Abstract
Patients with autonomic failure have disabling orthostatic hypotension due to impaired sympathetic activity. Norepinephrine transporter (NET) blockade with atomoxetine raises blood pressure in autonomic failure by increasing synaptic norepinephrine concentrations in postganglionic sympathetic neurons. This effect requires tonic release of norepinephrine, which is decreased in patients with low sympathetic tone. We hypothesized that increasing residual sympathetic outflow with the α-2 antagonist yohimbine would potentiate the pressor effect of NET blockade with atomoxetine, and improve orthostatic tolerance in peripheral autonomic failure. Seventeen patients received a single oral dose of either placebo, yohimbine 5.4 mg, atomoxetine 18 mg and the combination yohimbine and atomoxetine in a single blind, crossover study. Blood pressure was assessed while patients were seated and standing for up to 10 minutes before and 1 hour post-drug. Neither yohimbine nor atomoxetine significantly increased seated systolic blood pressure (SBP) or orthostatic tolerance compared to placebo. The combination, however, significantly increased seated SBP and orthostatic tolerance (P<0.001 and P=0.016, respectively) in a synergistic manner. The maximal increase in seated SBP seen with the combination was 31±33 mmHg at 60 minutes post-drug. Only the combination showed a significant improvement in orthostatic symptoms. In conclusion, the combination yohimbine and atomoxetine had a synergistic effect on blood pressure and orthostatic tolerance in peripheral autonomic failure, which may be explained by an increased release of norepinephrine in peripheral sympathetic neurons by α-2 antagonism combined with a reduced norepinephrine clearance by NET blockade. Safety studies are required to address the clinical usefulness of this pharmacological approach.
Keywords: autonomic failure, orthostatic hypotension, dysautonomia, atomoxetine, yohimbine, orthostatic tolerance
Severe orthostatic hypotension is one of the most disabling symptoms among patients with autonomic failure.1 Many patients require pharmacological therapy to reduce orthostatic symptoms and improve standing time. The current recommended first-line therapy is limited to medications that increase sodium reabsorption and expand plasma volume, such as fludrocortisone,2 or direct vasoconstrictors, such as midodrine.3 Their use, however, is frequently limited by the appearance of adverse effects, or by the lack of response in the most severe cases of autonomic failure. Thus, in many patients with severe orthostatic hypotension, alternative pharmacological approaches must be considered in addition to, or instead of these drugs.
Given that the loss of efferent sympathetic function may be incomplete in many patients with severe autonomic failure,4 medications manipulating norepinephrine metabolism can enhance residual sympathetic activity, and produce profound pressor responses in autonomic failure patients.5-7 Atomoxetine, a selective norepinephrine transporter (NET) inhibitor, raises blood pressure (BP) in autonomic failure patients by increasing synaptic norepinephrine concentrations in postganglionic sympathetic neurons. Patients with central autonomic impairment (multiple system atrophy [MSA]), who have intact postganglionic sympathetic fibers and residual sympathetic tone, had large pressor responses to atomoxetine; while patients with peripheral sympathetic denervation and low sympathetic tone such as pure autonomic failure (PAF) and Parkinson disease (PD) are less likely to have a pressor effect.7 It seems, therefore, that atomoxetine requires tonic release of norepinephrine from the nerve terminals to elicit a pressor effect in these patients.
In this study, we hypothesized that increasing residual sympathetic outflow with the selective α-2 adrenergic receptor antagonist, yohimbine,8, 9 would potentiate the pressor effect of NET blockade with atomoxetine, and improve orthostatic tolerance in patients with peripheral autonomic failure. In addition, we evaluated whether the effect of the combination on seated blood pressure and orthostatic tolerance is synergistic, and whether this interaction would translate into improvement of orthostatic symptoms.
Methods
Subjects
A total of 17 patients with severe peripheral autonomic failure (12 with pure autonomic failure [PAF] and 5 with Parkinson disease [PD]) were recruited from referrals to Vanderbilt University Autonomic Dysfunction Center. PAF and PD with autonomic failure were defined using the diagnostic criteria of the American Autonomic Society.10 Orthostatic hypotension was defined as ≥20-mmHg decrease in systolic blood pressure (SBP) or ≥10 mmHg of diastolic blood pressure (DBP) within 3 minutes on standing.10 Patients were excluded if they had secondary causes of autonomic failure (e.g., diabetes mellitus or amyloidosis), or if they had contraindications to administration of pressor agents (e.g. coronary artery disease). The Vanderbilt University Investigational Review Board approved this study, and written informed consent was obtained from each subject before initiating the study (ClinicalTrials.gov NCT00223691).
General Protocol
Patients were admitted to the Clinical Research Center at Vanderbilt University and were fed a low-monoamine, caffeine-free diet containing 150-mEq sodium and 70-mEq potassium per day. Medications affecting blood pressure, blood volume and the autonomic nervous system were withheld for ≥5 half-lives before testing. The screening consisted of a medical history, physical examination, 12-lead ECG, and laboratory assessments. Standardized autonomic function tests were performed to evaluate the severity of the autonomic impairment.11 These included orthostatic stress test, Valsalva maneuver, cold pressor test, isometric handgrip and sinus arrhythmia.12 Blood pressure and heart rate were obtained using an automated oscillometric sphygmomanometer (Dinamap, GE Medical Systems Information Technologies), finger photoplethysmography (Finometer, FMS, or Nexfin, BMEYE), and continuous ECG. During the orthostatic test, blood samples were obtained for catecholamines while patients were supine and upright, as described previously.7 Plasma catecholamines were determined by high-performance liquid chromatography with electrochemical detection.13
Acute Medication Trials
Medication trials were conducted in the morning, in a post-void state, and ≥2.5 hours after meals to avoid any acute hemodynamic effect from eating. Patients were seated on a chair with their feet on the floor. Blood pressure and heart rate were recorded every 5 minutes with an automated brachial blood pressure cuff (Dinamap; Critikon, Tampa, Florida), and digitally acquired into a custom designed database (Microsoft Access, Microsoft Corporation). Baseline parameters were measured for 30 minutes, and orthostatic tolerance was tested by measuring blood pressure and heart rate on standing for up to 10 minutes. After 5 minutes of drug administration, blood pressure and heart rate were measured for up to 60 minutes, and the assessment of orthostatic tolerance was repeated at the end of this period, as described above. Patients were asked to rate the severity of their orthostatic symptoms immediately after the orthostatic stress tests using an orthostatic symptom questionnaire.14 The questionnaire consisted of 6 items, including 1) lightheadedness, dizziness, feeling faint or like passing out; 2) blurring vision, seeing spots, tunnel vision; 3) trouble concentrating; 4) weakness; 5) fatigue; and 6) head, neck or shoulder discomfort. Each item was scored on a 0-10 scale (with 0 reflecting absence of symptoms), and the total scores (range 0-60) before and after treatment were used as a measure of symptom burden.
Patients were given a single oral dose of placebo, yohimbine 5.4 mg (Goldline, Ft. Lauderdale, Florida), atomoxetine 18 mg (Eli Lilly pharmaceuticals, Indianapolis, Ind) or the combination of yohimbine 5.4 mg and atomoxetine 18 mg in a single-blind, crossover fashion. Medication trials with placebo, yohimbine and atomoxetine were done in a random order; but for safety reasons the combination was given on the third or fourth study day. We were concerned that patients who had a good pressor response to either atomoxetine or yohimbine alone could have a larger and unsafe pressor response to the combination. Thus, if yohimbine and/or atomoxetine alone produced an increase in seated SBP ≥150 mmHg at 60 minutes post-drug, the study day with the combination was not performed.
Statistical Methods
We hypothesized that the combination had a greater effect on seated blood pressure compared to each drug alone, and that the combination had a synergistic effect on seated blood pressure compared to the sum of pressor effect of the two drugs individually. The primary outcome was the seated SBP at 60 minutes post-drug, and the seated SBP at baseline was adjusted along with age in the analysis. Both measurements were logarithmic transformed to reduce skewness in their distribution. A random effects model was used to examine whether the mean of log seated SBP 60 minutes after the combination was higher than that of each drug alone (placebo, atomoxetine and yohimbine), and than the sum effects of those during atomoxetine and yohimbine alone after adjusting for age and the log seated SBP at baseline. The model was also used to test whether the mean of log seated SBP at baseline was different between the treatment groups. A similar approach was used to test whether there is any difference in the mean of log heart rate (HR) 60 minutes after the treatment between the treatment groups, after adjusting for the log of average HR at baseline.
Secondary outcomes included orthostatic tolerance and orthostatic symptom score. The orthostatic tolerance was defined as the area under the curve of standing SBP calculated by the trapezoidal rule (AUCSBP; upright SBP multiplied by standing time). The AUCSBP at 60 minutes post-drug was considered as the outcome and the AUCSBP at baseline was adjusted along with age in the analysis. Both AUCSBP were logarithmic transformed due to skewed distribution. A random effects model was used to test differences in the orthostatic tolerance between the treatment groups, and between the combination vs. the sum of effects after atomoxetine and yohimbine alone. Comparisons were made only for patients who could stand after all active medications. Wilcoxon signed-rank test was used to test whether each treatment decreased the orthostatic symptom score compared to the baseline. Differences in post-drug total symptom scores between the treatment groups were analyzed by using a random effects model with adjustment of the baseline total symptom scores. Data are presented as mean ± SD unless otherwise noted. All of the tests were 2-tailed, and a P value of <0.05 was considered significant. Analyses were performed with STATA 11.0 (StataCorp, College Station, TX) and SPSS for Windows, version 17.0 (SPSS Inc., Chicago, IL).
Results
Patient Characteristics and Autonomic Testing
We studied 17 patients (7 men, 64±11 years) with severe peripheral autonomic failure, 12 met criteria for PAF and 5 for PD (Table 1). Supine SBP was similar in both groups (P=0.661), whereas upright SBP was higher in PD patients (P<0.01). Both groups had severe orthostatic hypotension (PAF −77±29 vs. PD −54±25; P=0.190). Supine and upright HR did not differ between groups (P=0.518 and P=0.949, respectively). Supine and upright plasma norepinephrine were similarly low in PAF and PD patients (supine, 100±85 vs. 78±46 pg/mL, P=0.792; upright, 186±216 vs. 121±53 pg/mL, P=0.673). There were no significant differences in age or body mass index between groups. The results of the orthostatic stress test and autonomic function testing of the whole group are shown in Table 2. The mean supine blood pressure and heart rate of the whole group was 143±31/82±14 mmHg and 72±7 bpm, respectively. On standing, all patients had a pronounced decrease in SBP (−69±29 mmHg) without an adequate compensatory heart rate increase (15±13 bpm). Sinus arrhythmia was markedly reduced in all patients. The decrease in SBP during phase II of the Valsalva maneuver was exaggerated compared to responses in normal controls, and the SBP overshoot during phase IV was absent. The Valsalva ratio was low, indicating inadequate compensatory changes of heart rate. Hyperventilation produced a substantial decrease in SBP. The pressor responses to pain stimuli (cold pressor test) and isometric exercise (handgrip) were absent. Thus, autonomic testing showed severe sympathetic and parasympathetic impairment in these patients.
Table 1. Patient Characteristics.
| Parameters | PAF (n=12) |
PD (n=5) |
All Patients (n=17) |
|---|---|---|---|
| Gender, male/female | 6/6 | 1/4 | 7/10 |
| Age, yr | 63 ± 11 | 66 ± 13 | 64 ± 11 |
| BMI, Kg/m2 | 25.0 ± 4.1 | 24.9 ± 4.0 | 24.9 ± 4.0 |
| Systolic blood pressure, mmHg | |||
| Supine | 140 ± 35 | 148 ± 25 | 143 ± 31 |
| Upright | 63 ± 13 | 94 ± 10 | 74 ± 19 |
| Heart rate, bpm | |||
| Supine | 71 ± 7 | 74 ± 7 | 72 ± 7 |
| Upright | 87 ± 17 | 86 ± 12 | 86 ± 15 |
| Norepinephrine, pg/mL | |||
| Supine | 100 ± 85 | 78 ± 46 | 93 ± 74 |
| Upright | 186 ± 216 | 121 ± 53 | 166 ± 184 |
Data are presented as mean±SD. PAF, pure autonomic failure; PD Parkinson disease; BMI, body mass index.
Table 2. Autonomic Function Tests and Orthostatic Stress Test.
| Parameters | All Patients | Normals* |
|---|---|---|
| Orthostatic change in systolic BP, mmHg | −69 ± 29 | ≤ 20 |
| Orthostatic change in heart rate, bpm | 15 ± 13 | 5 – 10 |
| Sinus arrhythmia ratio | 1.05 ± 0.03 | 1.2 ± 0.1 |
| Depressor response to Valsalva in phase II, mmHg | −61 ± 27 | ≤ 20 |
| BP response to Valsalva phase IV, mmHg† | −38 ± 14 | >20 |
| Valsalva ratio | 1.09 ± 0.1 | 1.5 ± 0.2 |
| Depressor response to hyperventilation, mmHg | −22 ± 17 | −5 ± 6.3 |
| Pressor response to cold pressor, mmHg | 1 ± 9.7 | 24 ± 13 |
| Pressor response to handgrip, mmHg | 0 ± 9.7 | 16 ± 6 |
Values are expressed as mean±SD. Pressor responses are given as changes in systolic BP.
Normal values are from the Autonomic Dysfunction Database at Vanderbilt University.
A negative value for phase IV of the Valsalva maneuver indicates that the blood pressure overshoot was absent.
Pressor Effect of Drugs
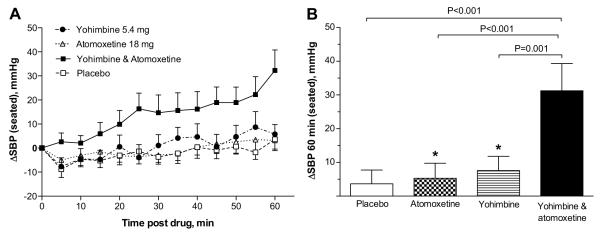
All participants (n=17) completed the four treatment arms. Average baseline seated SBP was similar among treatment groups (placebo 100±24 mmHg, yohimbine 103±25 mmHg, atomoxetine 99±25 mmHg and the combination 98±19 mmHg; P=0.691). Changes in seated SBP are shown in Figure 1. One hour after drug administration, the maximal increase in seated SBP was seen with the combination (31±33 mmHg [range −24 to 112 mmHg], 95% CI: 14 to 48 mmHg; Figure 1A and 1B), with an average SBP of 129±38 mmHg. The change from baseline in seated SBP produced by placebo (4±17 mmHg) was similar to that of yohimbine (7±17 mmHg) and atomoxetine (5±19 mmHg). For our primary outcome, we found that seated SBP was significantly higher 60 minutes after the combination than after each drug alone (placebo [P<0.001], atomoxetine [P<0.001] and yohimbine [P=0.001]); whereas the SBP after atomoxetine or yohimbine was not different from placebo (P> 0.05; Figure 1B). SBP changes were not accompanied by significant changes from baseline in heart rate (placebo 1±6 bpm, yohimbine −1±5 bpm, atomoxetine 0±6 bpm and the combination 0±7 bpm; all comparisons P >0.05).
Figure 1.
Changes from baseline in seated SBP (ΔSBP) during 1 hour after drug administration (A), and at 60 minutes post-drug (B). The combination significantly increased SBP at 60 minutes compared to each drug alone. There was no significant difference between placebo vs. atomoxetine, and placebo vs. yohimbine. Values are expressed as mean±SEM. The P values were generated by comparing the mean of log seated SBP 60 minutes after drug administration using random effects model.
* Comparisons between placebo vs. atomoxetine and placebo vs. yohimbine (P>0.05 by random effects model).
We found that the combination had a higher seated SBP 60 minutes after drug administration compared to the sum of the pressor effects produced by the two drugs individually (P=0.024, Figure 2), suggesting a synergistic, rather than an additive, pressor effect.
Figure 2.
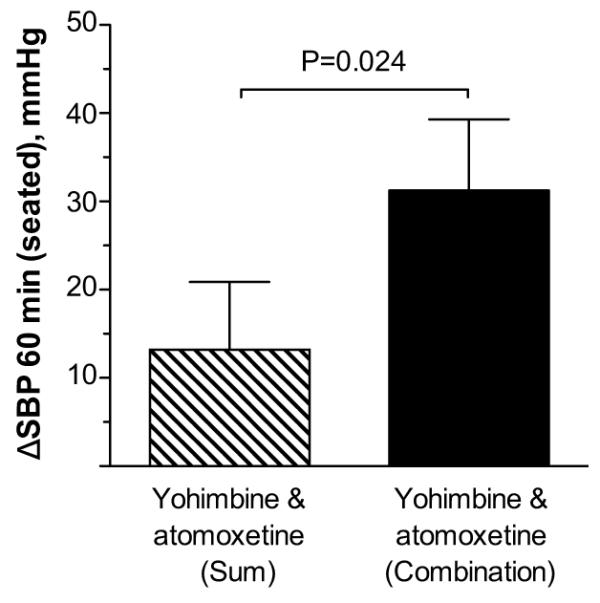
Changes from baseline in seated SBP (ΔSBP) at 60 minutes post-drug. The increase in SBP with the combination was significantly greater than the sum of the pressor effects produced by the two drugs individually. Values are expressed as mean±SEM. The P value was generated by comparing the mean of log seated SBP 60 minutes after the combination with the sum effects of those during the two drugs alone using random effects model.
Of the 17 patients studied, 12 were able to stand after all active arms and were included in the analysis of orthostatic tolerance. Compared to pre-drug administration, the increase in 1 minute standing SBP produced by placebo was 1±23 mmHg, atomoxetine 8±16 mmHg, yohimbine 9±22 mmHg and the combination 28±29 mmHg. Figure 3 shows changes from baseline in AUCSBP at 60 minutes post-drug. Only the combination showed an improvement in orthostatic tolerance, as indicated by a significantly higher AUCSBP (690±479) compared to that of each drug alone (placebo [AUCSBP 443±443; P=0.016], atomoxetine [AUCSBP 428±440; P=0.001] and yohimbine [AUCSBP 570±350; P=0.049]); whereas the AUCSBP after atomoxetine and yohimbine alone were not different from placebo (P>0.05). Moreover, the combination had a significantly higher AUCSBP compared with the sum of those during the two drugs alone (P=0.041), suggesting a synergistic effect of the combination on orthostatic tolerance.
Figure 3.
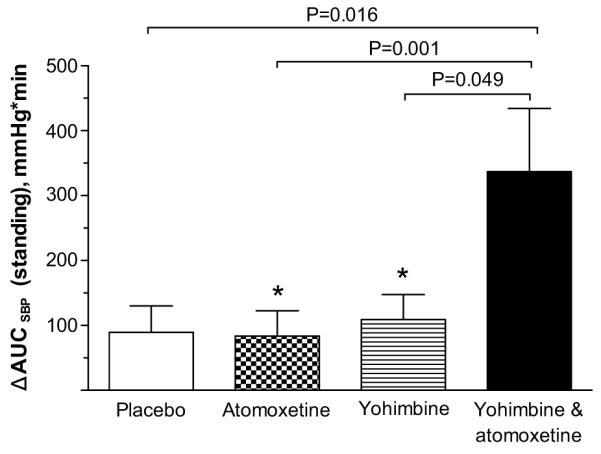
Changes from baseline in areas under the curve of standing SBP (Δ AUCSBP) at 60 minutes post-drug. The combination significantly increased AUCSBP at 60 minutes compared to each drug alone. There was no significant difference between placebo vs. atomoxetine, and placebo vs. yohimbine. Values are expressed as mean±SEM. The P values were generated by comparing the mean of log AUCSBP 60 minutes after drug administration using random effects model.
* Comparisons between placebo vs. atomoxetine and placebo vs. yohimbine (P>0.05 by random effects model).
Orthostatic Symptoms
Orthostatic symptom scores were obtained at baseline and 1 hour after placebo (n=7), atomoxetine (n=10), yohimbine (n=9) and the combination (n=10). The total orthostatic symptom burden after 1 hour post-drug significantly improved with the combination (lower scores) as compared to baseline (15.7±17.9 vs. 25.3±16.0, respectively; P=0.013; Figure 4). In contrast, the total symptom burden did not improve after atomoxetine (24.4±18.3 vs. 26.9±14.1 at baseline; P=0.799) or yohimbine alone (26.4±12.9 vs. 27.6±12.2 at baseline; P=0.905). Similar results were obtained if the analysis was restricted to patients with orthostatic symptom scores in all treatment arms.
Figure 4.
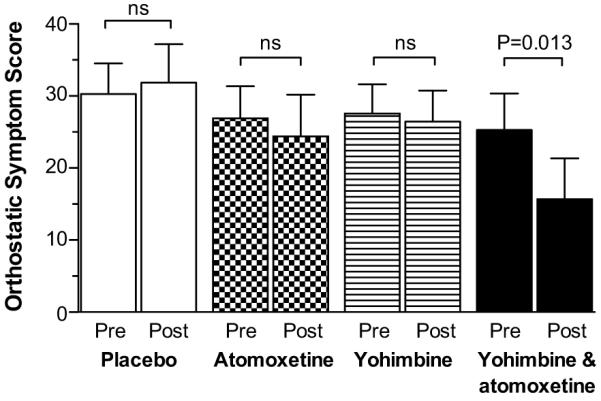
Orthostatic symptom score at baseline (pre) and after 1 hour of drug administration (post). The total score ranges from 0-60, with lower scores reflecting lower symptom burden. Values are expressed as mean±SEM. The P values were generated by Wilcoxon signed-rank test.
Only the combination showed a post-drug total symptom score significantly smaller than placebo after adjusting for the baseline total score (mean difference −11.1, 95% CI: −20.7 to −1.6; P = 0.022). On the other hand, the total symptom burden after atomoxetine or yohimbine alone were not significantly different from placebo (P> 0.05).
Discussion
The main findings of this study were that: 1) administration of yohimbine or atomoxetine alone did not produce a significant increase in blood pressure or orthostatic tolerance in this group of patients with severe peripheral autonomic failure; 2) the combination of yohimbine with atomoxetine, however, significantly increased seated systolic blood pressure and orthostatic tolerance in a synergistic manner; and that 3) the synergistic effect of the combination was associated with improvement in orthostatic symptoms. We propose that this interaction may be explained by an increased release of norepinephrine in peripheral sympathetic neurons by the α-2 antagonist combined with a reduced norepinephrine clearance by NET blockade.
Norepinephrine reuptake by the NET protein is the main mechanism by which the synaptic actions of norepinephrine are terminated. NET inhibition increases the synaptic concentrations of norepinephrine, and enhances the activation of pre- and post-synaptic adrenoreceptors. In the periphery, NET blockade would lead to increases in blood pressure and heart rate.15 This effect, however, seems to be partly counteracted by a central sympatholytic effect through activation of α-2 adrenoreceptors in the brain (“clonidine-like” effect).16-19 This central sympatholytic effect is likely to be significant in subjects with intact autonomic function, and probably accounts for the observation that NET inhibitors at therapeutic doses result in only minimal, if any, increases in blood pressure.20 Thus, the overall effect of systemic NET inhibition seems to depend on the balance between peripheral sympathetic stimulation and central sympathetic inhibition.15 In support of this hypothesis, patients with central autonomic impairment (MSA), who lack of central autonomic modulation but have intact peripheral sympathetic fibers and residual sympathetic tone,21 had a profound pressor response (54±26 mmHg) to pediatric doses (18mg PO) of atomoxetine.7 In contrast, we found that patients with peripheral autonomic impairment (PAF and PD), who have low sympathetic tone due to peripheral sympathetic denervation,22-24 showed no pressor response to atomoxetine, which is in agreement with our previous studies.7 Taken together, our findings suggest that the pressor effect of atomoxetine in autonomic failure requires a centrally-unrestrained tonic release of norepinephrine from peripheral sympathetic fibers, which is not seen in patients with peripheral autonomic failure and low sympathetic tone.
Yohimbine increases norepinephrine release from sympathetic nerves by augmenting central sympathetic outflow, via α-2 adrenoreceptor inhibition in the brain (an “anti-clonidine” effect), and by interfering with the inhibitory modulation of presynaptic α-2 adrenoreceptors on peripheral sympathetic nerves.8, 9 In autonomic failure patients, these actions result in a pressor response that appears to depend on the presence of residual sympathetic tone.22, 24, 25 This is supported by the observation that patients with autonomic failure due to a congenital absence of norepinephrine (dopamine-β-hydroxylase deficiency) had no pressor response to yohimbine;26 whereas patients with MSA, who have intact peripheral sympathetic nerves, showed profound increases in blood pressure with low oral doses (5.4 mg) of yohimbine.24, 27 Moreover, the pressor response to yohimbine in MSA patients positively correlated with the depressor response to ganglionic blockade with trimethaphan.24 On the other hand, the response to yohimbine in patients with peripheral autonomic failure is more heterogeneous. Some studies have reported similar and profound blood pressure increases in PAF and MSA patients;6, 28 while others have shown smaller, but still significant, pressor responses in PAF and PD patients.22, 27 Our results are in line with the latter findings. Furthermore, the blood pressure response to yohimbine in this study was not statistically different from placebo, suggesting that our cohort of patients were more severely affected by autonomic impairment than those recruited in previous studies.
While atomoxetine or yohimbine alone did not have any significant pressor effect, co-administration of both medications significantly increased seated SBP compared to placebo. Moreover, the magnitude of the pressor response to the combination was higher than the sum of the responses to each drug alone (Figure 2), suggesting a synergistic pressor effect. Given the lack of heart rate changes with the combination (0±7 bpm), which pointed to a significant cardiac autonomic denervation, it is tempting to speculate that the mechanism of the blood pressure increase by the combination was most likely due to sympathetically driven vasoconstriction. This was, however, not directly measured in this study.
We propose that yohimbine and atomoxetine act synergistically at two distinct and complementary levels to enhance residual sympathetic tone. In the central nervous system, α-2 adrenoreceptor inhibition with yohimbine would increase any residual central sympathetic outflow present in these patients, and counteract the “clonidine-like” effect of the NET inhibitor atomoxetine. In the neurovascular junction, norepinephrine concentrations would be further increased through attenuation of the α-2 adrenoreceptor-mediated feedback inhibition of norepinephrine release by yohimbine, and through reduced norepinephrine clearance by the NET blocker atomoxetine. Consistent with this hypothesis, Cohen et al. showed that yohimbine markedly reduced or abolished the dose-dependent central sympatholytic effect of desipramine, a tricyclic antidepressant with NET blockade actions, in rabbits with intact and impaired baroreflex function.29 Furthermore, previous studies in depressed patients with intact autonomic function taking clomipramine have shown that yohimbine, at doses (4 mg t.i.d.) that have no effect in normal subjects, induced significant increases in blood pressure.30 The present finding of a large pressor response to the combination, despite the lack of pressor response to each drug alone, supports the hypothesis that the loss of efferent sympathetic function is incomplete in many patients with severe peripheral autonomic failure. Residual sympathetic efferent fibers, therefore, may be pharmacologically engaged to treat orthostatic hypotension in some of these patients.
The main goal in the treatment of orthostatic hypotension is to reduce orthostatic symptoms and improve standing time (i.e., orthostatic tolerance). This is achieved by increasing and maintaining standing blood pressure within the range of cerebral autoregulation, in order to preserve adequate cerebral perfusion while standing. Orthostatic tolerance, therefore, may be better represented by the ability to maintain standing blood pressure above the threshold that overwhelms cerebral autoregulation rather than by absolute blood pressure levels measured at 1 minute of standing, which has been the efficacy endpoint in several orthostatic hypotension trials. This concept is supported by the observation that 24% of autonomic failure patients had severe cerebral autoregulatory failure, with a steep cerebral blood flow-blood pressure curve.31 For the same initial upright blood pressure, these patients would develop cerebral hypoperfusion sooner in response to small drops in blood pressure compared to patients with intact autoregulation. To address this issue, we defined orthostatic tolerance as the AUC of standing SBP. This approach would take into account not only blood pressure levels over time while standing, but also the total standing time. Our results indicated that only the combination significantly improved orthostatic tolerance and orthostatic symptom burden. Although yohimbine and atomoxetine alone tended to increase SBP at 1 minute of standing (9±22 mmHg and 8±16 mmHg, respectively), the orthostatic tolerance and orthostatic symptom burden did not improve. We propose that this synergistic interaction could be useful in the treatment of orthostatic hypotension in autonomic failure patients resistant to these drugs individually.
Our findings may also raise safety concerns. Medications with α-2 adrenoreceptor antagonism (e.g. mirtazapine) or NET blockade actions (e.g. bupropion, venlafaxine, duloxetine) are commonly used for depression and attention deficit disorder. Inadvertent co-administration of these medications may cause potentially dangerous blood pressure surges in subjects with impaired baroreflex function or in autonomic failure patients (e.g., diabetic neuropathy). It should be noted, however, that most of these medications have other mechanisms of action such as serotonin receptor antagonism, dopamine transporter blockade or serotonin reuptake inhibition that may have different effects on blood pressure regulation. Further research is required to test this hypothesis, and to determine whether these interactions may contribute to the cardiovascular risks associated with these drugs in the general population.
The primary limitation of this study was that plasma levels of atomoxetine and yohimbine were not measured. Because yohimbine and atomoxetine are metabolized through the cytochrome P450 (CYP2D6 and CYP3A4) pathway,32, 33 the impact of a pharmacokinetic interaction between these agents on the observed pressor effects cannot be excluded. In vivo studies have shown, however, that atomoxetine administration with substrates of the CYP2D6 and CYP3A did not result in clinically significant drug interactions. Co-administration of atomoxetine with desipramine or midazolam (a model compound for drugs metabolized by CYP2D6 and CYP3A, respectively) did not alter the plasma pharmacokinetics of any of these drugs.34 Another limitation was that we only assessed the pressor effects of these agents within 1 hour of drug administration. We did not systematically monitored blood pressure for longer periods. Thus, the duration of the pressor effects cannot be estimated from our results. Although we predicted that the peak pressor response to the combination would occur at 1 hour of drug administration based on our results and previous studies, 6, 7 it is possible that some patients may have a larger pressor response to the combination after 1 hour post-drug, particularly those patients who are poor metabolizers of CYP2D6 (about 7% of Caucasians and 2% of African Americans). Further research is required to address safety outcomes including the duration and magnitude of the pressor response to the combination beyond 1 hour of drug administration, and the pressor effect after multiple dosing. Furthermore, the effect on long-term outcomes such as frequency of falls, long-term safety and quality of life is unknown. Supine blood pressure was not assessed in our study. It is likely that the combination induces new or worsens preexistent supine hypertension in these patients; however, this would not be an adverse effect unique to these drugs. Indeed, supine hypertension is one of the most common adverse effects of medications used in the treatment of orthostatic hypotension, such as pressor agents (e.g., midodrine) or fludrocortisone. Most of these agents induce non-selective elevations of blood pressure regardless of the body position. Thus, patients taking these agents should avoid the supine position for 4-5 hours after drug administration, and omit a dose if supine or sitting blood pressure is 180/110 mmHg or greater. Finally, yohimbine is no longer sold as a medication because of its reduced sales market. However, it can be compounded by pharmacies for prescription use, and is readily available as a supplement in health stores and through Internet commerce.
Perspectives
Patients with autonomic failure offer a unique opportunity to explore human cardiovascular pharmacology, given that the hemodynamic effect of drugs are magnified or even “unmasked” in these patients because of the extreme sensitivity they have to any pressor or depressor stimuli. Medications that enhance sympathetic activity such as atomoxetine or yohimbine produce large pressor responses in patients with central autonomic failure. In contrast, patients with peripheral forms of autonomic failure are less likely to respond to these drugs, given the low residual sympathetic tone. Our results have shown that the combination of yohimbine and atomoxetine elicited a profound and synergistic pressor effect in peripheral autonomic failure patients, despite the lack of response to each drug alone. This synergistic interaction can be exploited in the treatment of orthostatic hypotension in autonomic failure patients who do not respond to these drugs individually. Our results also raise safety concerns. Medications with α-2 antagonism or NET blockade actions are commonly used for depression and attention deficit disorder. Co-administration of these drugs may cause potentially dangerous blood pressure surges in subjects with impaired baroreflex function or in autonomic failure patients (e.g., diabetic neuropathy), and could produce significant if less dramatic increases in blood pressure in normal subjects. Further research is required to test this hypothesis, and to determine whether these interactions may contribute to the cardiovascular risks associated with these drugs in the general population.
Acknowledgements
We acknowledge the patients who volunteered for these studies and the Clinical Research Center nurses who made this study possible.
Source of Funding
This work was supported by National Institutes of Health grants RO1 NS055670, PO1 HL56693, and UL1 RR024975 (Clinical and Translational Science Award) and the Paden Dysautomia Center.
Footnotes
Conflicts of Interest/Disclosures
None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shibao C, Okamoto L, Biaggioni I. Pharmacotherapy of autonomic failure. Pharmacol Ther. 2011 doi: 10.1016/j.pharmthera.2011.05.009. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frick MH. 9-alpha-fluorohydrocortisone in the treatment of postural hypotension. Acta Med Scand. 1966;179:293–299. doi: 10.1111/j.0954-6820.1966.tb05461.x. [DOI] [PubMed] [Google Scholar]
- 3.Low PA, Gilden JL, Freeman R, Sheng KN, McElligott MA. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. Midodrine study group. JAMA. 1997;277:1046–1051. [PubMed] [Google Scholar]
- 4.Jordan J, Shannon JR, Black BK, Lance RH, Squillante MD, Costa F, Robertson D. N(n)-nicotinic blockade as an acute human model of autonomic failure. Hypertension. 1998;31:1178–1184. doi: 10.1161/01.hyp.31.5.1178. [DOI] [PubMed] [Google Scholar]
- 5.Robertson D, Goldberg MR, Tung CS, Hollister AS, Robertson RM. Use of alpha 2 adrenoreceptor agonists and antagonists in the functional assessment of the sympathetic nervous system. J Clin Invest. 1986;78:576–581. doi: 10.1172/JCI112611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jordan J, Shannon JR, Biaggioni I, Norman R, Black BK, Robertson D. Contrasting actions of pressor agents in severe autonomic failure. Am J Med. 1998;105:116–124. doi: 10.1016/s0002-9343(98)00193-4. [DOI] [PubMed] [Google Scholar]
- 7.Shibao C, Raj SR, Gamboa A, Diedrich A, Choi L, Black BK, Robertson D, Biaggioni I. Norepinephrine transporter blockade with atomoxetine induces hypertension in patients with impaired autonomic function. Hypertension. 2007;50:47–53. doi: 10.1161/HYPERTENSIONAHA.107.089961. [DOI] [PubMed] [Google Scholar]
- 8.Grossman E, Rea RF, Hoffman A, Goldstein DS. Yohimbine increases sympathetic nerve activity and norepinephrine spillover in normal volunteers. Am J Physiol. 1991;260:R142–147. doi: 10.1152/ajpregu.1991.260.1.R142. [DOI] [PubMed] [Google Scholar]
- 9.Jie K, van Brummelen P, Vermey P, Timmermans PB, van Zwieten PA. Modulation of noradrenaline release by peripheral presynaptic alpha 2-adrenoceptors in humans. J Cardiovasc Pharmacol. 1987;9:407–413. doi: 10.1097/00005344-198704000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res. 1996;6:125–126. doi: 10.1007/BF02291236. [DOI] [PubMed] [Google Scholar]
- 11.Robertson D. Assessment of autonomic function. In: Baughman KL, Green BM, editors. Manual for house officers. Williams and Wilkins; Baltimore: 1981. pp. 86–131. [Google Scholar]
- 12.Mosqueda-Garcia R. Evaluation of autonomic failure. In: Robertson D, Biaggioni I, editors. Disorders of the autonomic nervous system. Harwood Academic Press; London: 1995. pp. 25–59. [Google Scholar]
- 13.Goldstein DS, Eisenhofer G, Stull R, Folio CJ, Keiser HR, Kopin IJ. Plasma dihydroxyphenylglycol and the intraneuronal disposition of norepinephrine in humans. J Clin Invest. 1988;81:213–220. doi: 10.1172/JCI113298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufmann H, Malamut R, Norcliffe-Kaufmann L, Rosa K, Freeman R. The orthostatic hypotension questionnaire (ohq): Validation of a novel symptom assessment scale. Clin Auton Res. 2011 doi: 10.1007/s10286-011-0146-2. In press. [DOI] [PubMed] [Google Scholar]
- 15.Mayer AF, Schroeder C, Heusser K, Tank J, Diedrich A, Schmieder RE, Luft FC, Jordan J. Influences of norepinephrine transporter function on the distribution of sympathetic activity in humans. Hypertension. 2006;48:120–126. doi: 10.1161/01.HYP.0000225424.13138.5d. [DOI] [PubMed] [Google Scholar]
- 16.Esler MD, Wallin G, Dorward PK, Eisenhofer G, Westerman R, Meredith I, Lambert G, Cox HS, Jennings G. Effects of desipramine on sympathetic nerve firing and norepinephrine spillover to plasma in humans. Am J Physiol. 1991;260:R817–823. doi: 10.1152/ajpregu.1991.260.4.R817. [DOI] [PubMed] [Google Scholar]
- 17.Birkenfeld AL, Schroeder C, Boschmann M, Tank J, Franke G, Luft FC, Biaggioni I, Sharma AM, Jordan J. Paradoxical effect of sibutramine on autonomic cardiovascular regulation. Circulation. 2002;106:2459–2465. doi: 10.1161/01.cir.0000036370.31856.73. [DOI] [PubMed] [Google Scholar]
- 18.Eisenhofer G, Saigusa T, Esler MD, Cox HS, Angus JA, Dorward PK. Central sympathoinhibition and peripheral neuronal uptake blockade after desipramine in rabbits. Am J Physiol. 1991;260:R824–832. doi: 10.1152/ajpregu.1991.260.4.R824. [DOI] [PubMed] [Google Scholar]
- 19.Tank J, Schroeder C, Diedrich A, Szczech E, Haertter S, Sharma AM, Luft FC, Jordan J. Selective impairment in sympathetic vasomotor control with norepinephrine transporter inhibition. Circulation. 2003;107:2949–2954. doi: 10.1161/01.CIR.0000072786.99163.FE. [DOI] [PubMed] [Google Scholar]
- 20.Stiefel G, Besag FM. Cardiovascular effects of methylphenidate, amphetamines and atomoxetine in the treatment of attention-deficit hyperactivity disorder. Drug Saf. 2010;33:821–842. doi: 10.2165/11536380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.Parikh SM, Diedrich A, Biaggioni I, Robertson D. The nature of the autonomic dysfunction in multiple system atrophy. J Neurol Sci. 2002;200:1–10. doi: 10.1016/s0022-510x(02)00126-0. [DOI] [PubMed] [Google Scholar]
- 22.Sharabi Y, Eldadah B, Li ST, Dendi R, Pechnik S, Holmes C, Goldstein DS. Neuropharmacologic distinction of neurogenic orthostatic hypotension syndromes. Clin Neuropharmacol. 2006;29:97–105. doi: 10.1097/01.WNF.0000220822.80640.0D. [DOI] [PubMed] [Google Scholar]
- 23.Sharabi Y, Imrich R, Holmes C, Pechnik S, Goldstein DS. Generalized and neurotransmitter-selective noradrenergic denervation in parkinson’s disease with orthostatic hypotension. Mov Disord. 2008;23:1725–1732. doi: 10.1002/mds.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shannon JR, Jordan J, Diedrich A, Pohar B, Black BK, Robertson D, Biaggioni I. Sympathetically mediated hypertension in autonomic failure. Circulation. 2000;101:2710–2715. doi: 10.1161/01.cir.101.23.2710. [DOI] [PubMed] [Google Scholar]
- 25.Onrot J, Goldberg MR, Biaggioni I, Wiley RG, Hollister AS, Robertson D. Oral yohimbine in human autonomic failure. Neurology. 1987;37:215–220. doi: 10.1212/wnl.37.2.215. [DOI] [PubMed] [Google Scholar]
- 26.Robertson D, Goldberg MR, Onrot J, Hollister AS, Wiley R, Thompson JG, Jr., Robertson RM. Isolated failure of autonomic noradrenergic neurotransmission. Evidence for impaired beta-hydroxylation of dopamine. N Engl J Med. 1986;314:1494–1497. doi: 10.1056/NEJM198606053142307. [DOI] [PubMed] [Google Scholar]
- 27.Shibao C, Okamoto LE, Gamboa A, Yu C, Diedrich A, Raj SR, Robertson D, Biaggioni I. Comparative efficacy of yohimbine against pyridostigmine for the treatment of orthostatic hypotension in autonomic failure. Hypertension. 2010;56:847–851. doi: 10.1161/HYPERTENSIONAHA.110.154898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biaggioni I, Robertson RM, Robertson D. Manipulation of norepinephrine metabolism with yohimbine in the treatment of autonomic failure. J Clin Pharmacol. 1994;34:418–423. doi: 10.1002/j.1552-4604.1994.tb04981.x. [DOI] [PubMed] [Google Scholar]
- 29.Cohen MD, Finberg J, Dibner-Dunlap M, Yuih SN, Thames MD. Effects of desipramine hydrochloride on peripheral sympathetic nerve activity. Am J Physiol. 1990;258:R876–882. doi: 10.1152/ajpregu.1990.258.4.R876. [DOI] [PubMed] [Google Scholar]
- 30.Lacomblez L, Bensimon G, Isnard F, Diquet B, Lecrubier Y, Puech AJ. Effect of yohimbine on blood pressure in patients with depression and orthostatic hypotension induced by clomipramine. Clin Pharmacol Ther. 1989;45:241–251. doi: 10.1038/clpt.1989.24. [DOI] [PubMed] [Google Scholar]
- 31.Novak V, Novak P, Spies JM, Low PA. Autoregulation of cerebral blood flow in orthostatic hypotension. Stroke. 1998;29:104–111. doi: 10.1161/01.str.29.1.104. [DOI] [PubMed] [Google Scholar]
- 32.Ring BJ, Gillespie JS, Eckstein JA, Wrighton SA. Identification of the human cytochromes p450 responsible for atomoxetine metabolism. Drug Metab Dispos. 2002;30:319–323. doi: 10.1124/dmd.30.3.319. [DOI] [PubMed] [Google Scholar]
- 33.Le Corre P, Parmer RJ, Kailasam MT, Kennedy BP, Skaar TP, Ho H, Leverge R, Smith DW, Ziegler MG, Insel PA, Schork NJ, Flockhart DA, O’Connor DT. Human sympathetic activation by alpha2-adrenergic blockade with yohimbine: Bimodal, epistatic influence of cytochrome p450-mediated drug metabolism. Clin Pharmacol Ther. 2004;76:139–153. doi: 10.1016/j.clpt.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Sauer JM, Long AJ, Ring B, Gillespie JS, Sanburn NP, DeSante KA, Petullo D, VandenBranden MR, Jensen CB, Wrighton SA, Smith BP, Read HA, Witcher JW. Atomoxetine hydrochloride: Clinical drug-drug interaction prediction and outcome. J Pharmacol Exp Ther. 2004;308:410–418. doi: 10.1124/jpet.103.058727. [DOI] [PubMed] [Google Scholar]