Background: The Treponema denticola FhbB protein binds FH, a complement regulator.
Results: The structure of FhbB was solved, and its interaction with FH was further defined.
Conclusion: The structurally unique FhbB protein interacts with CCP7 of FH through electrostatic interactions.
Significance: The T. denticola/FH interaction may perturb complement regulation resulting in conditions that favor the development of periodontal disease.
Keywords: Complement, Complement System, Microbial Pathogenesis, Periodontal Disease, X-ray Crystallography, Factor H, FhbB, Treponemes
Abstract
Periodontitis is the most common disease of microbial etiology in humans. Periopathogen survival is dependent upon evasion of complement-mediated destruction. Treponema denticola, an important contributor to periodontitis, evades killing by the alternative complement cascade by binding factor H (FH) to its surface. Bound FH is rapidly cleaved by the T. denticola protease, dentilisin. In this report, the structure of the T. denticola FH-binding protein, FhbB, was solved to 1.7 Å resolution. FhbB possesses a unique fold that imparts high thermostability. The kinetics of the FH/FhbB interaction were assessed using surface plasmon resonance. A KD value in the micromolar range (low affinity) was demonstrated, and rapid off kinetics were observed. Site-directed mutagenesis and sucrose octasulfate competition assays collectively indicate that the negatively charged face of FhbB binds within FH complement control protein module 7. This study provides significant new insight into the molecular basis of FH/FhbB interaction and advances our understanding of the role that T. denticola plays in the development and progression of periodontal disease.
Introduction
Complement evasion is a critical aspect of the molecular pathogenesis of bacteria that cause periodontal disease (1), the most common infection of middle-aged adults (2). The etiology of periodontal disease can be traced to several host-determined susceptibility factors and the population dynamics of the oral microflora community (3, 4). More than 70 species of anaerobic spirochetes (Treponemes) are found in the oral cavity. In the healthy subgingival crevice, they account for ∼1% of the total bacteria (5). With the progression of periodontitis, the abundance of oral treponemes increases dramatically and can reach 40% of the total bacterial population (6, 7). Disease severity correlates specifically with the outgrowth of Treponema denticola and other bacterial species of the red microbial complex (6, 8).
To survive in the subgingival crevice, bacteria must be able to evade immune-mediated destruction. Crevicular fluid and periodontal lesion exudate are rich in active complement (9–12). Pathogens or other “non-self” surfaces trigger a proteolytic cascade that results in the amplification of complement activation. Complement activity must be carefully regulated or significant damage to host cells and tissues can occur. Factor H (FH),2 a 155-kDa glycoprotein (400–800 μg ml−1 serum), regulates complement activation in serum and on host cell surfaces through several mechanisms (13). FH serves as a cofactor for the factor I-mediated cleavage of C3b, competes with factor B for binding to C3b (thereby preventing C3 convertase formation), and accelerates decay of preformed C3 convertase complex (14, 15). FH also contributes to the regulation of complement through its interaction with C-reactive protein (CRP), a positive regulator of the classical pathway that is negatively regulated by FH (16). FH consists of 20 imperfect, ∼60-amino acid repeat units, referred to as complement control protein (CCP) domains. Specific CCPs have been demonstrated to mediate intermolecular interactions and regulatory functions (17, 18). CCP1–4 are involved in fluid phase complement regulation, whereas CCP19–20 interact with cell surfaces facilitating self-discrimination. As detailed below, CCP6–8 and CCP19–20 have also been demonstrated to interact with surface proteins produced by numerous pathogens. Heritable polymorphisms in FH that disrupt its complement regulatory activity are the underlying basis for several important human diseases, including age-related macular degenerative disease (the most common cause of blindness in the elderly), atypical hemolytic uremic syndrome, and dense deposit disease (19).
T. denticola binds FH to its surface (20) via the FhbB protein (TDE0108). FhbB is the smallest (11.4 kDa) bacterially produced FH-binding protein identified to date (20, 21). FhbB binds and positions FH on the cell surface thus allowing FH cleavage by the T. denticola protease, dentilisin (22–24). It is our hypothesis that in vivo, FH cleavage leads to its depletion in the subgingival crevice resulting in local dysregulation of complement and conditions that favor the development and progression of periodontal disease.
Most characterized bacterial FH-binding proteins bind within CCP6–8 or CCP19–20 (17). Molecular structures have been determined for only two microbially produced FH-binding proteins, Borrelia burgdorferi CspA and Neisseria meningitidis fHbp (25, 26). These proteins do not share sequence or structural homology. The molecular or structural “signature” for bacterial recognition of FH remains unknown. The determination of additional FH-binding protein structures and the elucidation of the molecular basis of their interaction with FH will have significant implications for the development of preventive and therapeutic strategies for both infectious and inheritable diseases. Here, we report the atomic structure of the FhbB protein of the periodontal pathogen, T. denticola, and further define the molecular basis of its interaction with FH. FhbB possesses a previously undescribed protein fold that imparts high stability to the protein. Kinetic analyses of the FH/FhbB interaction revealed a KD value in the micromolar range with rapid on-off-rates. Site-directed mutagenesis led to the identification of FhbB and FH residues required for the FH/FhbB interaction. The binding site for FhbB on FH was localized to CCP7 and found to overlap with the binding site for glycosaminoglycans (GAG) and the GAG analog, sucrose octasulfate (SOS). These analyses represent a significant advancement in our understanding of the molecular interactions between FH and microbially produced FH-binding proteins (specifically those that bind to CCP6–8). In addition, the data allow for the development of a refined hypothesis regarding the biological role of FH binding and cleavage in T. denticola biology and the pathogenesis of periodontal disease.
EXPERIMENTAL PROCEDURES
Generation of Recombinant Proteins
Recombinant FhbB was generated as described previously (27). Site-directed mutations were introduced into the gene using mutagenic primers. All FhbB proteins were purified by immobilized metal affinity chromatography (HisTrap, GE Healthcare) according to the manufacturer's protocol. Proteins consisting of FH CCP6–8 and CCP19–20 were generated by amplification of the corresponding segment from human FH cDNA (Source BioScience imaGenes). The amplicons were annealed with pET46 Ek/LIC (Novagen). The proteins were expressed in Escherichia coli NovaBlue(DE3) cells overnight. The FH-derived proteins were purified from inclusion bodies by resuspension of the insoluble cell lysate in resolubilization buffer (0.1 m NaH2PO4, 0.5 m NaCl, 6 m guanidine hydrochloride, 5 mm β-mercaptoethanol, pH 8.0) for 1 h at room temperature. The proteins were purified by immobilized metal affinity chromatography (HisBind Resin, Novagen) and refolded using standard approaches.
Structure Determination, Data Collection, and Processing
Conditions for crystallization were as described previously (27). Native and selenomethionine single wavelength anomalous diffraction datasets were collected on an ADSC Q315 CCD x-ray detector at the X25 beamline of the National Synchrotron Light Source, Brookhaven National Laboratory. The diffraction images were processed using the HKL2000 software package (28). The data processing statistics are summarized in Table 1.
TABLE 1.
Data collection, phasing, and refinement statistics
| Nativea | Selenomethionine | |
|---|---|---|
| Data collection | ||
| Space group | P43212 | P43212 |
| Cell dimensions | ||
| a, b, and c | 46.98, 46.98, 168.95 Å | 46.99, 46.99, 168.96 Å |
| α, β, and γ | 90° | 90° |
| Wavelength | 1.5 | 0.9793 |
| Resolution | 40 to 1.7 Å | 50 to 1.77 |
| Rmerge | 0.06b (0.41) | 0.07 (0.28) |
| I/σI | 44 (3) | 20.8 (7.4) |
| Completeness | 99.6% (100%) | 99.4% (99.9%) |
| Redundancy | 9.8 (7%) | 9.3 (8.7%) |
| Refinement | ||
| Resolution | 36 to 1.7 Å | 45.2 to 1.77 Å |
| No. of reflections | 21,797 | 19,035 |
| Rwork/Rfree | 21/23.6 | 18.9/20.6 |
| No. of atoms | ||
| Protein | 1,222 | 1,252 |
| Ligand/ion | 6 | 12 |
| Water | 117 | 152 |
| B-factors | ||
| Protein | 21.4 | 23.1 |
| Ligand/ion | 19.7 | 31.3 |
| Water | 28.69 | 33.2 |
| Root mean square deviation | ||
| Bond lengths | 0.007 Å | 0.006 Å |
| Bond angles | 0.984° | 1.038° |
a A single crystal was used to collect each dataset.
b Values in parentheses are for highest resolution shell: native, 1.73 to 1.7; selenomethionine, 1.83 to 1.77.
Structure Determination and Refinement
Phases were determined from the 1.77-Å single wavelength anomalous diffraction dataset using the AutoSol module of PHENIX (29). The resultant model was further manually built in COOT (30, 31) and refined in PHENIX (29). The native structure was solved by the molecular replacement method using the PHASER (32) program as part of the CCP4 Software Suite (33) and the selenomethionine model as the search probe. Refinement statistics are given in Table 1. Models were evaluated by MolProbity (34, 35). Structural figures were made using PyMOL (Schrödinger Scientific). Electrostatic surface representations of FhbB were generated with APBS plugin in PyMOL. Structural similarity searches were completed using DALI (36).
Circular Dichroism (CD)
Recombinant wild type FhbB and lysozyme were used for thermal denaturation and renaturation studies. Proteins were extensively dialyzed into 10 mm NaH2PO4, 50 mm NaCl, pH 7.4, buffer and diluted to 20 mg ml−1 for analysis of CD spectra. Spectra (190–300 nm) were acquired on a Jasco J-720 spectropolarimeter at 4 °C in a 10-mm quartz cuvette. Replicate scans (5) were collected, averaged, and base-line subtracted. α-Helical content was monitored at 222 nm as the temperature was increased from 4 to 90 °C and returned to 4 °C (rate of 1 °C per minute). CD spectra were acquired prior to heating the protein and after cooling the protein.
Factor H Binding Overlay Assays
Overlay assays were performed as described previously (37). In brief, recombinant proteins were fractionated by SDS-PAGE, transferred to PVDF membrane by electroblotting, and the membranes incubated with purified FH (Complement Tech) or recombinant FH fragments consisting of CCP domains 6–8 or 19–20 (10 μg ml−1 in phosphate-buffered saline with 0.2% Tween 20 (PBST)). Binding was detected with goat anti-human FH (1:800 dilution; Complement Tech) with rabbit anti-goat IgG serving as the secondary (1:40,000 dilution; Calbiochem). Signal was detected by chemiluminescence with the Pierce ECL Western blotting substrate (Thermo).
FH-binding ELISAs
Recombinant proteins were immobilized in wells of ELISA plates (in triplicate; 1 μg per well in 100 mm NaHCO3, pH 9.6, overnight at 4 °C). Nonspecific binding was blocked for 1 h with 5% nonfat dry milk in PBST (PBSTM). FH (10 μg ml−1 in PBST) was added for 1 h, and the wells washed three times with PBST. Goat anti-human FH (1:800 in PBSTM; Complement Tech) was added for 1 h, and the plates were washed three times. Rabbit anti-goat IgG (1:20,000 in PBSTM; Calbiochem) was added for 1 h, washed, and signal was detected using 1-step ultra 3,3′,5,5′-tetramethylbenzidine substrate (Bio-Rad) as detailed by the supplier at 450 nm. The data were averaged across three plates. To determine whether SOS can inhibit FH binding, 1 μg of FhbB was immobilized in each well and non-specific binding was blocked with PBSTM for 1 h. FH (5 μg ml−1) was incubated with increasing concentrations of SOS (0–50 mm) in PBST for 1 h with constant gentle agitation. FhbB was overlaid with the FH/SOS solution for 1 h at RT. After 1 h, the FH was removed and binding was detected as described above. Data were averaged and normalized to FH binding with no SOS added.
Surface Plasmon Resonance
Surface plasmon resonance analyses were performed on a Biacore T100 and data evaluated using BIAEvaluation version 1.1 (Biacore, Uppsala, Sweden). Recombinant FhbB proteins (ligand) were immobilized to NiCl2-charged NTA chips via their N-terminal His6 tag. Low levels of immobilized protein (∼40 response units) were used to eliminate protein leaching and bulk contributions. Increasing concentrations of FH were passed over the chips in the fluid phase. Kinetic analyses were performed in NTA running buffer (0.01 m HEPES, 0.15 m NaCl, 50 μm EDTA, and 0.05% Tween 20 adjusted to pH 7.4). Nickel was immobilized to the sensor chip with NTA running buffer supplemented with 0.5 mm NiCl2 for 60 s at a rate of 10 μl/min. FH was extensively dialyzed in running buffer and diluted from 2.5 to 0.3125 μm. FH was passed over the immobilized FhbB protein for 30 s at 60 μl/min and allowed 600 s for dissociation. Flow cells were regenerated with running buffer supplemented with 0.35 m EDTA at pH 8.3 for 180 s at 30 μl/min and washed with running buffer under the same conditions. Data were fit to a Langmuir 1:1 binding model and averaged from three replicate experiments.
Analytical Ultracentrifugation
Sedimentation velocity experiments were performed in an XL-I analytical ultracentrifuge (Beckman Coulter), using adsorption optics at 280 nm and Rayleigh interference optics. Recombinant FhbB (3–821 μm in 10 mm HEPES, 100 mm NaCl, pH 7.0) was spun at 50,000 rpm (20 °C) in a An60Ti rotor (Beckman Coulter). Absorbance and interference scans were collected every 5 min for a 6.5 h. Distributions of sedimenting particles (c(s)) were calculated using SEDFIT (version 12.1c); isotherms were calculated for the apparent monomer-homodimer distribution by integrating the area under these distribution curves (38). SEDPHAT (version 8.2) was used to fit the isotherm data to the monomer-homodimer model (39).
RESULTS
FhbB Crystal Structure at 1.7 Å Resolution
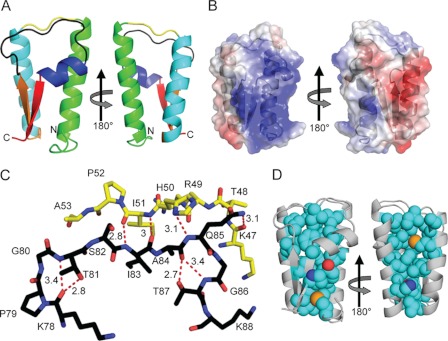
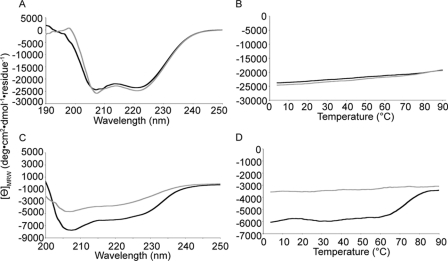
To determine the structure of the T. denticola FhbB protein (GenBankTM accession number EF032156), recombinant protein was generated. The leader peptide (residues 1–23) was replaced with a 1.7-kDa hexahistidine tag and enterokinase cleavage site. The crystallization conditions have been previously reported (27). Primary phasing was obtained by selenomethionyl single wavelength anomalous diffraction using a single derivatized crystal. The native structure was solved at 1.7 Å resolution by molecular replacement using the selenomethionine model as the search probe. Two molecules were present in the asymmetric unit with a root mean square deviation of 1.47 Å. Exclusion of the N-terminal six residues of FhbB, which exist in two conformations, resulted in a root mean square deviation of 0.347 Å. The structural model for residues 24–101 is in good agreement with the experimental data and conforms to expected geometric parameters (Table 1). The FhbB structure is highly ordered consisting of an α-α-β-α-β fold in which α1 and α2 form one face of the molecule and β1-α3-β2 form the opposing face (Fig. 1, A and B). The α1-α2 and β1-α3-β2 faces of FhbB are connected by turns 1 and 2 that span residues 47–52 and 77–88, respectively. The atomic interactions that occur between these turns are indicated in Fig. 1C. The core of the protein is largely defined by hydrophobic interactions (Fig. 1D) with a single hydrogen bond between residues His-34 and Tyr-72. Several C-terminal residues of FhbB are constituents of the core of the protein. DALI structural alignment analyses confirmed that the protein fold of FhbB is unique. The Thermoplasma acidophilum trehalose-6-phosphate phosphatase-related protein, a functionally unrelated protein, shared the highest homology (Z score = 4.2). To assess the stability of this unique fold, thermal denaturation-renaturation circular dichroism studies were performed. The CD spectrum of FhbB was measured at 4 °C before and after heating to 90 °C (Fig. 2A). Lysozyme served as a control (Fig. 2C). α-Helical content was measured at 222 nm over a temperature range of 4–90 °C (Fig. 2, B and D). The helical content of FhbB decreased only slightly at 90 °C and was fully restored upon cooling. In contrast, a large and irreversible loss of helical content was observed for lysozyme. These analyses demonstrate that FhbB is a highly thermostable protein.
FIGURE 1.
Structure and physiochemical properties of the T. denticola FH-binding protein, FhbB. A, FhbB (ribbon diagram) is color-coded as follows: α-helix 1, green; turn 1, yellow; α-helix 2, light blue; β-strand 1 (orange); turn 2, black; α-helix 3, dark blue; and β-strand 2, red. The N and C termini of the protein are indicated. The right image is rotated 180° around the y axis. B, electrostatic surface map of FhbB. The left and right images are oriented as in A. Positive and negative charges are indicated by blue and red, respectively. C, hydrogen bonding salt bridge network between turns 1 and 2 is depicted by dashed red lines with the distance indicated in angstroms. Residue numbering is based on the full-length FhbB sequence of T. denticola strain 35405. D, hydrophobic core of FhbB. The polypeptide backbone is indicated in gray, and the orientation of the protein is as in B. The atomic composition of the core is indicated as follows: carbon (teal), sulfur (orange), oxygen (red), and nitrogen (blue).
FIGURE 2.
FhbB is a highly thermostable protein. The thermostability of FhbB was assessed by circular dichroism. CD spectrum of FhbB (A) and lysozyme (C) was measured at 4 °C prior to heating and compared with the spectrum after heating the protein to 90 °C and returning to 4 °C (black, spectra prior to heating the protein; gray, spectra after heating and cooling the protein). α-Helical content of FhbB (B) and lysozyme (D) was monitored throughout the heating and cooling by measuring the CD at 222 nm (black, heating from 4 to 90 °C; gray, cooling from 90 to 4 °C).
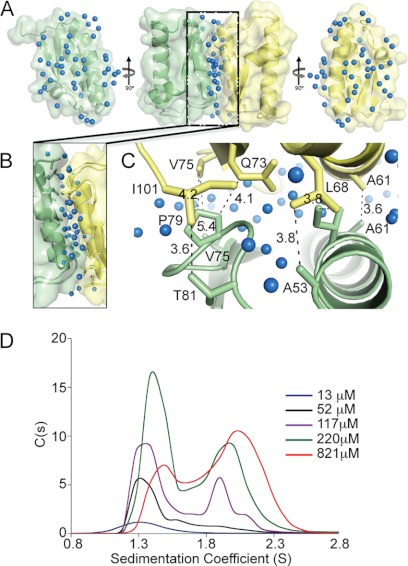
FhbB Forms a Weak Dimer
The FhbB asymmetric unit contains two subunits with an interface occurring along the α2-helices in an anti-parallel alignment (Fig. 3A). The dimer contains an extensive water network at its dimeric interface with no direct hydrogen bonding between the subunits (Fig. 3, A–C). To determine the subunit affinity, analytical ultracentrifugation was performed (Fig. 3D). As FhbB concentration was increased, the sedimentation coefficient shifted from ∼1.31 S to ∼2.15 S (monomer to dimer transition). The data conform well to a monomer-homodimer model (root mean square deviation of 0.005 fringes). The dissociation constant for the dimer was calculated to be 217 ± 40.5 μm indicative of a relatively weak interaction.
FIGURE 3.
Analysis of FhbB dimerization. A, center figure of A depicts the dimeric FhbB asymmetric unit with the water molecules at the interface indicated by blue spheres. To the left and right, the component monomers are shown, indicated in light green and yellow, after rotation 90° to allow for visualization of the buried interface and hydration patterns. B, interface is enlarged with interface residues indicated. C provides a top down view of the interactions at the interface. Residues involved in hydrophobic interactions are indicated with black dashed lines (distances in Å). D, sedimentation coefficients of recombinant FhbB over a concentration range of 13–821 μm were determined (monomer, 1.31 S; dimer, 2.2 S).
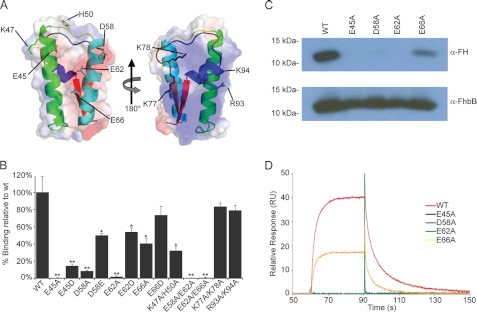
Mutagenesis Studies of the FhbB/FH Binding Interface
To identify FhbB residues involved in FH binding, solvent-accessible charged amino acids were substituted to yield recombinant proteins with single or double amino acid substitutions. The spatial placement of the targeted residues is indicated in Fig. 4A. Circular dichroism analyses revealed that the substitutions did not significantly alter secondary structure as no change in percent α-helical content was observed (data not shown). FH binding to the substituted proteins was assessed using ELISA-based and membrane overlay assay approaches (Fig. 4, B and C). Alanine substitution of Glu-45, Lys-47, His-50, Asp-58, Glu-62, and Glu-66 abolished or attenuated FH binding, whereas substitution of residues Lys-77, Lys-78, Arg-93, and Lys-94, which are on the positively charged opposite face of the protein, had no effect on FH binding.
FIGURE 4.
Site-directed mutagenesis of FhbB and quantification of FH binding. A, ribbon diagrams with superimposed transparent electrostatic surface charge maps are presented. The spatial placement of residues targeted for substitution are indicated on the appropriate face of the FhbB monomer. B, measurement of FH binding to wild type and single and double amino acid FhbB substitution mutants using ELISA. Recombinant proteins were immobilized in the wells, and purified human FH was added and binding assessed using HRP-conjugated anti-FH antibody. Each mutant is indicated by the x-axis, and the y axis indicates the percentage of binding relative to WT. Values represent the mean calculated from three independently performed experiments ± S.E. (*, p < 0.01; **, p < 0.001). C, analysis of FH binding to select FhbB substitution mutants using affinity ligand binding immunoblot assay approach. Purified recombinant proteins (indicated above each lane) were fractionated by SDS-PAGE and transferred to membranes. The membranes were incubated with FH, and then FH binding was assessed using HRP-conjugated anti-FH antibody. Equivalent loading of recombinant protein in each lane was verified by immunoblotting using anti-FhbB antiserum. D, determination of affinity constants through surface plasmon resonance. No binding of FH to the E45A, D58A, and E62A substitution mutants was detected, and hence affinity constants were not determined.
To assess the influence of side chain length on FH binding, select residues from the α1-α2 face of the protein that were identified to be important in FH binding were substituted with residues of similar charge but different side chain length (E45D, D58E, E62D, and E66D). These conservative substitution proteins bound FH at higher levels than the alanine substitutions, but binding was still greatly reduced relative to wild type. Collectively, the mutational analyses indicate that FH binding is mediated by the α1-α2 face of the protein and that amino acid charge and side chain length are important determinants in the interaction.
Determination of the Affinity of the FhbB/FH Interaction and Association-Dissociation Kinetics
The binding affinity and association-dissociation kinetics of the FH/FhbB interaction were assessed using surface plasmon resonance. The KD was determined to be 1.51 ± 0.02 μm (Fig. 4D). KD values for other FH CCP7/microbially produced FH-binding protein interactions have been reported to be in the nanomolar range (25, 26). Although the KD value for the FH/FhbB interaction indicates a weak interaction, it is similar to that reported for the interaction of FH with most host-derived ligands (40, 41). The KD values for select FhbB mutants that displayed attenuated FH binding were also assessed. With the E66A FhbB mutant, the KD increased to 2.97 ± 0.24 μm. No measurable binding of the E45A, D58A, and E62A substitution mutants to FH was detected, and therefore binding constants could not be determined. The association (ka) and dissociation (kd) constants of the FH/FhbB interaction were determined to be 7.56 ± 0.26 × 104 m−1s−1 and 0.11 ± 0.01 s−1, respectively. The off-rate is significantly faster than that calculated for the interaction of FH with other bacterially produced FH-binding proteins (25, 26, 42). As discussed in detail below, the biological role that FH binding plays in T. denticola pathogenesis may be facilitated by the rapid off kinetics of the FhbB/FH interaction.
FhbB Binds to a Glycosaminoglycan-binding (GAG) Site within FH CCP7
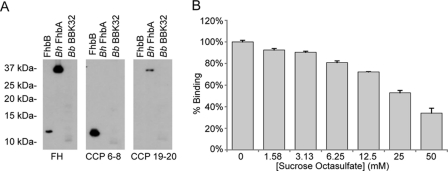
In a previous study (20), FhbB bound to a FH fragment consisting of CCP1–7 (designated as the H7 fragment) but not to the H7AB construct that has four amino acid substitutions within CCP7. These data and competitive binding studies with heparin, a GAG that binds within CCP7 and CCP19–20 (43), implicated CCP7 as the FhbB interaction site (20). To test this, recombinant FH proteins consisting of CCP6–8 or CCP19–20 (negative control) were generated. Binding was assessed using membrane overlay assays. Immobilized FhbB bound to the CCP6–8 construct but did not bind to the CCP19–20 construct (Fig. 5A). The Borrelia hermsii FhbA protein, which binds to CCP19–20 (44), and the B. burgdorferi BBK32 protein, a fibronectin-binding protein that does not bind to FH, served as controls (45). To further validate the FhbB/CCP6–8 interaction, competitive binding analyses were conducted using SOS. SOS is a GAG analog that interacts with a large positively charged groove within CCP6–8 (46). SOS inhibited FhbB binding to FH in a dose-dependent manner (Fig. 5B) suggesting overlapping binding sites within the CCP6–8 GAG-binding groove of FH.
FIGURE 5.
Interaction of FhbB with FH. A, membrane-bound recombinant FhbB, B. hermsii (Bh) FhbA (positive control for binding to CCPs19–20), and B. burgdorferi (Bb) BBK32 (negative control) were tested for their ability to bind full-length FH or fragments consisting of CCP6–8 and CCP19–20. Binding was assessed using an overlay assay. B, ability of SOS to inhibit FH binding to FhbB was assessed. ELISA demonstrates that FH binding to FhbB is inhibited by increasing concentrations of SOS.
DISCUSSION
Some bacterial pathogens evade complement-mediated destruction by binding FH, a negative regulator of the complement cascade (47). Although several bacterial binding proteins have been identified (17), the molecular basis of their interaction with FH is poorly defined. Identification of the minimal common determinants required for FH binding requires that structures of a diverse set of FH-binding proteins be solved. Prior to this report, atomic structures have been determined for only two bacterial FH-binding proteins: CspA (BBA68 or BbCRASP-1) of B. burgdorferi (25) and fHbp of N. meningitidis (26). Here, we report the structure of the FhbB protein of the periopathogen, T. denticola, and investigate the molecular basis of its interaction with FH.
The atomic structure of FhbB was determined at high resolution (1.7 Å). The protein has an α-α-β-α-β organization with well defined negatively (α1-α2) and positively charged (β1-α3-β2) faces. The tertiary fold, defined by extensive hydrophobic interactions within the protein core, imparts high thermostability to FhbB. Only minimal denaturation of FhbB, as assessed by circular dichroism, was observed after heating to 90 °C. DALI analyses revealed that the FhbB fold is unique, lacking structural homology with all other known FH-binding protein structures (25, 26). The asymmetric unit of the FhbB crystal consisted of a dimer. The surface area at the dimeric interface is low (<1000 Å2), and the interface is filled with solvent molecules. In addition, there are no hydrogen bonds between the monomers and minimal van der Waals interactions. The dissociation constant of the dimer is high (217 μm) with dimerization occurring only at nonphysiological protein concentrations (>100 μm). Collectively, these properties are indicative of a weak dimer. It can be concluded that FhbB is a structurally novel, highly stable protein that likely functions in vivo as a monomer.
Using site-directed mutagenesis, residues of FhbB involved in the FH/FhbB interaction were identified. Alanine substitution mutagenesis of surface-exposed residues (Glu-45, Asp-58, Glu-62, and Glu-66) on the α1-α2 face of FhbB eliminated or attenuated FH binding, whereas substitution of residues on the opposite face of the protein (Lys-77, Lys-78, Arg-93, and Lys-94) had no effect on binding. Although mutational analyses of FH were not conducted as part of this study, in an earlier analysis we demonstrated that specific residues within CCP7 contributed to FhbB binding. A recombinant CCP1–7 construct harboring alanine substitutions at residues Arg-387, Lys-388, Arg-404, and Lys-405, which are contained within CCP7(43), lost FhbB binding ability (20). Additional support for the involvement of Lys-405 in FhbB binding comes from FhbB-SOS competitive binding analyses. SOS inhibits the FH/FhbB interaction in a dose-dependent manner. Although SOS binds to at least three sites on FH, co-crystallization of SOS with a CCP6–8 construct revealed that SOS hydrogen bonds with K405FH (46).
The site-directed mutational analyses presented here, and in an earlier report (20), suggest that presentation of an electrostatic environment on the α1-α2 face of FhbB that is conducive to FH binding is dependent on intramolecular interactions within FhbB. Substitution analyses indicate that intramolecular hydrogen bonds between Asn-44–Lys-47 (α1-turn 1), Glu-45–Arg-49 (α1-turn 1), and His-50–Gln-85 (turn 1-turn 2) are important for FH binding. Other FhbB residues that have been demonstrated to influence FH binding include Ile-63, Leu-68, Phe-96, and the C-terminal seven amino acids (23). The placement of these residues on the FhbB structure suggests that their influence on FH binding is indirect as they are not surface-exposed. Loss of FH binding by an I63T substitution mutant most likely results from aberrant hydrophobic packing within α2. A Phe-96 substitution mutant and C-terminal 7-amino acid truncation mutant have also been shown to lack FH-binding ability (23). The structure of FhbB indicates that several C-terminal residues, including Phe-96, reside within the hydrophobic core of the protein forming a two-stranded β-sheet. C-terminal deletions or substitutions may disrupt FH binding by altering the core structure of the protein. An intact C-terminal domain has been demonstrated to be required for FH binding by other spirochetal FH-binding proteins (44, 48, 49).
As discussed above, a unique aspect of the FH/T. denticola interaction is the cleavage of FH by dentilisin. As the T. denticola population expands in periodontal pockets, FhbB-dentilisin-dependent cleavage of FH may deplete FH present in gingival crevicular fluid. FH depletion would dysregulate complement activation in the subgingival crevice. Decay of the C3bBb convertase complex and factor I-mediated cleavage of C3b would be inhibited resulting in C3b accumulation and deposition on host cell surfaces. This would disrupt normal self-recognition mechanisms resulting in destruction of the periodontium. Complement regulation mediated by CRP, which is elevated in crevicular fluid of periodontal disease patients (50), would also be affected. Unchecked complement activation by CRP would lead to activation of the C5 convertase complex and membrane attack complex formation. The FH-CRP complex also plays an important role in regulating pro-inflammatory cytokine production and in facilitating clearance of apoptotic and damaged cells by macrophages (51). In a FH limited microenvironment, clearance of damaged cells would be impaired, and pro-inflammatory cytokine production would be elevated resulting in inflammation and tissue destruction.
In summary, determination of the FhbB structure identified a unique fold that imparts high stability to the protein and presents distinct positive and negatively charged surfaces. Mutagenesis analyses demonstrated the negatively charged face of FhbB interacts with FH. It can be concluded that the basis of the FH/FhbB interaction is electrostatic. The inhibition of the FH/FhbB interaction by the GAG analog, SOS, is consistent with the primary interaction site for FhbB being within CCP7 of FH. Binding and kinetic analysis revealed that the FH/FhbB interaction is low affinity with a rapid off-rate. The kinetics of the interaction would favor rapid turnover and are consistent with the hypothesis that T. denticola may dysregulate complement control by depleting FH present in crevicular fluid leading to conditions that promote the progression of periodontal disease.
Acknowledgments
Data for this study were measured at beamline X25 of the National Synchrotron Light Source, supported principally by the Offices of Biological and Environmental Research and Basic Energy Sciences of the United States Department of Energy and by National Institutes of Health NCRR Grant P41RR012408.
This work was supported, in whole or in part, by National Institutes of Health Grant DE017401 from the NIAID-NIDCR (to R. T. M.), Grant 5K22CA122828-03, and Grant P30CA160589 (to SPR-Flow Cytometry Core, Virginia Commonwealth University). This work was also supported by American Chemical Society Grant IRG9922504 (to J. K. B.).
The atomic coordinates and structure factors (code 3QZ0) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
- FH
- factor H
- CCP
- complement control protein
- GAG
- glycosaminoglycan
- SOS
- sucrose octasulfate
- CRP
- C-reactive protein
- FhbB
- factor H-binding protein B
- NTA
- nitrilotriacetic acid.
REFERENCES
- 1. Krauss J. L., Potempa J., Lambris J. D., Hajishengallis G. (2010) Complementary tolls in the periodontium. How periodontal bacteria modify complement and Toll-like receptor responses to prevail in the host. Periodontol. 2000 52, 141–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Darveau R. P. (2010) Periodontitis. A polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 8, 481–490 [DOI] [PubMed] [Google Scholar]
- 3. Van Dyke T. E., Serhan C. N. (2003) Resolution of inflammation. A new paradigm for the pathogenesis of periodontal diseases. J. Dent. Res. 82, 82–90 [DOI] [PubMed] [Google Scholar]
- 4. Paster B. J., Olsen I., Aas J. A., Dewhirst F. E. (2006) The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol. 2000 42, 80–87 [DOI] [PubMed] [Google Scholar]
- 5. Paster B. J., Boches S. K., Galvin J. L., Ericson R. E., Lau C. N., Levanos V. A., Sahasrabudhe A., Dewhirst F. E. (2001) Bacterial diversity in human subgingival plaque. J. Bacteriol. 183, 3770–3783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ellen R. P., Galimanas V. B. (2005) Spirochetes at the forefront of periodontal infections. Periodontology 2000 38, 13–32 [DOI] [PubMed] [Google Scholar]
- 7. Dewhirst F. E., Tamer M. A., Ericson R. E., Lau C. N., Levanos V. A., Boches S. K., Galvin J. L., Paster B. J. (2000) The diversity of periodontal spirochetes by 16 S rRNA analysis. Oral Microbiol. Immunol. 15, 196–202 [DOI] [PubMed] [Google Scholar]
- 8. Socransky S. S., Haffajee A. D., Cugini M. A., Smith C., Kent R. L., Jr. (1998) Microbial complexes in subgingival plaque. J. Clin Periodontol. 25, 134–144 [DOI] [PubMed] [Google Scholar]
- 9. Boackle R. J. (1991) The interaction of salivary secretions with the human complement system. A model for the study of host defense systems on inflamed mucosal surfaces. Crit. Rev. Oral Biol. Med. 2, 355–367 [DOI] [PubMed] [Google Scholar]
- 10. Boackle R. J., Pruitt K. M., Silverman M. S., Glymph J. L., Jr. (1978) The effects of human saliva on the hemolytic activity of complement. J. Dent. Res. 57, 103–110 [DOI] [PubMed] [Google Scholar]
- 11. Schenkein H. A. (1991) The role of complement in periodontal diseases. Crit. Rev. Oral. Biol. Med. 2, 65–81 [DOI] [PubMed] [Google Scholar]
- 12. Schenkein H. A., Genco R. J. (1977) Gingival fluid and serum in periodontal diseases. I. Quantitative study of immunoglobulins, complement components, and other plasma proteins. J. Periodontol. 48, 772–777 [DOI] [PubMed] [Google Scholar]
- 13. Ruddy S., Austen K. F. (1971) C3b inactivator of man. II. Fragments produced by C3b inactivator cleavage of cell-bound or fluid phase C3b. J. Immunol. 107, 742–750 [PubMed] [Google Scholar]
- 14. Zipfel P. F., Skerka C. (2009) Complement regulators and inhibitory proteins. Nat. Rev. Immunol. 9, 729–740 [DOI] [PubMed] [Google Scholar]
- 15. Zipfel P. F., Skerka C., Hellwage J., Jokiranta S. T., Meri S., Brade V., Kraiczy P., Noris M., Remuzzi G. (2002) Factor H family proteins. On complement, microbes, and human diseases. Biochem. Soc. Trans. 30, 971–978 [DOI] [PubMed] [Google Scholar]
- 16. Jarva H., Jokiranta T. S., Hellwage J., Zipfel P. F., Meri S. (1999) Regulation of complement activation by C-reactive protein. Targeting the complement inhibitory activity of factor H by an interaction with short consensus repeat domains 7 and 8–11. J. Immunol. 163, 3957–3962 [PubMed] [Google Scholar]
- 17. Ferreira V. P., Pangburn M. K., Cortés C. (2010) Complement control protein factor H. The good, the bad, and the inadequate. Mol. Immunol. 47, 2187–2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schmidt C. Q., Herbert A. P., Hocking H. G., Uhrín D., Barlow P. N. (2008) Translational mini-review series on complement factor H. Structural and functional correlations for factor H. Clin. Exp. Immunol. 151, 14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holers V. M. (2008) The spectrum of complement alternative pathway-mediated diseases. Immunol. Rev. 223, 300–316 [DOI] [PubMed] [Google Scholar]
- 20. McDowell J. V., Lankford J., Stamm L., Sadlon T., Gordon D. L., Marconi R. T. (2005) Demonstration of factor H-like protein 1 binding to Treponema denticola, a pathogen associated with periodontal disease in humans. Infect. Immun. 73, 7126–7132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McDowell J. V., Frederick J., Stamm L., Marconi R. T. (2007) Identification of the gene encoding the FhbB protein of Treponema denticola, a highly unique factor H-like protein 1-binding protein. Infect. Immun. 75, 1050–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McDowell J. V., Frederick J., Miller D. P., Goetting-Minesky M. P., Goodman H., Fenno J. C., Marconi R. T. (2011) Identification of the primary mechanism of complement evasion by the periodontal pathogen, Treponema denticola. Mol. Oral Microbiol. 26, 140–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McDowell J. V., Huang B., Fenno J. C., Marconi R. T. (2009) Analysis of a unique interaction between the complement regulatory protein factor H and the periodontal pathogen Treponema denticola. Infect. Immun. 77, 1417–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Uitto V. J., Grenier D., Chan E. C., McBride B. C. (1988) Isolation of a chymotrypsin-like enzyme from Treponema denticola. Infect. Immun. 56, 2717–2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cordes F. S., Roversi P., Kraiczy P., Simon M. M., Brade V., Jahraus O., Wallis R., Skerka C., Zipfel P. F., Wallich R., Lea S. M. (2005) A novel fold for the factor H-binding protein BbCRASP-1 of Borrelia burgdorferi. Nat. Struct. Mol. Biol. 12, 276–277 [DOI] [PubMed] [Google Scholar]
- 26. Schneider M. C., Prosser B. E., Caesar J. J., Kugelberg E., Li S., Zhang Q., Quoraishi S., Lovett J. E., Deane J. E., Sim R. B., Roversi P., Johnson S., Tang C. M., Lea S. M. (2009) Neisseria meningitidis recruits factor H using protein mimicry of host carbohydrates. Nature 458, 890–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miller D. P., McDowell J. V., Bell J. K., Marconi R. T. (2011) Crystallization of the factor H-binding protein, FhbB, from the periopathogen Treponema denticola. Acta Crystallogr Sect. F Struct. Biol. Cryst. Commun. 67, 678–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Otwinowski Z., Minor W. (1997) Processing of x-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 29. Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., Richardson J. S., Terwilliger T. C., Zwart P. H. (2010) PHENIX. A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Emsley P., Cowtan K. (2004) Coot. Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 31. Emsley P., Lohkamp B., Scott W. G., Cowtan K. (2010) Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., Read R. J. (2007) Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Collaborative Computational Project, Number 4 (1994) The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 [DOI] [PubMed] [Google Scholar]
- 34. Chen V. B., Arendall W. B., 3rd, Headd J. J., Keedy D. A., Immormino R. M., Kapral G. J., Murray L. W., Richardson J. S., Richardson D. C. (2010) MolProbity. All-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Davis I. W., Leaver-Fay A., Chen V. B., Block J. N., Kapral G. J., Wang X., Murray L. W., Arendall W. B., 3rd, Snoeyink J., Richardson J. S., Richardson D. C. (2007) MolProbity. All-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 35, W375–W383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Holm L., Rosenström P. (2010) Dali server. Conservation mapping in 3D. Nucleic Acids Res. 38, W545–W549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McDowell J. V., Wolfgang J., Tran E., Metts M. S., Hamilton D., Marconi R. T. (2003) Comprehensive analysis of the factor H binding capabilities of Borrelia species associated with Lyme disease. Delineation of two distinct classes of factor H-binding proteins. Infect. Immun. 71, 3597–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schuck P. (2000) Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys. J. 78, 1606–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schuck P. (2003) On the analysis of protein self-association by sedimentation velocity analytical ultracentrifugation. Anal. Biochem. 320, 104–124 [DOI] [PubMed] [Google Scholar]
- 40. Morgan H. P., Schmidt C. Q., Guariento M., Blaum B. S., Gillespie D., Herbert A. P., Kavanagh D., Mertens H. D., Svergun D. I., Johansson C. M., Uhrín D., Barlow P. N., Hannan J. P. (2011) Structural basis for engagement by complement factor H of C3b on a self-surface. Nat. Struct. Mol. Biol. 18, 463–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu J., Wu Y. Q., Ricklin D., Janssen B. J., Lambris J. D., Gros P. (2009) Structure of complement fragment C3b-factor H and implications for host protection by complement regulators. Nat. Immunol. 10, 728–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rossmann E., Kraiczy P., Herzberger P., Skerka C., Kirschfink M., Simon M. M., Zipfel P. F., Wallich R. (2007) Dual binding specificity of a Borrelia hermsii-associated complement regulator-acquiring surface protein for factor H and plasminogen discloses a putative virulence factor of relapsing fever spirochetes. J. Immunol. 178, 7292–7301 [DOI] [PubMed] [Google Scholar]
- 43. Giannakis E., Jokiranta T. S., Male D. A., Ranganathan S., Ormsby R. J., Fischetti V. A., Mold C., Gordon D. L. (2003) A common site within factor H SCR 7 responsible for binding heparin, C-reactive protein, and streptococcal M protein. Eur. J. Immunol. 33, 962–969 [DOI] [PubMed] [Google Scholar]
- 44. Hovis K. M., Jones J. P., Sadlon T., Raval G., Gordon D. L., Marconi R. T. (2006) Molecular analyses of the interaction of Borrelia hermsii FhbA with the complement regulatory proteins factor H and factor H-like protein 1. Infect. Immun. 74, 2007–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Seshu J., Esteve-Gassent M. D., Labandeira-Rey M., Kim J. H., Trzeciakowski J. P., Höök M., Skare J. T. (2006) Inactivation of the fibronectin-binding adhesin gene bbk32 significantly attenuates the infectivity potential of Borrelia burgdorferi. Mol. Microbiol. 59, 1591–1601 [DOI] [PubMed] [Google Scholar]
- 46. Prosser B. E., Johnson S., Roversi P., Herbert A. P., Blaum B. S., Tyrrell J., Jowitt T. A., Clark S. J., Tarelli E., Uhrín D., Barlow P. N., Sim R. B., Day A. J., Lea S. M. (2007) Structural basis for complement factor H linked age-related macular degeneration. J. Exp. Med. 204, 2277–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zipfel P. F., Würzner R., Skerka C. (2007) Complement evasion of pathogens. Common strategies are shared by diverse organisms. Mol. Immunol. 44, 3850–3857 [DOI] [PubMed] [Google Scholar]
- 48. McDowell J. V., Harlin M. E., Rogers E. A., Marconi R. T. (2005) Putative coiled-coil structural elements of the BBA68 protein of Lyme disease spirochetes are required for formation of its factor H-binding site. J. Bacteriol. 187, 1317–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McDowell J. V., Wolfgang J., Senty L., Sundy C. M., Noto M. J., Marconi R. T. (2004) Demonstration of the involvement of outer surface protein E coiled coil structural domains and higher order structural elements in the binding of infection-induced antibody and the complement-regulatory protein, factor H. J. Immunol. 173, 7471–7480 [DOI] [PubMed] [Google Scholar]
- 50. Gomes-Filho I. S., Freitas Coelho J. M., da Cruz S. S., Passos J. S., Teixeira de Freitas C. O., Aragão Farias N. S., Amorim da Silva R., Silva Pereira M. N., Lima T. L., Barreto M. L. (2011) Chronic periodontitis and C-reactive protein levels. J. Periodontol. 82, 969–978 [DOI] [PubMed] [Google Scholar]
- 51. Kang Y. H., Urban B. C., Sim R. B., Kishore U. (2011) Immunobiology 217, 455–464 [DOI] [PubMed] [Google Scholar]