Abstract
Recent advances in molecular genetics combined with field manipulations are yielding new insight into the origin, evolutionary fate, and genetic architecture of phenotypic variation in natural plant populations, with two surprising implications for the evolution of plant genomes. First, genetic loci exhibiting antagonistic pleiotropy across natural environments appear rare relative to loci that are adaptive in one or more environments and neutral elsewhere. These ‘conditionally neutral’ alleles should sweep to fixation when they arise, yet genome comparisons find little evidence for such selective sweeps. Second, genes under biotic selection tend to be of larger effect than genes under abiotic selection. Recent theory suggests this may be a consequence of high gene flow among populations under selection for local adaptation.
Introduction
Modern plant biology seeks to understand the genetic basis, ecological significance, and evolutionary consequences of phenotypic variation. These interests underpin a number of important goals, including the improvement of crop yield to feed a growing human population, the sustainable harvest of natural resources, the preservation of endangered organisms, and the eradication of invasive species [1-3]; all of this must be achieved in the context of rapid, irreversible global climate change [4,5].
Genetic studies of phenotypic variation in natural populations can help to address these pressing issues by understanding how candidate genes and QTL characterized under relatively controlled environments translate to more natural field conditions, and how this in turn affects survival and reproduction. Here we review recent studies examining the genetic basis of ecologically relevant genetic variation, by which we mean genetic variation that affects survival and reproduction in natural environments. Several recent studies are adding insight into the number and magnitude of genetic effects underlying ecologically relevant alleles as well as the evolutionary origin and fate of these adaptive alleles in natural populations.
Which genes are ecologically relevant?
Following decades of elucidating genetic pathways in model plants, a next step is to ask which naturally occurring genetic polymorphisms affect ecologically relevant phenotypic traits. This is important because genes identified under controlled laboratory conditions may not translate to more natural conditions when genotype-by-environment effects are significant [6,7,8]. For example, developmental pathways affecting flowering time and other phenological traits in Arabidopsis thaliana are influenced by multiple environmental signals including vernalization time, temperature and photoperiod [9,10]. But despite extensive research on flowering time pathways in A. thaliana, a recent study examining over 200,000 SNPs from 13 independent recombinant inbred line (RIL) populations found only limited correspondence in flowering time QTL between field and glasshouse experiments [11]. In particular, 42 candidate loci were identified in this study, of which only two were associated with genes affecting flowering time in greenhouse studies (ETC3 and ATGA20X7). Similarly, there was little similarity between growth chamber and field experiments for genes affecting flowering time in Boechera stricta, a perennial relative of A. thaliana [12]. Importantly, these studies add to a growing literature on QTL-by-environment interactions [6] and show (i) strong candidate genes known from controlled environments are not detected under more natural conditions, as well as (ii) novel genetic loci affecting flowering time in the field but not greenhouse conditions.
In addition to field experiments using controlled crosses, genome wide association (GWA) studies can help to identify ecologically relevant genes under more natural conditions [13]. This approach was recently used to identify candidate genes for 107 traits in up to 192 Arabidopsis lines [14], and 682 individuals from 54 populations of Pinus taeda [15]. GWA analyses are a particularly valuable tool for the genetic study of trees and other long-lived perennials for which the creation of recombinant inbred lines is not feasible. However, the primary limitation with the GWA approach is the confounding of population genetic structure with locally adapted genes [13]. Statistical models must therefore balance false positive associations due to population structure against false negative (i.e., non-significant) results that fail to detect genes involved in local adaptation [16]. Therefore, an important next step will be moving from statistical association of QTL to gene characterization. For example, a major QTL affecting glucosinolate production, herbivore resistance and fitness in Boechera stricta has been identified for the purpose of positional cloning [17]. Future work will identify the specific gene(s) responsible, facilitating careful dissection of the exact genes and polymorphisms responsible for herbivore resistance and fitness variation.
Although positional cloning and direct experimentation on individual genes are preferable, recent modifications improve upon GWA analysis by coupling it with RILs, to provide an independent test of QTL associations (but see [18,19]). In Arabidopsis thaliana, for example, loci identified by GWA were validated using 13 independently derived mapping RILs, reducing false positive associations [11]. An alternative approach combines genome-wide SNP information with climate data to identify genomic regions associated with local adaptation in the native range of A. thaliana, reducing false negatives [15,20,21]. In addition to climate data, two of these studies measured fitness under field conditions and found widespread molecular evidence for local adaptation [20,21]. Hancock et al., [20] identified SNPs that were associated with a genotype's home climate. These putative adaptive SNPs show significant enrichment for functional, nonsynonymous changes, which is consistent with natural selection maintaining functional variation in genes responsible for local adaptation.
In addition to studying model systems in the field, studies of non-model organisms will provide complementary insight into ecologically relevant genetic variation. New methods associated with next-generation sequencing technology continue to decrease genotyping costs, facilitating ecological genetic studies of non-model organisms [22,23]. This, combined with a strong history of genetic studies in model systems, will accelerate understanding of ecologically relevant genetic variation. Recent work on Helianthus annuus, for example, examines homologues of flowering time genes identified in model systems [24,25]. Growth chamber experiments suggest these genes play a role in local adaptation to climate [24], and DNA polymorphism shows evidence of selection associated with domestication [25]. Modern advances in genetic and genomic technology are therefore having profound effects on the study of natural genetic variation. In the next section we consider what insights these studies might provide into the spread of adaptive alleles and the maintenance of genetic variation in natural populations.
What is the origin and evolutionary fate of adaptive alleles?
Natural selection causes advantageous alleles to spread (positive selection) while eliminating those that are deleterious or poorly adapted (negative selection). Ecologically relevant genetic variation that is favored by natural selection can originate from two sources – new advantageous mutations that eventually sweep to fixation (hard sweeps), or pre-existing alleles that become favored due to changes in natural selection (soft sweeps) [26]. Reports of hard sweeps have dominated evolutionary studies of DNA sequence data, probably because they are much easier to detect [27,28]. In contrast, limited information is available on the role of soft sweeps and polygenic adaptation, which leave little molecular signature of adaptive evolution. For example, a recent analysis of almost 5,000,000 SNPs in 80 A. thaliana genomes [29], and nine pairs of plant genomes [30] found that species-wide hard sweeps were rare, although the importance of soft sweeps could not be addressed. Yet, recent field studies using A. thaliana identified SNPs showing strong associations with climate [20], and the number of these local SNP alleles was a significant predictor of fitness in this field site. In parallel, Fournier-Level et al., [21] identified genomic regions responsible for adaptation to four European environments. Interestingly, these two studies reached contradictory conclusions regarding whether adaptation arose from pre-existing genetic variation or from new mutations. Nevertheless, results from both studies suggest that new adaptive mutations rarely sweep to fixation across the species range. What explains genetic variation at these adaptive loci?
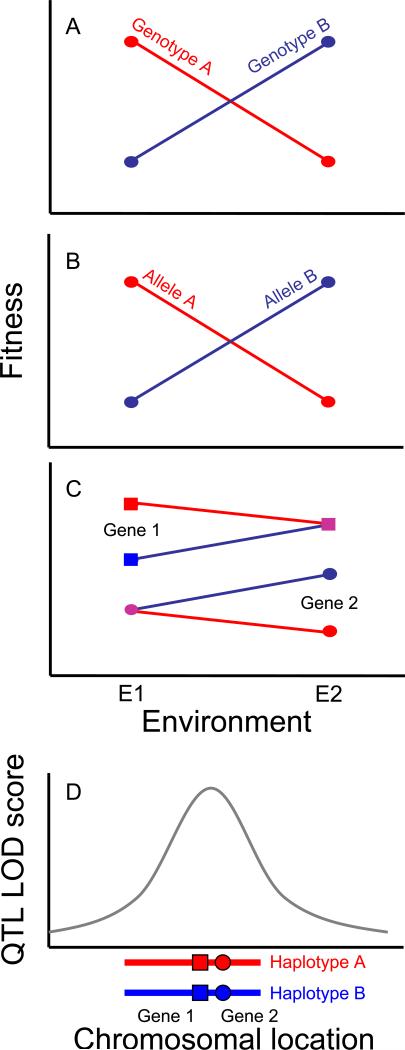
Antagonistic pleiotropy (AP) and conditional neutrality (CN) are two genetic phenomena that can cause plants to be locally adapted (Figure 1), but only AP can maintain genetic variation [6,8]. Conditional neutrality occurs when an allele is neutral in one environment and beneficial or deleterious in another whereas antagonistic pleiotropy occurs when the rank fitness of alleles changes across environments. Additionally, linkage disequilibrium between closely-linked CN loci may cause a QTL to exhibit genetic tradeoffs, resulting in “pseudo-antagonistic pleiotropy” (see Figure 1). Several recent experiments have identified conditionally neutral loci under field conditions, but evidence for antagonistic pleiotropy is rare. For example, Fournier-Level et al. [21] compared adaptive alleles across four common gardens and found different adaptive loci in different environments, consistent with conditional neutrality. In fact, only 12 of 797 loci statistically associated with local adaptation showed evidence of AP. Several genetic studies of local adaptation in other plant species have also identified CN, including studies of Avena barbata [31], Hordeum spontaneum [32], and Mimulus guttatus [33]. Exceptions include a tradeoff between pathogen resistance and growth rate due to the rpm1 locus in A. thaliana [34], and a large-effect QTL controlling life history variation in Boechera stricta, which shows genetic tradeoffs in the natural environments where this polymorphism originally evolved [35]. Overall, experimental evidence for AP is surprisingly rare.
Figure 1.
Local adaptation may be caused by tightly linked genes with reciprocal patterns of conditional neutrality (CN) or antagonistic pleiotropy (AP) at individual loci. (A and B) fitness reaction norms showing (A) genetic tradeoffs, across the entire genome and (B) at an individual locus (antagonistic pleiotropy, AP). (C) Reciprocal patterns of CN in two genetic loci. (D) Linkage disequilibrium between genes in panel C produces a pattern of ‘pseudo-AP’ at linked loci, resulting in a QTL that resembles panel B. Both AP and pseudo-AP with tight linkage disequilibrium can maintain genetic variation in natural populations.
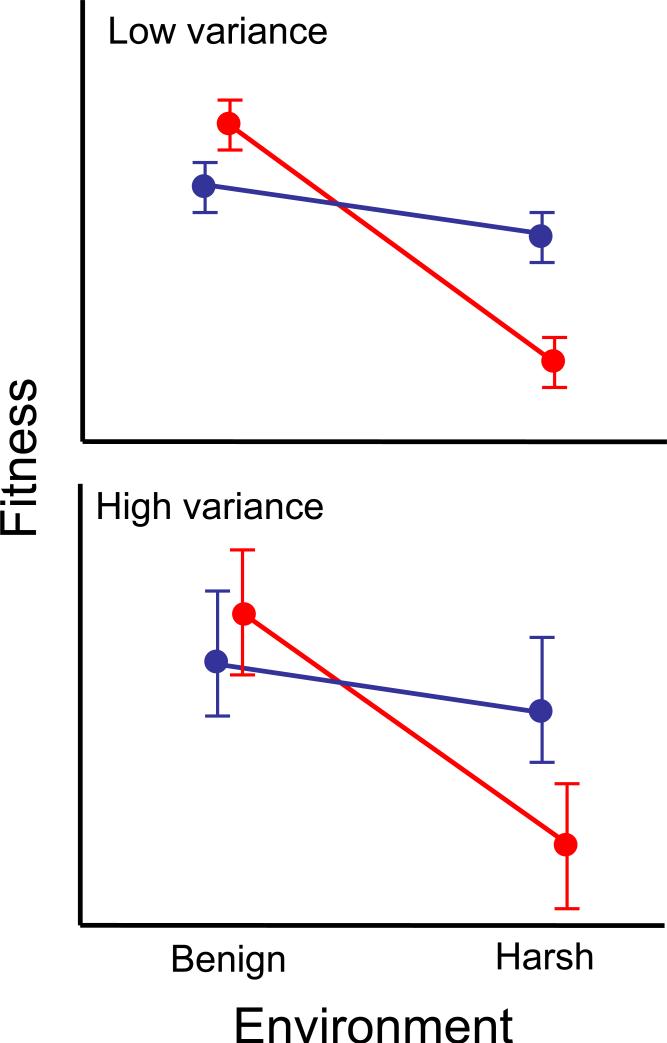
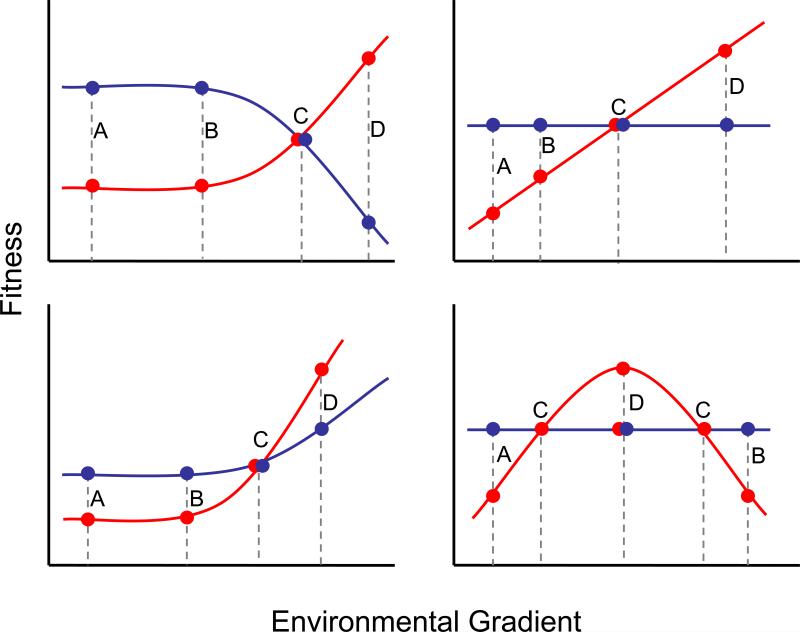
The large disparity between CN and AP loci is surprising because a conditionally beneficial allele should sweep to fixation, causing enrichment of AP loci among remaining functional polymorphisms. One explanation is that CN alleles arise frequently through mutation because many loci affect fitness, resulting in a number of CN polymorphisms on their way to fixation. However, this is not supported by the paucity of species-wide selective sweeps in genome-wide analysis of A. thaliana [29]. Instead, many AP polymorphisms may be mistaken as CN; this can occur for several reasons. First, AP is more difficult to detect statistically (Figure 2), as it requires rejection of the null hypothesis in two environments [7]. Second, fitness differences may be weaker and therefore more difficult to detect in benign environments (Figure 2), particularly for trade-offs involving energetic costs. For example, Todesco et al. [36] identify a QTL (ACD6) involved in microbial defenses that also reduces growth rate and biomass of A. thaliana, consistent with an AP locus. However, the difference in pathogen infection was orders of magnitude greater than the difference in growth, indicating a weaker trade-off in the absence of pathogens. Third, most field studies measuring fitness of QTL begin with established seedlings and examine fitness in a small number of environments – typically just two field sites. Detection of CN and AP can depend on the choice of experimental conditions when few environments are compared (Figure 3), providing an incomplete or inaccurate picture of the evolutionary significance of ecologically relevant genetic variation.
Figure 2.
Fitness reaction norms for two alleles at a locus (alleles Q1 in blue and Q2 in red) measured with low (top panel) or high (bottom panel) residual error in benign or harsh environments. Antagonistic pleiotropy is present in both panels but only detected in the top panel where the null hypothesis of equal means can be rejected in both environments.
Figure 3.
Hypothetical reaction norms for two alleles at a locus (alleles Q1 in blue and Q2 in red) are modeled as linear or quadratic fitness functions across an environmental gradient (e.g. temperature, moisture or herbivore damage). Reaction norms are drawn such that mean fitness across the environmental gradient is equal between the Q1 and Q2 alleles in each of the four panels, maintaining genetic variation across the gradient. Antagonistic pleiotropy is suggested when alleles are compared only in paired environments marked by A-D or B-D. Conditional neutrality is suggested when alleles are compared only in environments marked A-C, B-C or C-D. When only environments marked A-B are compared, the Q1 allele appears to have higher fitness in all four panels even though alleles have equal fitness when averaged across the gradient.
Selection on QTL with pleiotropic effects on unmeasured life stages (e.g., germination, seedling survival) in one or more environments could undermine estimates of fitness at other stages, resulting in inaccurate tests of AP and CN [37]. Additionally, spatial and temporal heterogeneity in biotic interactions and abiotic growing conditions could alter the strength of selection across a meta-population, resulting in AP that is not detected using current methods. Even when comprehensive survival and fecundity data are available, other factors such as mating success and offspring quality may affect fitness of QTL in natural populations. Given these difficulties, measuring changes in allele frequency at QTL of interest and at different life stages can improve field measurements of natural selection, increasing power to detect AP loci. Using this approach, Huang et al [38] identified germination traits under strong natural selection in field experiments. Direct estimates of fitness through changes in allele frequency are relatively straightforward using RILs [35], and this could help to identify pleiotropic effects operating at different life stages (e.g., seedling survival vs. adult fecundity) and at multiple field sites.
Both statistical and experimental difficulties have probably resulted in an under-reporting of AP relative to CN. However, these do not seem sufficient to account for the large disparity in identification of CN over AP loci. Instead, true CN polymorphisms may persist over large spatial scales and across other barriers to gene flow that greatly reduce the migration rate of adaptive alleles. This may in part explain the enrichment of CN loci found in many studies, as parental genotypes for QTL mapping lines often represent divergent genotypes due to geographical isolation [38], or isolated ecotypes occupying different habitats and with different life history strategies [31,33]. However, limited recombination in QTL mapping populations should also increase the number of “pseudo AP” loci due to linked genes having reciprocal patterns of CN (Figure 1).
Are adaptations caused by a few large-effect or many small-effect genes?
Recent theoretical work suggests that natural selection is likely to create fewer-and larger-effect QTL when selection favors local adaptation in populations subject to high gene flow [39]. Consistent with this model, large-effect QTL do appear to be common for plant traits associated with biotic selection. A recent meta-analysis found that QTL associated with traits involved in biotic interactions (e.g., herbivore resistance, pathogen damage) have larger effects than QTL for traits under abiotic selection (e.g., flowering time pathway) [39]. For example, flowering time in 5,000 Maize RILs is controlled by many QTL of small effect [41], while a single large QTL affects biochemistry and herbivore susceptibility in Boechera stricta [17]. In addition to differences in effect size, a recent analysis of A. thaliana genomes and transcriptomes from 18 natural accessions [42] found that genes under biotic selection were more polymorphic than genes under abiotic selection, consistent with stronger balancing selection on biotic traits.
The observation that QTL often have smaller effects under abiotic selection may be partly explained by the fact that abiotic conditions such as temperature or photoperiod often vary gradually over large geographical areas, whereas biotic interactions (e.g., herbivory, pathogen damage) are more likely to be patchily and distinctly distributed over small spatial scales. However, both can act as strong agents of natural selection (e.g., frost vs. herbivory), and indeed similar levels of antagonistic QTL in biotic and abiotic traits suggest that selection at individual QTL does not differ significantly [40]. Contrary to this result, QTL-level selection would have been higher for biotic traits if they typically involved fewer loci than abiotic traits. Interestingly, the Yeaman and Whitlock model also predicts that many large-effect QTL may be comprised of tightly linked loci of smaller effect, resulting in pseudo-AP. Although this is not the case for key QTL involved in maize domestication [43], additional studies will help to determine the ecological conditions under which this prediction holds.
Conclusion
QTL mapping of recombinant inbred lines, combined with GWA mapping is a powerful approach to identify QTL affecting ecologically-relevant traits, like flowering time. Positional cloning of candidate genes for field experiments are a logical next step to move from statistical association to manipulative experiments for robust testing of ecological relevance.
Trade-offs at individual loci (i.e., antagonistic pleiotropy; AP) should maintain genetic variation across a heterogeneous landscape and result in evolution of locally adapted genotypes. However, cases of AP have yet to be demonstrated [34] compared to loci that do not show evidence of a trade-off (conditional neutrality; CN). Moreover, CN at several linked loci in recombinant inbred lines could be mistaken for AP (i.e., pseudo-AP). The paucity of AP relative to CN loci is surprising because new mutations exhibiting CN should sweep to fixation when they arise, but these ‘hard sweeps’ appear to be rare. Methodological and statistical difficulties inherent in identifying AP, as well as environmental heterogeneity and limited gene flow among populations may explain the paucity of demonstrated examples of AP. In particular, most results come from A. thaliana, where high rates of self-fertilization limit gene flow and increase linkage disequilibrium among CN loci. Studies of additional plant taxa in more ecologically diverse environments, and measuring selection at multiple life stages, will shed further insight into the genomic basis of local adaptation.
Acknowledgements
The authors thank Drs. J.C. Pires and Y. Van de Peer helpful discussion and comments. Funding for this project came from an NSERC Postdoctoral Fellowship to RIC, a Hung Taiwan- Duke University Fellowship to CRL, and NSF grant (EF-0723447) and NIH grant (R01 GM086496) to TMO.
References Cited
- 1.Ceccarelli S, Grando S, Maatougui M, Michael M, Slash M, Haghparast R, Rahmanian M, Taheri A, Al-Yassin A, Benbelkacem A, et al. Plant breeding and climate changes. Journal of Agricultural Science. 2010;148:627–637. [Google Scholar]
- 2.Sexton JP, McIntyre PJ, Angert AL, Rice KJ. Annual Reviews, editor. Evolution and Ecology of Species Range Limits. Annual Review of Ecology Evolution and Systematics. 2009. pp. 415–436. Annual Review of Ecology Evolution and Systematics.
- 3.Chevin LM, Lande R, Mace GM. Adaptation, Plasticity, and Extinction in a Changing Environment: Towards a Predictive Theory. Plos Biology. 2010;8 doi: 10.1371/journal.pbio.1000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlenker W, Roberts MJ. Nonlinear temperature effects indicate severe damages to US crop yields under climate change. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15594–15598. doi: 10.1073/pnas.0906865106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heller NE, Zavaleta ES. Biodiversity management in the face of climate change: A review of 22 years of recommendations. Biological Conservation. 2009;142:14–32. [Google Scholar]
- 6.Mackay TFC. The genetic architecture of quantitative traits. Annual Review of Genetics. 2001;35:303–339. doi: 10.1146/annurev.genet.35.102401.090633. [DOI] [PubMed] [Google Scholar]
- 7.Weinig C, Ungerer MC, Dorn LA, Kane NC, Toyonaga Y, Halldorsdottir SS, Mackay TFC, Prugganan MD, Schmitt J. Novel loci control variation in reproductive timing in Arabidopsis thaliana in natural environments. Genetics. 2002;162:1875–11884. doi: 10.1093/genetics/162.4.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson JT, Willis JH, Mitchell-Olds T. Evolutionary genetics of plant adaptation. Trends in Genetics. 2011;27:258–266. doi: 10.1016/j.tig.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harmer SL. Annual Reviews, editor. The Circadian System in Higher Plants. Annual Review of Plant Biology. 2009. pp. 357–377. Annual Review of Plant Biology. [DOI] [PubMed]
- 10.Amasino R. Seasonal and developmental timing of flowering. Plant Journal. 2010;61:1001–1013. doi: 10.1111/j.1365-313X.2010.04148.x. [DOI] [PubMed] [Google Scholar]
- 11•.Brachi B, Faure N, Horton M, Flahauw E, Vazquez A, Nordborg M, Bergelson J, Cuguen J, Roux F. Linkage and Association Mapping of Arabidopsis thaliana Flowering Time in Nature. Plos Genetics. 2010;6 doi: 10.1371/journal.pgen.1000940. [This paper combines genome-wide association with multiple mapping pedigrees to examine genes controlling flowering time in A. thaliana. There is a striking disparity between the genes identified in lab and field, because it is difficult to mimic realistic field conditions in laboratory studies.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson JT, Lee CR, Mitchell-Olds T. LIFE-HISTORY QTLS AND NATURAL SELECTION ON FLOWERING TIME IN BOECHERA STRICTA, A PERENNIAL RELATIVE OF ARABIDOPSIS. Evolution. 2011;65:771–787. doi: 10.1111/j.1558-5646.2010.01175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ingvarsson PK, Street NR. Association genetics of complex traits in plants. New Phytologist. 2011;189:909–922. doi: 10.1111/j.1469-8137.2010.03593.x. [DOI] [PubMed] [Google Scholar]
- 14.Atwell S, Huang YS, Vilhjalmsson BJ, Willems G, Horton M, Li Y, Meng DZ, Platt A, Tarone AM, Hu TT, et al. Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature. 2010;465:627–631. doi: 10.1038/nature08800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckert AJ, Bower AD, Gonzalez-Martinez SC, Wegrzyn JL, Coop G, Neale DB. Back to nature: ecological genomics of loblolly pine (Pinus taeda, Pinaceae). Molecular Ecology. 2010;19:3789–3805. doi: 10.1111/j.1365-294X.2010.04698.x. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell-Olds T. Complex-trait analysis in plants. Genome Biology. 2010;11 doi: 10.1186/gb-2010-11-4-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schranz ME, Manzaneda AJ, Windsor AJ, Clauss MJ, Mitchell-Olds T. Ecological genomics of Boechera stricta: identification of a QTL controlling the allocation of methionine- vs branched-chain amino acid-derived glucosinolates and levels of insect herbivory. Heredity. 2009;102:465–474. doi: 10.1038/hdy.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergelson J, Roux F. Towards identifying genes underlying ecologically relevant traits in Arabidopsis thaliana. Nature Reviews Genetics. 2010;11:867–879. doi: 10.1038/nrg2896. [DOI] [PubMed] [Google Scholar]
- 19.Brachi B, Morris GP, Borevitz JO. Genome-wide association studies in plants: the missing heritability is in the field. Genome Biology. 2011;12:232. doi: 10.1186/gb-2011-12-10-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20•**.Hancock AM, Brachi B, Faure N, Horton MW, Jarymowycz LB, Sperone FG, Toomajian C, Roux F, Bergelson J. Adaptation to Climate Across the Arabidopsis thaliana Genome. Science. 2011;333:83–86. doi: 10.1126/science.1209244. [This reference and #17 provide complementary analyses of local adaptation to climate in A. thaliana, combining continental-scale climate data, genome-wide SNPs, and fitness estimates in the field.] [DOI] [PubMed] [Google Scholar]
- 21•**.Fournier-Level A, Korte A, Cooper MD, Nordborg M, Schmitt J, Wilczek AM. A Map of Local Adaptation in Arabidopsis thaliana. Science. 2011;333:86–89. doi: 10.1126/science.1209271. [See reference #16.] [DOI] [PubMed] [Google Scholar]
- 22.Ness RW, Siol M, Barrett SCH. De novo sequence assembly and characterization of the floral transcriptome in cross- and self-fertilizing plants. Bmc Genomics. 2011;12 doi: 10.1186/1471-2164-12-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wheat CW. Rapidly developing functional genomics in ecological model systems via 454 transcriptome sequencing. Genetica. 2010;138:433–451. doi: 10.1007/s10709-008-9326-y. [DOI] [PubMed] [Google Scholar]
- 24.Blackman BK, Michaels SD, Rieseberg LH. Connecting the sun to flowering in sunflower adaptation. Molecular Ecology. 2011;20:3503–3512. doi: 10.1111/j.1365-294X.2011.05166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blackman BK, Rasmussen DA, Strasburg JL, Raduski AR, Burke JM, Knapp SJ, Michaels SD, Rieseberg LH. Contributions of Flowering Time Genes to Sunflower Domestication and Improvement. Genetics. 2011;187:271–287. doi: 10.1534/genetics.110.121327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrett RDH, Schluter D. Adaptation from standing genetic variation. Trends in Ecology & Evolution. 2008;23:38–44. doi: 10.1016/j.tree.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 27•.Pritchard JK, Pickrell JK, Coop G. The Genetics of Human Adaptation: Hard Sweeps, Soft Sweeps, and Polygenic Adaptation. Current Biology. 2010;20:R208–R215. doi: 10.1016/j.cub.2009.11.055. [A nice review of methods for inferring natural selection from DNA sequence data.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhai WW, Nielsen R, Slatkin M. An Investigation of the Statistical Power of Neutrality Tests Based on Comparative and Population Genetic Data. Molecular Biology and Evolution. 2009;26:273–283. doi: 10.1093/molbev/msn231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao J, Schneeberger K, Ossowski S, Gunther T, Bender S, Fitz J, Koenig D, Lanz C, Stegle O, Lippert C, et al. Whole-genome sequencing of multiple Arabidopsis thaliana populations. Nature Genetics. 2011;43:956–U960. doi: 10.1038/ng.911. [DOI] [PubMed] [Google Scholar]
- 30.Gossmann TI, Song B-H, Windsor AJ, Mitchell-Olds T, Dixon CJ, Kapralov MV, Filatove DA, Eyre-Walker A. Genome Wide Analyses Reveal Little Evidence for Adaptive Evolution in Many Plant Species. Molecular Biology and Evolution. 2010;27:1822–1832. doi: 10.1093/molbev/msq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Latta RG, Gardner KM, Staples DA. Quantitative Trait Locus Mapping of Genes Under Selection Across Multiple Years and Sites in Avena barbata: Epistasis, Pleiotropy, and Genotype-by-Environment Interactions. Genetics. 2010;185:375–385. doi: 10.1534/genetics.110.114389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verhoeven KJF, Poorter H, Nevo E, Biere A. Habitat-specific natural selection at a flowering-time QTL is a main driver of local adaptation in two wild barley populations. Molecular Ecology. 2008;17:3416–3424. doi: 10.1111/j.1365-294X.2008.03847.x. [DOI] [PubMed] [Google Scholar]
- 33.Hall MC, Lowry DB, Willis JH. Is local adaptation in Mimulus guttatus caused by trade-offs at individual loci? Molecular Ecology. 2010;19:2739–2753. doi: 10.1111/j.1365-294X.2010.04680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian D, Traw MB, Chen JQ, Kreitman M, Bergelson J. Fitness costs of R-gene-mediated resistance in Arabidopsis thaliana. Nature. 2003;423:74–77. doi: 10.1038/nature01588. [DOI] [PubMed] [Google Scholar]
- 35.Anderson JT, Lee C-R, Rushworth C, Colautti RI, Mitchell-Olds T. Genetic tradeoffs and conditional neutrality contribute to local adaptation. Molecular Ecology. 2012 doi: 10.1111/j.1365-294X.2012.05522.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Todesco M, Balasubramanian S, Hu TT, Traw MB, Horton M, Epple P, Kuhns C, Sureshkumar S, Schwartz C, Lanz C, et al. Natural allelic variation underlying a major fitness trade-off in Arabidopsis thaliana. Nature. 2010;465:632–U129. doi: 10.1038/nature09083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakagawa S, Freckleton RP. Missing inaction: the dangers of ignoring missing data. Trends in Ecology & Evolution. 2008;23:592–596. doi: 10.1016/j.tree.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 38.Huang XQ, Schmitt J, Dorn L, Griffith C, Effgen S, Takao S, Koornneef M, Donohue K. The earliest stages of adaptation in an experimental plant population: strong selection on QTLS for seed dormancy. Molecular Ecology. 2010;19:1335–1351. doi: 10.1111/j.1365-294X.2010.04557.x. [DOI] [PubMed] [Google Scholar]
- 39.Yeaman S, Whitlock MC. THE GENETIC ARCHITECTURE OF ADAPTATION UNDER MIGRATION-SELECTION BALANCE. Evolution. 2011;65:1897–1911. doi: 10.1111/j.1558-5646.2011.01269.x. [DOI] [PubMed] [Google Scholar]
- 40.Louthan AM, Kay KM. Comparing the adaptive landscape across trait types: larger QTL effect size in traits under biotic selection. Bmc Evolutionary Biology. 2011;11 doi: 10.1186/1471-2148-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buckler ES, Holland JB, Bradbury PJ, Acharya CB, Brown PJ, Browne C, Ersoz E, Flint-Garcia S, Garcia A, Glaubitz JC, et al. The Genetic Architecture of Maize Flowering Time. Science. 2009;325:714–718. doi: 10.1126/science.1174276. [DOI] [PubMed] [Google Scholar]
- 42.Gan XC, Stegle O, Behr J, Steffen JG, Drewe P, Hildebrand KL, Lyngsoe R, Schultheiss SJ, Osborne EJ, Sreedharan VT, et al. Multiple reference genomes and transcriptomes for Arabidopsis thaliana. Nature. 2011;477:419–423. doi: 10.1038/nature10414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Studer AJ, Doebley JF. Do Large Effect QTL Fractionate? A Case Study at the Maize Domestication QTL teosinte branched1. Genetics. 2011;188:673–U257. doi: 10.1534/genetics.111.126508. [DOI] [PMC free article] [PubMed] [Google Scholar]