Abstract
Arabidopsis (Arabidopsis thaliana) Hexokinase-Like1 (HKL1) lacks glucose (Glc) phosphorylation activity and has been shown to act as a negative regulator of plant growth. Interestingly, the protein has a largely conserved Glc-binding domain, and protein overexpression was shown previously to promote seedling tolerance to exogenous 6% (w/v) Glc. Since these phenotypes occur independently of cellular Glc signaling activities, we have tested whether HKL1 might promote cross talk between the normal antagonists Glc and ethylene. We show that repression by 1-aminocyclopropane-1-carboxylic acid (ACC) of the Glc-dependent developmental arrest of wild-type Arabidopsis seedlings requires the HKL1 protein. We also describe an unusual root hair phenotype associated with growth on high Glc medium that occurs prominently in HKL1 overexpression lines and in glucose insensitive 2-1 (gin2-1), a null mutant of Hexokinase1 (HXK1). Seedlings of these lines produce bulbous root hairs with an enlarged base after transfer from agar plates with normal medium to plates with 6% Glc. Seedling transfer to plates with 2% Glc plus ACC mimics the high-Glc effect in the HKL1 overexpression line but not in gin2-1. A similar ACC-stimulated, bulbous root hair phenotype also was observed in wild-type seedlings transferred to plates with 9% Glc. From transcript expression analyses, we found that HKL1 and HXK1 have differential roles in Glc-dependent repression of some ethylene biosynthesis genes. Since we show by coimmunoprecipitation assays that HKL1 and HXK1 can interact, these two proteins likely form a critical node in Glc signaling that mediates overlapping, but also distinct, cellular responses to Glc and ethylene treatments.
In order to regulate growth and development, plants need to sense, transmit, and respond to internal hormonal signals as well as diverse environmental stimuli. Sugars generated through photosynthesis act not only as central energy molecules but also as hormone-like signaling molecules that modulate plant growth (Sheen et al., 1999; León and Sheen, 2003; Gibson, 2004). Plant sugar signaling interfaces with classical hormone signaling networks, including abscisic acid (ABA), ethylene, cytokinins, auxin, GAs, and brassinosteroids (Laxmi et al., 2004; Rolland and Sheen, 2005; Hartig and Beck, 2006; Rognoni et al., 2007). In most cases, these interactions have been identified initially by genetic studies, with corresponding proteins and regulatory mechanisms being much more slowly defined.
Plant Glc signaling is closely associated with ABA- and ethylene-mediated processes. Different ABA-deficient mutants (e.g. aba2) or ABA-insensitive mutants (e.g. abi4) have been identified as Glc-sensing mutants in phenotypic screens that monitor seedling development in the presence of high levels of exogenous sugars. This suggests that Glc signaling during early seedling development requires a number of ABA-associated genes and processes (Smeekens, 2000; Rolland et al., 2006). Transcript profiling studies further indicate that ABA and Glc treatments of Arabidopsis (Arabidopsis thaliana) seedlings affect the expression of about 130 common genes (Li et al., 2006), although the developmental context also can greatly influence the outcome of these processes (Yuan and Wysocka-Diller, 2006; Dekkers et al., 2008). For example, Glc treatment delays ABA catabolism in germinating seeds (Price et al., 2004; Zhu et al., 2009) but promotes ABA biosynthesis in developing seedlings (Cheng et al., 2002). In contrast to the overlapping and sometimes even synergistic interactions between Glc and ABA signaling (Li et al., 2006), Glc and ethylene often act in an antagonistic manner to each other. Some ethylene signaling mutants such as ein2 have a hypersensitive Glc phenotype, while treatment with the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC) can block the Glc-dependent developmental arrest of wild-type Arabidopsis seedlings (Zhou et al., 1998). Transcript profiling studies have shown that Glc represses a number of genes associated with ethylene biosynthesis (Price et al., 2004; Li et al., 2006). One key regulatory element in the interactions between Glc and ethylene responses is that Glc destabilizes the Ethylene Insensitive3 (EIN3) transcription factor through proteasome-mediated degradation, while ethylene stabilizes EIN3 (Guo and Ecker, 2003; Yanagisawa et al., 2003).
Available experimental evidence indicates that there are multiple Glc-signaling proteins in Arabidopsis, including some hexokinases (AtHXK1 and AtHXK2; Jang et al., 1997) and the regulator of G-protein signaling (AtRGS1; Temple and Jones, 2007). The latter occurs as a single seven-transmembrane protein and is proposed to act as a high [Glc] sensor that modulates AtGPA1 function and somehow represses cell division (Johnston et al., 2007). AtHXK sensors/transducers are thought to act as sensors of relatively lower [Glc] and to somehow affect cell growth (Moore et al., 2003). Arabidopsis HXKs show additional complexity, since they occur as a small gene family that encodes three HXK isoforms with catalytic activity and three hexokinase-like (HKL) isoforms that lack catalytic activity (Karve et al., 2008). The functions of HKL proteins are poorly understood, but homologs to AtHKL proteins likely occur in all plants (Karve et al., 2010). Their presence as multiple distinct plant lineages implies that there is a selective advantage in maintaining their functions. These proteins might represent a more general paradigm that expressed noncatalytic enzyme homologs have regulatory functions (Fucile et al., 2008; Vandesteene et al., 2010).
Since five of the six Arabidopsis HXK gene family members have a largely conserved Glc-binding domain, we have suggested that at least some of the HKL family proteins might also function as Glc sensor proteins (Karve et al., 2008). Initial studies of lines with altered AtHKL1 protein expression revealed that AtHKL1 is a negative regulator of plant growth (Karve and Moore, 2009). The overexpression of AtHKL1 resulted in reduced hypocotyl elongation in seedling assays and in greatly reduced plant growth under long-day conditions. A number of phenotypes of lines that overexpress AtHKL1 in a wild-type background were similar to those reported for an AtHXK1 null mutant, gin2-1 (Moore et al., 2003). Furthermore, the overexpression of AtHKL1 in gin2-1 often had no discernible phenotype (Karve and Moore, 2009). Thus, AtHKL1 might act in part as a dominant negative regulator of AtHXK1. Since both AtHXK1 and AtHKL1 occur at mitochondria (Heazlewood et al., 2004; Karve et al., 2008), these two proteins could interact with each other. However, AtHKL1 was neither required nor affected Glc signaling by AtHXK1, as shown by both protoplast and seedling assays (Karve and Moore, 2009). Therefore, some AtHKL1 phenotypes might result from Glc-dependent cross talk with plant hormone synthesis or response pathways.
In this study, we found that the Glc-dependent developmental arrest of hkl1-1 mutants could not be rescued in the presence of ACC. We then tested whether AtHKL1 might have a role in mediating cross talk between Glc and ethylene signaling pathways. In doing so, we describe an unusual Glc-dependent root hair morphology that is readily observed in Arabidopsis lines that overexpress HKL1 protein and in an HXK1-deficient line, gin2-1. Interestingly, in an HKL1 overexpression background, treatment with an ethylene biosynthesis inhibitor blocks this Glc-induced phenotype, while ACC treatment can mimic this morphology. We further discuss a scenario by which AtHKL1 and AtHXK1 form a critical signaling node that affects plant responses to Glc and ethylene.
RESULTS
AtHKL1 Protein Affects Cross Talk between Glc and Ethylene
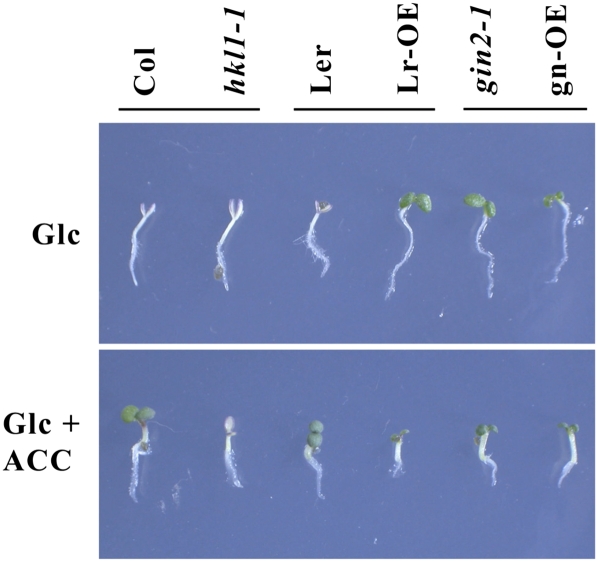
Throughout this study, we have used seedlings of different HKL1 transgenic and mutant lines as described previously (Karve and Moore, 2009). These include a knockout line, hkl1-1, which is in an ecotype Columbia (Col) background and two different AtHKL1 overexpression (OE) lines: HKL1-HA (for hemagglutinin) in a Landsberg erecta (Ler) background (line 52; Lr-OE) and HKL1-FLAG in a gin2-1 background (derived from Ler; line 79; gn-OE). The increased expression of HKL1 in different genetic backgrounds allows the evaluation of HKL1-dependent phenotypes in the presence or absence of the primary Glc sensor/transducer protein, HXK1. Both overexpression lines and gin2-1 typically have reduced growth (Supplemental Fig. S1; Karve and Moore, 2009). Since several previously described phenotypes of these transgenic and mutant lines could involve ethylene responses, we tested whether ACC can influence the Glc repression response of seedling growth among some of these lines. As described previously (Zhou et al., 1998), we also observed that the developmental arrest of wild-type Arabidopsis seedlings grown on agar plates with 6% Glc was blocked when the growth medium included 50 μm ACC (Fig. 1). That is, ACC treatment resulted in both Col and Ler seedlings having more normal growth on plates with 6% Glc, as seen by the green, expanded cotyledons. However, hkl1-1 seedlings still underwent the Glc-dependent developmental arrest in the presence of ACC (Fig. 1). Therefore, the HKL1 protein is required for the normal antagonistic effect of ACC on this response. As noted previously (Karve and Moore, 2009), seedlings of gin2-1 and the HKL1 overexpression lines did not undergo developmental arrest on 6% Glc plates. In these cases, ACC treatment still affected growth, as seen by reduced seedling organ sizes and increased hypocotyl diameters (radial swelling). Further experiments showed that the HKL1 transgenic and mutant lines had a normal triple response when grown in the dark in the presence of ACC (data not shown; Guzmán and Ecker, 1990). Thus, these data indicate that HKL1 has a role mediating cross talk between some Glc and ethylene growth responses.
Figure 1.
ACC blocks the Glc-dependent developmental arrest of wild-type seedlings but not of hkl1-1 seedlings. The bars indicate corresponding parental controls for modified lines. Lr-OE = HKL1 overexpression line 52 in the Ler background; gn-OE = HKL1 overexpression line 79 in the gin2-1 background. In the top panel, seedlings were grown 7 d on plates with 6% (w/v) Glc. In the bottom panel, seedlings were grown 7 d on plates with 6% (w/v) Glc + 50 μm ACC. [See online article for color version of this figure.]
Root Hair Development Is Influenced by Cross Talk between Glc and Ethylene
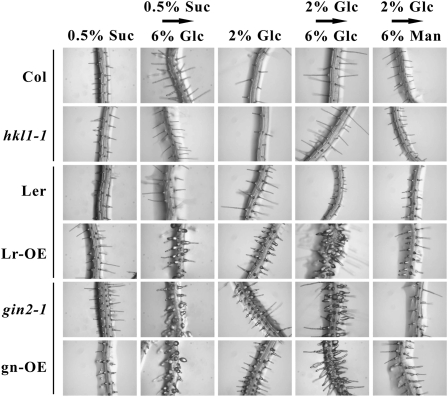
While examining seedling morphology under different Glc growth conditions, we observed an interesting root hair developmental phenotype among some lines. Developing root hairs make a good system to study cell growth, since these are single cells and can be examined with a light microscope without disturbance (Ringli et al., 2005). In our assay, seedlings were grown vertically on agar plates with either 0.5% Suc or 2% Glc under constant light for 7 d. Seedlings then were transferred to different plates with 6% Glc for 4 to 6 d. Newly developed root hairs were examined with a stereomicroscope. Both before and after transfer from either growth condition to 6% Glc, seedlings of Col, hkl1-1, and Ler lines produced slender, tubular root hairs without observable defects in morphology (Fig. 2). Seedlings of Lr-OE, gin2-1, and gn-OE also had normal root hairs when grown on 0.5% Suc. However, when these were grown on plates with 2% Glc, the root hairs appeared thicker than normal, with a considerable bulge at the base. Also in these cases, root hairs were often shorter. Transfer of seedlings of Lr-OE, gin2-1, and gn-OE from either initial growth condition to 6% Glc resulted in the enhanced formation of basally bulbous root hairs, which often failed to elongate. This response was less rapid when seedlings were transferred from 0.5% Suc plates rather than from 2% Glc plates (6 d versus 4 d). As an osmotic control, we also examined the morphology of new root hairs after seedling transfer from plates with 2% Glc to plates with 6% mannitol (Fig. 2). This treatment did not result in the formation of bulbous root hairs, although there was sometimes a slight enlargement of the base of some root hairs. Thus, the described prominently bulbous root hair phenotype is specifically induced by Glc. Furthermore, the observed growth responses indicate that HKL1 can be a negative regulator of root hair growth on Glc plates, while HXK1 promotes normal root hair growth on Glc plates. Notably, the overexpression of HKL1 in the gin2 background did not have an additive effect on root hair growth.
Figure 2.
Roots of several genotypes formed abnormal root hairs with basal bulges after seedling transfer to agar plates with 6% (w/v) Glc. At least 10 seedlings of each genotype were grown vertically under constant light (30 μmol m−2 s−1) and with different conditions, as follows: column 1, seedlings were grown continuously on 0.5% (w/v) Suc plates; column 2, seedlings were grown initially for 7 d on 0.5% (w/v) Suc plates and then transferred for 6 d to 6% (w/v) Glc plates; column 3, seedlings were grown continuously on 2% (w/v) Glc plates; column 4, seedlings were grown initially for 7 d on 2% (w/v) Glc plates and then transferred for 4 d to 6% (w/v) Glc plates; column 5, seedlings were grown initially for 7 d on 2% (w/v) Glc plates and then transferred for 6 d to 6% (w/v) mannitol (Man) plates.
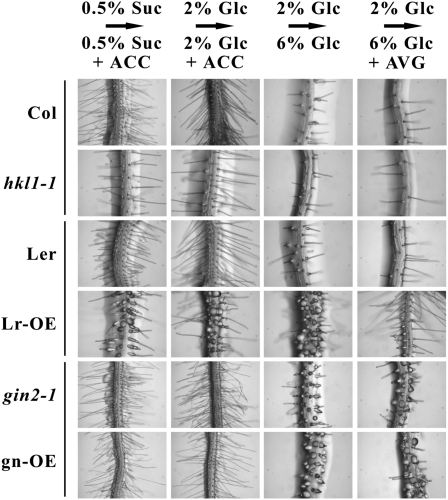
In Arabidopsis, root hair development and growth are controlled by multiple plant hormones, principally auxin and ethylene (Ringli et al., 2005). Therefore, we tested whether ethylene treatment might affect the Glc-dependent formation of the bulbous root hairs seen in the different HKL1 overexpression and gin2-1 lines. As described above, seedlings were grown in this experiment initially on agar plates with 0.5% Suc or with 2% Glc for 7 d but then were transferred to plates containing the respective carbon source plus 5 μm ACC. Transfer of Col or Ler seedlings to either Suc or Glc plates with ACC resulted in substantially increased numbers and lengths of root hairs (Fig. 3; compare with corresponding control images in Fig. 2). This response for Suc-grown seedlings was observed previously by Tanimoto et al. (1995). Seedlings of both gin2-1 and gn-OE lines also responded to ACC treatment on Suc or Glc plates by producing more root hairs, showing that HXK1 does not have a role in this response. The hkl1-1 seedlings, though, were less responsive to ACC treatment, in that they produced fewer root hairs on ACC plates with Suc or Glc compared with Col seedlings. Nonetheless, in all of these cases, the root hairs were slender and tube shaped. In contrast to these responses, seedlings of the Lr-OE line produced many bulbous root hairs after transfer to plates with ACC plus either 0.5% Suc or 2% Glc. These bulbous root hairs had a very similar morphology to that seen after transfer to plates with 6% Glc (Fig. 2). These data show that ACC promotes bulbous root hair formation at a relatively lower exogenous sugar level by a process that requires HXK1 protein and the overexpression of HKL1 protein.
Figure 3.
The abnormal root hair phenotype of Lr-OE occurs also by seedling transfer to plates with ACC. At least 10 seedlings of each genotype were grown vertically under constant light (30 μmol m−2 s−1) and with different conditions, as follows: column 1, seedlings were grown initially for 7 d on 0.5% (w/v) Suc plates and then transferred for 4 d to 0.5% (w/v) Suc plates with 5 μm ACC; column 2, seedlings were grown initially for 7 d on 2% (w/v) Glc plates and then transferred for 4 d to 2% (w/v) Glc plates with 5 μm ACC; column 3, for comparison and as a control, seedlings were grown as in Figure 2, initially for 7 d on 2% (w/v) Glc plates and then transferred for 4 d to 6% (w/v) Glc plates; column 4, seedlings were grown initially for 7 d on 2% (w/v) Glc plates and then transferred for 4 d to 6% (w/v) Glc plates with 1 μm AVG.
Since the hkl1-1 seedlings were less sensitive to increased root hair formation by ACC (Fig. 3), the formation of bulbous root hairs in the Lr-OE seedlings on ACC plates suggests that the latter might be hypersensitive to ethylene. In this case, blocking ethylene biosynthesis should rescue the root hair phenotype observed after transfer of seedlings to plates with 6% Glc. We tested this possibility by transferring seedlings of all the genotypes from 2% Glc plates to 6% Glc plates with 1 μm 2-amino-ethoxyvinyl-glycine (AVG), an ethylene biosynthesis inhibitor (Yang and Hoffman, 1984). AVG treatment did not alter the normal root hair morphology of Col, Ler, and hkl1-1 seedlings on plates with 6% Glc (Fig. 3). However, AVG treatment of the transferred Lr-OE seedlings resulted in the formation of rather normal, slender root hairs that were similar to those of wild-type Ler seedlings. Thus, the role of HKL1 in the formation of bulbous root hairs is due, in part, either to an increase in ethylene levels or, perhaps, an increase in ethylene perception or response. In contrast to this rescue, seedlings of gin2-1 and gn-OE continued to produce similar bulbous root hairs when transferred to 6% Glc plates with AVG. HXK1, therefore, acts downstream of ethylene and HKL1 in this response.
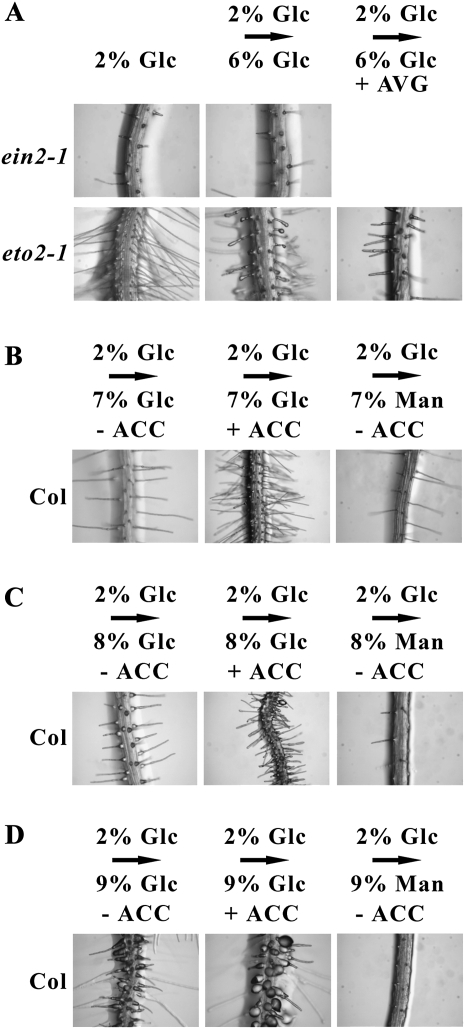
We next tested whether the formation of the bulbous root hairs might involve either ethylene signaling or ethylene production by examining the root hair development of two ethylene response mutants, ein2-1 and eto2-1. The former line is a strong ethylene signaling mutant (Alonso et al., 1999), and the latter is an ethylene overproduction mutant (Vogel et al., 1998). Seedlings of ein2-1 made very few root hairs when grown on 2% Glc plates or after transfer to 6% Glc plates (Fig. 4A). These root hairs were tubular, though relatively short, indicating that ethylene signaling likely has a role in the bulbous root hair phenotype. In contrast, seedlings of eto2-1 produced substantially greater numbers of root hairs when grown on plates with 2% Glc, although the individual root hair morphology was normal (Fig. 4A). However, after transfer to 6% Glc plates, the eto2-1 seedlings did produce unusual root hairs that had an expanded tip rather than an expanded base. Furthermore, transfer of eto2-1 seedlings to 6% Glc plates with AVG blocked the tip-expansion response, resulting instead in rather normal, slender root hairs without bulges. These responses show that even in a different genetic background, cross talk occurs between Glc and ethylene and affects root hair growth.
Figure 4.
Seedling root hair growth of ethylene mutants ein2-1 and eto2-1 and wild-type Col on plates with different media. A, Continuous growth of ethylene mutants on 2% (w/v) Glc plates or after seedling transfer to plates with 6% (w/v) Glc with or without 1 μm AVG. B, Wild-type Col seedlings were grown initially for 7 d on 2% (w/v) Glc plates and then transferred for 4 d to plates with 7% Glc, 7% Glc + 50 μm ACC, or 7% mannitol. C, Wild-type Col seedlings were transferred to plates with 8% Glc, 8% Glc + 50 μm ACC, or 8% mannitol. D, Wild-type Col seedlings were transferred to plates with 9% Glc, 9% Glc + 50 μm ACC, or 9% mannitol.
Since HKL1 affects the sensitivity of root hair growth to Glc and to ethylene, we tested whether increased Glc levels would similarly affect the root hair growth of wild-type seedlings. In this experiment, Col seedlings were transferred from 2% Glc plates to plates with 7%, 8%, or 9% Glc, all with or without ACC. In the absence of ACC, root hair morphology was rather normal on 7% Glc, showed basal bulge formation on 9% Glc, and had an intermediate morphology on 8% Glc (Fig. 4, B–D). The combination treatment of 9% Glc + ACC enhanced the formation of bulbous root hairs. These effects were not observed for Col seedlings transferred to equal levels of mannitol, although the increased mannitol inhibited root growth (Fig. 4). These data demonstrate that even in wild-type seedlings, Glc can act synergistically with ethylene to promote the formation of bulbous root hairs.
HKL1 and HXK1 Can Influence the Expression of Some Ethylene Metabolism Genes
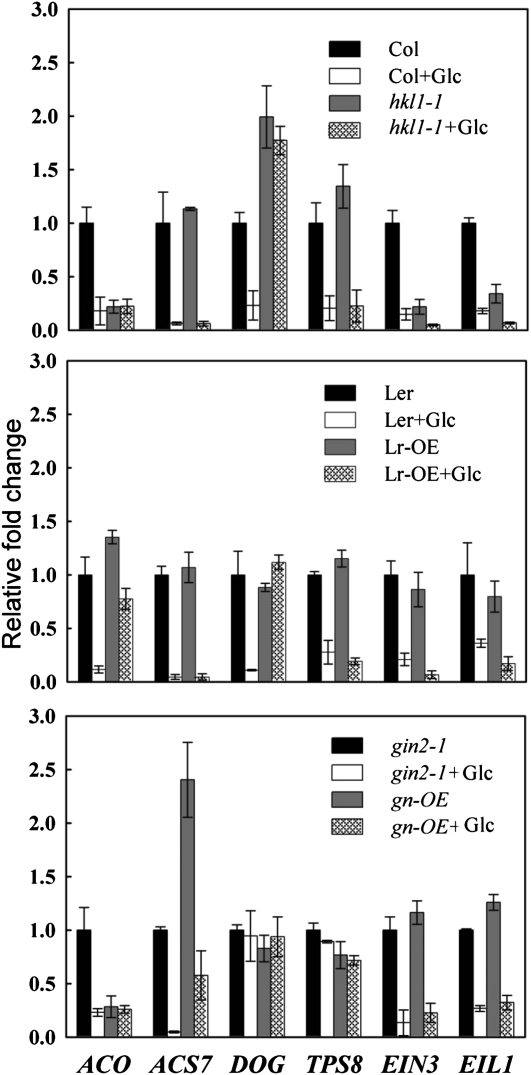
Transcript profiling experiments have shown that Glc represses the expression of a number of genes involved in ethylene metabolism and ethylene signaling (Price et al., 2004). Therefore, we examined whether some of this regulation might involve HKL1 or HXK1. For this experiment, seedlings were grown in liquid culture, challenged with 2% Glc, and examined by quantitative reverse transcription (qRT)-PCR for transcript abundance of most of the candidate genes identified by Price et al. (2004; see Table 2). Glc repression of the positive control Trehalose 6-Phosphate Synthase8 (At1g70290) was observed in both Col and Ler, requiring the presence of HXK1 but not HKL1, as shown previously (Fig. 5; Karve and Moore, 2009). The expression data confirm that wild-type Col and Ler showed strong Glc repression of several genes for ethylene metabolism (Fig. 5): ACS7, DOG, and ACO mRNA. In the hkl1-1 line, Glc treatment did not repress DOG and ACO mRNA but did repress ACS7 mRNA. In the HKL1 Lr-OE background, DOG mRNA was very slightly induced, although both ACS7 and ACO mRNAs were moderately repressed. Like wild-type Ler, Glc treatment repressed ACO and ACS7 mRNAs in gin2-1 seedling. However, unlike Ler seedlings, Glc treatment did not affect DOG mRNA level. Although Glc treatment repressed ACS7 mRNA strongly in gn-OE seedlings, ACO and DOG mRNA levels were not significantly affected. Interestingly, transcriptional responses of Glc to EIN3 (At3g20770) and Ethylene Insensitive3-Like1 (EIL1; At2g20750) mRNA were not affected among the different genotypes (Fig. 5). These responses of the two mutants do indicate that HKL1 and HXK1 have roles in the Glc repression of some genes associated with ethylene metabolism.
Figure 5.
The influence of Glc treatment on the expression of genes related to ethylene metabolism in HKL1 transgenic lines and mutants. Seedlings were grown initially in liquid MS medium, prior to dark adapting in sugar-free medium, followed by 8 h of treatment under light with or without 2% (w/v) Glc. qRT-PCR was used to determine seedling transcript levels of candidate genes identified previously by transcriptional profiling (Price et al., 2004): ACS7 (for ACC synthase 7; At4g26200), DOG (the homolog to tomato E8 dioxygenase protein; At5g26740), ACO (for ACC oxidase; At1g12010), EIN3 (At3g20770), and EIL (At2g27050). Transcript abundance of TPS8 (for trehalose 6-phosphate synthase 8; At1g70290) was monitored as a positive Glc-repressible control, and UBQ5 (At3g62250) was monitored as an internal control. The bars represent relative fold change relative to the respective control genotypes. Error bars represent se of two independent replicate samples.
HKL1 Interacts with HXK1 in a Coimmunoprecipitation Assay
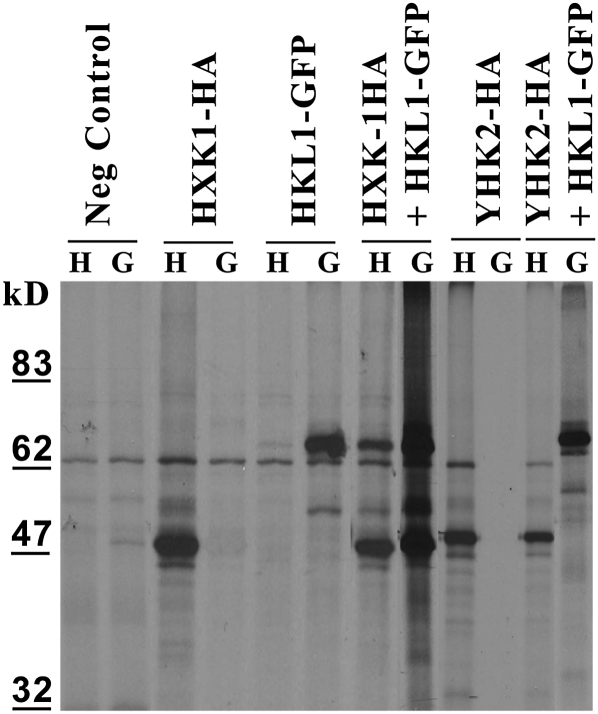
The described phenotypes indicate that HKL1 has a complex role in mediating cross talk between Glc and ethylene responses, which often depends on the presence of HXK1. Therefore, we tested by coimmunoprecipitation assays whether HKL1 and HXK1 can interact with each other to possibly form a functional association. This was done following the transient expression in maize (Zea mays) mesophyll protoplasts of plasmids with HXK1-HA and HKL1-GFP. Since both expressed proteins are about 53 kD, the addition of the GFP tag allowed us to resolve them by SDS-PAGE. In control assays, anti-GFP antibody did not capture HXK1-HA protein and anti-HA antibody did not capture HKL1-GFP protein, but each recognized the appropriate positive control (Fig. 6). However, from protoplasts that expressed both plasmids, anti-HA pulled down HKL1-GFP and anti-GFP pulled down HXK1-HA. As one control, we did comparable assays between cotransfected yeast HXK2-HA and HKL1-GFP (Fig. 6). The anti-HA antibody did precipitate ScHXK2-HA but did not pull down AtHKL1-GFP. Reciprocally, anti-GFP antibody did capture AtHKL1-GFP; however, it did not pull down ScHXK2-HA. As another approach, an alternate experiment was done using cotransfected HXK1-GFP and HKL1-HA. Once again, when both proteins were expressed together, anti-GFP pulled down HXK1-GFP as well as HKL1-HA (Supplemental Fig. S2). These data show that AtHKL1 potentially can interact with AtHXK1.
Figure 6.
Differentially tagged HXK1 and HKL1 can interact with each other by coimmunoprecipitation assay. Maize mesophyll protoplasts were transfected with the indicated combinations of AtHXK1-HA (approximately 54 kD), AtHKL1-GFP (approximately 82 kD), and/or yeast HXK2-HA (YHK2-HA; approximately 54 kD). Newly made proteins were labeled with [35S]Met and then pulled down using anti-HA antibody (H) or anti-GFP antibody (G). The negative control (Neg Control) was protoplasts transfected with an empty vector and then subjected to pull-down assay with the antibodies.
DISCUSSION
In Arabidopsis, the Glc response phenotypes of a number of mutants have been linked to the dysfunction of different proteins in the ethylene signaling pathway. For example, the ethylene-insensitive mutants etr1, ein2, and ein3 are hypersensitive to exogenous Glc, while the ethylene-hypersensitive mutants eto1 and ctr1 display a Glc-insensitive, or gin, phenotype (Zhou et al., 1998; Cheng et al., 2002; Yanagisawa et al., 2003). In many cases, mutants or transgenics that display a gin phenotype also have a dwarf growth phenotype (León and Sheen, 2003), including ctr1, gin2-1 (under high light), and HKL1 overexpression lines (under long photoperiods; Kieber et al., 1993; Moore et al., 2003; Karve and Moore, 2009).
Even though HKL1 lacks catalytic activity and does not directly modulate Glc signaling, its Glc-binding domain is largely conserved and HKL1 overexpression lines have Glc-dependent growth phenotypes (Figs. 1 and 2; Karve et al., 2008; Karve and Moore, 2009). Based on the following information, we suggest that the primary function of HKL1 is to mediate cross talk between plant Glc and ethylene responses. (1) HKL1 is required for ethylene-dependent repression of the gin phenotype (Fig. 1). This repression also requires ETR1, EIN2, and other positive effectors of ethylene signaling (León and Sheen, 2003). (2) HKL1 is required for ethylene-induced formation of ectopic root hairs (Figs. 2 and 3). The stimulation of root hair formation is a normal response to ACC treatment, which is mediated ultimately by auxin (Vandenbussche and Van Der Straeten, 2007). (3) HKL1 promotes the Glc-dependent formation of bulbous root hairs by a process that is attenuated by treatment with AVG (Figs. 2 and 3). AVG is a well-established inhibitor of ACC synthases and other pyridoxal-phosphate-utilizing enzymes (Yang and Hoffman, 1984). ACC treatment of even wild-type seedlings at very high levels of Glc can phenocopy this root hair growth response mediated by HKL1 (Fig. 4). (4) The HKL1 expression level was previously shown to modulate hypocotyl elongation at low light (Karve and Moore, 2009). Elongation was enhanced in hkl1-1 but reduced in HKL1 overexpression lines. Ethylene is well recognized for modulating organ elongation growth (Vandenbussche and Van Der Straeten, 2007), but the nature of this effect depends on the tissue context (Pierik et al., 2006). The hypocotyl growth responses seen previously in the overexpression lines are consistent with the idea that HKL1 is a positive effector of the ethylene repression of elongation. This also might account for the dwarf phenotype associated with HKL1 overexpression (Karve and Moore, 2009). (5) An increased HKL1 expression level was previously shown to attenuate auxin-induced lateral root formation (Karve and Moore, 2009). Recent experiments have shown that ethylene signaling perturbs auxin-induced lateral root formation by altering the root transport of auxin (Negi et al., 2008). Therefore, this HKL1 overexpression phenotype could be associated with enhanced ethylene signaling. (6) The absence of HKL1 prevents Glc-dependent repression of an Arabidopsis homolog of the tomato (Solanum lycopersicum) E8 protein, DOG (Fig. 5). While the function of this dioxygenase has not been defined, the tomato E8 protein is related to ACC oxidases, lacks their catalytic activity, but somehow is a negative regulator of ACC and ethylene accumulation (Giovannoni, 2004). We speculate that repression by HKL1 of the Arabidopsis E8 protein homolog might promote ethylene accumulation and thereby contribute to some of the apparent ethylene hyperrepsonse phenotypes shown by HKL1 overexpression lines. Overall, the described phenotypes identify HKL1 as a regulatory protein that, under conditions of elevated Glc, can attenuate plant growth by promoting plant responses to ethylene.
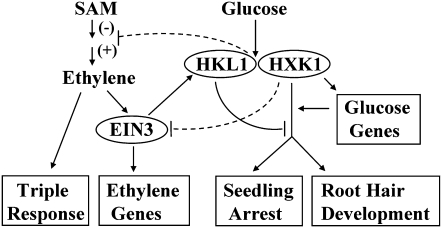
The major interactions between Glc, ethylene, HXK1, and HKL1 can be summarized in a model (Fig. 7). The model suggests that HKL1 can interact with HXK1. Presumably, this occurs at mitochondria, but whether this is a dynamic association is not known. Since HKL1 does not occur in nuclei, this could partly explain its lack of regulation of markers of HXK1-dependent gene expression in sugar response assays (Karve and Moore, 2009). Also, it is not known whether the recognized role of HXK1 in promoting root hair development and seedling arrest occurs by transcriptional or posttranscriptional processes. Nonetheless, the model accounts for the fact that HKL1 attenuates these responses, as does ethylene. The inhibitory role of ethylene toward these two Glc-dependent growth responses requires its signal transduction through EIN3 (Rolland et al., 2006). The current data are most simply accounted for by suggesting that this signaling output from ethylene also requires HKL1. Our model also suggests that HXK1 and HKL1 somehow directly regulate the expression of some ethylene biosynthesis genes. Glc is known to transcriptionally repress certain genes associated with ethylene biosynthesis (Price et al., 2004; Li et al., 2006) and to rapidly block the induction of ACC oxidase activity in treated tomato pericarp (Hong et al., 2004). Our gene expression data support a role of HXK1 and HKL1 in this Glc-dependent regulation (Fig. 5), but this aspect does require much more experimental work to establish its significance. The model also indicates that ethylene modulation of HKL1 or HXK1 could, in principle, form a feedback loop to influence its synthesis. While ethylene commonly inhibits its own biosynthesis in vegetative tissue (Argueso et al., 2007), we merely note that HXK1 could have a role in this feedback by repressing ACO expression.
Figure 7.
Proposed model for the roles of HKL1 and HXK1 in mediating cross talk between Glc and ethylene tissue responses. HKL1 is shown as a repressor of two Glc-dependent growth responses, which themselves are positively mediated by HXK1. HXK1-dependent growth responses might result from transcriptional or posttranscriptional regulation. Ethylene is shown as mediating an antagonistic role through HKL1 on these two Glc-dependent growth responses. Additionally, HKL1 and HXK1 are proposed to modulate, in a Glc-dependent fashion, the expression of either positive or negative effectors of ethylene biosynthesis. SAM, Shoot apical meristem.
The role of Glc in root hair growth is interesting to consider. In Arabidopsis, root hair growth is composed of five major phases: epidermal cell fate determination, initiation and site selection for outgrowth of the cell wall, protrusion of the epidermal cell wall, initiation of slow tip growth, and finally, rapid tip growth to full size (Dolan et al., 1994; Schiefelbein, 2000). While ethylene is thought to affect all stages of root hair development (Ringli et al., 2005), our experiments demonstrate that Glc specifically influences the formation of the basal bulge that occurs during the initial phase of root epidermal cell wall protrusion (Figs. 2–4). This abnormal expansion of the root hair base occurred in a Glc-dependent fashion in gin2-1, in both lines that overexpress AtHKL1 (Lr-OE and gn-OE), and even in wild-type Arabidopsis at very high Glc concentrations.
While HKL1 is a positive effector of root hair bulge formation and HXK1 is a negative effector, their mode of action is not clear. There are many described Arabidopsis mutants in root hair formation that impact all five of the major developmental events (Grierson et al., 2001). Among the mutants that have an expanded basal portion of the root hair, many are involved in cell wall synthesis and modifications, as part of the metabolic architecture by which cells implement root hair growth. These include rhd1 (UDP-Glc 4-epimerase; Schiefelbein and Somerville, 1990), prc1 (cellulose synthase6; Fagard et al., 2000), tip1 (S-acyl transferase; Hemsley et al., 2005), lrx1 (LRR-extension1, Baumberger et al., 2003), and, in a related sense, der1 (actin2; Gilliland et al., 2002; Ringli et al., 2002). Notably, a number of these proteins function in other stages of root hair growth or even elsewhere in the plant. The metabolic dysfunction associated with the bulbous root hair phenotype is evident also after seedling treatment with an arabinogalactan protein-binding reagent, which phenocopies this morphology (Willats and Knox, 1996; Ding and Zhu, 1997). These studies support the view that Glc-dependent formation of a root hair bulge is the result of excessive localized weakening of the epidermal cell wall.
Glc acting through HKL1 or HXK1 could affect cell wall metabolism, since profiling experiments show that the abundance of many cell wall gene transcripts is affected by Glc treatment (Price et al., 2004). However, in contrast to the numerous metabolic mutants associated with root hair bulge formation, fewer mutants have been described that are associated with the cellular control of growth of the root hair base. COBRA encodes a glycosylphosphatidylinositol-anchored extracellular protein that somehow orients cell expansion growth (Benfey et al., 1993; Roudier et al., 2005). MRH3 encodes an inositol-1,4,5-triphosphate phosphatase protein that has a role in establishing the basal width of root hairs (Jones et al., 2006). In general, these phosphatases terminate inositol triphosphate signaling, with some also having roles in sugar sensing (Ananieva et al., 2008). Another signaling protein with an associated role in basal root hair morphology is Rop2 GTPase. When overexpressed as a constitutively active form (CA-rop2), this can result in increased basal swelling of root hairs (Jones et al., 2002). This effect can be further enhanced by a mutation in MRH2 (a kinesin protein) in the CA-rop2 background (Yang et al., 2007). HKL1 and HXK1 might be involved in some of these processes, since increased Suc concentrations have been noted as enhancing the bulbous root hair phenotype in both act2-1 (1% Suc; Gilliland et al., 2002) and CA-rop2 (5% Suc; Yang et al., 2007). This sugar effect is interesting, since increased Suc would likely increase the availability of cellular Glc as a signaling molecule and since both Glc and Rop2 extensively modulate the actin cytoskeleton (Fu et el., 2002; Balasubramanian et al., 2007). In general, actin filaments are involved in root hair expansion, while microtubules are required for the directional orientation of the root hair (Sieberer et al., 2005). From a cellular control viewpoint, ethylene is a key regulator of root hair growth, but its mode of action is not entirely clear. Ethylene has been shown to suppress root hair bulging in rhd1 through a branch of ethylene signaling that involves an alternate receptor to ETR1 and an alternate transcription factor cascade to EIN3 (Seifert et al., 2004). This is different from its synergistic role with Glc that we have observed in strongly promoting root hair bulging in the Lr-OE line (Fig. 3). From the available evidence, we suggest that HKL1 is a control protein that modulates root hair growth.
Several previously described Arabidopsis phenotypes associated with altered HKL1 expression indicate that HKL1 might act as a dominant negative effector of HXK1 function (Karve and Moore, 2009). However, the present differentiation among root hair phenotypes and among even limited gene expression characteristics between HKL1 transgenic lines and gin2-1 indicate that, at least in these regards, HKL1 does not function strictly in this manner. For example, the function of HKL1 in root hair formation could represent a different branch for Glc and ethylene cross talk than the one that involves HXK1, because the root hairs of gin2-1 did not respond similarly to those of Lr-OE after ACC or AVG treatment (Fig. 3). Rather, since both proteins are located predominantly at the mitochondria and since they can potentially interact (Fig. 6), we suggest that these proteins form a critical node as an important junction in the transduction of a Glc signal. As defined by Taniguchi et al. (2006) in studies of the insulin signaling pathway, a critical node in a signaling pathway must fulfill three criteria. First, a critical node contains a group of related proteins in which two or more have a unique biological role within the signaling network. This criterion is clearly met by the differential, and opposite, effects of HKL1 and HXK1 expression on plant growth. Second, a critical node is subject to both positive and negative regulation. This criterion is less well supported for HKL1 and HXK1 at present, largely due to the lack of studies in this area. However, the recent identification of SIS3, a ubiquitin E3 ligase, as a positive effector of sugar signaling could be important in this context (Huang et al., 2010). Nonetheless, since the outputs from a possible HXK1-HKL1 signaling node can have either positive or negative effects on plant growth responses, it is reasonable to presume that the cellular activation of either, or both, also involves contrasting control inputs. Third, a critical node is a junction for potential cross talk. This criterion is well supported now with the recognition that both HKL1 and HXK1 influence tissue ethylene responses and that both proteins are widely expressed in Arabidopsis (Karve et al., 2008), as is the capacity for ethylene biosynthesis (Argueso et al., 2007). In the case of the insulin signaling pathway in mammals, the first of several critical nodes includes the different insulin receptor substrate proteins, which act to diversify response outputs. We are not aware of this concept having yet been applied to plant signaling networks, but this appears to be valid for HXK1 and HKL1 and might ultimately extend to some other HXK gene family members as well (Jang et al., 1997; Karve et al., 2008). One possible biochemical mechanism for HXK1 and HKL1 having diversified response outputs could occur if the two proteins are found to have different Glc-binding affinities, as we have suggested previously (Karve et al., 2008). Nonetheless, we surmise that the hexokinase node for plant Glc signaling is critical also, since protein homologs of both HXK1 and HKL1 likely are widespread among higher plants (Karve et al., 2010).
CONCLUSION
Plant responses to Glc are often antagonistic to responses to ethylene. Previous work links both the perception of ethylene and its transduction through EIN3 with the perception of Glc and its transduction by HXK1. By taking a functional genomics approach, we have shown that HKL1, which is a noncatalytic homolog of HXK1, is a positive effector of some plant Glc and ethylene interactions. In the future, it will be interesting to establish the targets and mechanisms by which HKL1 mediates at least certain aspects of cross talk between Glc and ethylene response pathways.
MATERIALS AND METHODS
Plant Material
Seeds of Arabidopsis (Arabidopsis thaliana) Col-0, Ler, and hkl1-1 were obtained from the Arabidopsis Biological Resource Center (Ohio State University). Seeds of gin2-1 were as described previously (Moore et al., 2003). The HKL1-HA and HKL1-FLAG overexpression lines were generated in the Ler and gin2-1 backgrounds and were designated Lr-OE and gn-OE, respectively (Karve and Moore, 2009). Two independent homozygous lines of each type were used in all the experiments, with data reported for Lr-OE line 52 and gn-OE line 79. Seeds of Arabidopsis eto2-1 and ein2-1 were a generous gift from Dr. J.-C. Jang. Seeds of maize (Zea mays) were purchased (line FR922 × FR967; Seed Genetics).
Plant Growth Conditions
Arabidopsis seeds were surface sterilized and stratified for 2 d at 4°C as described by Jang et al. (1997) and were grown on 1× Murashige and Skoog (MS) agar plates (basal medium modified with Gamborg vitamins; PhytoTechnology Laboratories) containing 0.7% (w/v) phytagar, pH 5.7, and different carbon sources under constant light (30 μmol m−2 s−1).
For Glc repression assays, seedlings were grown on 1× MS plates with 6% (w/v) Glc for 7 d under constant light. In some experiments, plate media were supplemented with 50 μm ACC (Sigma-Aldrich) or 1 μm AVG (Sigma-Aldrich).
To evaluate Glc-dependent regulation of candidate gene expression, seedlings were grown in a liquid culture medium. For this, 15 to 20 stratified seeds were placed in 125-mL flasks containing 50 mL of 1/2× MS basal medium supplemented with 1% (w/v) Suc. Seedlings were grown on a rotary shaker at 250 rpm under constant light (70 μmol m−2 s−1) at 22°C for 7 d. Seedlings were then washed with sugar-free 1/2× MS medium for 24 h in the dark while shaking and subsequently transferred to fresh sugar-free medium (control) or to medium supplemented with 2% (w/v) Glc. Seedlings were treated under constant light with shaking for 8 h and then harvested by quickly blotting with filter paper before freezing in liquid N2.
Assay for Root Hair Development
Seedlings were initially grown on 1/2× MS plates with 5% (w/v) Suc or 2% (w/v) Glc under constant light (30 μmol m−2 s−1). Seven-day-old seedlings were transferred to agar plates typically with 6% (w/v) Glc or 6% (w/v) mannitol for 4 to 6 d. In some experiments, seedlings were transferred to sugar plates with 5 μm ACC or 1 μm AVG. In one experiment, Col and mutant seedlings were transferred as indicated from plates with 2% (w/v) Glc to plates with 6% to 9% (w/v) Glc, sometimes including 1 μm AVG. Newly developed seedling root hairs were imaged directly from the agar plates using a Nikon SMZ1500 stereomicroscope with a MicroPublisher CCD cooled color camera and Image Pro Plus version 5.0 software.
RNA Isolation and qRT-PCR Analysis
The relative expression levels of several candidate shade-response genes were compared across treatments using qRT-PCR. Total RNA was isolated from whole seedlings using the RNeasy plant kit (Qiagen.) Following RNA extraction, an on-column DNase I treatment (Sigma-Aldrich) was performed according to the provided protocol to remove any potential genomic DNA contamination. One microgram of DNase-free RNA was used for cDNA synthesis using SuperScript III First-Strand Synthesis SuperMix for qRT-PCR (Invitrogen) according to the protocol provided.
Amplification reactions (20.0 μL) were carried out using iQ SYBR Green Supermix with 6-carboxy-X-rhodamine according to the instructions provided by Bio-Rad Laboratories. Each reaction contained a cDNA template (1.0 μL), SYBR Green Supermix (10 μL), sterile water (9 μL), and the appropriate forward and reverse primer mix (10 μm each; 1.0 μL). All PCR amplifications were performed in triplicate on the RNA extracted from the tissue of two independent biological experiments. The amplification was carried out using the StepOne Plus Real Time PCR detection system (Applied Biosystems) with the following amplification conditions: 5 min at 95°C, 40 cycles of 95°C for 15 s, 60°C for 20 s, and 72°C for 20 s, 1 min at 95°C, 80 cycles at 60°C for 10 s with a temperature increase of 0.3°C after each cycle, and then a hold a 4°C until the plates were removed from the machine. The PCR primer sequences for the candidate genes were generated using the AtRTPrimer public database (Han and Kim, 2006). The cDNA levels were normalized by an endogenous control gene, Arabidopsis Ubiquitin5 (UBQ5). The primer sequences and amplicon sizes of the candidate genes and control UBQ5 are reported in Supplemental Table S1.
Relative mRNA abundance was calculated using the method of Pfaffl (2001). For each cDNA, three technical replicates were performed and the values averaged. The efficiency of each primer pair was assessed by use of a dilution series. Across the biological replicates, threshold cycles for all products from the cDNAs fell within the valid range of the standard curves.
35S Labeling and Protein Interaction Assay by Coimmunoprecipitation
Protoplasts from greening maize leaves were transfected with 6 to 10 μg of cesium chloride-purified plasmids containing HKL1-GFP, HXK1-HA, or yeast HXK2-HA (YHK2; Moore et al., 2003; Karve et al., 2008) as described previously. Following transfection, protoplasts were incubated in the dark for 90 min, then [35S]Met (25 μCi; Perkin-Elmer) was added for 8 h. Transfection efficiencies were routinely greater than 60% as determined using WRKY-GFP (Balasubramanian et al., 2007). Harvested protoplasts were lysed and resuspended as described previously (Balasubramanian et al., 2007). Anti-HA (Roche) or anti-GFP (Sigma-Aldrich) antibodies were used with protein A-agarose beads (Roche) for immunoprecipitation assays (Karve et al., 2008). Washed beads were resuspended in 2× SDS sample buffer, and proteins then were separated on 10% (w/v) SDS-PAGE gels and visualized by fluorography.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Seedling growth of HKL1 overexpression lines and gin2-1.
Supplemental Figure S2. Differentially tagged HXK1 and HKL1 can interact with each.
Supplemental Table S1. List of primers used in qRT-PCR analysis.
Supplementary Material
Acknowledgments
We thank Dr. J.-C. Jang (Ohio State University) for providing seeds of eto2-1 and ein2-1 and for useful discussions. We also thank Dr. David Weston (Oak Ridge National Laboratory) for support and encouragement.
References
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148–2152 [DOI] [PubMed] [Google Scholar]
- Ananieva EA, Gillaspy GE, Ely A, Burnette RN, Erickson FL. (2008) Interaction of the WD40 domain of a myoinositol polyphosphate 5-phosphatase with SnRK1 links inositol, sugar, and stress signaling. Plant Physiol 148: 1868–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueso CT, Hansen M, Kieber JJ. (2007) Regulation of ethylene biosynthesis. J Plant Growth Regul 26: 92–105 [Google Scholar]
- Balasubramanian R, Karve A, Kandasamy M, Meagher RB, Moore B. (2007) A role for F-actin in hexokinase-mediated glucose signaling. Plant Physiol 145: 1423–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumberger N, Steiner M, Ryser U, Keller B, Ringli C. (2003) Synergistic interaction of the two paralogous Arabidopsis genes LRX1 and LRX2 in cell wall formation during root hair development. Plant J 35: 71–81 [DOI] [PubMed] [Google Scholar]
- Benfey PN, Linstead PJ, Roberts K, Schiefelbein JW, Hauser MT, Aeschbacher RA. (1993) Root development in Arabidopsis: four mutants with dramatically altered root morphogenesis. Development 119: 57–70 [DOI] [PubMed] [Google Scholar]
- Cheng WH, Endo A, Zhou L, Penney J, Chen HC, Arroyo A, León P, Nambara E, Asami T, Seo M, et al. (2002) A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and functions. Plant Cell 14: 2723–2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekkers BJ, Schuurmans JA, Smeekens SC. (2008) Interaction between sugar and abscisic acid signalling during early seedling development in Arabidopsis. Plant Mol Biol 67: 151–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Zhu J-K. (1997) A role for arabinogalactan-proteins in root epidermal cell expansion. Planta 203: 289–294 [DOI] [PubMed] [Google Scholar]
- Dolan L, Duckett CM, Grierson C, Linstead P, Schneider K, Lawson E, Dean C, Poething S, Roberts K. (1994) Clonal relationships and cell patterning in the root epidermis of Arabidopsis. Development 120: 2465–2474 [Google Scholar]
- Fagard M, Desnos T, Desprez T, Goubet F, Refregier G, Mouille G, McCann M, Rayon C, Vernhettes S, Höfte H. (2000) PROCUSTE1 encodes a cellulose synthase required for normal cell elongation specifically in roots and dark-grown hypocotyls of Arabidopsis. Plant Cell 12: 2409–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucile G, Falconer S, Christendat D. (2008) Evolutionary diversification of plant shikimate kinase gene duplicates. PLoS Genet 4: e1000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson SI. (2004) Sugar and phytohormone response pathways: navigating a signalling network. J Exp Bot 55: 253–264 [DOI] [PubMed] [Google Scholar]
- Gilliland LU, Kandasamy MK, Pawloski LC, Meagher RB. (2002) Both vegetative and reproductive actin isovariants complement the stunted root hair phenotype of the Arabidopsis act2-1 mutation. Plant Physiol 130: 2199–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni JJ. (2004) Genetic regulation of fruit development and ripening. Plant Cell (Suppl) 16: S170–S180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grierson CS, Parker JS, Kemp AC. (2001) Arabidopsis genes with roles in root hair development. J Plant Nutr Soil Sci 164: 131–140 [Google Scholar]
- Guo H, Ecker JR. (2003) Plant responses to ethylene gas are mediated by SCF(EBF1/EBF2)-dependent proteolysis of EIN3 transcription factor. Cell 115: 667–677 [DOI] [PubMed] [Google Scholar]
- Guzmán P, Ecker JR. (1990) Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2: 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Kim D. (2006) AtRTPrimer: database for Arabidopsis genome-wide homogeneous and specific RT-PCR primer-pairs. BMC Bioinformatics 7: 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartig K, Beck E. (2006) Crosstalk between auxin, cytokinins, and sugars in the plant cell cycle. Plant Biol (Stuttg) 8: 389–396 [DOI] [PubMed] [Google Scholar]
- Heazlewood JL, Tonti-Filippini JS, Gout AM, Day DA, Whelan J, Millar AH. (2004) Experimental analysis of the Arabidopsis mitochondrial proteome highlights signaling and regulatory components, provides assessment of targeting prediction programs, and indicates plant-specific mitochondrial proteins. Plant Cell 16: 241–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemsley PA, Kemp AC, Grierson CS. (2005) The TIP GROWTH DEFECTIVE1 S-acyl transferase regulates plant cell growth in Arabidopsis. Plant Cell 17: 2554–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JH, Cowan AK, Lee SK. (2004) Glucose inhibits ACC oxidase activity and ethylene biosynthesis in ripening tomato fruit. Plant Growth Regul 43: 81–87 [Google Scholar]
- Huang Y, Li CY, Pattison DL, Gray WM, Park S, Gibson SI. (2010) SUGAR-INSENSITIVE3, a RING E3 ligase, is a new player in plant sugar response. Plant Physiol 152: 1889–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JC, León P, Zhou L, Sheen J. (1997) Hexokinase as a sugar sensor in higher plants. Plant Cell 9: 5–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston CA, Taylor JP, Gao Y, Kimple AJ, Grigston JC, Chen J-G, Siderovski DP, Jones AM, Willard FS. (2007) GTPase acceleration as the rate-limiting step in Arabidopsis G protein-coupled sugar signaling. Proc Natl Acad Sci USA 104: 17317–17322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MA, Raymond MJ, Smirnoff N. (2006) Analysis of the root-hair morphogenesis transcriptome reveals the molecular identity of six genes with roles in root-hair development in Arabidopsis. Plant J 45: 83–100 [DOI] [PubMed] [Google Scholar]
- Jones MA, Shen J-J, Fu Y, Li H, Yang Z, Grierson CS. (2002) The Arabidopsis Rop2 GTPase is a positive regulator of both root hair initiation and tip growth. Plant Cell 14: 763–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karve A, Moore BD. (2009) Function of Arabidopsis hexokinase-like1 as a negative regulator of plant growth. J Exp Bot 60: 4137–4149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karve A, Rauh BL, Xia X, Kandasamy M, Meagher RB, Sheen J, Moore BD. (2008) Expression and evolutionary features of the hexokinase gene family in Arabidopsis. Planta 228: 411–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karve R, Lauria M, Virnig A, Xia X, Rauh BL, Moore B. (2010) Evolutionary lineages and functional diversification of plant hexokinases. Mol Plant 3: 334–346 [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. (1993) CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell 72: 427–441 [DOI] [PubMed] [Google Scholar]
- Laxmi A, Paul LK, Peters JL, Khurana JP. (2004) Arabidopsis constitutive photomorphogenic mutant, bls1, displays altered brassinosteroid response and sugar sensitivity. Plant Mol Biol 56: 185–201 [DOI] [PubMed] [Google Scholar]
- León P, Sheen J. (2003) Sugar and hormone connections. Trends Plant Sci 8: 110–116 [DOI] [PubMed] [Google Scholar]
- Li Y, Lee KK, Walsh S, Smith C, Hadingham S, Sorefan K, Cawley G, Bevan MW. (2006) Establishing glucose- and ABA-regulated transcription networks in Arabidopsis by microarray analysis and promoter classification using a Relevance Vector Machine. Genome Res 16: 414–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B, Zhou L, Rolland F, Hall Q, Cheng WH, Liu YX, Hwang I, Jones T, Sheen J. (2003) Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 300: 332–336 [DOI] [PubMed] [Google Scholar]
- Negi S, Ivanchenko MG, Muday GK. (2008) Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. Plant J 55: 175–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Tholen D, Poorter H, Visser EJW, Voesenek LA. (2006) The Janus face of ethylene: growth inhibition and stimulation. Trends Plant Sci 11: 176–183 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J, Laxmi A, St Martin SK, Jang JC. (2004) Global transcription profiling reveals multiple sugar signal transduction mechanisms in Arabidopsis. Plant Cell 16: 2128–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringli C, Baumberger N, Diet A, Frey B, Keller B. (2002) ACTIN2 is essential for bulge site selection and tip growth during root hair development of Arabidopsis. Plant Physiol 129: 1464–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringli C, Baumberger N, Keller B. (2005) The Arabidopsis root hair mutants der2-der9 are affected at different stages of root hair development. Plant Cell Physiol 46: 1046–1053 [DOI] [PubMed] [Google Scholar]
- Rognoni S, Teng S, Arru L, Smeekens SCM, Perata P. (2007) Sugar effects on early seedling development in Arabidopsis. Plant Growth Regul 52: 217–228 [Google Scholar]
- Rolland F, Baena-Gonzalez E, Sheen J. (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57: 675–709 [DOI] [PubMed] [Google Scholar]
- Rolland F, Sheen J. (2005) Sugar sensing and signalling networks in plants. Biochem Soc Trans 33: 269–271 [DOI] [PubMed] [Google Scholar]
- Roudier F, Fernandez AG, Fujita M, Himmelspach R, Borner GHH, Schindelman G, Song S, Baskin TI, Dupree P, Wasteneys GO, et al. (2005) COBRA, an Arabidopsis extracellular glycosyl-phosphatidyl inositol-anchored protein, specifically controls highly anisotropic expansion through its involvement in cellulose microfibril orientation. Plant Cell 17: 1749–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein JW. (2000) Constructing a plant cell: the genetic control of root hair development. Plant Physiol 124: 1525–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein JW, Somerville C. (1990) Genetic control of root hair development in Arabidopsis thaliana. Plant Cell 2: 235–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert GJ, Barber C, Wells B, Roberts K. (2004) Growth regulators and the control of nucleotide sugar flux. Plant Cell 16: 723–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J, Zhou L, Jang JC. (1999) Sugars as signaling molecules. Curr Opin Plant Biol 2: 410–418 [DOI] [PubMed] [Google Scholar]
- Sieberer BJ, Ketelaar T, Esseling JJ, Emons AM. (2005) Microtubules guide root hair tip growth. New Phytol 167: 711–719 [DOI] [PubMed] [Google Scholar]
- Smeekens S. (2000) Sugar-induced signal transduction in plants. Annu Rev Plant Physiol Plant Mol Biol 51: 49–81 [DOI] [PubMed] [Google Scholar]
- Taniguchi CM, Emanuelli B, Kahn CR. (2006) Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol 7: 85–96 [DOI] [PubMed] [Google Scholar]
- Tanimoto M, Roberts K, Dolan L. (1995) Ethylene is a positive regulator of root hair development in Arabidopsis thaliana. Plant J 8: 943–948 [DOI] [PubMed] [Google Scholar]
- Temple BRS, Jones AM. (2007) The plant heterotrimeric G-protein complex. Annu Rev Plant Biol 58: 249–266 [DOI] [PubMed] [Google Scholar]
- Vandenbussche F, Van Der Straeten D. (2007) One for all and all for one: cross-talk of multiple signals controlling the plant phenotype. J Plant Growth Regul 26: 178–187 [Google Scholar]
- Vandesteene L, Ramon M, Le Roy K, Van Dijck P, Rolland F. (2010) A single active trehalose-6-P synthase (TPS) and a family of putative regulatory TPS-like proteins in Arabidopsis. Mol Plant 3: 406–419 [DOI] [PubMed] [Google Scholar]
- Vogel JP, Woeste KE, Theologis A, Kieber JJ. (1998) Recessive and dominant mutations in the ethylene biosynthetic gene ACS5 of Arabidopsis confer cytokinin insensitivity and ethylene overproduction, respectively. Proc Natl Acad Sci USA 95: 4766–4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willats WGT, Knox JP. (1996) A role for arabinogalactan-proteins in plant cell expansion: evidence from studies on the interaction of β-glucosyl Yariv reagent with seedlings of Arabidopsis thaliana. Plant J 9: 919–925 [DOI] [PubMed] [Google Scholar]
- Yanagisawa S, Yoo S-D, Sheen J. (2003) Differential regulation of EIN3 stability by glucose and ethylene signalling in plants. Nature 425: 521–525 [DOI] [PubMed] [Google Scholar]
- Yang G, Gao P, Zhang H, Huang S, Zheng ZL. (2007) A mutation in MRH2 kinesin enhances the root hair tip growth defect caused by constitutively activated ROP2 small GTPase in Arabidopsis. PLoS ONE 2: e1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SF, Hoffman NE. (1984) Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol 35: 155–189 [Google Scholar]
- Yuan K, Wysocka-Diller J. (2006) Phytohormone signalling pathways interact with sugars during seed germination and seedling development. J Exp Bot 57: 3359–3367 [DOI] [PubMed] [Google Scholar]
- Zhou L, Jang JC, Jones TL, Sheen J. (1998) Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proc Natl Acad Sci USA 95: 10294–10299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Ye N, Zhang J. (2009) Glucose-induced delay of seed germination in rice is mediated by the suppression of ABA catabolism rather than an enhancement of ABA biosynthesis. Plant Cell Physiol 50: 644–651 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.