Abstract
OBJECTIVE
To estimate endophthalmitis incidence following cataract surgery nationally and at the state level in 2003–2004 and to explore risk factors.
DESIGN
Analysis of Medicare beneficiary claims data.
PARTICIPANTS
100% sample of Medicare recipients’ claims for endophthalmitis and outpatient cataract surgery services.
METHODS
Cataract surgeries were identified by procedure codes and merged with demographic information. Cataract annual surgical volume was calculated for all surgeons. Presumed post-operative endophthalmitis cases were identified by International Classification of Diseases-9 Clinical Modification Codes (ICD-9-CM) on claims within 42 days after surgery. Endophthalmitis rates and 95% confidence intervals were calculated at state and national levels. Logistic regression was used to investigate the association between developing endophthalmitis and surgery location and surgeon factors.
MAIN OUTCOME MEASURES
Endophthalmitis incidence and risk factors.
RESULTS
4,006 cases of presumed endophthalmitis occurred following 3,280,966 cataract surgeries. The national rate in 2003 was 1.33 per 1000 surgeries (95% confidence interval [CI]: 1.27–1.38) and decreased to 1.11 per 1000 (95% CI: 1.06–1.16) in 2004. Males (relative risk [RR] 1.23, 95% CI: 1.15–1.31), older individuals (RR 1.53, 95% CI 1.38–1.69; 85+ compared to 65–74 years), Blacks (RR 1.17, 95% CI 1.03–1.33) and Native Americans (RR 1.72, 95% CI 1.07–2.77) had increased risk of disease. After adjustment, surgeries by surgeons with low annual volume (RR 3.80, 95% CI 3.13–4.61 for 1–50 compared to 1001+annual surgeries) and less experience (RR 1.41, 95% CI 1.25–1.59 1–10 compared to 30+ years) and surgeries per formed in 2003 (RR 1.20, 95% CI 1.13–1.28) had increased endophthalmitis risk.
CONCLUSIONS
Endophthalmitis rates are lower than previous-year US estimates, but remain higher than rates reported from a series of studies from Sweden; patient factors or methodological differences may contribute to differences across countries. Patient age, gender and race, and surgeon volume and years of experience are important risk factors.
Introduction
Cataract is highly prevalent among older Americans, and surgery is a rapid and cost-effective means for restoring vision. Approximately 5% of individuals aged 70 years and older in the United States (US) undergo cataract surgery each year.1 While rare, postoperative endophthalmitis is a condition often associated with significant morbidity2;3 and treatment costs.4;5 Visual outcomes following endophthalmitis are often poor; approximately one third of individuals do not recover vision better than counting fingers,2 and 50% do not achieve vision better than 20/40.3
Identifying the true rate of endophthalmitis at a national level is difficult given the rare nature of the disease. Single centers with large surgical volumes are able to estimate endophthalmitis rates across time within their center; however, their rates may not be representative of the patient population at large, since they do not capture the variation in techniques utilized across the country and typically include only a few highly-skilled surgeons. Large, administrative billing databases have the advantage of including surgeries from a wide range of settings across a broad patient population, thereby making them more representative of the general population.
Government health insurance databases are a useful resource for evaluating surgical complications. Within the United States, Medicare is a federal health insurance plan for older and disabled Americans. Residents become eligible for Medicare at age 65 if they and/or their spouse have worked in the US. Researchers may obtain access to specific parts of Medicare claims billing data by requesting either a complete dataset of all claims for 5% of all beneficiaries (“5% sample”) or requesting specific types of claims data for all beneficiaries with a particular disease or treatment code (“100% sample”). These data have been used to calculate endophthalmitis incidence at the national level. Based on data from the early 1980’s, Javitt et al utilized a 100% sample of Medicare data and estimated the rate of endophthalmitis at 1.2 cases per 1000 extracapsular surgeries performed on an inpatient basis.6 This estimate is in concordance with a meta analysis of 90 studies from a similar time period, which estimated an endophthalmitis rate of 1.3 per 1000 cataract surgeries,7 while center-specific rates for that time period range from 1 per 300 cataract procedures and as low as no events over a several-year period.8–11 The wide variation in center-specific rates shows how rates may be influenced by focusing on one specific institution or geographic location.
More recently, West et al utilized the Medicare 5% sample to estimate rates for 1994–200112 and reported that annual rates increased during this time, reaching a maximum of 2.36 per 1000 surgeries in 2000, and this rate is similar to the rate found by Taban et al in a meta-analysis of 215 articles.13 While the 5% sample of Medicare beneficiaries allows a broad overview of the rate at the national level, it does not allow additional analyses at a more local level. The current study utilized administrative data from 100% of the cataract surgeries billed to Medicare in 2003–4, which provided the opportunity to examine more recent rates of endophthalmitis and to analyze data at the state level.
Materials and Methods
Participants
Medicare is the United States' federal health insurance plan which subsidizes healthcare costs for approximately 44 million elderly and disabled Americans. Estimates suggest that over 80% of cataract surgeries in the US are billed through Medicare.14 For rare but important complications of surgery, large administrative databases like Medicare are often the most comprehensive source of information, and are one of few sources that can provide precise estimates of incidence rates at the population level.
Medicare beneficiary claims data for the years 2003 and 2004 were obtained from the Centers for Medicare and Medicaid Services Research Data Distribution Center (CMS-RDDC) in the format of research-identifiable files (RIF). Data received included extracts from the standard analytic carrier files (formerly Physician/Supplier Part B file) containing all medical claims billed to Medicare for outpatient services (100% sample) under the fee-for-service plan (FFS). The data are person-specific and contain information on dates and place of service, diagnosis and procedure codes, the unique physician identification number (UPIN) for the provider submitting the claim, billing codes and associated charges and payments. In addition to individual provider claims, the carrier file also contains facility claims for procedures performed at ambulatory surgery centers (ASCs). In addition, we obtained the 2004 Medicare Physician Identification and Eligibility Registry (MPIER) file, which includes provider-specific information such as medical school graduation year, state of medical license, and specialty, and denominator files containing demographic information and type of Medicare coverage for all beneficiaries.
Identification of Study Cohort
CMS-RDDC provided a dataset that included all claims in the carrier file related to cataract surgery. These claims were identified by claims containing a line item procedure code for cataract extraction using the standard Current Procedural Terminology (CPT) codes15 for cataract surgery (Table 1). These data were merged with the MPIER file by physician UPIN and state where the cataract surgery was performed in order to identify the physician performing the procedure. Cataract surgery data were limited to allow a maximum of two cataract surgeries per beneficiary during the two-year study timeframe. Information within the line item claims was used to identify records that should be excluded. Records were excluded if data indicated the procedure was not performed, the procedure was a return to the operating room for a related procedure such as removal of retained lens material, the allowed billable amount was zero (non-covered service), or the line processing indicator code indicated an invalid or duplicate billing claim. Additionally, records were excluded when the only billing claim for a procedure was submitted by an optometrist, since optometrists are not licensed to perform cataract surgery in the United States.
Table 1.
Procedure and diagnostic codes used in analysis
| Description | |
|---|---|
| CPT code | |
| 66850 | Removal of lens material, phacofragmentation technique (mechanical or ultrasonic) (e.g., phacoemulsification) with aspiration |
| 66920 | Removal of lens material; intracapsular |
| 66930 | Removal of lens material; intracapsular, for dislocated lens |
| 66940 | Removal of lens material; extracapsular (other than 66840, 66850, 66852) |
| 66982 | Extracapsular cataract removal with insertion of IOL prosthesis (1-stage procedure), manual or mechanical technique (e.g., irrigation and aspiration or phacoemulsification) complex, requiring devices or techniques not generally used in routine cataract surgery |
| 66983 | Intracapsular extraction with insertion of IOL prosthesis (1-stage procedure) |
| 66984 | Extracapsular cataract removal with insertion of IOL prosthesis (1-stage procedure), manual or mechanical technique (e.g., irrigation and aspiration or phacoemulsification) |
| ICD-9-CM code | |
| 360.00 | Purulent endophthalmitis, unspecified |
| 360.01 | Acute endophthalmitis |
| 360.02 | Panophthalmitis |
| 360.03 | Chronic endophthalmitis |
| 360.04 | Vitreous abscess |
CPT=Current Procedural Terminology; IOL=intraocular lens; ICD-9-CM=International Classification of Diseases, 9th Revision, Clinical Modification
Once the final set of cataract surgeries was determined, these records were merged with each annual denominator file to obtain information on demographic data and information on Medicare beneficiary and enrollment status, including Health Maintenance Organization (HMO) participation. Claims were limited to those for aged Medicare beneficiaries (65 or older) with continuous part B (outpatient and physician services) coverage and no HMO coverage at any time during the calendar year. Approximately 15% of Medicare beneficiaries participated in a Medicare HMO during 2003–4. HMO services are provided on a contract basis, and providers are reimbursed on a fixed-fee schedule, regardless of the number of procedures they perform or the number of office visits completed. As such, no claims data are available for cataract surgery or endophthalmitis-related visits and treatments for beneficiaries while participating in an HMO.
Initial evaluations at the state level suggested potential coding inaccuracies with some claims from Alaska. Specifically, less than one quarter of the surgeries coded as having been performed in Alaska matched with surgeons practicing in Alaska and 92% of the surgeries that did not match up with a provider were coded as being performed in Alaska. In addition, for simplicity, Hawaii and DC were also removed from the analysis, allowing for an analysis of the 48 contiguous states.
Identification of cases with post-operative endophthalmitis
CMS-RDDC provided a dataset that included all claims billed to Medicare in 2003–4 that included a line item diagnosis code for endophthalmitis using the International Classification of Diseases-9 Clinical Modification Codes (ICD-9-CM)16 (Table 1). In order to identify presumed endophthalmitis that was a direct result of cataract surgery, these data were then manipulated as follows. First, claims were limited to the first claim for each individual, given that once an individual has been diagnosed with endophthalmitis, the treating physician is likely to perform a more extended exam at all follow up visits, and, therefore, may continue to code for endophthalmitis in the years after the acute event. These data were then merged onto the final cataract surgery dataset, and the cataract surgery date was compared with the first endophthalmitis claim date. If the first endophthalmitis claim occurred within 42 days following cataract surgery, the event was assumed to be secondary to cataract surgery, and the individual was classified as an endophthalmitis case for this analysis. The data request for this study was limited to data for 2003–2004, as these were the most recent data available when this project began. Thus, to allow for appropriate calculation of endophthalmitis incidence in the latter part of 2004, surgeries that occurred within the last 42 days of 2004 (November 20, 2004 onward) were excluded.
Analysis of incidence and measures of risk
Annual endophthalmitis rates were calculated for each state individually and for the 48 states overall. The carrier and denominator files provide information on a limited number of potential risk factors including age, race, gender and location of surgery (hospital outpatient center vs. ASC). Each surgeon’s annual Medicare surgical volume was calculated by summing the number of cataract surgeries for each individual UPIN number over the two-year period and dividing by two. Surgeons’ years of experience were calculated by subtracting the medical school graduation year from 2004. Logistic regression was used to investigate the association between developing endophthalmitis and each of these factors, adjusting for gender, age, and race. All analyses were performed using SAS software version 9.2 (SAS Institute, Cary, NC).
The Johns Hopkins Medicine Institutional Review Board and the Privacy Board at the Centers for Medicare and Medicaid Services approved the research protocol.
Results
The final analytic dataset contained 3,280,966 cataract surgeries within the two-year period, after excluding 165,452 surgeries which were performed in the last 42 days in 2004. Forty-four percent of individuals (n=1,005,826) undergoing cataract surgery had surgery on both eyes within the study period. In total, 4,006 patients developed endophthalmitis within 42 days of cataract surgery. The national endophthalmitis rate in 2003 was 1.32 per 1000 surgeries (95% confidence interval (CI): 1.27–1.38) and decreased to 1.11 per 1000 surgeries (95% CI: 1.06–1.16) in 2004 (Table 2). After adjusting for age, sex, race, surgical setting, surgeon years of experience and annual cataract surgery volume, surgeries performed in 2004 remained 13% less likely to develop endophthalmitis than surgeries performed in 2003.
Table 2.
Overall Post-cataract Surgery Endophthalmitis Rate by Year
| Year | Cataract Surgeries (N) |
Endophthalmitis Cases (N) |
Endophthalmitis Rate/1000 Surgeries within 42 days |
95% Confidence Interval |
|---|---|---|---|---|
| 2003 | 1,704,197 | 2,253 | 1.32 | 1.27–1.38 |
| 2004 | 1,576,769 | 1,753 | 1.11 | 1.06–1.16 |
| Overall | 3,280,966 | 4,006 | 1.22 | 1.18–1.26 |
Two-year average unadjusted state-based rates ranged from 0.4 – 2.1 per 1000 surgeries across states, with 50% of states having rates between 1.05 and 1.38 cases per 1000 surgeries (Tables 2 and 3). The annual number of surgeries performed varied substantially across states (4,500–280,000). Taking into account the wider confidence intervals associated with smaller numbers of surgeries and accompanying endophthalmitis cases, endophthalmitis rates were still statistically significantly different across states.
Table 3.
Post-Cataract Surgery Endophthalmitis Rates in 2003–2004 by State*
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| State* | # Cataract Surgeries |
# Endophthalmitis Cases |
Rate (per 1000 surgeries) |
95% CI¶ | Rate (per 1000 surgeries)§ |
95% CI§ |
| Alabama (AL) | 66602 | 71 | 1.07 | 0.8 -- 1.33 | 1.68 | 1.36 -- 1.99 |
| Arizona (AZ) | 54163 | 79 | 1.46 | 1.16 -- 1.75 | 1.87 | 1.53 -- 2.21 |
| Arkansas (AR) | 46193 | 35 | 0.76 | 0.44 -- 1.08 | 1.35 | 0.99 -- 1.71 |
| California | 225021 | 312 | 1.39 | 1.24 -- 1.53 | 1.58 | 1.37 -- 1.79 |
| Colorado (CO) | 31212 | 43 | 1.38 | 0.99 -- 1.77 | 1.46 | 1.04 -- 1.89 |
| Connecticut (CT) | 44581 | 62 | 1.39 | 1.07 -- 1.71 | 1.43 | 1.06 -- 1.8 |
| Delaware (DE) | 12329 | 17 | 1.38 | 0.76 -- 2 | 2.00 | 1.37 -- 2.64 |
| Florida (FL) | 284312 | 366 | 1.29 | 1.16 -- 1.42 | 1.79 | 1.58 -- 2.01 |
| Georgia (GA) | 91903 | 98 | 1.07 | 0.84 -- 1.29 | 1.53 | 1.25 -- 1.81 |
| Idaho (ID) | 17683 | 7 | 0.40 | 0.00 -- 0.91 | 0.81 | 0.25 -- 1.36 |
| Illinois (IL) | 136574 | 181 | 1.33 | 1.14 -- 1.51 | 1.65 | 1.4 -- 1.9 |
| Indiana (IN) | 101451 | 102 | 1.01 | 0.79 -- 1.22 | 1.60 | 1.33 -- 1.88 |
| Iowa (IA) | 50620 | 41 | 0.81 | 0.51 -- 1.11 | 1.32 | 0.97 -- 1.67 |
| Kansas (KS) | 44144 | 41 | 0.93 | 0.6 -- 1.25 | 1.45 | 1.08 -- 1.82 |
| Kentucky (KY) | 54921 | 68 | 1.24 | 0.95 -- 1.53 | 1.65 | 1.31 -- 1.99 |
| Louisiana (LA) | 55070 | 79 | 1.43 | 1.14 -- 1.73 | 1.88 | 1.54 -- 2.22 |
| Maine (ME) | 21420 | 24 | 1.12 | 0.65 -- 1.59 | 1.61 | 1.11 -- 2.11 |
| Maryland (MD) | 55171 | 72 | 1.31 | 1.01 -- 1.6 | 1.41 | 1.06 -- 1.75 |
| Massachusetts (MA) | 66132 | 87 | 1.32 | 1.05 -- 1.58 | 1.57 | 1.25 -- 1.89 |
| Michigan (MI) | 142570 | 149 | 1.05 | 0.86 -- 1.23 | 1.43 | 1.18 -- 1.68 |
| Minnesota (MN) | 53806 | 72 | 1.34 | 1.04 -- 1.63 | 1.54 | 1.19 -- 1.88 |
| Mississippi (MS) | 42866 | 90 | 2.10 | 1.77 -- 2.43 | 2.62 | 2.25 -- 3 |
| Missouri (MO) | 76214 | 96 | 1.26 | 1.01 -- 1.51 | 1.72 | 1.41 -- 2.02 |
| Montana (MT) | 14402 | 21 | 1.46 | 0.89 -- 2.03 | 1.81 | 1.22 -- 2.41 |
| Nebraska (NE) | 27172 | 30 | 1.10 | 0.69 -- 1.52 | 1.53 | 1.08 -- 1.98 |
| Nevada (NV) | 18884 | 19 | 1.01 | 0.51 -- 1.5 | 1.49 | 0.96 -- 2.02 |
| New Hampshire (NH) | 13378 | 11 | 0.82 | 0.23 -- 1.41 | 0.90 | 0.27 -- 1.53 |
| New Jersey (NJ) | 95981 | 149 | 1.55 | 1.33 -- 1.77 | 1.88 | 1.6 -- 2.16 |
| New Mexico (NM) | 16734 | 15 | 0.90 | 0.37 -- 1.43 | 1.38 | 0.83 -- 1.93 |
| New York (NY) | 168047 | 232 | 1.38 | 1.21 -- 1.55 | 1.51 | 1.28 -- 1.75 |
| North Carolina (NC) | 113504 | 123 | 1.08 | 0.88 -- 1.29 | 1.54 | 1.27 -- 1.8 |
| North Dakota (ND) | 14681 | 13 | 0.89 | 0.32 -- 1.45 | 1.30 | 0.71 -- 1.89 |
| Ohio (OH) | 140920 | 161 | 1.14 | 0.96 -- 1.32 | 1.53 | 1.28 -- 1.78 |
| Oklahoma (OK) | 52214 | 76 | 1.46 | 1.16 -- 1.76 | 1.97 | 1.64 -- 2.31 |
| Oregon (OR) | 30523 | 32 | 1.05 | 0.66 -- 1.44 | 1.19 | 0.76 -- 1.62 |
| Pennsylvania (PA) | 147454 | 186 | 1.26 | 1.08 -- 1.44 | 1.59 | 1.34 -- 1.84 |
| Rhode Island (RI) | 8807 | 16 | 1.82 | 1.09 -- 2.55 | 2.08 | 1.32 -- 2.84 |
| South Carolina (SC) | 56045 | 63 | 1.12 | 0.83 -- 1.41 | 1.52 | 1.18 -- 1.85 |
| South Dakota (SD) | 14255 | 8 | 0.56 | 0.00 -- 1.13 | 0.89 | 0.29 -- 1.49 |
| Tennessee (TN) | 86345 | 110 | 1.27 | 1.04 -- 1.51 | 1.71 | 1.43 -- 2 |
| Texas (TX) | 217056 | 227 | 1.05 | 0.9 -- 1.19 | 1.48 | 1.27 -- 1.7 |
| Utah (UT) | 24213 | 31 | 1.28 | 0.84 -- 1.72 | 1.60 | 1.12 -- 2.07 |
| Vermont (VT) | 6396 | 6 | 0.94 | 0.08 -- 1.79 | 1.19 | 0.31 -- 2.07 |
| Virginia (VA) | 80746 | 88 | 1.09 | 0.85 -- 1.33 | 1.47 | 1.17 -- 1.76 |
| Washington (WA) | 56952 | 60 | 1.05 | 0.77 -- 1.34 | 1.37 | 1.04 -- 1.7 |
| West Virginia (WV) | 24973 | 37 | 1.48 | 1.05 -- 1.91 | 1.94 | 1.47 -- 2.42 |
| Wisconsin (WI) | 71811 | 93 | 1.30 | 1.04 -- 1.55 | 1.57 | 1.26 -- 1.88 |
| Wyoming (WY) | 4515 | 7 | 1.55 | 0.53 -- 2.57 | 1.88 | 0.85 -- 2.92 |
State within the United States
CI: Confidence Interval
Adjusted for age, sex, race, surgery setting, surgeon years of experience and surgeon volume
An inverse dose-response relationship was seen between endophthalmitis rates and surgical volume, with a four-fold difference in endophthalmitis rates among surgeries performed by surgeons completing 50 or fewer surgeries per year compared with surgeries performed by surgeons whose annual volume was more than 1000 surgeries (Table 4). While 30% of surgeries were performed by surgeons who complete 200 or fewer surgeries per year, these surgeries accounted for 46% of endophthalmitis cases. The association between low surgical volume and higher endophthalmitis rates persisted in multivariate models (Table 5).
Table 4.
Endophthalmitis Rate by Annual Medicare Surgical Volume¶
| Annual Medicare Surgery Volume |
Total Physicians N(%) |
Total Surgeries N(%) |
Total Endophthalmitis Cases |
Overall Endophthalmitis Rate/1000 surgeries§ |
95% Confidence Interval |
|---|---|---|---|---|---|
| 1–50 | 4543 (38.3%) | 137,200 (4.2%) | 352 | 2.57 | (2.30 – 2.83) |
| 51–200 | 4529 (38.2%) | 975,370 (30.1%) | 1,455 | 1.49 | (1.42 – 1.57) |
| 201–500 | 2253 (19.0%) | 1,295,972 (39.9%) | 1,512 | 1.17 | (1.11 – 1.23) |
| 501–1000 | 451 (3.8%) | 565,158 (17.4%) | 454 | 0.80 | (0.73 – 0.88) |
| 1001+ | 97 (0.8%) | 272,198 (8.4%) | 168 | 0.62 | (0.52 – 0.71) |
Excludes 35,068 (1.1%) surgeries with unique physician identification numbers (UPIN) that cannot be attributed to a specific surgeon and surgeries for which surgeon characteristics data were missing.
Rate is overall for all surgeries within a specific annual volume category and does not reflect the average rate of endophthalmitis within each category.
Table 5.
Univariate and Multivariate Analyses of Risk Factors for Endophthalmitis after Cataract Surgery
| Unadjusted Relative Risk |
95% CI | Adjusted Relative Risk |
95% CI | |
|---|---|---|---|---|
| Age | ||||
| 65 – 74 years | 1.00 | Reference | 1.00 | Reference |
| 75 – 84 years | 1.12 | 1.04–1.19 | 1.11 | 1.04–1.19 |
| 85+ years | 1.58 | 1.43–1.74 | 1.53 | 1.38–1.69 |
| Sex | ||||
| Female | 1.00 | Reference | 1.00 | Reference |
| Male | 1.19 | 1.11–1.26 | 1.23 | 1.15–1.31 |
| Race | ||||
| Black | 1.22 | 1.08–1.38 | 1.17 | 1.03–1.33 |
| Hispanic | 1.13 | 0.89–1.42 | 1.09 | 0.86–1.38 |
| Asian | 1.16 | 0.91–1.50 | 0.99 | 0.76–1.29 |
| Native American | 1.52 | 0.96–2.42 | 1.72 | 1.07–2.77 |
| Others | 1.40 | 1.12–1.91 | 1.27 | 0.92–1.75 |
| White | 1.00 | Reference | 1.00 | Reference |
| Year | ||||
| 2003 | 1.19 | 1.12–1.27 | 1.20 | 1.13–1.28 |
| 2004 | 1.00 | Reference | 1.00 | Reference |
| Ambulatory Surgery Center | ||||
| No | 1.23 | 1.15–1.31 | 1.05 | 0.98–1.12 |
| Yes | 1.00 | Reference | 1.00 | Reference |
| Surgery volume | ||||
| 1–50 | 4.17 | 3.47–5.01 | 3.80 | 3.13–4.61 |
| 51–200 | 2.42 | 2.06–2.84 | 2.32 | 1.97–2.74 |
| 201–500 | 1.89 | 1.61–2.22 | 1.84 | 1.56–2.17 |
| 501–1000 | 1.30 | 1.09–1.55 | 1.30 | 1.09–1.56 |
| 1001+ | 1.00 | Reference | 1.00 | Reference |
| Surgeon experience | ||||
| 1–10 years | 1.55 | 1.38–1.74 | 1.41 | 1.25–1.59 |
| 11–20 years | 1.18 | 1.08–1.28 | 1.22 | 1.12–1.33 |
| 21–30 years | 1.06 | 0.97–1.15 | 1.10 | 1.01–1.20 |
| 30+ years | 1.00 | Reference | 1.00 | Reference |
CI: Confidence Interval
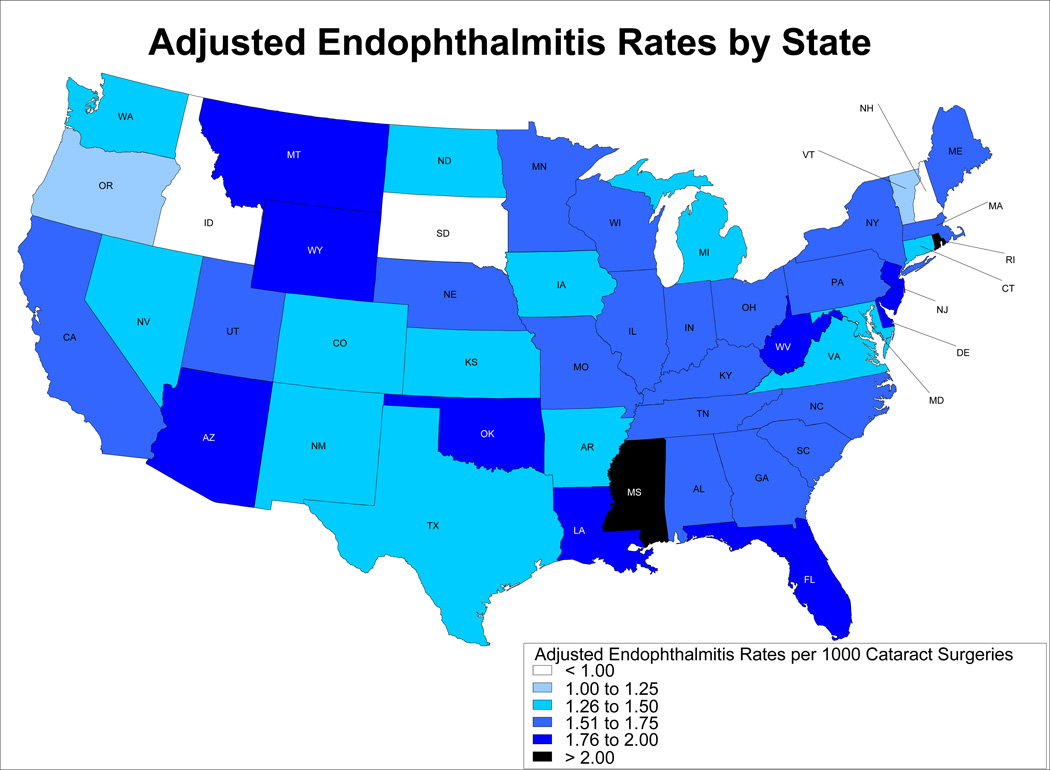
After adjustment for age, race, sex, year, surgical setting, surgeon experience and surgeon volume, differences between states were still apparent, with state-based adjusted rates ranging from 0.81 cases per 1000 surgeries to 2.62 (inter-quartile range 1.43–1.71) (Figure 1 and Table 3; Table 3 available at http://aaojournal.org.). No distinct geographic pattern in endophthalmitis rates was observed. In general, the lowest rates were seen in very northern states such as Idaho, South Dakota and New Hampshire and the highest rates were seen in southern states such as Mississippi and Oklahoma. However, rates at the upper end of the range also were seen in very northern states such as Montana and Wyoming, and several southerly states had adjusted rates below 1.5 per 1000 surgeries, suggesting that climate and latitude do not influence endophthalmitis rates.
Figure.
Rates of Endophthalmitis by State, Adjusted for age, sex, year of surgery, surgical setting, surgeon experience and surgeon volume
Fewer years of surgical experience correlated with increased endophthalmitis rates. In comparison to surgeries performed by surgeons with 30 years or more experience, surgeries performed by surgeons with fewer than 10 years of experience carried a 41% increased risk (relative risk (RR) 1.41; 95% CI: 1.25–1.59), even after adjusting for surgical volume, surgical setting and patient factors (Table 5).
Roughly fifty percent of all surgeries were performed in ASCs. In univariate analyses, surgeries performed in hospital outpatient centers had a 23% higher risk of endophthalmitis than surgeries performed in ASCs. However, after adjusting for age, gender, race, year of surgery and surgeon volume and experience, the risk associated with surgical setting was no longer apparent (adjusted RR 1.05; 95% CI 0.98–1.12). This finding is not surprising because while the age of patients receiving surgery was not different by setting, higher volume surgeons were more likely to work in ASCs. In ASCs, 13.1% of surgeries were performed by high volume surgeons (annual surgery volume >1000) compared to 3.7% of surgeries performed in hospitals.
Endophthalmitis risk increased with increasing age. Cataract surgery patients aged 85 years and older had a 53% increased risk of endophthalmitis compared with individuals aged 65–74 years (Table 4). Surgeries on males carried a 23% increased risk. Endophthalmitis risk varied by racial group, and surgeries on Black and Native Americans had increased risk compared to whites after adjustment (17% and 72% increased risk, respectively).
Discussion
These analyses report rates of endophthalmitis secondary to cataract surgery in the US for 2003–2004 using data from all surgeries performed on individuals with Medicare coverage in the fee-for-service setting. Over 3 million cataract surgeries were evaluated in this analysis, allowing calculation of precise annual estimates of endophthalmitis (1.22 per 1000 surgeries, on average). This dataset is larger by an order of magnitude than other population-based studies previously reported in Canada,17 Taiwan,18 Australia,19 the United Kingdom,20 Sweden,21–24 Norway,25 Finland,26 Denmark,27 and the Netherlands.28
Population-based studies can be generalized to the community more easily than center-specific studies, as they represent the broad range of conditions under which surgeries are conducted on diverse populations in a wide range of settings by many surgeons with varying levels of experience. In this analysis, we had the opportunity to access the complete Medicare billing dataset of cataract surgeries, while previous studies have been limited to 5% of the sample.12;29 Use of the full Medicare database allows not only precise estimates of endophthalmitis incidence, but also more detailed exploration of possible risk factors for disease. It also affords the opportunity to look at state-based rates, which is not feasible with smaller databases. Indeed we report differences in the rate of endophthalmitis between states, with adjusted rates ranging from 0.8–2.6. These differences are only partly accounted for by surgical factors and characteristics of patients accessing surgery in each state, suggesting that other factors may also play a role in this variation. Some potential factors that cannot be addressed in this paper include differences in socioeconomic status and general health of the population in each state.
As we and others have previously reported, we found that patient factors such as older age17;30;31 and male gender8;17 are associated with a higher risk of endophthalmitis after cataract surgery. Comorbidities, such as diabetes, hypertension and stroke are prevalent in cataract surgery populations and increase with age.32 While health-status data were not available, these variables likely would contribute to the age effect observed in this study. The increased risk of endophthalmitis in Blacks and Native Americans may be linked to social disadvantage and poorer general health status in these groups.
We found that fewer years of surgical experience increased the risk of post-operative endophthalmitis. With the exception of comparisons involving surgeons still in training,33 we believe this finding has not been previously reported. All of the surgeries included in this analysis were performed by physicians who were already in practice settings with their own UPINs. However, some of these surgeries surely were performed with an ophthalmology resident assisting, and we have not adjusted for this possibility. It is unlikely that surgeries including a resident would have a substantive influence on the outcome, given the small proportion of resident-assisted surgeries in the total volume of cataract surgery. Further, we have demonstrated that while experience is relevant, higher annual volume of surgery independently reduces risk of endophthalmitis. This finding is consistent with conclusions from Taiwan for endophthalmitis18 and in Canada for general post-surgical adverse events following cataract surgery.34 None of these studies, however, sheds light on what characteristics associated with higher volume (e.g., quicker surgery, more secure wounds, potential differences in anti-infection measures, etc.) are actually responsible for the reduced risk of endophthalmitis.
The Medicare billing database has been shown to be a valuable tool for evaluation of the apparent temporal trends in endophthalmitis across years in the US.12 We also find a temporal trend, where surgeries performed in 2003 had a higher risk of endophthalmitis compared to surgeries performed in 2004. It is unlikely that such a difference is a result of changes in procedure and diagnosis coding, as none of the codes utilized in this study changed during this time period, and the short duration makes it unlikely that a meaningful number of providers changed their billing practices during this time. This finding is in agreement with data from Canada17 and a single academic center in Florida,35 where more recent surgeries had lower risk. This risk reduction persisted after adjustment for available patient and surgical factors. However, information on surgical technique and prophylaxis were not available in this analysis, and it is possible that these factors have changed with time in the US. The authors are currently conducting further research in a nationally representative case-control study of endophthalmitis to address these questions.
The current study reports endophthalmitis rates that are lower than rates reported for prior years by our study team.12 In the prior study, the rate of endophthalmitis increased over a several-year period, peaking in 2000 and then showing a slight decline in 2001. Our study team did not have access to 2002 data for either analysis. Before making direct comparisons between the two studies, several factors should be noted. First, the earlier study did not have access to date-specific data for several years, but instead relied on the use of calendar quarter as the time unit. As a result, for comparison across years, that study utilized a 90-day rate for years in which date-specific data were available while the current study used six weeks as the cutoff. Although the vast majority of endophthalmitis occur within the first few weeks after surgery, one would expect a slightly lower rate when using a 6-week cutoff.
In addition, somewhat different methodology was used in the current study. In this study, more billing data were available, which allowed a more precise determination of which records were true cataract surgery data as compared to one-day post-operative data. In addition, CPT code 66840 was not used since it can reflect post-cataract surgery removal of retained lens material rather than an incident cataract surgery. Determining which direction such increased precision of cataract surgeries would alter the rates is difficult to predict. The current study also utilized the 100% sample data while the earlier study utilized 5% sample data. With such large databases, one would expect that the 5% sample would also give a reliable estimate, but with less precision than the 100% sample. One may argue that the switch from inpatient to nearly exclusively outpatient management of endophthalmitis cases may have affected case ascertainment. However, our methods are based on CPT coding, and it is unlikely that a substantial portion of tap and inject procedures are not billed. Therefore, we believe that our methods captured the vast majority of cases. Finally, endophthalmitis rates may indeed have declined during this time period. In the early 2000’ s, significant attention began being placed on examining whether wound construction has an effect on endophthalmitis rates, with particular emphasis on the use of clear corneal incisions.13;36–39 As a result, surgeons may have changed their practice to improve the quality and closure of their incisions, and these changes may have had an effect on endophthalmitis rates. Wound construction continues to be a popular area of research with numerous studies in recent years investigating this question.11;20;30;40–44
Internationally reported estimates of the incidence of endophthalmitis following cataract surgery range widely, with rates reported as high as 1 per 300 cataract procedures and as low as no events over a several-year period.8–11 The overall annual rate of endophthalmitis secondary to cataract surgery reported from this US-based study is substantially higher than the rate reported in Sweden (0.48 per 1000 surgeries) for the same time period,30 but very similar to the rate reported for the same time period in Ontario, Canada (1.4 per 1000 surgeries).45 The reason for differences between countries is uncertain, and may include differences in surgical procedures, prophylaxis practices, differences in racial or socioeconomic status, or methodological differences in measurement. Several researchers have debated the reasons for the differences in rates between Sweden and Ontario, with some proposing that the difference results from the use of intracameral antibiotics, while others suggest it is a difference in patient populations.46–48 A similar debate could be held with these data; however, as with the Ontario-based study, these data are based on billing claims and as such do not include information regarding the antibiotic prophylaxis used by each surgeon. Detailed medical record review would be required to determine which hypothesis holds true.
Recent surveys of surgeons in the US showed that a large and increasing proportion of procedures are performed at ASCs. In agreement with Leaming,49 we found that 50% of procedures were conducted at an ASC, and the majority of these surgeries were performed by higher-volume cataract surgeons. The lower rate of endophthalmitis in ASCs is likely explained by differences in surgeon characteristics in these settings, and not by the relative safety of the setting itself. Possible hypotheses to explain lower risk in high-volume surgeons might include differences in procedure including antibiotic prophylaxis, better wound construction, faster surgery and fewer intra-operative complications; however, these theories could not be explored in this dataset.
This analysis was based on carrier file claims data, and as such, only includes claims for fee-for-service (FFS) Medicare surgeries. In 2003–2004, 15% of Medicare participants in the 48 contiguous states had HMO coverage, and their surgeries are not included in the carrier file data. Therefore, our estimate of cataract surgeries does not provide a complete count of the cataract surgeries performed on Medicare beneficiaries during this time period. Furthermore, individuals enrolled in Medicare HMOs may differ from FFS individuals. Given the relatively small proportion of individuals enrolled in HMOs, it is unlikely that the inclusion of these individuals would substantially alter the interpretation of results.
Another limitation of the methodology is that identification of endophthalmitis cases relied on the accuracy of billing codes, which may be subject to misclassification. Li et al previously reported high rates of misclassification in coding both cataract surgery and endophthalmitis claims in Western Australia for 1980–1999.50 This rate of misclassification likely is not applicable to the US rate, given the differences in coding practices in the US, and the long-standing use of ICD-9-CM codes in the US. Tielsch et al previously reported a misclassification rate of approximately 34% for identifying retinal detachment post-cataract surgery using Medicare claims data from the early 1990’s.51 However, nearly half of this misclassification came from identifying retinal detachment procedures performed on the contra-lateral eye. In the current study, contra-lateral eye issues are less likely to be a problem, given the rare nature of endophthalmitis, and with cataract surgery being one of the primary risk factors in developing endophthalmitis. Additionally in this study, if an individual had surgery on both eyes within a short time period and developed endophthalmitis after one surgery, the eye to which the endophthalmitis would have been assigned is irrelevant. In either case, the individual would be considered to have endophthalmitis and would contribute one endophthalmitis case and two cataract surgeries to the analysis. Furthermore, several recent studies using Medicare data have reported improved accuracy in coding, with positive predictive values typically above 90% for diseases ranging from Alzheimer’s to kidney-cancer surgery.52–54
In summary, the 2003–2004 complete Medicare claims dataset of cataract surgeries has been used to make precise, population-based estimates of the risk of endophthalmitis secondary to cataract surgery in the US. Although the analysis demonstrated a decrease between 2003 and 2004, rates remain above published rates from Sweden, and it is likely that rates could be further reduced with changes in practice patterns. Although variation in endophthalmitis rates was seen across states, 50% of states had an adjusted rate between 1.43 and 1.71 cases per 1000 surgeries, and most states with higher or lower rates were those with smaller populations, which resulted in wider confidence intervals. Despite this, the size of variation suggests an area for future research to elucidate whether potential interventions might reduce disparities. Differences in socioeconomic status and health status across states may well explain part of the variation in rates. The analysis approach utilized here is valuable for ongoing monitoring of endophthalmitis rates, a disease which, though rare, is important given the large number of individuals who have cataract surgery each year in the US.
Acknowledgments
Funding: Support for this project was provided by the National Eye Institute: R01 EY016769. Dr. Keay is funded by an Australian National Health and Medical Research Council post-doctoral fellowship. Dr. Gower is the recipient of an Ernest and Elizabeth Althouse Special Scholar’s Award from Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Williams A, Sloan FA, Lee PP. Longitudinal rates of cataract surgery. Arch Ophthalmol. 2006;124:1308–1314. doi: 10.1001/archopht.124.9.1308. [DOI] [PubMed] [Google Scholar]
- 2.Karacal H, Kymes SM, Apte RS. Retrospective analysis of etiopathogenesis of all cases of endophthalmitis at a large tertiary referral center. Int Ophthalmol. 2007;27:251–259. doi: 10.1007/s10792-007-9068-3. [DOI] [PubMed] [Google Scholar]
- 3.Lalwani GA, Flynn HW, Jr, Scott IU, et al. Acute-onset endophthalmitis after clear corneal cataract surgery (1996–2005): clinical features, causative organisms, and visual acuity outcomes. Ophthalmology. 2008;115:473–476. doi: 10.1016/j.ophtha.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Schmier JK, Halpern MT, Covert DW, et al. Evaluation of Medicare costs of endophthalmitis among patients after cataract surgery. Ophthalmology. 2007;114:1094–1099. doi: 10.1016/j.ophtha.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 5.Colin X, Berdeaux G, Lafuma A, et al. Inpatient costs of endophthalmitis evaluated for the whole of France. Appl Health Econ Health Policy. 2010;8:53–60. doi: 10.1007/BF03256165. [DOI] [PubMed] [Google Scholar]
- 6.Javitt JC, Vitale S, Canner JK, et al. National outcomes of cataract extraction: endophthalmitis following inpatient surgery. Arch Ophthalmol. 1991;109:1085–1089. doi: 10.1001/archopht.1991.01080080045025. [DOI] [PubMed] [Google Scholar]
- 7.Powe NR, Schein OD, Gieser SC, et al. Cataract Patient Outcome Research Team Synthesis of the literature on visual acuity and complications following cataract extraction with intraocular lens implantation. Arch Ophthalmol. 1994;112:239–252. doi: 10.1001/archopht.1994.01090140115033. [DOI] [PubMed] [Google Scholar]
- 8.ESCRS Endophthalmitis Study Group. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33:978–988. doi: 10.1016/j.jcrs.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 9.Bohigian GM. A retrospective study of the incidence of culture-positive endophthalmitis after cataract surgery and the use of preoperative antibiotics. Ophthalmic Surg Lasers Imaging. 2007;38:103–106. doi: 10.3928/15428877-20070301-03. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Arumi J, Fonollosa A, Sararols L, et al. Topical anesthesia: possible risk factor for endophthalmitis after cataract extraction. J Cataract Refract Surg. 2007;33:989–992. doi: 10.1016/j.jcrs.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 11.Monica ML, Long DA. Nine-year safety with self-sealing corneal tunnel incision in clear cornea cataract surgery. Ophthalmology. 2005;112:985–986. doi: 10.1016/j.ophtha.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 12.West ES, Behrens A, McDonnell PJ, et al. The incidence of endophthalmitis after cataract surgery among the U.S. Medicare population increased between 1994 and 2001. Ophthalmology. 2005;112:1388–1394. doi: 10.1016/j.ophtha.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 13.Taban M, Behrens A, Newcomb RL, et al. Acute endophthalmitis following cataract surgery: a systematic review of the literature. Arch Ophthalmol. 2005;123:613–620. doi: 10.1001/archopht.123.5.613. [DOI] [PubMed] [Google Scholar]
- 14.Gray DT, Hodge DO, Ilstrup DM, et al. Concordance of Medicare data and population-based clinical data on cataract surgery utilization in Olmsted County, Minnesota. Am J Epidemiol. 1997;145:1123–1126. doi: 10.1093/oxfordjournals.aje.a009075. [DOI] [PubMed] [Google Scholar]
- 15.CPT: 2004 Current Procedural Terminology: Professional Edition. Chicago, IL: American Medical Association; 2003. [Google Scholar]
- 16.ICD 9-CM: International Classification of Diseases 9th Revision, Clinical Modification. 4th ed. Los Angeles, CA: Practical Management Information Corp.; 1994. [Google Scholar]
- 17.Freeman EE, Roy-Gagnon MH, Fortin E, et al. Rate of endophthalmitis after cataract surgery in Quebec, Canada, 1996–2005. Arch Ophthalmol. 2010;128:230–234. doi: 10.1001/archophthalmol.2009.380. [DOI] [PubMed] [Google Scholar]
- 18.Fang YT, Chien LN, Ng YY, et al. Association of hospital and surgeon operation volume with the incidence of postoperative endophthalmitis: Taiwan experience. Eye (Lond) 2006;20:900–907. doi: 10.1038/sj.eye.6702045. [DOI] [PubMed] [Google Scholar]
- 19.Semmens JB, Li J, Morlet N, Ng J teamEPSWA. Trends in cataract surgery and postoperative endophthalmitis in Western Australia (1980–1998): the Endophthalmitis Population Study of Western Australia. Clin Experiment Ophthalmol. 2003;31:213–219. doi: 10.1046/j.1442-9071.2003.00647.x. [DOI] [PubMed] [Google Scholar]
- 20.Mollan SP, Gao A, Lockwood A, et al. Postcataract endophthalmitis: incidence and microbial isolates in a United Kingdom region from 1996 through 2004. J Cataract Refract Surg. 2007;33:265–268. doi: 10.1016/j.jcrs.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 21.Lundstrom M, Stenevi U, Thorburn W. The Swedish National Cataract Register: a 9-year review. Acta Ophthalmol Scand. 2002;80:248–257. doi: 10.1034/j.1600-0420.2002.800304.x. [DOI] [PubMed] [Google Scholar]
- 22.Lundstrom M, Wejde G, Stenevi U, et al. Endophthalmitis after cataract surgery: a nationwide prospective study evaluating incidence in relation to incision type and location. Ophthalmology. 2007;114:866–870. doi: 10.1016/j.ophtha.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 23.Wejde G, Montan P, Lundstrom M, et al. Endophthalmitis following cataract surgery in Sweden: national prospective survey 1999–2001. Acta Ophthalmol Scand. 2005;83:7–10. doi: 10.1111/j.1600-0420.2005.00377.x. [DOI] [PubMed] [Google Scholar]
- 24.Montan P, Lundstrom M, Stenevi U, Thorburn W. Endophthalmitis following cataract surgery in Sweden: the 1998 national prospective survey. Acta Ophthalmol Scand. 2002;80:258–261. doi: 10.1034/j.1600-0420.2002.800305.x. [DOI] [PubMed] [Google Scholar]
- 25.Sandvig KU, Dannevig L. Postoperative endophthalmitis: establishment and results of a national registry. J Cataract Refract Surg. 2003;29:1273–1280. doi: 10.1016/s0886-3350(02)02048-5. [DOI] [PubMed] [Google Scholar]
- 26.Haapala TT, Nelimarkka L, Saari JM, et al. Endophthalmitis following cataract surgery in southwest Finland from 1987 to 2000. Graefes Arch Clin Exp Ophthalmol. 2005;243:1010–1017. doi: 10.1007/s00417-005-1190-1. [DOI] [PubMed] [Google Scholar]
- 27.Norregaard JC, Thoning H, Bernth-Petersen P, et al. Risk of endophthalmitis after cataract extraction: results from the International Cataract Surgery Outcomes study. Br J Ophthalmol. 1997;81:102–106. doi: 10.1136/bjo.81.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Versteegh MF, Van Rij G. Incidence of endophthalmitis after cataract surgery in the Netherlands: several surgical techniques compared. Doc Ophthalmol. 2000;100:1–6. doi: 10.1023/a:1001723124191. [DOI] [PubMed] [Google Scholar]
- 29.Javitt JC, Street DA, Tielsch JM, et al. Cataract Patient Outcomes Research Team. National outcomes of cataract extraction: retinal detachment and endophthalmitis after outpatient cataract surgery. Ophthalmology. 1994;101:100–105. doi: 10.1016/s0161-6420(13)31251-2. [DOI] [PubMed] [Google Scholar]
- 30.Lundstrom M, Wejde G, Stenevi U, et al. Endophthalmitis after cataract surgery: a nationwide prospective study evaluating incidence in relation to incision type and location. Ophthalmology. 2007;114:866–870. doi: 10.1016/j.ophtha.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Morlet N, Ng JQ, et al. Team EPSWA. Significant nonsurgical risk factors for endophthalmitis after cataract surgery: EPSWA fourth report. Invest Ophthalmol Vis Sci. 2004;45:1321–1328. doi: 10.1167/iovs.03-1000. [DOI] [PubMed] [Google Scholar]
- 32.Desai P, Minassian DC, Reidy A. National cataract surgery survey 1997–8: a report of the results of the clinical outcomes. Br J Ophthalmol. 1999;83:1336–1340. doi: 10.1136/bjo.83.12.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ravindran RD, Venkatesh R, Chang DF, et al. Incidence of post-cataract endophthalmitis at Aravind Eye Hospital: outcomes of more than 42,000 consecutive cases using standardized sterilization and prophylaxis protocols. J Cataract Refract Surg. 2009;35:629–636. doi: 10.1016/j.jcrs.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Bell CM, Hatch WV, Cernat G, Urbach DR. Surgeon volumes and selected patient outcomes in cataract surgery: a population-based analysis. Ophthalmology. 2007;114:405–410. doi: 10.1016/j.ophtha.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 35.Wykoff CC, Parrott MB, Flynn HW, Jr, et al. Nosocomial acute-onset postoperative endophthalmitis at a university teaching hospital (2002–2009) Am J Ophthalmol. 2010;150:392–398. doi: 10.1016/j.ajo.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Cooper BA, Holekamp NM, Bohigian G, Thompson PA. Case-control study of endophthalmitis after cataract surgery comparing scleral tunnel and clear corneal wounds. Am J Ophthalmol. 2003;136:300–305. doi: 10.1016/s0002-9394(03)00202-2. [DOI] [PubMed] [Google Scholar]
- 37.Nagaki Y, Hayasaka S, Kadoi C, et al. Bacterial endophthalmitis after small-incision cataract surgery: effect of incision placement and intraocular lens type. J Cataract Refract Surg. 2003;29:20–26. doi: 10.1016/s0886-3350(02)01483-9. [DOI] [PubMed] [Google Scholar]
- 38.Lertsumitkul S, Myers PC, O'Rourke MT, Chandra J. Endophthalmitis in the western Sydney region: a case-control study. Clin Experiment Ophthalmol. 2001;29:400–405. doi: 10.1046/j.1442-9071.2001.d01-20.x. [DOI] [PubMed] [Google Scholar]
- 39.Eifrig CW, Flynn HW, Jr, Scott IU, Newton J. Acute-onset postoperative endophthalmitis: review of incidence and visual outcomes (1995–2001) Ophthalmic Surg Lasers. 2002;33:373–378. [PubMed] [Google Scholar]
- 40.Ng JQ, Morlet N, Bulsara MK, Semmens JB. Reducing the risk for endophthalmitis after cataract surgery: population-based nested case-control study: endophthalmitis population study of Western Australia sixth report. J Cataract Refract Surg. 2007;33:269–280. doi: 10.1016/j.jcrs.2006.10.067. [DOI] [PubMed] [Google Scholar]
- 41.Colleaux KM, Hamilton WK. Effect of prophylactic antibiotics and incision type on the incidence of endophthalmitis after cataract surgery. Can J Ophthalmol. 2000;35:373–378. doi: 10.1016/s0008-4182(00)80124-6. [DOI] [PubMed] [Google Scholar]
- 42.Thoms SS, Musch DC, Soong HK. Postoperative endophthalmitis associated with sutured versus unsutured clear corneal cataract incisions. Br J Ophthalmol. 2007;91:728–730. doi: 10.1136/bjo.2006.109827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oshika T, Hatano H, Kuwayama Y, et al. Incidence of endophthalmitis after cataract surgery in Japan. Acta Ophthalmol Scand. 2007;85:848–851. doi: 10.1111/j.1600-0420.2007.00932.x. [DOI] [PubMed] [Google Scholar]
- 44.Miller JJ, Scott IU, Flynn HW, Jr, et al. Acute-onset endophthalmitis after cataract surgery (2000–2004): incidence, clinical settings, and visual acuity outcomes after treatment. Am J Ophthalmol. 2005;139:983–987. doi: 10.1016/j.ajo.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 45.Hatch WV, Cernat G, Wong D, et al. Risk factors for acute endophthalmitis after cataract surgery: a population-based study. Ophthalmology. 2009;116:425–430. doi: 10.1016/j.ophtha.2008.09.039. [DOI] [PubMed] [Google Scholar]
- 46.Anijeet D. Endophthalmitis after cataract surgery [letter] Ophthalmology. 2010;117:853. doi: 10.1016/j.ophtha.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Hatch W, Wong D, Devenyi R, Bell C. In reply to: Anijeet D. Endophthalmitis after cataract surgery [letter] Ophthalmology. 2010;117:853–854. [Google Scholar]
- 48.Montan P, Wejde G. In reply to: Anijeet D. Endophthalmitis after cataract surgery [letter]Author reply. Ophthalmology. 2010;117:854–855. doi: 10.1016/j.ophtha.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Leaming DV. Practice styles and preferences of ASCRS members--2002 survey. J Cataract Refract Surg. 2003;29:1412–1420. doi: 10.1016/s0886-3350(03)00405-x. [DOI] [PubMed] [Google Scholar]
- 50.Li J, Morlet N, Semmens J, et al. EPSWA Team. Coding accuracy for endophthalmitis diagnosis and cataract procedures in Western Australia. The Endophthalmitis Population Study of Western Australia (EPSWA): second report. Ophthalmic Epidemiol. 2003;10:133–145. doi: 10.1076/opep.10.2.133.13898. [DOI] [PubMed] [Google Scholar]
- 51.Tielsch JM, Legro MW, Cassard S, et al. Risk Factors for Retinal Detachment after Cataract Surgery: A Population-based Case-Control Study. Ophthalmology. 1996;103:1537–1545. doi: 10.1016/s0161-6420(96)30465-x. [DOI] [PubMed] [Google Scholar]
- 52.Eichler AF, Lamont EB. Utility of administrative claims data for the study of brain metastases: a validation study. J Neurooncol. 2009;95:427–431. doi: 10.1007/s11060-009-9943-z. [DOI] [PubMed] [Google Scholar]
- 53.Miller DC, Saigal CS, Warren JL, et al. External validation of a claims-based algorithm for classifying kidney-cancer surgeries. BMC Health Serv Res. 2009;9:92. doi: 10.1186/1472-6963-9-92. 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kiyota Y, Schneeweiss S, Glynn RJ, et al. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148:99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]