Abstract
Increasing the safety and the efficacy of existing HIV vaccines is one of the strategies that could help to promote the development of a vaccine for human use. We developed a HIV DNA vaccine (Δ4-SHIVku2) that has been shown to induce potent polyfunctional HIV-specific T cell responses following a single dose immunization of mice and macaques. Δ4-SHIVku2 also induced protection when immunized macaques were challenged with homologous pathogenic viruses. In the present study, our aim was to examine whether a chimeric HIV DNA vaccine (CAL-Δ4-SHIVku2) whose genome is driven by the LTR of the goat lentivirus, caprine arthritis encephalitis (CAEV) expresses efficiently the vaccine antigens and induces potent immune responses in animal models for HIV vaccine. Data of radioimmunoprecipitation assays clearly show that this chimeric genome drives efficient expression of all HIV antigens in the construct. In addition, evaluation of the p24 Gag protein in the supernatant of HEK-293-T cells transfected in parallel with Δ4-SHIVku2 and CAL-Δ4-SHIVku2 showed no difference suggesting that these two LTRs are inducing equally the expression of the viral genes. Immunization of mice and macaques using our single dose immunization regimen resulted in induction of similar IFN-γ ELISPOT responses in Δ4-SHIVku2- and CAL-Δ4-SHIVku2-treated mice. Similar profiles of T cell responses were also detected both in mice and macaques when multiparametric flow cytometry analyses were performed. Since CAEV LTR is not dependent of Tat to drive viral gene expression and is not functional for integration with HIV integrase, this new vector increases the safety and efficacy of our vaccine vectors and vaccination strategy.
Keywords: HIV, DNA vaccine, LTR, CD8+ T cells, animal models
1. Introduction
There is no doubt that developing efficient prophylactic and therapeutic vaccines against the human immunodeficiency virus (HIV) is critical to end the global continuously growing epidemic of this virus in humans. HIV continues to be a worldwide health problem with over 33 million individuals infected; over 2 million die and nearly 3 million get infected every year (http://www.unaids.org/globalreport/Global_report.htm, 2010).
Among the infected individuals, the identification of the small subsets of Long-Term Non-progressors (LTNP) and the Elite Suppressors (ES) of HIV-1 has provided the evidence that HIV-1 can be defeated [1–3]. The discovery that some of these patients have been infected with naturally attenuated HIV-1 variants by mutation in their nef gene (Live-attenuated) [4–7] has provided a possible strategy for derivation of a vaccine against the pathogenic HIV-1. In addition, the most provocative, convincing and reproducible results of protective vaccination in non-human primate model for HIV-1 are those obtained with live-attenuated SIV and SHIV [8–10]. However, these vaccines, derived from pathogenic strains of viruses were causing persistent infections with integration of their provirus into the genome of vaccinees and they were found to retain pathological properties in infants and reversion to pathogenic phenotype in some adult macaques [11–14]. Therefore, despite their high efficacy at inducing protective immune responses, this type of vaccines was excluded from possible use in humans because of the ethical as well as the safety issues. Nevertheless, like with defective HIV-1 genomes associated with some LTNP and ES, these data established strong evidences that live-attenuated viruses are efficient at inducing protective immunity against SIV/SHIV in non-human primates. Despite these critical observations, there was no attempt to explore the possible use of genetic elements from other lentiviruses that are well known to be not associated with AIDS-like disease in their hosts as bases to derive HIV live-attenuated vaccines.
We and others have developed non-replicating non-integrating HIV-based DNA vaccines and shown that they induce potent immune responses in rodents, but surprisingly these responses were found to be very weak in primates requiring therefore heterologous boost with proteins or viruses [15–19]. Efforts were also made to enhance the immunogenicity of these vaccines by optimizing their delivery, the expression of viral antigens or targeting particular compartment in expressing cells. We have recently increased the dose of delivered DNA and characterized the immune responses induced by our HIV-DNA vaccine alone (Δ4-SHIVku2) in immunized mice [15, 18] and macaques [20, 21]. We found, in both animal model studies that the responses to one high dose were made of long lasting and polyfunctional HIV-specific CD8+ T cells expressing Granzyme B endowed with high proliferating capacities. Interestingly, the immunological profile of ES individuals evidences the critical influence of a strong polyfunctional HIV specific-CD8+ T cell response resulting in effective Granzyme B-mediated killing of HIV-infected T cells [22].
Altogether these data provided the basic demonstrations that HIV-based DNA vaccines alone induce potent immune responses but they do not induce a protection equivalent to that induced by live-attenuated vaccines, especially when high pathogenic viruses were used for challenge. Therefore, it became evident that novel and provocative strategies were needed to reach this goal.
In the present work we focused on the optimization of our HIV DNA vaccine construct driven by the lentiviral SIV LTR (Δ4-SHIVku2). This construct used alone for immunization has proven its efficacy in preventing AIDS in macaques when challenged with homologous virus [21, 23]. To further enhance the safety and immunogenicity of this vaccine we removed the SIV 5'LTR from the construct and replaced it with the LTR from goat lentivirus, CAEV that does not induce AIDS in his host. This LTR was previously characterized to be Tat-independent therefore to have a constitutive promoter for gene expression [24]. This novel HIV vaccine was named CAL-Δ4- SHIVku2.
This replacement of the SIV LTR was initiated to primarily examine whether the LTR from animal lentiviruses other than primate can drive efficient expression of primate lentivirus proteins. This work represents a first step in our strategy to create a non-integrative, replication limited vaccine vector. Our data show that like the parental vaccine genome Δ4 SHIVku2, the new non-integrating, non-replicating vaccine CAL-Δ4-SHIVku2 expresses efficiently all the antigens encoded by the vaccine genome in transfected HEK-293T cells. DNA immunization of mice and macaques with this vaccine induced potent T cell immune responses specific to all HIV antigens in both models.
2. MATERIALS AND METHODS
2.1 DNA constructs
Details of construction of the Δ4-SHIVku2 plasmid DNA have been described in earlier reports [21, 25]. The inserted sequences were derived from SHIVku2 (GenBank data base accession #AY751799) and HIV-1SF2. The DNA is comprised of vpx and vpr genes from SIV-mac239, and gag, pro, vpu, tat, rev, env, nef and a portion of rt genes from HIV, all under the transcriptional control of the SIV 5'LTR promoter and the polyA sequences of SV40. The pol gene was truncated to remove 80% of rt, and the complete integrase and vif coding sequences together with the 3'LTR from SHIVku2. The CAL-Δ4-SHIVku2 was derived from the Δ4-SHIVku2 DNA by removing the 830 bp fragment containing the SIV 5'LTR sequences following double digestion with EcoR1 and Nar1 restriction enzymes and ligation of the 450 bp isolated from the 5'LTR of the molecularly cloned infectious clone of CAEV (CAEV-pBSCA) [29]. This fragment was obtained following PCR amplification and double digestion with EcoR1 and Nar1 enzymes. Both viral genomes were inserted into the pET9 plasmid (Novagen, EMD Chemicals, San Diego, CA)
2.2 Animals
Six week old female BALB/c mice were purchased from Harlan Laboratories. Two 3–5 year old Indian rhesus macaques were purchased and housed in the Laboratory Animal Resources of the University of Kansas Medical Center. All animals were used in accordance with National Institute of Health and the University of Kansas Medical Center Institutional Animal Care and Use Committee guidelines.
2.3 Transfection of HEK 293T cells for viral protein expression assessment
Transfections were performed using a cationic polymer polyethylenamine, ExGen™ 500 (Fermentas, according to the protocols provided by the manufacturer (Fermentas, Hanover, MD) for adherent cells. Supernatant fluids were harvested from HEK-293 T transfected cells 14 h and 24 h after transfection and assessed for p24 content. Transfected cells were then labeled with 100 μCi of 35S-methionine at 48 h post-transfection and used for immunoprecipitation of viral proteins from the cell lysate and supernatant compartments using a hyperimmune macaque serum that has antibodies against all the viral proteins as previously reported [25].
2.4 Quantification of Gag p24 release in the culture medium of transfected cells
Gag p24 was assessed by the highly sensitive capture enzyme-linked immunosorbent assay (ELISA) kit (Coulter laboratories, Hialeah, FL). A standard curve was prepared for each assay, as per the manufacturer's instructions. The concentrations of Gag p24 were determined from the OD450 plotted against a standard curve by linear regression analysis as previously reported [18].
2.5 Inoculation of mice and macaques
Endotoxin-free vaccine DNA was produced using a BIOFLO 110 modular Fermentor (New Brunswick Scientific, Edison, NJ) that routinely produced high yield of DNA following plasmid DNA extraction using the standard methods with Qiagen Giga kit.
Two groups of BALB/c mice were inoculated intramuscularly (IM) with a single dose of 200 μg of CAL-Δ4-SHIVku2 and Δ4-SHIVku2 DNA vaccine respectively. Each mouse was injected with a total of 100 μl of DNA solution prepared in phosphate buffer saline (PBS) at 2 μg/μl DNA; 50 μl in each gastrocnemius muscle.
Macaques were inoculated IM with a single dose of 30 mg of DNA vaccine at 6 mg/ml concentration. All DNAs used to inject the macaques and mice contained at least 90 % of the supercoiled (ccc) form of plasmid. DNA solution was prepared in 5 ml of PBS (0.1 M (pH 7.4)) and injected intramuscularly to macaques at ten different sites of the rear legs using a 21 gauge needle.
2.6 HIV peptides
Overlapping 15-mer peptides, with 11-aa overlaps, spanning the entire molecules of HIV Gag, Env, Tat, Rev, and Nef, proteins were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program (catalog nos. 8117, 6451, 5138, 6445, and 5189, respectively). These peptides are based on consensus sequences from clade B HIV genomes. Our HIV DNA vaccine encodes Gag, Pro and Nef from the SF2 HIV strain and Tat, Rev, Vpu and Env from the HXB2 HIV strain. These two strains are both clade B viruses.
2.7 Processing of spleen and blood for mononuclear cells isolations
Mice were killed at 2 and 4 weeks post-immunization, respectively, and spleen collected. Splenocytes were isolated in Hank's solution, treated with BD lysing solution to remove the erythrocytes, and mononuclear cells counted as previously described [18].
Peripheral blood samples were collected from vaccinated macaques by venipuncture in sodium heparin coated tubes. PBMCs were isolated from Buffy-coats by centrifugation through Ficoll-Hypaque density gradients. Cells were then used to perform multiparametric flow cytometry assays.
2.8 Assays for detection of HIV-specific immune T cells
Quantitative ELISPOT assay were performed on splenocytes to measure IFN-γ-producing splenocytes in response to groups of overlapping peptides used at a concentration of 2 μg /ml as previously described [18]. ConA stimulated cells (0.5 μg/ml) were used as positive controls and cells lacking stimulation with the peptides were used as negative controls. The cut off of positive ELISPOT was set up at 10 SFC/106 splenocytes, two times the background.
Polychromatic (six-color) flow cytometry analyses were performed on splenocytes [15] and PBMC [20] as we previously reported, using a three-laser BD LSRII instrument with standard setting. Data files were collected and analyzed using the FACSDiva software program (version 4.1.2; BD Biosciences, San Jose, CA). To monitor the expansion and proliferation of HIV-specific T cells, CFSE-labeled (107 cells/ml in 1 μM CFSE for 10 min at 37 °C, Molecular Probes, Invitrogen, Carlsbad, CA) splenocytes or PBMCs were seeded in 96-deep well tissue culture plates (Nunc, Fisher Scientific, Pittsburgh, PA) at a density of 2×106 cells/well in 1 ml of medium alone or loaded with 2 μg/ml of HIV peptides and incubated for 5 days at 37 °C. After 5 days of incubation, cells were restimulated for 6 h with medium only or by adding relevant HIV peptides in the presence of 0.5 μg/ml of costimulatory CD28, CD49 mAbs and 10 μg/ml of brefeldin A (Sigma-Aldrich, St Louis, MO). Cells were then washed and stained with anti-CD3, -CD8, -CD4 mAbs for 20 min at 4 °C. Additionally, ethidium monoazide (EMA; Molecular Probes, Invitrogen, Carlsbad, CA) was added at 0.5 μg/ml during the surface labeling step to allow exclusion of dead cells in samples that have been cultured for 5 days and restimulated for 6 h. In such a case, all samples were exposed to light for 15 min at room temperature to allow EMA to covalently link to the DNA in dead cells prior permeabilization. Then the cells were fixed/permeabilized (Cytofix/Cytoperm Plus; BD Biosciences, San Jose, CA) and stained with anti-IFN-γ and IL-2 mAbs for 30 min at room temperature. Cells were washed again (Perm/Wash; BD Biosciences, San Jose, CA), fixed in 1% paraformaldehyde in PBS, and stored at 4°C until flow cytometry analysis. All Abs were purchased from BD Biosciences and were listed in our previous work [15, 20]. For each experiment, unstained and all single-color controls were processed to allow proper compensation as well as all fluorescence-minus-one controls to determine proper population gates. Each analysis was gated on low forward and side scatter lymphocytes (FSC/SSC), EMA-, CD3+, and high CD8+ population to allow the collection of 25,000–50,000 CD8+ events (>106 total events). Data were displayed as two-color or density dot plots to measure the proportion of the single-positive or double-positive cells in the highly CD3+CD8+population. Bioexponential display was also used to show each population in its entirety.
Samples collected from each macaque before DNA injection were used to establish the threshold that helps to evaluate the random noise for PBMCs from each animal that were distinguished from the true proliferation values specific to the response against HIV antigens. The threshold of unspecific proliferative response was determined based on the analysis of data from samples cultured in medium for 5 days and then restimulated with HIV Ags for 6 h as well as samples cultured with HIV Ags for 5 days and then restimulated with HIV Ags for an additional 6 h from unvaccinated animals. The threshold of unspecific proliferative response was found to be 0.25 %, and that of unspecific IFN-γ secretion was 0.01 %. In our conditions, Ag-specific responses above these thresholds were considered positive.
In addition, at each time point, samples of cells from vaccinated animal were incubated with the medium alone (for 5 days) and then HIV Ags (for 6 h) and were used as internal controls to determine the background responses. The values of these background responses were then subtracted from those of measured Ag-specific proliferative responses.
Results
Construction of CAL-Δ4-SHIVku2 DNA
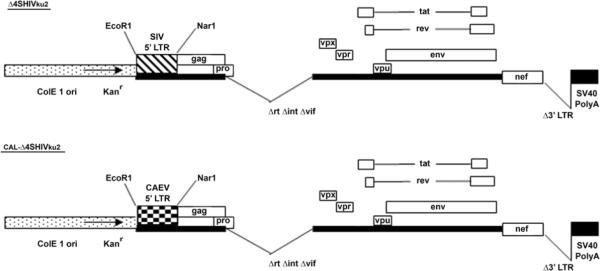
The schematic representation of the genomes of Δ4SHIVku2 and its derivative CAL-Δ4SHIVku2 is reported in Figure 1. Both genomes lacked the viral genes that encode RT, IN and Vif proteins. In addition the 3'LTR was replaced with the SV40 PolyA sequences. Therefore both constructs were deficient for replication and integration of the provirus. CAL-Δ4SHIVku2 genome lacked the SIV 5'LTR that was replaced with the 5'LTR of CAEV.
Figure 1.
Design of Δ4SHIVku2 and CAL-Δ4SHIVku2 DNA vaccine genomes.
CAL-Δ4SHIVku2 DNA was derived from Δ4SHIVku2 by removing the SIV 5' LTR sequences and ligating the CAEV 5' LTR. Oligonucleotide primers specific to the extremities of CAEV LTR were used to amplify the 0.45 kb PCR product from the molecular clone of CAEV. The resulting fragment was double digested with EcoR1 and Nar1 restriction enzymes and then used for ligation with the gel-purified EcoR1-Nar1-digested 9 kb fragment from Δ4SHIVku2. The resultant molecular construct was named CAL-Δ4SHIVku2.
Evaluation of viral protein expression in vitro
In order to evaluate the efficacy of protein expression, we first examined by ELISA the amount of Gag p24 antigen that was released by HEK-293T transfected cells with each of the two DNA vaccine constructs. Culture supernatants fluids were harvested at 14 h and 24 h following transfection. As shown in Fig. 2A both DNA vaccines produced similar amount of Gag p24 protein secreted at the two time points. In addition we evaluated the viral protein profiles in transfected HEK-293T cells by using an anti-SHIV monkey serum for the radioimmunoprecipitation assay of 35S-methionine labeled proteins. Results reported in Fig. 2B clearly show no substantial difference of viral proteins profiles both in the cell lysates and the supernatant fluids indicating that Δ4SHIVku2 or CAL-Δ4SHIVku2 vaccine constructs do express HIV viral proteins with similar efficiency.
Figure 2.
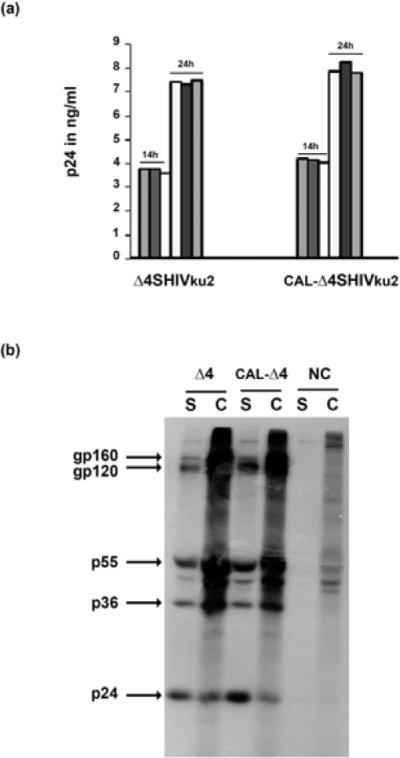
Viral protein expression in transfected HEK 293T cells
(A) Culture medium samples of cells transfected with HEK 293T with Δ4SHIVku2 and CAL-Δ4SHIVku2 constructs were harvested at 14 h and 24 h post-transfection and examined by ELISA for detection of HIV Gag p24. Triplicate measurements were performed and the results are representative of two independent experiments.
(B) After forty-eight hours, proteins of transfected HEK 293T cells were labeled with 35S-methionine and then viral proteins were immunoprecipitated from the cell lysate (C) and the supernatant fluid (S) with hyperimmune monkey anti-SHIV plasma. Sizes of the major proteins are indicated in kDa.
HIV specific -T cell responses in mice immunized with CAL-Δ-SHIVku2
We previously reported that 200 μg of the Δ4-SHIVku2 DNA injected in the gastrocnemius muscles of mice induced specific CD8+ T cell responses against all the HIV proteins expressed by the HIV DNA vaccine. The peak of these primary responses was within 2–4 weeks post-immunization [15]. In this study, we used the same conditions to determine whether the replacement of the SIV LTR by the CAEV LTR in the genome of Δ4-SHIVku2 construct to create CAL- Δ4-SHIVku2, had any effect on vaccine-induced T cells responses against expressed HIV antigens in injected mice. We first evaluated the IFN-γ ELISPOT responses within 2 to 4 weeks post-immunization. As shown in Fig. 3, at 2 weeks post-inoculation, mice immunized with CAL-Δ4-SHIVku2 developed IFN-γ-secreting splenocyte responses against Gag, Env, TRN (Tat+Rev+Nef), similar to those of mice immunized with Δ4-SHIVku2 DNA in injected mice.
Figure 3. IFN-γ ELISPOT responses in mice immunized with Δ4SHIVku2 and CAL-Δ4SHIVku2 DNA vaccines.
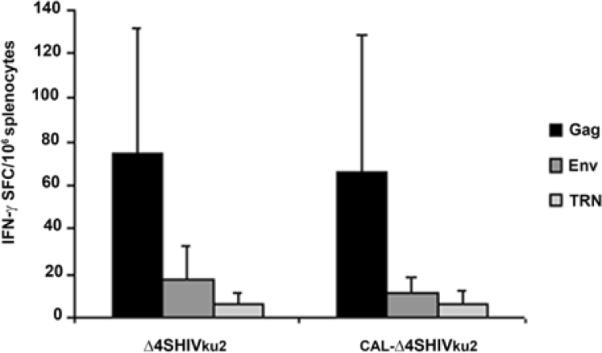
At two weeks post-immunization of BALB/c mice with a single dose of 200 μg of CAL-Δ4-SHIVku2 or Δ4-SHIVku2 DNA vaccine, splenocytes were isolated and examined for IFN-γ ELISPOT responses using pools of Gag, Env, TRN (Tat+Rev and Nef combined) HIV peptides. The data show the mean of the measurements and the standard deviation (represented by the errors bars) obtained by 5 immunized animals in each group. SFC: spot-forming cells. No significant difference was observed between the two different DNA vaccines.
To further examine the profile of the vaccine-induced immune responses, we used multiparametric flow cytometry to analyze antigen-specific proliferation (CFSE dilution) and IFN-γ secretion by T cells in response to HIV antigens. As shown in Figure 4, 0.3 % to 0.8 % of CD3+ CD8+ T cells produced IFN-γ and 0.9 % to 4 % underwent proliferation with Gag, Env and TRN peptides. Similarly to our previously reported data using the mouse model, the vast majority of the proliferating T cells did not produce detectable IFN-γ upon restimulation.
Figure 4. HIV-specific IFN-γ producing and proliferating CD8+ T cells in mice immunized with CAL-Δ4SHIVku2 DNA vaccine.
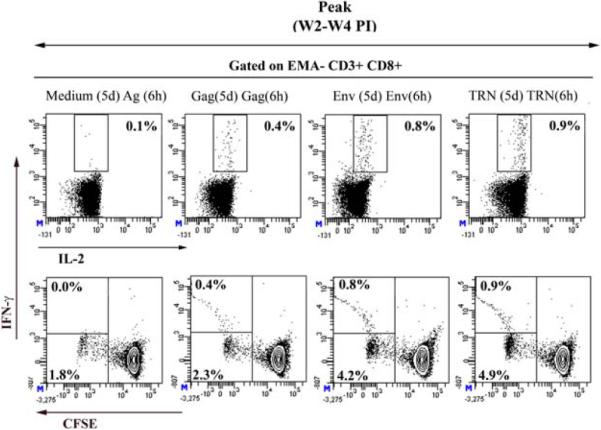
Pools of splenocytes from 3 mice immunized with a single dose of 200 μg of CAL-Δ4-SHIVku2 at two to four weeks post-immunization were used to detect and evaluate specific CD8+ T cell responses to HIV antigens. CFSE-labeled splenocytes were cultured for 5 days with specific HIV peptides (Gag, Env, TRN) or in absence of antigens (medium only). After 5 days, cells were harvested and restimulated for 6h with indicated antigens (Medium or Ag (5d) Ag (6h)) in the presence of co-stimulatory antibodies and Brefeldin A. Restimulated cells were surface labeled with anti-CD3, CD8, -CD4 antibodies in presence of EMA (for exclusion of dead cells) and subsequently fixed, permeabilized and stained with anti-IFN-γ and anti-IL-2 mAbs. Cells were gated on low FSC/SSC, EMA-, CD3+ and high CD8+ population and data were displayed as two color dot plots (IFN-γ vs IL-2 (upper row) and IFN-γ vs CFSE (lower row)). Frequencies of IFN-γ producing cells (upper number in each plot) and those of cells proliferating only (lower number in low row plots) are indicated. Results are representative of 2–3 independent experiments.
Thus immunization of mice with CAL- Δ4-SHIVku2 induced HIV-specific CD8+ T cell responses that qualitatively and quantitatively resembled the responses induced by the original Δ4-SHIVku2 DNA vaccine when injected in mice.
HIV specific -T cell responses in macaques immunized with CAL-Δ4-SHIVku2 DNA
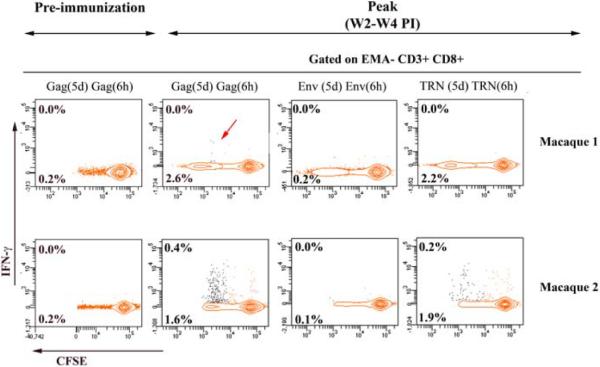
To further characterize the vaccine-induced T cell responses, we immunized two rhesus macaques with a single dose of 30 mg of CAL-Δ4-SHIVku2 using conditions similar to our previous immunization macaque study with Δ4-SHIVku2 DNA vaccine [20]. Using multiparametric flow cytometry-based assay the data shown in Fig. 5 demonstrate that both immunized animals developed HIV-specific CD3+ CD8+ T cells (1.9 % to 2 % of total CD3+ CD8+ T cells) that vigorously proliferated, but only 0.2 % of these cells produced IFN-γ in response to TRN mix of peptides. Similar responses were measured against Gag showing 1.8 % to 2.4 % of proliferative CD3+ CD8+ T cells with only up to 0.4 % IFN-γ-producing cells. No response against Env was detected at this early time-point but this response became detectable in the peripheral blood of these two macaques at a later time point (around 5 weeks) (data not shown).
Figure 5. HIV-specific IFN-γ producing and proliferating CD8+ T cells in macaque immunized with CAL-Δ4SHIVku2 DNA vaccine.
Macaque 1 and 2 were immunized with a single dose of 30 mg of CAL-Δ4SHIVku2 DNA vaccine. At the indicated pre-immunization and post-immunization times, PBMCs were collected, labeled with CFSE, cultured, restimulated and stained using the same procedure as that described for mouse in Fig. 4. The proportion of cells producing IFN-γ contour plot, upper number) and proliferating (CFSE dilution; contour plot, lower number) in response to specific HIV antigens are presented for the two macaques at the pre-immunization time point and at the peak of the primary vaccine induced-immune response (between 2 and 4 weeks post-immunization, PI). A red arrow is pointing the discrete proportion of cells producing IFN-γ and proliferating in response to Gag antigen in the macaque 1.
Discussion
The aim of the present study was to evaluate whether the replacement of the SIV 5'LTR with that of the naturally attenuated goat lentivirus (CAEV), in the genome of our vaccine Δ4SHIVku2 generates an HIV DNA vaccine that efficiently expresses the viral proteins and induces potent immune responses in animal models of HIV vaccines. Data presented clearly show that the new DNA vaccine driven by the 5'LTR of CAEV efficiently expresses all viral proteins included in the vaccine genome showing identical viral protein profiles expressed by the parental Δ4SHIVku2 and the new CAL-Δ4-SHIVku2 HIV DNA vaccines. Importantly, this new vaccine also induced CD8+ T cell responses against Gag, Env and TRN antigens comparable to those induced by the parental vaccine both in the mouse and macaque models used in this study.
Several DNA vaccine constructs encoding HIV proteins under a strong enhancer/promoter such as the human cytomegalovirus (CMV) have been developed and used, but none of the vaccination regimens using these DNA alone has yet induced potent immune responses associated with complete protection in absence of boost with recombinant vectors expressing viral proteins [26]. However, live attenuated vaccines have been shown to be the most effective vaccines against AIDS in non-human primate models [9]. These vaccines mimic natural infection and induction of immune responses by the host but they are not considered to be safe because of the associated risk of reversion to pathogenic virus. To maintain a similar viral gene expression to that of a live attenuated vaccine we and others have developed replication defective vaccines, Δ4SHIVku2 [18, 21] and pNL432-ZF1 [27] by introducing mutations/deletions into the full infectious viral genome. Plasmid DNA of these vectors produces non-infectious particles following expression of viral genes under control of the 5' HIV and SIV LTRs. In contrast to CMV driven vaccine constructs, injection of these LTR driven vaccines given alone induced immune responses that were protective against pathogenic challenge with homologous virus [21, 28]. To improve this vaccine regimen, Akahata and al, replaced the HIV LTR by the SIV LTR in their vaccine and reported better CTL induction in vaccinated macaques [28]. This increase of CTL is probably due to the higher antigen expression under control of the SIV LTR promoter in the simian host cells. To maintain the lentiviral composition and genome organization in our vaccine but also to include the constitutive expression of the viral genes, we choose to replace the SIV LTR by the CAEV LTR. We selected CAEV because it is a natural lentivirus of goat that is related to HIV but has no history of causing AIDS-like disease in his host. Unlike HIV/SIV, we have previously shown that the LTR promoter is highly active in a variety of human cells [29] and this promoter is Tat-independent, therefore constitutively expressing the controlled genes [24, 30]. The CMV promoter is well known to promote constitutive gene expression and has been shown to drive the production of infectious particles when used in replacement of the U3 region in the 5' HIV LTR. However, despite a constitutive and high expression efficacy, this promoter was associated with production of lower infectious titers than the wild-type virus genome [31], suggesting that this type of promoter is not sufficient to produce high yield of viral proteins that are efficiently assembled into infectious particles. Indeed, other studies have shown that the regulation of alternative splicing by viral LTRs result in accumulation of a balanced proportion of unspliced and unispliced RNA that are used for expression of structural genes of the virus and packaging of the genomic RNA into newly formed particles. In contrast, the replacement of the LTR promoter with the CMV promoter leads to excessive accumulation of multiply spliced viral RNA genome [31]. This regulation via alternative splicing process is used by the virus to maintain balanced gene expression [32]. Thus the conservation of a 5' LTR in a HIV DNA vaccine construct is important not only for efficient transcription of RNA genomes but also for the efficiency and specificity of post-transcriptional processes to closely mimic the dynamic and regulation of a live vaccine virus replication. However, there might be some reluctance to use plasmid DNAs bearing the full lentiviral genome as vaccines because of the potential reversion or recombination that may lead to emergence of pathogenic phenotype of virus. Interestingly, the use of CAEV LTR introduces a novel level of safety. Previous studies have established that HIV and SIV integrases (IN) are highly specific to their “att” sequences located in the terminal regions of U3 and U5 sequences [33]. Furthermore it has been demonstrated that the recombinant IN protein of CAEV does not allow in vitro integration of HIV LTR DNA [34, 35]. From these data we hypothesized that a SHIV genome, in which the SIV LTRs are replaced with those of CAEV, and used as DNA vaccine, will generate an integration-deficient viral genome but will retain the capacities to undergo one cycle of replication during which viral proteins will be expressed from the episomal unintegrated DNA.
Non-integrating lentiviral vectors, pseudotyped or trans-complemented with vesicular stomatitis virus glycoprotein have been recently used in a prime/boost protocol in NHP model and shown to promote strong control of early viral replication against a massive challenge with the highly pathogenic SIVmac251 and SIVmac239 [36, 37]. These results provided a strong evidence of the potential of this type of lentiviral vector system for vaccination. However, the structural organization of viral genome was fully remodeled so that they do not mimic virus infection and replication.
In conclusion, our results provide the demonstration that the replacement of SIV 5'LTR by the Tat-independent CAEV 5'LTR in the genome of our HIV DNA vaccine promotes efficient viral protein expression that is associated with successful priming of HIV-specific CD8+ T cell responses both in mice and macaque models. This study represents a first step in our vaccine design strategy whose main objective is to develop an integration-defective, replication limited (single cycle) HIV vaccine using the CAEV LTRs in replacement of SIV LTRs in our original infectious molecular clone, SHIVKU2. This vector will retain all the beneficial characteristics of a live attenuated vaccine without its disadvantages namely integration and persistence associated with the potential of reversion to a pathogenic phenotype.
Highlights
We used the LTR of the attenuated goat CAEV to express HIV gens in a DNA vaccine.
This vaccine expresses efficiently all driven genes in transfected cells.
This vaccine induces potent T cell responses in mice and macaques.
This vaccine is safer since this LTR does not integrate with HIV integrase.
Acknowledgements
We thank the National Institutes of Health AIDS research and Reference Reagent Program for providing HIV overlapping Gag, Env, Tat, Rev and Nef peptides (catalog# 8117, 6451, 5138, 6445, and 5189 respectively)
This work was supported by grants 2P20 RR016443-07 and R01 AI062340-04 from the National Institutes of Health and funding from INRA, Department of Animal Health.
Abbreviations
- CAEV
(Caprine Arthritis Encephalitis Virus)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006 Jun 15;107(12):4781–9. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Migueles SA, Laborico AC, Shupert WL, Sabbaghian MS, Rabin R, Hallahan CW, et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol. 2002 Nov;3(11):1061–8. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- [3].Theze J, Chakrabarti LA, Vingert B, Porichis F, Kaufmann DE. HIV controllers: a multifactorial phenotype of spontaneous viral suppression. Clin Immunol. 2011 Oct;141(1):15–30. doi: 10.1016/j.clim.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Casartelli N, Di Matteo G, Argentini C, Cancrini C, Bernardi S, Castelli G, et al. Structural defects and variations in the HIV-1 nef gene from rapid, slow and non-progressor children. Aids. 2003 Jun 13;17(9):1291–301. doi: 10.1097/00002030-200306130-00003. [DOI] [PubMed] [Google Scholar]
- [5].Saksena NK, Ge YC, Wang B, Xiang SH, Dwyer DE, Randle C, et al. An HIV-1 infected long-term non-progressor (LTNP): molecular analysis of HIV-1 strains in the vpr and nef genes. Ann Acad Med Singapore. 1996 Nov;25(6):848–54. [PubMed] [Google Scholar]
- [6].Schwartz DH, Viscidi R, Laeyendecker O, Song H, Ray SC, Michael N. Predominance of defective proviral sequences in an HIV + long-term non-progressor. Immunol Lett. 1996 Jun;51(1–2):3–6. doi: 10.1016/0165-2478(96)02547-3. [DOI] [PubMed] [Google Scholar]
- [7].Trible RP, Emert-Sedlak L, Wales TE, Ayyavoo V, Engen JR, Smithgall TE. Allosteric loss-of-function mutations in HIV-1 Nef from a long-term non-progressor. J Mol Biol. 2007 Nov 16;374(1):121–9. doi: 10.1016/j.jmb.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Genesca M, McChesney MB, Miller CJ. Antiviral CD8+ T cells in the genital tract control viral replication and delay progression to AIDS after vaginal SIV challenge in rhesus macaques immunized with virulence attenuated SHIV 89.6. J Intern Med. 2009 Jan;265(1):67–77. doi: 10.1111/j.1365-2796.2008.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Reynolds MR, Weiler AM, Weisgrau KL, Piaskowski SM, Furlott JR, Weinfurter JT, et al. Macaques vaccinated with live-attenuated SIV control replication of heterologous virus. J Exp Med. 2008 Oct 27;205(11):2537–50. doi: 10.1084/jem.20081524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yankee TM, Sheffer D, Liu Z, Dhillon S, Jia F, Chebloune Y, et al. Longitudinal study to assess the safety and efficacy of a live-attenuated SHIV vaccine in long term immunized rhesus macaques. Virology. 2009 Jan 5;383(1):103–11. doi: 10.1016/j.virol.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Baba TW, Jeong YS, Pennick D, Bronson R, Greene MF, Ruprecht RM. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science. 1995 Mar 24;267(5205):1820–5. doi: 10.1126/science.7892606. [DOI] [PubMed] [Google Scholar]
- [12].Baba TW, Liska V, Khimani AH, Ray NB, Dailey PJ, Penninck D, et al. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat Med. 1999 Feb;5(2):194–203. doi: 10.1038/5557. [DOI] [PubMed] [Google Scholar]
- [13].Desrosiers RC. Safety issues facing development of a live-attenuated, multiply deleted HIV-1 vaccine. AIDS Res Hum Retroviruses. 1994 Apr;10(4):331–2. doi: 10.1089/aid.1994.10.331. [DOI] [PubMed] [Google Scholar]
- [14].Hofmann-Lehmann R, Vlasak J, Williams AL, Chenine AL, McClure HM, Anderson DC, et al. Live attenuated, nef-deleted SIV is pathogenic in most adult macaques after prolonged observation. Aids. 2003 Jan 24;17(2):157–66. doi: 10.1097/00002030-200301240-00004. [DOI] [PubMed] [Google Scholar]
- [15].Arrode G, Hegde R, Mani A, Jin Y, Chebloune Y, Narayan O. Phenotypic and functional analysis of immune CD8+ T cell responses induced by a single injection of a HIV DNA vaccine in mice. J Immunol. 2007 Feb 15;178(4):2318–27. doi: 10.4049/jimmunol.178.4.2318. [DOI] [PubMed] [Google Scholar]
- [16].Barnett SW, Rajasekar S, Legg H, Doe B, Fuller DH, Haynes JR, et al. Vaccination with HIV-1 gp120 DNA induces immune responses that are boosted by a recombinant gp120 protein subunit. Vaccine. 1997 Jun;15(8):869–73. doi: 10.1016/s0264-410x(96)00264-2. [DOI] [PubMed] [Google Scholar]
- [17].Hanke T, Neumann VC, Blanchard TJ, Sweeney P, Hill AV, Smith GL, et al. Effective induction of HIV-specific CTL by multi-epitope using gene gun in a combined vaccination regime. Vaccine. 1999 Feb 12;17(6):589–96. doi: 10.1016/s0264-410x(98)00238-2. [DOI] [PubMed] [Google Scholar]
- [18].Hegde R, Liu Z, Mackay G, Smith M, Chebloune Y, Narayan O, et al. Antigen expression kinetics and immune responses of mice immunized with noninfectious simian-human immunodeficiency virus DNA. J Virol. 2005 Dec;79(23):14688–97. doi: 10.1128/JVI.79.23.14688-14697.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Letvin NL, Montefiori DC, Yasutomi Y, Perry HC, Davies ME, Lekutis C, et al. Potent, protective anti-HIV immune responses generated by bimodal HIV envelope DNA plus protein vaccination. Proc Natl Acad Sci U S A. 1997 Aug 19;94(17):9378–83. doi: 10.1073/pnas.94.17.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Arrode-Bruses G, Sheffer D, Hegde R, Dhillon S, Liu Z, Villinger F, et al. Characterization of T-cell responses in macaques immunized with a single dose of HIV DNA vaccine. J Virol. 2010 Feb;84(3):1243–53. doi: 10.1128/JVI.01846-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Singh DK, Liu Z, Sheffer D, Mackay GA, Smith M, Dhillon S, et al. A noninfectious simian/human immunodeficiency virus DNA vaccine that protects macaques against AIDS. J Virol. 2005 Mar;79(6):3419–28. doi: 10.1128/JVI.79.6.3419-3428.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Trono D, Van Lint C, Rouzioux C, Verdin E, Barre-Sinoussi F, Chun TW, et al. HIV persistence and the prospect of long-term drug-free remissions for HIV-infected individuals. Science. 2010 Jul 9;329(5988):174–80. doi: 10.1126/science.1191047. [DOI] [PubMed] [Google Scholar]
- [23].Liu Z, Singh DK, Sheffer D, Smith MS, Dhillon S, Chebloune Y, et al. Immunoprophylaxis against AIDS in macaques with a lentiviral DNA vaccine. Virology. 2006 Aug 1;351(2):444–54. doi: 10.1016/j.virol.2006.03.033. [DOI] [PubMed] [Google Scholar]
- [24].Villet S, Faure C, Bouzar BA, Morin T, Verdier G, Chebloune Y, et al. Lack of trans-activation function for Maedi Visna virus and Caprine arthritis encephalitis virus Tat proteins. Virology. 2003 Mar 15;307(2):317–27. doi: 10.1016/s0042-6822(02)00076-4. [DOI] [PubMed] [Google Scholar]
- [25].Arrode G, Hegde R, Jin Y, Singh DK, Narayan O, Chebloune Y. Nef modulates the immunogenicity of Gag encoded in a non-infectious HIV DNA vaccine. Vaccine. 2008 Jul 23;26(31):3795–804. doi: 10.1016/j.vaccine.2008.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat Rev Genet. 2008 Oct;9(10):776–88. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Akahata W, Ido E, Shimada T, Katsuyama K, Yamamoto H, Uesaka H, et al. DNA vaccination of macaques by a full genome HIV-1 plasmid which produces noninfectious virus particles. Virology. 2000 Sep 15;275(1):116–24. doi: 10.1006/viro.2000.0486. [DOI] [PubMed] [Google Scholar]
- [28].Akahata W, Ido E, Akiyama H, Uesaka H, Enose Y, Horiuchi R, et al. DNA vaccination of macaques by a full-genome simian/human immunodeficiency virus type 1 plasmid chimera that produces non-infectious virus particles. J Gen Virol. 2003 Aug;84(Pt 8):2237–44. doi: 10.1099/vir.0.19082-0. [DOI] [PubMed] [Google Scholar]
- [29].Mselli-Lakhal L, Favier C, Leung K, Guiguen F, Grezel D, Miossec P, et al. Lack of functional receptors is the only barrier that prevents caprine arthritis-encephalitis virus from infecting human cells. J Virol. 2000 Sep;74(18):8343–8. doi: 10.1128/jvi.74.18.8343-8348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Villet S, Bouzar BA, Morin T, Verdier G, Legras C, Chebloune Y. Maedi-visna virus and caprine arthritis encephalitis virus genomes encode a Vpr-like but no Tat protein. J Virol. 2003 Sep;77(17):9632–8. doi: 10.1128/JVI.77.17.9632-9638.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bohne J, Krausslich HG. Mutation of the major 5' splice site renders a CMV-driven HIV-1 proviral clone Tat-dependent: connections between transcription and splicing. FEBS Lett. 2004 Apr 9;563(1–3):113–8. doi: 10.1016/S0014-5793(04)00277-7. [DOI] [PubMed] [Google Scholar]
- [32].Bohne J, Schambach A, Zychlinski D. New way of regulating alternative splicing in retroviruses: the promoter makes a difference. J Virol. 2007 Apr;81(7):3652–6. doi: 10.1128/JVI.02105-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Masuda T, Kuroda MJ, Harada S. Specific and independent recognition of U3 and U5 att sites by human immunodeficiency virus type 1 integrase in vivo. J Virol. 1998 Oct;72(10):8396–402. doi: 10.1128/jvi.72.10.8396-8402.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Berger N, Heller AE, Stormann KD, Pfaff E. Characterization of chimeric enzymes between caprine arthritis--encephalitis virus, maedi--visna virus and human immunodeficiency virus type 1 integrases expressed in Escherichia coli. J Gen Virol. 2001 Jan;82(Pt 1):139–48. doi: 10.1099/0022-1317-82-1-139. [DOI] [PubMed] [Google Scholar]
- [35].Stormann KD, Schlecht MC, Pfaff E. Comparative studies of bacterially expressed integrase proteins of caprine arthritis-encephalitis virus, maedi-visna virus and human immunodeficiency virus type 1. J Gen Virol. 1995 Jul;76(Pt 7):1651–63. doi: 10.1099/0022-1317-76-7-1651. [DOI] [PubMed] [Google Scholar]
- [36].Beignon AS, Mollier K, Liard C, Coutant F, Munier S, Riviere J, et al. Lentiviral vector-based prime/boost vaccination against AIDS: pilot study shows protection against Simian immunodeficiency virus SIVmac251 challenge in macaques. J Virol. 2009 Nov;83(21):10963–74. doi: 10.1128/JVI.01284-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jia B, Ng SK, DeGottardi MQ, Piatak M, Yuste E, Carville A, et al. Immunization with single-cycle SIV significantly reduces viral loads after an intravenous challenge with SIV(mac)239. PLoS Pathog. 2009 Jan;5(1):e1000272. doi: 10.1371/journal.ppat.1000272. [DOI] [PMC free article] [PubMed] [Google Scholar]