SUMMARY
Post-transcriptional regulatory mechanisms superimpose “fine-tuning” control upon “on-off” switches characteristic of gene transcription. We have exploited computational modeling with experimental validation to resolve an anomalous relationship between mRNA expression and protein synthesis. Differential GAIT (Gamma-interferon Activated Inhibitor of Translation) complex activation repressed VEGF-A synthesis to a low, constant rate despite high, variable VEGFA mRNA expression. Dynamic model simulations indicated the presence of an unidentified, inhibitory GAIT element-interacting factor. We discovered a truncated form of glutamyl-prolyl tRNA synthetase (EPRS), the GAIT constituent that binds the 3’-UTR GAIT element in target transcripts. The truncated protein, EPRSN1, prevents binding of functional GAIT complex. EPRSN1 mRNA is generated by a remarkable polyadenylation-directed conversion of a Tyr codon in the EPRS coding sequence to a stop codon (PAY*). By low-level protection of GAIT element-bearing transcripts, EPRSN1 imposes a robust “translational trickle” of target protein expression. Genome-wide analysis shows PAY* generates multiple truncated transcripts thereby contributing to transcriptome expansion.
INTRODUCTION
The human genome encodes about 25,000 mRNAs that represent a lower bound of the expressed proteome in eukaryotic organisms due to expansion from upstream ORFs, alternative splicing, alternative polyadenylation, post-translational modification, and proteolysis. Alternative protein forms can acquire completely distinct activities, but more commonly exhibit a function related to the parental forms. One major protein family featuring a plethora of alternative structures and functions are the eukaryotic aminoacyl-tRNA synthetases (AARS). AARS are constitutive “house-keeping” enzymes, ubiquitous in the three kingdoms of life, and required for activation of cognate amino acids for interpretation of the genetic code (Ibba and Söll, 2000; Ribas de Pouplana and Schimmel, 2001). In addition to this ancient function, newly evolved noncanonical functions of multiple metazoan AARS depend on domains recently (in terms of evolutionary time) appended to the enzyme catalytic cores (Guo et al., 2010; Park et al., 2008). In previous studies of translational control of gene expression we have shown that the unique bifunctional AARS, glutamyl-prolyl tRNA synthetase (EPRS), exhibits an additional noncanonical function that is similarly dependent on a metazoa-specific appended domain (Mukhopadhyay et al., 2009; Ray et al., 2011).
IFN-γ treatment of myeloid cells robustly induces ceruloplasmin (Cp) and vascular endothelial growth factor-A (VEGF-A) mRNAs. However, synthesis of the proteins stops almost completely about 14–16 hr after IFN-γ treatment, despite undiminished mRNA level (Mazumder and Fox, 1999; Ray and Fox, 2007). Translational silencing requires binding of the GAIT (Interferon-Gamma-Activated Inhibitor of Translation) complex, consisting of EPRS as well as ribosomal protein L13a, NS1-associated protein 1 (NSAP1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), to structural elements in the target mRNA 3’-UTRs (Mazumder and Fox, 1999; Sampath et al., 2003) (Fig. 1A). GAIT complex assembly is driven by IFN-γ-inducible phosphorylation of EPRS and L13a, and consequent release from their parent macromolecular complexes, notably, the aminoacyl-tRNA multisynthetase complex (MSC) (Arif et al., 2011; Arif et al., 2009; Sampath et al., 2004) and the large ribosomal subunit, respectively (Mazumder et al., 2003; Mukhopadhyay et al., 2008). Assembly occurs in two stages. During the first 2-hr period, phospho-EPRS binds NSAP1 to form an inactive, pre-GAIT complex that does not bind GAIT-bearing target mRNA. About 12–14 hr later, phospho-L13a and GAPDH join the pre-GAIT complex to form the heterotetrameric GAIT complex that binds the 3’-UTR GAIT element of target mRNAs and blocks translation-initiation (Kapasi et al., 2007; Mazumder et al., 2001).
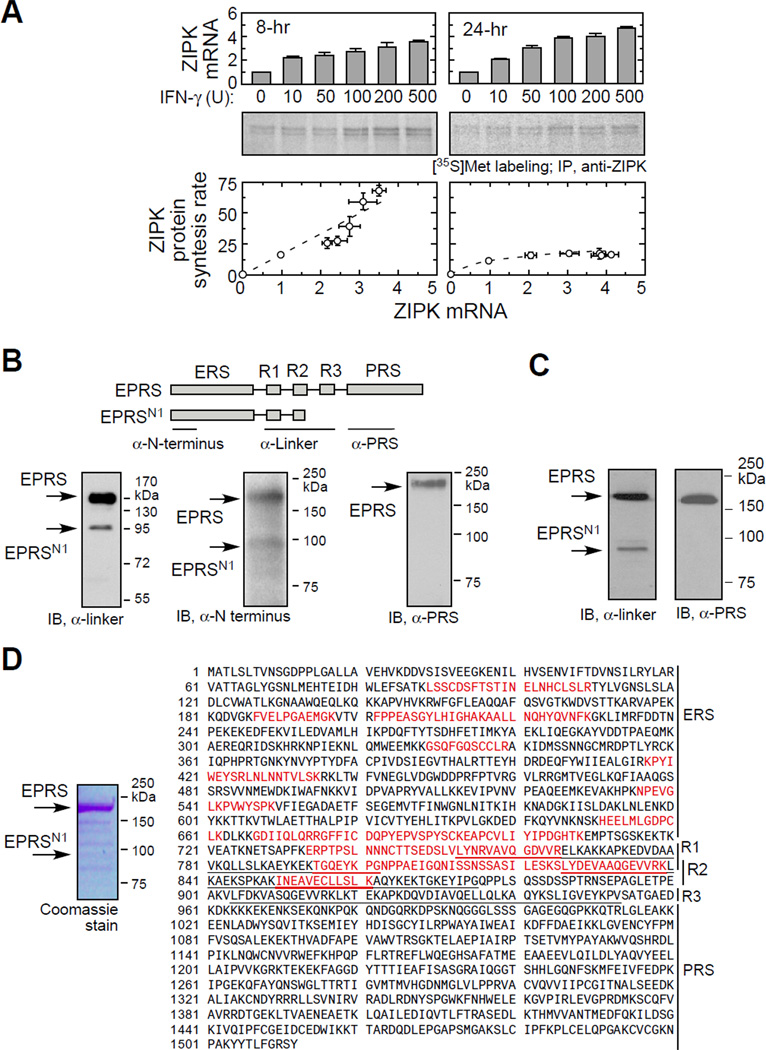
Figure 1. Differential Regulation of VEGF-A mRNA and Protein by the GAIT Pathway.
(A) Schematic of GAIT pathway of transcript-selective translational control. (B) IFN-γ-stimulated monocytic cells maintain low-level expression of VEGF-A. U937 cells were treated with IFN-γ at up to 500 U/ml for 8 (left) or 24 (right) hr. VEGF-A mRNA was determined by qRT-PCR (top), and VEGF-A protein and β-actin in cell lysates by immunoblot analysis (middle panels). VEGF-A protein as function of VEGF-A mRNA is shown (mean ± standard error, n = 3) (bottom).
EPRS has a special role in GAIT-mediated translational control, as it is solely responsible for recognition and interaction with GAIT elements in target mRNAs (Ray et al., 2009; Sampath et al., 2004). Metazoan EPRS is the only bifunctional AARS and catalyzes Glu and Pro ligation to cognate tRNAs (Ray et al., 2011). Human EPRS is a 172 kDa, 1512-amino acid, polypeptide consisting of three major domains. The N- and C-termini contain ERS and PRS catalytic domains, respectively, joined by a 300-amino acid linker containing 3 tandem WHEP-TRS (referred to as WHEP) domains. The WHEP domain is a 50-amino acid, helix-turn-helix structure (Cahuzac et al., 2000; Jeong et al., 2000) named after AARS’s containing them, i.e., WRS, HRS, and EPRS. The upstream WHEP repeat pair is essential for high-affinity binding to the GAIT RNA element, while the overlapping, downstream pair (and adjacent spacers) contain the phosphorylation sites essential for GAIT complex assembly (Jia et al., 2008).
L13a phosphorylation is a critical event determining the timing of GAIT system activation (Fig.1A). IFN-γ induces L13a phosphorylation by activation of a kinase cascade in which death-associated protein kinase-1 (DAPK) activates the proximal kinase, zipper-interacting protein kinase (ZIPK) (Mukhopadhyay et al., 2008). Remarkably, DAPK and ZIPK mRNAs contain functional 3’-UTR GAIT elements, and thus the inhibitory pathway activated by the kinases also suppresses their expression. Delayed feedback inhibition of DAPK and ZIPK restores the cell to the basal state and allows GAIT system re-activation by subsequent stimulation.
Here we exploit a dynamic modeling approach to understand the mechanism underlying a marked discrepancy in expression of GAIT system targets. Activation of monocytic cells for 24 hr by a wide range of concentrations of IFN-γ induces an equally broad range of expression of VEGF-A mRNA; however, differential mRNA expression does not correspond to differential protein expression, but rather to a constant, low-level “trickle” of synthesis of VEGF-A protein. Mathematical modeling of the known features of the GAIT system failed to replicate these observations. But the addition of a putative GAIT element-interacting factor to the model permitted a successful fit. The modeling was validated by discovery of a novel truncated EPRS mRNA and protein responsible for the observed aberrant relationship between VEGF-A mRNA and protein expression. Moreover, we have elucidated an RNA processing mechanism that generates the truncated EPRS mRNA and protein and contributes to transcriptome expansion.
RESULTS
Dynamic Modeling of GAIT System Predicts a GAIT Element-interacting Factor
During investigation of GAIT response dynamics, a dose-response experiment revealed an unexpected result. U937 monocytic cells were treated with a broad range of concentrations of IFN-γ for 8 hr, a time before induction of GAIT-mediated silencing activity, and for 24 hr, when GAIT silencing activity is in effect. At 8 hr, an increase in IFN-γ was associated with an increase of both VEGF-A mRNA and lysate protein (a relative measure of the rate of synthesis of the secreted protein) (Fig. 1B, left), and a near-linear relationship between VEGF-A mRNA and protein synthesis was observed. In contrast, at 24 hr VEGF-A protein was expressed at a low, near-constant amount over a wide range of IFN-γ treatment that was independent of the high, variable VEGF-A mRNA expression (Fig. 1B, right).
To understand the mechanism underlying this “translational trickle” of GAIT target protein expression observed at 24 hr, an initial mathematical model of the system dynamics was developed containing the key components that regulate gene expression. This mechanism-based model included delayed activation of the GAIT complex after 14 hr, and feedback inhibition of DAPK and ZIPK after 32 hr (Fig. 2A, without dashed compartment and vectors). Complete model details, initial conditions (Table S1), and parameters (Table S2) are described in Modeling Procedures in Supplemental Information. The maximum amount of GAIT complex formed was determined by the amount of free L13a in cells treated with IFN-γ for 24 hr, previously shown to be about 95% of total L13a (Mazumder et al., 2003). Total GAIT element-bearing target mRNA under the same condition was approximated by RNA-immunoprecipitation with anti-EPRS antibody. The ratio of GAIT complex to total target mRNA is about 50–100 in U937 cells and the upper limit value of 100 was used for model simulation. The kinetics and dose response of VEGF-A mRNA and protein following IFN-γ stimulation were taken as representative of GAIT targets as a whole. The model rate equations were represented by ordinary differential equations, and simulated results were compared to the experimental measurements. The simulation gave a linear relationship between target protein synthesis rate and mRNA at both 8 and 24 hr; moreover, protein synthesis at 24 hr was repressed almost completely (Fig. 2B, left). When the ratio of GAIT complex to target mRNA was varied (to influence the extent of silencing), the model still could not simulate the experimental non-linear relationship between total mRNA and protein synthesis rate. The best-fit value for the GAIT complex-to-target mRNA ratio was calculated by least-squares fitting of the model output to the experimental data. Although the fit was improved, the model failed to predict the observed non-linear relationship between VEGF-A mRNA and protein synthesis rate (Fig. 2B, right).
Figure 2. Dynamic Modeling of GAIT System Indicates a GAIT Element-Interacting Factor is Responsible for the “Translational Trickle” of VEGF-A Expression.
(A) Dynamic modeling of GAIT system. Key model features are: Gi, inactive (or unformed) GAIT complex; G, active GAIT complex; M, GAIT element-bearing mRNA; GM, GAIT complex-bound mRNA, P, newly synthesized GAIT target protein. Dashed compartment contains: F, GEIF; FM, GEIF-bound mRNA. Rate constants are indicated within triangles.
(B) Model simulations without GEIF. Rate of protein synthesis versus total mRNA before (8-hr) and after (24-hr) translational silencing using the initial model without GEIF (dashed lines); model simulations were done for range of IFN-γ concentrations (left). Model simulations at best-fit ratio of GAIT complex to target mRNA (ratio = 15) as determined by non-linear regression (right).
(C) Model simulations with GEIF. Simulations (dashed lines and curves) in presence of GEIF based on experimentally determined parameters (left), and in presence of a 2-fold higher or 75% lower amount of GEIF (right). See also Figure S1 and Tables S1 and S2.
We considered the potential role of a GAIT complex inhibitor that sequesters the complex from target mRNAs, but this model also generated a linear relationship between VEGF-A mRNA and protein synthesis rate at 24 hr (Fig. S1A-D). Likewise, a model featuring two inducible pools of mRNA with and without the GAIT element failed to simulate the non-linear relationship (Fig. S1E,F). Finally, we considered the possibility of a GAIT element-interacting factor (GEIF) that binds GAIT element-bearing mRNAs and prevents their interaction with the GAIT complex, while permitting translation (Fig. 2A, with dashed compartment and vectors). Upon inclusion of GEIF in an enhanced model, simulation produced the experimentally observed non-linear relationship between protein synthesis rate and mRNA at 24 hr, while maintaining the linear relationship at 8 hr (Fig. 2C, left). The species dynamics indicate the distribution of GAIT element-bearing mRNAs between the GAIT complex-bound, GEIF-bound, and unbound forms (Fig. S1G). The small amount of GEIF-bound, protected mRNA directs a constant “translational trickle” at 24 hr. Non-linear regression of the experimental data using the GEIF-containing model gave estimates of stoichiometry and dissociation constant of this putative binding factor (Table S2). We compared the squared residual (deviation of simulated protein synthesis rate from the experimental measurement) for various IFNγ0 inputs for several models. The best-fitting model, as indicated by the minimum value of the sum of squared residuals, was obtained for the enhanced model with GEIF (Fig. S1H). A p-value > 0.05 indicates no statistical difference between the model and experimental outputs, i.e., a good model fit. The enhanced model with GEIF (p = 0.23) satisfied this criterion, whereas the best-fit model without GEIF (p = 0.002) did not (see Supplemental Information for details). Increasing the amount of GEIF in the model increased the steady-state “trickle” level, whereas decreasing GEIF had the opposite effect (Fig. 2C, right).
Discovery of a Truncated EPRS Variant Containing Only the Upstream WHEP Domain Pair that Binds the GAIT Element
To determine the nature of the putative GEIF, i.e., RNA or protein, we examined whether expression of a second GAIT mRNA target exhibits similar dynamic characteristics. ZIPK mRNA contains a GAIT element with a predicted secondary structure similar to that of VEGF-A mRNA but with a completely unrelated primary sequence (Mukhopadhyay et al., 2008). U937 cells were treated with IFN-γ for 8 and 24 hr and ZIPK mRNA determined by RT-PCR and protein synthesis by [35S]methionine labeling followed by immunoprecipitation. The relationship between ZIPK mRNA expression and protein synthesis was similar to that observed for VEGF-A (Fig. 3A). The experimentally determined GAIT elements of VEGF-A and ZIPK have completely different mRNA sequences, but nearly identical calculated secondary structures (Mukhopadhyay et al., 2008; Ray and Fox, 2007). Thus the putative factor responsible for binding both elements is unlikely to be a sequence-specific microRNA (or other non-coding RNA), but rather an RNA-binding protein that recognizes the GAIT structural element common to all GAIT target mRNAs. Moreover, these results suggest that GEIF-directed relief of translational silencing might represent an additional layer of post-transcriptional regulation of the GAIT regulon.
Figure 3. Monocytic Cells Contain a C-terminal Truncated EPRS, EPRSN1.
(A) IFN-γ-stimulated monocytic cells maintain low-level synthesis of ZIPK. U937 cells were treated with IFN-γ as in (A). ZIPK mRNA was determined by qRT-PCR (top) and ZIPK synthesis determined by metabolic labeling with [35S]Met followed by immunoprecipitation (IP) with anti-ZIPK antibody (middle). ZIPK protein synthesis as function of ZIPK mRNA is shown (mean ± standard error, n = 3) (bottom).
(B) Detection of EPRSN1 protein in U937 cells with domain-specific antibodies. Cytosolic lysates from U937 cells were subjected to immunoblot analysis with antibodies directed against: EPRS linker domain, EPRS-N-terminal peptide, and PRS.
(C) Presence of EPRSN1 in human PBM. Immunoblot analysis with antibodies directed against the EPRS linker and PRS domains.
(D) Mass spectrometric analysis indicates EPRSN1 contains ERS, R1, and R2 domains, but lacks R3 and PRS domains. EPRSN1 was isolated by immunoprecipitation with anti-EPRS linker antibody and SDS-PAGE, and detected with Coomassie stain (left). Peptides detected by mass spectrometric analysis of 95-kDa EPRSN1 band (red) and WHEP domains (underlined) are shown (right).
To explore experimentally the presence of a GEIF, we considered previous studies showing the upstream pair of WHEP domains in the EPRS linker is sufficient for high-affinity binding to the GAIT RNA element, but does not contain the phosphorylation sites required for interaction with GAIT constituents (Arif et al., 2009; Jia et al., 2008). These findings suggest that a minor EPRS variant containing only the upstream pair of WHEP domains might be responsible for the observed trickle phenomenon. An approx. 95-kDa EPRS variant was detected in lysates from human monocytic U937 cells (Fig. 3B) and from primary human peripheral blood monocytes (PBM, Fig. 3C) by immunoblot with antibody against EPRS linker domain. Anti-N-terminus antibody also detected both proteins, but PRS-specific antibody recognized only full-length EPRS. The N-terminus-containing truncated form (designated EPRSN1) was analyzed by mass spectrometry following immunoprecipitation with anti-linker antibody and SDS-PAGE. Peptide coverage spanned ERS and the first two of three WHEP repeats in the linker domain (Fig. 3D).
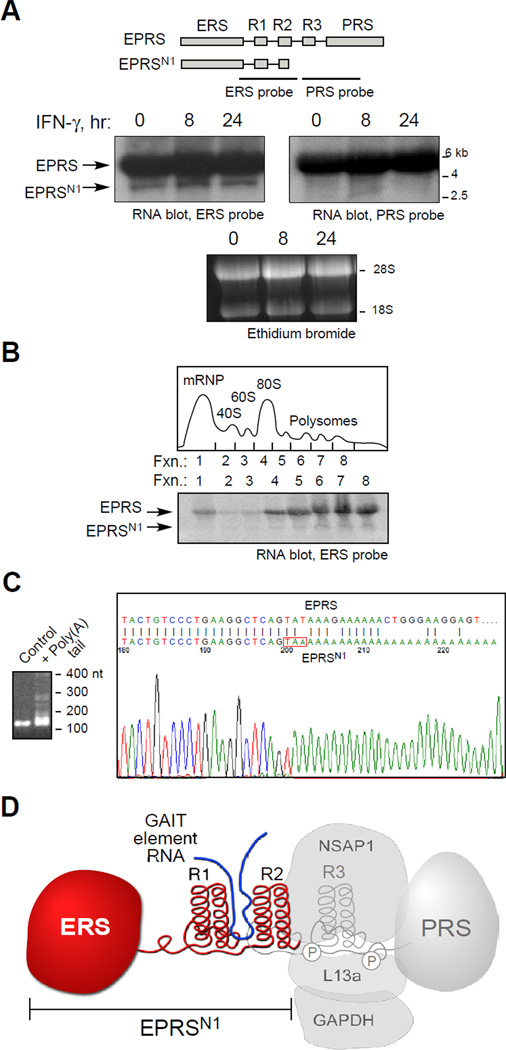
To investigate the mechanism underlying expression of truncated EPRS, total RNA from U937 cells was subjected to northern analysis using region-specific cDNA probes. Major 5.5-kb and minor 3-kb bands were detected with ERS-specific probe, but the smaller band was not detected by PRS-specific probe, suggesting transcript-directed synthesis of EPRSN1 (Fig. 4A). IFN-γ, which induces EPRS phosphorylation and release from the MSC (Arif et al., 2009), did not alter the amount of either transcript. Cytosolic lysates were fractionated on a sucrose gradient and total RNA from each fraction was subjected to Northern analysis with ERS-specific probe. Both full-length EPRS and EPRSN1 mRNAs were primarily found in the polysome fractions indicating efficient translation (Fig. 4B). Following G/I-tailing and RT-PCR, agarose gel electrophoresis detected polyadenylated products up to about 400 nt (Fig. 4C, left). Sequencing of the 200-nt fraction revealed a cDNA identical to the N-terminus of EPRS up to Tyr864-encoding UAU, which is replaced by a UAA stop codon followed by a poly(A) tail without an intervening 3’-UTR (Fig. 4C, right). EPRSN1 mRNA encodes ERS, WHEP R1, and the α-helices of WHEP R2, but not the last 12 amino acids of R2. The region containing R1 and R2 binds GAIT element RNA, whereas the linker region downstream of R2 contains both IFN-γ-dependent phosphorylation sites required for GAIT complex assembly and for GAIT function (Arif et al., 2009; Jia et al., 2008) (Fig. 4D).
Figure 4. Monocytic Cells Express EPRSN1 mRNA.
(A) EPRSN1 mRNA is expressed constitutively. Total RNA from U937 cells treated with IFN-γ was subjected to RNA blot analysis using ERS- and PRS-specific probes.
(B) EPRSN1 mRNA is translatable. U937 cell lysates were fractionated on a sucrose gradient and extracted RNA in each fraction was subjected to RNA blot analysis with ERS-specific probe.
(C) Nucleotide sequence of EPRSN1 mRNA. G/I stretches were added to poly(A)-tailed mRNA, RT-PCR was done using oligo-C and gene-specific upstream primers, and amplified fragment was cloned into T-vector and sequenced.
(D) Schematic of EPRSN1 structure in context of full-length EPRS and its phosphorylation sites and its binding partners.
EPRSN1 is Responsible for the Translational Trickle
To determine whether EPRSN1, like EPRS, resides in the MSC and is released upon stimulation, U937 cells were transfected with c-Myc-tagged EPRSN1 and incubated with IFN-γ for up to 24 hr. The MSC was collected by immunoprecipitation with antibody against KRS, an MSC constituent. As shown before, full-length EPRS is present in the MSC and released upon IFN-γ treatment; however, EPRSN1 is not detected in the MSC (Fig. 5A). The pre-GAIT and GAIT complexes were collected by immunoprecipitation with anti-NSAP1 antibody. As shown previously, IFN-γ induces translocation of full-length EPRS to the pre-GAIT complex (at 2 hr) where it is joined by other components to form the mature GAIT complex (at 14 hr), but neither complex contains EPRSN1. The inability to bind NSAP1 confirms that EPRSN1 does not form the functional, heterotetrameric GAIT complex.
Figure 5. EPRSN1 Binding to GAIT Element RNA Blocks Translational Repression.
(A) EPRSN1 exists as a free protein outside the MSC or GAIT complex. U937 cells were transfected with pcDNA3-EPRSN1-Myc plasmid and treated with IFN-γ for up to 24 hr. Cell lysates were immunoprecipitated with anti-KRS and anti-NSAP1 antibodies, and then subjected to immunoblot with anti-EPRS linker and anti-Myc tag antibodies.
(B) High-affinity binding of EPRSN1 to GAIT RNA element. Biotinylated, 29-nt Cp GAIT element RNA was immobilized on a streptavidin sensor chip. Binding of human EPRSN1 (left) and full-length rat EPRS (right) was determined by SPR and expressed as resonance units (RU).
(C) EPRSN1 interacts with VEGF-A mRNA in vivo. U937 cells were transfected with pcDNA3-EPRSN1-Myc or Myc vector control, and lysates immunoprecipitated (IP) with anti-Myc antibody. Extracted RNA was subjected to RT-PCR using primers specific for VEGF-A or β-actin mRNA.
(D) EPRSN1 inhibits GAIT complex binding to VEGF-A mRNA. U937 cells were transfected with pcDNA3-EPRSN1-Myc or vector control, and GAIT complex in lysates was immunoprecipitated with anti-L13a antibody. Extracted RNA was subjected to RTPCR as in (C).
(E) Recombinant EPRSN1 restores in vitro translation of GAIT element-bearing reporter. In vitro translation of Fluc reporter bearing the VEGF-A GAIT element (and Rluc control RNA) was determined in a rabbit reticulocyte lysate (RRL) in the presence of [35S]Met, cytosolic extracts from IFN-γ-treated U937 cells, and recombinant EPRSN1.
(F) EPRSN1 restores translation of endogenous GAIT element-bearing mRNAs. Lysates from U937 cells transfected with pcDNA3-EPRSN1-Myc were fractionated on a sucrose gradient, and total RNA subjected to RT-PCR with VEGF-A- and GAPDH-specific primers.
(G) EPRSN1 restores expression of GAIT element-bearing mRNAs. U937 cells were transfected with pcDNA3-EPRSN1-Myc and treated with IFN-γ for up to 24 hr. Cell lysates were subjected to immunoblot (left) and qRT-PCR (right) analyses as shown. See also Figure S2.
Based on the predicted binding activities, we considered that EPRSN1 might act as a dominant-negative inhibitor of the translational silencing activity directed by full-length EPRS. The interaction of EPRSN1 with GAIT element RNA was determined by surface plasmon resonance (SPR) spectrometry. Recombinant EPRSN1 bound Cp GAIT element RNA with a Kd of about 0.37 nM, whereas full-length EPRS bound with a Kd about 30-fold higher (Fig. 5B). The high binding affinity of EPRSN1 was primarily due to a very low off-rate, and suggests that EPRSN1 can effectively compete with GAIT complex for binding target mRNAs. To investigate binding in vivo, EPRSN1-c-Myc was transfected into cells, immunoprecipitated with anti-c-Myc antibody, and bound RNA detected by RT-PCR using mRNA-specific primers. Ectopically expressed EPRSN1 exhibited robust binding to VEGF-A but not β-actin mRNA, demonstrating specific interaction in cells as shown by RT-PCR (Fig. 5C), and more quantitatively by qRT-PCR (Fig. S2A). To determine its inhibitory activity, EPRSN1 was over-expressed in cells and the interaction of the GAIT complex constituent L13a with VEGF-A mRNA was measured by RNA immuonprecipitation with anti-L13a antibody. Indeed, ectopic expression of EPRSN1 blocked L13a binding to VEGF-A mRNA indicating effective competition, as shown by RT-PCR (Fig. 5D) and qRT-PCR (Fig. S2B).
To investigate the influence of EPRSN1 on GAIT target gene expression, we determined the effect of recombinant, His-tagged EPRSN1 on in vitro translation of a reporter RNA. Ceruloplasmin (Cp) GAIT element-bearing firefly luciferase (FLuc) and renilla Luc (RLuc) control RNAs were co-translated in rabbit reticulocyte lysate (RRL) in the presence of cytosolic lysate from IFN-γ-treated cells. As shown previously, 24-hr lysate specifically inhibited translation of GAIT element-bearing mRNA (Jia et al., 2008); however, EPRSN1 almost completely restored expression (Fig. 5E). To determine the mechanism of translational inhibition, lysates from c-Myc-tagged EPRSN1-transfected, IFN-γ-treated cells were fractionated on a sucrose gradient, and VEGF-A mRNA was determined by RT-PCR. A near-complete shift of VEGF-A mRNA from a translationally-silent mRNP pool to the translationally-active polysome fractions indicated EPRSN1 prevents GAIT complex-mediated inhibition of target mRNA translation (Fig. 5F). To show that EPRSN1 affects endogenous gene expression, transfected U937 cells were treated with IFN-γ and VEGF-A and Cp expression measured by immunoblot. Over-expression of EPRSN1 markedly increased in vivo expression of GAIT target proteins after 24-h IFN-γ treatment compared to transfection controls (Fig. 5G, left). VEGF-A mRNA expression was not significantly increased by ectopic expression of EPRSN1 (Fig. 5G, right). The nearly 2-fold increase in Cp mRNA is expected to have a small effect on protein expression as shown by an undetectable increase in protein following 4- to 8-fold increases in VEGF-A or ZIPK mRNA, respectively (Figs. 1B, 3A), and certainly cannot account for the observed 5-fold increase in Cp protein expression. These experiments demonstrate that EPRSN1, by high-affinity binding to GAIT target mRNAs, protects a small amount of mRNA from GAIT complex-mediated translational silencing, thereby maintaining low-level expression of target protein despite high-level induction of mRNA. Thus, EPRSN1 is the GEIF predicted by the modeling studies described above.
EPRSN1 is Generated by Polyadenylation-mediated Tyr-to-Stop Codon Conversion
Analysis of human genome sequence databases did not reveal a duplicated EPRS gene. Moreover, the substitution of a Tyr-encoding UAU codon in EPRS mRNA with a UAA stop codon followed by poly(A) in EPRSN1 mRNA was not consistent with an alternative splicing mechanism. We considered the possibility that EPRSN1 mRNA might be generated by an alternative polyadenylation (APA) event within the EPRS mRNA coding region that recodes a Tyr codon to a stop. Polyadenylation generally utilizes a C/UA cleavage site, an upstream hexanucleotide element (AAUAAA) or its variant (AUUAAA), and a downstream U- or GU-rich element, although these requirements are not absolute (Birnstiel et al., 1985). Inspection of EPRS mRNA sequence near the EPRSN1 3’-terminus revealed a UA cleavage site within the terminal Tyr codon, a consensus upstream hexanucleotide element at -40 (relative to the cleavage site), and a downstream U-rich element at +117 (Fig. 6A). To provide evidence for polyadenylation-mediated Tyr-to-stop codon conversion (PAY*), and verify the cis-acting signal elements, we subjected a [32P]UTP-labeled polyadenylation cassette to in vitro cleavage assay (Ahmed et al., 1991; Bar-Shira et al., 1991; Hans and Alwine, 2000; Wu and Alwine, 2004). The 240-nt APA cassette RNA, containing the cleavage site and putative upstream and downstream elements, was cleaved into a 109-nt 5’-RNA product by nuclear extract from U937 cells (Fig. 6B). Mutation of the UA cleavage site or the upstream or downstream elements prevented cleavage, as did immunodepletion of cleavage and polyadenylation stimulation factor-100 (CPSF100). In vitro polyadenylation of the pre-cleaved RNA cassette likewise was consistent with the in vitro cleavage results (Fig. 6C).
Figure 6. EPRSN1 mRNA is Generated from EPRS mRNA by the PAY* mechanism.
(A) EPRS mRNA cassette containing upstream (UE) and downstream (DE) APA signal elements and cleavage site (CS).
(B) In vitro cleavage assay for analysis of polyadenylation signal elements. [32P]UTP internal-labeled RNA cassettes from −109 to +131 relative to the cleavage site, containing the putative APA elements and mutants were generated by in vitro transcription and subjected to in vitro cleavage assay in presence of nuclear extracts from U937 cells.
(C) In vitro polyadenylation of EPRS mRNA. A pre-cleaved, [32P]UTP internal-labeled, 109-nt RNA was generated from the polyadenylation cassette by in vitro transcription, and used in an in vitro polyadenylation assay in which substrate RNA is mixed with nuclear extract and ATP.
(D) In vivo polyadenylation of EPRSN1 APA cassette. U937 cells were transfected with Renilla luciferase (Rluc) reporter plasmids containing wild-type and mutant APA cassettes and firefly luciferase (Fluc) reporter plasmid as transfection efficiency control. After 24 hr, luciferase activity of cell extracts was determined.
(E) Reduction of EPRSN1 expression diminishes the translational trickle of VEGF-A. U937 cells transfected with antisense morpholino oligomer targeting the cleavage site were treated with IFN-γ. EPRS and EPRSN1 mRNA were determined by RNA blot with ERS-specific probe, and protein by immunoblot with anti-linker antibody. VEGF-A and β-actin in cell lysates were determined by immunoblot.
(F) Truncated form of RRM1 mRNA is produced by in-CDS alternative polyadenylation mechanism. Total RNA from U937 cells was subjected to two-round nested RT-PCR using gene-specific primers (GSP). mRNA expression was determined by RT-PCR.
(G) Detection of RRM1N1 mRNA. Total RNA from U937 cells was subjected to RNA blot analysis using 5’-and 3’-specific RRM1 probes.
(H) Truncated mRNA of RRM1 is actively translatable. U937 cell lysates were fractionated by polysome profiling. The total RNA from various fractions was extracted and subjected to northern blot analysis with RRM1 5’-specific probe.
(I) Detection of RRM1N1 protein. Western blot was performed by using N-terminus and C-terminus-specific antibody against RRM1, respectively. See also Figure S3.
To investigate the role of the PAY* mechanism in vivo, a reporter plasmid was generated by sub-cloning the polyadenylation cassette downstream of the RLuc coding region to replace the SV40 late cassette in the pRL-SV40 plasmid. This assay is based on the requirement for transcript polyadenylation for efficient translation (McMahon et al., 2006). The reporter was co-transfected with a plasmid encoding FLuc as a control for transfection efficiency. The wild-type cassette was expressed much more efficiently than either of the upstream or downstream mutant consistent with in-CDS APA in cells (Fig. 6D). To verify the activity of endogenous EPRSN1, U937 cells (Fig. 6E) and human PBM (Fig. S3A) were transfected with antisense morpholino oligomer targeting the alternative cleavage site. The morpholino reduced EPRSN1 expression by about 75% and concomitantly reduced the VEGF-A “trickle level” by about the same amount (Fig. 6E), consistent with the simulation results (Fig. 2C, right, 0.25X GEIF). These data support the PAY* mechanism, and verify that EPRS N1 is responsible for maintaining the translational trickle of VEGF-A expression.
Transcriptome Expansion by the PAY* Mechanism
We used a bioinformatic approach to globally seek candidate mRNAs generated by in-CDS APA. The human expressed sequence tag (EST) and mRNA databases were queried using two criteria: (1) Coding RNA with a poly(A) tail immediately downstream of first UAA stop codon, and (2) the UAA stop codon replaces a Tyr-encoding UAU or UAC in a larger transcript that extends 3’ beyond the truncated mRNA (Fig. S3B,C). Seven candidate transcripts fulfilling both criteria were identified; among them only ribonucleotide reductase M1 (RRM1), an important cancer marker gene, exhibited a perfect consensus upstream element (at position -18) within the same exon as the cleavage site (Fig. S3D). Two-round nested RT-PCR of U937 cell RNA revealed the truncated form of RRM1 (RRM1N1) (Fig. 6F). We cannot exclude the possibility that other candidate truncated mRNAs might be found in other cell types or under other conditions. RNA blot analysis using 5’- and 3’-specific RRM1 probes verified the presence of the N-terminal truncated form (Fig. 6G). Sucrose gradient fractionation of cell lysate followed by Northern analysis with an N-terminus-specific RRM1 probe confirmed low-level expression of RRM1N1 (compared to the full-length mRNA), and also showed strong association with polysomes indicative of active translation (Fig. 6H). Immunoblot analysis of cell lysates with antibodies generated against the N- and Ctermini of RRM1 confirmed the presence of both full-length RRM1 and RRM1N1 in human PBM (data not shown) and U937 cells (Fig. 6I).
DISCUSSION
Dynamic modeling of the GAIT system, coupled with experimental validation, revealed two EPRS isoforms, a full-length form residing in the MSC and GAIT complex and a truncated free form, ERPSN1. ERPSN1 performs a function closely related to the noncanonical function of the parental form, namely, acting as a dominant-negative inhibitor that prevents complete translational silencing of target transcripts by the GAIT complex. Thus EPRS joins a select group of AARS that exhibit a noncanonical activity requiring a truncation event that reveals a cryptic stimulatory domain or removes an inhibitory peptide. For example, proteolytic removal of the EMAPII domain from YRS unmasks a tripeptide motif that confers pro-angiogenic activity (Wakasugi and Schimmel, 1999). Likewise, deletion of a WHEP domain from WRS by proteolysis or alternative splicing generates an anti-angiogenic protein (Wakasugi et al., 2002). RRS exhibits two isoforms produced by alternative translation initiation from a single mRNA; the larger isoform resides in the MSC whereas the smaller isoform is free (Kyriacou and Deutscher, 2008).
ERPSN1 is constitutively generated by an extraordinary PAY* mechanism within the coding region of EPRS. APA generally occurs in non-coding regions of mRNAs, most often distal to the stop codon generating mRNAs with a shorter 3’UTR thereby eliminating sites of post-transcriptional regulation (Danckwardt et al., 2008; Edwalds-Gilbert et al., 1997; Licatalosi and Darnell, 2010; Millevoi and Vagner, 2010). Less frequently, APA occurs distal to a stop codon in a cryptic intron to generate a truncated mRNA and protein, with an appended C-terminal sequence encoded by the intron (Fig. S4) (Di Giammartino et al., 2011). In mitochondria a poly(A) tail can be appended to a 3’ terminal U or UA to generate a UAA stop codon; however, unlike PAY* this mechanism does not generate an alternative mRNA or protein, but instead has evolved to maintain the small size of the mitochondrial genome by eliminating the 3’UTR (Fig. S4) (Anderson et al., 1981). APA within the coding sequence of several genes generates truncated transcripts lacking a stop codon and subjected to exosome-mediated, nonstop mRNA decay (Frischmeyer et al., 2002; van Hoof et al., 2002). Here, we show a unique example of generation of a stable truncated mRNA by APA within a coding region driven by recoding of an in-frame Tyr codon to a stop codon generating a 3’-truncated, 3’-UTR-less mRNA and a C-terminal truncated protein (Fig. 7A). The absence of any 3’-UTR in transcripts generated by the PAY* mechanism depletes posttranscriptional regulatory elements, including protein- and microRNA-binding sites (Mayr and Bartel, 2009). The relatively inefficient generation of EPRSN1 mRNA and protein might be due to sub-optimal location of the polyadenylation elements. About 0.7% of human mRNAs are predicted to contain APA signal elements within the CDS (Frischmeyer et al., 2002). In addition to myeloid cells, EPRSN1 is constitutively expressed by multiple types of human cells including umbilical vein and microvascular endothelial cells, hepatoma-derived HepG2 cells, embryonic kidney HEK293T cells, and cervical carcinoma HeLa cells (Fig. S5A). Inspection of EPRS mRNA sequences from several species shows that the required elements are conserved in primates, e.g., gorilla, chimpanzee, and macaque, but not in several other mammals, e.g., dog, cow, rabbit, pig, and mouse (Fig. S5B). Northern analysis confirmed the absence of a truncated EPRS mRNA in mouse RAW 264.7 cells (Fig. S5C). Thus, PAY*-mediated generation of EPRSN1 might be a recently evolved, species-selective mechanism of gene regulation.
Figure 7. Schematics of EPRSN1 Generation by PAY* and Function in Gene Expression.
(A) Pathways of EPRSN1 expression by PAY* (left) and EPRS expression by 3’-UTR-based polyadenylation (right). Exon segments of EPRSN1 (green) and EPRS (brown) upstream of the cleavage sites (underlined) are indicated.
(B) EPRSN1 function in maintaining a “translational trickle” of inflammatory gene expression in myeloid cells. See also Figure S4.
Our results reveal a unique negative-regulatory mechanism in which constitutive, low-level expression of a high-affinity competitor such as EPRSN1 shields a small, constant amount of GAIT target transcripts from translational repression. The GAIT complex can be considered as a closed gate that blocks translation of select transcripts following pro-inflammatory stimulation by IFN-γ, whereas, EPRSN1 can be considered as a “doorstop” that prevents complete closure of the gate, permitting a translational trickle of target expression (Fig. 7B). The GAIT target VEGF-A is a potent macrophage-derived angiogenic factor co-opted by tumors to induce blood vessel development and facilitate tumor growth and metastasis (Tammela et al., 2005). Anti-VEGF therapy successfully limits colorectal and renal cell carcinoma (Kamba and McDonald, 2007); however, adverse effects of these therapies, including blood vessel regression, suggests VEGF-A is essential for maintenance of healthy vessels as well as for development. Moreover, recent studies show that conditional deletion of macrophage-derived VEGF-A results in accelerated tumor growth (Stockmann et al., 2008). We suggest that a major function of EPRSN1 is to maintain VEGF-A, and other pro-inflammatory proteins, at basal levels required for tissue health and organismal advantage. The possible function of RRM1N1 is less clear. RRM1 is the catalyst of deoxyribonuclotide synthesis during DNA synthesis and cell division, and is the molecular target of gemcitabine for treatment of cancer (Jordheim et al., 2011). RRM1N1 contains the effector binding site and partial catalytic active site but lacks the C-terminus required for high-affinity binding to its regulated partner RRM2, which conceivably could reduce RRM1 activity or contribute to gemcitabine resistance. The discovery of multiple potential PAY* targets, and the validation of RRM1, indicates PAY* might be a fundamental mechanism of transcriptome and proteome expansion.
Organisms have evolved multiple mechanisms to compel low-level or basal amounts of important molecules under conditions in which there is both inductive synthetic pressure as well as negative regulation to prevent excessive expression. The most common regulatory mechanisms involve negative-feedback loops. For example, the inducible cfos transcription factor binds and represses its own promoter, thereby maintaining intermediate levels of the transcription factor and its inducible targets (Sassone-Corsi et al., 1988). More complex systems involving multiple feedback loops can also maintain gene expression within defined limits. As one example, delayed proteasomal degradation of the bHLH transcription suppressor Hes1 results in oscillatory expression of the factor.
The oscillation (and frequency) is established by a pair of temporally displaced regulatory loops, a feature common to circadian and other oscillatory circuits (Hirata et al., 2002). MicroRNAs exert post-transcriptional control of gene expression by diverse mechanisms (Leung and Sharp, 2010). Every microRNA targets multiple mRNAs, and most mRNAs bind multiple regulatory miRNAs. The net output is determined by the relative cellular concentration of the miRNA ensemble and their target mRNAs. Although individual microRNAs generally inhibit protein production by small amounts, usually much less than 50%, the sum of targeting microRNAs can completely switch off gene expression when and where necessary. In other cases, microRNAs perform subtle “tuning” functions (Bartel, 2009). For example, atrophin is repressed by miR-8 in Drosophila, but not to the low level detrimental to cell viability (Karres et al., 2007). The mechanism by which appropriate microRNA-to-target stoichiometry is controlled to maintain optimal protein output is not well understood, but negative-feedback loops might be important. For example, stress-inducible, pro-inflammatory activation of the NF-κB transcription in macrophages induces a family of microRNAs that target and repress proinflammatory signaling molecules, including NF-κB (Leung and Sharp, 2010). The timescale of induction, maturation, and accumulation of these microRNAs is about 24 hr. This delayed temporal response is suggested to permit a vigorous inflammatory defense against pathogens while constraining the response duration to minimize host injury. To our knowledge, there are not any reports of systems comparable to the GAIT system in which endogenous, transcript-selective RNA-binding proteins act as dominant-negative repressors. In the Pseudomonas RsmA-RsmZ regulatory system, the primary inhibitor, RsmA, binds the 5'-UTR of target mRNAs and inhibits their translation (Heeb et al., 2002). The secondary regulator, RsmZ, is a small RNA that acts as an RNA decoy, binding RsmA and reducing its translational repression activity. This system is different from the EPRS/EPRSN1 system in which the secondary regulator, i.e., EPRSN1, binds target mRNAs, not the GAIT complex primary inhibitor. Our modeling studies show that modulation of RsmA by RsmZ will indeed relieve translational repression, but will not generate the non-linear “translational trickle” characteristic of our newly described mechanism (Fig. S1A-D).
We propose that the GAIT complex exerts a protective function as a delayed translational repressor of inflammatory gene expression, while EPRSN1 prevents repression below basal amounts. Thus, regulation is broadly exerted on a post-transcriptional regulon united by a common structural RNA element. Importantly, this regulatory mechanism is superimposed on the identical regulon controlled by the GAIT complex. We envision the presence of similar regulatory systems that generate “translational trickles” of distinct mRNA families. The system requirements include low-level expression of a fragment of an RNA-binding protein that binds RNA elements in a post-transcriptional mRNA regulon. The fragment is likely to bind the element with an affinity substantially higher than the parental RNA-binding protein. Also, the fragment must be defective with respect to translational silencing activity, possibly by preventing holo-complex formation or by direct inactivation of the inhibitory function. Our results have not yet revealed regulation of EPRSN1 production; however, this is not a necessary system attribute, and indeed the level of the “trickle” might be regulated in the GAIT system (e.g., by condition-dependent regulation of PAY* activity) and in other systems. The GAIT/EPRSN1 system is uniquely characterized by constitutive, low-level expression of EPRSN1, established by an intrinsically inefficient PAY* mechanism, and the absence of any 3’-UTR in EPRSN1 contributes to the lack of regulation. The EPRSN1-driven regulatory system represents an unusual “bottom-up” mechanism. Instead of the usual mechanism in which low-level amounts of protein products are maintained by restricting the inhibitory activity, EPRSN1 exerts a stimulatory activity that is totally independent of other stimulatory or inhibitory activities, including feedback inhibition. In total, these attributes result in a unique regulatory mechanism that can ensure a constant low-level, trickle of target protein expression even under strong negative pressure.
EXPERIMENTAL PROCEDURES
Modeling Procedures
Development and application of dynamic models to simulate the GAIT system are described in Supplemental Information.
Cell Culture
Human U937 monocytic cells (ATCC, Rockville, MD) and primary human peripheral blood monocytes (PBM, from healthy clinical donors) were cultured in RPMI 1640 medium containing 10% fetal bovine serum (FBS). For preparation of cytosolic extracts, the cells were incubated for 1 hr in medium containing 0.5% FBS and then incubated with IFN-γ for an additional 8 or 24 hr.
Plasmids, Site-directed Mutagenesis, and Recombinant Protein Expression
EPRSN1 (Met1 to Gln863) was cloned into pET-28 expression vector between SacI and SalI restriction sites and expressed as described (Jia et al., 2008). pcDNA3-EPRSN1-c-Myc was generated by using pET28-EPRSN1 as template and cloned between NotI and XbaI restriction sites. The DNA template for in vitro transcription of EPRSN1 polyadenylation cassette RNA was cloned into PSP64 vector (PSP64-PAC) between SalI and EcoRI restriction sites. Specific mutations for EPRSN1 polyadenylation cassette RNA were introduced into PSP64-PAC by PCR-based mutagenesis. The reporter plasmids were constructed by subcloning polyadenylation cassettes into pRLSV40 reporter plasmid between XbaI and BamHI sites. The SV40 polyadenylation cassette was replaced by EPRSN1 polyadenylation cassette.
Immunoprecipitation and Immunodepletion
For immunoprecipitation, cell lysates prepared in Phospho-safe extraction buffer were pre-cleared with protein A/G-agarose beads. After centrifugation the supernatants were combined with protein A/G beads and antibody, and incubated with rotation at 4 °C for 4 hr. The beads were washed with cold cell lysis buffer. Protein gel loading dye was added and the samples boiled before gel-loading. For immunodepletion, nuclear extracts from U937 cells were incubated with rabbit anti-CPSF100 antibody coupled to protein A/G-agarose beads in extraction buffer. The beads were pelleted, the supernatants subjected to two additional rounds of depletion, and immunodepletion established by immunoblot with mouse anti-CPSF100 antibody.
RNA Blot Analysis
For RNA blot analysis, total RNA was extracted from 108 U937 cells using Trizol reagent. RNA (20 µg) was fractionated on 1% agarose-formaldehyde gel and transferred to Zeta-Probe GT membrane. The blot was hybridized with random primerlabeled cDNA probes, the signal scanned, and quantified by Phosphoimager. qRT-PCR determinations of VEGF-A, Cp, and GAPDH mRNA were done using the Taqman Gene Expression Assays for various targets and the StepOnePlus Real-Time PCR System.
Ribonucleoprotein Immunoprecipitation (RIP)-RT-PCR
Cell lysates were immunoprecipitated with mouse anti-c-Myc antibody or pre-immune IgG as above. Total and immunoprecipitated RNA were extracted with Trizol reagent separately. Immunoprecipitated and total RNA were subjected to RT-PCR with Taq DNA polymerase and visualized by 1.5% agarose gel.
Mass Spectrometry
Proteins immunoprecipitated by anti-EPRS linker antibody from U937 cell lysates were resolved by 4–20% SDS-PAGE gel. The 95 kDa, Coomassie-stained band was in-gel digested with trypsin and analyzed by capillary LC-tandem MS analysis. CID spectra were queried against the human reference sequence database using Mascot software, and matching spectra verified manually and by using the Sequest and Blast as needed.
G/I Tailing and cDNA Cloning
Total RNA from U937 cells was extracted with Trizol reageat and subjected to G/I tailing following the protocol from Poly(A) Tail-Length Assay Kit. Poly(G/I)-tailed RNA product was subjected to RT-PCR and the reaction product loaded onto a 2.5% agarose TBE gel. The band with lowest molecular weight was removed, purified, and ligated into pGEM-T vector for DNA sequencing.
Polysome Profiling
Cells were pre-incubated with cycloheximide (CHX, 100 µg/ml) for 15 min, and washed twice with CHX-containing cold PBS. Cells were suspended in TMK lysis buffer, the lysates were centrifuged and the supernatant collected. Sucrose gradient solutions (10% and 50%) containing RNase inhibitor and CHX were freshly prepared. The cytosolic lysates were loaded on the sucrose gradient, centrifuged at 29,000 rpm for 4 hr, and subjected to gradient fractionation.
In Vitro Translation
Capped, poly(A)-tailed template mRNAs were prepared using mMESSAGE mMACHINE SP6 and T7 kits. Firefly luciferase (FLuc)-Cp GAIT element-poly(A) and Renilla luciferase (RLuc) reporter RNAs were incubated with U937 cytosolic extract in the presence of rabbit reticulocyte lysate and [35S]methionine for 90 min at 30 °C, resolved by 10% SDS-PAGE gel, and visualized by Phosphorimager.
RNA-protein Interaction by Surface Plasmon Resonance (SPR)
Protein binding to Cp GAIT element RNA was determined by SPR in a Biacore 3000 system (Jia et al., 2008). Biotinylated, wild-type and mutant GAIT element RNA (U87C) were separately immobilized on a streptavidin sensor chip in HBS-P buffer. The analyte flow rate was 5 µl/min in the same buffer containing 5 mM MgCl2. Dissociation constants were calculated for a range of protein concentrations using Biaevaluation software.
In Vitro Cleavage and Polyadenylation Assays
The polyadenylation cassette RNA was transcribed using MAXIscript SP6 in vitro transcription kit. Transcribed RNA was dephosphorylated with calf intestinal phosphatase (CIP) at 37 °C for 30 min, and CIP removed by phenol/chloroform extraction. The RNA product was 5’-end labeled with 32P-γ-ATP catalyzed by polynucleotide kinase at 37 °C for 1 hr, and purified using a Micro Bio-Spin 30 chromatography column. To measure cleavage reaction, the 32P-labeled polyadenylation cassette RNA was incubated with U937 nuclear extract (50%, v/v), KCl (50 mM), MgCl2 (1 mM), EDTA (0.1 mM), glycerol (10%), DTT (0.2 mM), creatine phosphate (20 mM), and polyvinyl alcohol (2%). For in vitro polyadenylation assay, 1 mM ATP was added to the same reaction solution in the presence of pre-cleaved polyadenylation cassette RNA.
Cell Transfection and Dual Luciferase Reporter Assay
U937 cells were transiently co-transfected with 0.5 µg pRL-SV40 plasmid (containing polyadenylation cassette) and 1 µg pCD-FLuc (for normalization of transfection efficiency) using human monocyte nucleofector kit. After 24 hr, transfected cells were lysed and luciferase activity of the lysates was measured by using the Dual Luciferase Reporter assay system. To knock-down endogenous EPRSN1, U937 cells were transfected with morpholino antisense oligomers or a control morpholino oligomer by using human monocyte nucleofector kit.
Global Detection of Candidate PAY* mRNAs
We examined a collection of 19,629 human reference genes (RefGene) in the University of California at Santa Cruz (UCSC) Genome Browser, including genomic information on start and stop codon positions and 3’- and 5’-splice sites. 3’ UTR-less, PAY* transcripts were sought using sequence and genomic alignment information of human ESTs and mRNAs available in the UCSC Genome Browser. Sequences with tracts (defined as 10 or more contiguous nt) of poly(A) in the 3’ end or poly(T) in the 5’ end (for reverse-complemented ESTs or mRNAs) were identified as poly(A) ESTs or mRNAs. The first A or T in the poly(A) or poly(T) tract was considered to be the transcript poly(A) site, and is required to be upstream or downstream 5 nt from the nearest alignment site on the genome. The poly(A) tail of ESTs or mRNAs was required to be absent (less than 18 A’s within 20 bp downstream or upstream of the poly(A) site) in the corresponding genome region to eliminate internal priming sites. When a poly(A)-containing EST or mRNA shared all internal splice sites with a RefGene, that RefGene was assigned as reference, and the start codon position was inferred by the open-reading frame of its RefGene. If the first stop codon coincides with the start of the poly(A) tail, then the poly(A) sequence is considered as a 3’-UTR-less, in-CDS EST or mRNA. From this group, PAY* candidates were selected by replacement of TAT or TAC Tyr codons with a TAA stop codon in 3’ UTR-less in-CDS ESTs or mRNAs.
Nested RT-PCR for Detecting Candidate mRNAs Generated by PAY*
Total RNA was extracted from U937 cells and subjected to reverse transcription using oligo-dT primer. Two-round nested PCR was performed with the cDNA as template.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Richard Padgett for helpful discussions. This work was supported in part by National Institutes of Health grants P01 HL029582, P01 HL076491, R01 GM086430, and R01 DK083359 to P.L.F. P.Y. was supported by a Postdoctoral Fellowship from the American Heart Association, Great Rivers Affiliate, and A.A. by National Center Scientist Development Grant 10SDG3930003 from the American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahmed YF, Gilmartin GM, Hanly SM, Nevins JR, Greene WC. The HTLV-I Rex response element mediates a novel form of mRNA polyadenylation. Cell. 1991;64:727–737. doi: 10.1016/0092-8674(91)90502-p. [DOI] [PubMed] [Google Scholar]
- Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Arif A, Jia J, Moodt RA, DiCorleto PE, Fox PL. Phosphorylation of glutamyl-prolyl tRNA synthetase by cyclin-dependent kinase 5 dictates transcriptselective translational control. Proc Natl Acad Sci U S A. 2011;108:1415–1420. doi: 10.1073/pnas.1011275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif A, Jia J, Mukhopadhyay R, Willard B, Kinter M, Fox PL. Twosite phosphorylation of EPRS coordinates multimodal regulation of noncanonical translational control activity. Mol Cell. 2009;35:164–180. doi: 10.1016/j.molcel.2009.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shira A, Panet A, Honigman A. An RNA secondary structure juxtaposes two remote genetic signals for human T-cell leukemia virus type I RNA 3'- end processing. J Virol. 1991;65:5165–5173. doi: 10.1128/jvi.65.10.5165-5173.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnstiel ML, Busslinger M, Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985;41:349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Cahuzac B, Berthonneau E, Birlirakis N, Guittet E, Mirande M. A recurrent RNA-binding domain is appended to eukaryotic aminoacyl-tRNA synthetases. EMBO J. 2000;19:445–452. doi: 10.1093/emboj/19.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danckwardt S, Hentze MW, Kulozik AE. 3' end mRNA processing: molecular mechanisms and implications for health and disease. EMBO J. 2008;27:482–498. doi: 10.1038/sj.emboj.7601932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giammartino DC, Nishida K, Manley JL. Mechanisms and consequences of alternative polyadenylation. Mol Cell. 2011;43:853–866. doi: 10.1016/j.molcel.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwalds-Gilbert G, Veraldi KL, Milcarek C. Alternative poly(A) site selection in complex transcription units: means to an end? Nucleic Acids Res. 1997;25:2547–2561. doi: 10.1093/nar/25.13.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischmeyer PA, van Hoof A, O'Donnell K, Guerrerio AL, Parker R, Dietz HC. An mRNA surveillance mechanism that eliminates transcripts lacking termination codons. Science. 2002;295:2258–2261. doi: 10.1126/science.1067338. [DOI] [PubMed] [Google Scholar]
- Guo M, Yang XL, Schimmel P. New functions of aminoacyl-tRNA synthetases beyond translation. Nat Rev Mol Cell Biol. 2010;11:668–674. doi: 10.1038/nrm2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans H, Alwine JC. Functionally significant secondary structure of the simian virus 40 late polyadenylation signal. Mol Cell Biol. 2000;20:2926–2932. doi: 10.1128/mcb.20.8.2926-2932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeb S, Blumer C, Haas D. Regulatory RNA as mediator in GacA/RsmA-dependent global control of exoproduct formation in Pseudomonas fluorescens CHA0. J Bacteriol. 2002;184:1046–1056. doi: 10.1128/jb.184.4.1046-1056.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Yoshiura S, Ohtsuka T, Bessho Y, Harada T, Yoshikawa K, Kageyama R. Oscillatory expression of the bHLH factor Hes1 regulated by a negative feedback loop. Science. 2002;298:840–843. doi: 10.1126/science.1074560. [DOI] [PubMed] [Google Scholar]
- Ibba M, Söll D. Aminoacyl-tRNA synthesis. Annu Rev Biochem. 2000;69:617–650. doi: 10.1146/annurev.biochem.69.1.617. [DOI] [PubMed] [Google Scholar]
- Jeong EJ, Hwang GS, Kim KH, Kim MJ, Kim S, Kim KS. Structural analysis of multifunctional peptide motifs in human bifunctional tRNA synthetase: Identification of RNA-binding residues and functional implications for tandem repeats. Biochemistry. 2000;39:15775–15782. doi: 10.1021/bi001393h. [DOI] [PubMed] [Google Scholar]
- Jia J, Arif A, Ray PS, Fox PL. WHEP domains direct noncanonical function of glutamyl-prolyl tRNA synthetase in translational control of gene expression. Mol Cell. 2008;29:679–690. doi: 10.1016/j.molcel.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordheim LP, Seve P, Tredan O, Dumontet C. The ribonucleotide reductase large subunit (RRM1) as a predictive factor in patients with cancer. Lancet Oncol. 2011;12:693–702. doi: 10.1016/S1470-2045(10)70244-8. [DOI] [PubMed] [Google Scholar]
- Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96:1788–1795. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapasi P, Chaudhuri S, Vyas K, Baus D, Komar AA, Fox PL, Merrick WC, Mazumder B. L13a blocks 48S assembly: Role of a general initiation factor in mRNA-specific translational control. Mol Cell. 2007;25:113–126. doi: 10.1016/j.molcel.2006.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karres JS, Hilgers V, Carrera I, Treisman J, Cohen SM. The conserved microRNA miR-8 tunes atrophin levels to prevent neurodegeneration in Drosophila. Cell. 2007;131:136–145. doi: 10.1016/j.cell.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Kyriacou SV, Deutscher MP. An important role for the multienzyme aminoacyl-tRNA synthetase complex in mammalian translation and cell growth. Mol Cell. 2008;29:419–427. doi: 10.1016/j.molcel.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AK, Sharp PA. MicroRNA functions in stress responses. Mol Cell. 2010;40:205–215. doi: 10.1016/j.molcel.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licatalosi DD, Darnell RB. RNA processing and its regulation: global insights into biological networks. Nat Rev Genet. 2010;11:75–87. doi: 10.1038/nrg2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr C, Bartel DP. Widespread shortening of 3'UTRs by alternative cleavage and polyadenylation activates oncogenes in cancer cells. Cell. 2009;138:673–684. doi: 10.1016/j.cell.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder B, Fox PL. Delayed translational silencing of ceruloplasmin transcript in gamma interferon-activated U937 monocytic cells: Role of the 3' untranslated region. Mol Cell Biol. 1999;19:6898–6905. doi: 10.1128/mcb.19.10.6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder B, Sampath P, Seshadri V, Maitra RK, DiCorleto P, Fox PL. Regulated release of L13a from the 60S ribosomal subunit as a mechanism of transcript-specific translational control. Cell. 2003;115:187–198. doi: 10.1016/s0092-8674(03)00773-6. [DOI] [PubMed] [Google Scholar]
- Mazumder B, Seshadri V, Imataka H, Sonenberg N, Fox PL. Translational silencing of ceruloplasmin requires the essential elements of mRNA circularization: Poly(A) tail, poly(A)-binding protein, and eukaryotic translation initiation factor 4G. Mol Cell Biol. 2001;21:6440–6449. doi: 10.1128/MCB.21.19.6440-6449.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon KW, Hirsch BA, MacDonald CC. Differences in polyadenylation site choice between somatic and male germ cells. BMC Mol Biol. 2006;7:35. doi: 10.1186/1471-2199-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millevoi S, Vagner S. Molecular mechanisms of eukaryotic pre-mRNA 3' end processing regulation. Nucleic Acids Res. 2010;38:2757–2774. doi: 10.1093/nar/gkp1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay R, Jia J, Arif A, Ray PS, Fox PL. The GAIT system: a gatekeeper of inflammatory gene expression. Trends Biochem Sci. 2009;34:324–331. doi: 10.1016/j.tibs.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay R, Ray PS, Arif A, Brady AK, Kinter M, Fox PL. DAPK-ZIPK-L13a axis constitutes a negative-feedback module regulating inflammatory gene expression. Mol Cell. 2008;32:371–382. doi: 10.1016/j.molcel.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SG, Schimmel P, Kim S. Aminoacyl tRNA synthetases and their connections to disease. Proc Natl Acad Sci U S A. 2008;105:11043–11049. doi: 10.1073/pnas.0802862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray PS, Fox PL. A post-transcriptional pathway represses monocyte VEGF-A expression and angiogenic activity. EMBO J. 2007;26:3360–3372. doi: 10.1038/sj.emboj.7601774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray PS, Jia J, Yao P, Majumder M, Hatzoglou M, Fox PL. A stressresponsive RNA switch regulates VEGFA expression. Nature. 2009;457:915–919. doi: 10.1038/nature07598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray PS, Sullivan JC, Jia J, Francis J, Finnerty JR, Fox PL. Evolution of function of a fused metazoan tRNA synthetase. Mol Biol Evol. 2011;28:437–447. doi: 10.1093/molbev/msq246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas de Pouplana L, Schimmel P. Aminoacyl-tRNA synthetases: potential markers of genetic code development. Trends Biochem Sci. 2001;26:591–596. doi: 10.1016/s0968-0004(01)01932-6. [DOI] [PubMed] [Google Scholar]
- Sampath P, Mazumder B, Seshadri V, Fox PL. Transcript-selective translational silencing by gamma interferon is directed by a novel structural element in the ceruloplasmin mRNA 3' untranslated region. Mol Cell Biol. 2003;23:1509–1519. doi: 10.1128/MCB.23.5.1509-1519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath P, Mazumder B, Seshadri V, Gerber CA, Chavatte L, Kinter M, Ting SM, Dignam JD, Kim S, Driscoll DM, Fox PL. Noncanonical function of glutamyl-prolyl-tRNA synthetase: gene-specific silencing of translation. Cell. 2004;119:195–208. doi: 10.1016/j.cell.2004.09.030. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P, Sisson JC, Verma IM. Transcriptional autoregulation of the proto-oncogene fos. Nature. 1988;334:314–319. doi: 10.1038/334314a0. [DOI] [PubMed] [Google Scholar]
- Stockmann C, Doedens A, Weidemann A, Zhang N, Takeda N, Greenberg JI, Cheresh DA, Johnson RS. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature. 2008;456:814–818. doi: 10.1038/nature07445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005;65:550–563. doi: 10.1016/j.cardiores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- van Hoof A, Frischmeyer PA, Dietz HC, Parker R. Exosome-mediated recognition and degradation of mRNAs lacking a termination codon. Science. 2002;295:2262–2264. doi: 10.1126/science.1067272. [DOI] [PubMed] [Google Scholar]
- Wakasugi K, Schimmel P. Two distinct cytokines released from a human aminoacyl-tRNA synthetase. Science. 1999;284:147–151. doi: 10.1126/science.284.5411.147. [DOI] [PubMed] [Google Scholar]
- Wakasugi K, Slike BM, Hood J, Otani A, Ewalt KL, Friedlander M, Cheresh DA, Schimmel P. A human aminoacyl-tRNA synthetase as a regulator of angiogenesis. Proc Natl Acad Sci U S A. 2002;99:173–177. doi: 10.1073/pnas.012602099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Alwine JC. Secondary structure as a functional feature in the downstream region of mammalian polyadenylation signals. Mol Cell Biol. 2004;24:2789–2796. doi: 10.1128/MCB.24.7.2789-2796.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.