Abstract
There was established evidence that silencing the attenuator and activating the TLRs could activate the dendritic cells in synergic effects. In this study, we constructed a plasmid, namely pshS1NH, which encodes SOCS1-shRNA, NY-ESO-1-MAGE3 (HLA-A2*0201) fusion antigen and secretory HMGB1, an agent used to modify dendritic cells (DCs), aiming to generate potent DC vaccine against tumors. The SOCS1-shRNA could efficiently downregulate the expression of SOCS1, as indicated by real-time RT-PCR and Western blot. The fusion antigen was detected in the pshS1NH–DCs by PCR and Western blot. Simultaneously, HMGB1 level in the pshS1NH–DCs culture media was significantly higher than that in the control DCs culture media. Levels of Th1 cytokines in pshS1NH–DCs culture media, such as IL-1β, IL-6, TNF-α and IL-12p70, were dramatically higher than those in control DCs culture media. In addition, lymphocytes co-cultured with pshS1NH–DCs secreted dramatically higher level of IFN-γ, whereas no difference was detected in IL-4 levels. Taken together, these data suggest that pshS1NH–DCs may be a potential adjuvant immunotherapy for cancers in clinical applications.
Keywords: Dendritic cells, Tumor vaccine, SOCS1, HMGB1
Introduction
Dendritic cells (DCs), the most potent antigen-presenting cells in vivo due to their superior capacity for acquiring and processing antigens to T cells, are involved in the adaptive and innate immune responses. DC-based antitumor vaccines are widely investigated for their potential application in immune therapy of cancer. Notably, the first phase III clinical trial based on gene-modified DCs vaccine was recently approved, opening a new avenue to develop and improve immune therapies using gene-modified DCs vaccine against tumors [1].
To efficiently activate naïve T cells, DCs need to be fully matured. TLR ligands, among the several stimuli including the proinflammatory stimuli CD40L and TLR ligands, have showed superior capability and synergistic effects in activating DCs. HMGB1 was initially recognized as a member of nonhistone, chromatin-associated high-mobility family of proteins and was passively released by cells upon necrosis. In extracellular environment, however, it interacts with TLR2/4/9 and RAGE on DCs and behaves as a cytokine promoting inflammatory responses. HMGB1 has been demonstrated to be a potent immune adjuvant, indicated by the higher level of co-stimulatory molecules and secretion of IL-12, as well as the enhanced migration ability of DCs, both in vitro and in vivo [2–4].
However, there are more pathways and receptors involved in controlling immune responses than in inducing immune responses. It is critical for health maintenance to have a highly evolved process to turn off immune responses when there are too many inflammatory challenges [5–8]. Therefore, a number of negative regulatory mechanisms are known to attenuate TLR and cytokine signaling and maintain the immunological balance. Among them, SOCS1 is a classic molecule that inhibits the TLR signaling pathway mainly through two different mechanisms. First, SOCS1 can directly inhibit the TLR signaling pathway through binding to Mal, IRAK, p65 or JAK2 for degradation [9–14]. Second, SOCS1 can inhibit the JAK–STATs signaling pathway, which indirectly attenuates the TLR signaling that induces the expression of cytokines acting through the JAK–STATs signaling pathway [9, 14]. Thus, SOCS1 activation, via negative feedback, results in the inhibition NF-κB signaling through both direct and indirect mechanisms [9, 14].
Due to the precise regulatory mechanism to maintain the immune balance, there is no autoimmune response occurrence in healthy body. However, for inducing antitumor immunity, it is necessary to disrupt the immune balance and induce the immunity toward the cellular immune responses that is critical to destroy the tumor cells. Thus, when SOCS1 is silenced in DCs, the associated loss of negative feedback results in increased activity of NF-κB, thereby blunting the antigen presentation attenuation effects of SOCS1 [5]. Simultaneously, if we give DCs another stimulus, such as HMGB1, it will enhance the NF-κB signaling in a synergic effect with the inhibition of SOCS1, resulting in potent activation of DCs [5]. This has been verified by SOCS1 knockdown in DCs using siRNA, which leads to a similar hyperactivation as that observed in DCs derived from SOCS1-deficient mice, both of which are more responsive to LPS, another ligand of TLR4 [6]. Therefore, activated DCs will be prone to induce cellular immunity when modified as described above.
The principle behind DCs vaccines is to express a target antigen (or antigens) followed by DC modification. Peptide processed by DCs can bind to MHC-I molecular forming peptide–MHC-I complex, which interacts with TCR, resulting in the stimulation of CD8+T cell responses. NY-ESO-1 and MAGE3 both belong to cancer-testis antigens (CTA) due to their unique expression in tumors but not in normal tissue except testis, making them good candidates for tumor vaccine because there is low risk in inducing autoimmunity.
Here, we constructed a plasmid co-expressing the SOCS1-shRNA, NY-ESO-1-MAGE3 (HLA-A2*0201) fusion protein and secretory HMGB1, in order to modify DCs in one step. A new DC vaccine was generated and exhibited improved activity in functional assessment.
Materials and methods
Construction of recombinant plasmids
pIRES-EGFP vector was purchased from BD Bioscience. The NY-ESO-1 (GenBank: AJ003149) and HMGB1 (GenBank: BC003378) ORF were purchased from Invitrogen. BLOCK-iT™ U6 RNAi Entry Vector kit was purchased from Invitrogen. All restriction enzymes were purchased from New England Biolabs (China). HMGB1 was subcloned from the ORF with primers P1 and P2 (Table 1). Internal ribosome entry site (IRES) was subcloned from the pIRES-EGFP vector with primers P3 and P4. The two fragments were then linked together by overlap extension PCR, which was subcloned into pIRES-EGFP vector to replace the EGFP gene at the sites of EcoRI and NotI to generate the pH vector. Primers P5 and P6 were designed to amplify NY-ESO-1 from the ORF. The PCR product and pIRES-EGFP vector were both digested by EcoRI and NotI and linked together to generate a plasmid expressing NY-ESO-1-MAGE3 (epitope). Next, NY-ESO-1-MAGE3 (epitope) was subcloned from this plasmid with primers P7 and P8, and inserted into pH at the sites of EcoRI and BglII, to generate a plasmid expressing CMV-NY-ESO-1-MAGE3 (epitope)-IRES-Sig-HMGB1, namely pNH. The RNA interference sequence [15] and the negative control sequence that contained no interfering function of SOCS1 were CTGGTTGTTGTAGCAGCTTAA and CCTAA GGTTA AGTCG CCCTC GC, respectively, which were converted to shRNA sequences and inserted into pENTR/U6. U6-shSOCS1 was subcloned from shSOCS1-U6-shRNA using primers P9 and P10 and inserted into pIRES-EGFP vector to generate pshS1. U6-shNC was subcloned from shNC-U6-shRNA using primers P9 and P11 and inserted into pIRES-EGFP at the sites of BfaI and NotI, resulting in pshNC. The subcloned U6-shSOCS1 from pshS1 using primers P9 and P12, as well as CMV-NY-ESO-1-IRES-Sig-HMGB1 from pNH using P2 and P14 was linked together by T4 DNA Ligase and inserted into pIRES-EGFP vector by AseI and XbaI to generate pshS1NH. The subcloned U6-NC from pshNC using P9 and P15 as well as CMV-GFP from pEGFP-N1 using P16 and P17 were linked together and inserted into pIRES-EGFP vector as the negative control, namely pshNC-GFP.
Table 1.
Primers used in constructing plasmids
| Primers | Sequences (5′→3′)a,b |
|---|---|
| P1 | 5′-ATGGCCCTGTGGATGCGCCTCCTGCCCCTGCTGGCGCTGCTGGCCCTCTGGGGACCTGACCCAGCCGCAGCCGGCAAAGGAGATCCTAAGAAGC-3′ (signal peptide) |
| P2 | 5′-GCGTTTGCGGCCGCTTATTCATCATCATCATCTTCTTCTTC-3′ (NotI) |
| P3 | 5′-AGCTCAAGCTTCGAATTCTGC-3′ (EcoRI) |
| P4 | 5′-CCAGAGGGCCAGCAGCGCCAGCAGGGGCAGGAGGCGCATCCACAGGGCCATGGTTGTGGCCATATTATCATCG-3′ (IRES) |
| P5 | 5′-TTTAAAGAATTCATGCAGGCCGAAGGCCGG-3′ (EcoRI) |
| P6 | 5′-AAAGGGGCGGCCGCTTAAACGAGGGCCCTTGGACCCCACAGGAAGCGCCTCTGCCCTGAGGGA-3′ (NotI, MAGE3 (HLA-A2*0201)) |
| P7 | 5′-GGGTTAAGATCTATGCAGGCCGAAGGCCG-3′ (BglII) |
| P8 | 5′-AAATTTGAATTCTTAAACGAGGGCCCTTGGA-3′ (EcoRI) |
| P9 | 5′-GGGCAACTAGAAGGTCGGGCAGGAAGAGG-3′ (BfaI) |
| P10 | 5′-GGTTCCGCGGCCGCAAAAAACTGGTTGTTGTAGCAGC-3′ (NotI) |
| P11 | 5′-GGTTCCGCGGCCGCAAAAAACCTAAGGTTAAGTCGCC-3′ (NotI) |
| P12 | 5′-tttccgccGGTCTCAACTAAAAAAACTGGTTGTTGTAGCAGC-3′ (BsaI) |
| P13 | 5′-tttccgccGGTCTCATAGTTATTAATAGTAATCAATTACGGGGTC-3′ (BsaI) |
| P14 | 5′-TTTCCGCCGGTCTCAACTAAAAAAACCTAAGGTTAAGTCGCC-3′ (BsaI) |
| P15 | 5′-TTTCCGCCGGTCTCATAGTTATTAATAGTAATCAATTACGGGGTC-3′ (BsaI) |
| P16 | 5′-TTTAAAGCGGCCGCTTACTTGTACAGCTCGTCCATGC-3′ (NotI) |
aUnderlined nucleotides are restriction sites of the enzymes indicated in the brackets at the ends
bThe shaded nucleotides are representing the sequence indicated in the brackets at the ends
Double enzyme digestion and PCR
To confirm the construct of the plasmid, BglII and EcoRI were used to digest pshS1NH. Digested DNA was subject to electrophoresis. pshS1NH was used to amplify NY-ESO-1 and HMGB1. The primers were as follows: NY-ESO-1:PF:CGGCAACATACTGACTATCCGACTG. PR:GAAAGACTGCGTGATCCACATC. HMGB1:PF:AGATATGGCAAAAGCGGACAA. PR:GGGCGATACTCAGA GCAGAAG.
Generation of monocyte-derived DCs
Peripheral blood was collected from volunteers. PBMCs were isolated by centrifugation in Ficoll–Hypaque gradient (Institute of hematology, Chinese academy of medical sciences). Briefly, 4 ml blood diluted 1:2 in endotoxin-free PBS (Bioroc, China) was layered on 4 ml gradient and centrifuged at 1,800 rpm for 20 min. PBMCs were collected, washed and allowed to adhere to 75 cm2 culture flasks from Falcon (Corning, China) at a density of 5 × 106 cells/ml for 2 h at 37°C in RPMI 1640 (Bioroc, China). Suspension cells were collected for generating immunological effector cells. The adherent cells were incubated in RPMI1640 supplemented with 10% fetal bovine serum (Hyclone, USA), 1,000 U/ml rhGM-CSF and 1,000 U/ml rhIL-4 (Peprotech, USA). Cells were incubated at 37°C in a humidified atmosphere of 5% CO2 and replenished every three days with GM-CSF and IL-4. Cells were harvested on day 6.
Nucleofection
DCs were harvested at day 6 of culture, washed once in PBS/1%BSA and resuspended in specified nucleofection buffer from Amaxa (Germany) at a final concentration of 2 × 107 cells/ml. Plasmid DNA (2.5 μg) was mixed with 0.1 ml of cell suspension and transferred into electroporation cuvette (Amaxa). DCs were nucleofected with the Amaxa Nucleofector apparatus following the manufacturer’s protocol. After nucleofection, cells were immediately transferred to 2 ml of complete pre-warmed medium and cultured in six-well plates for 24 h or 48 h.
RT-PCR and real-time PCR
Total RNA was isolated from DCs using TRIzol (Invitrogen, China) according to the manufacturer’s instructions. The cDNA was synthesized using 1 μg total RNA using RT kit (Takara, Dalian, China) and used as the template to amplify NY-ESO-1 using the primers described above. Primers used for real-time PCR amplification were 5′-ATGCAGTCTCCACAGCAGCAGAG-3′ and 5′-CGAACGGAATGTGCGGAAGTG-3′ (SOCS1); and 5′-GAAGGTGAAGGTCGGAGTC-3′ and 5′-GAAGATGGTGATGGGATTTC-3′ (GAPDH). Real-time PCR amplification from cDNA was performed in a final volume of 25 μl containing 2.5 mM magnesium dichloride, 1.25 U Ex Taq polymerase (Takara, Dalian, China) and 1 M specific primers. Cycling conditions were 94°C for 30 s, and then 63.4°C for 30 s (7500 Real time PCR System, ABI, USA) for 40 cycles.
Western blot
At 48 h after nucleofection, DCs were harvested and homogenized with cell lysis buffer containing protease inhibitor mixture (Sigma-Aldrich) according to the instruction buffer. The lysates were subjected to SDS-PAGE on 10% polyacrylamide gel and were then transferred onto PVDF membranes (Millipore). After the reaction was blocked with 5% skim milk in PBS containing 0.1% Tween 20 at room temperature for 1 h, the membranes were incubated with mouse anti-SOCS1 Ab (Millipore), mouse anti-NY-ESO-1 Ab (Abcam, Hong Kong) or mouse anti-β-actin Ab (Santa Cruz Biotechnology) at 4°C overnight, followed by reaction with HRP-conjugated anti-mouse IgG (Zhongshan Golden Bridge Bio, China) at room temperature for 1 h. The band was visualized by ECL plus Western blotting detection reagents (Thermo Scientific, USA).
Flow cytometry
Harvested DCs were washed with PBS supplemented with 1% FBS. Immunofluorescence staining was performed by incubation of DCs for 30 min at 4°C with each mAb diluted to the optimal concentration according to the manufacturer’s instructions. The following mAbs were used: anti-CD80-PE, anti-CD83-PE and anti-HLA-DR-FITC. Relevant isotype controls were always used. All mAb and isotype are purchased from eBioscience. Cells were then washed twice and analyzed by FACS (BD FACSArill).
Cytokine assay
At 48 h after the nucleofection, the culture supernatants were collected for cytokines measurements. IL-1β, TNF-α, IL-6 and IL-12p70 were measured using the ELISA kits (Sizhengbai, China) following the instructions. The co-culture supernatants were collected for measuring the levels of IFN-γ and IL-4 (Sizhengbai, China). HMGB1 in the DCs culture supernatants is also measured (Yueyan biological technology Co. Ltd, China).
To prime T cells
After nucleofection, DCs were continuously cultured in complete medium containing GM-CSF and IL-4 for 24 h at 37°C in a humidified atmosphere containing 5% CO2. Subsequently, the nucleofected DCs were used as stimulator cells to co-culture with lymphocyte cells for 7 days in the presence of IL-2 (50 U/ml). The cultures were supplemented with recombinant human IL-2 (Peprotech, USA) every 3 days. The supernatants were collected for measuring INF-γ and IL-4.
Statistical analysis
One-way ANOVA was used to calculate the significance of statistical comparisons. The overall significant level was set at 5%. Results are presented as mean ± SE. Data were calculated using SPSS 16.0 (SPSS Inc., Chicago, IL) software package.
Results
Design, construction and analysis of recombinant plasmid expressing multiple gene products
pshS1NH encodes the three genes of interest, SOCS1-shRNA, NY-ESO-1-MAGE3 (HLA-A2*0201) fusion antigen and secretory HMGB1. The RNAi sequence was inserted into the plasmid vector as the form of shRNA under the control of U6 promoter to silence SOCS1. The tumor-associated antigens, NY-ESO-1-MAGE3 (HLA-A2*0201) and HMGB1, were transcripted under the control of CMV promoter, and the IRES between the two genes allows independent expression of NY-ESO-1-MAGE3 (HLA-A2*0201) and HMGB1. Importantly, the plasmid-encoded HMGB1 was engineered to be secreted, as we added a secretion signal sequence in front of HMGB1 ORF (Fig. 1). Thus, we constructed a plasmid encoding three different genes with individual functions. Silencing the attenuator, SOCS1, and enhancing the TLRs could activate the DCs in a synergistic effect and represents a form of potent DC vaccine against tumors expressing targeted tumor-associated antigens.
Fig. 1.
Schematic representation of pshS1NH
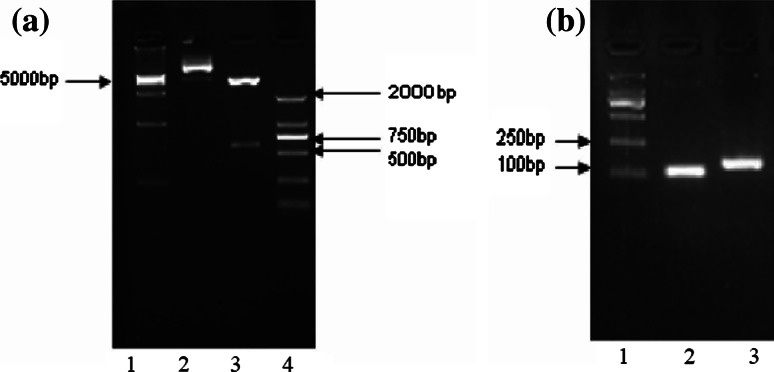
Due to the complexity of constructing the triple-gene plasmid, which involved several steps as described in the “Materials and methods”, we verified the sequences of the plasmids we constructed by DNA sequencing (date not shown). We got two fragments after double enzyme digestion with expected lengths (Fig. 2a). In addition, two pairs of primers were used to amplify the NY-ESO-1 and HMGB1 from the pshS1NH template, respectively. The results showed that the fragments of interest were the same as expected (Fig. 2b). As such, we confirmed that the pshS1NH we constructed indeed carried the three genes of interest.
Fig. 2.
Verification of the structure of pshS1NH. a Identification by restriction enzyme digestion. Lane 1 DL15000 marker; lane 2 pshS1NH without double enzyme digestion (6,191 bp); lane 3 pshS1NH with double enzyme digestion by BglII and EcoRI (576 bp and 5,614 bp); lane 4 DL2000 marker. b Amplification of NY-ESO-1 and HMGB1 from pshS1NH. Lane 1 DL2000 marker; lane 2 NY-ESO-1 (113 bp); lane 3 HMGB1 (150 bp). All of the fragments exhibited expected sizes
The expression of transgenes in DCs
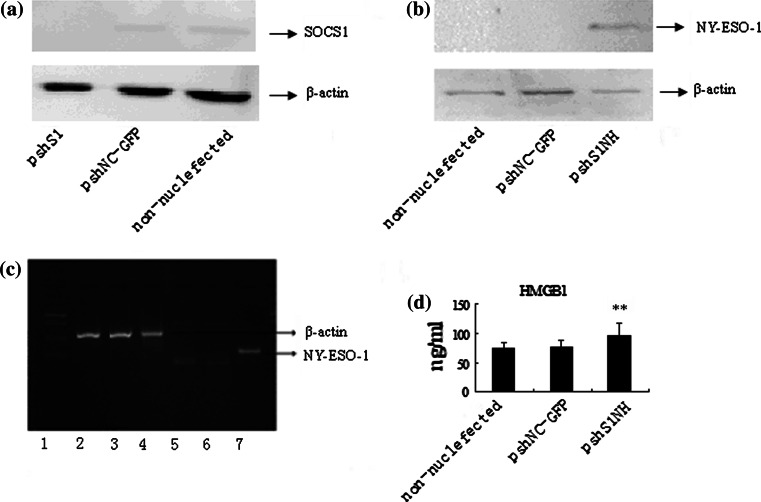
The plasmid we constructed needs to be efficiently transfected into DCs to display a specific function. Plasmid pshNC-GFP was efficiently nucleofected into PBMC-derived DCs ex vivo in the presence of GM-CSF and IL-4 by Amaxa Nucleofector with a transfection efficiency of approximately 52%. Compared to that in pshNC-GFP–DCs that cannot downregulate SOCS1, the level of SOCS1 mRNA in the total pshS1-DCs population was specifically decreased by approximately 80% as indicated by real-time PCR, consistent with the result indicated by Western blot (Fig. 3a). NY-ESO-1 and MAGE3 (HLA-A2*0201) genes were cloned together as a fusion protein, in which only a 9-amino acid peptide from MAGE3 (HLA-A2*0201 epitope) was retained, making the epitope impossible to be detected by PCR. Therefore, we verified the expression of NY-ESO-1 to confirm the expression of the fusion protein. PCR and Western blot were used to verify the expression of NY-ESO-1 in pshS1NH–DCs at the mRNA and protein levels, respectively (Fig. 3b, c). HMGB1 constitutively expressed in cell is mainly present in nucleus. In contrast, the additional amount of HMGB1 was designed to be secreted to the extracellular environment after nucleofection with pshS1NH. Indeed, we observed (by ELISA analyses) a significant increase in HMGB1 protein secreted from the pshS1NH–DCs, which produced 96 ng/ml of HMGB1 protein, compared to the low secretion level by pshNC-GFP–DCs (75 ng/ml). These data illustrated that pshS1NH increased the secretion of HMGB1 protein compared to the pshNC-GFP vector control (Fig. 3d). Thus, all transgenes are expressed and functionally active in nucleofected DCs. SOCS1-siRNA can efficiently downregulate the expression of SOCS1, and HMGB1 could indeed secrete to outside of the cell under the signal peptide-directed transportation. The fusion antigen was also efficiently express in pshS1NH–DCs.
Fig. 3.
The expression of transgenes in DCs. a Western blot to verify the downregulation of SOCS1 in pshS1-DCs. SOCS1 could not be detected in pshS1NH–DCs. b Western blot confirmation of NY-ESO-1 in pshS1NH–DCs. c PCR confirmation of the presence of NY-ESO-1 in pshS1NH–DCs. 1 DL2000 marker; 2, 5 non-nucleofected-DCs; 3, 6 pNC-GFP-DCs; 4, 7 pshS1NH–DCs. It was obviously that NY-ESO-1 was only expressed in pshS1NH–DCs. d Secretion of HMGB1 in the supernatants. All transgenes are expressed as expected and exhibited their specific functions in DCs
The phenotype of DCs after gene modification
The co-stimulatory molecules on the surface of DCs were analyzed by FACS. Compared to non-nucleofected DCs, nucleofected DCs expressed slightly lower level of CD80 and CD83. Meanwhile, CD80 and CD83 on pshS1NH–DC were slightly higher than that on pshNC-GFP–DCs, but there was no significant difference. HLA-DR, however, was comparable in any group. The results are consistent with the prior studies related to the nucleofection technique [16–18] (Fig. 4).
Fig. 4.
Phenotypic analysis of DCs. At 24 h after nucleofection, DCs were harvested and stained with mAbs against DCs surface markers or appropriate isotype control antibody as described in “Materials and methods”. Percentage of positive DCs is indicated. One representative experiment of five is shown
The Th1 cytokines in the pshS1NH–DC culture media were dramatically higher than those in control media
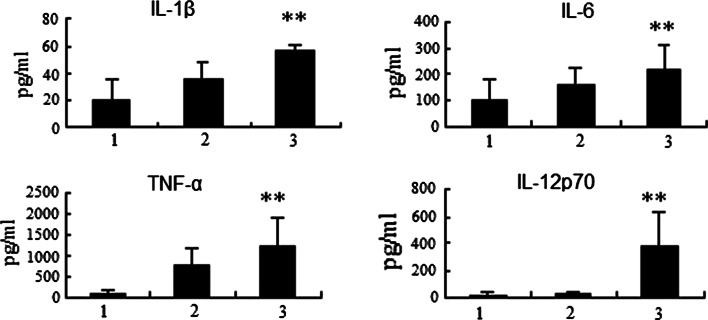
ELISA kits were used to detect the secretion of IL-1β, IL-6, IL-12p70 and TNF-α. Compared to non-nucleofected DC, nucleofected DC secreted higher levels of all tested cytokines, which indicated the nonspecific activation of DCs by nucleofection. However, the pshS1NH–DCs secreted significantly higher levels of IL-1β, IL-6, TNF-α and IL-12p70, compared to pshNC-GFP–DCs (Fig. 5). All these cytokines are known as Th1 cytokines, especially IL-12, which plays a critical role in differentiating T cells to cellular immunity-dominant state.
Fig. 5.
Th1 cytokines secreted by pshS1NH–DCs were higher than those secreted by non-nucleofected DCs and pNC-GFP-DCs. 1 non-nucleofected DCs; 2 pNC-GFP-DCs; 3 pshS1NH–DCs. At 48 h after nucleofection, supernatants were collected for TNF-α, IL-6, IL-1β and IL-12p70 ELISA analyses. The pshS1NH–DCs secreted significantly higher level of Th1 cytokines than the pNC-GFP-DCs and non-nucleofected DCs (P < 0.05). Data shown are the means ± SEM and representative of six independent experiments. The significant differences between pshS1NH–DCs and control were determined by ANOVA
pshS1NH–DCs were more potent in stimulating Th1 cell responses
We assessed by ELISA the Th1/Th2 cytokine balance in media of syngeneic naive T cells co-cultured with differentially treated DCs for 7 days at a responder/stimulator ratio of 10 in the presence of 50 U/ml recombinant human IL-2. pshS1NH–DCs could markedly enhance Th1-skewing IFN-γ secretion from syngeneic naive T cells when compared to non-nucleofected DCs, whereas only a slight increase in IFN-γ levels was observed during co-cultivation with pshNC-GFP–DCs. On the other hand, secretion of the Th2 cytokine IL-4 was comparable in all syngeneic T cells, either cultured alone or stimulated by pshS1NH–DCs or pshNC-GFP–DCs (Fig. 6). These results suggested that psh1NH–DCs more efficiently differentiated sensitized T cells at the Th1-biasing state (the cellular immunity-dominant state), a process required for the induction of effective tumor immunity.
Fig. 6.
The Th1/Th2 cytokine balance in media of syngeneic naive T cells co-cultured with modified DCs. 1 T cells alone; 2 T cells co-cultured with pNC-GFP-DC; 3 T cells co-cultured with pshS1NH–DCs. The IFN-γ secreted by T cells co-cultured with pshS1NH–DCs was significantly higher than that in control groups (P < 0.05). However, there was no significant difference in IL-4 levels between the two groups (P > 0.05). Data shown are the means ± SEM and representative of six independent experiments. The significant differences between T cells co-cultured with pshS1NH–DCs and T cells alone and T cells co-cultured with pNC-GFP-DC were determined by ANOVA
Discussion
Here, we introduced an absolutely new DC vaccine against cancers that sheds new light on optimizing antitumor vaccines. Based on the previous reports that silencing SOCS1 in DCs could enhance the antigen presentation capacity of DCs [7, 19–21], we added another stimulus, HMGB1, to activate DCs in a synergistic effect, in order to further improve the antigen presentation capacity of DCs [5, 22].
SOCS1 shares similar characteristics with A20, both of which are the terminator of TLRs activation in DCs. Karine Breckpot [8] had reported that silencing A20 in PBMC-derived DCs markedly increased the efficiency of double-strand RNA-activated DCs as an anticancer vaccine. Consistent with this report, attenuation of SOCS1 and activation of TLRs by HMGB1 also had synergistic effect in activating DCs as an anticancer vaccine. Here, we proposed to modify DCs in one step to achieve the purpose based on the following considerations. First, due to the feedback regulation of SOCS1 by TLR signaling pathway, it was expected that only when silencing SOCS1 and activating TLRs are achieved simultaneously in the same cell that presents the TAAs on its surface, the DCs can exhibit the most potent antigen presentation capacity. It was, therefore, more efficient to have all transgenes expressed by the same plasmid. Another reason for the development and use of such a multigene construct involves the conservation of energy and resources, which must be considered in translational efforts to develop practical clinical agents. For example, the development of pshS1, pH vector and purified antigens, as opposed to the pshS1NH vector, would result in triple or even more efforts in terms of GMP production of agents and quality control issues. Although they may not be consider "scientific issues," they are often the bottleneck or even the barrier between good scientific and intellectual efforts and clinical benefits in patients with terminal diseases [23].
pshS1NH was constructed by linking the subcloned U6-shSOCS1 with CMV-NY-ESO-1-MAGE3 (epitope)-IRES-Sig-HMGB1, which was then inserted into pIRES2-eGFP. CMV in pshS1NH could initiate the transcription of the inserted genes. And, the IRES, which recruited the ribosomes without using the standard protein translation initiation mechanism, can initiate the translation of HMGB1, assuring the expression of both NY-ESO-1-MAGE3 (epitope) and HMGB1. If simply replace EGFP with HMGB1, HMGB1 can only be expressed in cytoplasm, which will limit its activity. Therefore, we added the insulin signal peptide in front of the HMGB1 coding region, forcing HMGB1 to be secreted out of the transfected cell. Plasmid pshS1 containing DNA template for the synthesis of siRNA-SOCS1 under the control of U6 promoter was constructed in our experiment, which was effective in downregulating the expression of SOCS1 through sequence-specific post-transcriptional gene silencing. The silencing efficiency was almost 80% as indicated by real-time PCR. However, the RNAi sequence in the pshS1NH did not show comparable efficiency as pshS1, and only inhibited gene expression by 40%. We speculated that the activation of TLRs by HMGB1 upregulated the expression of SOCS1, which abated the silencing efficiency and indirectly reflected the expected expression of the plasmid in DCs, as indicated by real-time PCR [8, 10, 11]. The fusion antigen was verified by PCR and Western blot, which was consistent with the expected results. Meanwhile, HMGB1 level in the experimental group of media that activated DCs by auto- or paracrine secretion was higher than that in control groups as indicated by ELISA.
pshS1NH was designed to modify DCs, aiming to activate DCs in general. The co-stimulatory molecules on the surface of DCs were slightly downregulated after gene modification. The result is consistent with the prior studies, which have shown that nucleofection can impair the expression of co-stimulatory molecules on DCs, though it is effective in transfection. Cytokines secreted by DCs are critical markers for evaluating the immune state of DCs. Here, levels of IL-1β, IL-12p70, TNF-α and IL-6 in pshS1NH–DCs culture media were all dramatically higher than those in the control groups, indicating the activation of DCs after modification. This was most significant with IL-12, a key factor that drives the immune balance toward a Th1 response and promotes a switch from an established Th2 to a Th1 response [24]. In addition, Evel-Kabler et al. [21] had proven that SOCS1 restricted the ability of DCs to induce antitumor immunity by regulating the production of IL-12. Thus, pshS1NH–DCs may induce stronger T-cell responses in vitro and may break self-tolerance in vivo. Consistent with our expectation, the T cells co-cultured with pshS1NH–DCs secreted extremely high level of IFN-γ compared to that secreted by T cells co-cultured with control DCs or cultured alone. Meanwhile, there was no significant difference in IL-4 secretion among the groups. These data reflected the shift of immune balance to cellular immunity by pshS1NH–DCs, a process required for destroying tumor cells. In addition, both the upregulation of co-stimulatory molecules and the secretion of Th1 cytokines are indicative of the activation of DCs. However, the Th1 cytokines secretion may be more significant than co-stimulatory molecules in priming of T cells in our study, suggesting that it is not proper to justify the activation state of DCs only depending on the level of co-stimulatory molecules.
IFN-γ is mainly secreted by CD4+T cells and plays a critical role in licensing CD8+T cells and generating memory CTLs responses [25–27]. To test the overall function of pshS1NH–DCs in regulating T cells, we did not separate CD4+ or CD8+T cells from the lymphocyte cells to better mimic the microenvironment in vivo. Vaccines against single antigen may induce the antigen drift of tumors or have no efficiency resulting from the antigen drift after other therapy [23]. Vaccines expressing two different antigens may therefore have a broader spectrum of antitumor activity by avoiding antigen drift. MAGE3 is expressed on most tumors, and MAGE3 (HLA-A2*0201) is the most common epitope in Asians. We therefore selected MAGE3 for the plasmid constructed herein. As indicated by IHC, NY-ESO-1 is overexpressed in 82% of neuroblastoma [28], 80% of Synovial Sarcoma [29], 46% of melanoma [30], as well as in epithelial ovarian cancer and esophageal cancer, etc. In order to increase the epitopes presented by DCs, the full-length NY-ESO-1 was constructed in our vaccine to ensure the activity in non-HLA-A2 patients.
Encouraged by the positive results of DCs modified by plasmid, we have made a plan for constructing viral vector as a future direction. Overall, in this study, we explored the strategy to silence SOCS1 and activate TLRs simultaneously and confirmed the enhanced antigen presentation capacity of DCs. This novel approach provides a new tool for anticancer immune therapy for future clinical studies.
Acknowledgments
This work was supported by Tianjin Natural Science Foundation of China (09JCZDJC20400) and Innovation Founding for Graduate of Tianjin Medical University (2010GSI18).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Madan RA, Gulley JL. Sipuleucel-T: harbinger of a new age of therapeutics for prostate cancer. Expert Rev Vaccines. 2011;10:141–150. doi: 10.1586/erv.10.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saenz R, Souza CS, Huang CT, Larsson M, Esener S, Messmer D. HMGB1-derived peptide acts as adjuvant inducing immune responses to peptide and protein antigen. Vaccine. 2010;28:7556–7562. doi: 10.1016/j.vaccine.2010.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang D, Chen Q, Yang H, Tracey KJ, Bustin M, Oppenheim JJ. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol. 2007;81:59–66. doi: 10.1189/jlb.0306180. [DOI] [PubMed] [Google Scholar]
- 4.Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, Giazzon M, Dumitriu IE, Muller S, Iannacone M, Traversari C, et al. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 2004;5:825–830. doi: 10.1038/sj.embor.7400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham BS. New approaches to vaccine adjuvants: inhibiting the inhibitor. PLoS Med. 2006;3:e57. doi: 10.1371/journal.pmed.0030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi T, Yoshimura A. Keeping DCs awake by putting SOCS1 to sleep. Trends Immunol. 2005;26:177–179. doi: 10.1016/j.it.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Gilboa E. Knocking the SOCS1 off dendritic cells. Nat Biotechnol. 2004;22:1521–1522. doi: 10.1038/nbt1204-1521. [DOI] [PubMed] [Google Scholar]
- 8.Breckpot K, Aerts-Toegaert C, Heirman C, Peeters U, Beyaert R, Aerts JL, Thielemans K. Attenuated expression of A20 markedly increases the efficacy of double-stranded RNA-activated dendritic cells as an anti-cancer vaccine. J Immunol. 2009;182:860–870. doi: 10.4049/jimmunol.182.2.860. [DOI] [PubMed] [Google Scholar]
- 9.Fujimoto M, Naka T (2010) SOCS1, a negative regulator of cytokine signals and TLR responses, in Human Liver Diseases. Gastroenterol Res Pract 2010:470468. doi:10.1155/2010/470468 [DOI] [PMC free article] [PubMed]
- 10.Nakagawa R, Naka T, Tsutsui H, Fujimoto M, Kimura A, Abe T, Seki E, Sato S, Takeuchi O, Takeda K, et al. SOCS-1 participates in negative regulation of LPS responses. Immunity. 2002;17:677–687. doi: 10.1016/S1074-7613(02)00449-1. [DOI] [PubMed] [Google Scholar]
- 11.Mansell A, Smith R, Doyle SL, Gray P, Fenner JE, Crack PJ, Nicholson SE, Hilton DJ, O’Neill LA, Hertzog PJ. Suppressor of cytokine signaling 1 negatively regulates toll-like receptor signaling by mediating mal degradation. Nat Immunol. 2006;7:148–155. doi: 10.1038/ni1299. [DOI] [PubMed] [Google Scholar]
- 12.Ryo A, Suizu F, Yoshida Y, Perrem K, Liou YC, Wulf G, Rottapel R, Yamaoka S, Lu KP. Regulation of NF-kappaB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol Cell. 2003;12:1413–1426. doi: 10.1016/S1097-2765(03)00490-8. [DOI] [PubMed] [Google Scholar]
- 13.Kimura A, Naka T, Muta T, Takeuchi O, Akira S, Kawase I, Kishimoto T. Suppressor of cytokine signaling-1 selectively inhibits LPS-induced IL-6 production by regulating JAK-STAT. Proc Natl Acad Sci USA. 2005;102:17089–17094. doi: 10.1073/pnas.0508517102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Li H, Yu JP, Wang SE, Ren XB (2011) Role of SOCS1 in tumor progression and therapeutic application. Int J Cancer [Epub ahead of print] [DOI] [PubMed]
- 15.Zimmerer JM, Lesinski GB, Kondadasula SV, Karpa VI, Lehman A, Raychaudhury A, Becknell B, Carson WR. IFN-alpha-induced signal transduction, gene expression, and antitumor activity of immune effector cells are negatively regulated by suppressor of cytokine signaling proteins. J Immunol. 2007;178:4832–4845. doi: 10.4049/jimmunol.178.8.4832. [DOI] [PubMed] [Google Scholar]
- 16.Lenz P, Bacot SM, Frazier-Jessen MR, Feldman GM. Nucleoporation of dendritic cells: efficient gene transfer by electroporation into human monocyte-derived dendritic cells. FEBS Lett. 2003;538:149–154. doi: 10.1016/S0014-5793(03)00169-8. [DOI] [PubMed] [Google Scholar]
- 17.Melhem NM, Gleason SM, Liu XD, Barratt-Boyes SM. High-level antigen expression and sustained antigen presentation in dendritic cells nucleofected with wild-type viral mRNA but not DNA. Clin Vaccine Immunol. 2008;15:1337–1344. doi: 10.1128/CVI.00154-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Artusio E, Hathaway B, Stanson J, Whiteside TL. Transfection of human monocyte-derived dendritic cells with native tumor DNA induces antigen-specific T-cell responses in vitro. Cancer Biol Ther. 2006;5:1624–1631. doi: 10.4161/cbt.5.12.3353. [DOI] [PubMed] [Google Scholar]
- 19.Shen L, Evel-Kabler K, Strube R, Chen SY. Silencing of SOCS1 enhances antigen presentation by dendritic cells and antigen-specific anti-tumor immunity. Nat Biotechnol. 2004;22:1546–1553. doi: 10.1038/nbt1035. [DOI] [PubMed] [Google Scholar]
- 20.Hong B, Ren W, Song XT, Evel-Kabler K, Chen SY, Huang XF. Human suppressor of cytokine signaling 1 controls immunostimulatory activity of monocyte-derived dendritic cells. Cancer Res. 2009;69:8076–8084. doi: 10.1158/0008-5472.CAN-09-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evel-Kabler K, Song XT, Aldrich M, Huang XF, Chen SY. SOCS1 restricts dendritic cells’ ability to break self tolerance and induce antitumor immunity by regulating IL-12 production and signaling. J Clin Invest. 2006;116:90–100. doi: 10.1172/JCI26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang D, Chen Q, Yang H, Tracey KJ, Bustin M, Oppenheim JJ. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol. 2007;81:59–66. doi: 10.1189/jlb.0306180. [DOI] [PubMed] [Google Scholar]
- 23.Tsang KY, Palena C, Yokokawa J, Arlen PM, Gulley JL, Mazzara GP, Gritz L, Yafal AG, Ogueta S, Greenhalgh P, et al. Analyses of recombinant vaccinia and fowlpox vaccine vectors expressing transgenes for two human tumor antigens and three human costimulatory molecules. Clin Cancer Res. 2005;11:1597–1607. doi: 10.1158/1078-0432.CCR-04-1609. [DOI] [PubMed] [Google Scholar]
- 24.Okada N, Iiyama S, Okada Y, Mizuguchi H, Hayakawa T, Nakagawa S, Mayumi T, Fujita T, Yamamoto A. Immunological properties and vaccine efficacy of murine dendritic cells simultaneously expressing melanoma-associated antigen and interleukin-12. Cancer Gene Ther. 2005;12:72–83. doi: 10.1038/sj.cgt.7700772. [DOI] [PubMed] [Google Scholar]
- 25.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 26.Daniel D, Chiu C, Giraudo E, Inoue M, Mizzen LA, Chu NR, Hanahan D. CD4+T cell-mediated antigen-specific immunotherapy in a mouse model of cervical cancer. Cancer Res. 2005;65:2018–2025. doi: 10.1158/0008-5472.CAN-04-3444. [DOI] [PubMed] [Google Scholar]
- 27.Wang RF. The role of MHC class II-restricted tumor antigens and CD4+T cells in antitumor immunity. Trends Immunol. 2001;22:269–276. doi: 10.1016/S1471-4906(01)01896-8. [DOI] [PubMed] [Google Scholar]
- 28.Rodolfo M, Luksch R, Stockert E, Chen YT, Collini P, Ranzani T, Lombardo C, Dalerba P, Rivoltini L, Arienti F, et al. Antigen-specific immunity in neuroblastoma patients: antibody and T-cell recognition of NY-ESO-1 tumor antigen. Cancer Res. 2003;63:6948–6955. [PubMed] [Google Scholar]
- 29.Jungbluth AA, Antonescu CR, Busam KJ, Iversen K, Kolb D, Coplan K, Chen YT, Stockert E, Ladanyi M, Old LJ. Monophasic and biphasic synovial sarcomas abundantly express cancer/testis antigen NY-ESO-1 but not MAGE-A1 or CT7. Int J Cancer. 2001;94:252–256. doi: 10.1002/ijc.1451. [DOI] [PubMed] [Google Scholar]
- 30.Barrow C, Browning J, MacGregor D, Davis ID, Sturrock S, Jungbluth AA, Cebon J. Tumor antigen expression in melanoma varies according to antigen and stage. Clin Cancer Res. 2006;12:764–771. doi: 10.1158/1078-0432.CCR-05-1544. [DOI] [PubMed] [Google Scholar]