The determinants of antibody responses to adjuvanted influenza A/H1N1/09 vaccine were examined in a prospective, single-center field study in cancer outpatients.
Abstract
Purpose.
To identify the determinants of antibody responses to adjuvanted influenza A/H1N1/09 vaccines in a cohort of cancer outpatients.
Patients and Methods.
Patients with cancer and controls were enrolled in a prospective single-center field study. Two doses of AS03-adjuvanted pandemic influenza vaccine were administered to patients and one dose was administered to controls. Antibody responses were measured using hemagglutination inhibition and confirmed by microneutralization. Geometric mean titers (GMTs) and seroprotection rates (defined as GMTs ≥40) were compared.
Results.
Immunizations were safe and well tolerated in 197 cancer patients (lymphoma, 57; glioma, 26; lung or head and neck, 37; gastrointestinal, 41; breast, 36) and 138 controls. Similar seroprotection rates (82.3% versus 87%) and GMTs (336.9 versus 329.9) were achieved after two doses of adjuvanted vaccine in cancer patients and one dose in controls. Univariate analyses identified older age, prior immunization against seasonal influenza, lymphoma, CD4 count, active chemotherapy, and rituximab and steroid treatments as being associated with weaker antibody responses. However, only age and chemotherapy plus rituximab remained independent determinants of vaccine responses in multivariate analyses.
Conclusions.
Two doses of AS03-adjuvanted influenza vaccine elicited potent antibody responses in most cancer patients despite ongoing chemotherapy, with the exception of rituximab-induced B-cell depletion. Oncology patients treated in an outpatient setting benefit from preventive vaccination against influenza with adjuvanted vaccines.
Introduction
In cancer patients, influenza infections cause a high risk for morbidity and mortality resulting from disease-related or treatment-induced immunosuppression [1–4]. Influenza may also delay anticancer treatments, worsening the oncologic outcome [5, 6].
Several studies have demonstrated that seasonal influenza immunization is safe in cancer patients [2]. However, the efficacy and thus benefits of influenza vaccines remain controversial, with some studies suggesting lower seroprotection rates than in healthy controls [7–10] and others finding no differences [11–14]. Such contrasting results may reflect differences in past immunizations, exposure to influenza virus, or the relative antigenic distance from previously circulating influenza strains, resulting in heterogeneous baseline immunity levels against influenza [2]. Moreover, seroresponses could differ according to cancer type and treatment intensity. For instance, patients with hematological malignancies were shown to reach lower seroconversion and seroprotection rates than patients with solid tumors [10, 15]. Finally, a direct comparison among studies is limited by several factors, including the heterogeneity of cohorts, use of different end points and assays to assess vaccine responses, and differences among vaccine strains [16].
In 2009, the rapid spread of a new influenza A/H1N1 viral strain led to fears of a pandemic with a potential for high morbidity and mortality, especially in immunocompromised patients. In September 2009, the European Medical Agency approved novel oil-in-water squalene-based adjuvanted pandemic influenza vaccines [17] based on immunogenicity and safety data obtained in a few hundred healthy volunteers [18, 19]. This provided a unique opportunity to prospectively assess the immunogenicity and safety profiles of the novel AS03-adjuvanted influenza A/H1N1/09 vaccine in cancer patients who were unlikely to have been previously exposed to this new viral strain [18]. Although the influenza A/H1N1/09 pandemic is now over and this virus now circulates as a seasonal strain, these questions remain critical for defining the potential role of squalene-based adjuvanted seasonal influenza vaccines in cancer patients.
Patients and Methods
This study was a single-center, prospective, controlled, open-label field trial, approved by the institution's ethical committee (ID, CER-09-234), registered prior to patient enrollment (ClinicalTrials.gov identifier, NCT01022905), and conducted in accordance with the principles of the Declaration of Helsinki, the standards of Good Clinical Practice, and Swiss regulatory requirements.
Study Design and Participants
Patients were recruited in November and December 2009 from among cancer patients who were undergoing active treatment or were in follow-up at the Center of Oncology as part of a multiple parallel cohort study performed in the University Hospitals of Geneva, Switzerland. Eligible patients were individuals aged >18 years to whom the influenza A/H1N1/09 vaccination was medically indicated according to official recommendations. Patients with hematological conditions other than lymphoma, those undergoing allogeneic stem cell transplantation, those scheduled to start chemotherapy during the study period, and those whose life expectancy was <3 months were not included in this study. Family members (mostly spouses) without chronic disease or treatment known to affect immune competence were recruited as controls. Patients who failed to comply with the study protocol were excluded from the immunological analyses.
Vaccine and Immunizations
According to Swiss recommendations, patients at risk for compromised immunity received 2 i.m. doses of the AS03-adjuvanted split influenza A/H1N1/09 vaccine (Pandemrix®; GlaxoSmithKline, Brentford, U.K.) with a 3- to 4-week interval, whereas controls received one dose. Each dose of Pandemrix® contained the influenza A/H1N1/09 antigen (3.75 μg), squalene (10.69 mg), dl-α-tocopherol (11.86 mg), and polysorbate 80 (4.86 mg) [17]. A single vaccine lot was used and administered in the deltoid muscle with a 25-mm needle.
Data Collection
Medical information was retrieved through a detailed questionnaire upon enrollment and completed through the patient's medical records. Blood was collected on the day of the first dose, 34 weeks after dose 1 (optional blood sampling), and 3–4 weeks after the last vaccine dose. Sera were prepared and stored at −20°C until used.
Hemagglutination Inhibition Assay
Sera were decomplemented and treated with a receptor-destroying enzyme. Hemagglutination inhibition (HAI) assays were performed as described elsewhere [20–22]. In brief, sera were subjected to twofold serial dilutions (starting at 1:8) and incubated with four HA units of the influenza A/California/7/09 (A/H1N1/09) virus and 0.4% glutaraldehyde-fixed turkey RBCs. Results are expressed as the reciprocal of the highest dilution showing positive HAI. Samples with an HAI titer <1:8 were considered as negative and were assigned a titer of 1:4 for computational purposes.
Microneutralization Assay
Microneutralization (MN) assays were performed as described elsewhere [20]. Briefly, decomplemented sera were serially diluted twofold (starting at 1:10) in flat-bottomed, 96-well microtiter plates. Virus was added and neutralization allowed prior to the addition of Madin-Darby canine kidney cells, incubation, fixation, and staining for influenza A nucleoproteins. The endpoint neutralization titer was expressed as the reciprocal of the highest dilution of serum with an optical density value of <x, where x = [(average of virus and cell wells) − (average of cell-only wells)]/2 + (average of cell-only wells). Negative samples were assigned a titer of 1:5 for computational purposes.
Immunological Endpoints
The coprimary immunogenicity endpoints were measured using HAI according to the conventional criteria used to assess influenza vaccines: (a) antibody titers prior to and after vaccination as described by the geometric mean titers (GMTs) ± 95% confidence interval (CI), (b) seroprotection rate (defined as a postvaccination HAI titer ≥1:40), and (c) seroconversion rate (defined as a postvaccination HAI titer ≥1:40 and a fourfold increase in GMTs). The secondary immunogenicity endpoints were similarly defined using the MN assay in a subset of 33% randomly selected samples. Only patients with two immunizations were included in the final analysis of the immunological endpoints.
Safety Monitoring
All patients who received at least one vaccine dose were monitored for drug safety. Safety endpoints were defined as injection-site (local pain, erythema, and swelling) and systemic (fever, fatigue, headache, and anorexia) reactions. Adverse events were recorded in self-completed diaries for 7 days after each immunization. Serious adverse events (SAEs) were defined according to Swiss regulatory requirements. The nature of the SAE and its relationship to immunization were assessed by our institution's pharmacovigilance center.
Statistical Analyses
The GMTs and the corresponding 95% CI describe antibody titers. For each possible value of the titer (abscissa), reverse cumulative distributions were obtained by plotting the proportion of subjects with a titer greater than this value. A comparison of the titers between the strata of categorical variables was performed using a Kruskal–Wallis test. A multivariate regression model was used to analyze the association between potential factors (factors with p < .10 in the univariate analyses) and the titer. Because the distribution of the titer was not Gaussian, we modeled log10-transformed titers. The normality of the residuals was checked (Shapiro–Wilks test). The parameter of the linear model indicated the variation in the log10-tranformed titer. To aid interpretation, we derived the percentage difference compared with the category of reference (for categorical factors) or corresponding to the increment of one unit (for continuous factors like age) from the regression model. A multivariate linear model combining patients and controls was also used. Factors common to both groups (age, gender, immunization in 2009) were introduced in the model, along with the group variable.
The percentages of patients and controls who experienced adverse events were assessed with the 95% CI obtained by the exact Clopper–Pearson method and compared using Fisher's exact test. The significance level was 0.05. Data were analyzed using S-PLUS 8.0 (Insightful Corp., Seattle, WA).
Results
Characteristics of Patients and Controls
The cancer patient cohort consisted of 197 patients with lymphoma (n = 57), glioma (n = 26), lung or head and neck cancer (n = 37), gastrointestinal cancer (n = 41), or breast cancer (n = 36). One hundred thirty-eight healthy controls were also enrolled. The two groups were well balanced except for the slightly older age of the cancer cohort (median, 59.6 years versus 50.8 years; p < .001) (Table 1). Similar proportions of cancer patients and controls had been immunized against the 2009–2010 seasonal influenza prior to enrollment (57.9% versus 51.4%; p = .29). The performance status of cancer patients was good, with only 15% showing a Karnofsky performance status score of 70%–80% and none <70%. Most patients (80.4%) showed normal serum albumin levels (median, 38 g/L; range, 19–46 g/L).
Table 1.
Clinical characteristics of patients
Abbreviations: F, female; IQR, interquartile range; KPS, Karnofsky performance status; M, male.
At baseline, 34.5% of patients were receiving active chemotherapy, at proportions and with regimens that differed among cancer groups (Table 2). During the period of the study, 9.1% of the patients received chronic steroid treatment at a median dose of 19.5 mg/day prednisone equivalent (range, 5–40 mg/day). Of the lymphoma patients, 12 of 57 (21%) were on anti-CD20 therapy (rituximab) and five other patients had received autologous cell stem transplants (5, 6, 50, 52, and 54 months earlier). The remaining 65.5% of patients were not receiving active chemotherapy treatment at the time of the study. In total, 17 patients (13.2%) did not receive any chemotherapy before and during inclusion in this study. For the remaining patients, the mean time from the last chemotherapy was 23 months (Table 2).
Table 2.
Treatment characteristics of patients
aOne patient being treated with rituximab was receiving rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; all others were on maintenance rituximab treatment.
Abbreviations: NA, not available; SD, standard deviation.
Seven controls were unreachable at time of the last study visit. Fifteen patients did not complete the study: six died, three were unreachable, and six withdrew from the study because of loss of motivation. In summary, 182 of 197 (92.4%) patients and 131 of 138 (94.9%) controls were included in the evaluation of vaccine-induced antibody responses.
Immunogenicity of H1N1 Influenza Immunization
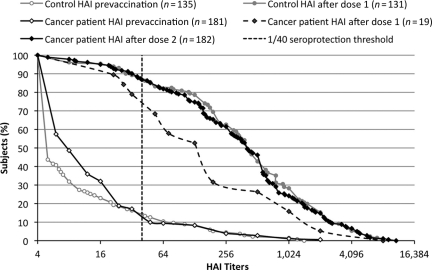
Baseline antibodies to influenza A/H1N1/09 were available for 181 of 197 (91.8%) patients and 135 of 138 (97.8%) controls. Prior to immunization, seroprotection rates (patients: 10.5%; 95% CI, 6.4%–15.9%; controls: 14.8%; 95% CI, 9.3%–21.9%; p = .33) and GMTs (patients: 10.4; 95% CI, 8.6–12.5; controls: 9.2; 95% CI, 7.3–11.7; p = .047) were low, indicating that most had not been exposed to influenza A/H1N1/09 (Fig. 1 and Table 3).
Figure 1.
Reverse cumulative distribution of anti–influenza A/H1N1 antibodies in cancer patients and controls. Blood was collected prior to immunization and 3–4 weeks after one (controls and 19 patients) or two (181 patients) vaccine doses. The curves represent the distribution of individual antibody levels measured by a hemagglutination inhibition (HAI) assay. The vertical dotted line represents the 1:40 seroprotective threshold.
Table 3.
Determinants of anti-influenza H1N1 vaccine antibody responses in patients and controls
p-values set in bold font indicate statistical significance.
Abbreviations: CI, confidence interval; GMT, geometric mean titers; KPS, Karnofsky performance status.
In controls, a single vaccine dose elicited strong responses, with seroprotection and seroconversion rates of 87% (95% CI, 80%–92.3%) and GMTs of 339.9 (95% CI, 254.9–453.2). Only 19 of 197 (9.6%) patients provided an optional sample after dose 1: a single dose elicited seroprotection and seroconversion rates of 73.7% and GMTs of 144.8 (95% CI, 63.4–330.8) (Table 4). After two immunizations, a significantly higher GMTs (423.5; 95% CI, 219.6–816.8; p = .049) was observed and the seroprotection and seroconversion rates reached 94.7% and 89.5%, respectively (p = .13 and p = 0.25, respectively, compared with the rates after the first immunization). After two immunizations, cancer patients achieved similar seroprotection (87.4%; 95% CI, 81.6%–91.8%; p = 0.16) and seroconversion (82.3%; 95% CI, 76.0%–87.6%; p = .33) rates and similar GMTs (326.9; 95% CI, 256.7–416.3; p = .74) as controls did after a single dose. In order to validate the use of HAI, a subset of 33% of patient samples was randomly selected to compare HAI and MN titers. Close correlation was observed (online supplemental Fig. 1), confirming that HAI titers could be reliably used as a surrogate for seroprotection and as the primary endpoint for statistical analyses.
Table 4.
Influence of age, ongoing chemotherapy, and number of vaccine doses on HAI GMT and SP and SC rates
Abbreviations: GMT, geometric mean titers; HAI, hemagglutination inhibition; SC, seroconversion; SP, seroprotection.
Determinants of Vaccine Responses
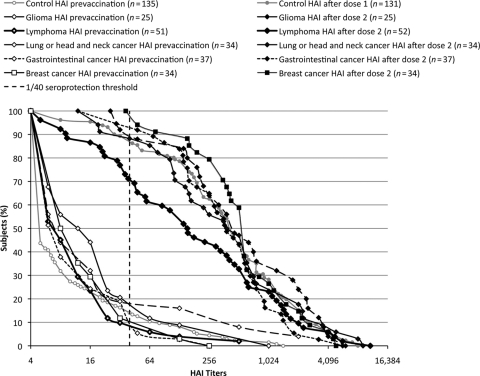
Next, we assessed various clinical indices that might predict vaccine responses (Table 3). Age, gender, and prior immunization against 2009–2010 seasonal influenza were assessed in both cohorts. In cancer patients, performance status, cancer type, serum albumin level, active chemotherapy, use of rituximab, and use of steroids were added to the analyses. Gender showed no influence, but age strongly impacted influenza A/H1N1/09 responses in both groups (p < .001). In univariate analyses, antibody responses tended to be lower in individuals who were previously immunized against seasonal influenza, for both cancer patients and healthy controls (p = .004 and p = .09, respectively). Active chemotherapy (p = .01), lymphoma (p = .03), the use of rituximab (p < .001), and steroid treatment (p = .02) were associated with lesser antibody responses in cancer patients (Table 3 and Fig. 2). CD4 levels were determined in 50 of 57 lymphoma patients and 25 of 26 glioma patients at time of first immunization. Median CD4 levels were 484/mm3 (interquartile range, 320–724/mm3). Only five patients (8.8%) had CD4 levels <200/mm3 and 17 (29.8%) had levels of 200–400/mm3. Patients with CD4 counts <400/mm3 had lower GMT values (119.0; 95% CI, 52.4–269.9) than those with higher CD4 counts (GMTs, 348.0; 95% CI, 199.9–605.9; p = .04). Patients with the lowest CD4 counts showed the lowest vaccine responses (Table 3).
Figure 2.
Reverse cumulative distribution of anti–influenza A/H1N1 antibody titers in patients with different cancer types and controls, before and after immunization. Blood was collected as described in Figure 1 caption. The curves represent the distribution of individual antibody levels measured by a hemagglutination inhibition (HAI) assay. The vertical dotted line represents the 1:40 seroprotective threshold.
A multivariate analysis including age, gender, performance status, type of cancer, prior vaccination against seasonal influenza, serum albumin level, active chemotherapy, rituximab, and steroid use indicated that vaccine responses were primarily influenced by age (p = .002 in controls and p = .003 in cancer patients) and the use of rituximab (p < .001), but not by chemotherapy when it was not combined with rituximab (p = .81). Age played a major role: each additional 10 years resulted in 30% lower antibody titers in both patients and controls (Table 5). Antibody titers were 80% lower in individuals treated with rituximab. Previous immunization against seasonal influenza exerted no significant influence on the GMTs when age and treatment groups were controlled for, confirming the hypothesis of interference between seasonal and H1N1 immunization. All other factors, including type of cancer and steroid use, had no significant impact on vaccine response.
Table 5.
Multivariate analyses of humoral responses in cancer patients and controls
p-values set in bold font indicate statistical significance.
Abbreviations: KPS, Karnofsky performance status; SE, standard error.
Safety
Immunization was generally well tolerated: injection-site pain and swelling occurred at a rather lower frequency in patients than in controls (supplemental online Table 1). Nineteen SAEs were reported in cancer patients, mostly related to tumor progression (9 of 11 hospitalizations and six deaths). Two patients developed prolonged (up to 1 week) flu-like symptoms following immunization. Upon careful review, all other adverse events and SAEs were considered unrelated to immunization by the physicians in charge.
Discussion
Whether or not influenza vaccination should be systematically offered to cancer patients remains controversial, mainly because the quality of immune responses elicited by seasonal influenza vaccines is difficult to interpret as a result of previous immunizations, previous infections, and patient heterogeneity [2, 8, 23–26]. Because the majority of adults are unlikely to have been exposed to or immunized against the new H1N1 strain of influenza, we postulated that the influenza A/H1N1 pandemic observed in 2009 provided a unique opportunity to unravel this controversy. Thus, this study was designed to explore the safety and humoral immunogenicity of the new squalene-based adjuvanted H1N1 vaccine in a large population of oncology patients followed in an outpatient setting.
The safety of the novel H1N1 vaccine was confirmed in oncology patients, as in other patients [20, 27]. Side effects were similar or even lower (for local reactions and swelling) in the cohort of cancer patients than in controls. It is noteworthy that this excellent tolerance was observed despite two doses of squalene-based adjuvanted vaccine, which had been reported to induce more side effects in certain populations, including younger and elderly patients [28, 29].
Immunogenicity was excellent for the entire cohort, with antibody responses being achieved by cancer patients after two doses of AS03-adjuvanted H1N1 vaccine similar to those of healthy controls after one dose. Although the HAI assay only measured the phase of virus attachment to a host cell, the MN assay confirmed that vaccine-induced antibodies prevented viral infection. Thus, most cancer patients were likely protected against influenza A/H1N1/09, although T-cell responses were not assessed [30, 31]. The high seroprotection and seroconversion rates observed in our study contrasted with recent data showing poor immune responses (i.e., seroprotection and seroconversion rates <50%) in cancer patients [26, 32, 33]. Several factors may have contributed to such discrepancies.
First, the population of cancer patients included in the analysis impacts the quality of the immune response. Whereas previous studies focused on patients receiving aggressive chemotherapy [7, 26] or having recently undergone allogeneic hematopoietic stem cell transplantation (HSCT) [33], our study cohort mainly included patients with a good performance status and normal levels of albumin, and was representative of a typical outpatient oncology clinic, with the majority of patients in follow-up (65%) and ∼35% receiving active chemotherapy. However, we observed similarly good responses in a parallel cohort of patients after HSCT [27].
A second important factor is the number of vaccine doses. Although our study was not designed to compare immune responses elicited by one or two doses, antibody titers and seroprotection and seroconversion rates were lower after a single dose than after two doses, with seroconversion rates of 44% after one dose and 73% after two doses, as observed in other studies with patients on active chemotherapy [26]. Thus, the number of doses may be a critical factor for cancer patients, which may explain the very low seroconversion rates reported for patients with solid (45%) and hematological (19%) tumors in a Canadian study that used a single dose of the same vaccine [32]. Given the limited number of patients assessed after one dose, a formal demonstration of the impact of the second dose of adjuvanted vaccine could not be generated.
The precise role of the adjuvant is more difficult to definitively assess, because nonadjuvanted H1N1/09 vaccines were not licensed in Switzerland and were thus unavailable for head-to-head comparisons. Previous studies showed that cancer patients not undergoing active treatment showed responses to a nonadjuvanted vaccine against seasonal influenza that were similar to those of healthy controls [14, 34, 35], whereas patients undergoing chemotherapy did not [7, 10, 15, 35–38]. In this context, it is noteworthy that active chemotherapy (not combined with rituximab) was not identified as a risk factor for poor seroconversion in our multivariate analysis. We therefore presume that the use of the potent squalene adjuvant may have overcome the limited immune competence in these patients, as observed in our parallel cohorts of immunocompromised patients with inflammatory autoimmune diseases [20], in cancer patients immunized after allogeneic HSCT [27], and in patients receiving docetaxel [39].
The large number of cancer patients recruited in this study allowed multivariate analyses, identifying independent critical factors for the quality of vaccine responses. In the multivariate analysis, age and the use of rituximab were the only factors associated with lower antibody responses. Humoral responses are known to be affected by aging [40], and our study indicated that even two doses of a potent adjuvanted vaccine might not circumvent the influence of immune senescence. This could suggest that the best way to protect elderly patients is to vaccinate their close family members, younger patients, and health care professionals.
The most important negative factor identified in our study was rituximab. This has also been reported by others, mainly with nonadjuvanted seasonal influenza vaccines [32, 33, 41], probably as a result of the profound depletion of memory B cells that may persist for years after rituximab treatment [41]. Our data clearly show that an appropriate humoral response is not achievable, even with an adjuvanted vaccine. However, rituximab does not impair cellular immunity, and recent observations in the field of rheumatoid arthritis suggest that patients may be protected via the preservation of T-cell immunity [42]. In the oncology setting, patients at highest risk for insufficient protection are therefore those with combined B-cell (resulting from rituximab) and T-cell defects, which could be related to the underlying disease (e.g., chronic lymphocytic leukemia) or other drugs (e.g., fludarabine) [41]. In this regard, it is interesting to note that patients with low CD4 counts (mainly as a result of the use of temozolomide in our study) failed to seroconvert following vaccination, although the number of events was too low to reach significance in the multivariate analysis.
A subgroup analysis showed lower seroconversion rates to influenza A/H1N1 in patients previously immunized against seasonal influenza, as previously described [43]. This may reflect the original antigenic sin hypothesis, which stipulates that new influenza strains evade surveillance when memory B cells reactive to previous influenza strains dominate the serological response [44, 45]. However, this effect disappeared when controlling for other factors in multivariate analyses, indicating that seasonal immunization was preferentially proposed to or accepted by a subset of patients at higher risk for underlying immunosuppression. However, the fact that vaccination rates for seasonal influenza were quite low in our cohort, despite strong recommendations for vaccination against seasonal influenza in this high-risk group, remains a matter of concern.
In conclusion, our study demonstrated that a two-dose regimen of the AS-03 adjuvanted pandemic vaccine was safe and highly immunogenic in most cancer patients treated in an outpatient setting, with the exception of reduced humoral responses in older and rituximab-treated patients. Whether one or two doses of adjuvanted vaccine could improve the response rate of cancer patients to seasonal influenza vaccines is now open for study.
Acknowledgments
The H1N1 Study Group of the Geneva University Hospitals consists of the following individuals: C.A. Siegrist, K. Posfay-Barbe, S. Meier, M. Bel, S. Grillet, G. Sealy (Center for Vaccinology); J. Demeules, S. Charvat, M. Verdon, C. Combescure (Clinical Research Center); B. Hirschel, A. Calmy, A. Nguyen, C. Delhumeau-Cartier, J. Ambrosioni (Division of Infectious Diseases); C. Gabay, P.A. Guerne (Division of Rheumatology); J. Seebach, C. Ribi, J. Villard (Division of Immunology and Allergology); P.Y. Dietrich, A.C. George, L. Favet, A.F. Hottinger (Division of Oncology); C. van Delden, I. Morard, G. Mentha, E. Giostra (Division of Transplantation); K. Hadaya, P.Y. Martin (Division of Nephrology); P. Soccal (Division of Thoracic Surgery); T. Berney (Division of Visceral Surgery); S. Noble (Division of Cardiology); B. Mohty, M. Nagy, Y. Chalandon, E. Roosnek, J. Passweg (Division of Hematology); L. Kaiser, S. Yerly, Y. Thomas, W. Wunderli (Laboratory of Virology).
Anne-Claude George, Andreas F. Hottinger, and Michael Bel contributed equally as first authors, Claire-Anne Siegrist and Pierre-Yves Dietrich contributed equally as last authors.
The study was funded by the Center for Clinical Research, Fondation Louis Jeantet, and Center for Vaccinology (Geneva University Hospitals and Medical School), La Ligue Genevoise contre le Cancer, La Fondation Artêres.
Claire-Anne Siegrists's institution received grants and travel and accommodation expenses from GlaxoSmithKline. GlaxoSmithKline did not provide support for this study.
Author Contributions
Conception/Design: Pierre-Yves Dietrich, Andreas F. Hottinger, Anne-Claude C. George, Michael Bel, Laurence Favet, Christophe Combescure, Claire-Anne Siegrist
Provision of study material or patients: Pierre-Yves Dietrich, Andreas F. Hottinger, Anne-Claude C. George, Laurence Favet, Claire-Anne Siegrist
Collection and/or assembly of data: Pierre-Yves Dietrich, Andreas F. Hottinger, Anne-Claude C. George, Michael Bel, Laurence Favet, Claire-Anne Siegrist
Data analysis and interpretation: Pierre-Yves Dietrich, Andreas F. Hottinger, Anne-Claude C. George, Michael Bel, Christophe Combescure, Sara Meier, Stéphane Grillet, Claire-Anne Siegrist, Klara Posfay-Barbe, Laurent Kaiser
Manuscript writing: Pierre-Yves Dietrich, Andreas F. Hottinger, Anne-Claude C. George, Michael Bel, Laurence Favet, Christophe Combescure, Claire-Anne Siegrist
Final approval of manuscript: Pierre-Yves Dietrich, Andreas F. Hottinger, Anne-Claude C. George, Michael Bel, Laurence Favet, Christophe Combescure, Sara Meier, Stéphane Grillet, Claire-Anne Siegrist, Klara Posfay-Barbe, Laurent Kaiser
References
- 1.Hajjar LA, Mauad T, Galas FR, et al. Severe novel influenza A (H1N1) infection in cancer patients. Ann Oncol. 2010;21:2333–2341. doi: 10.1093/annonc/mdq254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pollyea DA, Brown JM, Horning SJ. Utility of influenza vaccination for oncology patients. J Clin Oncol. 2010;28:2481–2490. doi: 10.1200/JCO.2009.26.6908. [DOI] [PubMed] [Google Scholar]
- 3.Liu C, Schwartz BS, Vallabhaneni S, et al. Pandemic (H1N1) 2009 infection in patients with hematologic malignancy. Emerg Infect Dis. 2010;16:1910–1917. doi: 10.3201/eid1612.100772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couch RB, Englund JA, Whimbey E. Respiratory viral infections in immunocompetent and immunocompromised persons. Am J Med. 1997;102:2–9. doi: 10.1016/S0002-9343(97)00003-X. discussion 25–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldman S, Webster RG, Sugg M. Influenza in children and young adults with cancer: 20 cases. Cancer. 1977;39:350–353. doi: 10.1002/1097-0142(197701)39:1<350::aid-cncr2820390153>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 6.Ozdemir N, Celkan T, Midilli K, et al. Novel influenza a (H1N1) infection in a pediatric hematology oncology clinic during the 2009–2010 pandemia. Pediatr Hematol Oncol. 2011;28:288–293. doi: 10.3109/08880018.2010.550986. [DOI] [PubMed] [Google Scholar]
- 7.Meerveld-Eggink A, de Weerdt O, van der Velden AM, et al. Response to influenza virus vaccination during chemotherapy in patients with breast cancer. Ann Oncol. 2011;22:2031–2035. doi: 10.1093/annonc/mdq728. [DOI] [PubMed] [Google Scholar]
- 8.Mazza JJ, Yale SH, Arrowood JR, et al. Efficacy of the influenza vaccine in patients with malignant lymphoma. Clin Med Res. 2005;3:214–220. doi: 10.3121/cmr.3.4.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuzaki A, Suminoe A, Koga Y, et al. Immune response after influenza vaccination in children with cancer. Pediatr Blood Cancer. 2005;45:831–837. doi: 10.1002/pbc.20470. [DOI] [PubMed] [Google Scholar]
- 10.Stiver HG, Weinerman BH. Impaired serum antibody response to inactivated influenza A and B vaccine in cancer patients. Can Med Assoc J. 1978;119:733–735. 738. [PMC free article] [PubMed] [Google Scholar]
- 11.Feery BJ, Sullivan JR, Hurley TH, et al. Immunization with influenza vaccine in patients with haematological malignant disease. Med J Aust. 1977;1:292–294. doi: 10.5694/j.1326-5377.1977.tb130704.x. [DOI] [PubMed] [Google Scholar]
- 12.Gribabis DA, Panayiotidis P, Boussiotis VA, et al. Influenza virus vaccine in B-cell chronic lymphocytic leukaemia patients. Acta Haematol. 1994;91:115–118. doi: 10.1159/000204315. [DOI] [PubMed] [Google Scholar]
- 13.Nordy̸ T, Aaberge IS, Husebekk A, et al. Cancer patients undergoing chemotherapy show adequate serological response to vaccinations against influenza virus and Streptococcus pneumoniae. Med Oncol. 2002;19:71–78. doi: 10.1385/MO:19:2:71. [DOI] [PubMed] [Google Scholar]
- 14.Centkowski P, Brydak L, Machala M, et al. Immunogenicity of influenza vaccination in patients with non-Hodgkin lymphoma. J Clin Immunol. 2007;27:339–346. doi: 10.1007/s10875-007-9073-3. [DOI] [PubMed] [Google Scholar]
- 15.Shildt RA, Luedke DW, Kasai G, et al. Antibody response to influenza immunization in adult patients with malignant disease. Cancer. 1979;44:1629–1635. doi: 10.1002/1097-0142(197911)44:5<1629::aid-cncr2820440514>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 16.Kunisaki KM, Janoff EN. Influenza in immunosuppressed populations: A review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect Dis. 2009;9:493–504. doi: 10.1016/S1473-3099(09)70175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Medicines Agency. Pandemic Influenza (H1N1). Summary of Product Characteristics. [accessed September, 2011]. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Report/2010/02/
- 18.Clark TW, Pareek M, Hoschler K, et al. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med. 2009;361:2424–2435. doi: 10.1056/NEJMoa0907650. [DOI] [PubMed] [Google Scholar]
- 19.Schwarz TF, Horacek T, Knuf M, et al. Single dose vaccination with AS03-adjuvanted H5N1 vaccines in a randomized trial induces strong and broad immune responsiveness to booster vaccination in adults. Vaccine. 2009;27:6284–6290. doi: 10.1016/j.vaccine.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 20.Gabay C, Bel M, Combescure C, et al. Impact of synthetic and biologic disease-modifying antirheumatic drugs on antibody responses to the AS03-adjuvanted pandemic influenza vaccine: A prospective, open-label, parallel-cohort, single-center study. Arthritis Rheum. 2011;63:1486–1496. doi: 10.1002/art.30325. [DOI] [PubMed] [Google Scholar]
- 21.Ikram H, Prince AM. A method for coupling cytomegalovirus antigens to aldehyde-fixed erythrocytes for use in passive hemagglutination. J Virol Methods. 1983;7:127–134. doi: 10.1016/0166-0934(83)90002-2. [DOI] [PubMed] [Google Scholar]
- 22.Miller E, Hoschler K, Hardelid P, et al. Incidence of 2009 pandemic influenza A H1N1 infection in England: A cross-sectional serological study. Lancet. 2010;375:1100–1108. doi: 10.1016/S0140-6736(09)62126-7. [DOI] [PubMed] [Google Scholar]
- 23.Kempe A, Hall CB, MacDonald NE, et al. Influenza in children with cancer. J Pediatr. 1989;115:33–39. doi: 10.1016/s0022-3476(89)80325-7. [DOI] [PubMed] [Google Scholar]
- 24.Barker WH, Mullooly JP. Pneumonia and influenza deaths during epidemics: Implications for prevention. Arch Intern Med. 1982;142:85–89. [PubMed] [Google Scholar]
- 25.Elting LS, Whimbey E, Lo W, et al. Epidemiology of influenza A virus infection in patients with acute or chronic leukemia. Support Care Cancer. 1995;3:198–202. doi: 10.1007/BF00368891. [DOI] [PubMed] [Google Scholar]
- 26.Rousseau B, Loulergue P, Mir O, et al. Immunogenicity and safety of the influenza A H1N1v 2009 vaccine in cancer patients treated with cytotoxic chemotherapy and/or targeted therapy: The VACANCE study. Ann Oncol. 2011;23:450–457. doi: 10.1093/annonc/mdr141. [DOI] [PubMed] [Google Scholar]
- 27.Mohty B, Bel M, Vukicevic M, et al. Graft-versus-host disease is the major determinant of humoral responses to the AS03-adjuvanted influenza A/09/H1N1 vaccine in allogeneic hematopoietic stem cell transplant recipients. Haematologica. 2011;96:896–904. doi: 10.3324/haematol.2011.040386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waddington C, Andrews N, Hoschler K, et al. Open-label, randomised, parallel-group, multicentre study to evaluate the safety, tolerability and immunogenicity of an AS03(B)/oil-in-water emulsion-adjuvanted (AS03(B)) split-virion versus non-adjuvanted whole-virion H1N1 influenza vaccine in UK children 6 months to 12 years of age. Health Technol Assess. 2010;14:1–130. doi: 10.3310/hta14460-01. [DOI] [PubMed] [Google Scholar]
- 29.Minutello M, Senatore F, Cecchinelli G, et al. Safety and immunogenicity of an inactivated subunit influenza virus vaccine combined with MF59 adjuvant emulsion in elderly subjects, immunized for three consecutive influenza seasons. Vaccine. 1999;17:99–104. doi: 10.1016/s0264-410x(98)00185-6. [DOI] [PubMed] [Google Scholar]
- 30.Jefferson TO, Rivetti D, Di Pietrantonj C, et al. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2007;(2):CD001269. doi: 10.1002/14651858.CD001269.pub3. [DOI] [PubMed] [Google Scholar]
- 31.Ortqvist A, Berggren I, Insulander M, et al. Effectiveness of an adjuvanted monovalent vaccine against the 2009 pandemic strain of influenza A(H1N1)v, in Stockholm County, Sweden. Clin Infect Dis. 2011;52:1203–1211. doi: 10.1093/cid/cir182. [DOI] [PubMed] [Google Scholar]
- 32.Mackay HJ, McGee J, Villa D, et al. Evaluation of pandemic H1N1 (2009) influenza vaccine in adults with solid tumor and hematological malignancies on active systemic treatment. J Clin Virol. 2011;50:212–216. doi: 10.1016/j.jcv.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 33.Issa NC, Marty FM, Gagne LS, et al. Seroprotective titers against 2009 H1N1 influenza A virus after vaccination in allogeneic hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2011;17:434–438. doi: 10.1016/j.bbmt.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson H, Petrie K, Berrisford C, et al. Seroconversion after influenza vaccination in patients with lung cancer. Br J Cancer. 1999;80:219–220. doi: 10.1038/sj.bjc.6690342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brydak LB, Guzy J, Starzyk J, et al. Humoral immune response after vaccination against influenza in patients with breast cancer. Support Care Cancer. 2001;9:65–68. doi: 10.1007/s005200000186. [DOI] [PubMed] [Google Scholar]
- 36.Ramanathan RK, Potter DM, Belani CP, et al. Randomized trial of influenza vaccine with granulocyte-macrophage colony-stimulating factor or placebo in cancer patients. J Clin Oncol. 2002;20:4313–4318. doi: 10.1200/JCO.2002.02.041. [DOI] [PubMed] [Google Scholar]
- 37.Ortbals DW, Liebhaber H, Presant CA, et al. Influenza immunization of adult patients with malignant diseases. Ann Intern Med. 1977;87:552–557. doi: 10.7326/0003-4819-87-5-552. [DOI] [PubMed] [Google Scholar]
- 38.Schafer AI, Churchill WH, Ames P, et al. The influence of chemotherapy on response of patients with hematologic malignancies to influenza vaccine. Cancer. 1979;43:25–30. doi: 10.1002/1097-0142(197901)43:1<25::aid-cncr2820430103>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 39.Loulergue P, Alexandre J, Iurisci I, et al. Low immunogenicity of seasonal trivalent influenza vaccine among patients receiving docetaxel for a solid tumour: Results of a prospective pilot study. Br J Cancer. 2011;104:1670–1674. doi: 10.1038/bjc.2011.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ademokun A, Wu YC, Dunn-Walters D. The ageing B cell population: Composition and function. Biogerontology. 2010;11:125–137. doi: 10.1007/s10522-009-9256-9. [DOI] [PubMed] [Google Scholar]
- 41.Bedognetti D, Zoppoli G, Massucco C, et al. Impaired response to influenza vaccine associated with persistent memory B cell depletion in non-Hodgkin's lymphoma patients treated with rituximab-containing regimens. J Immunol. 2011;186:6044–6055. doi: 10.4049/jimmunol.1004095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arad U, Tzadok S, Amir S, et al. The cellular immune response to influenza vaccination is preserved in rheumatoid arthritis patients treated with rituximab. Vaccine. 2011;29:1643–1648. doi: 10.1016/j.vaccine.2010.12.072. [DOI] [PubMed] [Google Scholar]
- 43.Andrews N, Waight P, Yung CF, et al. Age-specific effectiveness of an oil-in-water adjuvanted pandemic (H1N1) 2009 vaccine against confirmed infection in high risk groups in England. J Infect Dis. 2011;203:32–39. doi: 10.1093/infdis/jiq014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fazekas de St Groth, Webster RG. Disquisitions of original antigenic sin. I. Evidence in man. J Exp Med. 1966;124:331–345. doi: 10.1084/jem.124.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim JH, Skountzou I, Compans R, et al. Original antigenic sin responses to influenza viruses. J Immunol. 2009;183:3294–3301. doi: 10.4049/jimmunol.0900398. [DOI] [PMC free article] [PubMed] [Google Scholar]