Background: Rac is a central regulator of cancer cell migration/invasion and metastasis.
Results: EHop-016 inhibits Rac activity with an IC50 of 1 μm. EHop-016 blocks Rac interaction with the Rac exchange factor Vav2, lamellipodia extension, and cell migration.
Conclusion: EHop-016 is an effective Rac inhibitor.
Significance: EHop-016 has potential as a metastasis therapeutic and for investigations of Rac-regulated cellular responses.
Keywords: Actin; Cdc42; Enzyme Inhibitors; GTPase; Guanine Nucleotide Exchange Factor (GEF); Rac; Rho; Rho GTPases; Rac Inhibitor, Ehop-016, Rac GEFs
Abstract
The Rho GTPase Rac regulates actin cytoskeleton reorganization to form cell surface extensions (lamellipodia) required for cell migration/invasion during cancer metastasis. Rac hyperactivation and overexpression are associated with aggressive cancers; thus, interference of the interaction of Rac with its direct upstream activators, guanine nucleotide exchange factors (GEFs), is a viable strategy for inhibiting Rac activity. We synthesized EHop-016, a novel inhibitor of Rac activity, based on the structure of the established Rac/Rac GEF inhibitor NSC23766. Herein, we demonstrate that EHop-016 inhibits Rac activity in the MDA-MB-435 metastatic cancer cells that overexpress Rac and exhibits high endogenous Rac activity. The IC50 of 1.1 μm for Rac inhibition by EHop-016 is ∼100-fold lower than for NSC23766. EHop-016 is specific for Rac1 and Rac3 at concentrations of ≤5 μm. At higher concentrations, EHop-016 inhibits the close homolog Cdc42. In MDA-MB-435 cells that demonstrate high active levels of the Rac GEF Vav2, EHop-016 inhibits the association of Vav2 with a nucleotide-free Rac1(G15A), which has a high affinity for activated GEFs. EHop-016 also inhibits the Rac activity of MDA-MB-231 metastatic breast cancer cells and reduces Rac-directed lamellipodia formation in both cell lines. EHop-016 decreases Rac downstream effects of PAK1 (p21-activated kinase 1) activity and directed migration of metastatic cancer cells. Moreover, at effective concentrations (<5 μm), EHop-016 does not affect the viability of transformed mammary epithelial cells (MCF-10A) and reduces viability of MDA-MB-435 cells by only 20%. Therefore, EHop-016 holds promise as a targeted therapeutic agent for the treatment of metastatic cancers with high Rac activity.
Introduction
Rho family GTPases (Rho, Rac, Cdc42) are important intracellular signaling proteins that control diverse cellular functions, including actin cytoskeletal organization, migration and invasion, transcriptional regulation, cell cycle progression, apoptosis, vesicle trafficking, and cell-to-cell and cell-to-extracellular matrix adhesions (1, 2). Consequently, Rho GTPases have been implicated in cancer and in the progression of other diseases by a large number of studies (3–9). Of the Rho family GTPases, Rac1 and Rac3, the isoforms expressed in non-hematopoietic cells, have been specifically associated with the rearrangement of the actin cytoskeleton into cell surface protrusions called lamellipodia or invadopodia that are specific for forward migration during invasion (10). Therefore, Racs have been linked to promotion of metastasis (11–14). Racs have also been shown to be essential for Ras and other oncogene-mediated transformation (15, 16). We and others have implicated hyperactive Rac1 and Rac3 with increased survival, proliferation, and invasion of breast and brain cancers (13, 17–21). Recent reports have shown a role for Rac in mammalian target of rapamycin (mTOR)4-mediated regulation of cancer malignancy (22, 23) and anti-breast cancer therapy resistance (24). Moreover, Rac1 was shown to increase estrogen receptor α-mediated transcriptional activity in breast cancer (25). Studies have also demonstrated a cancer-promoting role for the constitutively active Rac1b splice variant that is overexpressed in breast and colorectal cancer (21, 26–29). Because the malignant phenotype of Rac is associated with activation of its direct downstream effectors p21-activated kinases (PAKs) (30, 31), much effort has been focused on the development of PAK inhibitors as anti-cancer therapeutics (32, 33). However, in addition to PAK, Racs have multiple downstream effectors, such as WAVE (Wiskott-Aldrich syndrome protein family member) and Mena/VASP (vasodilator-stimulated phosphoprotein) that contribute to cancer (34, 35). Therefore, targeting Rac is a more viable approach for the development of anticancer drugs (36).
Unlike the related small GTPase Ras, Racs are not mutated in malignant cancers but rather overexpressed or hyperactivated (21). Racs are activated by GTP/GDP exchange catalyzed by guanine nucleotide exchange factors (GEFs) that are regulated via a myriad of cell surface receptors (37). So far, over 60 potential Rac-GEFs have been identified (38–40). Of these, Dbl family GEFs, such as Tiam-1 (T-cell invasion and metastasis gene product) and Vavs 1–3 have been implicated in cancer progression (41–44). Vav oncogenes play a central role in cancer and are differentially regulated from Tiam-1 and Trio that contain the Dbl homology (DH), pleckstrin homology (PH) domains essential for GEF activity (45, 46). Vavs have additional Src homology and cysteine-rich domains and activate Rac, Rho, and Cdc42, whereas Tiam-1 and Trio are specific for Rac1 activation (47–50). Recent reports have also shown that phosphatidylinositol 1,4,5-trisphosphate-dependent Rac exchanger 1 (p-Rex1) is up-regulated in breast cancer cells and breast cancer patients with poor prognosis (51, 52). Elevation of Rac-GEF expression and/or activity appears to be a common phenomenon during cancer progression. Therefore, targeting the binding of Rac to GEFs is a rational strategy to inhibit Rac activity and thus cancer invasion.
NSC23766 was identified as a small molecule that binds to a putative binding pocket in the surface groove of Rac1 that interacts with a subset of Rac-GEFs Trio and Tiam-1 but not Vav (48, 53, 54). In breast cancer cells, inhibition of Rac activity by NSC23766 was shown to induce G1 cell cycle arrest or apoptosis (20). NSC23766 has been shown to inhibit the anchorage-independent growth and invasion of human prostate cancer PC-3 cells, Rac activation, and Rac-dependent aggregation of platelets stimulated by thrombin, Rac1, and Rac2 activities of hematopoietic stem/progenitor cells and migration from mouse bone marrow to peripheral blood (55, 56). NSC23766 has also been shown to inhibit invasion of chronic myelogenous leukemia cells in vitro and in vivo in a mouse model (53). Thus, such structure-function-based rational design appears to represent a new avenue for generating small molecule inhibitors of Rac and its functions. However, NSC23766 is a moderately active Rac inhibitor with a relatively high IC50 of 50–100 μm in fibroblasts, which limits its potential use as a therapeutic agent (48). In addition, we have found that in the highly metastatic cancer cell line MDA-MB-435, NSC23766 inhibits Rac1 by only ∼20% at a concentration of 50 μm and that at this concentration, there is no significant effect on lamellopodia formation (57). Therefore, there is a need for more effective inhibitors of Rac activity in highly metastatic cancer cells.
The identification of novel inhibitors of Rac that function via different inhibitory mechanisms has been the subject of several studies. Whereas NSC23766 inhibits the interaction of Rac1 with several of its GEFs, the Rac inhibitor EHT 1864 interferes with the interaction of Rac with its downstream effectors at concentrations of 10–50 μm (58, 59). A virtual screening of a selected subset of compounds from the ZINC data base for binding affinity to Rac1 based on the crystal structure of Rac1 with NSC23766 identified several novel Rac1 inhibitors with experimental IC50 values ranging from 12.2 to 57 μm (60). In addition, a high-throughput flow cytometry bead-based multiplex assay identified MLS000532223 as a compound that prevents GTP binding to Rac. However, other Rho GTPases, such as Cdc42, are also affected by this compound. Small molecule compounds have also been synthesized to specifically inhibit the Rac1b splice variant (61). Another report identified ITX3 as a GEF inhibitor that targeted Rac and RhoG interaction with Trio; however, this compound is effective at high 50–100 μm concentrations (62). In an endeavor to develop novel more potent Rac inhibitors with possible clinical applications, we demonstrated that NSC23766 could be utilized as a lead structure for the design of compounds with 2–3 times enhanced potency (57). We now report the identification and characterization of the biological activity of EHop-016, a novel NSC23766 derivative that inhibits Rac1 100-fold more effectively than the parent compound.
EXPERIMENTAL PROCEDURES
Synthesis of EHop-016
All chemicals were purchased from Sigma-Aldrich. The synthesis of EHop-016 was performed in two steps according to the reaction scheme provided in Fig. 1 and carried out analogous to the procedure described previously (58). (2-Chloro-pyrimidin-4-yl)-(9-ethyl-9H-carbazol-3-yl)-amine was obtained as a pure compound in a yield of 53%. The product was identified with TLC, NMR, and GC/MS. Rf = 0.23 (3:1, hexane-ethyl acetate); 1H NMR (DMSO-d6, 400 MHz) δ 1.32 (t, J = 6.9 Hz, 3H), 4.45 (q, J = 6.6 Hz, 2H), 6.72 (s, 1H), 7.20 (t, J = 7.36 Hz, 1H), 7.47 (t, J = 7.30 Hz, 1H), 7.56 (s, 1H), 7.62 (t, J = 8.68 Hz, 1H), 8.11 (t, J = 7.36 Hz, 1H), 8.27 (s, 1H), 10.1 (s, 1H); 13C (DMSO-d6, 100 MHz) δ 13.7, 37.0, 109.2, 109.4, 115.0, 118.7, 120.3, 121.3, 121.9, 122.3, 125.9, 129.9, 136.9, 140.0, 156.9, 159.6, 162.4; LRGC-MS m/z (rel%):[M]+ 276 (100), [M-Cl]+ 241 (40), [M-C5H5N3Cl]+ 134 (26). N4-(9-Ethyl-9H-carbazol-3-yl)-N2-(3-morpholin-4-yl propyl)-pyrimidine-2,4-diamine (EHop-016) was obtained as a pure compound in a yield of 93%. The product was identified to be essentially pure by TLC and NMR: Rf = 0.34 (9:1, CH2Cl2-MeOH); 1H NMR (DMSO-d6, 400 MHz) δ 1.31 (t, J = 7.0 Hz, 3H), 1.73 (m, 2H), 2.32 (m, 2H), 2.34 (t, J = 6.89 Hz, 8H), 3.52 (m, 2H), 4.42 (q, J = 7.0 Hz, 2H), 5.98 (d, J = 5.7 Hz, 1H), 6.69 (t, J = 5.3 Hz, 1H), 7.16 (t, J = 7.4, 1H), 7.43 (t, J = 7.2 Hz, 1H), 7.53 (t, J = 9.0 Hz, 4H), 7.81 (d, J = 5.4 Hz, 1H), 8.10 (s, 1H), 8.66 (s, 1H), 9.1 (s, 1H); 13C (DMSO-d6, 100 MHz) δ 13.7, 26.2, 36.9, 53.4, 56.3, 66.2, 108.9, 109.0, 118.2, 119.7, 120.2, 122.0, 122.2, 125.6, 132.5, 135.5, 139.9, 159.8, 160.9, 162.1. 1H and 13C NMR spectra were recorded on a Bruker 400-MHz spectrometer. Mass spectra were obtained on a Hewlett Packard 6890N GC/MS spectrometer.
FIGURE 1.
Synthesis and docking of EHop-016 into the putative GEF binding pocket of Rac1. A, synthetic scheme for the preparation of EHop-016. The synthesis was performed by a two-step approach as described (57). B, EHop-016 docked into the GEF binding pocket of Rac1 and its comparison with the position of NSC23766 in the crystal structure of the Rac1-NSC23766 complex.
Docking of EHop-16 into Crystal Structure of Rac1
For molecular docking, Autodock 4.0 with AutodockTools 1.5.4 as the graphical user interface was used (63, 64). The coordinates of the crystal structure from the Rac1-NSC23766 complex were obtained from Ref. 65. EHop-016 was drawn using ChemDraw Ultra 7.0 and energy-minimized with MOPAC AM1 in Chem3D Ultra 7.0. After removing NSC23766 from the crystal structure, AutodockTools was used to prepare the GEF-interacting region of Rac and EHop-016 for docking and to create a grid of 60 × 60 × 60 Å with a grid spacing of 0.375 Å centered on the original position of NSC23766. A flexible docking of 100 GA runs was performed with the number of individuals in the population set to 200 and the maximum number of energy evaluations set to 25,000,000, with other parameters accepted as suggested by AutodockTools, which was also used for clustering (root mean square deviation = 2 Å) of the results obtained.
Rac Activity Assays
Rac activity was determined from lysates of the MDA-MB-435 and MDA-MB-231 human metastatic cancer cell lines (from ATCC). Cancer cells in culture medium (DMEM, 10% FBS, pH 7.5) were treated with vehicle (0.1% DMSO) or varying concentrations of EHop-016 (0–10 μm) for 24 h. Rac1 activity was determined as described previously (57), using the G-LISA Rac1 activation assay kit (Cytoskeleton, Inc., Denver, CO).
For generation of IC50 curves for each inhibitor (EHop-016 or NSC23766), data from three independent duplicate experiments were pooled, and four-parameter dose-response curves were fitted using the non-linear regression function of GraphPad Prism®.
Rho GTPase Activity Assays
Rho, Rac, and Cdc42 activities were analyzed from MDA-MB-435 and MDA-MB-231 cell lysates by pull-down assays following treatment with EHop-016 for 24 h. The GST-Rho binding domain from rhotekin was used to isolate active GTP-bound Rho, and a GST-Cdc42 and Rac interactive binding (CRIB) domain of PAK1 was used to isolate active Rac-GTP or Cdc42-GTP, as described previously (17, 18). Active and total Rho GTPases were identified by Western blotting with specific antibodies.
Western Blotting
Cell lysates or pull-downs were Western blotted using routine laboratory procedures as described previously (17, 18). Anti-RhoA, -Rac (Rac1, -2, and -3), -Cdc42, and -phospho-PAKThr-423 antibodies were from Cell Signaling Technology, Inc. (Danvers, MA). Anti-Vav2 antibodies were from Zymed Laboratories Inc., and Rac3, PAK1, Tiam-1, and Trio antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Precipitation of Activated GEFs with Recombinant Mutant Rac1(G15A) Protein
MDA-MB-435 cells in culture medium were lysed in 1% Triton X-100, 20 mm HEPES, pH 7.4, 150 mm NaCl, 5 mm MgCl2, and protease inhibitors and processed as described (66). Equal amounts of protein from cleared lysates were incubated for 1 h at 4 °C with glutathione-agarose beads conjugated to GST-Rac1(G15A) nucleotide-free mutant (Cell Biolabs, San Diego, CA) that were preincubated (for 1 h) with vehicle or 2 or 4 μm EHop-016. The beads were washed, and the lysates and pull-downs were immunoblotted for Tiam-1, Trio, or Vav2.
Interaction of Tiam-1 DH/PH Domain with Rac1(G15A)
His-tagged Tiam-1 DH-PH pET construct (a kind gift of Dr. Ernesto Fuentes, University of Iowa, Iowa City, IA) was transformed into Rosetta DE3 Escherichia coli cells, and clarified lysates were purified by batch affinity chromatography using His-Select nickel affinity gel (Sigma). Tiam-1 was eluted with 300 mm imidazole and separated by an FPLC size exclusion Superdex 200 column. Purity of the Tiam-1 fraction at 1.7 mg/ml was observed to be >95% by SDS-PAGE. GST-Rac1(G15A) glutathione-agarose or glutathione-agarose beads alone were preincubated with varying concentrations of EHop-016 or NSC23766 for 1 h in lysis buffer (1% Igepal, 20 mm HEPES, 150 mm NaCl, 5 mm MgCl2, pH 7.5). Purified His-Tiam-1 DH/PH domain was added at a concentration of 2:1 Rac1(G15A)/Tiam-1 and incubated for another 1 h at 4 °C. Pull-downs were washed three times in 1% Igepal buffer and 1 time in HEPES buffer and Western blotted with an anti-His antibody to visualize His-Tiam-1 DH/PH domain protein.
Fluorescence Microscopy
As described previously (57), MDA-MB-435 or MDA-MB-231 cells in culture medium were treated with vehicle (0.1% DMSO) or EHop-016 at 2 and 4 μm for 24 h. Cells were fixed, permeabilized, and stained with rhodamine phalloidin to visualize F-actin. Fluorescence micrographs were acquired at ×600 magnification in an Olympus BX40 fluorescence microscope using a Spot digital camera.
Cell Migration Assays
As described previously (57), quiescent MDA-MB-435 cells were treated with vehicle or varying concentrations of EHop-016 (0–5 μm) for 24 h. Exactly 2 × 105 cells were placed on the top well of Transwell chambers (Corning Glass) with culture medium containing 10% FBS in the bottom well. The number of cells that migrated to the underside of the membrane following a 4-h incubation was quantified for each treatment. Fixed cells were stained with propidium iodide to visualize nuclei. For each treatment (three biological experiments with two technical replicates each), cells in 20 microscopic fields were quantified at ×200 magnification in a Olympus CKX41 inverted fluorescence microscope.
Cell Viability Assays
As described previously (57), MDA-MB-231, MDA-MB-435, or MCF-10A mammary epithelial cells (from ATCC) were incubated in vehicle (0.1% DMSO) or varying concentrations of EHop-016 (0–10 μm) for 24 h. Cell viability was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide cell survival and proliferation kit (Millipore, Inc., Billerica, MA) according to the manufacturer's instructions.
RESULTS
Synthesis of EHop-016
The inhibition of Rac1 activity has been proposed as a strategy for the prevention of cancer metastasis. However, the frequently utilized small molecule Rac1 inhibitor NSC23766 only has a moderate biological effect on the highly metastatic cancer cell line MDA-MB-435, even at high concentrations, thus illustrating the need for more potent and effective inhibitors. We recently reported the synthesis of novel NSC23766 derivatives that were more efficient than the parent compound in MDA-MB-435 cells with a considerable reduction of cell functions regulated by Rac (57). Further optimization of the NSC23766 lead structure led to the identification of EHop-016, which was synthesized according to the procedure described in the legend to Fig. 1.
Briefly, 2,4-dichloropyrimidine (1) was reacted with 3-amino-9-ethylcarbazole (2) by heating in isopropyl alcohol in the presence of N,N-diisopropylethylamine. Separation of the regioisomers provided the pure 4-substituted pyrimidine derivative (3), which was reacted with 4-(3-aminopropyl)morpholine (4) in sec-butanol under microwave heating in the presence of N,N-diisopropylethylamine to provide EHop-016 (5).
Molecular Docking of EHop-016
NSC23766 was designed to prevent the activation of Rac1 by binding to the region where several GEFs interact with Rac1, thus inhibiting its activation. This mode of action was recently confirmed via analysis of the crystal structure of Rac1 with NSC23766. For the design of novel, more potent inhibitors, we wished to improve the activity and maintain the selectivity profile of NSC23766. Therefore, in order to obtain a similar binding interaction for the new inhibitors, the core structural features of NSC23766 were maintained, including a central pyrimidine core with an aromatic 4-substituent and an amino group-containing 2-substituent, both connected to the pyrimidine core via nitrogen atoms. Molecular docking, to study the binding interactions of EHop-016 with Rac1, demonstrated that EHop-016 can bind to Rac1 in different orientations into the cleft formed by amino acids Pro-34, Thr-35, Val-36, Phe-37, Asp-38, Asn-39, Trp-56, Asp-57, Thr-58, Ala-59, Tyr-64, Leu-67, Arg-68, Leu-70, and Ser-71. Clustering of the docking results revealed that the largest cluster (size = 29) also had the docking conformation with the lowest energy (−7.91 kcal/mol), and the most favorable conformation is illustrated in Fig. 1B, together with the position of NSC23766 in the crystal structure of the Rac1-NSC23766 complex. Whereas NSC23766 is stretched over the surface of Rac1, similar to the other novel Rac1 inhibitors that were recently identified (60), EHop-016 appears to favor a bent conformation that binds to a deeper binding pocket similar to the N,N-diethylamino group of NSC23766. In its energetically most favorable conformation, the binding of EHop-016 is strengthened by two hydrogen bonding interactions with residues Asp-38 and Asn-39. Furthermore, EHop-016 has a close interaction with Trp-56, which has been shown to be critical for binding of Rac to its GEFs (48, 53, 54). Two smaller clusters (sizes = 13 and 21) with lowest energy conformations of −7.61 and −7.41 kcal/mol, respectively, dock into the same cleft, albeit in a somewhat different mode (supplemental Fig. S1). Based on the above molecular docking results, structural similarity to NSC23766, and the biological activity profile described herein, it is reasonable to postulate that EHop-016 also interferes with binding of Rac1 with its GEFs via binding to the three-way junction site that contains the switch I, switch II, and β loops of the effector region of Rac that interacts with the DH domain of Rac GEFs (67). The crystal structures of a complex of the binding domains of the homologous GEF Vav1 with Rac1 were described recently (49, 68). Using this information, analysis of binding interactions indicates that unlike NSC23766, EHop-016 interacts with several of the amino acid residues that form the putative binding pocket of Vav1 with Rac1. More specifically, of the residues that are calculated to interact with EHop-016, Vav1 interacts with residues Thr-35, Val-36, and Asn-39 of switch I and with residues Ala-59 and Tyr-64 of switch II (Fig. 1B). Therefore, it is suggested that EHop-016 binds tightly to key amino acid residues of Rac1, potentially inhibiting interaction with Vav.
EHop-016 Is Potent Inhibitor of Rac1
The potential of EHop-016 to inhibit Rac activity was determined in the highly metastatic human cancer cell line MDA-MB-435, which was previously reported by us to contain high endogenous Rac activity (18). MDA-MB-435 cells were treated for 24 h with varying concentrations of EHop-016 and, for comparison, NSC23766. Rac activity from cell lysates was measured using the G-LISA Rac1 activation assay. The concentration curves in Fig. 2 demonstrate that EHop-016 inhibits Rac1 activity in MDA-MB-435 cells with an IC50 of 1.1 μm, whereas the IC50 for NSC23766 in the same cell line was 95 μm. Thus, EHop-016 is ∼100 times more potent than NSC23766 and 10–50 times more potent than other currently available Rac inhibitors.
FIGURE 2.
Effect of EHop-016 and NSC23766 on Rac activity. MDA-MB-435 cells were treated with vehicle (0.1% DMSO) or varying concentrations of EHop-016 (0–10 μm) or NSC23766 (0–100 μm) for 24 h. Cell lysates were subjected to the G-LISA Rac1 activation assay (Cytoskeleton, Inc.). IC50 curves for percentage Rac activity are relative to vehicle from three biological replicates each with two technical replicates. Error bars, S.D. Four-parameter dose-response curves generated using GraphPad Prism® are shown.
Effect of EHop-016 on Activity of Other Rho Family GTPases
In order to investigate the selectivity of EHop-016, we studied its effect on other Rho family GTPases. Similar to Rac1, the Rac isoform Rac3 that is also overexpressed in cancer cells was inhibited by 58% at a concentration of 1 μm EHop-016 (Fig. 3A). This is expected because Rac1 and Rac3 demonstrate significant structure similarity and are activated by the same GEFs. EHop-016 did not affect the activity of the Rac homolog Cdc42 at 2 or 4 μm but inhibited Cdc42 activity by 28% at 5 μm and 74% at 10 μm (Fig. 3B). Therefore, EHop-016 may not bind the similar DH-interacting domain of Cdc42 as tightly as Rac but is able to inhibit Cdc42 activity at higher concentrations. In contrast, the activity of the closely related Rho GTPase RhoA was increased ∼1.3-fold in response to 2, 4, 5, or 10 μm EHop-016. Increased Rho activity, when Rac is being inhibited by EHop-016, may be a compensatory mechanism. This is expected to increase Rho-regulated assembly of F-actin into stress fibers and further inhibit Rac-mediated lamellipodia formation and directed migration (1, 4, 10).
FIGURE 3.
Effect of EHop-016 on Rho GTPase activity. A and B, MDA-MB-435 cells were treated with vehicle (0) or EHop-016 at the indicated concentrations (1, 2, 4, 5, or 10 μm) for 24 h. Cell lysates were subjected to a pull-down assay using a GST-CRIB domain of PAK and Western blotted for Rac3 or Cdc42. C, a GST-RBD domain of rhotekin was used to pull down Rho-GTP and detected by Western blotting with anti-RhoA antibody. The average percentage of Rho GTPase activity was calculated from the integrated density of positive bands of Rho GTPase-GTP from a pull-down/total Rho GTPase in cell lysate for each treatment and each Rho GTPase (Rac3, Cdc42, or RhoA) relative to vehicle controls. A, representative Western blot of pull-downs immunostained for Rac3-GTP (top row) or total Rac3 in cell lysate (bottom row) (n = 3). B, representative Western blots (from the same experiment) of pull-downs immunostained for Cdc42-GTP (top row) or total Cdc42 in cell lysate (bottom row) (n = 2). C, representative Western blots of pull-downs immunostained for RhoA-GTP (top row) or total RhoA in cell lysate (bottom row), n = 3. Western blots show positive bands for Rho GTPases at ∼21 kDa.
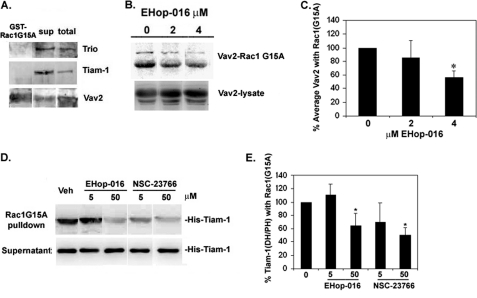
EHop-016 Inhibits Association of Active Vav2 with Rac1(G15A) Mutant Fusion Protein
To investigate a mechanism for the inhibition of Rac by EHop-016, the active Rac GEFs in MDA-MB-435 cells were detected from pull-downs of a glutathione-agarose-conjugated GST fusion protein of a Rac1 nucleotide-free mutant Rac1(G15A) that has a high affinity for activated GEFs (66, 69). As shown in Fig. 4A, in the highly metastatic MDA-MB-435 cell line with elevated Rac activity, Vav2 appears to be more abundant compared with Tiam-1 and Trio. Although equal amounts of total protein were used for Western blotting, it is possible that the differences in GEF expression may reflect the relative affinities with their specific antibodies. Moreover, in MDA-MB-435 cells growing in 10% serum, only active Vav2 was pulled down with the GST-Rac1(G15A) and not Tiam-1 or Trio (Fig. 4A). We show that the association of Vav2 with Rac1(G15A) was inhibited by 4 μm EHop-016 to ∼50% compared with controls in a statistically significant manner (p < 0.005) (Fig. 4, B and C). Because we did not detect association of Tiam-1 with Rac1(G15A) under our experimental conditions, the effect of EHop-016 on the interaction of Rac1 and Tiam-1 could not be measured in vivo.
FIGURE 4.
Effect of EHop-016 on Rac/Rac GEF interaction. MDA-MB-435 cells were lysed and incubated with glutathione-agarose-coupled GST-Rac1(G15A) beads to pull down activated Rac GEFs. A, a representative Western blot (from the same experiment) of GST-Rac1(G15A) pull-down, supernatant (sup) from the incubation, and cell lysates, immunostained for Trio, Tiam-1, or Vav2. B, GST-Rac1(G15A) beads were preincubated with vehicle (0), or 2 or 4 μm EHop-016 prior to incubation with MDA-MB-435 cell lysates. A representative Western blot (from the same experiment) (n = 3) immunostained for Vav2 is shown. Top row, pull-down; bottom row, total cell lysate. C, quantification of the percentage of average Vav2 (two bands at ∼100 kDa) associated with the Rac1(G15A) beads from pull-down assays in the presence or absence of EHop-016. ImageJ software was used to quantify the integrated density of positive bands (∼100 kDa) from Vav2 Western blots of pull-downs. Data are represented relative to vehicle controls (100%). D and E, purified His-tagged Tiam-1 DH/PH domain was added at a concentration of 2:1 to Rac1(G15A) beads that were preincubated with vehicle (Veh) or Ehop-016 or NSC23766 at the indicated concentrations for 1 h at 4 °C. Pull-downs were washed and immunoblotted with an anti-His antibody to detect His-Tiam-1 as a ∼45 kDa band. D, a representative Western blot (from the same experiment). Top, GST-Rac1(G15A) pull-downs; bottom, supernatants. E, quantification of percentage of Tiam-1 (DH/PH) domain associated with Rac1(G15A) beads in the presence or absence of EHop-016 or NSC23766. n = 3. Error bars, S.D. *, statistical significance compared with vehicle controls (p ≤ 0.05).
When Rac1(G15A) beads were incubated with a purified Tiam-1 DH/PH domain as described previously (67, 70), the active Tiam-1 was associated with the Rac1(G15A) beads. However, EHop-016 inhibited the interaction of Tiam-1 DH/PH domain with Rac1(G15A) at concentrations of ≥40 μm (supplemental Fig. S2). At 5 μm, EHop-016 did not affect the interaction of Tiam-1 DH/PH domain with Rac1(G15A) beads. At 50 μm EHop-016, there was a statistically significant 64% inhibition of Tiam-1/Rac1(G15A) interaction. In contrast, the parent compound NSC23766 inhibited the Rac1(G15A) interaction with purified Tiam-1 DH/PH domain at both 5 and 50 μm. Therefore, the concentrations at which EHop-016 inhibited Tiam-1/Rac1 interaction were 10 times higher than the physiological concentrations (2–4 μm) at which EHop-016 inhibited Rac activity or the interaction of Rac1(G15A) with Vav2 in MDA-MB-435 cell lysates.
EHop-016 Reduces Rac-regulated Cell Functions
Rac is a central regulator of lamellipodia and invadopodia that control directed mesenchymal migration and invasion of cancer cells (4). Therefore, we investigated the effect of EHop-016 on Rac activity and lamellipodia formation in the metastatic breast cancer cell line MDA-MB-231 and the highly metastatic variant of MDA-MB-435. As shown in Fig. 5A, for equal amounts of total protein, Rac expression and activity in MDA-MB-231 cells were less when compared with MDA-MB-435 cells. EHop-016 at 2 μm (double the IC50 for Rac inhibition) inhibited the Rac activity of MDA-MB-435 cells by 79% and by 93% at 4 μm. The Rac activity of MDA-MB-231 cells was also inhibited by EHop-016 with an IC50 of ∼3 μm (Fig. 5A). Therefore, EHop-016 is more efficient at inhibiting MDA-MB-435 cells with elevated Rac activity. Lamellipodia extension was determined in cells stained with rhodamine phalloidin to localize F-actin. Treatment for 24 h with EHop-016 at 2 and 4 μm inhibited lamellipodia formation in both MDA-MB-231 and MDA-MB-435 cells. Results show that close to 100% of control cells demonstrated lamellipodia and membrane ruffles. Following EHop-016 treatment, at 4 μm in MDA-MB-231 cells and both 2 and 4 μm in MDA MB-435 cells, only ∼30% of the cells extended lamellipodia. Therefore, EHop-016 at concentrations that inhibit Rac activity significantly inhibited lamellipodia extension to a similar extent, indicating a direct regulatory effect of Rac activity on lamellipodia extension (Fig. 5B). However, EHop-016 did not affect the extension of filopodia that are regulated by Cdc42 (71). These data indicate a specific role for EHop-016 in inhibiting Rac-directed actin structures.
FIGURE 5.
Effect of EHop-016 on Rac activity and the actin cytoskeleton of metastatic cancer cells. A, MDA-MB-231 or MDA-MB-435 cells were treated with vehicle (0) or EHop-016 at the indicated concentrations (2 or 4 μm) for 24 h. Cell lysates were subjected to a pull-down assay using a GST-CRIB domain of PAK and Western blotted for Rac (Rac1, -2, and -3). Representative Western blot (from the same experiment) of pull-downs immunostained for Rac-GTP (top row) or total Rac in cell lysate (bottom row). n = 3. B, MDA-MB-231 or MDA-MB-435 metastatic breast cancer cells were treated with vehicle or EHop-016 at 2 or 4 μm for 24 h to determine changes in actin cytoskeletal structures. Cells were fixed, permeabilized, and stained with rhodamine phalloidin to visualize F-actin. Top, representative micrographs are shown at ×600 magnification. Arrows, lamellipodia; arrowheads, filopodia. Bottom, percentage of cells that demonstrated lamellipodia was quantified for each treatment from 10 representative microscopic fields. Error bars, S.E. *, statistical significance compared with vehicle controls (p ≤ 0.001).
The effect of EHop-016 on Rac action was also determined by analyzing the activity of the Rac downstream effector PAK that regulates Rac-mediated lamellipodia extension and directed cell migration (4). By Western blotting with a specific antibody to the kinase active form of PAK1 with a phospho-Thr-423 (72), we show that EHop-016 dramatically inhibits PAK activity at both 2 and 4 μm (Fig. 6A). Because Rac/PAK signaling regulates directed cell migration, the effect of EHop-016 at 0–5 μm was determined on migration of MDA-MB-435 cells by using a Transwell assay. As shown in Fig. 6B, treatment with 2 and 5 μm EHop-016 reduced directed cell migration by ∼60% at concentrations that reduced Rac activity by ∼80% (Figs. 2 and 4). It is possible that Rac may not be the only regulator of cell migration in this highly invasive cancer cell line. Because equal cell numbers were placed on the top wells of the Transwell chambers and the migration assays were conducted for only 4 h, we do not expect effects of reduced cell viability or cell death to affect data interpretation.
FIGURE 6.
Effect of EHop-016 on Rac-regulated PAK activity and directed cell migration. A, MDA-MB-435 cells were treated with vehicle (0) or 2 or 4 μm EHop-016 for 24 h, and the cells were lysed and Western blotted for active phospho-PAKThr-423 (p-PAK; upper band) or total PAK (lower band). B, quantification of positive bands from Western blots (65 kDa) using ImageJ software. The integrated density of the phospho-PAK band was divided by the integrated density of total PAK band from the same sample. EHop-016 treatments are presented relative to vehicle controls (100%). n = 3. Error bars, S.D. *, statistical significance compared with vehicle controls (p ≤ 0.05). C, MDA-MB-435 cells treated with vehicle (0) or EHop-016 (1–5 μm) for 24 h were subjected to a Transwell migration assay. The number of cells that migrated to the underside of the top well in response to serum in the bottom well was quantified for each treatment. Top, representative micrographs of propidium iodide-stained cells for each treatment at ×200 magnification. Bottom, percentage of cells that migrated to the underside of a membrane with 8-μm diameter pores, relative to vehicle (100%). Results are shown for three biological replicates with two technical replicates per experiment. Error bars, S.E. *, statistical significance compared with vehicle controls (p ≤ 0.05).
These experiments were conducted in culture medium containing 10% FBS to recapitulate the endogenous cellular environment. Because serum contains activators of Rac, Rho, and Cdc42, this experimental design does not allow for the analysis of Rac regulation in the absence of other cytoskeletal regulators. Experiments testing the effect of Rac-specific regulators, such as epidermal growth factors (EGFR and HER2), are in progress.
Effect of EHop-016 on Cell Viability
We tested the viability of MDA-MB-231 and MDA-MB-435 cancer cells and MCF10A transformed mammary epithelial cells in the presence of EHop-016. EHop-016 at an IC50 of 1 or 2 μm did not significantly affect mammary epithelial or cancer cell viability (Fig. 7). At 5 μm, EHop-016 decreased MDA-MB-435 and MCF-10A cell number by 30% compared with controls, which was further decreased to 50% at 10 μm. Therefore, further inhibition of Rac and Cdc42 activity by EHop-016 at concentrations above 5 μm may lead to additional inhibition of Rac-mediated effects on cell cycle progression and growth.
FIGURE 7.
Effect of EHop-016 on cell viability. Cell viability of MDA-MB-231, MDA-MB-435, or MCF-10A cells was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide cell survival and proliferation kit (Millipore, Inc.). The mean values ± S.E. (error bars) (n = 3) are presented relative to vehicle (100%). *, statistical significance compared with vehicle controls (p ≤ 0.05).
DISCUSSION
Herein, we describe the synthesis and characterization of a potent and specific small molecule inhibitor of Rac, a key signaling protein that regulates cancer progression and metastasis. Recent studies have demonstrated that inhibition of Rac activity and thus cancer cell invasion is a viable strategy for the treatment of breast cancer metastasis (73, 74). Current small molecule inhibitors of Rac activity, such as NSC23766 and EHT 1864, are effective at high concentrations, ∼50–100 μm for NSC23766 and 10 μm for EHT 1864, and the inhibitory efficiency appears to be dependent on cell type. As we have previously shown, high metastatic variants of MDA-MB-435 demonstrate high endogenous Rac activity, without changes in Rac expression (18). Therefore, high Rac-GEF activity may explain the moderate biological activity of NSC23766 in metastatic cancer cells.
Our objective was to utilize the structure of NSC23766 as a lead for the development of a novel, more potent inhibitor that, similar to NSC23766, binds specifically into a surface groove of Rac1 known to be critical for GEF interaction (75). Docking studies demonstrated that EHop-016 binds to the effector domain of Rac1 with a deeper interaction in the switch 1 and switch II regions than NSC23766. Therefore, EHop-016 has the potential to block Rac GEFs distinct from the action of NSC23766. Moreover, EHop-016 was found to be a ∼100-fold more potent inhibitor of Rac activity than NSC23766 in the metastatic MDA-MB-435 cancer cell line. EHop-016 is also a 10–50-fold more effective inhibitor of Rac1 than the recently reported NSC23766 derivatives (60). Our data demonstrate that EHop-016 is specific for Rac isoforms Rac1 and Rac3. Cdc42, a close homolog that is not activated by the Rac GEFs Tiam-1, Trio, or P-Rex1 but is activated by Vav2 (46, 51, 67), is inhibited by EHop-016 at higher concentrations. Therefore, EHop-016 may be specific for Rac at lower physiologically relevant concentrations when Rac1 is the preferred partner of Vav2. Because of its structural similarity to NSC23766 as well as its biological activity profile and molecular docking findings, it is proposed that EHop-016 also interacts with the effector region of Rac, where it inhibits the binding of GEFs (48, 67, 70). In fact, we found that EHop-016 at 50 μm, a concentration much higher than its physiologically effective 2 μm concentration, may impede the interaction of active Tiam-1 with Rac1(G15A) in vitro. However, the Tiam-1-specific inhibitor NSC23766 blocked the interaction of Tiam-1 and Rac1(G15A) to a similar extent at a lower (5 μm) concentration compared with EHop-016, thus indicating that EHop-016 is not specific to Tiam-1. Similarly, EHop-016 is probably not specific to Trio, a GEF that shares the amino acid residue Trp-56 in Rac1 with Tiam-1, as a critical determinant for their activity (75).
The significant finding that EHop-016 inhibits the interaction of Vav2 with Rac1 at physiologically relevant concentrations is central to the further development of this compound as an inhibitor of cancer malignancy. Both Vav2 and Tiam-1 have been implicated in Rac-mediated transformation and invasion/metastasis (76, 77). To our knowledge, no specific inhibitors for Vav2/Rac interaction have been described. Using an established method for detecting active Rac GEFs from cell lysates (66, 69), we found that Vav2, and not Tiam-1 or Trio, was active in the MDA-MB-435 cell line in culture. It is possible that low active levels of Tiam-1 and Trio were not detected due to the limited sensitivity of immunoblotting. Another alternative is that the serum in culture medium activated Vav2 preferentially, and future studies will be conducted in quiescent cells following epidermal growth factor receptor/human epidermal growth factor receptor 2 stimulation, which is expected to activate both Vav2 and Tiam-1.
Vav2 has been shown to act as an exchange factor for Rac, Rho, and Cdc42 in vitro (50, 78). The increased Rho activity following EHop-016 treatment may be due to a complex biological response to Rac inhibition. Studies have shown that Rac activation can inhibit Rho activity and vice versa (1, 4, 10). Indeed, disparate roles have been proposed for Vav2-activated Rho and Rac in mammary epithelial cells (79), thus suggesting that EHop-016-mediated inhibition of Rac/Vav2 interaction may result in enhanced Vav2 availability for Rho activation. Binding studies of Rho and EHop-016 will be conducted in future studies to rule out a role for EHop-016 in direct activation of Rho.
We also show that cancer cell viability is not affected by EHop-016 at concentrations that inhibit Rac and actin cytoskeletal changes. Racs have been shown to affect cell survival via a number of signaling pathways, including phosphoinositide 3-kinase (PI3K), nuclear factor κB (NF-κB), and Jun kinase (JNK)/p38 mitogen-activated protein kinases (MAPK) (80). However, at the concentrations that inhibit Rac-mediated lamellipodia extension, we do not see significant effects of EHop-016 on cell viability. Cell viability may be affected at higher concentrations (≥5 mm), when EHop-016 inhibits both Rac and Cdc42 activities. This is expected because in addition to regulation of cell migration, Rho GTPases have also been implicated in regulation of the cell cycle (10). Our data demonstrating that EHop-016 does not affect the viability of mammary epithelial cells at effective concentrations suggest its utility as a viable anti-metastatic cancer therapeutic agent with only minor toxicity to normal cells. In preliminary studies, we have tested the effect of oral administration of EHop-016 to athymic nude mice at 1 mg/kg body weight once a week for 9 weeks and found no change in body weight or gross indications of toxicity (supplemental Fig. S3). Therefore, EHop-016 holds promise as a non-toxic and specific Rac inhibitor for further development as an anti-cancer metastasis therapeutic.
Results show that EHop-016 reduced lamellipodia and directed cell migration by 60–70% at concentrations that do not affect cell viability but inhibit Rac activity by ∼80%. At a concentration of 2 μm, EHop-016 exerted a dramatic reduction in lamellipodia formation without affecting Cdc42-induced filopodia extension. The concentration at which EHop-016 inhibited lamellipodia extension and cell migration is ∼10–50-fold less than the reported concentrations of NSC23766 and EHT 1864 required to inhibit lamellipodia extension and cell migration (48, 57, 81). This result is similar to our previous report of decreased MDA-MB-435 cell migration in the presence of other NSC23766 derivatives that inhibit Rac activity (57). Although a single report demonstrated increased migration of MDA-MB-435 and MDA-MB-231 metastatic breast cancer cells following 50–100 μm NSC23766, in our hands, treatment with 100 μm NSC23766 for 24 h resulted in a 75% decrease in MDA-MB-435 cell migration (data not shown). Moreover, there are several reports of decreased cell migration in metastatic breast cancer cells following direct inhibition of Rac1 and Rac3 by expression of dominant negative Rac and siRNA knockdown of Rac expression or by indirect inhibition of Rac by blocking upstream effector activity (13, 18, 57, 82–87).
In conclusion, we have shown that EHop-016 is an effective Rac-specific inhibitor at micromolar concentrations. EHop-016 substantially inhibits Vav2 interaction with Rac, Rac-activated PAK1, lamellipodia formation, and cell migration. Because Rac/PAK activity is central to cancer cell migration and invasion (30), EHop-016 appears to be a promising candidate for further development as a pharmacological inhibitor of Rac activity in metastatic cancer cells. In addition, EHop-016 could prove to be a valuable, more potent probe for the study of Rac-regulated cellular processes.
Supplementary Material
Acknowledgment
We thank Dr. José R. Rodríguez-Medina for use of the Olympus BX40 fluorescence microscope.
This work was supported, in whole or in part, by National Institutes of Health, NCRR, Grants G12RR035051 (to the University of Puerto Rico Medical Sciences Campus), 5U54CA096297 (to the University of Puerto Rico Medical Sciences Campus), and G12RR03035 (to Universidad Central del Caribe). This work was also supported by Department of Defense/United States Army Breast Cancer Research Program Grants W81XWH-07-1-0330 and 3SC3GM084824-02S1 (to S. D.). A provisional patent application, number 61/536,069, “Novel Small-Molecule Inhibitors of Rac 1 in Metastatic Breast Cancer,” is pending.

This article contains supplemental Figs. S1–S3.
- mTOR
- mammalian target of rapamycin
- IC50
- half maximal inhibitory concentration
- GTP
- guanosine-5′-trisphosphate
- Cdc42
- cell division cycle 42
- PAK
- p21-activated kinase
- GEF
- guanine nucleotide exchange factor
- p-Rex 1
- phosphatidylinositol 1,4,5-trisphosphate-dependent Rac exchanger 1
- DH
- Dbl homology
- PH
- pleckstrin homology
- CRIB
- Cdc42 and Rac interactive binding.
REFERENCES
- 1. Ridley A. J. (2006) Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 16, 522–529 [DOI] [PubMed] [Google Scholar]
- 2. Mack N. A., Whalley H. J., Castillo-Lluva S., Malliri A. (2011) The diverse roles of Rac signaling in tumorigenesis. Cell Cycle 10, 1571–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lazer G., Katzav S. (2011) Guanine nucleotide exchange factors for RhoGTPases. Good therapeutic targets for cancer therapy? Cell. Signal. 23, 969–979 [DOI] [PubMed] [Google Scholar]
- 4. Parri M., Chiarugi P. (2010) Rac and Rho GTPases in cancer cell motility control. Cell Commun. Signal. 8, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mulloy J. C., Cancelas J. A., Filippi M. D., Kalfa T. A., Guo F., Zheng Y. (2010) Rho GTPases in hematopoiesis and hemopathies. Blood 115, 936–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pai S. Y., Kim C., Williams D. A. (2010) Rac GTPases in human diseases. Dis. Markers 29, 177–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vega F. M., Ridley A. J. (2008) Rho GTPases in cancer cell biology. FEBS Lett. 582, 2093–2101 [DOI] [PubMed] [Google Scholar]
- 8. Tang Y., Olufemi L., Wang M. T., Nie D. (2008) Role of Rho GTPases in breast cancer. Front. Biosci. 13, 759–776 [DOI] [PubMed] [Google Scholar]
- 9. Karlsson R., Pedersen E. D., Wang Z., Brakebusch C. (2009) Rho GTPase function in tumorigenesis. Biochim. Biophys. Acta 1796, 91–98 [DOI] [PubMed] [Google Scholar]
- 10. Hall A. (2005) Rho GTPases and the control of cell behavior. Biochem. Soc. Trans. 33, 891–895 [DOI] [PubMed] [Google Scholar]
- 11. Lin M., van Golen K. L. (2004) Rho-regulatory proteins in breast cancer cell motility and invasion. Breast Cancer Res. Treat. 84, 49–60 [DOI] [PubMed] [Google Scholar]
- 12. Kleer C. G., Griffith K. A., Sabel M. S., Gallagher G., van Golen K. L., Wu Z. F., Merajver S. D. (2005) RhoC-GTPase is a novel tissue biomarker associated with biologically aggressive carcinomas of the breast. Breast Cancer Res. Treat. 93, 101–110 [DOI] [PubMed] [Google Scholar]
- 13. Chan A. Y., Coniglio S. J., Chuang Y. Y., Michaelson D., Knaus U. G., Philips M. R., Symons M. (2005) Roles of the Rac1 and Rac3 GTPases in human tumor cell invasion. Oncogene 24, 7821–7829 [DOI] [PubMed] [Google Scholar]
- 14. Burbelo P., Wellstein A., Pestell R. G. (2004) Altered Rho GTPase signaling pathways in breast cancer cells. Breast Cancer Res. Treat. 84, 43–48 [DOI] [PubMed] [Google Scholar]
- 15. Qiu R. G., Chen J., Kirn D., McCormick F., Symons M. (1995) An essential role for Rac in Ras transformation. Nature 374, 457–459 [DOI] [PubMed] [Google Scholar]
- 16. Renshaw M. W., Lea-Chou E., Wang J. Y. (1996) Rac is required for v-Abl tyrosine kinase to activate mitogenesis. Curr. Biol. 6, 76–83 [DOI] [PubMed] [Google Scholar]
- 17. Azios N. G., Krishnamoorthy L., Harris M., Cubano L. A., Cammer M., Dharmawardhane S. F. (2007) Estrogen and resveratrol regulate Rac and Cdc42 signaling to the actin cytoskeleton of metastatic breast cancer cells. Neoplasia 9, 147–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baugher P. J., Krishnamoorthy L., Price J. E., Dharmawardhane S. F. (2005) Rac1 and Rac3 isoform activation is involved in the invasive and metastatic phenotype of human breast cancer cells. Breast Cancer Res. 7, R965–R974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mira J. P., Benard V., Groffen J., Sanders L. C., Knaus U. G. (2000) Endogenous, hyperactive Rac3 controls proliferation of breast cancer cells by a p21-activated kinase-dependent pathway. Proc. Natl. Acad. Sci. U.S.A. 97, 185–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yoshida T., Zhang Y., Rivera Rosado L. A., Chen J., Khan T., Moon S. Y., Zhang B. (2010) Blockade of Rac1 activity induces G1 cell cycle arrest or apoptosis in breast cancer cells through down-regulation of cyclin D1, survivin, and X-linked inhibitor of apoptosis protein. Mol. Cancer Ther. 9, 1657–1668 [DOI] [PubMed] [Google Scholar]
- 21. Schnelzer A., Prechtel D., Knaus U., Dehne K., Gerhard M., Graeff H., Harbeck N., Schmitt M., Lengyel E. (2000) Rac1 in human breast cancer. overexpression, mutation analysis, and characterization of a new isoform, Rac1b. Oncogene 19, 3013–3020 [DOI] [PubMed] [Google Scholar]
- 22. Saci A., Cantley L. C., Carpenter C. L. (2011) Rac1 regulates the activity of mTORC1 and mTORC2 and controls cellular size. Mol. Cell 42, 50–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gulhati P., Bowen K. A., Liu J., Stevens P. D., Rychahou P. G., Chen M., Lee E. Y., Weiss H. L., O'Connor K. L., Gao T., Evers B. M. (2011) mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 71, 3246–3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dokmanovic M., Hirsch D. S., Shen Y., Wu W. J. (2009) Rac1 contributes to trastuzumab resistance of breast cancer cells. Rac1 as a potential therapeutic target for the treatment of trastuzumab-resistant breast cancer. Mol. Cancer Ther. 8, 1557–1569 [DOI] [PubMed] [Google Scholar]
- 25. Rosenblatt A. E., Garcia M. I., Lyons L., Xie Y., Maiorino C., Désiré L., Slingerland J., Burnstein K. L. (2011) Inhibition of the Rho GTPase, Rac1, decreases estrogen receptor levels and is a novel therapeutic strategy in breast cancer. Endocr. Relat. Cancer 18, 207–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Singh A., Karnoub A. E., Palmby T. R., Lengyel E., Sondek J., Der C. J. (2004) Rac1b, a tumor-associated, constitutively active Rac1 splice variant, promotes cellular transformation. Oncogene 23, 9369–9380 [DOI] [PubMed] [Google Scholar]
- 27. Stallings-Mann M., Radisky D. (2007) Matrix metalloproteinase-induced malignancy in mammary epithelial cells. Cells Tissues Organs 185, 104–110 [DOI] [PubMed] [Google Scholar]
- 28. Jordan P., Brazåo R., Boavida M. G., Gespach C., Chastre E. (1999) Cloning of a novel human Rac1b splice variant with increased expression in colorectal tumors. Oncogene 18, 6835–6839 [DOI] [PubMed] [Google Scholar]
- 29. Matos P., Jordan P. (2008) Increased Rac1b expression sustains colorectal tumor cell survival. Mol. Cancer Res. 6, 1178–1184 [DOI] [PubMed] [Google Scholar]
- 30. Whale A., Hashim F. N., Fram S., Jones G. E., Wells C. M. (2011) Signaling to cancer cell invasion through PAK family kinases. Front. Biosci. 16, 849–864 [DOI] [PubMed] [Google Scholar]
- 31. Dummler B., Ohshiro K., Kumar R., Field J. (2009) Pak protein kinases and their role in cancer. Cancer Metastasis Rev. 28, 51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kichina J. V., Goc A., Al-Husein B., Somanath P. R., Kandel E. S. (2010) PAK1 as a therapeutic target. Expert Opin. Ther. Targets 14, 703–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yi C., Maksimoska J., Marmorstein R., Kissil J. L. (2010) Development of small-molecule inhibitors of the group I p21-activated kinases, emerging therapeutic targets in cancer. Biochem. Pharmacol. 80, 683–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bishop A. L., Hall A. (2000) Rho GTPases and their effector proteins. Biochem. J. 348, 241–255 [PMC free article] [PubMed] [Google Scholar]
- 35. Baranwal S., Alahari S. K. (2011) Curr. Drug Targets 12, 1194–1201 [DOI] [PubMed] [Google Scholar]
- 36. Deacon S. W., Peterson J. R. (2008) Chemical inhibition through conformational stabilization of Rho GTPase effectors. Handb. Exp. Pharmacol. 186, 431–460 [DOI] [PubMed] [Google Scholar]
- 37. Jaffe A. B., Hall A. (2005) Rho GTPases. Biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21, 247–269 [DOI] [PubMed] [Google Scholar]
- 38. Rossman K. L., Der C. J., Sondek J. (2005) GEF means go. Turning on RHO GTPases with guanine nucleotide exchange factors. Nat. Rev. Mol. Cell Biol. 6, 167–180 [DOI] [PubMed] [Google Scholar]
- 39. Schmidt A., Hall A. (2002) Guanine nucleotide exchange factors for Rho GTPases. Turning on the switch. Genes Dev. 16, 1587–1609 [DOI] [PubMed] [Google Scholar]
- 40. Bernards A. (2003) GAPs galore! A survey of putative Ras superfamily GTPase-activating proteins in man and Drosophila. Biochim. Biophys. Acta 1603, 47–82 [DOI] [PubMed] [Google Scholar]
- 41. Palmby T. R., Abe K., Karnoub A. E., Der C. J. (2004) Vav transformation requires activation of multiple GTPases and regulation of gene expression. Mol. Cancer Res. 2, 702–711 [PubMed] [Google Scholar]
- 42. Miller S. L., DeMaria J. E., Freier D. O., Riegel A. M., Clevenger C. V. (2005) Novel association of Vav2 and Nek3 modulates signaling through the human prolactin receptor. Mol. Endocrinol. 19, 939–949 [DOI] [PubMed] [Google Scholar]
- 43. Sastry S. K., Rajfur Z., Liu B. P., Cote J. F., Tremblay M. L., Burridge K. (2006) PTP-PEST couples membrane protrusion and tail retraction via VAV2 and p190RhoGAP. J. Biol. Chem. 281, 11627–11636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Minard M. E., Kim L. S., Price J. E., Gallick G. E. (2004) The role of the guanine nucleotide exchange factor Tiam1 in cellular migration, invasion, adhesion, and tumor progression. Breast Cancer Res. Treat. 84, 21–32 [DOI] [PubMed] [Google Scholar]
- 45. Brantley-Sieders D. M., Zhuang G., Vaught D., Freeman T., Hwang Y., Hicks D., Chen J. (2009) Host deficiency in Vav2/3 guanine nucleotide exchange factors impairs tumor growth, survival, and angiogenesis in vivo. Mol. Cancer Res. 7, 615–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zugaza J. L., López-Lago M. A., Caloca M. J., Dosil M., Movilla N., Bustelo X. R. (2002) Structural determinants for the biological activity of Vav proteins. J. Biol. Chem. 277, 45377–45392 [DOI] [PubMed] [Google Scholar]
- 47. Liu B. P., Burridge K. (2000) Vav2 activates Rac1, Cdc42, and RhoA downstream from growth factor receptors but not β1 integrins. Mol. Cell Biol. 20, 7160–7169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gao Y., Dickerson J. B., Guo F., Zheng J., Zheng Y. (2004) Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc. Natl. Acad. Sci. U.S.A. 101, 7618–7623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chrencik J. E., Brooun A., Zhang H., Mathews I. I., Hura G. L., Foster S. A., Perry J. J., Streiff M., Ramage P., Widmer H., Bokoch G. M., Tainer J. A., Weckbecker G., Kuhn P. (2008) Structural basis of guanine nucleotide exchange mediated by the T-cell essential Vav1. J. Mol. Biol. 380, 828–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Abe K., Rossman K. L., Liu B., Ritola K. D., Chiang D., Campbell S. L., Burridge K., Der C. J. (2000) Vav2 is an activator of Cdc42, Rac1, and RhoA. J. Biol. Chem. 275, 10141–10149 [DOI] [PubMed] [Google Scholar]
- 51. Sosa M. S., Lopez-Haber C., Yang C., Wang H., Lemmon M. A., Busillo J. M., Luo J., Benovic J. L., Klein-Szanto A., Yagi H., Gutkind J. S., Parsons R. E., Kazanietz M. G. (2010) Identification of the Rac-GEF P-Rex1 as an essential mediator of ErbB signaling in breast cancer. Mol. Cell 40, 877–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Montero J. C., Seoane S., Ocaña A., Pandiella A. (2011) P-Rex1 participates in Neuregulin-ErbB signal transduction and its expression correlates with patient outcome in breast cancer. Oncogene 30, 1059–1071 [DOI] [PubMed] [Google Scholar]
- 53. Thomas E. K., Cancelas J. A., Chae H. D., Cox A. D., Keller P. J., Perrotti D., Neviani P., Druker B. J., Setchell K. D., Zheng Y., Harris C. E., Williams D. A. (2007) Rac guanosine triphosphatases represent integrating molecular therapeutic targets for BCR-ABL-induced myeloproliferative disease. Cancer Cell 12, 467–478 [DOI] [PubMed] [Google Scholar]
- 54. Binker M. G., Binker-Cosen A. A., Gaisano H. Y., Cosen-Binker L. I. (2008) Inhibition of Rac1 decreases the severity of pancreatitis and pancreatitis-associated lung injury in mice. Exp. Physiol. 93, 1091–1103 [DOI] [PubMed] [Google Scholar]
- 55. Akbar H., Cancelas J., Williams D. A., Zheng J., Zheng Y. (2006) Rational design and applications of a Rac GTPase-specific small molecule inhibitor. Methods Enzymol. 406, 554–565 [DOI] [PubMed] [Google Scholar]
- 56. Nassar N., Cancelas J., Zheng J., Williams D. A., Zheng Y. (2006) Structure-function-based design of small molecule inhibitors targeting Rho family GTPases. Curr. Top. Med. Chem. 6, 1109–1116 [DOI] [PubMed] [Google Scholar]
- 57. Hernández E., De La Mota-Peynado A., Dharmawardhane S., Vlaar C. P. (2010) Novel inhibitors of Rac1 in metastatic breast cancer. P. R. Health Sci. J. 29, 348–356 [PubMed] [Google Scholar]
- 58. Onesto C., Shutes A., Picard V., Schweighoffer F., Der C. J. (2008) Characterization of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases. Methods Enzymol. 439, 111–129 [DOI] [PubMed] [Google Scholar]
- 59. Shutes A., Onesto C., Picard V., Leblond B., Schweighoffer F., Der C. J. (2007) Specificity and mechanism of action of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases. J. Biol. Chem. 282, 35666–35678 [DOI] [PubMed] [Google Scholar]
- 60. Ferri N., Corsini A., Bottino P., Clerici F., Contini A. (2009) Virtual screening approach for the identification of new Rac1 inhibitors. J. Med. Chem. 52, 4087–4090 [DOI] [PubMed] [Google Scholar]
- 61. Beausoleil E., Chauvignac C., Taverne T., Lacombe S., Pognante L., Leblond B., Pallares D., Oliveira C. D., Bachelot F., Carton R., Peillon H., Coutadeur S., Picard V., Lambeng N., Désiré L., Schweighoffer F. (2009) Structure-activity relationship of isoform-selective inhibitors of Rac1/1b GTPase nucleotide binding. Bioorg. Med. Chem. Lett. 19, 5594–5598 [DOI] [PubMed] [Google Scholar]
- 62. Bouquier N., Vignal E., Charrasse S., Weill M., Schmidt S., Léonetti J. P., Blangy A., Fort P. (2009) A cell active chemical GEF inhibitor selectively targets the Trio/RhoG/Rac1 signaling pathway. Chem. Biol. 16, 657–666 [DOI] [PubMed] [Google Scholar]
- 63. Morris G. M., Goodsell D. S., Halliday R. S., Huey R., Hart W. E., Belew R. K., Olson A. J. (1998) Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 19, 1639–1662 [Google Scholar]
- 64. Huey R., Morris G. M., Olsen A. J., Goodsell D. S. (2007) A semi-empirical free energy force field with charge-based desolvation. J. Comput. Chem. 28, 1145–1152 [DOI] [PubMed] [Google Scholar]
- 65. Zheng Y., Nassar N., Skowronek K. R. (2010) United States Patent 17,826,982
- 66. García-Mata R., Wennerberg K., Arthur W. T., Noren N. K., Ellerbroek S. M., Burridge K. (2006) Analysis of activated GAPs and GEFs in cell lysates. Methods Enzymol. 406, 425–437 [DOI] [PubMed] [Google Scholar]
- 67. Worthylake D. K., Rossman K. L., Sondek J. (2000) Crystal structure of Rac1 in complex with the guanine nucleotide exchange region of Tiam1. Nature 408, 682–688 [DOI] [PubMed] [Google Scholar]
- 68. Rapley J., Tybulewicz V. L., Rittinger K. (2008) Crucial structural role for the PH and C1 domains of the Vav1 exchange factor. EMBO Rep. 9, 655–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Garrett T. A., Van Buul J. D., Burridge K. (2007) VEGF-induced Rac1 activation in endothelial cells is regulated by the guanine nucleotide exchange factor Vav2. Exp. Cell Res. 313, 3285–3297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Karnoub A. E., Worthylake D. K., Rossman K. L., Pruitt W. M., Campbell S. L., Sondek J., Der C. J. (2001) Molecular basis for Rac1 recognition by guanine nucleotide exchange factors. Nat. Struct. Biol. 8, 1037–1041 [DOI] [PubMed] [Google Scholar]
- 71. Fukata M., Nakagawa M., Kaibuchi K. (2003) Roles of Rho-family GTPases in cell polarization and directional migration. Curr. Opin. Cell Biol. 15, 590–597 [DOI] [PubMed] [Google Scholar]
- 72. Dharmawardhane S., Schürmann A., Sells M. A., Chernoff J., Schmid S. L., Bokoch G. M. (2000) Regulation of macropinocytosis by p21-activated kinase-1. Mol. Biol. Cell 11, 3341–3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sun D., Xu D., Zhang B. (2006) Rac signaling in tumorigenesis and as target for anticancer drug development. Drug Resist. Updat. 9, 274–287 [DOI] [PubMed] [Google Scholar]
- 74. Yamazaki D., Kurisu S., Takenawa T. (2005) Regulation of cancer cell motility through actin reorganization. Cancer Sci. 96, 379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gao Y., Xing J., Streuli M., Leto T. L., Zheng Y. (2001) Trp56 of Rac1 specifies interaction with a subset of guanine nucleotide exchange factors. J. Biol. Chem. 276, 47530–47541 [DOI] [PubMed] [Google Scholar]
- 76. Servitja J. M., Marinissen M. J., Sodhi A., Bustelo X. R., Gutkind J. S. (2003) Rac1 function is required for Src-induced transformation. Evidence of a role for Tiam1 and Vav2 in Rac activation by Src. J. Biol. Chem. 278, 34339–34346 [DOI] [PubMed] [Google Scholar]
- 77. Patel V., Rosenfeldt H. M., Lyons R., Servitja J. M., Bustelo X. R., Siroff M., Gutkind J. S. (2007) Persistent activation of Rac1 in squamous carcinomas of the head and neck. Evidence for an EGFR/Vav2 signaling axis involved in cell invasion. Carcinogenesis 28, 1145–1152 [DOI] [PubMed] [Google Scholar]
- 78. Movilla N., Dosil M., Zheng Y., Bustelo X. R. (2001) How Vav proteins discriminate the GTPases Rac1 and RhoA from Cdc42. Oncogene 20, 8057–8065 [DOI] [PubMed] [Google Scholar]
- 79. Duan L., Chen G., Virmani S., Ying G., Raja S. M., Chung B. M., Rainey M. A., Dimri M., Ortega-Cava C. F., Zhao X., Clubb R. J., Tu C., Reddi A. L., Naramura M., Band V., Band H. (2010) Distinct roles for Rho versus Rac/Cdc42 GTPases downstream of Vav2 in regulating mammary epithelial acinar architecture. J. Biol. Chem. 285, 1555–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Murga C., Zohar M., Teramoto H., Gutkind J. S. (2002) Rac1 and RhoG promote cell survival by the activation of PI3K and Akt, independently of their ability to stimulate JNK and NF-κB. Oncogene 21, 207–216 [DOI] [PubMed] [Google Scholar]
- 81. Nethe M., Anthony E. C., Fernandez-Borja M., Dee R., Geerts D., Hensbergen P. J., Deelder A. M., Schmidt G., Hordijk P. L. (2010) Focal-adhesion targeting links caveolin-1 to a Rac1 degradation pathway. J. Cell Sci. 123, 1948–1958 [DOI] [PubMed] [Google Scholar]
- 82. Chan A., Akhtar M., Brenner M., Zheng Y., Gulko P. S., Symons M. (2007) The GTPase Rac regulates the proliferation and invasion of fibroblast-like synoviocytes from rheumatoid arthritis patients. Mol. Med. 13, 297–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kirui J. K., Xie Y., Wolff D. W., Jiang H., Abel P. W., Tu Y. (2010) Gβγ signaling promotes breast cancer cell migration and invasion. J. Pharmacol. Exp. Ther. 333, 393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Han G., Fan B., Zhang Y., Zhou X., Wang Y., Dong H., Wei Y., Sun S., Hu M., Zhang J., Wei L. (2008) Positive regulation of migration and invasion by vasodilator-stimulated phosphoprotein via Rac1 pathway in human breast cancer cells. Oncol. Rep. 20, 929–939 [PubMed] [Google Scholar]
- 85. Shi H. Y., Stafford L. J., Liu Z., Liu M., Zhang M. (2007) Maspin controls mammary tumor cell migration through inhibiting Rac1 and Cdc42, but not the RhoA GTPase. Cell Motil. Cytoskeleton 64, 338–346 [DOI] [PubMed] [Google Scholar]
- 86. Sala G., Dituri F., Raimondi C., Previdi S., Maffucci T., Mazzoletti M., Rossi C., Iezzi M., Lattanzio R., Piantelli M., Iacobelli S., Broggini M., Falasca M. (2008) Phospholipase Cγ1 is required for metastasis development and progression. Cancer Res. 68, 10187–10196 [DOI] [PubMed] [Google Scholar]
- 87. Schunke D., Span P., Ronneburg H., Dittmer A., Vetter M., Holzhausen H. J., Kantelhardt E., Krenkel S., Müller V., Sweep F. C., Thomssen C., Dittmer J. (2007) Cyclooxygenase-2 is a target gene of Rho GDP dissociation inhibitor β in breast cancer cells. Cancer Res. 67, 10694–10702 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.