Abstract
BACKGROUND AND OBJECTIVE:
Delayed cord clamping (DCC) has been advocated during preterm delivery to improve hemodynamic stability during the early neonatal period. The hemodynamic effects of DCC in premature infants after birth have not been previously examined. Our objective was to compare the hemodynamic differences between premature infants randomized to either DCC or immediate cord clamping (ICC).
METHODS:
This prospective study was conducted on a subset of infants who were enrolled in a randomized controlled trial to evaluate the effects of DCC versus ICC. Entry criteria included gestational ages of 240 to 316 weeks. Twins and infants of mothers with substance abuse were excluded. Serial Doppler studies were performed at 6 ± 2, 24 ± 4, 48 ± 6, and 108 ± 12 hours of life. Measurements included superior vena cava blood flow, right ventricle output, middle cerebral artery blood flow velocity (BFV), superior mesenteric artery BFV, left ventricle shortening fraction, and presence of a persistent ductus arteriosus.
RESULTS:
Twenty-five infants were enrolled in the DCC group and 26 in the ICC group. Gestational age, birth weight, and male gender were similar. Admission laboratory and clinical events were also similar. DCC resulted in significantly higher superior vena cava blood flow over the study period, as well as greater right ventricle output and right ventricular stroke volumes at 48 hours. No differences were noted in middle cerebral artery BFV, mean superior mesenteric artery BFV, shortening fraction, or the incidence of a persistent ductus arteriosus.
CONCLUSIONS:
DCC in premature infants is associated with potentially beneficial hemodynamic changes over the first days of life.
KEY WORDS: delayed cord clamping, premature infants
What’s Known on This Subject:
Delayed umbilical cord clamping in premature infants has been associated with decreased rates of intraventricular hemorrhage; however, the mechanisms that explain this finding have not been described.
What This Study Adds:
Premature infants with delayed umbilical cord clamping have improved superior vena cava blood flow over the first days of life. This may provide one of the mechanism(s) by which this technique reduces the incidence in intraventricular hemorrhage in this at-risk population.
Delayed cord clamping (DCC) has been previously shown to result in significant placental transfusion in term and premature infants.1,2 The latter received ∼10% to 15% additional blood volume,3 which may provide beneficial effects during the transition from fetal to neonatal life. Immediate cord clamping (ICC) deprives the premature infant of this potential additional blood volume, which could result in hypovolemia and hypotension, leading to serious morbidities, such as periventricular or intraventricular hemorrhage (P/IVH) and periventricular leukomalacia.4 Although there are multiple risk factors for the development of P/IVH, a strong association exists with decreased blood flow measured in the superior vena cava (SVC).5
We have previously shown that DCC for 45 seconds after birth resulted in decreased rates of IVH in premature infants.6 This finding was recently confirmed by a meta-analysis.7 As a result of these findings, the International Consensus on Cardio-Pulmonary Resuscitation has endorsed DCC for premature infants not needing immediate intervention.8 However, the mechanisms that account for an association between DCC and the reduced incidence of P/IVH have not been investigated. One possibility is that DCC improves circulating blood volume, including brain blood flow resulting in increased blood flow in the SVC.
The objective of the current study was to perform ultrasound Doppler measurements to evaluate the hemodynamic changes associated with DCC in premature infants over the first few days of life. Our hypothesis was that DCC would lead to greater SVC and middle cerebral artery (MCA) blood flow velocity (BFV).
METHODS
Study Design
The study was performed between May 2009 and July 2010 at Women & Infants Hospital of Rhode Island. The study was a prospective cohort study nested within a randomized control trial. The latter is an ongoing National Institutes of Health–funded single-center study to randomly assign premature infants to DCC for 45 seconds or to ICC (Clinical Trials NCT00818220). Entry criteria included a gestational age of 240/7 to 316 /7 weeks. Exclusion criteria included twin infants and infants of mothers with substance abuse. All infants from the main trial were eligible and parental informed consent was obtained separately. This study was approved by the institutional review boards of Women & Infants Hospital of Rhode Island and the University of Rhode Island. Cord-clamp timing was recorded by a research nurse present at the delivery who was responsible for infant enrollment and group allocation.
Ultrasound Studies
Sequential hemodynamic studies were performed at 6 ± 2, 24 ± 4, 48 ± 6, and 108 ± 12 hours of life by using a Toshiba Aplio XG ultrasound Doppler machine (Tustin, CA) by a single investigator (R.S.) who was not aware of the infant’s main trial enrollment status, except in 4 cases when he was the responsible neonatologist at the delivery. A subset of 10 infants underwent repeated testing to determine intraobserver reliability. The hemodynamic variables measured included SVC blood flow, incidence of low SVC flow, right ventricle output (RVO), MCA-BFV, superior mesenteric artery BFV (SMA-BFV), and left ventricle shortening fraction. If a persistent ductus arteriosus (PDA) was visualized from the parasternal short axis view, the size and direction of blood flow were documented. SVC blood flow and RVO (mL/kg/min) were calculated by using previously described techniques,9,10 after calculating respective stroke volumes from the velocity time integral and by using the infant’s heart rate and body weight. Low SVC flow was considered as any measurement ≤45 mL/kg/min. Mean BFV measurements were calculated by tracing the Doppler curve for 3 sequential heartbeats and averaging the values. The primary outcome was the SVC blood flow and MCA-BFV over the course of the study. Secondary outcomes included all other ultrasound measurements.
Patient Data
Maternal data, including age, placental complications (placenta previa, abruption, intrauterine growth restriction, premature prolonged rupture of membranes), clinical chorioamnionitis, antenatal steroid administration, perinatal magnesium administration, and mode of delivery, were obtained from medical records. Infant data at birth, including gestational age, gender, race, weight, Apgar scores at 1 and 5 minutes, need of intubation after delivery, initial blood gas values, hematocrit, and blood pressure, were also obtained from medical records.
Statistical Analysis
Intraobserver reliability was determined by the median variance and repeatability coefficient. Demographic and clinical continuous variables were compared by using a 2-tailed Student’s t test or Wilcoxon rank-sum test. Categorical variables were compared by using χ2 or Fisher’s exact test as appropriate. Two-way analysis of variance (ANOVA) for repeated measures (rANOVA) was performed with treatment group and time as the repeated factors. Logistic regression models controlling for differences in categorical variables were performed if present. If the global ANOVA F tests were significant (P < .05), post hoc testing with the Fisher least significant difference test was used to detect differences between the groups at specific time points (P < .05). Dichotomous outcome variables were evaluated similarly by using robust variance logistic regression. Data were analyzed by using Stata 10 (StataCorp, College Station, TX).
RESULTS
Over the course of the study, 188 mothers were enrolled, with 78 of them delivering infants who were randomized. Sixty of these women were approached for enrollment to the secondary hemodynamic study. There were 9 refusals, leaving 51 infants undergoing examinations. The groups consisted of 25 infants receiving DCC and 26 infants receiving ICC. Maternal variables were similar (Table 1). Infants with DCC were 3 days older than the infants exposed to ICC (P = .4) and had birth weights that were 88 g heavier than the ICC group (P = .5). These differences were controlled for in all analyses of hemodynamic measurements. Thirty-two percent of infants with DCC were younger than 27 weeks’ gestational age and, similarly, 38% of infants with ICC were younger than 27 weeks’ gestational age. More black infants were exposed to DCC, whereas more Hispanic infants were exposed to ICC. All other demographic variables were similar between the 2 groups (Table 2). The initial events at delivery and admission laboratory values were similar between the 2 groups. The initial severity of illness, as determined by neonatal acute physiology scores, was similar. Cord-clamping time was longer in the DCC than the ICC group (41 ± 9 vs 5 ± 7 seconds, Table 2). There were no differences between groups in heart rate, mean blood pressure, and respiratory variables, including percentage of infants intubated and mean airway pressure values over the course of the studies. The examiner exhibited a median intraobserver variability of 10% and a repeatability coefficient for the SVC flow measurement of 24 mL/kg/min.
TABLE 1.
Maternal Demographic and Clinical Characteristics
| DCC, n = 25 | ICC, n = 26 | P | |
|---|---|---|---|
| Maternal age, mean (SD) | 28.1 (7.0) | 28.2 (5.3) | .9 |
| Placental complications, n (%) | .9 | ||
| Previa | 1 (4.0) | 1 (3.9) | |
| Abruption | 1 (4.0) | 3 (11.5) | |
| IUGR | 1 (4.0) | 2 (7.7) | |
| PPROM | 5 (20.0) | 5 (19.2) | |
| Chorioamnionitis, n (%) | 2 (8.0) | 4 (15.4) | .7 |
| Antenatal steroid doses, n (%) | .1 | ||
| 0 | 1 (4.0) | 3 (11.5) | |
| 1 | 9 (36.0) | 3 (11.5) | |
| 2 | 15 (60.0) | 20 (76.9) | |
| Perinatal magnesium, n (%) | 17 (68.0) | 18 (69.2) | .9 |
IUGR, intrauterine growth restriction; PPROM, premature prolonged rupture of membranes.
TABLE 2.
Infants’ Demographic and Clinical Characteristics after Delivery
| DCC, n = 25 | ICC, n = 26 | P | |
|---|---|---|---|
| Gestation wk, mean (SD) | 28.3 (2.3) | 27.7 (2.0) | .4 |
| Birth weight, g, mean (SD) | 1204 (394) | 1116 (467) | .5 |
| Cesarean delivery, n (%) | 9 (36.0) | 14 (53.9) | .3 |
| Race, n (%) | .01 | ||
| Black | 6 (24.0) | 2 (7.7) | |
| White | 15 (60.0) | 16 (61.5) | |
| Hispanic | 1 (4.0) | 8 (30.8) | |
| Other | 3 (12.0) | 0 | |
| Cord clamping time, s, mean (SD) | 41 (9) | 5 (7) | <.01 |
| Apgar 1 min, median (range) | 7 (2–9) | 7 (2–9) | .6 |
| Apgar 5 min, median (range) | 8 (3–9) | 8 (5–9) | .6 |
| Intubated, n (%) | 10 (40.0) | 12 (46.1) | .8 |
| SNAP score, mean (SD) | 13.6 (6.0) | 14.0 (9.8) | .8 |
| pH, mean (SD) | 7.3 (0.07) | 7.3 (0.09) | .7 |
| pCO2 mm Hg, mean (SD) | 44.3 (12.3) | 46.9 (17.5) | .6 |
| pO2 mm Hg, mean (SD) | 57.4 (29.0) | 63.5 (21.4) | .4 |
| BE mmol/L, mean (SD) | −1.3 (3.5) | −1.4 (2.4) | .9 |
| HCT, mean (SD) | 47.6 (7.9) | 46.6 (6.4) | .7 |
BE, base excess; HCT, hematocrit; SNAP, score of neonatal acute physiology.
Hemodynamic Data
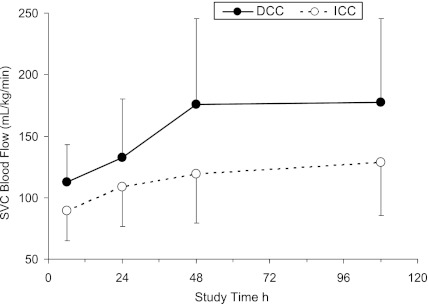
Infants exposed to DCC exhibited higher SVC blood flow over the course of the study compared with the infants exposed to ICC after controlling for gestational age and birth weight (ANOVA: Groups, P = .003, Table 3 and Fig 1). Infants exposed to DCC had higher RVO and right ventricle stroke volumes (RV-SV) than infants exposed to ICC at 48 hours of life (ANOVA: Interactions, P < .003, Fisher least significant difference, P < .04). Infants exposed to DCC trended to have higher RV-SV over the course of the study (ANOVA: Groups, P = .1). Only 1 infant in the ICC group at the 6-hour measurement had low SVC flow of 44 mL/kg/min. There were no differences identified between the groups for mean MCA BFV, SMA-BFV, shortening fraction, or in the incidence of a PDA.
TABLE 3.
Hemodynamic Measurements Over Course of Study
| Age, h | 6 | 24 | 48 | 108 | P | |
|---|---|---|---|---|---|---|
| SVC blood flow, mL/kg/min | DCC | 112 (30) | 132 (49) | 176 (72) | 178 (85) | .003 |
| ICC | 89 (24) | 108 (31) | 119 (39) | 129 (38) | ||
| RVO, mL/kg/min | DCC | 282 (93) | 379 (103) | 426 (116) | 405 (115) | .5 |
| ICC | 295 (98) | 342 (100) | 350 (109) | 432 (153) | ||
| RV-SV, mL/beat | DCC | 9.5 (4.6) | 11.8 (5.0) | 12.9 (4.7) | 11.8 (4.8) | .1 |
| ICC | 8.8 (4.4) | 10.1 (4.7) | 9.2 (4.4) | 10.9 (4.4) | ||
| MCA-BFV, cm/s | DCC | 12.2 (4.0) | 15.9 (5.0) | 18.2 (4.1) | 19.1 (5.3) | NS |
| ICC | 11.9 (3.2) | 15.4 (4.5) | 17.4 (6.1) | 19.5 (7.3) | ||
| SMA-BFV, cm/s | DCC | 17.7 (9.6) | 18.2 (7.9) | 18.6 (5.9) | 23.0 (11.1) | |
| ICC | 16.5 (4.9) | 19.2 (7.3) | 18.1 (5.3) | 23.3 (7.8) | ||
| SF % | DCC | 31.6 (8.8) | 28.3 (9.4) | 33.6 (6.2) | 33.3 (10.9) | |
| ICC | 31.8 (5.7) | 31.1 (9.4) | 33.8 (9.6) | 32.2 (7.9) | ||
| PDA % | DCC | 75 | 47.6 | 38.5 | 14.3 | |
| ICC | 72.2 | 42.8 | 36.8 | 0 |
Results are mean (SD) unless otherwise indicated. P values are for overall group difference by ANOVA (means). NS, non significant; SF, shortening fraction.
FIGURE 1.
SVC blood flow over the course of the study, mean ± SD (ANOVA: Groups, P = .003).
There were 2 protocol violations consisting of 2 infants who were enrolled into the ICC group but had cord-clamping durations that were >30 seconds. Data analysis by actual received treatment revealed that infants exposed to DCC exhibited higher RV-SV than infants exposed to ICC (ANOVA: Groups, P = .04).
Discussion
This study demonstrates that premature infants exposed to DCC have higher SVC blood flow during the first 4 days of life when compared with infants exposed to ICC. Furthermore, the differences in SVC flow between the DCC and ICC groups at 48 and 108 hours (53 and 54 mL/kg/min, respectively) were greater than at 6 and 24 hours (26 and 23 mL/kg/min), suggesting that the effects of DCC persist beyond the immediate postnatal period when P/IVH frequently occurs. We speculate that, in premature infants exposed to DCC, the higher SVC blood flow values are the result of increased blood volumes. Increased SVC blood flow in preterm infants may attenuate P/IVH rates by facilitating a more efficient cerebral vascular regulation in the presence of pathophysiological events, such as hypoxia and hypercarbia.
In a recent observational nonrandomized study of 30 infants with an average gestational age of 26.5 weeks, Meyer and Mildenhall11 identified greater median SVC blood flow values at 24 hours of age for 13 infants exposed to DCC (91 mL/kg/min) compared with 17 infants exposed to ICC (52 mL/kg/min). Their observed SVC blood flow values at 24 hours of age for infants exposed to DCC were 70% greater than that of the infants exposed to ICC. These differences were greater than the 20% difference between the DCC and ICC groups that we observed in the current study. Although interobserver variability could explain some of the difference between the studies, it is also possible that DCC has a greater effect on SVC flow at lower gestational ages, as the referenced infants were about 1 week younger than in the current study.
In a separate study, investigators evaluated cerebral oxygenation values by using near-infrared spectroscopy in 39 infants with a mean gestational age of 30.4 weeks, who were randomized to DCC or ICC. They found higher regional tissue oxygenation values of about 4% at 4 and 24 hours after birth; however, this finding did not persist at the evaluations 72 hours after birth.12 Although these findings are suggestive of improved physiologic outcomes resulting from DCC, it is not possible to discern how much of the improved oxygenation could be related to the higher hematocrit values that they observed in the infants exposed to DCC and/or differences in SVC blood flow. Previous work has suggested that changes in cerebral oxygenation correlate with changes in SVC blood flow.13
SVC blood flow is a measurement of systemic blood flow that is independent of blood pressure and the fetal shunts, such as a PDA or patent foramen-ovale.9 Therefore, infants may have low systemic blood pressure without low SVC flow, or vice versa. The meta-analysis and our study did not find differences in blood pressure or inotropic use for low blood pressure in the infants exposed to ICC compared with those exposed to DCC.7 Although there appears to be a strong correlation between low SVC flow and the development of P/IVH,5 low SVC blood flow does not appear to consistently correlate with low blood pressure.5 Although some large studies have identified an association between low blood pressure values and the development of P/IVH,14 more recent studies have not observed this association.15 The differences in the results in these reports14–16 could be related to variability in the definitions of hypotension in extremely premature infants, variability in the levels of blood pressure that triggered treatment,17 and the lack of correlation between blood pressure and cerebral blood flow values.18
In theory, treatments that increase SVC blood flow could attenuate rates of IVH; however, there are no data to support this contention.19 High-dose dobutamine and dopamine in the presence of a PDA have been shown to increase SVC blood flow without affecting the incidence of IVH.20,21 We speculate that DCC could potentially represent an important intervention that could increase SVC blood flow and, consequently, provide a hemodynamic explanation for the reduced incidence of IVH after DCC.6,11
Early SVC blood flow measurements in the infants exposed to ICC were higher than previously reported.9 An increase in SVC blood flow values over time in infants not receiving any intervention has been previously observed.19 Previous explanations have included improvements in neonatal care.19 The infants in our study were of an older gestational age than the previously referenced studies, which may limit direct comparisons between absolute values of SVC blood flow; moreover, the relatively higher absolute SVC values would not affect comparisons between the DCC and ICC groups. It is important to note, however, that we observed similar SVC blood flow values at 6 and 24 hours of age to those in a cohort of infants with gestational ages that were similar to those in the current study.22 Although there is less information regarding SVC blood flow values after the third day of life, we found that the values in our ICC group at a mean age of 4.5 days (125 ± 38 mL/kg/min) were very similar to a recent prospective observational study of 17 infants with a mean gestational age of 28 weeks who were studied at a median age of 5 days (130 ± 40 mL/kg/min).20
Although infants with low SVC blood flow have also been reported to have low MCA blood velocity values,9 contrary to our hypothesis, the elevated SVC blood flow values were not associated with increased MCA-BFV measurements. These findings could be related to the fact that the former measurement is actual blood flow, whereas the MCA velocity measurement is only velocity and does not reflect changes in vessel size.
The finding of the higher RVO values at 48 hours, as well as increased stroke volumes over the course of the study in the infants exposed to DCC, supports the contention that stable premature infants respond to increased SVC blood flow by increasing their RVO because of increased stroke volume, rather than increased heart rate. Although in response to stress and pathophysiological conditions premature infants have been reported to increase cardiac output by increasing heart rate,23 less is known regarding the ability of stable premature infants to respond to increases in preload (SVC blood flow) and, hence, cardiac output by increasing stroke volume. Based on our findings, it is not feasible to discern whether DCC improves stroke volume by increasing total blood volume and preload or by decreasing afterload. The mechanism by which DCC facilitates improved stroke volume is intriguing and merits further investigation.
Although this secondary study was nested in a randomized control trial, the 2 groups were equally matched except for race (more black infants and fewer Hispanic infants in DCC group); however, it is unlikely that this difference would affect the hemodynamic outcomes that result from the study intervention. The interobserver variability is far greater than intraobserver variability of SVC blood flow measurements.22 To control for these potential differences, we limited these measurements to 1 examiner (R.S.), whose intraobserver variability was comparable with published findings.22
In summary, the results of our study have categorized the hemodynamic effects of DCC in premature infants, which include increased SVC blood flow, and increased RVO and RV-SV at 48 hours of life. These adaptive cardiovascular changes may provide one of the mechanism(s) by which DCC reduces the incidence in IVH in premature infants.
Acknowledgments
The primary author acknowledges the Department of Pediatrics of Women & Infants Hospital for allowing him to use their ultrasound equipment, the families of studied infants for their participation, as well as his family for their support during this project.
Glossary
- ANOVA
analysis of variance
- BFV
blood flow velocity
- DCC
delayed cord clamping
- ICC
immediate cord clamping
- MCA
middle cerebral artery
- PDA
persistent ductus arteriosus
- P/IVH
periventricular or intraventricular hemorrhage
- RVO
right ventricle output
- RV-SV
right ventricle stroke volume
- SMA
superior mesenteric artery
- SVC
superior vena cava
Footnotes
This trial has been registered at www.clinicaltrials.gov (identifier NCT00818220).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This work was supported by National Institutes of Health, National Institute for Nursing Research grant 5R01NR010015.
References
- 1.Oh W, Fanaroff AA, Carlo WA, Donovan EF, McDonald SA, Poole WK, Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network Effects of delayed cord clamping in very-low-birth-weight infants. J Perinatol. 2011;31(suppl 1):S68–S71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao AC, Moinian M, Lind J. Distribution of blood between infant and placenta after birth. Lancet. 1969;2(7626):871–873 [DOI] [PubMed] [Google Scholar]
- 3.Aladangady N, McHugh S, Aitchison TC, Wardrop CA, Holland BM. Infants’ blood volume in a controlled trial of placental transfusion at preterm delivery. Pediatrics. 2006;117(1):93–98 [DOI] [PubMed] [Google Scholar]
- 4.Volpe JJ. Brain injury in the premature infant. Neuropathology, clinical aspects, pathogenesis, and prevention. Clin Perinatol. 1997;24(3):567–587 [PubMed] [Google Scholar]
- 5.Kluckow M, Evans N. Low superior vena cava flow and intraventricular haemorrhage in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2000;82(3):F188–F194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mercer JS, Vohr BR, McGrath MM, Padbury JF, Wallach M, Oh W. Delayed cord clamping in very preterm infants reduces the incidence of intraventricular hemorrhage and late-onset sepsis: a randomized, controlled trial. Pediatrics. 2006;117(4):1235–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabe H, Reynolds G, Diaz-Rossello J. A systematic review and meta-analysis of a brief delay in clamping the umbilical cord of preterm infants. Neonatology. 2008;93(2):138–144 [DOI] [PubMed] [Google Scholar]
- 8.Perlman JM, Wyllie J, Kattwinkel J, et al. Neonatal Resuscitation Chapter Collaborators Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Pediatrics. 2010;126(5). Available at: www.pediatrics.org/cgi/content/full/126/5/e1319 [DOI] [PubMed] [Google Scholar]
- 9.Evans N, Kluckow M, Simmons M, Osborn D. Which to measure, systemic or organ blood flow? Middle cerebral artery and superior vena cava flow in very preterm infants. Arch Dis Child Fetal Neonatal Ed. 2002;87(3):F181–F184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yanowitz TD, Yao AC, Pettigrew KD, Werner JC, Oh W, Stonestreet BS. Postnatal hemodynamic changes in very-low-birthweight infants. J Appl Physiol. 1999;87(1):370–380 [DOI] [PubMed] [Google Scholar]
- 11.Meyer MP, Mildenhall L. Delayed cord clamping and blood flow in the superior vena cava in preterm infants: an observational study Arch Dis Child Fetal Neonatal Ed 2011 [DOI] [PubMed] [Google Scholar]
- 12.Baenziger O, Stolkin F, Keel M, et al. The influence of the timing of cord clamping on postnatal cerebral oxygenation in preterm neonates: a randomized, controlled trial. Pediatrics. 2007;119(3):455–459 [DOI] [PubMed] [Google Scholar]
- 13.Moran M, Miletin J, Pichova K, Dempsey EM. Cerebral tissue oxygenation index and superior vena cava blood flow in the very low birth weight infant. Acta Paediatr. 2009;98(1):43–46 [DOI] [PubMed] [Google Scholar]
- 14.Perlman JM, Risser R, Broyles RS. Bilateral cystic periventricular leukomalacia in the premature infant: associated risk factors. Pediatrics. 1996;97(6 pt 1):822–827 [PubMed] [Google Scholar]
- 15.Logan JW, O’Shea TM, Allred EN, et al. ELGAN Study Investigators Early postnatal hypotension is not associated with indicators of white matter damage or cerebral palsy in extremely low gestational age newborns. J Perinatol. 2011;31(8):524–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dammann O, Allred EN, Kuban KC, et al. Developmental Epidemiology Network Systemic hypotension and white-matter damage in preterm infants. Dev Med Child Neurol. 2002;44(2):82–90 [DOI] [PubMed] [Google Scholar]
- 17.Dempsey EM, Barrington KJ. Treating hypotension in the preterm infant: when and with what: a critical and systematic review. J Perinatol. 2007;27(8):469–478 [DOI] [PubMed] [Google Scholar]
- 18.Lightburn MH, Gauss CH, Williams DK, Kaiser JR. Cerebral blood flow velocities in extremely low birth weight infants with hypotension and infants with normal blood pressure. J Pediatr. 2009;154(6):824–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paradisis M, Evans N, Kluckow M, Osborn D. Randomized trial of milrinone versus placebo for prevention of low systemic blood flow in very preterm infants. J Pediatr 2009;154(2):189–195 [DOI] [PubMed]
- 20.Bouissou A, Rakza T, Klosowski S, Tourneux P, Vanderborght M, Storme L. Hypotension in preterm infants with significant patent ductus arteriosus: effects of dopamine. J Pediatr. 2008;153(6):790–794 [DOI] [PubMed] [Google Scholar]
- 21.Osborn D, Evans N, Kluckow M. Randomized trial of dobutamine versus dopamine in preterm infants with low systemic blood flow. J Pediatr. 2002;140(2):183–191 [DOI] [PubMed] [Google Scholar]
- 22.Groves AM, Kuschel CA, Knight DB, Skinner JR. Echocardiographic assessment of blood flow volume in the superior vena cava and descending aorta in the newborn infant. Arch Dis Child Fetal Neonatal Ed. 2008;93(1):F24–F28 [DOI] [PubMed] [Google Scholar]
- 23.Rudolph AM, Heymann MA. Cardiac output in the fetal lamb: the effects of spontaneous and induced changes of heart rate on right and left ventricular output. Am J Obstet Gynecol. 1976;124(2):183–192 [DOI] [PubMed] [Google Scholar]