Abstract
The Center for Magnetic Resonance (CMRR) at the University of Minnesota was one of laboratories where the work that simultaneously and independently introduced functional magnetic resonance imaging (fMRI) of human brain activity was carried out. However, unlike other laboratories pursuing fMRI at the time, our work was performed at 4 Tesla magnetic field and coincided with the effort to push human magnetic resonance imaging to field strength significantly beyond 1.5 Tesla which was the high-end standard of the time. The human fMRI experiments performed in CMRR were planned between two colleagues who had known each other and had worked together previously in Bell Laboratories, namely Seiji Ogawa and myself, immediately after the Blood Oxygenation Level Dependent (BOLD) contrast was developed by Seiji. We were waiting for our first human system, a 4 Tesla system, to arrive in order to attempt at imaging brain activity in the human brain and these were the first experiments we performed on the 4 Tesla instrument in CMRR when it became marginally operational. This was a prelude to a subsequent systematic push we initiated for exploiting higher magnetic fields to improve the accuracy and sensitivity of fMRI maps, first going to 9.4 Tesla for animal model studies and subsequently developing a 7 Tesla human system for the first time. Steady improvements in high field instrumentation and ever expanding armamentarium of image acquisition and engineering solutions to challenges posed by ultrahigh fields has brought fMRI to submillimeter resolution in the whole brain at 7 Tesla, the scale necessary to reach cortical columns and laminar differentiation in the whole brain. The solutions that emerged in response to technological challenges posed by 7 Tesla also propagated and continues to propagate to lower field clinical systems, a major advantage of the ultrahigh fields effort that is underappreciated. Further improvements at 7T are inevitable. Further translation of these improvements to lower field clinical systems to achieve new capabilities and to magnetic fields significantly higher than 7 Tesla to enable human imaging is inescapable.
Keywords: High field, Ultrahigh field, 4 Tesla, 7 Tesla, 7T, functional imaging, fMRI, neuroimaging, brain imaging, neuroimaging, MRI, BOLD, Multiband, Slice Accelerated Imaging, Slice Acceleration
INTRODUCTION
In this article, I have taken the opportunity to reflect on some of the events that shaped my career and ultimately my intense interest and involvement with ultrahigh field magnetic resonance and functional brain imaging (fMRI) at such high fields1. The two are intricately tied for me and the laboratory that I lead at the University of Minnesota, the Center for Magnetic Resonance Research (CMRR); one cannot separate them since as soon as our 4 Tesla system, one of the first three to be installed at about the same time circa 1990, became operational we initiated a project we had been planning for a while, namely imaging of brain activity using the BOLD contrast described by my colleague Seiji Ogawa (see also Ugurbil, this issue). Of course, this did not happen suddenly and in isolation; the path to that development started a long time before then.
EARLY YEARS LEADING to HIGH FIELD MR
I finished high school in Istanbul. Having received a bilingual education from age twelve on, I was able to explore the possibility of studying in the USA and came to Columbia University in New York City for my undergraduate studies. Initially, I was not sure what I wanted to study. But I decided to major in physics after taking a course in electricity and magnetism in the first semester of my second year, a course which used a text book titled Electricity and Magnetism (at the time known as the Vol.2 of the Berkeley Physics Course) written by Ed Purcell, who as many would know, shared the Nobel Prize with Felix Bloch for the discovery of Nuclear Magnetic Resonance (NMR) in 1952. That was my first encounter with magnetic resonance I suppose, though I did not know it at the time. But clearly, the pleasure of learning about electricity and magnetism from this wonderful textbook is one of the reasons I decided to study physics. However, by the time I was about to finish my undergraduate studies, the bottom fell out of the support for physics research in the USA. Many physicists were getting laid off and new job opportunities were scarce. I joined the rush of physicist abandoning physics and turning to biology both because of the excitement that engulfed biological sciences in the seventies and because of funding and employment opportunities.
After getting my bachelors degree in 1971, I worked for a year in the lab of Cyrus Levinthal, well known for, among other things, the Levinthal’s Paradox. Cyrus was a high-energy physicist turned successful biological researcher who, after working in molecular biology for a while, had switched his attention to neurosciences, in particular to connectivity in the developing nervous system. He was interested in the use of graphical computational tools to reconstruct, by tracing of electron microscopy serial sections, the growth and initial connections of the optic nerve axons in the small microcrustacean Daphnia (commonly referred to as the water flea) (e.g. (Lopresti et al. 1973)). In a way, this was a high-energy-physics inspired approach since, at the time, laboriously digitizing pathways of particles created in accelerator experiments was already a common practice. Cyrus had one of the first computers with a graphical interface in his lab and I worked that year as a programmer on this platform. I find it interesting that after many years, I now work towards tackling analogous problems, but with high and ultrahigh2 field MR imaging and humans in the Human Connectome Project (http://humanconnectome.org/consortia/) that was recently awarded by NIH to a consortium lead by Washington University and University of Minnesota with David Van Essen and I as the principal investigators.
Despite my exposure to biology and neurosciences early on, I still did not pursue graduate studies in these topics. Instead, I drifted back to more physics with some biology included, doing my PhD with Richard Bersohn in the Chemistry Department at Columbia. Richard was a physical chemist who had done a lot of theoretical and experimental work with molecular beams, but was getting interested in biological problems at the time. During my PhD, I studied the structure and function of a cupper-containing electron transfer protein from bacteria. My PhD experience was quite broad. I carried out many aspects of the work alone, isolating and purifying the protein of interest and conducting studies of that protein with techniques like NMR, optical detection of triplet states, and fluorescence; I even worked on and with nanosecond lasers to measure rotational correlation times of the protein in solution, a parameter that is important for relaxation mechanisms in NMR, though this work was never published.
My exposure to biological problems at the cellular level came when I joined Robert (Bob) Shulman, Seiji Ogawa and Truman Brown at Bell Laboratories in the effort Bob had initiated in the department he led, the Biophysics Department, to apply MR spectroscopy to study intracellular processes in intact cells. Later, Jan den Hollander, Sheila Cohen and Bob Gillies would join us, and Gil Navon as well early in the effort. We employed 31P and 13C NMR spectroscopy to study energetics and metabolism in E. coli and yeast cells in suspension (e.g. (Ugurbil et al. 1978; Ugurbil et al. 1978; Shulman et al. 1979; Ugurbil et al. 1982)). The work from this lab together with the contemporaneous effort from the laboratory of George Radda at Oxford pioneered in vivo magnetic resonance spectroscopy or MRS that many employ today to study metabolism in the human body using high and ultrahigh magnetic fields. Led by Bob, who is one of the greatest talents I have known in recognizing an important new scientific direction, we were immersed in, totally excited about, and energized by the realization that we were pushing the boundaries of NMR. This atmosphere, together with presence of superb colleagues in Bell Labs, created an extremely rich intellectual environment and a rigorous scientific “culture”.
Bell Labs in general was truly a unique place3; in this large laboratory, owned and operated by a telephone company (AT&T), basic science research thrived with immense support given without demanding short-term returns. I particularly remember our lunches because they were almost always accompanied with long and interesting discussions among colleagues with diverse backgrounds, pursuing interesting questions, untethered with expectations of immediate return. Biological research flourished in Bell Labs because Bob Shulman had pointed out that a telephone company is ultimately interested in “information” and biological systems stored and utilized immense amount of information. Indeed a very long-term view in investment! This approach ultimately paid off not only in the great number Nobel prizes awarded to Bell Labs scientists, but also in the immense number of practical new technologies and consequent commercial returns. Max Perutz is quoted as saying “Creativity in science, as in arts, cannot be organized. It arises spontaneously from individual talent. Well-run laboratories can foster it but hierarchical organization, inflexible bureaucratic rules, and mounds of futile paperwork can kill it. Discoveries cannot be planned; they pop up, like Puck in unexpected corners”. Bell Labs knew about these principles from its inception; it was a well-run laboratory that fostered creativity. It is a model that department chairmen, deans, directors of labs etc. and especially those who formulate science and funding policy should be mindful of, especially these days when we appear to be rapidly sacrificing a long-term vision for possible short-term gains and, at the same time, increasing “inflexible bureaucratic rules and mounds of futile paperwork”. My Bell Labs experience was probably the most formative with respect to the development of my scientific interests and my approach to science, and is ultimately responsible for the push towards very high magnetic fields for human studies.
When I later moved to Columbia University as a faculty member and, subsequently, to the University of Minnesota in 1982, I continued the work I started in Bell Labs with in vivo spectroscopy but I switched from using cells in suspension to ex vivo perfused hearts and subsequently to whole animal models, in the latter case employing spatially localized spectroscopy, the introduction of which also dates back to Bell Labs years (Brown et al. 1982). I was, of course, always interested in expanding this effort ultimately to humans. However, these spectroscopy studies were being conducted at very high fields in order to compensate for the inherently poor signal-to-noise ratio (SNR) due to the very low concentrations of intracellular metabolites; chemical shift resolution also played a role, although with some nuclei increasing field strength does not always lead to improved chemical shift resolution for intracellular metabolites. Cell suspension and perfused organ studies utilized ~8.4 Tesla (8.4T) (360 MHz 1H frequency) vertical bore magnets intended originally for solution studies. Our whole animal investigations were performed at 4.7T using a magnet with a 40 cm horizontal bore that became available in the early nineteen eighties. At the time, human MR research was being carried out mainly at 1.5T. I did not see spectroscopy succeeding at this low magnetic field and was not interested in pursuing it, despite the fact that there was a significant effort on human 1H spectroscopy at the time using 1.5T in other laboratories. What was potentially interesting to me, however, was the 4T projects launched by the three major manufacturers of clinical MRI instruments in the nineteen eighties.
DEVELOPMENT of 4 TESLA MR for HUMANS
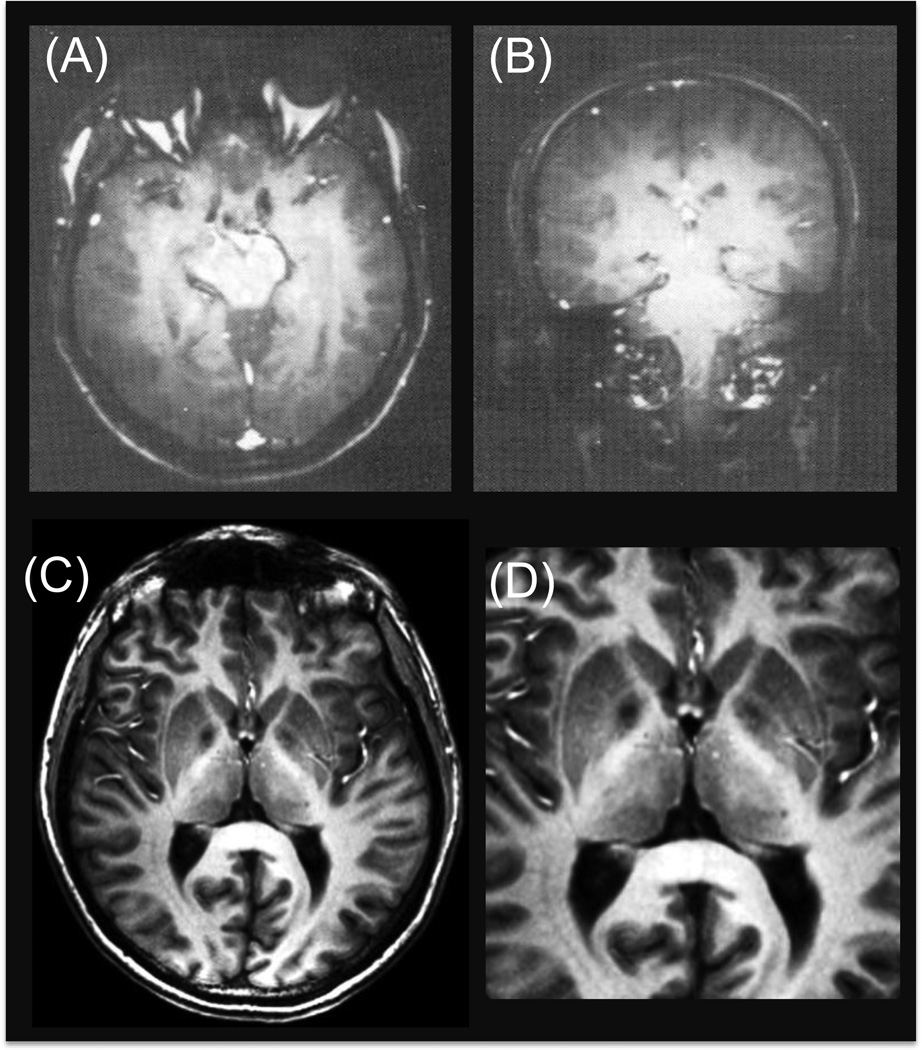
In the mid eighties, two 4T magnets, one with an amazing 125 cm diameter bore and the other with a more conventional 100 cm diameter bore, were built by Siemens in Erlangen, Germany. General Electric and Philips had also commissioned Oxford Magnet Technologies to build two 4T magnets, one for each company. Initial efforts in the research laboratories of these companies produced terrible 4T images (Figure 1A and 1B) and highlighted more problems than advantages at this high field (Barfuss et al. 1988; Barfuss et al. 1990). It was clear that going to 4T would require significant new investment in research and development; it was not going to be as simple as changing the frequency of the electronics and the RF coils and beautiful images would just fall out of the scanner. Most likely because of this discouraging conclusion, the industry abandoned the high field effort and more or less “dumped” their 4T systems on academic laboratories that were interested in them and even pay something towards them. In my opinion, this made commercial sense because there was already a large market for 1.5T and lower field instruments, which provided an opportunity to recover the initial R&D investment in MRI; so, why not reap the benefits of this initial R&D rather than invest more in new technology that appeared difficult and that did not promise immediate and easy returns.
FIGURE 1.
(A) and (B): Early 4 Tesla brain images published from Siemens (Barfuss et al. 1988; Barfuss et al. 1990). (C) and (D): 4 Tesla MDEFT images of human brain obtained in CMRR (Ugurbil et al. 1993; Lee et al. 1995).
I was not at all convinced about the early 4T results from industry. Since I had worked at fields up to 8.4T with spectroscopy in intact, albeit ex vivo tissues, I was not necessarily biased against high fields and saw the need for increasing the field strength. Consequently, I pursued the acquisition of one of the 4T magnets, often traveling and visiting manufacturers with Gerald Pohost, who had been one of the pioneers in promoting MRI in clinical cardiac applications, and who was interested also in acquiring a 4T system for the University of Alabama in Birmingham. We visited Philips, first in Hamburg, Germany and then in Best, Netherlands in April 1987; we marked the occasion in Best with a few Heinekens (Figure 2). Ultimately, Gerald Pohost acquired the Philips 4T system for the University of Alabama; we in University of Minnesota got the 125 cm bore magnet from Siemens (and electronics from SISCO, a short-lived joint venture of Varian and Siemens). The General Electric 4T system went to Bob Balaban at NIH intramural research. These systems were installed and became operational approximately two decades ago. Our magnet was not really designed for shipping. The strategy for shipping had to be carefully thought out. A brand new, specially equipped Mercedes truck was used. The entire truck was shipped to the USA by sea and the magnet did not leave this truck on its journey from Erlangen, Germany to Minneapolis. Nevertheless, the magnet was damaged. Luckily it was repairable and was repaired; however, we suffered a delay in the start of the project.
FIGURE 2.
The “Heineken” coaster that I and others signed 1-April-1987 as we enjoyed a beer on the occasion of a visit to Philips by me, Gerald Pohost and some of his colleagues from the University of Alabama to discuss 4 Tesla.
During the several years we waited for the 4T system to arrive, we were confronted repeatedly with significant skepticism about the high field human effort. One colleague even described the 4T effort as scientific suicide. This skepticism was probably fueled by the early work at 4T from industry and possibly by the earlier dire predictions about high fields for human imaging based on modeling studies. Two seminal thinkers in the field, Hoult and Lauterbur, concluded in a 1979 paper (Hoult and Lauterbur 1979) that “Effects associated with the radiofrequency penetration depth suggests that the frequency of operation of the spectrometer should be less than about 10 MHz”. They were proven to be wrong about 10 MHz long before the 4T magnets came into existence but their arguments against the higher fields lingered at least as qualitative harbingers of difficulties to be expected with ever increasing magnetic fields. However, my experience over the years with many reviewers of manuscripts and grants suggests also that there is an inherent conservatism prevalent in the MR imaging community. Clearly not all colleagues fall in this category, and luckily as we struggled to fund our work we found supporters as well. But very often we encountered an attitude centered on the notion that the best magnetic field is the prevalent one at the time. In countless grant reviews to this day, we see and continue to see comments to the effect that this or that is done best at 3T, or should be done at 3T because 3T is “clinical” and 7T is not or because 3T is more commonly available than the 7T. Ironically, the 3T “clinical” field strength that many seem to be content with these days itself was not “clinical” or commonly available for a long time while 1.5T reigned as the beloved high-end platform; in fact, MRI itself at any field strength was not “clinical” not too long ago. It would not be unfair to say we developed 4T and later 7T despite the resistance and conservatism from many members of the MR imaging community. I am perplexed and bemused by this conservatism; how does one enlarge the boundaries of knowledge and even diagnostic capability with such thinking, without taking risks, without exploring the unconventional? Every research field needs efforts at the fringes and, sometimes, as I fully believe to be the case for high/ultrahigh field MR, such fringe efforts indeed succeed and define new directions and capabilities and/or provide new insights, solutions, and improvements to mainstream technologies. To quote Kurt Vonnegut, a well-known American novelist, at times “we should all jump from cliffs and develop our wings on the way down” or at least we should all tolerate and even support those who do “jump from cliffs” and seek to develop their “wings on the way down”. Of course, in science (as well as in literature), one does not blindly jump over cliffs and expect a miracle to happen. But when grounds to question conventional wisdom exists and a flicker of light for new developments is perceivable, we should embark on unconventional paths that may appear to many as being equivalent to “jumping from cliffs”. In that precarious journey, human creativity will often prevail and overcome impending hurdles; thus, the effort will grow “wings” and take off. Many will undoubtedly crash and be destroyed. But others will develop their “wings on the way down” and lead us in new directions.
“Jumping from a cliff” in the view of many in the MRI field is more or less what we and the two other sites did when we decided to pursue 4T in the late eighties for human MR. Had any of these sites relied on NIH funding through reviews by our peers, 4T development would never have happened. The conservatism of the peer-review system would have prevented it. At the University of Minnesota, we had never worked with human imaging at any magnetic field before and 4T was uncharted, potentially treacherous territory. This would have been a toxic combination for the “peer review” system. But we were fortunate enough to encounter an opportunity in the mid eighties when the central administration of the University of Minnesota announced an interest in investing in new research directions. We applied for these funds4 and received them with the specific aim of developing a high field MR imaging center, later named the Center for Magnetic Resonance Research (CMRR), with 4T human MR as the principal focus.
Our 4T system was developed in the manufacturing plant of SISCO in Freemont California, using the Siemens 100 cm bore 4T magnet that was not destined for CMRR. The electronics were shipped to us from California while the 125 cm bore 4T magnet was shipped from Erlangen (Germany) to Minneapolis, to be integrated on site in CMRR. Just before the system was taken apart at SISCO, Mike Garwood from CMRR went to Freemont and produced beautiful T1 weighted anatomical images of the human brain on this 4T system with Tom Buddinger, a long proponent of ultrahigh fields, volunteering for the studies; these were the first 4T images ever showing image quality superior to contemporaneous 1.5T images, and certainly far superior than the earlier 4T images coming from industry laboratories. These images, obtained with a sequence named MDEFT, were reported much later in a review article (Ugurbil et al. 1993) and in a paper (Lee et al. 1995) but were first presented at a conference in Whistler in the fall of 1990. Figure 1C and 1D illustrates a slice and expanded view from that slice, respectively, acquired at 4T with this sequence. The success of the MDEFT approach came from the relative B1 insensitivity of the sequence, thus avoiding the problems arising from nonuniform B1’s at 4T and higher field strengths. Immediately after the 4T system was installed in Minneapolis, I pursued with it functional brain imaging, together with my former Bell Labs colleague Seiji Ogawa and my post-docs at the time Ravi Menon and Jutta Ellermann; this work became one of the studies that simultaneously and independently introduced fMRI (Ogawa et al. 1992). Shortly afterwards, Seong-Gi Kim and Xiaoping Hu joined the effort leading rapidly to many publications on high field fMRI. Armed with these early results, we applied for and received a grant from National Centers for Research Resources (NCRR), NIH (P41 RR008079) to support the development of fMRI, MRI and MR spectroscopy at 4T as a Biotechnology Research Resource (nowadays called Biotechnology Research Center), which is now in its twentieth year of funding. It was not a bad start for a field strength that was abandoned by the manufacturers and an instrument that was far from technologically mature. This 125 cm bore 4T magnet is now a garden “sculpture” in the courtyard of our MR center, the CMRR (see Figure 2 of Ugurbil in this issue).
FUNCTIONAL IMAGING
The fMRI effort came about because of the work Seiji did in Bell Labs introducing the BOLD effect (Ogawa et al. 1990; Ogawa et al. 1990; Ogawa and Lee 1990). These early experiments conducted on rats did not show functional mapping; rather, they demonstrated that metabolic perturbations such as hypoglycemia and graded levels of oxygen in the inhaled gas mixture affected the visibility of venous blood vessels. However, in his Proceedings of the National Academy of Science (PNAS) paper (Ogawa et al. 1990), Seiji and his coworkers suggested the use of this method to study oxygen metabolism and even possibly achieve functional imaging in the brain, in a way analogous to the PET functional imaging approach but, unlike PET, using an endogenous contrast mechanism. The link to oxygen metabolism, at the time my research focus, is what initially attracted my attention to this work as I heard Seiji present it in conferences. Even before Seiji’s 1990 PNAS paper appeared in press, we started talking about pursuing functional imaging together in the human brain using the 4T system I was waiting to receive in Minneapolis. Had the 4T system been delivered earlier or had it functioned right away, we would have achieved fMRI earlier. Although the 4T system was initially envisioned because of our spectroscopy studies, I was not “married” to spectroscopy; rather, I was, and all of us in CMRR still are, interested in obtaining unique biological information using MR techniques, what ever that technique may be. Thus, with the elucidation of the BOLD mechanism, fMRI became my highest priority project on the 4T even before this magnet arrived in Minnesota (also see Ugurbil, in this issue).
As we waited for the 4T to be operational, we did not think of pursuing fMRI at 1.5T because we were focused on BOLD contrast, which is a susceptibility effect. As such, we did not think BOLD fMRI would work adequately at low fields like 1.5T. In principle, we were right but our understating of functional signals was incomplete. Today, we know that the fMRI signal is quite complex and has numerous contributions; such contributions include inflow effects, mostly originating from flow increases in large blood vessels (e.g. (Duyn et al. 1994; Segebarth et al. 1994)), intravascular effects due to changes in blood T2 and T2* (e.g. (Duong et al. 2003; Silvennoinen et al. 2003)), or signal changes associated with large veins that occupy a large fraction of the voxel volume (e.g. (Hoogenraad et al. 1999)). We did not fully consider these effects at the time. Rather, we were focused on BOLD effect arising from intracortical veins and capillaries, which is relatively small, if there at all, at 1.5T (e.g. (Hoogenraad et al. 2001)).
Our 4T fMRI maps rapidly evolved to look different than what was being produced at 1.5T at the time. They revealed extensive gray-white matter structure that coincided well with the anatomical images, following the contours of cortical gray matter and, to some extend, overlapping the sulcal space (see Ugurbil, in this issue). I think this came about largely because of the high field, but also because we were able to progress towards relatively high resolution using gradient recalled echo sequence (e.g. FLASH), avoiding some of the limitations of Echo Planar Imaging (EPI); especially of concern at high fields is the effective resolution degradation due to the long echo trains and distortions caused by magnetic field inhomogeneities. At 4T, EPI was not available or even thinkable at the time; most of our sequences had to be painstakingly implement from scratch or from rudimentary implementations provided by SISCO. EPI was ultimately developed completely in house by Ravi Menon and Xiaoping Hu. It took many years, significant improvements in gradient performance and the development of parallel imaging before exquisite, high resolution EPI based fMRI images at high and ultrahigh fields would be obtained. Clearly, sequences like FLASH is unsuitable for whole brain fMRI studies that are becoming routine even at 7T today and EPI has other important advantages for fMRI because of the speed with which a slice image can be acquired. But the high field more than compensated for the disadvantages we had with FLASH type sequences. These early 4T FLASH images gave us the first hint that we can go far beyond the diffuse activations maps provided by PET, the dominant technology utilized for functional brain imaging prior to discovery of fMRI or by the contemporaneous 1.5T EPI based fMRI data, and led us to even dream of column level mapping; for us, these FLASH images set the standard for what EPI had to meet and ultimately exceed at high fields, with other added benefits such as whole brain coverage.
Our enthusiasm encountered a major hurdle when we became aware of the ”draining vein” problem. We realized the existence of this problem in a study where we employed a sagittal slice; in the resulting functional map we saw clearly the outline of the sagittal sinus as if looking at an angiogram. It was somewhat of a depressing moment as the realization and implications sank in. We published the existence of this confound in 1993 (Menon et al. 1993), and later in 1994 (Kim et al. 1994), for the first time calling attention to it. Nevertheless, modeling studies from our effort (Ogawa et al. 1993) and others at the time also suggested that the superiority of the 4T functional maps may originate from increased contributions from intracortical veins and micovasculature at the higher fields. In particular, the recognition that microvascular BOLD effect would increase supralinearly with magnetic field5 and the consequent expectation of high resolution functional mapping with improved fidelity to sites of neuronal activity, drove us to seek even higher magnetic fields for fMRI.
7 TESLA for HUMAN STUDIES
In the endeavor to achieve even higher fields for human fMRI (as well as spectroscopy) a critical partnership evolved with David Rayner and his magnet company Magnex. David was willing to assume the financial risk for the development of ultrahigh fields; he agreed to build and install ultrahigh field magnets in CMRR before the funding was in place, in exchange for a promise on our part to seek funding through grant applications. In each case, the magnets were installed before the funding ultimately materialized from NIH and the Keck Foundation. It is commendable that these organizations were willing to fund these magnets even though they were already in place. But without David’s contribution, the development of ultrahigh fields would have been significantly delayed.
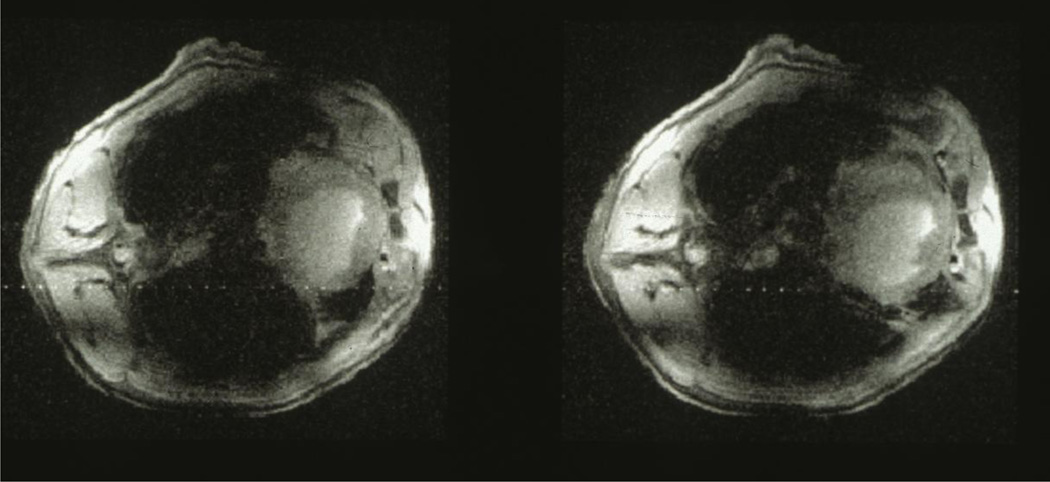
Our first project with David Rayner was a 9.4T/33 cm bore magnet for animal model studies. This was the first magnet at such a high field with such a bore size. It was successfully built and provided a plethora of fMRI as well spectroscopy data to warrant the exploration of similar fields for human studies. We also used this magnet to obtain an image of an intact porcine chest, approximating the size of the human head, demonstrating that RF problems can be tackled to achieve human head imaging even at such high fields (Figure 3). This may be one of the first uses of multichannel transmit and receive concept since we essentially employed a four loop coil to make a volume coil (arranged as two “quadrature” coils each of which was made up of two loops coupled through a 90 degree hybrid); we did not have multiple transmitter or receive channels but obtained images first with one quadrature pair and then the other, and subsequently combined them. Multichannel transmit is of course a bit more complex due to the simultaneity of the transmission; nonetheless, the approach anticipated the multichannel transmit and receive array technology we developed many years later for 7 and 9.4T human imaging (e.g. (Adriany et al. 2005; Vaughan et al. 2006)). I showed this porcine chest data that year (1995) in a plenary session at the annual meeting of the Radiological Society of North America (RSNA) to demonstrate the feasibility of imaging the human brain at even higher magnetic field strength than 4T, in anticipation of such a development.
FIGURE 3.
Image of two slices obtained in intact anesthetized porcine torso 9.4 Tesla with four loops, somewhat mimicking a multichannel transmits and receive coil (built by Hellmut Merkle in CMRR); the images were shown in a plenary talk at the 1995 annual meeting of the RSNA to demonstrate the feasibility of (and our intent to pursue) imaging human heads at such high field strengths.
David Rayner and I started discussing this prospect in the ISMAR meeting in Sydney in 1995, where I gave a talk. We looked at many designs, starting conservatively with a small-bore “head only” human magnet (Figure 4); this first exploration was completed by August 1995 (Figure 4). Subsequently, we expanded the search up to 7 Tesla/90 cm bore. David reported the results to us on 1 April 1996 (Figure 4, lower Fax). In 1996, we decided ultimately on a 7 Tesla/90 cm bore magnet because we did not want to compromise on the bore size; we anticipated that gradients will be a major challenge at this field strength and we did not want the bore size to limit gradient design. This system was installed in 1999 in CMRR. The installation was problematic because of an asymmetric passive shield design that was used to contain the stray field, leading to many months of delays. The complete system was put together by our group from parts we obtained from various manufacturers, with the “console” coming from Varian, gradient amplifiers donated by Siemens (arranged by Franz Schmitt), RF amplifiers from CPC, etc.
FIGURE 4.
Two Faxes from David Rayner dated 03 August 1995 and 01 April 96; discussing the 7T initiative. The approximate pricing supplied is blanked out. The color markings in the 1996 Fax identify our deliberations in CMRR at the time. Ultimately we decided on the 7.0/900 magnet; this design was employed on all 7T systems installed until 2011 when two new, actively shielded 7T magnets with 830 and 900 cm bore diameters were recently installed.
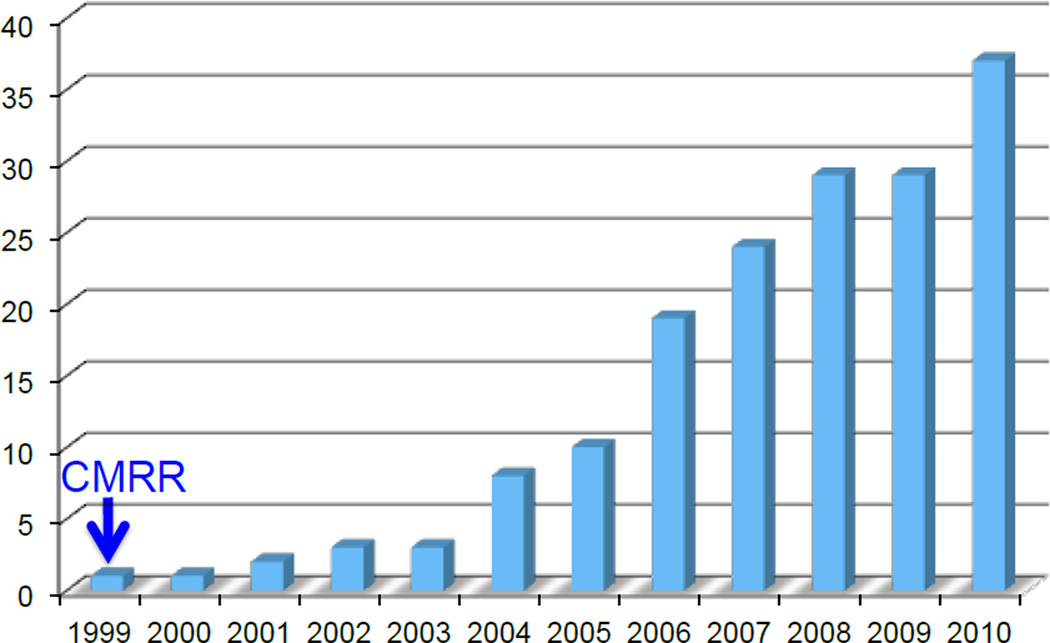
This “lego” 7T system is the first ever 7T MR instrument established for human studies; it did not emerge because a manufacturer one day decided to offer a 7T system. It came about based on a large body of work, first with 4T human studies and subsequently with 9.4T animal model experiments, mainly driven by functional imaging and in vivo spectroscopy. If you like, the CMRR 7T is the “mother” of all 7T human systems. Until 2011, all 7T magnets were based on the exact magnet that David Rayner and we had finally agreed upon. The system we put together was far less than optimal and significantly inferior to contemporary 7T instruments offered by the three major manufacturers; but, before another 7T paper on humans from another lab appeared in press, approximately thirty papers came from this “lego” 7T demonstrating the unusual physics of ultrahigh fields in the human body (i.e. the traveling wave behavior) (e.g. (Collins et al. 2002; Yang et al. 2002)), the signal-to noise gains (Vaughan et al. 2001), feasibility of excellent anatomical imaging (Vaughan et al. 2001), and of course the unique advantages for fMRI in the human brain (e.g., (Yacoub et al. 2001; Duong et al. 2002; Pfeuffer et al. 2002; Pfeuffer et al. 2002; Shmuel et al. 2002; Duong et al. 2003; Yacoub et al. 2003)), starting the trend for rapidly increasing number of 7T systems (Figure 5).
FIGURE 5.
Number of 7T human systems ordered as of April 2010. Data compiled from information obtained from Magnex/Varian (now Agilent) and Siemens.
An 8 Tesla/80 cm bore human magnet was delivered and installed at Ohio State shortly before our 7T was delivered. Beautiful gradient recalled echo images of the brain were produced from this system (e.g. (Abduljalil et al. 1999; Bourekas et al. 1999; Burgess et al. 1999; Norris et al. 1999; Robitaille et al. 2000)). Ultimately, however, it was the 7T work that defined the path of the ultrahigh field MR. On the way, several new technologies and image acquisition methods were developed that impact not only 7T but also lower field strength clinical instruments. This benefit of pushing the ultrahigh field technology is generally underappreciated. Parallel transmit was developed for overcoming the 7T problems of unusual B1 fields in the human body and is now used for improved torso imaging at 3T. Slice accelerated ultrafast whole brain imaging, i.e. the Multiband technique, that is becoming increasingly popular at all field strengths has found its first driving biological utility in ultrahigh field fMRI, thus starting its current popularity; the gains at 7 Tesla permitted us to aspire for obtaining whole brain functional maps with submillimeter isotropic resolution but it took too long to cover the entire human brain at such high resolutions. To overcome this problem, we proposed the Multiband fMRI approach for the competitive renewal application of our P41 Biotechnology Research Resource grant in 2007 and reported it first at the 2008 ISMRM annual meeting (Moeller et al. 2008), later publishing it at the beginning of 2010 (Moeller et al. 2010). Only subsequently, the Multiband technique and a version of it where we combined it with the SIR approach for greater acceleration (Feinberg et al. 2010) became the solution to the needs of the human connectome project at 3T, both in our and in the MGH-UCLA consortium (Setsompop et al. 2011).
Interestingly, we came on the Multiband technique because of breast imaging and spectroscopy studies that Mike Garwood and his colleagues were pursuing in CMRR at 4T. They employed a separate coil for each breast, and aimed to collect data from both simultaneously using a dual band pulse to excite both breasts but using a field-of-view adjusted only for one breast in order to gain speed of acquisition. They expected to distinguish the images from the two breasts simply by the spatial separation of the two coils; however, there was residual coil coupling and consequently cross-contamination. The fMRI group in CMRR was at the time very much into regular parallel imaging (i.e. with reduction of field-of-view along the phase encode direction) (e.g. (Wiesinger et al. 2004; Adriany et al. 2005; Van de Moortele et al. 2005)) and following this line of thought we took care of Mike’s problem by unaliasing the slices using the spatial sensitivity profile of the two coils. Then, we realized we could do something analogous in the brain to increase the speed of high resolution, whole brain multi-slice fMRI. At the time, we were not aware of the work by Larkman (Larkman et al. 2001) or improved versions of that work which came somewhat later (Breuer et al. 2005; Breuer et al. 2006) (shows that we were not always good at following the field or recognizing important contributions). Only later, searching the literature for prior work, we found these papers. Larkman, thus, deserves the credit for the original discovery. Nevertheless, there is great merit in showing the power of a method that had otherwise failed to be recognized and failed to make it into mainstream imaging; in case of Multiband imaging, this came about by the aspirations made possible by ultrahigh field fMRI. The episode also underscores the importance of a diverse scientific environment where cross-pollination of ideas can occur, as we intentionally try to garner in CMRR.
Functional imaging in the human brain, one of the main reasons we pushed to ultrahigh fields, reached new levels of spatial resolution and specificity at 7T, yielding for the first time tonotopic maps of the human primary auditory cortex, revealing its mirror symmetric organization (Formisano et al. 2003), presence of negative signal changes surrounding regions of increased neuronal activity (Shmuel et al. 2002), robust functional maps associated largely with the microvasculature detected using spin-echo (SE) fMRI (Duong et al. 2002; Yacoub et al. 2003; Yacoub et al. 2005), images of orientation columns (Yacoub et al. 2008) defining the organizational relationship between these elementary computational units and the ocular dominance columns, and 3D functional maps of the axis-of-motion selective features in human area MT with laminar resolution (Zimmermann et al. 2011). We worked towards the goal of imaging with columnar resolution from the very start of fMRI, and I personally felt fulfilled after the paper on orientation columns (Yacoub et al. 2008) finally appeared in press.
At the present, whole brain fMRI at isotropic columnar and laminar resolution, conducted at 7T with gradient echo EPI is feasible and in the near future we will see papers reporting studies with this capability with matching whole brain anatomy of exquisite detail. Critical technologies that make this possible are parallel imaging to accelerate along the phase encoding direction (Pruessmann et al. 1999; Sodickson et al. 1999; Griswold et al. 2002), improved EPI methods (e.g. (Zaitsev et al. 2004; Speck et al. 2008; Chung et al. 2011)), the use of slice accelerated whole brain imaging (Moeller et al. 2008; Feinberg et al. 2010; Moeller et al. 2010), parallel transmission (e.g. (Adriany et al. 2005; Van de Moortele et al. 2005; Vaughan et al. 2006; Metzger et al. 2008; Setsompop et al. 2008; Setsompop et al. 2008; Setsompop et al. 2008; Setsompop et al. 2009)) biased field correction methods in anatomical imaging (e.g. (Duyn et al. 2007; Van de Moortele et al. 2009)), and improved anatomical contrast (e.g. (Duyn et al. 2007; Rooney et al. 2007; Budde et al. 2011; Henry et al. 2011)). I believe when combined with recently developed decoding and encoding approaches for fMRI (e.g. (Kamitani and Tong 2005; Chaimow et al. 2010; Shmuel et al. 2010; Naselaris et al. 2011)) the ability to obtain such detailed functional images together with the corresponding anatomical information will elevate to new heights the methods of studying brain function as well as our understanding of it.
Closing the circle on what was started in Bell Labs, spectroscopy experiments first performed on E. coli cells in suspension are now being performed in the human brain thanks to the gains provided by ultrahigh fields. The very same magnetization transfer experiment Truman Brown and I introduced to measure enzymatic rates in E. coli (Brown et al. 1977), was finally accomplished approximately two and a half decades later in the human brain at 7T, yielding a quantitative measure of the oxidative ATP synthesis rate (Lei et al. 2003), while spectroscopy with 1H and low gyromagnetic ratio nuclei increasingly provide neurochemical and metabolic information in the human brain with unprecedented detail and biomedical relevance (e.g. (Tkac et al. 2001; Mangia et al. 2006; Mangia et al. 2007; Mangia et al. 2007; Avdievich et al. 2009; Tkac et al. 2009; Atkinson and Thulborn 2010; Oz et al. 2010)) with more improvements expected as a consequence of the recent focus on higher order shimming techniques (e.g. (Hetherington et al. 2006; Juchem et al. 2010). Until recently, all the high field studies were in the brain, but recently our group also showed for the first time that ultrahigh field imaging in the human torso, a more challenging goal due to the relative dimensions of the object versus the RF wavelength, is feasible at 7T (Metzger et al. 2008; Snyder et al. 2009; Vaughan et al. 2009; Metzger et al. 2010), thus starting a new burgeoning activity in several laboratories. New peaks are expected at ultrahigh field human MR as 9.4T systems mature and improve and the 10.5T and 11.7T systems planned for installation in 2011 and 2012 come into operation, hopefully in 2012.
At the 2002 annual meeting of the European Society of Magnetic Resonance in Medicine and Biology (ESMRMB) at Cannes, France, I was asked to take part in a “Hot Topics Debate” and argue against the motion that “there is only a niche market beyond 3T” with David Norris acting as the proponent. The exact motion may have been worded slightly differently. As expected in a debate, we had to argue the position we were assigned irrespective of our true conviction. In my case this was simple; I defended my convictions. We debated with humor and scientific data. David Norris naturally focused on all the difficulties faced at high fields, particularly at 7T, as evident in the sparse 7T data available at the time. I argued based on the 4T accomplishments and promises of 7T as suggested even by the early data. I reproduce verbatim below my concluding summary slide from this debate. The text in brackets [ ] are added for clarification. My slide read:
To CLAIM that BEYOND 3 T there is only but a NICHE MARKET is to
Deny all the experimentally demonstrated gains with [increasing] magnetic field magnitude
Deny that we are capable of creativity [to solve the problems of ultrahigh fields]
Deny the dynamism of MR research & development, and applications
Deny that there is clinical utility beyond todays clinical applications
Deny history
Deny that humans are fundamentally greedy!
At the end, the audience voted and I won the debate. I believe that since that debate, a decade of fantastic technological developments and increasing number of biological results, especially in fMRI, on the 7T platform has proven that I was right.
Critical to the work on high and ultrahigh field MR conducted at CMRR is, of course, the large number of talented individuals I had and continue to have the pleasure of working with; some of these individuals have moved on to establish and lead high field centers of their own in the USA and abroad. They are too numerous to list here but their names appear in the large number of papers we have published on this journey. The contributions of our numerous collaborators throughout the world have also been indispensible and should be recognized; again, they are too numerous to list here but can bee seen in our publications. Of course, without the funding from NIH (particularly our P41 Biotechnology Research Center grant (P41RR08079) and several High-end and Shared Instrumentation grants from the National Centers for Research Resources (NCRR)), the Keck Foundation and the University of Minnesota, this development would not have been possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Although, this article covers some of the literature on functional magnetic resonance imaging and ultrahigh fields, it is by no means meant to be a comprehensive scientific review of these topics or the relevant literature.
The terminology is based on that used for classifying radiofrequency (RF) bands. The frequency range 300 MHz to 3 GHz is defined as Ultra High Frequency (UHF) (see http://en.wikipedia.org/wiki/Ultra_high_frequency). The hydrogen nucleus resonance frequency at 7 Tesla is ~300 MHz i.e. in UHF band and hence, 7T can be defined as Ultra High Field (UHF).
Bell Labs, as it was in those days, disappeared with the break-up of AT&T.
I partnered with the Radiology Department chairman, William Thompson, for this project even though I was not a member of his Department at the time. His support, as well as the support of the Medical School administration, over the years was indispensable for our effort to develop CMRR, centered on high and ultrahigh field imaging.
Early models examined the field dependence over a limited range and predicted a quadratic dependence with magnetic field magnitude for capillary size vessels. More recent models covering field strengths up to ~17T demonstrate that supralinear dependence has a limit and persists to about ~7 to 10T (Uludag et al. 2009).
REFERENCES
- Abduljalil AM, Kangarlu A, Zhang X, Burgess RE, Robitaille PM. Acquisition of human multislice MR images at 8 Tesla. J Comput Assist Tomogr. 1999;23(3):335–340. doi: 10.1097/00004728-199905000-00001. [DOI] [PubMed] [Google Scholar]
- Adriany G, Van de Moortele PF, Wiesinger F, Moeller S, Strupp JP, Andersen P, Snyder C, Zhang X, Chen W, Pruessmann KP, Boesiger P, Vaughan T, Ugurbil K. Transmit and receive transmission line arrays for 7 Tesla parallel imaging. Magn Reson Med. 2005;53(2):434–445. doi: 10.1002/mrm.20321. [DOI] [PubMed] [Google Scholar]
- Atkinson IC, Thulborn KR. Feasibility of mapping the tissue mass corrected bioscale of cerebral metabolic rate of oxygen consumption using 17-oxygen and 23-sodium MR imaging in a human brain at 9.4 T. Neuroimage. 2010;51(2):723–733. doi: 10.1016/j.neuroimage.2010.02.056. [DOI] [PubMed] [Google Scholar]
- Avdievich NI, Pan JW, Baehring JM, Spencer DD, Hetherington HP. Short echo spectroscopic imaging of the human brain at 7T using transceiver arrays. Magn Reson Med. 2009 doi: 10.1002/mrm.21970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barfuss H, Fischer H, Hentschel D, Ladebeck R, Oppelt A, Wittig R, Duerr W, Oppelt R. In vivo magnetic resonance imaging and spectroscopy of humans with a 4 T whole-body magnet. NMR Biomed. 1990;3(1):31–45. doi: 10.1002/nbm.1940030106. [DOI] [PubMed] [Google Scholar]
- Barfuss H, Fischer H, Hentschel D, Ladebeck R, Vetter J. Whole-body MR imaging and spectroscopy with a 4-T system. Radiology. 1988;169(3):811–816. doi: 10.1148/radiology.169.3.3187004. [DOI] [PubMed] [Google Scholar]
- Bourekas EC, Christoforidis GA, Abduljalil AM, Kangarlu A, Chakeres DW, Spigos DG, Robitaille PM. High resolution MRI of the deep gray nuclei at 8 Tesla. J Comput Assist Tomogr. 1999;23(6):867–874. doi: 10.1097/00004728-199911000-00009. [DOI] [PubMed] [Google Scholar]
- Breuer FA, Blaimer M, Heidemann RM, Mueller MF, Griswold MA, Jakob PM. Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2005;53(3):684–691. doi: 10.1002/mrm.20401. [DOI] [PubMed] [Google Scholar]
- Breuer FA, Blaimer M, Mueller MF, Seiberlich N, Heidemann RM, Griswold MA, Jakob PM. Controlled aliasing in volumetric parallel imaging (2D CAIPIRINHA) Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2006;55(3):549–556. doi: 10.1002/mrm.20787. [DOI] [PubMed] [Google Scholar]
- Brown TR, Kincaid BM, Ugurbil K. NMR chemical shift imaging in three dimensions. Proc Natl Acad Sci U S A. 1982;79(11):3523–3526. doi: 10.1073/pnas.79.11.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TR, Ugurbil K, Shulman RG. 31P nuclear magnetic resonance measurements of ATPase kinetics in aerobic Escherichia coli cells. Proc Natl Acad Sci U S A. 1977;74(12):5551–5553. doi: 10.1073/pnas.74.12.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde J, Shajan G, Hoffmann J, Ugurbil K, Pohmann R. Human imaging at 9.4 T using T(2) *-, phase-, and susceptibility-weighted contrast. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2011;65(2):544–550. doi: 10.1002/mrm.22632. [DOI] [PubMed] [Google Scholar]
- Burgess RE, Yu Y, Abduljalil AM, Kangarlu A, Robitaille PM. High signal-to-noise FLASH imaging at 8 Tesla. Magn Reson Imaging. 1999;17(8):1099–1103. doi: 10.1016/s0730-725x(99)00072-7. [DOI] [PubMed] [Google Scholar]
- Chaimow D, Yacoub E, Ugurbil K, Shmuel A. Modeling and analysis of mechanisms underlying fMRI-based decoding of information conveyed in cortical columns. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JY, In MH, Oh SH, Zaitsev M, Speck O, Cho ZH. An improved PSF mapping method for EPI distortion correction in human brain at ultra high field (7T) MAGMA. 2011;24(3):179–190. doi: 10.1007/s10334-011-0251-1. [DOI] [PubMed] [Google Scholar]
- Collins CM, Yang QX, Wang JH, Zhang X, Liu H, Michaeli S, Zhu XH, Adriany G, Vaughan JT, Anderson P, Merkle H, Ugurbil K, Smith MB, Chen W. Different excitation and reception distributions with a single-loop transmit-receive surface coil near a head-sized spherical phantom at 300 MHz. Magn Reson Med. 2002;47(5):1026–1028. doi: 10.1002/mrm.10153. [DOI] [PubMed] [Google Scholar]
- Duong TQ, Yacoub E, Adriany G, Hu X, Ugurbil K, Kim SG. Microvascular BOLD contribution at 4 and 7 T in the human brain: Gradientecho and spin-echo fMRI with suppression of blood effects. Magn Reson Med. 2003;49(6):1019–1027. doi: 10.1002/mrm.10472. [DOI] [PubMed] [Google Scholar]
- Duong TQ, Yacoub E, Adriany G, Hu X, Ugurbil K, Vaughan JT, Merkle H, Kim SG. High-resolution, spin-echo BOLD, and CBF fMRI at 4 and 7 T. Magn Reson Med. 2002;48(4):589–593. doi: 10.1002/mrm.10252. [DOI] [PubMed] [Google Scholar]
- Duyn JH, Moonen CT, van Yperen GH, de Boer RW, Luyten PR. Inflow versus deoxyhemoglobin effects in BOLD functional MRI using gradient echoes at 1.5 T. NMR in biomedicine. 1994;7(1–2):83–88. doi: 10.1002/nbm.1940070113. [DOI] [PubMed] [Google Scholar]
- Duyn JH, van Gelderen P, Li TQ, de Zwart JA, Koretsky AP, Fukunaga M. High-field MRI of brain cortical substructure based on signal phase. Proc Natl Acad Sci U S A. 2007;104(28):11796–11801. doi: 10.1073/pnas.0610821104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Glasser MF, Miller KL, Ugurbil K, Yacoub E. Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PLoS ONE. 2010;5(12):e15710. doi: 10.1371/journal.pone.0015710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formisano E, Kim DS, Di Salle F, van de Moortele PF, Ugurbil K, Goebel R. Mirror-symmetric tonotopic maps in human primary auditory cortex. Neuron. 2003;40(4):859–869. doi: 10.1016/s0896-6273(03)00669-x. [DOI] [PubMed] [Google Scholar]
- Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002;47(6):1202–1210. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- Henry TR, Chupin M, Lehericy S, Strupp J, Sikora MA, Sha Z-Y, Ugurbi K, Van de Moortele PF. Hippocampal Sclerosis in Temporal Lobe Epilepsy: New Findings at 7 Tesla. Radiology. 2011 doi: 10.1148/radiol.11101651. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington HP, Chu WJ, Gonen O, Pan JW. Robust fully automated shimming of the human brain for high-field 1H spectroscopic imaging. Magn Reson Med. 2006;56(1):26–33. doi: 10.1002/mrm.20941. [DOI] [PubMed] [Google Scholar]
- Hoogenraad FG, Hofman MB, Pouwels PJ, Reichenbach JR, Rombouts SA, Haacke EM. Sub-millimeter fMRI at 1.5 Tesla: correlation of high resolution with low resolution measurements. J Magn Reson Imaging. 1999;9(3):475–482. doi: 10.1002/(sici)1522-2586(199903)9:3<475::aid-jmri17>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Hoogenraad FG, Pouwels PJ, Hofman MB, Reichenbach JR, Sprenger M, Haacke EM. Quantitative differentiation between BOLD models in fMRI. Magn Reson Med. 2001;45(2):233–246. doi: 10.1002/1522-2594(200102)45:2<233::aid-mrm1032>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Hoult DI, Lauterbur PC. The sensitivity of the Zeugmatographic experiment involving human samples. J Magn Reson. 1979;34:425–433. [Google Scholar]
- Juchem C, Nixon TW, McIntyre S, Rothman DL, de Graaf RA. Magnetic field homogenization of the human prefrontal cortex with a set of localized electrical coils. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2010;63(1):171–180. doi: 10.1002/mrm.22164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamitani Y, Tong F. Decoding the visual and subjective contents of the human brain. Nat Neurosci. 2005;8(5):679–685. doi: 10.1038/nn1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SG, Hendrich K, Hu X, Merkle H, Ugurbil K. Potential pitfalls of functional MRI using conventional gradient-recalled echo techniques. NMR Biomed. 1994;7(1–2):69–74. doi: 10.1002/nbm.1940070111. [DOI] [PubMed] [Google Scholar]
- Larkman DJ, Hajnal JV, Herlihy AH, Coutts GA, Young IR, Ehnholm G. Use of multicoil arrays for separation of signal from multiple slices simultaneously excited. J Magn Reson Imaging. 2001;13(2):313–317. doi: 10.1002/1522-2586(200102)13:2<313::aid-jmri1045>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Lee JH, Garwood M, Menon R, Adriany G, Andersen P, Truwit CL, Ugurbil K. High contrast and fast three-dimensional magnetic resonance imaging at high fields. Magn Reson Med. 1995;34(3):308–312. doi: 10.1002/mrm.1910340305. [DOI] [PubMed] [Google Scholar]
- Lei H, Ugurbil K, Chen W. Measurement of unidirectional Pi to ATP flux in human visual cortex at 7 T by using in vivo 31P magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 2003;100(24):14409–14414. doi: 10.1073/pnas.2332656100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopresti V, Macagno ER, VLevinthal C. Structure and development of the neuronal connections in isogeneic Organisms: Cellular interactionsin the development of the ptic lamina of Daphnia. Proc. Natl. acad. Sci. USA. 1973;70(2):433–437. doi: 10.1073/pnas.70.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangia S, Tkac I, Gruetter R, Van De Moortele PF, Giove F, Maraviglia B, Ugurbil K. Sensitivity of single-voxel 1H-MRS in investigating the metabolism of the activated human visual cortex at 7 T. Magn Reson Imaging. 2006;24(4):343–348. doi: 10.1016/j.mri.2005.12.023. [DOI] [PubMed] [Google Scholar]
- Mangia S, Tkac I, Gruetter R, Van de Moortele PF, Maraviglia B, Ugurbil K. Sustained neuronal activation raises oxidative metabolism to a new steady-state level: evidence from 1H NMR spectroscopy in the human visual cortex. J Cereb Blood Flow Metab. 2007;27(5):1055–1063. doi: 10.1038/sj.jcbfm.9600401. [DOI] [PubMed] [Google Scholar]
- Mangia S, Tkac I, Logothetis NK, Gruetter R, Van de Moortele PF, Ugurbil K. Dynamics of lactate concentration and blood oxygen level-dependent effect in the human visual cortex during repeated identical stimuli. J Neurosci Res. 2007 doi: 10.1002/jnr.21371. [DOI] [PubMed] [Google Scholar]
- Menon RS, Ogawa S, Tank DW, Ugurbil K. 4 Tesla gradient recalled echo characteristics of photic stimulation- induced signal changes in the human primary visual cortex. Magn Reson Med. 1993;30(3):380–386. doi: 10.1002/mrm.1910300317. [DOI] [PubMed] [Google Scholar]
- Metzger GJ, Snyder C, Akgun C, Vaughan T, Ugurbil K, Van de Moortele PF. Local B1+ shimming for prostate imaging with transceiver arrays at 7T based on subject-dependent transmit phase measurements. Magn Reson Med. 2008;59(2):396–409. doi: 10.1002/mrm.21476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger GJ, van de Moortele PF, Akgun C, Snyder CJ, Moeller S, Strupp J, Andersen P, Shrivastava D, Vaughan T, Ugurbil K, Adriany G. Performance of external and internal coil configurations for prostate investigations at 7 T. Magn Reson Med. 2010;64(6):1625–1639. doi: 10.1002/mrm.22552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller S, Auerbach E, van de Moortele P-F, Adriany G, Ugurbil K. fMRI with 16 fold reduction using multibanded multislice sampling. Proc. Int. Soc. Magn. Reson. in Med. 2008;16:2366. [Google Scholar]
- Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, Ugurbil K. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med. 2010;63(5):1144–1153. doi: 10.1002/mrm.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naselaris T, Kay KN, Nishimoto S, Gallant JL. Encoding and decoding in fMRI. Neuroimage. 2011;56(2):400–410. doi: 10.1016/j.neuroimage.2010.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris DG, Kangarlu A, Schwarzbauer C, Abduljalil AM, Christoforidis G, Robitaille PM. MDEFT imaging of the human brain at 8 T. MAGMA. 1999;9(1–2):92–96. doi: 10.1007/BF02634598. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee T-M, Kay AR, Tank DW. Brain Magnetic Resonance Imaging with Contrast Dependent on Blood Oxygenation. Proc Natl Acad Sci USA. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Lee T-M, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990;14:68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM. Magnetic Resonance Imaging of Blood Vessels at High Fields: in Vivo and in Vitro Measurments and Image Simulation. Magn Reson Med. 1990;16:9–18. doi: 10.1002/mrm.1910160103. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Menon RS, Tank DW, Kim S-G, Merkle H, Ellermann JM, Ugurbil K. Functional Brain Mapping by Blood Oxygenation Level- Dependent Contrast Magnetic Resonance Imaging. Biophys J. 1993;64:800–812. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992;89(13):5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz G, Iltis I, Hutter D, Thomas W, Bushara KO, Gomez CM. Distinct Neurochemical Profiles of Spinocerebellar Ataxias 1, 2, 6, and Cerebellar Multiple System Atrophy. Cerebellum. 2010 doi: 10.1007/s12311-010-0213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeuffer J, Adriany G, Shmuel A, Yacoub E, Van De Moortele PF, Hu X, Ugurbil K. Perfusion-based high-resolution functional imaging in the human brain at 7 Tesla. Magn Reson Med. 2002;47(5):903–911. doi: 10.1002/mrm.10154. [DOI] [PubMed] [Google Scholar]
- Pfeuffer J, van de Moortele PF, Yacoub E, Shmuel A, Adriany G, Andersen P, Merkle H, Garwood M, Ugurbil K, Hu X. Zoomed functional imaging in the human brain at 7 Tesla with simultaneous high spatial and high temporal resolution. Neuroimage. 2002;17(1):272–286. doi: 10.1006/nimg.2002.1103. [DOI] [PubMed] [Google Scholar]
- Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42(5):952–962. [PubMed] [Google Scholar]
- Robitaille PM, Abduljalil AM, Kangarlu A. Ultra high resolution imaging of the human head at 8 tesla, 2K×2K for Y2K. J Comput Assist Tomogr. 2000;24(1):2–8. doi: 10.1097/00004728-200001000-00002. [DOI] [PubMed] [Google Scholar]
- Rooney WD, Johnson G, Li X, Cohen ER, Kim SG, Ugurbil K, Springer CS., Jr Magnetic field and tissue dependencies of human brain longitudinal 1H2O relaxation in vivo. Magn Reson Med. 2007;57(2):308–318. doi: 10.1002/mrm.21122. [DOI] [PubMed] [Google Scholar]
- Segebarth C, Belle V, Delon C, Massarelli R, Decety J, Le Bas JF, Decorps M, Benabid AL. Functional MRI of the human brain: predominance of signals from extracerebral veins. Neuroreport. 1994;5(7):813–816. doi: 10.1097/00001756-199403000-00019. [DOI] [PubMed] [Google Scholar]
- Setsompop K, Alagappan V, Gagoski B, Witzel T, Polimeni J, Potthast A, Hebrank F, Fontius U, Schmitt F, Wald LL, Adalsteinsson E. Slice-selective RF pulses for in vivo B1+ inhomogeneity mitigation at 7 tesla using parallel RF excitation with a 16-element coil. Magn Reson Med. 2008;60(6):1422–1432. doi: 10.1002/mrm.21739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setsompop K, Alagappan V, Gagoski BA, Potthast A, Hebrank F, Fontius U, Schmitt F, Wald LL, Adalsteinsson E. Broadband slab selection with B1+ mitigation at 7T via parallel spectral-spatial excitation. Magn Reson Med. 2009;61(2):493–500. doi: 10.1002/mrm.21834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setsompop K, Alagappan V, Zelinski AC, Potthast A, Fontius U, Hebrank F, Schmitt F, Wald LL, Adalsteinsson E. High-flip-angle slice-selective parallel RF transmission with 8 channels at 7 T. J Magn Reson. 2008;195(1):76–84. doi: 10.1016/j.jmr.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planer imaging with reduced g-factor penalty. Magn Reson Med. 2011 doi: 10.1002/mrm.23097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setsompop K, Wald LL, Alagappan V, Gagoski BA, Adalsteinsson E. Magnitude least squares optimization for parallel radio frequency excitation design demonstrated at 7 Tesla with eight channels. Magn Reson Med. 2008;59(4):908–915. doi: 10.1002/mrm.21513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Chaimow D, Raddatz G, Ugurbil K, Yacoub E. Mechanisms underlying decoding at 7 T: ocular dominance columns, broad structures, and macroscopic blood vessels in V1 convey information on the stimulated eye. Neuroimage. 2010;49(3):1957–1964. doi: 10.1016/j.neuroimage.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Yacoub E, Pfeuffer J, Van de Moortele PF, Adriany G, Hu X, Ugurbil K. Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron. 2002;36(6):1195–1210. doi: 10.1016/s0896-6273(02)01061-9. [DOI] [PubMed] [Google Scholar]
- Shulman RG, Brown TR, Ugurbil K, Ogawa S, Cohen SM, den Hollander JA. Cellular applications of 31P and 13C nuclear magnetic resonance. Science. 1979;205(4402):160–166. doi: 10.1126/science.36664. [DOI] [PubMed] [Google Scholar]
- Silvennoinen MJ, Clingman CS, Golay X, Kauppinen RA, van Zijl PC. Comparison of the dependence of blood R2 and R2* on oxygen saturation at 1.5 and 4.7 Tesla. Magn Reson Med. 2003;49(1):47–60. doi: 10.1002/mrm.10355. [DOI] [PubMed] [Google Scholar]
- Snyder CJ, DelaBarre L, Metzger GJ, van de Moortele PF, Akgun C, Ugurbil K, Vaughan JT. Initial results of cardiac imaging at 7 Tesla. Magn Reson Med. 2009;61(3):517–524. doi: 10.1002/mrm.21895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodickson DK, Griswold MA, Jakob PM. SMASH imaging. Magn Reson Imaging Clin N Am. 1999;7(2):237–254. vii-viii. [PubMed] [Google Scholar]
- Speck O, Stadler J, Zaitsev M. High resolution single-shot EPI at 7T. MAGMA. 2008;21(1–2):73–86. doi: 10.1007/s10334-007-0087-x. [DOI] [PubMed] [Google Scholar]
- Tkac I, Andersen P, Adriany G, Merkle H, Ugurbil K, Gruetter R. In vivo (1)H NMR spectroscopy of the human brain at 7 T. Magn Reson Med. 2001;46(3):451–456. doi: 10.1002/mrm.1213. [DOI] [PubMed] [Google Scholar]
- Tkac I, Oz G, Adriany G, Ugurbil K, Gruetter R. In vivo (1)H NMR spectroscopy of the human brain at high magnetic fields: Metabolite quantification at 4T vs. 7T. Magn Reson Med. 2009 doi: 10.1002/mrm.22086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurbil K, Brown TR, den Hollander JA, Glynn P, G. Shulman R. High-resolution 13C nuclear magnetic resonance studies of glucose metabolism in Escherichia coli. Proc Natl Acad Sci U S A. 1978;75(8):3742–3746. doi: 10.1073/pnas.75.8.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurbil K, Garwood M, Ellermann J, Hendrich K, Hinke R, Hu X, Kim SG, Menon R, Merkle H, Ogawa S, Salmi R. Imaging at high magnetic fields: initial experiences at 4 T. Magn Reson Q. 1993;9(4):259–277. [PubMed] [Google Scholar]
- Ugurbil K, Rottenberg H, Glynn P, Shulman RG. 31P nuclear magnetic resonance studies of bioenergetics and glycolysis in anaerobic Escherichia coli cells. Proc Natl Acad Sci U S A. 1978;75(5):2244–2248. doi: 10.1073/pnas.75.5.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurbil K, Rottenberg H, Glynn P, Shulman RG. Phosphorus-31 nuclear magnetic resonance studies of bioenergetics in wild-type and adenosinetriphosphatase(1-) Escherichia coli cells. Biochemistry. 1982;21(5):1068–1075. doi: 10.1021/bi00534a038. [DOI] [PubMed] [Google Scholar]
- Uludag K, Muller-Bierl B, Ugurbil K. An integrative model for neuronal activity-induced signal changes for gradient and spin echo functional imaging. Neuroimage. 2009;48(1):150–165. doi: 10.1016/j.neuroimage.2009.05.051. [DOI] [PubMed] [Google Scholar]
- Van de Moortele PF, Akgun C, Adriany G, Moeller S, Ritter J, Collins CM, Smith MB, Vaughan JT, Ugurbil K. B(1) destructive interferences and spatial phase patterns at 7 T with a head transceiver array coil. Magn Reson Med. 2005;54(6):1503–1518. doi: 10.1002/mrm.20708. [DOI] [PubMed] [Google Scholar]
- Van de Moortele PF, Auerbach EJ, Olman C, Yacoub E, Ugurbil K, Moeller S. T1 weighted brain images at 7 Tesla unbiased for Proton Density, T2* contrast and RF coil receive B1 sensitivity with simultaneous vessel visualization. Neuroimage. 2009;46(2):432–446. doi: 10.1016/j.neuroimage.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan JT, Garwood M, Collins CM, Liu W, DelaBarre L, Adriany G, Andersen P, Merkle H, Goebel R, Smith MB, Ugurbil K. 7T vs. 4T: RF power, homogeneity, and signal-to-noise comparison in head images. Magn Reson Med. 2001;46(1):24–30. doi: 10.1002/mrm.1156. [DOI] [PubMed] [Google Scholar]
- Vaughan JT, Snyder CJ, DelaBarre LJ, Bolan PJ, Tian J, Bolinger L, Adriany G, Andersen P, Strupp J, Ugurbil K. Whole-body imaging at 7T: preliminary results. Magn Reson Med. 2009;61(1):244–248. doi: 10.1002/mrm.21751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan T, DelaBarre L, Snyder C, Tian J, Akgun C, Shrivastava D, Liu W, Olson C, Adriany G, Strupp J, Andersen P, Gopinath A, van de Moortele PF, Garwood M, Ugurbil K. 9.4T human MRI: preliminary results. Magn Reson Med. 2006;56(6):1274–1282. doi: 10.1002/mrm.21073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesinger F, Van de Moortele PF, Adriany G, De Zanche N, Ugurbil K, Pruessmann KP. Parallel imaging performance as a function of field strength--an experimental investigation using electrodynamic scaling. Magn Reson Med. 2004;52(5):953–964. doi: 10.1002/mrm.20281. [DOI] [PubMed] [Google Scholar]
- Yacoub E, Duong TQ, Van De Moortele PF, Lindquist M, Adriany G, Kim SG, Ugurbil K, Hu X. Spin-echo fMRI in humans using high spatial resolutions and high magnetic fields. Magn Reson Med. 2003;49(4):655–664. doi: 10.1002/mrm.10433. [DOI] [PubMed] [Google Scholar]
- Yacoub E, Harel N, Ugurbil K. High-field fMRI unveils orientation columns in humans. Proc Natl Acad Sci U S A. 2008;105(30):10607–10612. doi: 10.1073/pnas.0804110105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub E, Shmuel A, Pfeuffer J, Van De Moortele PF, Adriany G, Andersen P, Vaughan JT, Merkle H, Ugurbil K, Hu X. Imaging brain function in humans at 7 Tesla. Magn Reson Med. 2001;45(4):588–594. doi: 10.1002/mrm.1080. [DOI] [PubMed] [Google Scholar]
- Yacoub E, Van De Moortele PF, Shmuel A, Ugurbil K. Signal and noise characteristics of Hahn SE and GE BOLD fMRI at 7 T in humans. Neuroimage. 2005;24(3):738–750. doi: 10.1016/j.neuroimage.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Yang QX, Wang J, Zhang X, Collins CM, Smith MB, Liu H, Zhu XH, Vaughan JT, Ugurbil K, Chen W. Analysis of wave behavior in lossy dielectric samples at high field. Magn Reson Med. 2002;47(5):982–989. doi: 10.1002/mrm.10137. [DOI] [PubMed] [Google Scholar]
- Zaitsev M, Hennig J, Speck O. Point spread function mapping with parallel imaging techniques and high acceleration factors: fast, robust, and flexible method for echo-planar imaging distortion correction. Magn Reson Med. 2004;52(5):1156–1166. doi: 10.1002/mrm.20261. [DOI] [PubMed] [Google Scholar]
- Zimmermann J, Goebel R, De Martino F, van de Moortele PF, Feinberg D, Adriany G, Chaimow D, Shmuel A, Ugurbil K, Yacoub E. Mapping the Organization of Axis of Motion Selective Features in Human Area MT Using High-Field fMRI. PLoS ONE. 2011;6(12):e28716. doi: 10.1371/journal.pone.0028716. [DOI] [PMC free article] [PubMed] [Google Scholar]