Abstract
Outer surface protein A (OspA) is a crucial protein in the infection of Borrelia burgdorferi causing Lyme disease. We studied conformational fluctuations of OspA with high-pressure 15N/1H two-dimensional NMR along with high-pressure fluorescence spectroscopy. We found evidence within folded, native OspA for rapid local fluctuations of the polypeptide backbone in the nonglobular single layer β-sheet connecting the N- and C-terminal domains with τ << ms, which may give the two domains certain independence in mobility and thermodynamic stability. Furthermore, we found that folded, native OspA is in equilibrium (τ >> ms) with a minor conformer I, which is almost fully disordered and hydrated for the entire C-terminal part of the polypeptide chain from β8 to the C-terminus. Conformer I is characterized with ΔG0 = 32 ± 9 kJ/mol and ΔV0 = −140 ± 40 mL/mol, populating only ∼0.001% at 40°C at 0.1 MPa, pH 5.9. Because in the folded conformer the receptor binding epitope of OspA is buried in the C-terminal domain, its transition into conformer I under in vivo conditions may be critical for the infection of B. burgdorferi. The formation and stability of the peculiar conformer I are apparently supported by a large packing defect or cavity located in the C-terminal domain.
Introduction
High-energy conformers, including low-lying excited states within the folded manifold and intermediates with an open structure or partial disorder, are key elements of protein structure that could be crucially important for function. However, the high-energy conformers are rarely detected directly by spectroscopy under physiological conditions due usually to their extremely low populations. Combining variable pressure techniques with NMR spectroscopy is a suitable method for direct detection of high-energy conformers of proteins, allowing investigation of their structures with atomic resolution (1–7). Pressure is a fundamental thermodynamic variable for defining protein conformational states in solution. The structure of a protein is closely coupled with its partial molar volume, and typically, as it unfolds under conditions close to physiological, there is a corresponding decrease in partial molar volume due to the hydration of internal cavities and the solvent contraction around the freed charged residues. Thus, in general, pressure can cause a shift in population among the ensemble of conformers of a protein from the highest volume conformer, which is usually the energetically most stable, most ordered conformer N, through various lower volume conformers with less ordered conformation, and finally to the lowest volume conformer, which is the energetically most unstable, fully unfolded conformer U. Therefore, pressure that shifts the population under isothermal conditions without increasing the kinetic energy of the system is a perturbation that can depict most subtle conformational fluctuations requiring little thermal energy.
Outer surface protein A (OspA) is a 31-kDa immunogenic lipoprotein expressed on the outer surface of Borrelia burgdorferi, the causative agent of Lyme disease. As disclosed by x-ray crystallography (8,9), the folded structure of OspA (Fig. 1) is quite unusual. It has an extended β-sheet structure made up of 21 consecutive antiparallel β-strands with only one C-terminal α-helix, as shown in Fig. 1. Both the N- and C-terminal ends are globular, but the two domains are connected by a linker consisting of an unusual nonglobular antiparallel single-layer β-sheet (Strands β8-β9-β10-β11). Furthermore, the C-terminal domain has an unusually large cavity (∼200 Å3 or 130 mL/mol), whereas the N-terminal domain has no significant cavities.
Figure 1.

Schematic representation of the secondary structure elements of OspA. (Sticks) Side chains of Leu-109, Val-199, and Trp-216. Strands 8–10 comprising the single layer β-sheet are labeled. The internal cavities (shaded spheres) of the protein (x-ray structure, PDB:1OSP) are shown with the program MOLMOL (43), as water inaccessible volumes.
OspA is responsible for the attachment of the spirochete to the uninfected host (e.g., a tick), through its binding to a receptor protein (10–12). Although the full-length OspA was once used as a vaccine against Lyme disease, a second-generation vaccine based on an OspA fragment (recombinant of OspA-vaccine) has been shown to effectively block the transmission of Lyme disease in mice (13). The second-generation vaccine is based on the C-terminal region of the protein (14) and includes several mutations to restore stability; without a stable conformation, the antibodies produced were not effective in conveying full immunity (13).
If we consider the need for preparing a useful vaccine for the Lyme disease, a fundamental question is how such a unique static structure as in Fig. 1 is needed for OspA to perform its function. Previous physicochemical studies of OspA were conducted on backbone dynamics using H/D exchange NMR (15–17), and NMR spin relaxation (18) on thermodynamics by calorimetry (19) and on mechanics using single molecular force spectroscopy (20). Interestingly, they have consistently identified multiple conformational states of the protein. The most stable conformer in solution, herewith designated as native state conformer (N), has been characterized by NMR spectroscopy (18,21,22) and small angle x-ray scattering in solution (23), which are essentially the same as that in Fig. 1 (8,9). By chemical, thermal, and mechanical perturbations, less stable conformers were identified whose structures are distinctly different from conformer N, and show a highly instable C-terminal relative to the N-terminal. The H/D exchange NMR analysis, coupled with the chemical perturbation experiments, also supports the presence of multiple conformations in the C-terminal region.
In this study, we use variable-pressure NMR spectroscopy to gain insight into the intrinsic conformational fluctuations of OspA in a wide range of conformational space from the basic folded to the highly disordered conformers in an effort to find clues to the molecular mechanism for infection. We utilize 1H one-dimensional and 15N/1H two-dimensional NMR spectroscopy between 0.3 and 250 MPa to investigate the conformational fluctuations of OspA under conditions close to physiological without chemical, thermal, or mechanical perturbations. Previously, we have conducted similar experiments on proteins such as dihydrofolate reductase, ubiquitin, and ubiquitin-like proteins, and were able to identify multiple biologically relevant, high-energy conformations (1–6). In addition to NMR analysis, we utilize high-pressure fluorescence spectroscopy between 0.3 and 400 MPa to monitor a major conformational change of the C-terminal domain with a single Trp residue at position 216 as monitor (Fig. 1).
Materials and Methods
Sample preparation
Uniformly 15N and 2H-labeled OspA was prepared as previously described, in which the 2H incorporation was ∼70% (23). The final protein concentration for the NMR study was 1.5 mM in 10 mM phosphate buffer, 50 mM NaCl, 0.02% NaN3, 10% 2H2O, 1 mM 2,2-dimethyl-2-silapentane-5-sulfonate (DSS), pH 5.9. For the Trp fluorescence study, the final protein concentration was 22 μM in 10 mM phosphate buffer, 50 mM NaCl, pH 5.9.
Variable pressure fluorescence measurements
Tryptophan emission spectra of OspA were collected from 0.1 MPa to 400 MPa at ∼50 MPa steps at temperatures of 10, 20, 30, and 40°C. The excitation wavelength from a xenon arc lamp was set at 295 nm with a slit-width of 5 nm and the emission at 310–500 nm was collected with a slit-width of 10 nm at a scanning speed of 500 nm/min. The shutter was kept open only when the emission was recorded.
Because the wavelength of Trp fluorescence emission is quite sensitive to its environment, the wavelength of maximum emission was followed as a function of pressure. The change in maximum wavelength was fitted to a two-state transition from the native state N to an intermediate state I (N ⇄ I) with an equilibrium constant K,
| (1) |
which changes by pressure according to
| (2) |
in which we assume a negligible change in differential compressibility between the two conformers with pressure. Here, λg(f) and λg(i) are the maximum wavelength of emission for native and intermediate, respectively, and λg(p) is the maximum wavelength of emission at pressure p. Here R is the gas constant, T is the absolute temperature, K is the equilibrium constant, and the values ΔGp and ΔG0 are the Gibbs free energy differences at pressure p and p0 (= 1 bar), respectively. The value ΔV0 is the partial molar volume difference between the two conformers.
Variable pressure NMR experiments
NMR experiments were performed at 40°C and pH 5.9 at pressures from 3 to 250 MPa on a DRX 800 spectrometer (Bruker BioSpin, Billerica, MA) combined with the on-line high-pressure NMR cell technique reported (25). The quantity of 3 MPa was chosen instead of 0.1 MPa just to avoid undesirable effects by bubbles in the sample solution on the spectrum.
Proton one-dimensional NMR spectra were measured with water-suppression of a 3-9-19 pulsed field gradient at a proton frequency of 800.16 MHz. 15N/1H transverse relaxation-optimized spectroscopy (TROSY) spectra (26) were measured combined with a water flip-back technique at a proton frequency of 800.16 MHz and a 15N frequency of 81.08 MHz. The 15N dimension was acquired with 256 increments and for the proton dimension 2048 complex points were collected with 80 scans at each increment. A delay was set to 1.5 s at each scan. At all pressures, 1H chemical shifts were referenced to the methyl signal of DSS and 15N chemical shifts were indirectly referenced to DSS (0 ppm for 1H). Data were processed with the XWIN-NMR (Bruker BioSpin), NMRPipe (27), and NMR View (28). The data points were extended to 2 k × 512 and 90° shifted sine-square window function was applied in both dimensions. With increasing pressure above 150 MPa, either a local or an overall unfolding takes place into another conformer I, then the original signal intensity I0 for site i may decrease to Ip. The decrease in the intensity, I0 – Ip, divided by the intensity Ip represents the equilibrium constant [I]/[N] at site i. Namely,
| (3) |
In actual applications, Ip may represent individual crosspeak intensities in two-dimensional TROSY spectra. The Gibbs energy difference ΔG between the two conformers is expressed as a function of pressure p by Eq. 2 under the assumption of a negligible change in differential compressibility between conformers N and I. By combining Eqs. 2 and 3, Ip is expressed as a function of pressure by
| (4) |
Ip values are fitted to Eq. 4 by two parameters, ΔG0 and ΔV0.
Results
Conformational fluctuation of OspA detected by variable-pressure Trp fluorescence
The single Trp residue of OspA is located at a strategic position (residue 216), facing the large cavity in the C-terminal domain (see Fig. 1). To gain insight into a global conformational fluctuation of OspA, we first carried out Trp fluorescence measurements of OspA at pH 5.9 in 10 mM phosphate buffer under variable pressure from 0.1 to 400 MPa at temperatures of 10, 20, 30, and 40°C. A dramatic spectral change was observed as pressure was increased from 0.1 to 400 MPa at 40°C (Fig. 2 a). Namely, the fluorescence emission shifted from 331 nm at 0.1 MPa to 354 nm at 400 MPa, showing that the single Trp residue (residue 216), completely buried at 0.1 MPa, becomes almost fully exposed to the solvent at 400 MPa, the midpoint of the transition being at ∼200 MPa (Fig. 2 b). The result indicated that OspA has a conformational fluctuation, by which a single Trp residue becomes fully exposed to the solvent at 40°C. Because the spectral change is reversible with pressure, we have performed thermodynamic analysis of the conformational transition, as will be described in Thermodynamic Characterization of the Partially Folded Conformer, below.
Figure 2.
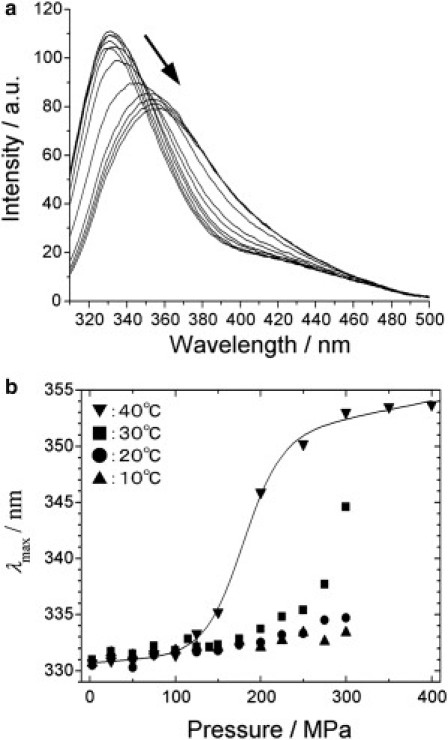
Variable-pressure fluorescence spectroscopy of OspA. (a) Tryptophan fluorescence spectra of OspA at 40°C at different pressures from 0.1 to 400 MPa. The protein concentration was 22 μM in 10 mM phosphate buffer and 50 mM NaCl with a pH of 5.9. (b) Plots of the maximum wavelength of emission at 10, 20, 30, and 40°C as a function of pressure.
Conformational fluctuations within the folded manifold: evidence from pressure-dependent 1H and 15N chemical shifts
15N/1H two-dimensional NMR experiments were carried out under variable pressure to investigate conformational fluctuations of OspA in site-specific detail. Fig. 3 shows a series of 15N/1H TROSY experiments at 40°C and pH 5.9 at pressures from 3 to 250 MPa. At 3 MPa, the 15N/1H crosspeaks corresponding to the folded structure are well dispersed in both 15N and 1H dimensions, and undergo continuous changes in chemical shifts with increasing pressure up to ∼150 MPa without the appearance of new peaks, showing that the spectral changes up to ∼150 MPa depict conformational changes within the folded conformer (N). Above 150 MPa, intensities of original crosspeaks are gradually lost, and a group of new peaks appear in the central part of the spectrum (Fig. 3). The entire spectral changes up to 250 MPa are reversible with pressure, showing that the spectral changes arise primarily from the shift of population among various conformers of OspA in solution.
Figure 3.

15N TROSY spectra of the OspA at 3, 50, 100, 150, 200, and 250 MPa at 40°C. (Inset) Region details the change in chemical shift of Val-199 that lies outside the plot.
Fig. 4, a and b, summarize 1H and 15N chemical shift changes (ΔδH and ΔδN), respectively, caused by pressure at 150 MPa against the residue number of OspA. Because the 15N/1H TROSY crosspeaks shifted continuously with pressure, assignments of 1H and 15N crosspeaks to specific residues at varying pressure were made simply by following the assignments preformed previously at ambient pressure (13). From the statistical thermodynamics relation between the compressibility and the volume fluctuation (29), the local conformational changes represented by these shifts in response to pressure may be taken to represent part of the microscopic volume fluctuation of OspA. Thus, the larger pressure shifts would generally indicate larger conformational fluctuations. We note that the majority of the shifts are positive, meaning high-frequency shifts, the value averaged over residues (±RMSD) being 0.05 ± 0.06 ppm/150 MPa and 0.4 ± 0.3 ppm/150 MPa for 1H and 15N, respectively. Pressure-induced high-frequency shifts are generally observed for hydrogen-bonded NH protons, which are mainly attributable to the shortening of the NH-O hydrogen bonds in addition to relatively minor effects from changes in secondary and tertiary structures (30). The average 1H shift (0.05 ± 0.06 ppm/150 MPa) is comparable to the average shift (0.059 ± 0.056 ppm/200 MPa, corresponding to 0.044 ± 0.042 ppm/150 MPa) for 1H hydrogen-bonded to carbonyls in BPTI (30), for which the average NH-O=C bond distance shortened is estimated to be 0.029 ± 0.117 Å at 200 MPa (31). Thus, we expect the average shrinkage of NH-O=C hydrogen bonds in folded OspA are comparable to that in BPTI, despite its unique 21 consecutive antiparallel β-strands.
Figure 4.

(a and b) Changes in 1H (a) and 15N (b) chemical shifts between 3 and 150 MPa at 40°C. The average changes in 1H and15N shifts between 3 and 150 MPa are 0.05 ± 0.06 ppm and 0.4 ± 0.3 ppm, respectively. (Solid line) Average value. (First and second broken lines) Average values of average ± RMSD and ± 2 × RMSD, respectively. (c and d) Residues showing anomalous 1H (c) or 15N (d) chemical shift changes are marked. (Red) Residues with the deviation from the average larger than 2|RMSD|; (yellow) deviation between |RMSD| and 2|RMSD|.
On the other hand, we found in Fig. 4, a and b, that the 1H and 15N shifts are considerably heterogeneous over residues. Of particular interest is the finding that a number of crosspeaks showed large deviations from the average. They originate from residues 52, 57, 59, 61, 85, 109, 126, 135, 199, 206, 212, 232, 243, 262, and 264 for 1H and from residues 57, 59, 60, 101, 110, 115, 123, 126, 139, 171, 213, 239, and 243 for 15N, which are marked by red (>2|RMSD|) and yellow (>|RMSD|) in the crystal structure in Fig. 4, c and d, respectively. Larger deviations of 1H shifts from the average are observed mainly at the edge of a β-strand, suggesting that larger conformational changes may take place locally at β-strand ends. However, these changes may include changes in secondary and tertiary structures in addition to changes in the NH-O hydrogen-bond distances (31).
We now focus on 15N shifts, which, in addition to changes in hydrogen bond, are considered to be sensitive to changes in backbone torsion angles ϕ, ψ, and χ1 (4). In fact, in functional proteins such as DHFR (4), ubiquitin (5), and hen lysozyme (32), we have detected distinctly large 15N shifts for selected residues, attributable to torsional motions or hingelike motions of the polypeptide chain in the time range of μs ∼ ms (4,5,32). Incidentally, we detected no such anomalous 15N shifts in BPTI, whose folded structure is relatively rigid as a proteinase inhibitor (33). In Fig. 4 d, we find the anomalous 15N shifts that deviate considerably from the average for residues 59 and 60 between β3 and β4, for a number of residues of β7-β10 located in the linker region (β8-β9-β10-β11), and also for residues 239 and 243 in the receptor binding site close to the hydrophobic cavity. Strands β7–β10 belong to the linker region consisting of an unusual nonglobular antiparallel single-layer β-sheet (strands β8-β9-β10-β11), showing the possibility that these are the sites of somewhat unusual conformational fluctuations. Because 1H and 15N signals shift continuously with the variation of pressure, any fluctuations causing the shift must be rapid in the NMR timescale, i.e., τ << ms. The local internal motions found in the nonglobular linker region connecting the two globular domains are expected to contribute to the independence of the two domains in their mobility and stability.
Conformational fluctuations involving a partially folded conformer: Evidence from pressure-dependent 1H/15N crosspeak intensities
As the pressure is increased above 150 MPa, approximately two-thirds of the TROSY crosspeaks of the folded conformer lose their intensities significantly, whereas the intensities of the rest remain unaffected even at 250 MPa (Fig. 3). Concomitantly with the decrease of the crosspeaks from the folded conformer (N), new crosspeaks appear in a narrow spectral region (1H: 8–9 ppm, 15N: 110–130 ppm), typical for a disordered and mobile polypeptide chain (Fig. 3, 8–8.5 ppm for proton). The intensities of individual crosspeaks (measured as peak volumes) of the conformer N are plotted as a function of pressure in Fig. 5 a, which show variations for different residues, among which the rapidly decaying group (blue) are distinct against the slowly or no-decay group (red).
Figure 5.

(a) Changes in resonance intensities versus pressure. (Red) Residues unperturbed by pressure; (blue) residues losing intensity; (green) residues showing a mountain-like transition. (Solid circles) Averages for the three groups. ΔG0 and ΔV0 values are obtained from the average transition curves. (b) Resonance intensities relative to those at atmospheric pressure as a function of residue number at 225 and 250 MPa. (Green stars) Residues showing mountain-like transitions with pressure. (c) Mapping of the blue and red residues onto a crystal structure of OspA. (Dark-blue spheres) Cavities. (d) Protection factors PF versus residue number. Errors were estimated from the standard deviation of noise on the base plane.
In Fig. 5 b, intensities of individual crosspeaks at 225 MPa and 250 MPa are shown as histograms normalized to those at 3 MPa. The histogram shows clearly that the crosspeak intensity is decreased for most residues spanning from β8 to β21, covering most of the nonglobular single-layer β-sheet (strands 8–10) and the C-terminal domain of the protein (marked blue in Fig. 5 c), except for a few residues (235–237) in the C-terminal. On the other hand, the crosspeak intensities from the rest of the residues, belonging to the N-terminal domain (marked red in Fig. 5 c), are almost unchanged. These spectral features clearly indicate that we have a distinct subensemble of conformers, conformer I, in which the N-terminal domain remains folded but the central β-sheet region and the C-terminal domain are largely disordered. The continuous population change from N to I with increasing pressure indicates that conformer I is in equilibrium with conformer N at all pressures: N ⇄ I. Because the crosspeaks for N and I are separately observed for the same residues, the fluctuation between N and I must be slow in the NMR timescale, meaning τ >> ms.
In Fig. 5 a, we note in the low-pressure range (below 150 MPa) that, in addition to the blue and red groups, there is another group of crosspeaks (marked in green), showing marked initial gain in intensity with pressure, followed by a decrease in intensity as the pressure increases, giving clear mountain-like intensity profiles (Fig. 5, a and b). The green group residues, at least 20 in number, belong to β8–β11 and β16–β19, namely the single-layer β-sheet in the nonglobular linker region and part of the C-terminal domain, which eventually become disordered above 150 MPa. Slight mountain-like intensity profiles (measured as peak volumes) are occasionally encountered in high pressure two-dimensional 15N/1H NMR experiments of proteins (3), but such a strong anomaly in intensities as that shown in Fig. 5 a is not common, requiring special attention. Phenomenologically, this means that, at low-pressure 3 MP, the crosspeak intensities of the green group residues are lower than they should be at 3 MPa for some reason, but increase to their normal values with the increase of pressure to ∼150 MPa. Above ∼150 MPa, as the polypeptide chain becomes disordered in the β8–β11 and β16–β19 regions, the corresponding crosspeak intensities of the folded conformation will decrease again, as observed in Fig. 5 a.
The reason why, at 3 MPa, the crosspeak intensities of the green group residues are low is likely to be correlated to the broadening of the crosspeaks of the β8–β11 and β16–β19 segments due to the conformational dynamics in μs ∼ ms range, as predicted for the same region from 15N pressure shifts. In fact, large exchange contribution to the 15N transverse relaxation has been detected for many residues in the central β-sheet and the C-terminal domain at 0.1 MPa (18), in support of this 15N pressure-shift study. It is conceivable that the loss of crosspeak intensities from these regions caused by such relatively slow fluctuations has been overlooked at 0.1 MPa. Pressurization up to ∼150 MPa (Fig. 5 a) would then have recovered the loss by effectively changing the rate of fluctuation.
On the other hand, the loss of crosspeak intensity in the HSQC spectrum could also arise from self-association of OspA molecules in the relatively high protein concentration of OspA molecules (1.5 mM OspA or 46 mg/mL) employed in the high-pressure NMR experiment. Indeed, substantial broadening of signals due to self-association was clearly shown even for one-dimensional 1H NMR spectrum in a protein Streptomyces subtilisin inhibitor (23 kDa) in the concentration range of 5–40 mg/mL (34). Intriguingly, for OspA, the self-association could be more than just the nuisance in NMR spectroscopy. Pal et al. (11) showed experimental evidence that OspA interacts preferentially with other OspA molecules, which, as they suggested, could be an important step in the adherence of spirochete to the tick gut. In this regard, the peculiar mountain-like intensity profile of OspA may represent an important clue to a specific molecular association for function. In either case, elucidation of the true mechanism must await future studies.
Structural information from pressure-dependent 1H NMR signals of side chains
Investigation of the 1H one-dimensional NMR spectra shows the appearance of two methyl peaks at ∼−0.3 and −1.0 ppm at 3 MPa (Fig. 6). These methyl protons arise from Leu-109 and Val-199, which are located in separate domains of the protein, Leu-109 (β8) in a hydrophobic patch in the central β-sheet region and Val-199 in the C-terminal domain (see Fig. 1). The transition of the two side-chain signals occurs in the same pressure range, giving direct evidence for the cooperative transition of the C-terminal domain and the central β-sheet region into the conformer I with increasing pressure. In addition, because β-strands 16 and 17 interact with Trp-216 via a single unusual hydrogen bond between the backbone amide of Val-199 and the aromatic ring of Trp-216 (18), the transition of the former must be directly related to the transition of Trp-216 at high pressure. The pressure-induced changes in the fluorescence emission of Trp-216 are consistent with the above interpretation of the 1H one-dimensional NMR data. Taken together, the pressure-stabilized conformer I appears to have a highly disordered polypeptide chain at the C-terminal side of the protein (β8-C-terminus) with the rest of the molecule largely intact.
Figure 6.

1H one-dimensional NMR spectra of 1.5 mM OspA at pressures ranging from 3 MPa to 250 MPa. Two methyl peaks corresponding to residues L109 (a) and V199 (b) can be seen upfield of 0 ppm.
Thermodynamic characterization of the partially folded conformer
Our high-pressure NMR experiments have clearly shown that the folded conformer (designated by N) of OspA is in equilibrium with the partially folded conformer (designated by I), in which the N-terminal domain (β1–β8) is structured, whereas the rest (β8-C-terminus) is highly disordered. To characterize the partially folded conformer I thermodynamically with respect to N, we carried out the analysis of the pressure-dependent changes of 1H/15N TROSY crosspeak intensity according to Eqs. 3 and 4.
The free energy change ΔG0 and the volume change ΔV0 for the N to I transition were evaluated from the crosspeak intensity changes in Fig. 5 a (blue circles) of the rapidly decaying group (blue, residues in the C-terminal domain and the central β-sheet region), excluding those showing the anomalous (i.e., mountain-like) behavior. We determined ΔG0 and ΔV0 values to be 32 ± 9 kJ/mol and −140 ± 40 mL/mol, respectively (see Materials and Methods). The large volume decrease (ΔV0 = −140 ± 40 mL/mol) is noted, which appears consistent with the notion that approximately two-thirds of the polypeptide chain, namely the central β-sheet region and the C-terminal domain, are disordered and well hydrated in conformer I. Typically, as a protein unfolds, there is a corresponding decrease in partial molar volume due to the hydration of the cavities and the solvent contraction around the freed charged residues. The volume decrease of −140 ± 40 mL/mol for the disorder and hydration of roughly 160 residues may be compared with the typical volume decrease in the unfolding transition of a small globular protein (∼50–100 mL/mol for 10 kDa protein) (35).
The ΔG0 and ΔV0 values obtained from the fluorescence analysis of Trp-216 in the C-terminal domain are 21 ± 3 kJ/mol and −120 ± 20 mL/mol, respectively. The fairly large values of these parameters indicate that the conformational fluctuation involving Trp-216 is not local, but is correlated with a global transition of the protein from N to I. Table 1 summarizes the thermodynamic parameters ΔG0 and ΔV0 obtained from pressure perturbations both in NMR and fluorescence. For comparison, thermodynamic parameters obtained previously from other methods are also listed.
Table 1.
Thermodynamic parameters for transitions from the folded to intermediate conformers in OspA studied by different methods
| Conditions (protein concentration) | Method | Unfolding of structural segments | ΔG0 kJ/mol | ΔV0 mL/mol | ΔH0 kJ/mol |
|---|---|---|---|---|---|
| pH5.9, 40°C∗ (1.5 mM) | Pressure NMR | β8-C-terminus | 32 ± 9 | −140 ± 40 | |
| pH5.9, 40°C∗ (0.02 mM) | Pressure fluorescence | β8-C-terminus | 21 ± 3 | −120 ± 20 | |
| pH6.0, 37°C† (1 mM) | H/D exchange NMR | β8-C-terminus | 20–30§ | ||
| N-terminus-β8 | 45–60§ | ||||
| pH6.0, 40°C‡ (∼0.1 mM) | DSC | β8-C-terminus | 36¶ | 456‖ | |
| Total | 66¶ | 735‖ |
10 mM sodium phosphate buffer, 50 mM NaCl and 10% 2H2O (only for NMR).
10 mM sodium phosphate buffer, 650 mM NaCl and 100% 2H2O.
10 mM sodium phosphate buffer and 50 mM NaCl.
Gibbs free energy for underlying structural opening reaction for the backbone amide groups.
Gibbs free energy changes calculated from ΔH and ΔCp (19). Details are described in Nakagawa et al. (19).
Enthalpy changes ΔH of protein unfolding obtained from differential scanning calorimetry (DSC) studies extrapolated to 40°C by using the heat capacity changes ΔCp, which is assumed to be constant (19).
In the limited pressure range of the experiment (<250 MPa for NMR), we could not evaluate thermodynamic parameters (ΔG0 and ΔV0) for the transition from the partially folded conformer I to the totally unfolded conformer U. Above 200 MPa, a slight tendency of losing intensities for all 1H/15N HSQC crosspeaks (Fig. 3) appears to depict the onset of total unfolding of OspA (U). Thus, the simplest scheme for the overall conformational equilibrium of OspA at 40°C would be represented by an equilibrium N ⇌ I ⇌ U, schematically shown in Fig. 7, with estimated populations from Eqs. 2 and 3. We note that conformer I (∼0.001%) is indeed a minor conformer at 0.1 MPa.
Figure 7.

Conformational equilibrium of OspA depicted from high-pressure NMR. The folded conformer (N, left) has relatively large and rapid local fluctuations (τ < ms) in the nonglobular part of the protein consisting of a single-layer β-sheet (strands 8–10) connecting the N- and C-terminal domains. The folded conformer (N) also undergoes a large-amplitude fluctuation (ms << τ) into an intermediate conformer (I, middle) and the unfolded conformer (U, right). In the intermediate, approximately two-thirds of the polypeptide chain in the C-terminal side (β8-C-terminus) are disordered and hydrated, whereas the N-terminal side (β1–β8) remains largely intact. The overall equilibrium (with populations estimated from high-pressure NMR experiments at 40°C, pH 5.9, 0.1 MPa) is N (∼99.999%) ⇌ I (∼0.001%) ⇌ U (<<0.001%).
Discussion
Comparison of the present result with those from other methods
The low stability of the central and C-terminal region of the protein was also demonstrated using 1H/2H exchange NMR spectroscopy at pH 6.0 and 37°C, replotted in Fig. 5 d (15,17). In case that 1H/2H exchange experiments have been performed at the EX2 limit condition, the hydrogen exchange rates are related to the stability (ΔG0 or protection factor) underlying the structural opening reaction. Thus, the amide regions showing lower stability than the rest are thought to be involved in a transition from the basic folded conformer to the partially folded conformer. Most residues in the N-terminal domain (residues 20–108) show protection factors on the order of 105–7, ∼100 times larger than those of the rest (104–5), giving ΔG0 values of 25 kJ/mol (the C-terminal domain), 30 kJ/mol (the single-layer β-sheet), very close to the value obtained from the present variable-pressure NMR study (32 ± 9 kJ/mol, see Table 1).
The close identity not only of the structure but also of the thermodynamic stability of the partially folded conformers revealed from the two independent methods, pressure (1,2) and 1H/2H exchange (36,37), provides solid evidence that NMR spectroscopy under variable pressure and 1H/2H exchange NMR spectroscopy at 0.1 MPa are closely related methods useful for delineating rare conformers of proteins that exist under physiological conditions of 0.1 MPa. 1H/2H exchange NMR is more widely used, but a merit of high-pressure NMR is that it allows direct NMR detection and structural analysis of rare conformers (2,5,7).
In related experiments, differential scanning calorimetry (DSC) was used to study thermal unfolding of OspA at pH 6.0 (19). Two transition peaks were observed, one from N to an intermediately folded conformer and the other from the intermediately folded conformer to the fully unfolded conformer U (19). The ΔG0 value for the intermediately folded conformer as extrapolated to 40°C is 36 kJ/mol, close to the value (32 ± 9 kJ/mol) obtained for the partially unfolded conformer I from this study (see also Table 1). Single molecular atomic force microscopy also identified two mechanical unfolding steps (20). All these different experiments persistently show the existence of intermediate conformers whose N-terminal domain is intact and the rest is unfolded, structures closely related to the partially folded conformer I characterized in this work—giving a hint that conformer I may have an important role in the function of OspA.
Structural design and functional strategy of outer surface protein A
Thus far, the function of OspA has been discussed based on the conformational fluctuation largely within the folded conformer N (9,14,18,22–24). Here we consider a likely mechanism involving the partially folded conformer I.
OspA, the causative agent of Lyme disease, has an impressive folded structure with an array of 21 β-strands (Fig. 1). Both the N- and C-terminal ends are globular, but the two domains are connected by a linker consisting of an unusual nonglobular antiparallel single-layer β-sheet (strands β8-β9-β10-β11). We have found, in addition to the unique internal motions in the polypeptide backbone (τ << ms) in the single-layer β-sheet part, the unique array of 21 β-strands is not fully stable, but exists in equilibrium with a peculiar conformer I with its N-terminal domain folded but the entire segment from β8 to the C-terminal nearly fully unstructured.
Importantly, the C-terminal domain β18–β20 (residues 228–247) of OspA has been shown to contain the binding sites for the receptor in the tick gut and the binding sites for neutralizing antibodies (11,13). Although we have detected internal motions in μs ∼ ms timescale by spin relaxation around the receptor binding region (18) and by15N pressure shift specifically for residues 239 and 243, the residues responsible for receptor binding (236–237, 242–244) are largely buried in conformer N (8,11). On the other hand, the receptor binding residues would be largely exposed to the solvent if OspA assumes conformer I. This will greatly accelerate the binding of OspA to the receptor protein in the tick gut and thus accelerate the infection of B. burgdorferi to the tick. In support of this notion, it has been found that the unstructured OspA peptides (∼20 residues) incorporating receptor binding residues can compete with the full-length OspA in receptor binding (11).
The functional importance of the disordered segments of a protein for macromolecular recognition has long been recognized (38–40). Our estimate of the equilibrium population of conformer I under conditions close to physiological (e.g., at 0.1 MPa, 40°C, pH 5.9) gives only 10−2 ∼ 10−4% of the total population of OspA based on ΔG0 = 32 ± 9 kJ/mol, prohibiting even the recognition of its presence. It may appear quite unlikely that the minor conformer I plays an essential role in the receptor binding. However, some of the free energy gap 32 ± 9 kJ/mol may vary in the biological environment, and moreover the ΔG0 may easily be compensated by the extra stability gained by the binding of OspA with the receptor protein. If, on the contrary, the conformer I was more readily populated, the highly disordered C-terminus would most likely cause the protein to be subjected to enzymatic proteolysis, or certainly in danger for amyloid fibrils or other aggregate formation. Thus, we assume that the rarely populated conformer I may play a crucial role in the function of OspA, by allowing the protein, through a partial unfolding, to bind with critical receptors.
The distinct differential stability between the N-terminal domain (β1–β8) and the rest of the protein (β8-C terminus) in conformer I appears to be a rare motif relative to other proteins under study, making it an interesting protein target to investigate. In addition to the partially disordered C-terminus, looking at the crystal structure of OspA (Fig. 1), large cavities are present only in the C-terminal domain, but none in the N-terminal domain (Fig. 1). The combined cavity volume in the C-terminal domain (PDB: 1OSP) is ∼130 mL/mol (200 Å3) (which was estimated using a 1.4 Å scanning probe sizes using the computational program MOLMOL (41)), the hydration of which would cause a considerable loss of volume. In addition, the disruption of several Glu-Lys salt bridges in the nonglobular single-layer β-sheet region (β8–β11) could bring a significant decrease in volume, because the elimination of one salt-bridge is expected to contribute ∼−10–20 mL/mol by the electrostriction of the solvent (42). Indeed, in a previous high-pressure NMR study, Urbauer et al. (43) estimated a volume decrease of −60 mL/mol for a collapse of a salt-bridge and corresponding changes in void volumes in apo-calmodulin. It appears certain that the cavities and salt-bridges present in the folded structure N (Fig. 1) are the important structural elements that produce the peculiar conformer I when disrupted. In fact, it has recently been shown in hen lysozyme (44) that hydration of cavities can be a decisive factor for producing a selective disorder of a domain. Moreover, the size and the location of cavities are very likely to be a result of evolutional selection (44).
In conclusion, the most stable conformer in solution with the unique 21 consecutive antiparallel β-strands is not all that OspA has acquired through evolution. Hydration of the cavities dominating in the C-terminal domain would cause the selective unfolding of the C-terminal domain, assisted by the somewhat flexible linker in between the domains. This would expose the receptor binding site of OspA to interact efficiently with the receptor in the tick gut. The delicate interplay of the unique folded structure, the localized conformational dynamics, and the controlled thermodynamic stability is manifested in OspA for its function.
Acknowledgments
The authors thank reviewers for the constructive comments and Dr. Peter F. Flynn for discussions.
This research was supported by the U.S. National Institutes of Health, the National Science Foundation, the Japan Society for the Promotion of Science, the East Asia Summer Pacific Institute Fellowship, and the Academic Frontier Program (No. 07F010) of the Ministry of Education, Culture, Sports, Science and Technology of Japan. R.K. was supported by a fellowship from the Japan Society for the Promotion of Science.
References
- 1.Akasaka K., Yamada H. On-line cell high-pressure nuclear magnetic resonance technique: application to protein studies. Methods Enzymol. 2001;338:134–158. doi: 10.1016/s0076-6879(02)38218-1. [DOI] [PubMed] [Google Scholar]
- 2.Akasaka K. Probing conformational fluctuation of proteins by pressure perturbation. Chem. Rev. 2006;106:1814–1835. doi: 10.1021/cr040440z. [DOI] [PubMed] [Google Scholar]
- 3.Kitahara R., Akasaka K. Close identity of a pressure-stabilized intermediate with a kinetic intermediate in protein folding. Proc. Natl. Acad. Sci. USA. 2003;100:3167–3172. doi: 10.1073/pnas.0630309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitahara R., Sareth S., Akasaka K. High pressure NMR reveals active-site hinge motion of folate-bound Escherichia coli dihydrofolate reductase. Biochemistry. 2000;39:12789–12795. doi: 10.1021/bi0009993. [DOI] [PubMed] [Google Scholar]
- 5.Kitahara R., Yokoyama S., Akasaka K. NMR snapshots of a fluctuating protein structure: ubiquitin at 30 bar-3 kbar. J. Mol. Biol. 2005;347:277–285. doi: 10.1016/j.jmb.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 6.Kitahara R., Yamaguchi Y., Akasaka K. Evolutionally conserved intermediates between ubiquitin and NEDD8. J. Mol. Biol. 2006;363:395–404. doi: 10.1016/j.jmb.2006.07.074. [DOI] [PubMed] [Google Scholar]
- 7.Wilton D.J., Kitahara R., Williamson M.P. Pressure-dependent structure changes in barnase on ligand binding reveal intermediate rate fluctuations. Biophys. J. 2009;97:1482–1490. doi: 10.1016/j.bpj.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li H., Dunn J.J., Lawson C.L. Crystal structure of Lyme disease antigen outer surface protein A complexed with an Fab. Proc. Natl. Acad. Sci. USA. 1997;94:3584–3589. doi: 10.1073/pnas.94.8.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makabe K., Tereshko V., Koide S. Atomic-resolution crystal structure of Borrelia burgdorferi outer surface protein A via surface engineering. Protein Sci. 2006;15:1907–1914. doi: 10.1110/ps.062246706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Philipp M.T. Studies on OspA: a source of new paradigms in Lyme disease research. Trends Microbiol. 1998;6:44–47. doi: 10.1016/S0966-842X(97)01201-8. [DOI] [PubMed] [Google Scholar]
- 11.Pal U., de Silva A.M., Fikrig E. Attachment of Borrelia burgdorferi within Ixodes scapularis mediated by outer surface protein A. J. Clin. Invest. 2000;106:561–569. doi: 10.1172/JCI9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pal U., Li X., Fikrig E. TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell. 2004;119:457–468. doi: 10.1016/j.cell.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 13.Koide S., Yang X., Luft B.J. Structure-based design of a second-generation Lyme disease vaccine based on a C-terminal fragment of Borrelia burgdorferi OspA. J. Mol. Biol. 2005;350:290–299. doi: 10.1016/j.jmb.2005.04.066. [DOI] [PubMed] [Google Scholar]
- 14.Ding W., Huang X., Lawson C.L. Structural identification of a key protective B-cell epitope in Lyme disease antigen OspA. J. Mol. Biol. 2000;302:1153–1164. doi: 10.1006/jmbi.2000.4119. [DOI] [PubMed] [Google Scholar]
- 15.Pham T.N., Koide A., Koide S. A stable single-layer β-sheet without a hydrophobic core. Nat. Struct. Biol. 1998;5:115–119. doi: 10.1038/nsb0298-115. [DOI] [PubMed] [Google Scholar]
- 16.Yan S., Gawlak G., Koide S. Hydrophobic surface burial is the major stability determinant of a flat, single-layer β-sheet. J. Mol. Biol. 2007;368:230–243. doi: 10.1016/j.jmb.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan S., Kennedy S.D., Koide S. Thermodynamic and kinetic exploration of the energy landscape of Borrelia burgdorferi OspA by native-state hydrogen exchange. J. Mol. Biol. 2002;323:363–375. doi: 10.1016/s0022-2836(02)00882-3. [DOI] [PubMed] [Google Scholar]
- 18.Pawley N.H., Koide S., Nicholson L.K. Backbone dynamics and thermodynamics of Borrelia outer surface protein A. J. Mol. Biol. 2002;324:991–1002. doi: 10.1016/s0022-2836(02)01146-4. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa T., Shimizu H., Tamura A. Calorimetric dissection of thermal unfolding of OspA, a predominantly β-sheet protein containing a single-layer β-sheet. J. Mol. Biol. 2002;323:751–762. doi: 10.1016/s0022-2836(02)00974-9. [DOI] [PubMed] [Google Scholar]
- 20.Hertadi R., Gruswitz F., Ikai A. Unfolding mechanics of multiple OspA substructures investigated with single molecule force spectroscopy. J. Mol. Biol. 2003;333:993–1002. doi: 10.1016/j.jmb.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Koide S., Bu Z., Engelman D.M. Multistep denaturation of Borrelia burgdorferi OspA, a protein containing a single-layer β-sheet. Biochemistry. 1999;38:4757–4767. doi: 10.1021/bi982443+. [DOI] [PubMed] [Google Scholar]
- 22.Pham T.N., Koide S. NMR studies of Borrelia burgdorferi OspA, a 28 kDa protein containing a single-layer β-sheet. J. Biomol. NMR. 1998;11:407–414. doi: 10.1023/a:1008246908142. [DOI] [PubMed] [Google Scholar]
- 23.Bu Z., Koide S., Engelman D.M. A solution SAXS study of Borrelia burgdorferi OspA, a protein containing a single-layer β-sheet. Protein Sci. 1998;7:2681–2683. doi: 10.1002/pro.5560071223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang X., Yang X., Koide S. NMR identification of epitopes of Lyme disease antigen OspA to monoclonal antibodies. J. Mol. Biol. 1998;281:61–67. doi: 10.1006/jmbi.1998.1930. [DOI] [PubMed] [Google Scholar]
- 25.Yamada H., Nishikawa K., Akasaka K. Pressure-resisting cell for high-pressure, high-resolution nuclear magnetic resonance measurements at very high magnetic fields. Rev. Sci. Instrum. 2001;72:1463–1471. [Google Scholar]
- 26.Pervushin K., Riek R., Wüthrich K. Attenuated T2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc. Natl. Acad. Sci. USA. 1997;94:12366–12371. doi: 10.1073/pnas.94.23.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delaglio F., Grzesiek S., Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 28.Johnson B.A., Blevins R.A. NMR View: a computer program for the visualization and analysis of NMR data. J. Biomol. NMR. 1994;4:603–614. doi: 10.1007/BF00404272. [DOI] [PubMed] [Google Scholar]
- 29.Cooper A. Thermodynamic fluctuations in protein molecules. Proc. Natl. Acad. Sci. USA. 1976;73:2740–2741. doi: 10.1073/pnas.73.8.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H., Yamada H., Akasaka K. Effect of pressure on individual hydrogen bonds in proteins. Basic pancreatic trypsin inhibitor. Biochemistry. 1998;37:1167–1173. doi: 10.1021/bi972288j. [DOI] [PubMed] [Google Scholar]
- 31.Williamson M.P., Akasaka K., Refaee M. The solution structure of bovine pancreatic trypsin inhibitor at high pressure. Protein Sci. 2003;12:1971–1979. doi: 10.1110/ps.0242103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamatari Y.O., Yamada H., Smith L.J. Response of native and denatured hen lysozyme to high pressure studied by 15N/1H NMR spectroscopy. Eur. J. Biochem. 2001;268:1782–1793. [PubMed] [Google Scholar]
- 33.Akasaka K., Li H., Woodward C.K. Pressure response of protein backbone structure. Pressure-induced amide 15N chemical shifts in BPTI. Protein Sci. 1999;8:1946–1953. doi: 10.1110/ps.8.10.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inoue T., Akasaka K. Self association of Streptomyces subtilisin inhibitor: sedimentation equilibrium and 1H NMR studies. J. Biochem. 1987;102:1371–1378. doi: 10.1093/oxfordjournals.jbchem.a122183. [DOI] [PubMed] [Google Scholar]
- 35.Royer C.A. Revisiting volume changes in pressure-induced protein unfolding. Biochim. Biophys. Acta. 2002;1595:201–209. doi: 10.1016/s0167-4838(01)00344-2. [DOI] [PubMed] [Google Scholar]
- 36.Li R., Woodward C. The hydrogen exchange core and protein folding. Protein Sci. 1999;8:1571–1590. doi: 10.1110/ps.8.8.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maity H., Lim W.K., Englander S.W. Protein hydrogen exchange mechanism: local fluctuations. Protein Sci. 2003;12:153–160. doi: 10.1110/ps.0225803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jardetzky O., Akasaka K., Holmes K.C. Unusual segmental flexibility in a region of tobacco mosaic virus coat protein. Nature. 1978;273:564–566. doi: 10.1038/273564a0. [DOI] [PubMed] [Google Scholar]
- 39.Huber R., Bennett W.S., Jr. Functional significance of flexibility in proteins. Pure Appl. Chem. 1982;54:2489–2500. [Google Scholar]
- 40.Dyson H.J., Wright P.E. Intrinsically unstructured proteins and their functions. Nat. Rev. Mol. Cell Biol. 2005;6:197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- 41.Koradi R., Billeter M., Wüthrich K. MOLMOL: a program for display and analysis of macromolecular structures. J. Mol. Graph. 1996;14:51–55. doi: 10.1016/0263-7855(96)00009-4. 29–32. [DOI] [PubMed] [Google Scholar]
- 42.Asano T., le Noble W.J. Activation and reaction volumes in solution. Chem. Rev. 1978;78:407–489. doi: 10.1021/cr970461b. [DOI] [PubMed] [Google Scholar]
- 43.Urbauer J.L., Ehrhardt M.R., Wand A.J. High-resolution triple-resonance NMR spectroscopy of a novel calmodulin-peptide complex at kilobar pressures. J. Am. Chem. Soc. 1996;118:11329–11330. [Google Scholar]
- 44.Kamatari Y.O., Smith L.J., Akasaka K. Cavity hydration as a gateway to unfolding: an NMR study of hen lysozyme at high pressure and low temperature. Biophys. Chem. 2011;156:24–30. doi: 10.1016/j.bpc.2011.01.009. [DOI] [PubMed] [Google Scholar]


