT-expressing cells were incubated with ASP+ yielded an uptake km between 9.9 and 20 μm (54). Our previous research also indicated ASP+ was very weak at labeling hSERT-expressing cells (29). Therefore, it was unknown what the utility of ASP+ as a fluorescent hSERT reporter would be in real-time measurements, and the development for improved fluorescent hSERT substrates was evident.
In this work, we identified APP+ from a series of synthesized fluorescent MPP+ analogs and thoroughly characterized its utility as an hSERT substrate. The fluorescent APP+ uptake rate by hSERT resembles the ASP+ uptake phase by hNET observed previously (29). Furthermore, affinity values for APP+ uptake by hSERT are 5 to 10 times better than the reported km for ASP+ as an hSERT substrate (54). This difference in affinity is consistent with TEVC recordings where APP+ induces hSERT-mediated inward currents with a km that is roughly 10 times better than the km for ASP+-induced hSERT-mediated outward currents. In agreement, the radiolabeled substrate uptake competition assay that we performed shows APP+ has nearly a 10-fold better ki than ASP+ when inhibiting [3H]5HT uptake through hSERT. By all of our measures, APP+ is superior to ASP+ at targeting hSERT.
Action of APP+ on hSERT
Consistent with activity as an hSERT substrate, APP+ behaves similarly to the endogenous hSERT substrate 5HT. The km for APP+ fluorescence uptake by hSERT is comparable with the reported km for [3H]5HT uptake (55, 56). In agreement, 5HT and APP+ display similar affinity when evoking hSERT-mediated currents. Because inward currents by hSERT are observed only in response to transported substrates, such as 5HT, MPP+, or MDMA the presence of APP+-induced hSERT-mediated inward currents supports that APP+ is a substrate of hSERT. Although the km values for APP+ fluorescence uptake and APP+-induced hSERT currents are comparable (∼2.3 and 1.13 μm, respectively), the APP+ ki for [3H]5HT uptake inhibition is much weaker (nearly 20 μm). This discrepancy in affinity can be explained by the assay employed. Both APP+ uptake and APP+-induced currents are measured with only the substrate present; on the other hand, the [3H]5HT inhibition assay requires the presence of 5HT, which could confer an alternate conformational state of hSERT, and in turn alter the interaction between APP+ and hSERT.
Source of APP+ Fluorescence
To perform biophysical studies for hSERT similar to ones we previously performed for hNET, the fluorescent substrate for hSERT should display fluorescence on the plasma membrane while interacting with the transporter. Hence, we sought to identify APP+ fluorescence on the plasma membrane of hSERT-HEK cells but were unsuccessful in all our attempts, which included co-localization studies using DiI and additional APP+ analogs, and experiments employing an hSERT mutant. The absence of fluorescence of APP+ and its analogs while interacting with the transporter may be explained by the physical properties of this class of compounds. APP+ and its analogs are “twisted” intramolecular charge transfer state-forming (TICT) compounds (57), which display energy emission under specific structural conditions. For APP+ to be fluorescent, the phenyl and pyridyl rings must assume a co-planar conformation. Molecular modeling studies have suggested that the lowest energy conformer of APP+ is in the twisted conformation (Fig. 1A, and supplemental Fig. 7), and it is likely that this conformation is the most abundant conformer in an aqueous environment. Because the co-planar conformer may become more energetically favorable when the molecule binds to intracellular biomolecules such as proteins or DNA, APP+ will fluoresce only after entering cells. It is likely that the co-planar APP+ conformer intercalates between the base pairs of DNA and RNA because this conformer would produce π-π stacking interactions with nucleic acid base pairs and confer π-π stacking interactions among nucleic acid base pairs. Intriguingly, the lack of APP+ fluorescence on the plasma membrane of hSERT-expressing cells suggests the co-planar fluorescent state of APP+ is not achieved during transport by hSERT. Instead, APP+ fluorescence accumulation in hSERT-HEK cells seems diffuse in the cytosol and gathers preferentially at mitochondria and nucleoli. It is well known that MPP+ and its analogs exert their neurotoxic effects by disrupting the electron transport chain at mitochondria (58–60), which supports the preference APP+ (an MPP+ analog) has to stain mitochondria. In addition, because the membrane potential of mitochondria is highly negative (61, 62), positively charged compounds such as APP+ would display favorable mitochondrial accumulation. Nucleoli are the most dense compartments in the nucleus consisting of tightly packed DNA, RNA, and proteins and were recently shown to exhibit the slowest rate of protein diffusion, which implies limited mobility (63). Perhaps this dense and spatially restricted environment within nucleoli favors APP+ accumulation and subsequent binding to nucleic acids in its fluorescence-emitting conformation.
It is worth noting that we observed minimal reversal of APP+ signal in response to 5HT application (Fig. 3C). As mentioned, for APP+ to display fluorescence, it must abide to a rigid co-planar conformation, which occurs when it binds to specific regions in the cell. Thus, for transported hSERT substrates such as 5HT, or releasing agents such as MDMA to induce hSERT-mediated APP+ efflux, they would need to displace bound APP+, which requires affinity at the same binding sites. Furthermore, if APP+ intercalates the base pairs in DNA and RNA, it would form strong hydrophobic interactions between the base pairs of DNA and RNA. The intrinsic positive charge of APP+ might form charge-charge interactions between the positively charged pyridyl nitrogen and the phosphate backbone of these polymers. Because these interactions are energetically favorable, they would result in strong binding, making it very difficult for APP+ to be displaced from nucleic acids. Lastly, if bound APP+ could be displaced, it might bind to other subcellular compartments before undergoing hSERT-mediated efflux. Further studies employing strong releasing compounds are warranted.
The Hill coefficient for APP+ fluorescence accumulation through hSERT ranged from 2.5 to 2.9 (depending on accumulation rate), which is in contrast to the Hill coefficient obtained from TEVC recordings and the [3H]5HT uptake inhibition assay (1.23). APP+ fluorescence accumulation consists of several processes that could influence the Hill coefficient, including rate of uptake through hSERT, distinct accumulation sites within the cell that are at different distances from the entry point (besides displaying fluorescence at nucleoli and mitochondria, APP+ emits diffuse fluorescence in the cytosol), manner of APP+ incorporation into subcellular compartments, and the mechanism whereby APP+ abides to the co-planar conformation. On the other hand, TEVC recordings and the [3H]5HT uptake inhibition assay measure single processes by hSERT (currents mediated primarily by Na+ or [3H]5HT uptake), which are the limiting step. Because these measures are solely dependent on hSERT activity, a Hill coefficient near unity seems reasonable.
Action of ASP+ on hSERT
Although ASP+ does not report specific hSERT uptake or binding, our study demonstrates ASP+ interacts with hSERT. Most striking is the result in TEVC oocyte recordings showing ASP+ induces hSERT-mediated outward currents, which resemble the outward currents induced by hSERT inhibitors such as fluoxetine (25) and are usually interpreted as the inhibition of the constitutive inward leak current seen by many transporters (24, 64). However, ASP+ proves to be very poor when acting as an inhibitor, as demonstrated by the high ASP+ concentrations required to inhibit 5HT-induced hSERT currents, and the poor ki obtained for ASP+ inhibition of [3H]5HT uptake. Still, we cannot rule out that ASP+ is transported through hSERT and that its interaction with hSERT is distinct from bona fide hSERT inhibitors. ASP+ could even serve dual functions at hSERT, as both an inhibitor (in electrophysiology measurements) and a substrate (in uptake measurements), albeit likely having a very slow uptake rate. To determine whether a fraction of ASP+ is taken up through hSERT, radiolabeled ASP+ could be employed in uptake assays. Interestingly, ASP+ is effectively transported by hNET and electrophysiology experiments show ASP+ elicits inward currents through hNET (30). The distinct effect of ASP+ as an inhibitor on hSERT and as a substrate on hNET highlights the structural and functional differences between these two monoamine transporters.
The difference observed in affinity for ASP+ on hSERT in the [3H]5HT competition assay versus TEVC oocyte recordings could be explained by the existence of two distinct binding sites, the established substrate-binding active region and the recently discovered secondary antidepressant binding site in the extracellular vestibule of the homologous LeuT (65–67), which has been further substantiated for SERT (56, 68). ASP+ might not be readily accessible to the substrate-binding site, and hence, it is weak at displacing 5HT, whereas it can induce an hSERT-mediated outward current at a much lower concentration possibly because in the absence of 5HT, hSERT is at a conformation that provides easy access for ASP+ at the outer antidepressant-binding site. Supporting this possibility are recent studies showing that two structurally dissimilar classes of drugs, the tricyclic antidepressants and the selective serotonin reuptake inhibitors, interact at this promiscuous binding site (67, 68).
Docking to hSERT Homology Model
To understand the interaction of APP+ and ASP+ with hSERT, we performed a docking study, which yielded favorable docking scores for 5HT, APP+, and ASP+ at the active region of hSERT, and agrees well with the electrophysiology data, in which, when applied individually, 5HT, APP+, and ASP+ induce visible hSERT-mediated currents at low μm concentrations. The hSERT model predicts amino acid side chains in proximity to docked substrates within the active region. Of interest are residues that displayed interactions with the three compounds tested in our model that are involved in substrate or antidepressant affinity to hSERT, including Tyr-95, Ile-172, Ala-169, and Ser-438 (26, 56, 69, 70). Additionally, although 5HT shares 80% of hSERT interacting residues with APP+, it only shares ∼57% residues to docked ASP+ (supplemental Table 2). The similarity between 5HT and APP+ interaction with hSERT is consistent with comparable affinity at hSERT and their action as transported substrates. On the other hand, ASP+, which interacts with distinct residues than 5HT in the hSERT model, has the weakest affinity for hSERT in all the assays performed in this study, and it exhibits minimal transport. We speculate that the interactions a docked compound makes to residues in this model can help predict whether the compound will function as a substrate or inhibitor.
In conclusion, we have established that APP+ is a suitable fluorescent substrate to study hSERT uptake activity in single cells, which may introduce new protocols to study hSERT transport in real-time.
Supplementary Material
Acknowledgment
We thank Dr. Jose M. Eltit for valuable input in the writing of this work.
This work was supported by National Institutes of Health Grants 1RC1DA028112-01, DA026947-01A1, EB003728, and 5RO3MH61874-02.
- 5HT
- serotonin
- MPP+
- 1-methyl-4-phenylpyridinium
- APP+
- 4-(4-(dimethylamino) phenyl)-1-methylpyridinium
- ASP+
- 4-(4-(dimethylamino) styryl)-N-methylpyridinium
- AFU
- arbitrary fluorescent units
- SERT
- serotonin transporter
- hSERT
- human serotonin transporter
- hNET
- human norepinephrine transporter
- hDAT
- human dopamine transporter
- DiI
- 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- TEVC
- two-electrode voltage-clamp.
REFERENCES
- 1. Schloss P., Williams D. C. (1998) The serotonin transporter: A primary target for antidepressant drugs. J. Psychopharmacol. 12, 115–121 [DOI] [PubMed] [Google Scholar]
- 2. Stahl S. M. (1998) Mechanism of action of serotonin-selective reuptake inhibitors. Serotonin receptors and pathways mediate therapeutic effects and side effects. J. Affect Disord. 51, 215–235 [DOI] [PubMed] [Google Scholar]
- 3. Budygin E. A., John C. E., Mateo Y., Jones S. R. (2002) Lack of cocaine effect on dopamine clearance in the core and shell of the nucleus accumbens of dopamine transporter knock-out mice. J. Neurosci. 22, RC222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murphy D. L., Lerner A., Rudnick G., Lesch K. P. (2004) Serotonin transporter: Gene, genetic disorders, and pharmacogenetics. Mol. Interv. 4, 109–123 [DOI] [PubMed] [Google Scholar]
- 5. Feighner J. P. (1994) Clinical effects of serotonin reuptake inhibitors–a review. Fortschr. Neurol. Psychiatr. 62, 9–15 [PubMed] [Google Scholar]
- 6. Vaswani M., Kalra H. (2004) Selective serotonin re-uptake inhibitors in anorexia nervosa. Expert Opin. Investig. Drugs 13, 349–357 [DOI] [PubMed] [Google Scholar]
- 7. Vaswani M., Linda F. K., Ramesh S. (2003) Role of selective serotonin reuptake inhibitors in psychiatric disorders: A comprehensive review. Prog. Neuropsychopharmacol. Biol. Psychiatry 27, 85–102 [DOI] [PubMed] [Google Scholar]
- 8. Hoffman B. J., Hansson S. R., Mezey E., Palkovits M. (1998) Localization and dynamic regulation of biogenic amine transporters in the mammalian central nervous system. Front Neuroendocrinol. 19, 187–231 [DOI] [PubMed] [Google Scholar]
- 9. Richelson E. (1996) J. Clin. Psychopharmacol. 16, 1S-7S; discussion 7S-9S [DOI] [PubMed] [Google Scholar]
- 10. Amara S. G., Sonders M. S. (1998) Neurotransmitter transporters as molecular targets for addictive drugs. Drug Alcohol. Depend. 51, 87–96 [DOI] [PubMed] [Google Scholar]
- 11. White K. J., Walline C. C., Barker E. L. (2005) Serotonin transporters: Implications for antidepressant drug development. AAPS J. 7, E421–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kahlig K. M., Binda F., Khoshbouei H., Blakely R. D., McMahon D. G., Javitch J. A., Galli A. (2005) Amphetamine induces dopamine efflux through a dopamine transporter channel. Proc. Natl. Acad. Sci. U.S.A. 102, 3495–3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ramamoorthy S., Bauman A. L., Moore K. R., Han H., Yang-Feng T., Chang A. S., Ganapathy V., Blakely R. D. (1993) Antidepressant- and cocaine-sensitive human serotonin transporter: Molecular cloning, expression, and chromosomal localization. Proc. Natl. Acad. Sci. U.S.A. 90, 2542–2546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gether U., Andersen P. H., Larsson O. M., Schousboe A. (2006) Neurotransmitter transporters: Molecular function of important drug targets. Trends Pharmacol. Sci. 27, 375–383 [DOI] [PubMed] [Google Scholar]
- 15. Hahn M. K., Blakely R. D. (2007) The functional impact of SLC6 transporter genetic variation. Annu. Rev. Pharmacol. Toxicol. 47, 401–441 [DOI] [PubMed] [Google Scholar]
- 16. Rudnick G. (1998) Ion-coupled neurotransmitter transport: Thermodynamic versus kinetic determinations of stoichiometry. Methods Enzymol. 296, 233–247 [DOI] [PubMed] [Google Scholar]
- 17. Rudnick G. (1998) Bioenergetics of neurotransmitter transport. J. Bioenerg. Biomembr. 30, 173–185 [DOI] [PubMed] [Google Scholar]
- 18. DeFelice L. J., Blakely R. D. (1996) Pore models for transporters? Biophys. J. 70, 579–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Keyes S. R., Rudnick G. (1982) Coupling of transmembrane proton gradients to platelet serotonin transport. J. Biol. Chem. 257, 1172–1176 [PubMed] [Google Scholar]
- 20. Rudnick G., Wall S. C. (1993) Non-neurotoxic amphetamine derivatives release serotonin through serotonin transporters. Mol. Pharmacol. 43, 271–276 [PubMed] [Google Scholar]
- 21. Petersen C. I., DeFelice L. J. (1999) Ionic interactions in the Drosophila serotonin transporter identify it as a serotonin channel. Nat. Neurosci. 2, 605–610 [DOI] [PubMed] [Google Scholar]
- 22. Ramsey I. S., DeFelice L. J. (2002) Serotonin transporter function and pharmacology are sensitive to expression level: Evidence for an endogenous regulatory factor. J. Biol. Chem. 277, 14475–14482 [DOI] [PubMed] [Google Scholar]
- 23. Adams S. V., DeFelice L. J. (2002) Flux coupling in the human serotonin transporter. Biophys. J. 83, 3268–3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adams S. V., DeFelice L. J. (2003) Ionic currents in the human serotonin transporter reveal inconsistencies in the alternating access hypothesis. Biophys. J. 85, 1548–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li C., Zhong H., Wang Y., Wang H., Yang Z., Zheng Y., Liu K., Liu Y. (2006) Voltage and ionic regulation of human serotonin transporter in Xenopus oocytes. Clin. Exp. Pharmacol. Physiol. 33, 1088–1092 [DOI] [PubMed] [Google Scholar]
- 26. Henry L. K., Field J. R., Adkins E. M., Parnas M. L., Vaughan R. A., Zou M. F., Newman A. H., Blakely R. D. (2006) Tyr-95 and Ile-172 in transmembrane segments 1 and 3 of human serotonin transporters interact to establish high affinity recognition of antidepressants. J. Biol. Chem. 281, 2012–2023 [DOI] [PubMed] [Google Scholar]
- 27. Haunsø A., Buchanan D. (2007) Pharmacological characterization of a fluorescent uptake assay for the noradrenaline transporter. J. Biomol. Screen. 12, 378–384 [DOI] [PubMed] [Google Scholar]
- 28. Jørgensen S., Nielsen E. Ø., Peters D., Dyhring T. (2008) Validation of a fluorescence-based high-throughput assay for the measurement of neurotransmitter transporter uptake activity. J. Neurosci. Methods 169, 168–176 [DOI] [PubMed] [Google Scholar]
- 29. Schwartz J. W., Blakely R. D., DeFelice L. J. (2003) Binding and transport in norepinephrine transporters. Real-time, spatially resolved analysis in single cells using a fluorescent substrate. J. Biol. Chem. 278, 9768–9777 [DOI] [PubMed] [Google Scholar]
- 30. Schwartz J. W., Novarino G., Piston D. W., DeFelice L. J. (2005) Substrate binding stoichiometry and kinetics of the norepinephrine transporter. J. Biol. Chem. 280, 19177–19184 [DOI] [PubMed] [Google Scholar]
- 31. Mason J. N., Farmer H., Tomlinson I. D., Schwartz J. W., Savchenko V., DeFelice L. J., Rosenthal S. J., Blakely R. D. (2005) Novel fluorescence-based approaches for the study of biogenic amine transporter localization, activity, and regulation. J. Neurosci. Methods 143, 3–25 [DOI] [PubMed] [Google Scholar]
- 32. Buck K. J., Amara S. G. (1994) Chimeric dopamine-norepinephrine transporters delineate structural domains influencing selectivity for catecholamines and 1-methyl-4-phenylpyridinium. Proc. Natl. Acad. Sci. U.S.A. 91, 12584–12588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bryan-Lluka L. J., Siebert G. A., Pond S. M. (1999) Potencies of haloperidol metabolites as inhibitors of the human noradrenaline, dopamine, and serotonin transporters in transfected COS-7 cells. Naunyn Schmiedebergs Arch. Pharmacol. 360, 109–115 [DOI] [PubMed] [Google Scholar]
- 34. Wright A. M., Bempong J., Kirby M. L., Barlow R. L., Bloomquist J. R. (1998) Effects of haloperidol metabolites on neurotransmitter uptake and release: Possible role in neurotoxicity and tardive dyskinesia. Brain Res. 788, 215–222 [DOI] [PubMed] [Google Scholar]
- 35. Tsuruda P. R., Yung J., Martin W. J., Chang R., Mai N., Smith J. A. (2010) Influence of ligand binding kinetics on functional inhibition of human recombinant serotonin and norepinephrine transporters. J. Pharmacol. Toxicol. Methods 61, 192–204 [DOI] [PubMed] [Google Scholar]
- 36. Bolan E. A., Kivell B., Jaligam V., Oz M., Jayanthi L. D., Han Y., Sen N., Urizar E., Gomes I., Devi L. A., Ramamoorthy S., Javitch J. A., Zapata A., Shippenberg T. S. (2007) D2 receptors regulate dopamine transporter function via an extracellular signal-regulated kinases 1 and 2-dependent and phosphoinositide 3 kinase-independent mechanism. Mol. Pharmacol. 71, 1222–1232 [DOI] [PubMed] [Google Scholar]
- 37. Schwartz J. W., Piston D., DeFelice L. J. (2006) Molecular microfluorometry: Converting arbitrary fluorescence units into absolute molecular concentrations to study binding kinetics and stoichiometry in transporters. Handb. Exp. Pharmacol. 175, 23–57 [DOI] [PubMed] [Google Scholar]
- 38. Zapata A., Kivell B., Han Y., Javitch J. A., Bolan E. A., Kuraguntla D., Jaligam V., Oz M., Jayanthi L. D., Samuvel D. J., Ramamoorthy S., Shippenberg T. S. (2007) Regulation of dopamine transporter function and cell surface expression by D3 dopamine receptors. J. Biol. Chem. 282, 35842–35854 [DOI] [PubMed] [Google Scholar]
- 39. Yamashita A., Singh S. K., Kawate T., Jin Y., Gouaux E. (2005) Crystal structure of a bacterial homologue of Na+/Cl-dependent neurotransmitter transporters. Nature 437, 215–223 [DOI] [PubMed] [Google Scholar]
- 40. Machaca K., Hartzell H. (1998) Asymmetrical distribution of Ca-activated Cl channels in Xenopus oocytes. Biophys. J. 74, 1286–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Iwamoto H., Blakely R. D., De Felice L. J. (2006) Na+, Cl−, and pH dependence of the human choline transporter (hCHT) in Xenopus oocytes: The proton inactivation hypothesis of hCHT in synaptic vesicles. J. Neurosci. 26, 9851–9859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang H. W., Li C. Z., Yang Z. F., Zheng Y. Q., Zhang Y., Liu Y. M. (2006) Electrophysiological effect of fluoxetine on Xenopus oocytes heterologously expressing human serotonin transporter. Acta Pharmacol. Sin. 27, 289–293 [DOI] [PubMed] [Google Scholar]
- 43. Henry L. K., Adkins E. M., Han Q., Blakely R. D. (2003) Serotonin and cocaine-sensitive inactivation of human serotonin transporters by methanethiosulfonates targeted to transmembrane domain I. J. Biol. Chem. 278, 37052–37063 [DOI] [PubMed] [Google Scholar]
- 44. Barker E. L., Moore K. R., Rakhshan F., Blakely R. D. (1999) Transmembrane domain I contributes to the permeation pathway for serotonin and ions in the serotonin transporter. J. Neurosci. 19, 4705–4717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chang A. S., Frnka J. V., Chen D. N., Lam D. M. (1989) Characterization of a genetically reconstituted high affinity system for serotonin transport. Proc. Natl. Acad. Sci. U.S.A. 86, 9611–9615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nelson N. (1998) The family of Na+/Cl− neurotransmitter transporters. J. Neurochem. 71, 1785–1803 [DOI] [PubMed] [Google Scholar]
- 47. Talvenheimo J., Nelson P. J., Rudnick G. (1979) Mechanism of imipramine inhibition of platelet 5-hydroxytryptamine transport. J. Biol. Chem. 254, 4631–4635 [PubMed] [Google Scholar]
- 48. Field J. R., Henry L. K., Blakely R. D. (2010) Transmembrane domain 6 of the human serotonin transporter contributes to an aqueously accessible binding pocket for serotonin and the psychostimulant 3,4-methylene dioxymethamphetamine. J. Biol. Chem. 285, 11270–11280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Busch A. E., Quester S., Ulzheimer J. C., Waldegger S., Gorboulev V., Arndt P., Lang F., Koepsell H. (1996) Electrogenic properties and substrate specificity of the polyspecific rat cation transporter rOCT1. J. Biol. Chem. 271, 32599–32604 [DOI] [PubMed] [Google Scholar]
- 50. Gorboulev V., Ulzheimer J. C., Akhoundova A., Ulzheimer-Teuber I., Karbach U., Quester S., Baumann C., Lang F., Busch A. E., Koepsell H. (1997) Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol. 16, 871–881 [DOI] [PubMed] [Google Scholar]
- 51. Hohage H., Stachon A., Feidt C., Hirsch J. R., Schlatter E. (1998) Regulation of organic cation transport in IHKE-1 and LLC-PK1 cells. Fluorometric studies with 4-(4-dimethylaminostyryl)-N-methylpyridinium. J. Pharmacol. Exp. Ther. 286, 305–310 [PubMed] [Google Scholar]
- 52. Mehrens T., Lelleck S., Cetinkaya I., Knollmann M., Hohage H., Gorboulev V., Bokník P., Koepsell H., Schlatter E. (2000) The affinity of the organic cation transporter rOCT1 is increased by protein kinase C-dependent phosphorylation. J. Am. Soc. Nephrol. 11, 1216–1224 [DOI] [PubMed] [Google Scholar]
- 53. Daws L. C. (2009) Unfaithful neurotransmitter transporters: Focus on serotonin uptake and implications for antidepressant efficacy. Pharmacol. Ther. 121, 89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fowler A., Seifert N., Acker V., Woehrle T., Kilpert C., de Saizieu A. (2006) A nonradioactive high throughput/high content assay for measurement of the human serotonin reuptake transporter function in vitro. J. Biomol. Screen 11, 1027–1034 [DOI] [PubMed] [Google Scholar]
- 55. Hilber B., Scholze P., Dorostkar M. M., Sandtner W., Holy M., Boehm S., Singer E. A., Sitte H. H. (2005) Serotonin transporter-mediated efflux: A pharmacological analysis of amphetamines and non-amphetamines. Neuropharmacology 49, 811–819 [DOI] [PubMed] [Google Scholar]
- 56. Andersen J., Taboureau O., Hansen K. B., Olsen L., Egebjerg J., Strømgaard K., Kristensen A. S. (2009) Location of the antidepressant binding site in the serotonin transporter: Importance of Ser-438 in recognition of citalopram and tricyclic antidepressants. J. Biol. Chem. 284, 10276–10284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Murali S., Rettig W. (2006) TICT formation in para- and meta-derivatives of N-phenylpyrrole. J. Phys. Chem. A 110, 28–37 [DOI] [PubMed] [Google Scholar]
- 58. Gluck M. R., Youngster S. K., Ramsay R. R., Singer T. P., Nicklas W. J. (1994) Studies on the characterization of the inhibitory mechanism of 4′-alkylated 1-methyl-4-phenylpyridinium and phenylpyridine analogs in mitochondria and electron transport particles. J. Neurochem. 63, 655–661 [DOI] [PubMed] [Google Scholar]
- 59. Krueger M. J., Sablin S. O., Ramsay R., Singer T. P. (1993) Reactivation of NADH dehydrogenase (complex I) inhibited by 1-methyl-4-(4′-alkylphenyl)pyridinium analogs: A clue to the nature of the inhibition site. J. Neurochem. 61, 1546–1548 [DOI] [PubMed] [Google Scholar]
- 60. Desai V. G., Feuers R. J., Hart R. W., Ali S. F. (1996) MPP+-induced neurotoxicity in mouse is age-dependent: Evidenced by the selective inhibition of complexes of electron transport. Brain Res. 715, 1–8 [DOI] [PubMed] [Google Scholar]
- 61. Johnson L. V., Walsh M. L., Bockus B. J., Chen L. B. (1981) Monitoring of relative mitochondrial membrane potential in living cells by fluorescence microscopy. J. Cell Biol. 88, 526–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Johnson L. V., Walsh M. L., Chen L. B. (1980) Localization of mitochondria in living cells with rhodamine 123. Proc. Natl. Acad. Sci. U.S.A. 77, 990–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bancaud A., Huet S., Daigle N., Mozziconacci J., Beaudouin J., Ellenberg J. (2009) Molecular crowding affects diffusion and binding of nuclear proteins in heterochromatin and reveals the fractal organization of chromatin. EMBO J. 28, 3785–3798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mager S., Min C., Henry D. J., Chavkin C., Hoffman B. J., Davidson N., Lester H. A. (1994) Conducting states of a mammalian serotonin transporter. Neuron 12, 845–859 [DOI] [PubMed] [Google Scholar]
- 65. Singh S. K., Yamashita A., Gouaux E. (2007) Antidepressant binding site in a bacterial homolog of neurotransmitter transporters. Nature 448, 952–956 [DOI] [PubMed] [Google Scholar]
- 66. Zhou Z., Zhen J., Karpowich N. K., Goetz R. M., Law C. J., Reith M. E., Wang D. N. (2007) LeuT-desipramine structure reveals how antidepressants block neurotransmitter reuptake. Science 317, 1390–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhou Z., Zhen J., Karpowich N. K., Law C. J., Reith M. E., Wang D. N. (2009) Antidepressant specificity of serotonin transporter suggested by three LeuT-SSRI structures. Nat. Struct. Mol. Biol. 16, 652–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sarker S., Weissensteiner R., Steiner I., Sitte H. H., Ecker G. F., Freissmuth M., Sucic S. (2010) The high affinity binding site for tricyclic antidepressants resides in the outer vestibule of the serotonin transporter. Mol. Pharmacol. 78, 1026–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Barker E. L., Blakely R. D. (1996) Identification of a single amino acid, phenylalanine 586, that is responsible for high affinity interactions of tricyclic antidepressants with the human serotonin transporter. Mol. Pharmacol. 50, 957–965 [PubMed] [Google Scholar]
- 70. Celik L., Sinning S., Severinsen K., Hansen C. G., Møller M. S., Bols M., Wiborg O., Schiøtt B. (2008) Binding of serotonin to the human serotonin transporter. Molecular modeling and experimental validation. J. Am. Chem. Soc. 130, 3853–3865 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.