Abstract
Recently, several cases of intraductal oncocytic papillary neoplasm (IOPN) of the liver and hepatic bile ducts have been reported. The author herein reports the first case of IOPN of the common bile duct (CBD). A 78-year-old man was admitted to our hospital because of jaundice. Imaging modalities including US, CT, MRI revealed an intraductal tumor of the middle CBD and biliary dilation distal to the tumor. A partial resection of the CBD was performed. Grossly, a papillary tumor measuring 20 × 15 mm was found within the CBD. Mucus is absent. Histologically, the papillary tumor was composed of atypical oncocytes. The atypia was enough to be diagnosed as adenocarcinoma. No invasive features were noted. Immunohistochemically, the tumor cells were positive for pancytokeratins (CK), CK 7, CK 18, CK19, EMA, CA19-9, CEA, mitochondria, p53 protein, C-erbB2, Ki-67 (labeling = 80%), MUC2, MUC5AC and MUC-6,. The tumor cells were negative for CK8, CK20, chromogranin, synaptophysin, neuron-specific enolase, S100 protein, CD56, MUC1, CD10 and CDX2. These immunohistochemical findings were compatible with IOPN. The patient died of other non-tumorous disease 7 year after the operation. In summary, the author presented the first case of IOPN of the CBD.
Keywords: Common bile duct, intraductal oncocytic papillary neoplasm, immunohistochemistry, histopathology
Introduction
Recently, several cases of intraductal oncocytic papillary neoplasms (IOPN), previously reported as biliary papillomatosis [1] have been reported in the intrahepatic bile ducts of the liver [2-7]. It is a liver counterpart of the well known IOPN of the pancreas [8]. The author herein reports a case of IOPN of the common bile duct (CBD). This is the first case of IOPN occurring in the CBD.
Case report
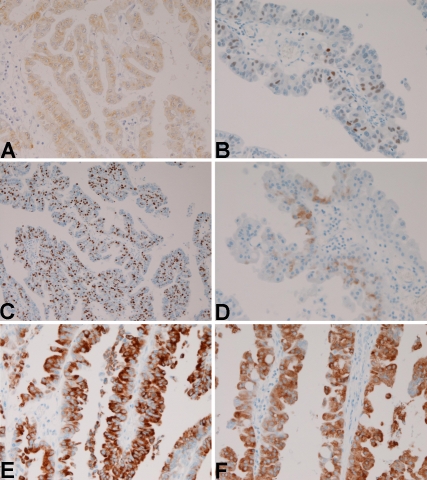
A 78-year-old man was admitted to our hospital because of jaundice. Imaging modalities including US, CT and MRI revealed an intraductal tumor of the CBD and biliary dilation distal to the tumor. A partial resection of the common bile duct was performed. Grossly, papillary tumor measuring 20 × 15 mm was found within the middle CBD (Figure 1). No mucus is present. Histologically, the papillary tumor consisted of atypical oncocytes (Figures 2A and 2B). The atypia was enough to be diagnosed as adenocarcinoma (Figure 2B). No invasive features were noted (Figure 2A). Mucin stains revealed no mucus hypersecretion. The surgical margins were negative for tumor cells. An immunohistochemical study was performed by Dako's Envision method (Dako, Glostrup, Denmark), as previously described [9-17]. The evaluation of immunohistochemistry was categorized as follows: -, less than 1% positive; +, 2-33% positive; 2 + , 33-67% positive; and 3 + , 68-100% positive. The tumor cells were positive for pancytokeratins (CK), CK 7, CK 18, CK19, epithelial membrane antigen, carbohydrate antigen19-9 (CA19-9), carcinoembryonic antigen (CEA), mitochondria, p53 protein, C-erbB2, Ki-67 (labeling = 80%), epidermal growth factor receptor (Figure 3A), MUC2 (Figure 3D), MUC5AC (Figure 3E), and MUC (Figure 3F). The tumor cells were negative for CK8, CK20, chromogranin, synaptophysin, neuron-specific enolase, S100 protein, CD56, CD10, HER2/ neu, MUC1, and CDX2. The patient died of other non-tumorous disease 7 year after the operation.
Figure 1.
Gross features. The resected common bile duct shows papillary tumors measuring 20×15 mm. The common bile duct is dilated.
Figure 2.
Microscopic picture. A: Low power view demonstrates papillary proliferation of tumor cells. HE, x40. B: High power view shows that the papillary tumor is composed only of atypical oncocytes, which are regarded as malignant cells in view of the structural and cytological atypia. HE, x200
Figure 3.
Immunohistochemical findings. A: The tumor cells are positive for cytokeratin. × 200. B: The tumor cells are positive for p53 protein. x200. C: The tumor cells are positive for Ki-67 antigen. The labeling is 80%. x100. D: The tumor cells are positive for MUC2. x200. E: The tumor cells are positive for MUC5AC., x200. F: The tumor cells are positive for MUC6, x200.
Discussion
The present tumor was located in the CBD and showed papillary proliferation of oncocytes. The oncocytes showed cellular and structural atypia regarded as adenocarcinoma. The oncocytes may be due to a large number of mitochondria, as suggested by the immunohistochemistry. The positive reactions for p53 protein, CA19-9 and CEA and high Ki-67 labeling (80%) highly suggest the malignant nature of the present tumor [18-20]. The positive C-erbB2 also suggests malignant nature of the present tumor [21]. Therefore, the present CBD tumor is a malignant IOPN of the CBD. This is the first report of IOPM occurring in the CBD.
In the past, the authors studied intraductal papillary mucinous neoplasms (IPMN) of the pancreas and intraductal tubular neoplasm (ITN) of the pancreas [22-27]. The present tumor is not IPMN or ITN because of the lack of mucin hypersecretion and of the lack of tubular structures.
Neuroendocrine differentiation may occur in some tumors. In the present tumor showed no reactions for CD56, chromogranin, synaptophysin, and neuron-specific enolase. The findings indicate that the present tumor is not neuroendocrine tumor or tumor with neuroendocrine differentiation.
Recently, IPMN of the liver has been reported [28, 29]. Pancreas and hepatobiliary organs share many phenotypes. Both arise from the common embryonic bud [30]. The similarity of the bile ducts and pancreatic ducts are evident. For example, the pancreas show pancreatic hepatocytes [31] and hepatobiliary organs show pancreatic acinar cells [32]. Both pancreatic ducts and bile ducts have been demonstrated to contain pancreatic digestive enzymes [33-40]. In addition, pancreatic acinar cells and bile ducts having pancreatic digestive enzymes have been observed in fetal organogenesis of bile ducts [41-47]. These findings highly suggest the common phenotypes and biologic characters between pancreatic ducts and bile ducts. Thus, it is not strange that IPMN and IOPM occur in the biliary tracts, as is well established in the pancreas. The present case also supports the above statements.
IPMN of the pancreas is classified by mucus type into gastric type, oncocytic type, pancreatobiliary type and intestinal type [48]. In the present study, MUC2, MUC5AC and MUC6 were positive, being compatible with oncocytic type [48]. The negative immunostaining of CD10 and CDX2 suggest that the present tumor have no intestinal phenotypes.
The present tumor was characterized by a little or no cytoplasmic mucins and no mucus secretion. Since MUC apomucins were present in the tumor cell cytoplasm, it is possible that glycosylation is not operative in the cytoplasm. The negative mucus secretion may indicate that mucus secretory process is impaired in the present IOPN.
The CK profile of the normal pancreatic ducts and intrahepatic bile ducts is CK7 +, CK8 +, CK18 +, CK19 + [49, 50]. In the present IOPM, the CK profile was CK7 +, CK8 -, CK18 +, CK19 +. This suggests that the expression of CK8 diminishes and disappears during the tumorigenesis of IOPM in the present case. In summary, the author presented the first case of IOPN of the CBD.
Interest of conflict
The author declares that he has no conflict of interest
References
- 1.Tearda T, Mitsui T, Nakanuma Y, Miura S, Toya D. Intrahepatic biliary papillomatosis arising in non-obstructive intrahepatic biliary dilations confined to the hepatic left lobe. Am J Gastroenterol. 1991;86:1523–1526. [PubMed] [Google Scholar]
- 2.Sudo Y, Harada K, Tsuneyama K, Katayanagi K, Zen Y, Nakanuma Y. Oncocytic biliary cystademnoma is a form of intraductal oncocytic papillary neoplasm of the liver. Mod Pathol. 2001;14:1304–1309. doi: 10.1038/modpathol.3880479. [DOI] [PubMed] [Google Scholar]
- 3.Martin RC, Klimstra DS, Schwarz L, Yilmaz A, Blumgard LH, Jarnagin W. Hepatic intraductal oncocytic papillary carcinoma. Cancer. 2002;95:2180–2187. doi: 10.1002/cncr.10934. [DOI] [PubMed] [Google Scholar]
- 4.Terada T, Taniguchi M. Intraductal oncocytic papillary neoplasm of the liver. Pathol Int. 2004;54:116–123. doi: 10.1111/j.1440-1827.2004.01594.x. [DOI] [PubMed] [Google Scholar]
- 5.Rouzbahman M, Serra S, Adsay NV, Bejarano PA, Nakanuma Y, Chetty R. Oncocytic papillary neoplasms of the biliary tract: a clinicopathological, mucin core and Wnt pathway protein analysis of four cases. Pathology. 2007;39:413–418. doi: 10.1080/00313020701444531. [DOI] [PubMed] [Google Scholar]
- 6.Tabibian JH, Lassman CR, Margolis DJ, Lanverde C, Busuttil RW, Durazo FA. Intraductal oncocytic papillary neoplasm of the liver: case report and review of a rare variant. Ann Hepatol. 2008;7:168–1737. [PubMed] [Google Scholar]
- 7.Spector SA, Bejarano PA, Amortegui PA, Renfrow MR, Livingstone AS. Intraductal oncocytic papillary neoplasm of the extrahepatic biliary tree: first report. Am Surg. 2004;70:55–58. [PubMed] [Google Scholar]
- 8.Adsay NV, Adair CF, Heffes CS, Klimstra DS. Intraductal oncocytic papillary neoplasm of the pancreas. Am J Surg Pathol. 1996;20:980–994. doi: 10.1097/00000478-199608000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Terada T, Kawaguchi M, Furukawa K, Sekido Y, Osamura RY. Minute mixed ductalendocrine carcinoma of the pancreas with predominant intraductal growth. Pathol Int. 2002;52:740–746. doi: 10.1046/j.1440-1827.2002.01416.x. [DOI] [PubMed] [Google Scholar]
- 10.Terada T, Kawaguchi M. Primary clear cell adenocarcinoma of the peritoneum. Tohoku J Exp Med. 2005;206:271–275. doi: 10.1620/tjem.206.271. [DOI] [PubMed] [Google Scholar]
- 11.Terada T. Primary multiple extragastrointestinal stromal tumors of the omentum with different mutations of c-kit gene. Would J Gastroenterol. 2008;14:7256–7259. doi: 10.3748/wjg.14.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terada T. Gastrointestinal stromal tumor of the uterus: A case report with genetic analyses of c-kit and PDGFRA genes. Int J Gynecol Pathol. 2009;28:29–34. doi: 10.1097/PGP.0b013e3181808000. [DOI] [PubMed] [Google Scholar]
- 13.Terada T. Large endocervical polyp with cartilaginous and osseous metaplasia: a hitherto unreported entity. Int J Gynecol Pathol. 2009;28:98–100. doi: 10.1097/PGP.0b013e31817eb796. [DOI] [PubMed] [Google Scholar]
- 14.Terada T. Ductal adenoma of the breast: Immunohistochemistry of two cases. Pathol Int. 2008;58:801–805. doi: 10.1111/j.1440-1827.2008.02315.x. [DOI] [PubMed] [Google Scholar]
- 15.Terada T. Gall bladder adenocarcinoma arising in Rokitansky-Schoff sinuses. Pathol Int. 2008;58:806–809. doi: 10.1111/j.1440-1827.2008.02316.x. [DOI] [PubMed] [Google Scholar]
- 16.Terada T. Primary extragastrointestinal stromal tumors of the transverse mesocolon without c-kit mutations but with PDGFRA mutations. Med Oncol. 2009;26:233–237. doi: 10.1007/s12032-008-9092-9. [DOI] [PubMed] [Google Scholar]
- 17.Terada T. Primary small cell carcinoma of the mediastinum: A case report with immunohistochemical and molecular genetic analyses of KIT and PDGFRA genes. Med Oncol. 2009;26:247–250. doi: 10.1007/s12032-008-9116-5. [DOI] [PubMed] [Google Scholar]
- 18.Terada T, Nakanuma Y, Ohta T, Nagakawa T. Histological features and interphase nucleolar organizer regions in hyperplastic, dysplastic and neoplastic epithelium in hepatolithiasis. Histopathology. 1992;21:233–240. doi: 10.1111/j.1365-2559.1992.tb00381.x. [DOI] [PubMed] [Google Scholar]
- 19.Terada T, Nakanuma Y. Cell kinetic analyses and expression of carcinoembryonic antigen, carbohydrate antigen 19-9 and DU-PAN-2 in hyperplastic, preneoplastic and neoplastic lesions of intrahepatic bile ducts in livers with hepatoliths. Virchows Arch [A] 1992;420:327–335. doi: 10.1007/BF01600212. [DOI] [PubMed] [Google Scholar]
- 20.Terada T, Shimizu K, Izumi R, Nakanuma Y. p53 expression in formalin-fixed, paraffin-embedded specimens of intrahepatic cholangiocarcinoma: Retrieval of p53 antigenicity by microwave oven heating of tissue sections. Mod Pathol. 1994;7:249–252. [PubMed] [Google Scholar]
- 21.Terada T, Ashida K, Endo K, Horie S, Maeta Y, Matsunaga Y, Takashima K, Ohta T, Kitamura Y. C-erbB-2 protein is expressed hepatolithiasis and cholangiocarcinoma. Histopathology. 1998;33:325–331. doi: 10.1046/j.1365-2559.1998.00496.x. [DOI] [PubMed] [Google Scholar]
- 22.Terada T, Ohta T, Sasaki M, Nakanuma Y, Kim YS. Expression of MUC apomucins in normal pancreas and pancreatic tumours. J Pathol. 1996;180:160–165. doi: 10.1002/(SICI)1096-9896(199610)180:2<160::AID-PATH625>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 23.Terada T, Nakanuma Y. Expression of mucin carbohydrate antigens (T, Tn, and syalyl-Tn) and MUC-1 gene product in intraductal papillary mucinous neoplasm of the pancreas. Am J Clin Pathol. 1996;105:613–620. doi: 10.1093/ajcp/105.5.613. [DOI] [PubMed] [Google Scholar]
- 24.Terada T, Ohta T, Nakanuma Y. Expression of oncogene products and anti-oncogene products and oncofetal antigens in intraductal papillary-mucinous neoplasm of the pancreas. Histopathology. 1996;29:355–361. doi: 10.1111/j.1365-2559.1996.tb01419.x. [DOI] [PubMed] [Google Scholar]
- 25.Terada T, Ohta T, Kitamura Y, Ashida K, Matsunaga Y, Kato M. Endocrine cells in intraductal papillary-mucinous neoplasm of the pancreas. Virchows Archiv. 1997;431:31–36. doi: 10.1007/s004280050066. [DOI] [PubMed] [Google Scholar]
- 26.Terada T, Ohta T, Kitamura Y, Ashida K, Matsunaga Y. Cell proliferative activity in intraductal papillary-mucinous neoplasm and invasive ductal adenocarcinoma of the pancreas. Arch Pathol Lab Med. 1998;122:42–46. [PubMed] [Google Scholar]
- 27.Terada T. Intraductal tubular carcinoma, intestinal type, of the pancreas. Pathol Int. 2009;59:53–58. doi: 10.1111/j.1440-1827.2008.02325.x. [DOI] [PubMed] [Google Scholar]
- 28.Shibahara H, Tamada S, Goto M, Oda K, Nagino M, Nagasaka T, Batra SK, Hollingsworth MA, Imai K, Nimura Y, Yonezawa S. Pathologic features of mucin-producing bile duct tumors. Am J Surg Pathol. 2004;28:327–338. doi: 10.1097/00000478-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Zen Y, Fujii T, Itatsu K, Nakamura K, Minato H, Kashima S, Kurumaya H, Katayanagi K, Kawashima A, Matsuda S, Niwa H, Mitsui T, Asada Y, Miura S, Ohta T, Nakanuma Y. Biliary papillary tumors share pathological features with intraductal papillary-mucinous neoplasm of the pancreas. Hepatology. 2006;44:1333–1343. doi: 10.1002/hep.21387. [DOI] [PubMed] [Google Scholar]
- 30.Terada T, Kitamura Y, Nakanuma Y. Normal and abnormal development of the intrahepatic biliary system: A review. Tohoku J Exp Med. 1997;181:19–32. doi: 10.1620/tjem.181.19. [DOI] [PubMed] [Google Scholar]
- 31.Terada T, Nakanuma Y, Kakita A. Pathologic observations of intrahepatic peribiliary glands in 1,000 consecutive autopsy livers: Heterotopic pancreas in the liver. Gastroenterology. 1990;98:1333–1337. doi: 10.1016/s0016-5085(12)90353-4. [DOI] [PubMed] [Google Scholar]
- 32.Reddy JK, Rao MS, Qureshi SA, Reddy MK, Scarpelli DG, Lalwani ND. Induction and origin of hepatocytes in rat pancreas. J Cell Biol. 1984;98:2082–2090. doi: 10.1083/jcb.98.6.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terada T, Kida T, Nakanuma Y. Extrahepatic peribiliary glands express α-amylase isozymes, trypsin and pancreatic lipase: An immunohistochemical analysis. Hepatology. 1993;18:803–808. doi: 10.1002/hep.1840180409. [DOI] [PubMed] [Google Scholar]
- 34.Terada T, Nakanuma Y. Immunohistochemical demonstration of pancreatic α-amylase and trypsin in intrahepatic bile ducts and peribiliary glands. Hepatology. 1991;14:1129–1135. [PubMed] [Google Scholar]
- 35.Terada T, Kono N, Nakanuma Y. Immunohistochemical and immunoelectron microscopical analyses of α-amylase isozymes in the intrahepatic biliary epithelium and hepatocytes. J Histochem Cytochem. 1992;40:1627–1635. doi: 10.1177/40.11.1431051. [DOI] [PubMed] [Google Scholar]
- 36.Terada T, Nakanuma Y. Expression of α-amylase isoenzymes and trypsin by proliferating epithelium of large intrahepatic bile ducts and intrahepatic peribiliary glands in hepatolithiasis. Histopathology. 1993;22:467–473. doi: 10.1111/j.1365-2559.1993.tb00160.x. [DOI] [PubMed] [Google Scholar]
- 37.Terada T, Nakanuma Y. Pancreatic lipase is a useful phenotypic marker of large and septal bile dusts, peribiliary glands, and their malignant counterparts. Mod Pathol. 1993;6:419–426. [PubMed] [Google Scholar]
- 38.Ohta T, Terada T, Nagakawa T, Ito H, Tajima H, Miyazaki I. Presence of pancreatic α-amylase, trypsinogen, and lipase immunoreactivity in normal human pancreatic ducts. Pancreas. 1994;9:382–386. doi: 10.1097/00006676-199405000-00016. [DOI] [PubMed] [Google Scholar]
- 39.Terada T, Morita T, Hoso M, Nakanuma Y. Pancreatic enzymes in epithelium of intrahepatic large bile ducts and in hepatic bile in patients with extrahepatic bile duct obstruction. J Clin Pathol. 1994;47:924–927. doi: 10.1136/jcp.47.10.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terada T, Ohta T, Minato H, Nakanuma Y. Expression of pancreatic trypsinogen/trypsin and cathepsin B in human cholangiocarcinomas and hepatocellular carcinomas. Hum Pathol. 1995;26:746–752. doi: 10.1016/0046-8177(95)90222-8. [DOI] [PubMed] [Google Scholar]
- 41.Terada T, Nakanuma Y. Development of human intrahepatic peribiliary glands: Histological, keratin immunohistochemical and mucus histochemical analyses. Lab Invest. 1993;68:261–269. [PubMed] [Google Scholar]
- 42.Terada T, Nakanuma Y. Development of human peribiliary capillary plexus: A lectinhistochemical and immunohistochemical study. Hepatology. 1993;18:529–536. [PubMed] [Google Scholar]
- 43.Terada T, Nakanuma Y. Profiles of expression of carbohydrate chain structures during human intrahepatic bile duct development and maturation: A lectinhistochemical and immunohistochemical study. Hepatology. 1994;20:388–397. [PubMed] [Google Scholar]
- 44.Terada T, Nakanuma Y. Detection of apoptosis and expression of apoptosis-related proteins during human intrahepatic bile duct development. Am J Pathol. 1995;146:67–74. [PMC free article] [PubMed] [Google Scholar]
- 45.Terada T, Nakanuma Y. Expression of pancreatic enzymes (α-amylase, trypsinogen and lipase) during human liver development and maturation. Gastroenterology. 1995;108:1236–1245. doi: 10.1016/0016-5085(95)90225-2. [DOI] [PubMed] [Google Scholar]
- 46.Terada T, Okada Y, Nakanuma Y. Expression of matrix proteinases during human intrahepatic bile duct development: A possible role in biliary cell migration. Am J Pathol. 1995;147:1207–1213. [PMC free article] [PubMed] [Google Scholar]
- 47.Terada T, Ashida K, Kitamura Y, Matsunaga Y, Takashima K, Kato M, Ohta T. Expression of E-cadherin, alpha-catenin and beta-catenin during human intrahepatic bile duct development. J Hepatol. 1998;28:263–268. doi: 10.1016/0168-8278(88)80013-8. [DOI] [PubMed] [Google Scholar]
- 48.Furukawa T, Kloppel G, Adsay V, Albores-Saavedra J, Fukushima N, Horii A, Hruban RH, Kato Y, Klimstra DS, Longnecker DS, Lutteges J, Offerhaus GA, Shimizu M, Sunamura M, Suriawinata A, Takaori K, Yonezawa S. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 2005;447:794–799. doi: 10.1007/s00428-005-0039-7. [DOI] [PubMed] [Google Scholar]
- 49.Van Eyken P, Sciot R, Callea F, Van der Steen K, Moerman P, Desmet VJ. The development of the intrahepatic bile ducts in man: a keratin immunohistochemical study. Hepatology. 1988;8:1586–1595. doi: 10.1002/hep.1840080619. [DOI] [PubMed] [Google Scholar]
- 50.Shussler MH, Skoudy A, Raemakers F, Real FX. Intermediate filaments as differentiation markers of normal pancreas and pancreas cancer. Am J Pathol. 1992;140:559–568. [PMC free article] [PubMed] [Google Scholar]